Abstract
A preperitoneal abscess is an uncommon manifestation of extraperitoneal collection. We present a case of an anterior abdominal wall preperitoneal abscess in a 30-year-old Nigerian female with abdominal pain and purulent abdominal wall discharge ten days after an initial admission for spontaneous bacterial peritonitis. This report underscores the role of ultrasound in diagnosis and follow-up and percutaneous ultrasound-guided continuous percutaneous catheter drainage and management of an extraperitoneal abscess.
Keywords: Pre-peritoneal abscess, Ultrasound-guided, Continuous drainage
INTRODUCTION
Preperitoneal space is an uncommon site in the extraperitoneal space for an abscess to develop. Abscess in the extraperitoneal space can result from an infectious, inflammatory, traumatic, or neoplastic process1, 2.
While contrast-enhanced computed tomographic examination with gastrointestinal tract contrast administration will depict excellently intraabdominal abscess in any abdominal compartment3, ultrasonography has evolved as an ionizing radiation-free, accurate, cheap, and repeatable imaging modality in intraabdominal abscess management.3 We reported this case to emphasize the need to have a high index of suspicion for the diagnosis of preperitoneal abscess using an ultrasound scan and emphasize the benefit of ultrasound-guided percutaneous catheter drainage of the abscess using the Seldinger technique.
CASE REPORT
A 30-year-old Nigerian female initially presented with progressive abdominal swelling and pain of a one-month duration with associated intermittent high-grade fever and yellowness of eyes of 5 days before presentation. Abdominal pain was insidious in onset, dull, and generalized but worse at the right upper quadrant of the abdomen, non-radiating, and no aggravating or relieving factor was noted by the patient. There were associated episodes of vomiting and loose stools three days before presentation. She had scarification marks on her abdomen and visited a private hospital from where she was referred to our hospital for expert management. Past medical history was insignificant; she was not a known hypertensive, diabetic, asthmatic, or peptic ulcer patient. She was admitted and managed for spontaneous bacteria peritonitis with background chronic liver disease. She was also transfused with two units of blood due to severe anaemia (Haematocrit then was 16%). She had intravenous rocephin. metronidazole, omeprazole, ringers’ lactate, intravenous febramol, intramuscular pentazocine, and significantly improved, and she was discharged home in a stable condition. She was readmitted during her first follow-up visit at the medical outpatient clinic, ten days after being discharged from the hospital, because of a 2-day history of abdominal pain with associated purulent discharge from the anterior abdominal wall. The purulent discharge was from her supraumbilical region. There was no associated fever, vomiting, or constipation.
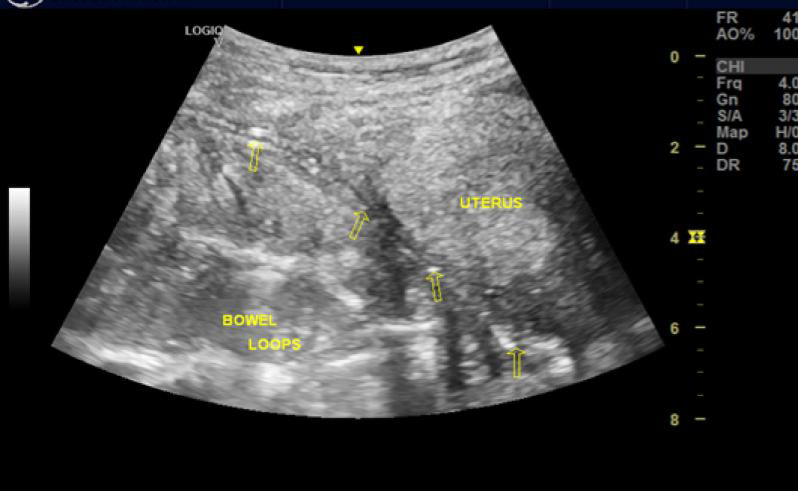
Clinical examination at the follow-up visit revealed a chronically ill-looking, pale, febrile (Temperature = 39°C), and an anicteric young woman with tachycardia. The abdomen was full, moved with respiration but had a supraumbilical sinus draining foul-smelling purulent fluid. There was generalized abdominal tenderness and hepatomegaly. The respiratory and central nervous system examination findings were not contributory. Some laboratory tests were not done due to a lack of funds, and social workers were called in to help out. A clinical diagnosis of the intraabdominal abscess was made, she was referred to the interventional radiology unit to confirm the diagnosis and possible image-guided drainage of the suspected intra- abdominal abscess. Abdomino-pelvic ultrasound scan, however, revealed extensive low-level echo collections with internal echogenic foci in the preperitoneal layer of the abdominal wall, worse in the left quadrants, in keeping with gas-forming infection of this abdominal wall layer [Figure 1]. Furthermore, the abscess’s maximum depth (anteroposterior) was measured at 14.3 mm in the left lumbar region [Figure 1], and the collection extends inferiorly over the dome of the distended urinary bladder and the retroverted uterus. Besides, an infra umbilical herniation of small bowel loops into the transversalis fascial plane of the abdominal wall was noted in the suprapubic region. The visualized bowel loops, however, showed normal caliber and peristalsis.
Figure 1:
Sonogram showing pre-peritoneum hypoechoic collection with echogenic foci from gas forming organism, in keeping with preperitoneal abscess
Laboratory Test Results
The patient’s haematocrit was 25%. The white blood cell count was 9,600/µl, lymphocytes were 54% (increased count), and neutrophils were 39%. Her serum was non-reactive for Human Immunodeficiency Virus (HIV I and II). In preparation for percutaneous drainage, the clotting profile was normal, the international normalized ratio was 1.01, and the prothrombin time was 13.1s. Unfortunately, C-reactive protein, serial white cell count, and anaerobic blood culture could not be done due to limitations of funds, as the patient was not on the National health insurance scheme and has to pay out of pocket.
Percutaneous Ultrasound-guided Drainage
She had ultrasound-guided drainage of the extraperitoneal abscess with local analgesia using a Mindray Diagnostic Ultrasound (Model: DC-30, manufactured by Mindray Bio-medical electronic Company Limited, PR China, Year 2019-03) with a high frequency (7.5MHz) linear array transducer. The largest pool of abscess collection was noted in the left lumbar region, and site marking was done. Under the aseptic technique, the anterior abdominal wall over the left lumbar region was infiltrated with 2% lidocaine down to the capsule of the extraperitoneal abscess. Using the Seldinger technique, with an 18-gauge trocar needle, the abscess cavity was punctured. A specimen of frank pus was aspirated for microscopy, culture, and sensitivity (MCS). A 0.035-inch guidewire was inserted, guided by ultrasound (Figure 2). After gradual serial dilatation of the track with dilators, a size 10- French pigtail drainage catheter with side holes was passed over the guidewire into the cavity [Figure 3a and 3b]. The catheter was connected to an external drainage bag and sutured to the skin for continuous drainage. The procedure lasted approximately 30 minutes and was well tolerated. She had intravenous ceftriaxone for 48 hours and afterward had intravenous ciprofloxacin and metronidazole, pending the result of the aspirate MCS.
Figure 2:
Sonogram depicting a linear echogenic structure in keeping with an 18-guage trochar needle with the needle-tip on the wall of the preperitoneal abscess.
Figure 3a:
Longitudinal paramedian B-mode ultrasound (US) in the suprapubic region shows residual collection in preperitoneal space with catheter seen in the longitudinal view in-situ.
Figure 3b:
Midline Sagittal pelvic US shows residual abscess collection in preperitoneal space extending over the fundal aspect of the retroverted uterus. The urinary bladder was poorly distended.
Daily irrigation of the cavity was done using 10 ml of saline, and the drainage was monitored. In addition, serial post-procedure ultrasound scans were done, which showed a progressive reduction in the abscess volume. The patient also had an ultrasound-guided adjustment of the pigtail drain to facilitate complete drainage of the abscess. By the 17th day post-procedure, the drainage had become significantly minimal, with a follow-up scan showing a near-complete resolution of the abscess. The catheter was thereafter removed under aseptic conditions (Figure 4).
Figure 4:
Picture showing the percutaneous drainage tube in-situ at removal.
Altogether, 629 ml of pus (including fluid that could not be completely withdrawn at irrigation) was drained. The pus Aspirate M/C/S result showed gram-negative Bacilli, puss cells ++, culture yielded moderate Klebsiella pneumoniae, resistant to ampicillin/sulbactam, cefuroxime, ceftriaxone, levofluxacin but sensitive to amikacin, piperacilin/tazocatam.
Due to financial constraints, she was continued on ciprofloxacin and metronidazole with the resolution of the pre-peritoneal abscess confirmed on ultrasonography. She was discharged on oral ciprofloxacin and metronidazole and haematinics (oral multivite, folate, vitamin C, and vitamin B complex) and was on antibiotics for about four weeks in total. A Follow-up ultrasound scan revealed no recurrence of the collection two months after discharge. At discharge from the interventional Radiology unit, her PCV was 29%. However, a repeat FBC and WBC could not be done before discharge due to the aforementioned financial constraints.
DISCUSSION
Extraperitoneal space is a potential space that surrounds the peritoneal cavity, being defined by the parietal peritoneum internally and by transversalis fascia externally. It comprises the retroperitoneum, posteriorly, and the less clinically significant preperitoneum, including the peri-vesical and the larger pre-vesical spaces, anteriorly4. Extraperitoneal tissues do not react acutely to bacterial contamination, as does the peritoneal cavity5. This may result in delayed diagnosis of an extraperitoneal abscess for days after admission. The aforementioned reason might be the case in this patient who presented with purulent discharge from the abdominal wall ten days after being discharged home following initial treatment for spontaneous bacterial peritonitis. Unfortunately, laboratory blood tests that could have suggested bacterial infection and Klebsiella infection were not done due to fund availability.
An extraperitoneal abscess can result from various infectious, inflammatory, neoplastic, or traumatic processes1, 2. Cases of preperitoneal abscess originating from ischiorectal infection6-9 or complicating a retained appendicolith have been reported though uncommon10. The abscess in this index case might be a consequence of sepsis, for which the patient was initially treated. However, the history of scarifications on the abdomen mostly carried out with non-sterilized sharp objects could also be a potential source.
Although Ultrasonography, CT, or MRI could easily be used to diagnose intraperitoneal abscesses, percutaneous drainages are commonly performed under US or CT guidance.11 While CT guided drainages are particularly useful in deep and posterior abscesses or those obscured by bowel gases on ultrasound, its drawbacks of not being truly dynamic, high ionization radiation exposure, larger numbers of staff needed, and difficulty in its use in uncooperative patient12 have made ultrasound-guided drainages a commonly used technique.11,12 Very few cases of MRI-guided percutaneous drainages have also been reported 13, but the need for compatible guidewires, high cost of MRI, little room for maneuver, and not being easily accessible in third world countries have limited its use.
Most of the reported cases of preperitoneal abscess were managed surgically with a lower midline incision, laparotomy, multiple stab incisions, or even by laparoscopy.7-10 However, percutaneous drainages are favored over surgical interventions.7 Generally, advantages of image-guided percutaneous drainages include the reliability of the procedures, no need for general anaesthesia, better tolerated with much lower morbidity and mortality compared with other surgical techniques14.
To the best of our knowledge, there is no published study on preperitoneal abscess occurring in an African patient, and which was treated by percutaneous catheter drainage. However, percutaneous catheter drainage and antibiotics are the standard treatment for many abdominal, and pelvic abscesses in the absence of immediate surgical indication as this avoids the risks of general anesthesia and complications that might result from surgery15-16.
CONCLUSION
Ultrasonography is a good imaging modality for the evaluation of pre-peritoneal abscess, particularly in low-income settings.
Ultrasound-guided percutaneous catheter drainage of the preperitoneal abscess is a safe, effective, and less invasive treatment option and eliminates the need for surgery in most of these cases.
REFERENCES
- 1.Tirkes T, Sandrasegaran K, Patel AA, et al. Peritoneal and retroperitoneal anatomy and its relevance for cross-sectional imaging. Radiographics. 2012;32(2): 437–451. doi: 10.1148/rg.322115032. [DOI] [PubMed] [Google Scholar]
- 2.Gore RM, Balfe DM, Aizenstein RI, Silverman PM. The great escape: interfascial decompression planes of the retroperitoneum. American Journal of Roentgenology. 2000; 175(2):363–370. doi: 10.2214/ajr.175.2.1750363. [DOI] [PubMed] [Google Scholar]
- 3. Saber AA, LaRaja RD. Abdominal abscess workup. emedicine. https://emedicine.medscape.com/ article/1979032-workup#c12 . [last accessed on 16 June 2021].
- 4.Horta M, Neto N, Couceiro C, Martins L. Extraperitoneal Space: Anatomic and Radiologic Overview. Eur Congr Radiol. 2014. Mar 6, [Last accessed on 16 June, 2021].
- 5. Meyers M. The Extraperitoneal Spaces: Normal and Physiologic Anatomy. In: Dynamic Radiology of the Abdomen: Normal and Pathologic Anatomy. 6th Edition. New York: Springer-Verlag ; 2005. [Google Scholar]
- 6.Hamza E, Saeed MF, Salem A, Mazin I. Extraperitoneal abscess originating from an ischiorectal abscess. BMJ Case Rep. 2017;2017:bcr2016218229. doi: 10.1136/bcr-2016-218229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mentzer CJ, Yon JR, King R, Warren JA. Complex perirectal abscess extending to the preperitoneum and space of retzius. GHS Proc. 2016;1(1):49–51. [Google Scholar]
- 8.Darlington CD, Anitha GF. A rare case of ischiorectal abscess presenting with extensive abdominal wall abscess. Int Surg J . 2016;3:963–4. [Google Scholar]
- 9.Tayaran A, Boccola MA, Vanyai J. Preperitoneal abscess secondary to a retained appendicolith: an uncommon complication in an uncommon location. ANZ journal of surgery. 2018; 88(6):648–649. doi: 10.1111/ans.13457. [DOI] [PubMed] [Google Scholar]
- 10.Das DK, Patra RK, Mishra S, Panigrahi SK. Ultrasound guided percutaneous catheter drainage of an appendicular perforation with large intraperitoneal abscess formation: an effective modality of management in selected cases. International Surgery Journal. 2019; 6(6):2219–2221. [Google Scholar]
- 11. Bulut MD, Yavuz A, Batur A, et al. Ultrasoundguided percutaneous treatment of abscess foci in different localizations of the body: Results of three year-experience. Eastern J Med. 2015; 20(4): 182–186. [Google Scholar]
- 12.Radiopedia. CT guided percutaneous drainage. https://radiopaedia.org/articles/ct-guidedpercutaneous-drainage?lang=gb- [ Last accessed 16, June, 2021].
- 13.Seyfer P, Jansen M, Mahnken AH. MR-Guided Percutaneous Abscess Drainage in Pregnancy. Journal of Vascular and Interventional Radiology. 2016;27(11):1767–1768. doi: 10.1016/j.jvir.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Akhan O, Durmaz H, Balc' S, et al. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020; 26:124–130. doi: 10.5152/dir.2019.19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abusedera MA, Khalil M, Ali AM, Hassan AE. Percutaneous image-guided aspiration versus catheter drainage of abdominal and pelvic collections. The Egyptian Journal of Radiology and Nuclear Medicine. 2013; 44(2):223–230. [Google Scholar]
- 16.Maher MM, Gervais DA, Kalra MK, et al. The inaccessible or undrainable abscess: how to drain it. Radiographics. 2004; 24(3):717–735. doi: 10.1148/rg.243035100. [DOI] [PubMed] [Google Scholar]