Abstract
Background
Intravenous (IV) golimumab, a TNFi, is approved for treating rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS). We analyzed pooled safety results from three phase 3 IV golimumab trials in these rheumatologic diseases and hypothesized that the safety profile of IV golimumab would be similar to that established for other TNFi, including subcutaneous golimumab.
Methods
Data from three double-blind, randomized trials of IV golimumab in patients with RA, PsA, and AS, each with a placebo-controlled period and an extension of active treatment, were included. Golimumab 2 mg/kg was administered at weeks 0 and 4, then every 8 weeks through week 100 (RA) or week 52 (PsA, AS). Concomitant low-dose, oral corticosteroids were permitted. Concomitant methotrexate was required in the RA trial and permitted in the PsA and AS trials; placebo patients crossed over to golimumab at weeks 24 (RA, PsA) and 16 (AS), respectively. Adverse events (AEs), including infections, serious infections, malignancies, and major adverse cardiovascular events (MACE), were assessed through week 112 (RA) or week 60 (PsA, AS).
Results
In total, 539 patients were randomized to placebo, and 740 patients were randomized to golimumab; 1248 patients received ≥ 1 golimumab administration. Among the placebo and golimumab patients, respectively, during the placebo-controlled periods, 40.6% and 50.3% had an AE, 2.4% and 3.8% had a serious AE, and 0.4% and 0.8% had a serious infection. Among all golimumab-treated patients, the numbers of events/100 patient-years (95% CI) were as follows: AEs, 175.2 (169.0, 181.6); serious AEs, 12.7 (11.0, 14.5); serious infections, 3.4 (2.5, 4.4); active tuberculosis, 0.4 (0.1, 0.8); opportunistic infection, 0.2 (0.1, 0.6); malignancies, 0.4 (0.2, 0.9), and MACE, 0.5 (0.2, 1.0). There were no cases of lymphoma. Three (0.6%) placebo-treated patients and 6 (0.5%) golimumab-treated patients died during the studies. Concomitant methotrexate was associated with increased occurrence of elevated alanine transaminase levels and lower incidence of antibodies to golimumab. During the placebo-controlled periods, serious infections in the placebo and golimumab groups were more common in patients receiving concomitant low-dose oral corticosteroids vs. those not receiving corticosteroids.
Conclusions
IV golimumab demonstrated a safety profile that was broadly consistent across these rheumatologic indications and with other TNFi, including subcutaneous golimumab. Concomitant methotrexate or corticosteroids were associated with an increase in specific AEs.
Trial registrations
ClinicalTrials.gov, NCT00973479. Registered on September 9, 2009. ClinicalTrials.gov, NCT02181673. Registered on July 4, 2014. ClinicalTrials.gov, NCT02186873. Registered on July 10, 2014.
Keywords: Intravenous golimumab, TNF inhibitor, Rheumatoid arthritis, Psoriatic arthritis, Ankylosing spondylitis, Safety, Adverse event
Introduction
Golimumab is a human monoclonal antibody that binds and inhibits the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα). Intravenous (IV) golimumab is approved for the treatment of rheumatoid arthritis (RA) (in combination with methotrexate [MTX]), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) [1]. Efficacy and safety of IV golimumab have been assessed in three double-blind, randomized, placebo-controlled, phase 3 trials of patients with RA (GO-FURTHER) [2–4], PsA (GO-VIBRANT) [5, 6], and AS (GO-ALIVE) [7, 8]. Patients with immune-mediated diseases such as RA, PsA, and AS are likely to require ongoing treatment. Thus, the long-term safety of treatments for these conditions is of particular interest for both health care providers and patients. Possible associations with the risk of serious infections, opportunistic infections, tuberculosis (TB), demyelinating disorders, and lymphoma have been reported for other TNF inhibitors (TNFi) [9–14].
Intravenous golimumab has demonstrated significantly greater improvements, compared with placebo, in signs and symptoms in patients with RA [2], PsA [5, 15], and AS [7]. In addition, IV golimumab treatment has slowed radiographic progression compared with placebo in patients with RA and PsA [3, 15]. Trial extensions of active treatment with golimumab demonstrated that improvements were maintained over time [4, 6, 8].
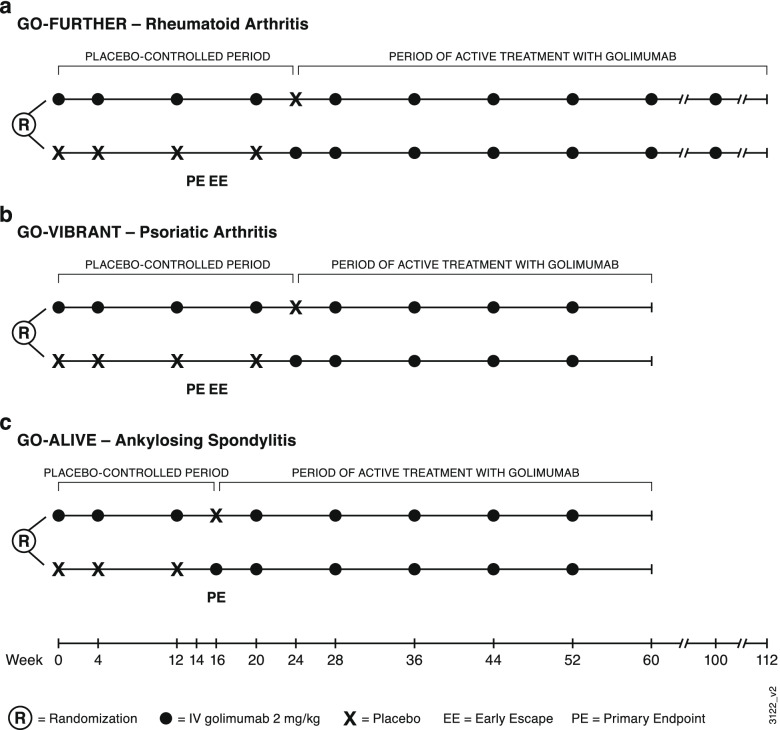
Here, we report pooled safety results from the three double-blind, randomized, placebo-controlled, phase 3 trials of IV golimumab in patients with RA [2–4], PsA [5, 6], and AS [7, 8], each with a placebo-controlled period and an extension of active treatment with golimumab (Fig. 1). Safety results are presented for all treated patients, as well as for patient subgroups defined by baseline use of MTX and low-dose oral corticosteroids.
Fig. 1.
Study designs of three phase 3 trials of golimumab in patients with a rheumatoid arthritis (GO-FURTHER), b psoriatic arthritis (GO-VIBRANT), and c ankylosing spondylitis (GO-ALIVE)
Methods
Patient populations and study designs
These analyses utilized data from three double-blind, randomized, placebo-controlled, phase 3 trials of IV golimumab: GO-FURTHER (NCT00973479), GO-VIBRANT (NCT02181673), and GO-ALIVE (NCT02186873) (Fig. 1). In GO-FURTHER, eligible adults with active RA (≥ 6/66 swollen joints and ≥ 6/68 tender joints) despite MTX therapy (≥ 3 months) were randomized (2:1) to receive IV golimumab 2 mg/kg at weeks 0 and 4 and every 8 weeks thereafter through week 100 or placebo infusions at weeks 0 and 4 and every 8 weeks with crossover to golimumab at week 24 [2]. Patients in the placebo group who had < 10% improvement in swollen and tender joint counts entered early escape (double-blind) at week 16 and received IV golimumab 2 mg/kg. Patients were required to continue their stable doses of concomitant MTX (15–25 mg/week) and were permitted to continue oral low-dose corticosteroid therapy (≤ 10 mg/day of prednisone or equivalent) throughout the trial.
In GO-VIBRANT, adult patients with active PsA (≥ 5/66 swollen joints, ≥ 5/68 tender joints, and C-reactive protein ≥ 0.6 mg/dL) were eligible [5]. Patients were randomized (1:1) to receive IV golimumab 2 mg/kg at weeks 0 and 4 and every 8 weeks thereafter through week 52 or placebo infusions at weeks 0 and 4 and every 8 weeks with crossover to golimumab at week 24. Concomitant MTX (≤ 25 mg/week) was permitted for those patients who had been receiving MTX for ≥ 3 months at a stable dose for ≥ 4 weeks. At week 16, patients in both groups who had < 5% improvement in swollen and tender joint counts could enter early escape and were allowed to have one of the following treatment changes at the investigators’ discretion: an increase in corticosteroid dose (total dose ≤ 10 mg/day prednisone or equivalent), MTX dose (total dose ≤ 25 mg/week), or non-steroidal anti-inflammatory drugs (NSAIDs) dose; or initiation of NSAIDs, corticosteroids (≤ 10 mg/day prednisone or equivalent), MTX (≤ 25 mg/week), sulfasalazine (≤ 3 g/day), hydroxychloroquine (≤ 400 mg/day), or leflunomide (≤ 20 mg/day).
The GO-ALIVE trial included adults with active AS (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] ≥ 4 and a visual analog scale [0–10 cm] score for total back pain of ≥ 4) [7]. Patients were randomized (1:1) to receive IV golimumab 2 mg/kg at weeks 0 and 4 and every 8 weeks thereafter through week 52 or placebo infusions at weeks 0 and 4 with crossover to golimumab at week 16. Patients could have received prior therapy with one TNFi other than golimumab (limited to 20% of enrollment) ≥ 3 months before the first study agent administration. Stable doses of concomitant MTX (≤ 25 mg/week), sulfasalazine, hydroxychloroquine, NSAIDs, other analgesics, and low-dose oral corticosteroids (dose equivalent to ≤ 10 mg prednisone/day) were permitted.
In all three trials, infusions were administered over approximately 30 min. Placebo-treated patients received only saline solution that did not contain excipients found in the golimumab formulation. Infusion reactions were defined as adverse events (AEs) that occurred within 1 h following infusion of the study agent. Patients were permitted prophylactic drugs (excluding corticosteroids) prior to infusion; pre-treatment was at the discretion of the investigator and typically included acetaminophen, antihistamines, and/or NSAIDs.
The trials were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practices. Protocols were approved by an Institutional Review Board or ethics committees for each site, and all patients provided written informed consent.
Assessments
Safety was monitored throughout the trials, with final safety assessments at week 112 in GO-FURTHER and week 60 in GO-VIBRANT and GO-ALIVE or at specified times after early discontinuation (~ 12 weeks in the RA trial and 8 weeks in the PsA and AS trials). Serum samples were collected for laboratory analyses and for the detection of antibodies to golimumab. A highly sensitive, drug-tolerant, enzyme immunoassay was used to detect antibodies to golimumab [16]. Immunogenicity analyses included all golimumab-treated patients who had a baseline sample and at least one post-baseline golimumab infusion.
Statistical methods
Adverse events are summarized by treatment group within each study and pooled across the studies for both the placebo-controlled period and open-label follow-up. Due to the differences in the lengths of follow-up between the three IV golimumab trials, time-adjusted incidences of AEs (events per 100 patient-years [100 PY]) with 95% confidence intervals are also provided across indications by treatment groups. Adverse events of interest included serious infections, malignancies, major adverse cardiovascular events (MACE, defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke), and infusion reactions.
Results
Patients
In the combined RA, PsA, and AS trials, 1279 patients were randomized and received ≥ 1 administration of placebo (n = 539) or IV golimumab (n = 740). A total of 1248 patients received ≥ 1 golimumab administration, including those who were randomized to placebo and initiated IV golimumab at pre-specified time points for crossover or early escape (Fig. 1). Baseline demographic and disease characteristics have been reported in each study [2, 5, 7] with selected characteristics shown in Table 1 for reference. Patients with RA had a higher mean age than patients with PsA or AS, and there were fewer men than women in the RA trial and fewer women than men in the AS trial. MTX use varied by trial; at baseline, 75% of patients were receiving MTX and 45% were receiving oral corticosteroids. All patients received MTX in the RA trial by design, while 70% in the PsA trial were receiving MTX at baseline. A minority (18%) of patients in the AS trial were receiving MTX at baseline. Corticosteroid use at baseline was also higher in the RA trial (65.0%) compared with the PsA (27.7%) and AS (26.4%) trials (Table 1).
Table 1.
Baseline demographics and disease characteristics for patients enrolled in studies of IV golimumab in RA, PsA, and AS*
| RA trial | PsA trial | AS trial | ||||
|---|---|---|---|---|---|---|
| Placebo | Golimumab | Placebo | Golimumab | Placebo | Golimumab | |
| Patients, N | 197 | 395 | 239 | 241 | 103 | 105 |
| Demographics and disease characteristics | ||||||
| Mean age, years (range) | 51.4 (19, 78) | 51.9 (18, 83) | 46.7 (18, 79) | 45.7 (19, 69) | 39.2 (20, 67) | 38.4 (19, 64) |
| Male, n (%) | 40 (20.3) | 69 (17.5) | 121 (50.6) | 128 (53.1) | 77 (74.8) | 86 (81.9) |
| BMI, kg/m2 | 27.0 (5.7) | 26.8 (5.5) | 28.9 (6.2) | 28.9 (6.4) | 26.8 (6.4) | 27.2 (5.9) |
| Disease duration, years | 7.0 (7.2) | 6.9 (7.0) | 5.3 (5.9) | 6.2 (6.0) | 5.5 (5.9) | 5.6 (6.6) |
| Swollen Joint count (0–66) | 14.8 (8.5) | 15.0 (8.2) | 14.1 (8.2) | 14.0 (8.4) | – | – |
| Tender joint count (0–68) | 25.9 (14.1) | 26.4 (13.9) | 26.1 (14.4) | 25.1 (13.8) | – | – |
| CRP, mg/dL | 2.2 (1.9) | 2.8 (2.9) | 2.0 (2.0) | 1.9 (2.5) | 1.9 (1.7) | 2.0 (1.8) |
| BASDAI, N | – | – | 53** | 56** | 103 | 105 |
| Score | – | – | 6.4 (1.9) | 6.5 (1.8) | 7.1 (1.2) | 7.0 (1.2) |
| Methotrexate, n (%) | 197 (100) | 395 (100) | 173 (72.4) | 163 (67.6) | 21 (20.4) | 16 (15.2) |
| Dose, mg/week | 16.6 (2.8) | 16.8 (2.9) | 14.9 (4.8) | 14.8 (4.7) | 13.7 (5.0) | 16.7 (4.9) |
| Oral corticosteroids, n (%) | 134 (68.0) | 251 (63.5) | 67 (28.0) | 66 (27.4) | 23 (22.3) | 32 (30.5) |
| Dose***, mg/day | 7.0 (2.5) | 7.0 (2.5) | 7.6 (2.5) | 7.4 (2.6) | 6.1 (2.5) | 7.8 (2.7) |
| NSAIDs, n (%) | 156 (79.2) | 323 (81.8) | 167 (69.9) | 173 (71.8) | 90 (87.4) | 94 (89.5) |
AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BMI body mass index, CRP C-reactive protein, IV intravenous, NSAID non-steroidal anti-inflammatory drug, PsA psoriatic arthritis, RA rheumatoid arthritis, SD standard deviation
*Data presented as mean (SD) unless otherwise noted
**Among patients with investigator-assessed spondylitis in addition to peripheral arthritis as their primary presentation of PsA
***Prednisone or equivalent
Detailed patient disposition through the end of the studies has been previously reported [2–8]. In total, 1104 (86%) patients who received ≥ 1 golimumab administration (including 468 placebo ➔ golimumab patients) completed study treatment through week 52/100. In these pooled analyses, the mean duration of follow-up ranged from 16 to 23 weeks for placebo and 47 to 96 weeks for golimumab. The number of IV administrations per patient (mean ± SD) was 3.9 ± 0.8 for placebo and 9.0 ± 3.6 for golimumab. For golimumab-treated patients, the total PY of exposure used to determine the incidence/100 PY was 1077 in the RA trial, 417 in the PsA trial, and 203 in the AS trial for a pooled total PY of 1697.
Adverse events during the placebo-controlled periods of the IV golimumab trials
Across the trials, during the placebo-controlled periods, 40.6% of placebo-treated patients and 50.3% of IV golimumab-treated patients experienced ≥ 1 AE, and 2.4% and 3.8% respectively had ≥ 1 serious AE (SAE) (Table 2). Details for each study have been previously reported [2, 5, 7]. Across the three studies, a greater proportion of golimumab-treated patients discontinued treatment because of an AE (2.3%) compared with placebo (0.7%; Table 2). Infections were the most common type of AE. Through the placebo-controlled periods, infections and serious infections, respectively, occurred in 17.3% and 0.4% of placebo patients and in 23.8% and 0.8% of golimumab-treated patients (Table 2). Adverse events reported in ≥ 3% of patients in any treatment group included upper respiratory tract infection, nasopharyngitis, and increases in serum aminotransferases (Table 2). Most SAEs were singular events, with no clear relationship to the underlying conditions or study treatment across the three trials. Three deaths occurred during the placebo-controlled periods of the trials, all among patients receiving placebo and none in the golimumab groups: in the RA trial, one death due to stroke; in the PsA trial, one each due to acute cardiac failure subsequent to pneumonia and cardiorespiratory insufficiency due to metastasis (esophageal neoplasm) [2, 5].
Table 2.
Adverse events during the placebo-controlled periods of studies of IV golimumab in patients with RA, PsA, and AS*
| RA trial, weeks 0–24 | PsA trial, weeks 0–24 | AS trial, weeks 0–16 | Pooled | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Golimumab | Placebo | Golimumab | Placebo | Golimumab | Placebo | Golimumab | |
| Patients, N | 197 | 395 | 239 | 240 | 103 | 105 | 539 | 740 |
| Mean follow-up, weeks | 20.9 | 23.6 | 23.2 | 23.9 | 16.0 | 16.1 | 21.0 | 22.6 |
| Patients who discontinued due to an AE | 1 (0.5) | 12 (3.0) | 3 (1.3) | 5 (2.1) | 0 | 0 | 4 (0.7) | 17 (2.3) |
| Patients with ≥ 1 AE | 98 (49.7) | 227 (57.5) | 97 (40.6) | 111 (46.3) | 24 (23.3) | 34 (32.4) | 219 (40.6) | 372 (50.3) |
| Patients with ≥ 1 infection | 48 (24.4) | 119 (30.1) | 37 (15.5) | 45 (18.8) | 8 (7.8) | 12 (11.4) | 93 (17.3) | 176 (23.8) |
| Patients with ≥ 1 SAE | 5 (2.5) | 19 (4.8) | 8 (3.3) | 7 (2.9) | 0 | 2 (1.9) | 13 (2.4) | 28 (3.8) |
| Patients with ≥ 1 serious infection | 0 | 4 (1.0) | 2 (0.8) | 1 (0.4) | 0 | 1 (1.0) | 2 (0.4) | 6 (0.8) |
| Patients with malignancy | 0 | 1 (0.3) | 2 (0.8) | 0 | 0 | 0 | 2 (0.4) | 1 (0.1) |
| Deaths | 1 (0.5) | 0 | 2 (0.8) | 0 | 0 | 0 | 3 (0.6) | 0 |
| Patients with ≥ 1 infusion reaction | 1 (0.5) | 14 (3.5) | 0 | 4 (1.7) | 0 | 3 (2.9) | 1 (0.2) | 21 (2.8) |
| Common AEs** | ||||||||
| Upper respiratory tract infection | 15 (7.6) | 29 (7.3) | 3 (1.3) | 7 (2.9) | 1 (1.0) | 3 (2.9) | 19 (3.5) | 39 (5.3) |
| Increased ALT | 7 (3.6) | 11 (2.8) | 5 (2.1) | 19 (7.9) | 0 | 3 (2.9) | 12 (2.2) | 33 (4.5) |
| Headache | 5 (2.5) | 20 (5.1) | 5 (2.1) | 5 (2.1) | 1 (1.0) | 4 (3.8) | 11 (2.0) | 29 (3.9) |
| Nasopharyngitis | 5 (2.5) | 10 (2.5) | 13 (5.4) | 8 (3.3) | 1 (1.0) | 6 (5.7) | 19 (3.5) | 24 (3.2) |
| Increased AST | 3 (1.5) | 7 (1.8) | 5 (2.1) | 13 (5.4) | 0 | 0 | 8 (1.5) | 20 (2.7) |
| Hypertension | 4 (2.0) | 14 (3.5) | 4 (1.7) | 4 (1.7) | 0 | 1 (1.0) | 8 (1.5) | 19 (2.6) |
| Bronchitis | 2 (1.0) | 12 (3.0) | 4 (1.7) | 1 (0.4) | 0 | 0 | 6 (1.1) | 13 (1.8) |
| Urinary tract infection | 6 (3.0) | 12 (3.0) | 0 | 0 | 1 (1.0) | 0 | 7 (1.3) | 12 (1.6) |
| Worsening rheumatoid arthritis | 12 (6.1) | 9 (2.3) | 0 | 0 | 0 | 0 | 12 (2.2) | 9 (1.2) |
| Neutropenia | 0 | 0 | 5 (2.1) | 9 (3.8) | 0 | 0 | 5 (0.9) | 9 (1.2) |
AE adverse event, AS ankylosing spondylitis, ALT alanine aminotransferase, AST aspartate aminotransferase, IV intravenous, PsA psoriatic arthritis, RA rheumatoid arthritis, SAE serious adverse event
*Data presented as n (%) unless otherwise noted
**AEs occurring in ≥ 3% of patients in any treatment group
Adverse events in IV golimumab-treated patients over the course of treatment
Through completion of the studies, the numbers/100 PY of AEs, SAEs, and discontinuations due to AEs were numerically greater in the RA trial, followed by the PsA and then AS trials (Table 3). Altogether, discontinuation due to an AE during treatment with IV golimumab occurred at a rate of 5% over the course of all trials.
Table 3.
Adverse events through study completion in patients with RA, PsA, and AS who received ≥ 1 administration of IV golimumab*
| RA trial | PsA trial | AS trial | Pooled golimumab | |
|---|---|---|---|---|
| Patients, N | 584 | 460 | 204 | 1248 |
| Mean follow-up, weeks (SD) | 95.9 (25.7) | 47.2 (13.0) | 51.8 (9.0) | 70.7 (30.7) |
| Patient years of follow-up | 1077 | 417 | 203 | 1697 |
| Patients who discontinued due to an AE | 41 (7.0) | 17 (3.7) | 4 (2.0) | 62 (5.0) |
| AEs | ||||
| Patients with ≥ 1 AE | 462 (79.1) | 234 (50.9) | 113 (55.4) | 809 (64.8) |
| Events/100 patient-years (95% CI) | 195.4 (187.2, 204.0) | 143.5 (132.3, 155.5) | 133.0 (117.6, 149.8) | 175.2 (169.0, 181.6) |
| Infections | ||||
| Patients with ≥ 1 infection | 287 (49.1) | 105 (22.8) | 67 (32.8) | 459 (36.8) |
| Events/100 patient-years (95% CI) | 59.9 (55.4, 64.7) | 34.0 (28.7, 40.1) | 49.8 (40.5, 60.5) | 52.3 (48.9, 55.9) |
| SAEs | ||||
| Patients with ≥ 1 SAE | 106 (18.2) | 24 (5.2) | 7 (3.4) | 137 (11.0) |
| Events/100 patient-years (95% CI) | 16.1 (13.8, 18.7) | 8.2 (5.6, 11.4) | 3.9 (1.7, 7.8) | 12.7 (11.0, 14.5) |
| Serious infections | ||||
| Patients with ≥ 1 serious infection | 36 (6.2) | 10 (2.2) | 3 (1.5) | 49 (3.9) |
| Events/100 patient-years (95% CI) | 4.0 (2.9, 5.4) | 2.6 (1.3, 4.7) | 1.5 (0.3, 4.3) | 3.4 (2.5, 4.4) |
| MACE | ||||
| Patients with ≥ 1 MACE | 9 (1.5) | 0 | 0 | 9 (0.7) |
| Events/100 patient-years (95% CI) | 0.8 (0.4, 1.6) | 0 (0.0, 0.7) | 0 (0.0, 1.5) | 0.5 (0.2, 1.0) |
| Malignancies | ||||
| Patients | 5 (0.9) | 2 (0.4) | 0 | 7 (0.6) |
| Events/100 patient-years (95% CI) | 0.5 (0.2, 1.1) | 0.5 (0.1, 1.7) | 0 (0.0, 1.5) | 0.4 (0.2, 0.9) |
| Deaths | ||||
| Patients | 5 (0.9) | 1 (0.2) | 0 | 6 (0.5) |
| Events/100 patient-years (95% CI) | 0.5 (0.2, 1.1) | 0.2 (0.0, 1.3) | 0 (0.0, 1.5) | 0.4 (0.1, 0.8) |
| Opportunistic infections | ||||
| Patients with ≥ 1 opportunistic infection | 4 (0.7) | 0 | 0 | 4 (0.3) |
| Events/100 patient-years (95% CI) | 0.4 (0.1, 1.0) | 0 (0.0, 0.7) | 0 (0.0, 1.5) | 0.2 (0.1, 0.6) |
| Active TB | ||||
| Patients | 3 (0.5) | 2 (0.4) | 1 (0.5) | 6 (0.5) |
| Events/100 patient-years (95% CI) | 0.3 (0.1, 0.8) | 0.5 (0.1, 1.7) | 0.5 (0.0, 2.7) | 0.4 (0.1, 0.8) |
| Infusion reaction | ||||
| Patients with ≥ 1 infusion reaction | 27 (4.6) | 4 (0.9) | 3 (1.5) | 34 (2.7) |
| Events/100 patient-years (95% CI) | 3.7 (2.7, 5.1) | 1.4 (0.5, 3.1) | 2.0 (0.5, 5.0) | 3.0 (2.2, 3.9) |
AE adverse event, AS ankylosing spondylitis, CI confidence interval, IV intravenous, MACE major adverse cardiovascular event, PsA psoriatic arthritis, RA rheumatoid arthritis, SAE serious adverse event, SD standard deviation, TB tuberculosis
*Data presented as n (%) unless otherwise noted
Six deaths occurred in golimumab-treated patients, and all occurred after the placebo-controlled periods: pneumonia with subsequent presumed myocardial infarction, acute abdominal syndrome (abscess fluid removed during laparotomy was positive for TB), septic shock secondary to a pyogenic lung abscess, dehydration due to Clostridium difficile, and one of unknown cause in the RA trial [2–4] and one due to acute hepatitis of mixed etiology in the PsA trial [6]. No deaths occurred in the AS trial [7, 8].
Adverse events of interest
Malignancies occurred in 2 (0.4%) patients receiving placebo (non-small cell lung cancer and esophageal neoplasm in the PsA trial; Table 2). In addition, one case of non-treatment-emergent lung adenocarcinoma occurred in a patient randomized to placebo in the RA trial. Among IV golimumab-treated patients, eight malignancies occurred in seven (0.6%) patients: basal cell carcinoma, breast cancer, cervical carcinoma in situ, and chronic lymphocytic leukemia, and one patient with both Bowen’s disease and basal cell carcinoma (all in the RA trial) and gastric cancer and colon cancer (one patient each) in the PsA trial. The incidence of malignancies/100 PY (95% CI) across the three studies was 0.4 (0.2, 0.9) (Table 3). There were no cases of lymphoma.
In total, 49 (3.9%) of all golimumab-treated patients had a serious infection; the incidence/100 PY (95% CI) was 3.4 (2.5, 4.4) across the three trials (4.0 [2.9, 5.4] in the RA trial; 2.6 [1.3, 4.7] in the PsA trial, and 1.5 [0.3, 4.3] in the AS trial; Table 3). Serious infections included pneumonia (n = 11), urinary tract infection (n = 5), sepsis (n = 4), appendicitis (n = 2), empyema (n = 2), and erysipelas (n = 2); other serious infections were singular events and included infected dermal cyst, acute pyelonephritis, periodontitis, and acute hepatitis of mixed etiology [4, 6, 8]. Among the golimumab-treated patients, six cases of active TB occurred (RA trial, n = 3; PsA trial, n = 2; AS trial, n = 1) (Table 3)), all in patients who screened negative for TB at baseline and lived in countries endemic for TB (Argentina, Lithuania, Malaysia, Mexico, and Ukraine) [4, 6, 8]. No opportunistic infections occurred in the PsA or AS trials. Four opportunistic infections occurred among golimumab-treated patients in the RA trial: cryptococcal pneumonia (n = 1) and localized vertebral candidiasis (n = 1) (both classified as serious and led to discontinuation of golimumab) [4], and two golimumab-treated patients were diagnosed with non-serious esophageal candidiasis infection.
One demyelinating event (non-infectious encephalomyelitis) occurred in a patient receiving golimumab in the PsA trial [5]. Two events of uveitis occurred in patients who were receiving placebo (both in the AS trial). One golimumab-treated patient had de novo uveitis (RA trial); the patient had a concurrent herpes simplex infection of the eye. Two patients reported pregnancy during the trial, both randomized to golimumab: one patient (RA trial) discontinued study treatment with no additional information provided, and the other patient (PsA trial) discontinued study treatment and had an elective termination. There were no cases of drug-induced lupus or aplastic anemia.
Two patients experienced heart failure (both in the PsA trial): one patient in the placebo group developed acute heart failure that had a fatal outcome, and one patient in the golimumab group had a non-serious AE of chronic heart failure (hypertension and ischemic heart disease ongoing at baseline; the patient discontinued study treatment). Two patients experienced deep vein thrombosis: one golimumab-treated patient in the RA trial and one placebo-treated patient in the PsA trial. The incidence (95% CI) of MACE among all golimumab-treated patients was 0.5/100 PY (0.2, 1.0), all occurring in the RA trial (Table 3): two deaths (one due to unknown cause and the aforementioned presumed myocardial infarction secondary to pneumonia), three non-fatal myocardial infarctions, and four non-fatal strokes. The incidence (95% CI) of MACE among patients receiving placebo was 1.4/100 PY (0.3, 4.0): two deaths (the aforementioned stroke [RA trial] and acute cardiac failure subsequent to pneumonia [PsA trial]) and one non-fatal stroke (PsA trial) [2, 5].
During the placebo-controlled periods, infusion reactions were seen in 2.8% of all IV golimumab-treated patients (Table 2); the incidence remained low over the course of the trials (3.0/100 PY; Table 3). No infusion reaction was considered serious or severe. One patient in the PsA trial receiving golimumab discontinued study agent due to chest tightness associated with infusion.
Adverse events in patients with and without concomitant methotrexate or oral corticosteroids
Across the three trials, 965 (placebo, n = 391; golimumab, n = 574) were receiving concomitant MTX at baseline (Table 4). Concomitant MTX treatment was mandatory in the RA trial and optional in the PsA and AS trials. During the placebo-controlled periods of the PsA and AS trials, infections, serious infections, and SAEs occurred in comparable proportions of patients receiving vs. not receiving concomitant MTX (Table 4). Pooling data across the three trials, infections appeared to be more common in patients with vs. without MTX use at baseline during the placebo-controlled periods and through trial completion. However, interpretation of these findings is limited by the requirement for all patients in the RA trial to use concomitant MTX. Through the end of the PsA trial, concomitant MTX treatment was associated with elevations in serum hepatic alanine aminotransferase (ALT) from ≥ 3 to < 5 times the upper limit of normal (ULN); the occurrence of ALT elevations ≥ 5× ULN was similar between patients with and without MTX use (Table 4). No clear relationship was seen between MTX use and aspartate aminotransferase elevation (data not shown).
Table 4.
Adverse events, including clinical laboratory abnormalities, summarized by methotrexate use at baseline in patients with RA, PsA, and AS*
| Occurrence during placebo-controlled periods, n (%) | ||||||||||||||
| RA trial | PsA trial | AS trial | Pooled | |||||||||||
| PBO | Golimumab | PBO | Golimumab | PBO | Golimumab | PBO | Golimumab | |||||||
| Methotrexate use at baseline | + | + | + | − | + | − | + | − | + | − | + | − | + | − |
| Treated patients, N | 197 | 395 | 173 | 66 | 163 | 77 | 21 | 82 | 16 | 89 | 391 | 148 | 574 | 166 |
| Mean follow-up, weeks | 20.9 | 23.6 | 23.2 | 23.1 | 23.8 | 24.0 | 15.9 | 16.0 | 16.1 | 16.1 | 21.6 | 19.2 | 23.5 | 19.7 |
| ≥ 1 infection | 48 (24.4) | 119 (30.1) | 26 (15.0) | 11 (16.7) | 26 (16.0) | 19 (24.7) | 2 (9.5) | 6 (7.3) | 2 (12.5) | 10 (11.2) | 76 (19.4) | 17 (11.5) | 147 (25.6) | 29 (17.5) |
| ≥ 1 SAE | 5 (2.5) | 19 (4.8) | 4 (2.3) | 4 (6.1) | 5 (3.1) | 2 (2.6) | 0 | 0 | 0 | 2 (2.2) | 9 (2.3) | 4 (2.7) | 24 (4.2) | 4 (2.4) |
| ≥ 1 serious infection | 0 | 4 (1.0) | 1 (0.6) | 1 (1.5) | 1 (0.6) | 0 | 0 | 0 | 0 | 1 (1.1) | 1 (0.3) | 1 (0.7) | 5 (0.9) | 1 (0.6) |
| Baseline ALT ≤ ULN, n | 182 | 357 | 144 | 60 | 135 | 74 | 18 | 76 | 15 | 83 | 344 | 136 | 507 | 157 |
| ALT ≥ 3 to < 5× ULN | 2 (1.1) | 4 (1.1) | 1 (0.7) | 0 | 2 (1.5) | 1 (1.4) | 0 | 0 | 0 | 1 (1.2) | 3 (0.9) | 0 | 6 (1.2) | 2 (1.3) |
| ALT ≥ 5× ULN | 0 | 3 (0.8) | 0 | 1 (1.7) | 0 | 1 (1.4) | 0 | 1 (1.3) | 0 | 0 | 0 | 2 (1.5) | 3 (0.6) | 1 (0.6) |
| Baseline ALT > ULN, n | 14 | 34 | 25 | 4 | 27 | 3 | 3 | 4 | 1 | 6 | 42 | 8 | 62 | 9 |
| ALT ≥ 3 to < 5× ULN | 3 (21.4) | 4 (11.8) | 0 | 0 | 3 (11.1) | 1 (33.3) | 0 | 0 | 0 | 0 | 3 (7.1) | 0 | 7 (11.3) | 1 (11.1) |
| ALT ≥ 5× ULN | 0 | 0 | 0 | 0 | 3 (11.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (4.8) | 0 |
| Events/100 patient-years through study completion** | ||||||||||||||
| RA trial | PsA trial | AS trial | Pooled | |||||||||||
| Golimumab | Golimumab | Golimumab | Golimumab | |||||||||||
| Methotrexate use at baseline | + | + | − | + | − | + | − | |||||||
| Patients, N | 584 | 323 | 137 | 36 | 168 | 943 | 305 | |||||||
| Total PY of follow-up | 1077 | 290 | 127 | 35 | 168 | 1402 | 295 | |||||||
| Serious infections | 4.0 (2.9, 5.4) | 3.8 (1.9, 6.8) | 0 (0.0, 2.4) | 0 (0.0, 8.5) | 1.8 (0.4, 5.2) | 3.9 (2.9, 5.0) | 1.0 (0.2, 3.0) | |||||||
| ALT ≥ 3 to < 5× ULN | 3.5 (2.5, 4.8) | 21.0 (16.1, 27.0) | 7.9 (3.8, 14.5) | 2.8 (0.1, 15.8) | 2.4 (0.7, 6.1) | 7.1 (5.8, 8.7) | 4.8 (2.6, 8.0) | |||||||
| ALT ≥ 5× ULN | 2.0 (1.3 ,3.1) | 3.1 (1.4, 5.9) | 3.2 (0.9, 8.1) | 0 (0.0, 8.5) | 1.2 (0.1, 4.3) | 2.2 (1.5, 3.1) | 2.0 (0.8, 4.4) | |||||||
| Patients positive for antibodies to golimumab***, n/N (%) | 129/552 (23.4) | 60/316 (19.0) | 39/134 (29.1) | 4/36 (11.1) | 37/167 (22.2) | 193/904 (21.3) | 76/301 (25.2) | |||||||
“+” indicates with methotrexate; “−” indicates without methotrexate
AE adverse event, ALT alanine aminotransferase, AS ankylosing spondylitis, CI confidence interval, PBO placebo, PsA psoriatic arthritis, PY patient-years, RA rheumatoid arthritis, SAE serious adverse event, ULN upper limit of normal
*Data presented as n (%), n/N (%), or events /100 PY (95% CI), unless otherwise noted
**The three trials ranged from 60 to 112 weeks in duration (Fig. 1). Time-adjusted incidence of AEs (events/100 PY) are shown
***Antibodies to golimumab were assessed using a drug-tolerant enzyme immunoassay at 52 weeks for all three trials
A total of 573 (placebo, n = 224; golimumab, n = 349) patients were receiving concomitant low-dose oral corticosteroids at baseline (Table 5). Concomitant treatment with oral corticosteroids was associated with an increased rate of SAEs during the placebo-controlled period of the RA trial (Table 5). However, the trend did not continue through the extended course of golimumab treatment in the RA trial, and SAEs were not more common in patients receiving corticosteroid treatment in the PsA and AS trials. Among the pooled golimumab-treated patients, the incidence of serious infections per 100 PY (95% CI) through the end of the studies was similar between patients who received concomitant oral corticosteroids (3.4 [2.2, 4.8]) and those who did not (3.4 [2.2, 4.9]).
Table 5.
Adverse events summarized by corticosteroid use at baseline in patients with RA, PsA, and AS*
| Occurrence during placebo-controlled periods, n (%) | ||||||||||||||||
| RA trial | PsA trial | AS trial | Pooled | |||||||||||||
| PBO | Golimumab | PBO | Golimumab | PBO | Golimumab | PBO | Golimumab | |||||||||
| Corticosteroid use at baseline | + | - | + | − | + | − | + | − | + | − | + | − | + | − | + | − |
| Treated patients, N | 134 | 63 | 251 | 144 | 67 | 172 | 66 | 174 | 23 | 80 | 32 | 73 | 224 | 315 | 349 | 391 |
| Mean follow-up, weeks | 21.1 | 20.4 | 23.5 | 23.9 | 22.7 | 23.3 | 23.4 | 24.0 | 15.9 | 16.0 | 16.0 | 16.1 | 21.0 | 20.9 | 22.8 | 22.5 |
| ≥ 1 infection | 34 (25.4) | 14 (22.2) | 79 (31.5) | 40 (27.8) | 9 (13.4) | 28 (16.3) | 13 (19.7) | 32 (18.4) | 0 | 8 (10.0) | 2 (6.3) | 10 (13.7) | 43 (19.2) | 50 (15.9) | 94 (26.9) | 82 (21.0) |
| ≥ 1 SAE | 5 (3.7) | 0 | 16 (6.4) | 3 (2.1) | 3 (4.5) | 5 (2.9) | 1 (1.5) | 6 (3.4) | 0 | 0 | 0 | 2 (2.7) | 8 (3.6) | 5 (1.6) | 17 (4.9) | 11 (2.8) |
| ≥ 1 serious infection | 0 | 0 | 3 (1.2) | 1 (0.7) | 2 (3.0) | 0 | 1 (1.5) | 0 | 0 | 0 | 0 | 1 (1.4) | 2 (0.9) | 0 | 4 (1.1) | 2 (0.5) |
| Events/100 patient-years through study completion** | ||||||||||||||||
| RA trial | PsA trial | AS trial | Pooled | |||||||||||||
| Golimumab | Golimumab | Golimumab | Golimumab | |||||||||||||
| Corticosteroid use at baseline | + | − | + | − | + | − | + | − | ||||||||
| Patients, N | 381 | 203 | 126 | 334 | 55 | 149 | 562 | 686 | ||||||||
| Total PY of follow-up | 697 | 379 | 112 | 306 | 57 | 146 | 865 | 832 | ||||||||
| SAE | 16.4 (13.5, 19.6) | 15.6 (11.8, 20.1) | 4.5 (1.5, 10.5) | 9.5 (6.4, 13.6) | 0 (0.0, 5.3) | 5.5 (2.4, 10.8) | 13.8 (11.4, 16.5) | 11.5 (9.4, 14.1) | ||||||||
| Serious infections | 3.6 (2.3, 5.3) | 4.7 (2.8, 7.5) | 3.6 (1.0, 9.2) | 2.3 (0.9, 4.7) | 0.0 (0.0, 5.3) | 2.1 (0.4, 6.0) | 3.4 (2.2, 4.8) | 3.4 (2.2, 4.9) | ||||||||
“+” indicates with corticosteroids; “−” indicates without corticosteroids
AE adverse event, AS ankylosing spondylitis, CI confidence interval, PBO placebo, PsA psoriatic arthritis, PY patient-years, RA rheumatoid arthritis, SAE serious adverse event
*Data presented as n (%) or events/100 PY (95% CI), unless otherwise noted
**The three trials ranged from 60 to 112 weeks in duration (Fig. 1). Time-adjusted incidence of AEs (events/100 PY) is shown
Immunogenicity
In total, 1205 patients received ≥ 1 administration of IV golimumab and had appropriate samples for evaluating immunogenicity. Through week 52 of all three trials, 269 (22%) patients were positive for antibodies to golimumab (RA trial, 23%; PsA trial, 22%; AS trial, 20%), including 71 (6%) who were positive for neutralizing antibodies. Infusion reactions occurred in 20 (2.1%) patients who tested negative for antibodies to golimumab and in 11 (4.1%) who tested positive for antibodies to golimumab; the proportions of infusions with an infusion reaction were 0.4% and 0.8%, respectively. In the PsA and AS trials, in which not all patients received MTX, the occurrence of antibodies to golimumab was lower in patients who were receiving MTX at baseline compared with patients who were not (Table 4); a similar trend was observed for neutralizing antibodies (data not shown).
Discussion
Patients with RA [17], PsA [18, 19], and AS [20, 21] typically require life-long treatment; therefore, long-term safety is an important consideration for both health care providers and patients when selecting treatment. In these pooled analyses, we combined the safety results of the TNFi IV golimumab 2 mg/kg from three phase 3 studies, GO-FURTHER (RA), GO-VIBRANT (PsA), and GO-ALIVE (AS). The proportion of patients who completed the safety follow-up (through week 112 in the RA trial and week 60 in the PsA and AS trials) was relatively high (86%), resulting in a total of 1697 PY of exposure. During these trials, IV golimumab was generally well-tolerated, and AEs were consistent with the known safety profile of other TNFi, including subcutaneous golimumab [9, 11, 13, 14, 22].
During the placebo-controlled periods, SAEs were uncommon in both the placebo group (2.4%) and the combined golimumab group (3.8%). Infections were the most common type of AE in the pooled results, which was consistent with the results reported individually from each of these studies. The incidence of serious infections per 100 PY was highest in the RA trial, followed by the PsA and AS trials; the pooled incidence of 3.4 serious infections per 100 PY in all golimumab-treated patients was comparable to that reported for other TNFi agents [23–25]. Other AEs of interest, including malignancy, MACE, opportunistic infections, and uveitis, were also uncommon through the end of the trials. Malignancy occurrence was comparable between placebo (0.4%) and golimumab (0.1%) during the placebo-controlled periods; no cases of lymphoma were reported through trial completion. While a small number of golimumab patients (n = 6 [0.4%]) acquired active TB in countries where TB is endemic (Argentina, Lithuania, Malaysia, Mexico, and Ukraine), no case of latent TB converted to active TB.
Among the three studies, AEs tended to be more common in patients with RA, with infections being the most common class of AE reported. Previous analyses of patients receiving TNFi have shown a higher incidence of serious infections in RA patients than in patients with AS or PsA [26, 27]. In the current study, patients in the RA study were, on average, older and had longer disease duration and were more likely to be receiving concomitant corticosteroids relative to patients in the PsA and AS studies. These factors have been previously associated with an increased risk of serious infection among RA patients treated with TNFi [28]. In addition, concomitant MTX use was required in the RA trial, but not in the other trials. In the PsA and AS trials, there was no clear pattern of differences in the rate of SAEs or serious infections between patients who were receiving concomitant MTX and those who were not. Elevations in ALT were more frequent in patients receiving concomitant MTX in the PsA trial, which was not unexpected considering that hepatic toxicity is commonly observed with MTX therapy. This pattern was not seen in the AS trial, although the proportion of patients receiving MTX in that trial was considerably lower.
Fewer than 3% of all golimumab-treated patients had an infusion reaction; none was serious or severe. Approximately 22% of patients treated with golimumab developed antibodies to golimumab, although only 6% of patients tested positive for neutralizing antibodies. The proportion of patients with antibodies to golimumab and neutralizing antibodies was numerically lower in patients who received concomitant MTX than in those who received golimumab monotherapy; however, interpretation of these results is limited by the lack of a golimumab monotherapy population in the RA study and the relatively small number of patients receiving golimumab+MTX in the AS study. Infusion reactions were more common in patients with antibodies to golimumab than those without antibodies to golimumab.
Oral corticosteroid use has been associated with an increased risk of serious infections in patients with RA, even with doses ≤ 5 mg/day (prednisone or equivalent) [29]. In the current pooled analyses, the proportions of patients with a serious infection during the placebo-controlled periods were numerically higher in patients receiving concomitant oral corticosteroids compared with patients not receiving these medications in both the placebo and golimumab groups. Among all golimumab-treated patients, the number of serious infections per 100 PY through study completion was similar for patients who did and did not receive oral corticosteroids. Previous research has shown that the highest risk of serious infection with TNFi tends to be within the first 6 months of treatment [28], and the risk of serious infections associated with corticosteroids is dose-dependent. The mean corticosteroid dose in the treatment groups across the three trials ranged from 6.1 to 7.9 mg/day (prednisone or equivalent), although patients could have received doses up to 10 mg/day. Also, it should be noted that there may have been imbalances in age, disease duration, and concomitant medication use between the patients who did and did not receive concomitant corticosteroids, as the studies were not designed to compare outcomes by corticosteroid use.
With data from over 700 golimumab-treated patients and approximately 71 patient-years of follow-up, these results represent the largest pooled analysis, to date, from phase 3 studies of IV golimumab in patients with RA, PsA, and AS. The studies included in the current analysis were limited to 1 or 2 years in duration, and the studies were not powered to detect rare events. Differences in study design also limited some of the analyses on concomitant medications. However, the totality of the pooled data across three rheumatologic indications demonstrated consistency with each of the individual trials.
Conclusions
These pooled analyses from three double-blind, randomized, placebo-controlled, phase 3 trials of patients with RA, PsA, and AS included safety data from 1280 patients for a total of 1697 PY of follow-up. Infusion reactions were uncommon across the three studies. Concomitant use of MTX and of low-dose oral corticosteroids was associated with a higher occurrence of ALT elevations and serious infections, respectively. Overall, most elevations in liver transaminases were mild and transient, and few patients experienced a serious infection. The safety profile of IV golimumab was generally consistent across the RA, PsA, and AS trials, as well as with other TNFi.
Acknowledgements
Medical writing support was provided by Rebecca Clemente, PhD (Janssen Scientific Affairs, LLC) and Elizabeth Rosenberg, PhD (Kelly Services, funded by Janssen) under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2015;163:461-4). The authors thank Diane D. Harrison, MD, MPH (consultant funded by Janssen Research & Development, LLC) and Shelly Kafka, MD (formerly of Janssen Scientific Affairs, LLC) for substantive review.
Abbreviations
- AE
Adverse event
- ALT
Alanine aminotransferase
- AS
Ankylosing spondylitis
- AST
Aspartate aminotransferase
- BASDAI
Bath Ankylosing Spondylitis Disease Activity Index
- BMI
Body mass index
- CI
Confidence interval
- CRP
C-reactive protein
- EE
Early escape
- GLM
Golimumab
- IV
Intravenous
- MACE
Major adverse cardiovascular events
- MTX
Methotrexate
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PBO
Placebo
- PE
Primary endpoint
- PsA
Psoriatic arthritis
- PY
Patient years
- R
Randomization
- RA
Rheumatoid arthritis
- SAE
Serious adverse event
- SD
Standard deviation
- TB
Tuberculosis
- TNFα
Tumor necrosis factor alpha
- TNFi
Tumor necrosis factor inhibitor
- ULN
Upper limit of normal
Authors’ contributions
Study design: MEH, AD, ECH, KHL, and AK. Data collection: JHL and YZ. Data interpretation: MEH, AD, SS, SDC, ECH, JHL, YZ, KHL, and AK. All authors revised the manuscript and gave final approval for submission.
Funding
This study was funded by Janssen Research & Development, LLC.
Availability of data and materials
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Declarations
Ethics approval and consent to participate
The GO-FURTHER, GO-VIBRANT, and GO-ALIVE trials were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocols were approved by the Institutional Review Boards. All patients gave written informed consent before any study-related procedures were performed.
Consent for publication
Not applicable.
Competing interests
MEH has received honoraria for participation in advisory boards (AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Regeneron, UCB).
AD has participated in advisory boards (AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, UCB), received research grants (AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, UCB), and participated in speakers’ bureaus (AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, UCB).
SS has received consulting fees, research grants, speakers’ bureau activity, or ownership or partnership (AbbVie, Amgen, Boston Scientific, Crescendo Bioscience, Dermtech, Eli Lilly, Genentech, Gilead Sciences, Janssen, Medtronic, Myriad Genetics, National Psoriasis Foundation, Novartis, Pfizer, Regeneron, Samsung, Sanofi, UCB) and participated in advisory boards (Jubilant, Myriad, Stelexis).
AK has received research support and/or consulting fees (AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Genentech, Janssen, Merck, Novartis, Pfizer, UCB)
SDC is an employee of Janssen Scientific Affairs, LLC, and owns stock in Johnson & Johnson.
ECH, JHL, YZ, and KHL are or were employees of Janssen Research & Development, LLC, at the time this work was performed and own stock in Johnson & Johnson.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simponi ARIA. Package insert. Horsham: Janssen Biotech, Inc; 2021. [Google Scholar]
- 2.Weinblatt ME, Bingham CO, 3rd, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis. 2013;72(3):381–389. doi: 10.1136/annrheumdis-2012-201411. [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Westhovens R, Mendelsohn AM, Kim L, Lo KH, Sheng S, et al. Radiographic benefit and maintenance of clinical benefit with intravenous golimumab therapy in patients with active rheumatoid arthritis despite methotrexate therapy: results up to 1 year of the phase 3, randomised, multicentre, double blind, placebo controlled GO-FURTHER trial. Ann Rheum Dis. 2014;73(12):2152–2159. doi: 10.1136/annrheumdis-2013-203742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingham CO, 3rd, Mendelsohn AM, Kim L, Xu Z, Leu J, Han C, et al. Maintenance of clinical and radiographic benefit with intravenous golimumab therapy in patients with active rheumatoid arthritis despite methotrexate therapy: week-112 efficacy and safety results of the open-label long-term extension of a phase III, double-blind, randomized, placebo-controlled trial. Arthritis Care Res (Hoboken). 2015;67(12):1627–1636. doi: 10.1002/acr.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol. 2017;69(11):2151–2161. doi: 10.1002/art.40226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husni ME, Kavanaugh A, Murphy F, Rekalov D, Harrison DD, Kim L, et al. Efficacy and safety of intravenous golimumab through 1 year in patients with active psoriatic arthritis. Arthritis Care Res (Hoboken). 2020;72(6):806–813. doi: 10.1002/acr.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deodhar A, Reveille JD, Harrison DD, Kim L, Lo KH, Leu JH, et al. Safety and efficacy of golimumab administered intravenously in adults with ankylosing spondylitis: results through week 28 of the GO-ALIVE study. J Rheumatol. 2018;45(3):341–348. doi: 10.3899/jrheum.170487. [DOI] [PubMed] [Google Scholar]
- 8.Reveille JD, Deodhar A, Caldron PH, Dudek A, Harrison DD, Kim L, et al. Safety and efficacy of intravenous golimumab in adults with ankylosing spondylitis: results through 1 year of the GO-ALIVE Study. J Rheumatol. 2019;46(10):1277–1283. doi: 10.3899/jrheum.180718. [DOI] [PubMed] [Google Scholar]
- 9.Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Mack M, et al. Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann Rheum Dis. 2015;74(3):538–546. doi: 10.1136/annrheumdis-2013-204195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(9990):258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Zhou Y, et al. Five-year safety data from 5 clinical trials of subcutaneous golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2016;43(12):2120–2130. doi: 10.3899/jrheum.160420. [DOI] [PubMed] [Google Scholar]
- 12.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-a blockers. Curr Neurol Neurosci Rep. 2017;17(4):36. doi: 10.1007/s11910-017-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, van der Heijde D, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1093–1101. doi: 10.1136/annrheumdis-2016-210708. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelo S, Carriero A, Gilio M, Ursini F, Leccese P, Palazzi C. Safety of treatment options for spondyloarthritis: a narrative review. Expert Opin Drug Saf. 2018;17(5):475–486. doi: 10.1080/14740338.2018.1448785. [DOI] [PubMed] [Google Scholar]
- 15.Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Noonan L, et al. Radiographic progression inhibition with intravenous golimumab in psoriatic arthritis: week 24 results of a phase III, randomized, double-blind, placebo-controlled trial. J Rheumatol. 2019;46(6):595–602. doi: 10.3899/jrheum.180681. [DOI] [PubMed] [Google Scholar]
- 16.Leu JH, Adedokun OJ, Gargano C, Hsia EC, Xu Z, Shankar G. Immunogenicity of golimumab and its clinical relevance in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Rheumatology (Oxford). 2019;58(3):441–446. doi: 10.1093/rheumatology/key309. [DOI] [PubMed] [Google Scholar]
- 17.Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–1471. doi: 10.1136/annrheumdis-2019-215920. [DOI] [PubMed] [Google Scholar]
- 18.Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(3):351–360. doi: 10.1016/j.semarthrit.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;48(1):28–34. doi: 10.1016/j.semarthrit.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Garrido-Cumbrera M, Poddubnyy D, Gossec L, Galvez-Ruiz D, Bundy C, Mahapatra R, et al. The European map of axial spondyloarthritis: capturing the patient perspective-an analysis of 2846 patients across 13 countries. Curr Rheumatol Rep. 2019;21(5):19. doi: 10.1007/s11926-019-0819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol. 2019;38(3):625–634. doi: 10.1007/s10067-018-4397-3. [DOI] [PubMed] [Google Scholar]
- 22.Wronski J, Fiedor P. The safety profile of tumor necrosis factor inhibitors in ankylosing spondylitis: are TNF inhibitors safer than we thought? J Clin Pharmacol. 2019;59(4):445–462. doi: 10.1002/jcph.1348. [DOI] [PubMed] [Google Scholar]
- 23.Burmester GR, Gordon KB, Rosenbaum JT, Arikan D, Lau WL, Li P, et al. Long-term safety of adalimumab in 29,967 adult patients from global clinical trials across multiple indications: an updated analysis. Adv Ther. 2020;37(1):364–380. doi: 10.1007/s12325-019-01145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furst DE, Shaikh SA, Greenwald M, Bennett B, Davies O, Luijtens K, et al. Two dosing regimens of certolizumab pegol in patients with active rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67(2):151–160. doi: 10.1002/acr.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh JA, Gottlieb AB, Hoepken B, Nurminen T, Mease PJ. Efficacy of certolizumab pegol with and without concomitant use of disease-modifying anti-rheumatic drugs over 4 years in psoriatic arthritis patients: results from the RAPID-PsA randomized controlled trial. Clin Rheumatol. 2018;37(12):3285–3296. doi: 10.1007/s10067-018-4227-7. [DOI] [PubMed] [Google Scholar]
- 26.Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–524. doi: 10.1136/annrheumdis-2011-201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen IE, Lillegraven S, Mielnik P, Bakland G, Loli L, Sexton J, et al. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis. 2022;81:398–401. doi: 10.1136/annrheumdis-2021-221007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford). 2011;50(1):124–131. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George MD, Baker JF, Winthrop K, Hsu JY, Wu Q, Chen L, et al. Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med. 2020;173(11):870–878. doi: 10.7326/M20-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.