Abstract
Renal neoplasia occurring as a second malignancy following childhood cancer has been most closely associated with neuroblastoma and Wilms tumor. While some cases have been associated with a genetic predisposition, nearly all are thought to result from “late effects” of therapy-related toxicity that involves chemotherapy or radiation. It is unclear if these tumors are enriched for specific molecular or morphologic characteristics. A query of our institutional nephrectomy registry of 8,295 patients for renal neoplasia occurring post-treatment for childhood cancer revealed 6 patients with Wilms tumor, 4 with neuroblastoma and 1 with acute lymphoblastic leukemia (ALL). Three additional cases of MiT family translocation renal cell carcinoma (RCC), from 2 patients, following chemotherapy for neuroblastoma and systemic lupus erythematosus and another of clear cell RCC post-ALL were included. The most common tumor type was clear cell RCC: 9/19 cases (47.4%), followed by metanephric adenoma and MiT family translocation RCC (3/19, 15.8%). There were no characteristic features to indicate a unique renal neoplasia subtype. Potential syndromic renal neoplasia occurred in 2 patients; metanephric adenomas and oncocytoma in a patient with hyperparathyroidism-jaw tumor syndrome post-treatment of Wilms tumor and a fumarate hydratase (FH)-deficient RCC in a patient post-treatment for ALL. The mean age at diagnosis of childhood neoplasia or treatment with chemotherapy or radiation was 4.7 years, and the average time to subsequent renal neoplasia was 31 years. Five (of 14) patients developed metastatic RCC and there were 2 RCC-related deaths. These results indicate the need for extended clinical follow-up of these patients.
Keywords: Renal cell carcinoma, Chemotherapy, Radiation, Wilms Tumor, Neuroblastoma
1.0. Introduction
Renal tumors occurring as a second malignancy following pediatric neoplasia is most commonly associated with neuroblastoma and Wilms tumor and less frequently following other tumor types such as primitive neuroectodermal tumors, acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma, Hodgkin lymphoma, rhabdomyosarcoma, leiomyosarcoma and osteosarcoma (1-7). Syndromal associations have been rarely identified in this context (6, 8, 9). Notably, most of these patients have been documented to have received chemotherapy or radiation either for neoplasia or other indications including systemic lupus erythematosus (SLE) and Hurler syndrome in early childhood (2, 4-6).
In contrast to patients with low risk neuroblastoma who are often managed with surgery alone (with limited use of chemotherapy), patients with intermediate risk neuroblastoma receive multiple cycles of chemotherapy (and radiation, if needed), while patients with high risk neuroblastoma typically receive multimodality therapy including high-dose chemotherapy and radiation (2). The latter patient population is therefore at significant risk of therapy-related toxicity including “late effects” in the form of subsequent malignancy (2). This is supported by a review of 5,987 patients with neuroblastoma which revealed that the 10-year cumulative incidence of a second malignant neoplasm was significantly higher in high risk patients (1.8%) compared to lower risk patients (0.38%, p=0.01) (2, 10). It is unclear if renal neoplasia occurring after the treatment of neuroblastoma has characteristic morphologic features and behavior. In the 2004 WHO classification of renal neoplasia, renal cell carcinoma (RCC) associated with neuroblastoma was a unique subtype that was subsequently not included in the 2016 classification due to limited data (11).
Similarly, RCC has been reported to occur both synchronously and metachronously following Wilms tumor (3, 12). It is unclear if embryonal nephrogenic rests that do not spontaneously regress contribute to the development of RCC (3). However, similar to neuroblastoma, a significant number of Wilms tumor patients with a subsequent malignant renal neoplasm have a prior history of chemotherapy or radiation (3, 4, 13).
In the most comprehensive study of childhood cancer survivors (Childhood Cancer Survivor Study, CCSS; conducted at 26 institutions in the United States and Canada between 1970 and 1986), 26 patients were diagnosed with a subsequent RCC among 14,358 survivors, with a median time to diagnosis of 22.6 years (4). The standardized incidence ratio (SIR) of RCC amongst childhood cancer survivors was 8.0, and the corresponding risk of developing RCC among neuroblastoma survivors was a staggering 85.8-fold higher compared to the general population (4). The SIR for Wilms tumor (7.4) and acute leukemia (4.2) were comparatively lower (4). This risk was attributed to chemotherapy with agents such as cisplatin (univariable analysis, relative risk: 3.8; multivariable analysis, relative risk: 3.5) and radiation therapy of 5 Gy or higher to one or both kidneys (univariable analysis, relative risk: 4.1; multivariable analysis, relative risk: 3.8) (4).
Some studies have shown that a subset of renal tumors occurring in this context represent MiT family translocation RCC (with rearrangements of the TFE3 or TFEB genes); however, it is unclear if this is secondary to a higher incidence of this tumor type amongst pediatric compared to adult patients (1, 4-6, 14).
Herein, we have primarily summarized our single institution experience of renal neoplasia occurring in the context of prior chemotherapy or radiation in pediatric patients.
2.0. Materials and Methods
2.1. Patient specimens
Following approval from the Institutional Review Board at Mayo Clinic (Rochester, Minnesota), the nephrectomy registry (8,295 patients; 1970 to 2020) was queried for patients with a prior documented history of pediatric neoplasia including the following: hematologic neoplasia (acute leukemia, lymphoma), neuroblastoma, Wilms tumor and germ cell tumors. Clinical and pathologic features collected at nephrectomy for this registry include year, age, sex, tumor size, histologic subtype, the 2016 primary tumor, regional lymph node, and distant metastases classifications (TNM), coagulative tumor necrosis, rhabdoid differentiation and sarcomatoid differentiation (15). All clinical information by chart abstraction and yearly follow-up communications with patients are provided by a nurse abstractor and all pathology-related data are provided by a slide review by a designated urologic pathologist using contemporary (2016 WHO/ISUP) classification and grading criteria (15, 16). A single case (case 13) was received in consultation by the Mayo Clinic urologic pathology service and included in this series. Finally, two additional (previously reported) patients who presented with renal neoplasia following chemotherapy for neuroblastoma (case 7) and SLE (case 14, treated with cyclophosphamide and prednisone) were included in this series (5, 17).
2.2. Histopathology and Immunohistochemistry
All cases were subjected to histopathologic review. In addition, immunohistochemistry was performed on whole slide sections for carbonic anhydrase IX (clone EP161, prediluted, Ventana, Tucson, AZ), CD117 (clone YR145, prediluted; Cell Marque, Rocklin, CA, USA), CK7 (clone OV-TL 12/30, 1:50 dilution; DAKO, Santa Clara, CA, USA), melan A (clone A103, 1:50 dilution; DAKO, Santa Clara, CA, USA), FH (clone: J-13, 1:50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), BRAF (mutated V600 E; clone: VE1, 1:100 dilution, Abcam, Cambridge, UK), Parafibromin (clone: 2H1, 1:50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) and S-(2-succino)-cysteine (2SC; rabbit polyclonal directed against KLH conjugated synthetic peptide crb1200002e antigen, 1:250 dilution, Discovery antibodies, Cambridge Research Biochemicals, England, United Kingdom).
2.3. Next Generation Sequencing
Formalin fixed paraffin embedded (FFPE) samples were extracted using the QiaAmp DSP DNA FFPE kit (Qiagen) and 50ng of DNA was used for library prep with the KAPA HyperPrep kit (Roche). Libraries underwent enrichment using a custom xGen hybrid capture panel (IDT) consisting of 57 genes commonly altered in solid tumors, including the entire coding region of BRAF. Final libraries were sequenced on a NextSeq instrument (Illumina) with 2x151 read lengths and raw data was aligned to hg19 using a BWA-MEM based custom bioinformatics pipeline.
3.0. Results
3.1. Clinical Features
Of 8,295 patients in the Mayo Clinic nephrectomy registry, 11 patients were found to have a renal neoplasm occurring in the context of a prior pediatric malignancy. This included 6 patients with a prior diagnosis of Wilms tumor (cases 1 to 6), 4 patients with a prior diagnosis of neuroblastoma (cases 8 to 11) as well as 1 patient with a prior diagnosis of ALL (case 12). A second case (received in consultation) of a renal neoplasm occurring post-chemotherapy for ALL was also included in this series (case 13). The relevant clinical features for these patients have been listed in Table 1. Of these 12 patients, all had been treated with chemotherapy or radiation therapy for the initial tumor. Two additional cases included a patient with a TFEB-rearranged RCC occurring after an interval of 11 years following chemotherapy for neuroblastoma (case 7), and a patient (case 14) presenting 8 years following initiation of cyclophosphamide and prednisone therapy for SLE with a right-sided ASPL-TFE3 rearranged tumor and a subsequent contralateral PRCC-TFE3 rearranged tumor that was diagnosed 5 years later (5, 17). Overall, prior chemotherapy or radiation in the pediatric age group was documented for all 14 cases reported herein.
Table 1.
Clinical Features.
| Patie nt No. |
Prior Diagnosis & Year |
Age at Prior Diagnosis (y) |
Therapy | Renal Neoplasm |
Age at Renal Neoplasm Diagnosis (y) |
Time Interval (y) |
Other Associated Neoplasia |
Age at Last Follow Up (f/u) |
|---|---|---|---|---|---|---|---|---|
| 1 | Wilms Tumor (1956) | 1y | Radiation | Clear Cell RCC | 58y | 57y | - | NED (64y) |
| 2 | Wilms Tumor (1964) | 2y | Actinomycin D and Radiation | Clear Cell RCC | 54y | 52y | Papillary thyroid carcinoma | AWD (58y); Metastatic RcC on f/u |
| 3 | Wilms Tumor (1949) | 2y | Radiation | Tumor1: Clear Cell RCC; Tumor2: Oncocytoma (Synchronous) | 56y | 54y | - | AWD (72y); Metastatic RcC on f/u |
| 4 | Wilms Tumor (1957) | 5y | Radiation | Clear Cell RCC | 57y | 52y | Breast ductal carcinoma in situ | DoD (59y); Metastatic RCC on f/u |
| 5 | Wilms Tumor (1986) | 6y | Chemotherapy (Regimen unknown) and Radiation | Metanephric Adenoma | 27y | 21y | Esophageal squamous cell carcinoma | NED (40y) |
| 6 | Wilms Tumor (1988) | 20y | Chemotherapy (Regimen unknown) and Radiation | Tumor1 (left): Metanephric Adenoma; Tumor2 (left): Oncocytoma (Synchronous) | 39y | 19y | Ossifying fibromas (jaw), Uterine adenofibroma; Parathyroid adenoma (Familial Hyperparathyroidism) | NED (51y) |
| Tumor3 (left): Metanephric Adenoma (biopsy/cryoablation) | 51y | 32y | - | |||||
| Tumor4 (left): Unknown (cryoablation) | 51y | 32y | - | |||||
| 7 | Neuroblastoma (2005, *Previously Reported) | <1y | Carboplatin, Etoposide, Cyclophospha mide and Doxorubicin | t(6;11) RCC | 12y | 11y | - | - |
| 8 | Neuroblastoma (1972) | 1y | Radiation (1800 cGy in 17 fractions; Recurrence: 2500 cGy in 30 fractions and cyclophospha mide) | Clear Cell RCC | 26y | 25y | Basal cell carcinoma (vulva); Hepatic adenoma | NED (49y) |
| 9 | Neuroblastoma (1980) | 2y | Radiation (2500 cGy) | Clear Cell RCC | 27y | 25y | Ganglioneuroma at L1 (6y); Ganglioneuroma at the time of partial nephrectomy (27y) | NED (41y) |
| 10 | Neuroblastoma (1994) | 5y | Chemotherapy (Regimen unknown) and Radiation | Clear Cell RCC | 27y | 22y | - | NED (30y) |
| 11 | Neuroblastoma (1975) | 6y | Chemotherapy (Regimen unknown) and Radiation | Clear Cell RCC | 50y | 44y | Urothelial carcinoma (pT3bN0), Prostatic adenocarcinoma (Gleason score 3+3=6, grade group1; pT2N0); Pancreatic intraductal papillary mucinous neoplasm | AWD (50y) |
| 12 | ALL (1973) | 3y | Chemotherapy (Regimen unknown) and Radiation | FH-Deficient RCC (no family history significant for HLRCC) | 43y | 40y | Hurthle Cell thyroid carcinoma; Follicular thyroid carcinoma; Schwannoma | DoD (45y); Metastatic RCC on f/u |
| 13 | ALL (2001) | 3y | Chemotherapy (Regimen unknown) | Clear Cell RCC | 22y | 19y | - | NED (22y) |
| 14 | SLE (**Previously Reported) | 9y | Cyclophosphamide and Prednisone | Tumor1 (right): ASPL-TFE3 rearranged RCC2 | 17y | 8y | - | - |
| Tumor2 (left): PRCC-TFE3 rearranged RCC | 22y | 13y | - | AWD (22y); Metastatic RCC |
cGy: centigray; ALL: acute lymphoblastic leukemia; SLE: systemic lupus erythematosus; y: years; RCC: renal cell carcinoma; FH: fumarate hydratase; HLRCC: hereditary leiomyomatosis and renal cell cancer; NED: no evidence of disease; AWD: alive with disease; DoD: dead of disease.
Patient 7 was reported previously by Gupta et al (2019)
Patient 14 was reported previously by Argani et al (2001 and 2006).
The mean age at diagnosis of the initial neoplasm or initiation of chemotherapy or radiation was 3 years for neuroblastoma, 6 years for Wilms tumor and 4.7 years for the entire cohort of 14 patients. The mean age at diagnosis of the (second) renal neoplasm as well as the average time interval to the development of the second neoplasm was 28 and 26 years for neuroblastoma, 49 and 43 years for Wilms tumor and 36 and 31 years for the entire cohort. Of the 12 patients for whom clinical follow-up was available, 7 had no evidence of recurrent or metastatic renal tumor. Five patients had evidence of metastatic RCC and 2 of these patients died of disease-related complications.
While many of these patients had other (non-renal) tumor types on long-term follow-up and no recurrent patterns were identified. Two patients had a clinical course that suggests a syndromic background. The manifestation of Wilms tumor, uterine adenofibromas, ossifying fibromas of the jaw and parathyroid adenoma in case 6 were consistent with a clinical diagnosis of hyperparathyroidism-jaw tumor syndrome (metanephric adenoma and oncocytoma occurring 19 years post-Wilms tumor and metanephric adenoma occurring 32 years post-Wilms tumor); germline testing was not pursued due to a lack of consent (18-20). In addition, 1 patient (case 12) developed a lethal fumarate hydratase (FH)-deficient RCC as a second malignancy following an initial diagnosis of ALL; germline testing results were not available for this patient due to lack of consent.
3.2. Histopathology and Immunohistochemistry
A total of 19 renal tumors (in 14 patients) were documented to occur as a second neoplasm post-malignancy that was treated with chemotherapy or radiation in the pediatric age group (Table 2). This included 9 clear cell RCCs (47.4%; Figure 1 A-D), an FH-deficient RCC (5.3%; tumor from patient no. 12 depicted in Figure 2 A-D), 2 oncocytomas (10.5%, tumors from patient nos. 3 and 6 depicted in Figure 3 A-B), 3 metanephric adenomas (15.8%, tumor from patient no. 5 depicted in Figure 3 C) and 3 MiT family translocation RCCs (15.8%; TFE3 rearrangement: 2, TFEB-rearrangement: 1; tumor from patient no. 7 depicted in Figure 3 D) (5, 17). The most common renal tumor occurring as a second neoplasm following Wilms tumor and neuroblastoma was clear cell RCC (post-Wilms tumor: 4 of 10, 40%; post-neuroblastoma: 4 of 5, 80%). The second most common tumor type in this context included metanephric adenomas and MiT family translocation RCCs, each (3 of 19, 15.8%). Of note, 2 patients had synchronous renal tumors (patient 3 and 6) and 2 patients had metachronous renal tumors (patient 6 and 14).
Table 2.
Histopathology.
| Patie nt No. |
Prior Diagnosis |
Renal Neoplasm | Size (cm) |
Other Features | Grade (WHO/ISUP) |
pT | N | M |
|---|---|---|---|---|---|---|---|---|
| 1 | Wilms Tumor (R) | Clear Cell RCC (L) | 5.2 | Rhabdoid+, Sarcomatoid+, Necrosis+ | 4 | pT3a | NX | MX |
| 2 | Wilms Tumor (R) | Clear Cell RCC (L) | 6.6 | Rhabdoid+, Necrosis+ | 4 | pT1b | NX | MX |
| 3 | Wilms Tumor (R) | Tumor1: Clear Cell RCC (L) | 5.0 | - | 2 | pT1b | NX | MX |
| Tumor2: Oncocytoma (L) | 2.5 | (Synchronous) | NA | NA | NA | NA | ||
| 4 | Wilms Tumor (L) | Clear Cell RCC (R) | 8.5 | Rhabdoid+, Necrosis+ | 4 | pT3a | NX | MX |
| 5 | Wilms Tumor (R) | Metanephric Adenoma (L) | 1.2 | - | NA | NA | NX | MX |
| 6 | Wilms Tumor (R) | Tumor1 (L): Metanephric Adenoma | 1.2 | - | NA | NA | NX | MX |
| Tumor2 (L): Oncocytoma | 1.5 | (Synchronous) | NA | NA | NA | NA | ||
| Tumor3 (L): Metanephric Adenoma | 2.3* | (Metachronous; Biopsy/Cryoablation) | NA | NA | NA | NA | ||
| Tumor4 (L): Unknown | 2.1* | (Metachronous; Cryoablation) | NA | NA | NA | NA | ||
| 7 | Neuroblastoma (left) | t(6;11) RCC* (R) | 5.4 | - | 3 | pT1b | N0 | M0 |
| 8 | Neuroblastoma (right) | Clear Cell RCC (R) | 1.5 | - | 1 | pT1a | NX | MX |
| 9 | Neuroblastoma (right) | Clear Cell RCC (R) | 2.4 | - | 2 | pT1a | NX | MX |
| 10 | Neuroblastoma (left) | Clear Cell RCC (L) | 2.7 | Cystic | 1 | pT1a | NX | MX |
| 11 | Neuroblastoma (laterality unknown) | Clear Cell RCC (L) | 9.7 | - | 2 | pT2a | NX | MX |
| 12 | ALL | FH-Deficient RCC (L) | 13.5 | LVI+, (3/7 lymph nodes+), adrenal metastasis+ | 3 | pT3a | N1 | M1 |
| 13 | ALL | Clear Cell RCC (R) | 12.5 | - | 2 | pT3a | NX | MX |
| 14 | SLE | Tumor1 (R): ASPL-TFE3** rearranged RCC2 | 8.0 | - | - | pT3a | NX | MX |
| Tumor2 (L): PRCC-TFE3** rearranged RCC | 5.0 | Associated with 14.0 cm retroperitoneal mass and involvement of 1 (of 2) perinephric lymph nodes | - | pT2a | N1 | M1 |
L: left kidney; R: right kidney; ALL: acute lymphoblastic leukemia; SLE: systemic lupus erythematosus; RCC: renal cell carcinoma; FH: fumarate hydratase; LVI: lymphovascular invasion; WHO: World Health Organization; ISUP: International Society of Urologic Pathology.
Patient 7 was reported previously by Gupta et al (2019)
Patient 14 was reported previously by Argani et al (2001 and 2006).
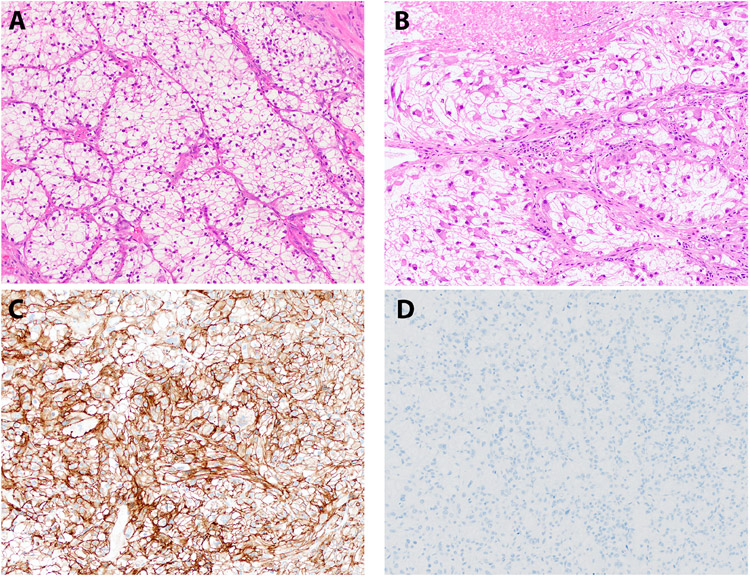
Figure 1. Histopathology: Clear Cell RCC.
Representative H&E stained images of a clear cell RCC, occurring as a second neoplasm, are depicted (A - B); panel B shows areas of rhabdoid transformation with accompanying necrosis. Corresponding immunohistochemistry for carbonic anhydrase IX (C) and melan A (D) are shown.
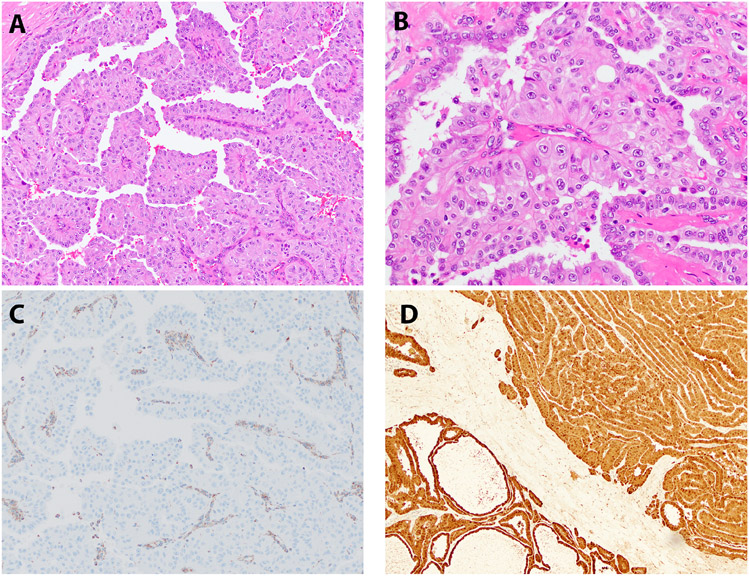
Figure 2. Histopathology: FH-Deficient RCC.
Representative H&E stained images of an FH-deficient RCC (Case 12) is depicted (A-B), with corresponding immunohistochemistry for FH (lost, C) and S-(2-succino)-cysteine (2SC; elevated, D).
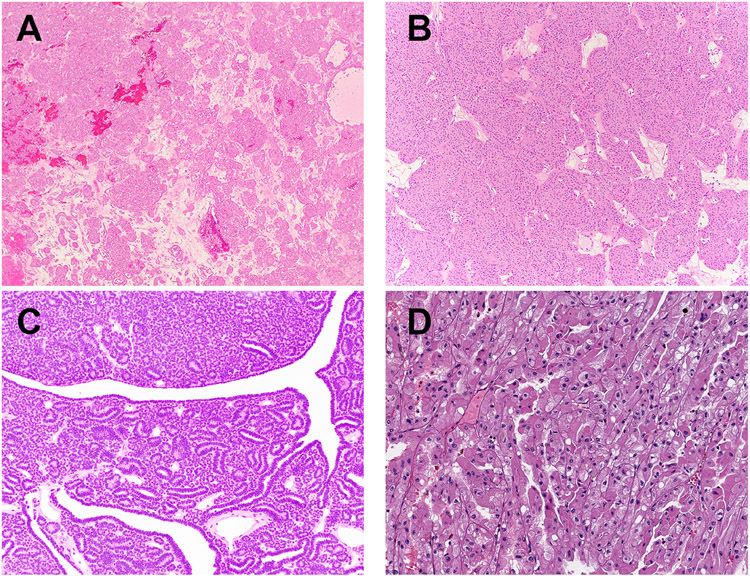
Figure 3. Histopathology.
Representative H&E stained images of oncocytomas (A-B) and a metanephric adenomas (C; patient 5), all occurring as a second neoplasm, have been shown. A t(6; 11) (TFEB-rearranged, D) RCC occurring as a second neoplasm has also been depicted.
Renal tumors with aggressive clinical behavior included 3 clear cell RCCs, 1 FH-deficient RCC and 1 TFE3-rearranged RCC. All exhibited metastatic behavior, and death from disease-related complications was documented for 1 patient with clear cell RCC (case 4, post-Wilms tumor) and 1 patient with an FH-deficient RCC (case 12, post-ALL).
The diagnosis of clear cell RCC was confirmed based on a combination of histopathologic review and immunophenotype. Specifically, these tumors exhibited diffuse “box-like” membranous localization of carbonic anhydrase 9 (CAIX) and absence of melan A, consistent with clear cell RCC (Table 3, Figure 1 A-D). The FH-deficient RCC was characterized by the presence of papillary morphology, marked nucleolar enlargement and focal perinuclear clearing (Figure 2 A-B). This tumor exhibited lymphovascular invasion and multiple lymph node metastases as well as adrenal metastasis at the time of nephrectomy (Table 2). In addition, this tumor demonstrated loss of CK7 as has been previously reported for FH-deficient RCCs (not shown) (21). The diagnosis was confirmed based on absence of FH immunostaining in the presence of appropriate internal positive controls (Table 3, Figure 2 C), as well as elevated levels of aberrant protein succination and high levels of covalent modifications of cysteine residues, as evidenced by increased accumulation of S-(2-succino)-cysteine (2SC; Figure 2 D) (22). Both oncocytomas (Table 3, Figure 3 A-B) and metanephric adenomas (Table 3, Figure 3 C, 4 A-F) exhibited classic morphologic features. The former were positive for CD117 and negative for CK7, while the latter were diffusely positive for WT1. All 3 metanephric adenomas showed absence of staining with BRAF V600E and no BRAF alterations were identified by next generation sequencing in 2 of 3 metanephric adenomas that were tested. Finally, rearrangements of the TFEB and TFE3 genes were confirmed using a combination of next generation sequencing, RNA seq, FISH and conventional cytogenetics (Table 3, Figure 3 D) (5, 17).
Table 3.
Immunohistochemistry.
| Patient No. |
Prior Diagnosis | Renal Neoplasm | Immunophenotype |
|---|---|---|---|
| 1 | Wilms Tumor | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 2 | Wilms Tumor | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 3 | Wilms Tumor | Tumor1: Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| Tumor2: Oncocytoma | CD117+, CK7− | ||
| 4 | Wilms Tumor | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 5 | Wilms Tumor | Metanephric Adenoma | WT1+, BRAF V600E− (NGS: negative for BRAF alterations) |
| 6 | Wilms Tumor | Tumor1 (left): Metanephric Adenoma | WT1+, BRAF V600E− (NGS: negative for BRAF alterations), Parafibromin loss |
| Tumor2 (left): Oncocytoma | CD117+, CK7− | ||
| Tumor3 (left): Metanephric Adenoma | WT1+, BRAF V600E− (NGS: insufficient amplifiable DNA), Parafibromin loss | ||
| Tumor4 (left): Unknown | Unknown | ||
| 7 | Neuroblastoma | t(6; 11) RCC* | Cathepsin K+, Melan A+, HMB45+ |
| 8 | Neuroblastoma | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 9 | Neuroblastoma | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 10 | Neuroblastoma | Clear Cell RCC | - |
| 11 | Neuroblastoma | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 12 | Acute Lymphoblastic Leukemia | FH-Deficient RCC | CK7−, FH-deficient, increased 2SC |
| 13 | Acute Lymphoblastic Leukemia | Clear Cell RCC | Diffuse "box-like" CAIX, Melan A− |
| 14 | SLE | Tumor1 (right): ASPL-TFE3** rearranged RCC2 | Diffuse: TFE3, CD10; Focal: CK AE/AE3, Cam5.2 |
| Tumor2 (left): PRCC-TFE3** rearranged RCC | Diffuse: TFE3, CD10; Focal: CK AE/AE3, Cam5.2 |
RCC: renal cell carcinoma; FH: fumarate hydratase; NGS: next generation sequencing; 2SC: S-(2-succino)-cysteine.
Patient 7 was reported previously by Gupta et al (2019) and
Patient 14 was reported previously by Argani et al (2001 and 2006).
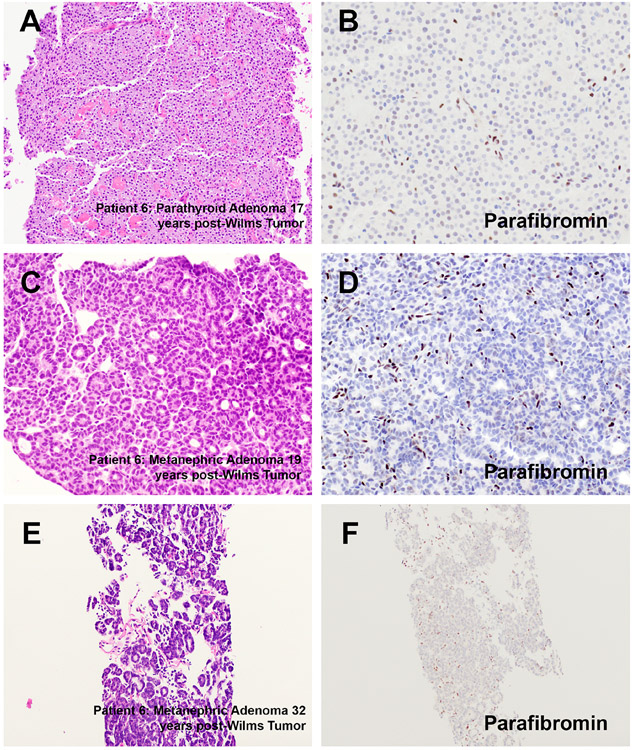
Figure 4. Histopathology.
Representative H&E stained images of a parathyroid adenoma and metanephric adenomas (A, C, E), that occurred 17, 19 and 32 years post-Wilms tumor (patient 6) have been depicted along with representative immunohistochemistry results for parafibromin (B, D and F).
Of note, patient 6 developed a metanephric adenoma in the context of hyperparathyroidism-jaw tumor syndrome 19 years post-Wilms tumor. On further clinical follow up, this patient was recently found to have two separate renal masses (32 years post-Wilms tumor, 2.3cm and 2.1cm). Both lesions were cryoablated and a biopsy of the larger lesion revealed a metanephric adenoma with a similar immunophenotype (WT1+, BRAF V600E−). While sufficient material was not available to perform next generation sequencing for BRAF mutational status for the most recent metanephric adenoma in this patient, next generation sequencing confirmed an absence of BRAF alterations in the first metanephric adenoma. In addition, immunohistochemistry for parafibromin confirmed loss of protein expression in a parathyroid adenoma (Figure 4 A-B) and both metanephric adenomas (Table 3, Figure 4 C-F).
3.3. Literature Review
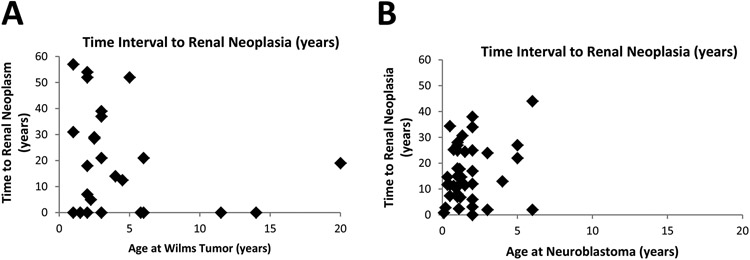
A review of the current English language literature, when combined with cases reported in our current study, led to the identification of 29 cases of Wilms tumor with associated renal neoplasia (3-5, 12, 23-35). The mean age at diagnosis of Wilms tumor and time to subsequent renal neoplasia was 4.3 years and 18.3 years, respectively, for all cases combined (Table 4; Figure 5 A). Ten of the previously reported cases presented with synchronous renal neoplasia and the remainder had documented evidence of subsequent chemotherapy or radiation prior to the second renal neoplasm (Table 4). For cases where the histologic subtype was specified, clear cell RCC was most common and two TFE3 rearranged RCCs were identified in this cohort (Table 4). Interestingly, a female predisposition was identified for all cases combined (20 of 29, 69%); however, given the limited number of cases the significance of this finding is uncertain.
Table 4.
Literature Review.
| Wilms Tumor (n=29) | Neuroblastoma (n=46) | |
|---|---|---|
| Age at Diagnosis (years) | Mean: 4.3; Range: 1 to 20 | Mean: 1.7; Range: 0.1 to 6 |
| Time to Renal Neoplasia (years) | Mean: 18.3; Range: 0 to 57 | Mean: 15.9; Range: 0 to 45 |
| Synchronous Renal Neoplasia | 10/29 (34%) | 1/46 (2%) |
| Renal Neoplasia (Post Chemotherapy or Radiation) | 19/29 (66%) | 41/46 (89%) |
| Male (M) : Female (F) | 9M : 20F | 22M : 24F |
| Histologic Subtype | Clear Cell RCC: 12 cases; MiT Family RCC: 2 cases | Clear Cell RCC: 17 cases; MiT Family RCC: 7 cases |
RCC: renal cell carcinoma.
Figure 5. Time to Renal Neoplasia.
The time interval (in years) to the development of renal neoplasia (y-axis) is shown relative to the age at diagnosis of Wilms tumor (x-axis; A) and neuroblastoma (x-axis; B).
Similarly, 46 cases of neuroblastoma associated with renal neoplasia have been reported, including the patients in our current study (1, 4, 6, 8, 9, 35-50). The mean age at diagnosis of neuroblastoma and time to subsequent renal neoplasia was 1.7 years and 15.9 years, respectively, for all cases combined (Table 4; Figure 5 B). In contrast to Wilms tumor, only a single case of synchronous renal neoplasia was identified and 40 (of 44) of the remainder of the cases had documented evidence of chemotherapy or radiation prior to the second renal neoplasm (Table 4) (44). No sex predilection was identified and for cases where the histologic subtype was specified, clear cell RCC was the most common. Of note, 7 cases of MiT family translocation RCCs were identified in this cohort (Table 4).
4.1. Discussion
Renal cell carcinoma occurring before 45 years of age is rare and has often been reported to occur either in the context of hereditary predisposition syndromes or as a second malignancy following childhood cancer (2, 4). In the latter scenario, this has been primarily documented for Wilms tumor and neuroblastoma that have been treated with chemotherapy or radiation (3, 4). However, it must be noted that a significant number of cases of renal neoplasia following Wilms tumor (and at least 1 case associated with neuroblastoma) were reported to occur synchronously, and some cases have been reported to occur without intervening therapy (6, 12). With regards to patients with Wilms tumor, some underlying factors that were considered in previous studies included a genetic predisposition to renal tumor formation due to mutations in WT1 and WT2 genes (Beckwith-Wiedemann syndrome, Denys-Drash syndrome, and Wilms Tumor-Aniridia-Genitourinary Anomalies-Mental Retardation, WAGR, syndrome), while the contributing role of embryonal nephrogenic rests that do not spontaneously regress is unclear (3, 29, 34).
Of note, the SIR of developing RCC among neuroblastoma, Wilms tumor and acute leukemia survivors was 85.8, 7.4 and 4.2, respectively, compared to the general population (4). In some studies, this risk has been attributed to cytotoxic chemotherapy with platinum based regimens (multivariable analysis, relative risk: 3.5) and radiation therapy of 5 Gy or higher to one or both kidneys (multivariable analysis, relative risk: 3.8) (4). Prior studies have therefore hypothesized that the increased rates of proliferation of renal tissue in early childhood “may render it more sensitive to the mutagenic effects” of chemotherapy or radiation resulting in a higher risk of subsequent renal neoplasia in patients with neuroblastoma when presentation with the pediatric tumor occurs at an earlier age (5).
Initial studies had suggested that RCCs occurring post-neuroblastoma may have specific morphologic and genetic features and based on this, it was considered an unique subtype of RCC under the 2004 World Health Organization (WHO) classification scheme (6, 44). Such a subtype is not currently recognized. Some less common morphologic and molecular subtypes of RCC have been reported in this context and these include carcinomas with oncocytic features, MiT family translocation RCCs, hybrid oncocytic-chromophobe tumors and SDH-deficient RCCs (5, 6, 8, 9, 17, 36, 37, 44, 48). Of these tumors, some have argued that RCCs with oncocytoid features may, in contemporary studies, be reclassified as eosinophilic solid and cystic RCCs that have been described to occur in association with somatic or germline alterations of TSC1 and TSC2 genes (6, 51-53). This suggests that at least a small subset of these cases may occur in the context of a germline predisposition syndrome, for instance associated with tuberous sclerosis complex or SDH-deficiency (8, 9). Of the 19 tumors reported in our study: clear cell RCC was the most common (9 of 19, 47.4%) followed by 3 metanephric adenomas and MiT family translocation RCCs, each (15.8%). No cases with “oncocytoid” morphology or SDH-deficiency were identified; but, at least 2 cases raised the possibility of an underlying syndromal association.
Specifically, the manifestation of Wilms tumor, ossifying fibromas of the jaw and parathyroid adenoma were consistent with a clinical diagnosis of hyperparathyroidism-jaw tumor syndrome for case 6. Although germline alterations of the CDC73 gene (also known as HRPT2, encoding parafibromin) have been implicated in hyperparathyroidism-jaw tumor syndrome, germline testing was not pursued due to a lack of consent (18-20). However, loss of parafibromin in the metanephric adenomas occurring at an interval of 19 and 32 years post-Wilms tumor (based on immunohistochemistry results), is highly suggestive of a syndromal association in this case (54). In addition, 1 patient (case 12) developed a fumarate hydratase (FH)-deficient RCC as a second malignancy following an initial diagnosis of ALL. Neither ALL nor follicular thyroid carcinoma seen in this patient have been described as part of the clinical spectrum of hereditary leiomyomatosis and RCC (HLRCC), secondary to germline pathogenic alterations of the FH gene. Germline testing was not performed for this patient due to a lack of consent. However, the possibility of this FH-deficient RCC being secondary to a somatic alteration of the FH gene cannot be entirely excluded. It must be noted that this case was initially diagnosed as a WHO/ISUP grade 3 papillary renal cell carcinoma and this diagnosis was revised to that of a fumarate hydratase (FH)-deficient RCC based on contemporary pathologic review. Therefore, the exact role of a syndromic background in these patients, if any, in the development of the second neoplasm needs to be weighed against the potential role of chemotherapy or radiation. Consistent with prior studies, all patients (14 of 14) had received prior chemotherapy or radiation (3, 4, 6).
The mean age at diagnosis of the initial neoplasm was 4.7 years for all cases in our study, and overall, neuroblastoma was diagnosed earlier compared to Wilms tumor (neuroblastoma, n=5, 3 years; current study combined with cases in reviewed literature, n=46, 1.7 years; Wilms tumor, n=6, 6 years; current study combined with cases in reviewed literature, n=29, 4.3 years). The average time interval to the development of the second neoplasm was 31 years for all cases reported herein. When looking at a combination of cases in the current study and those reported in the current English language literature, although the time to second renal neoplasm was similar for neuroblastoma (15.9 years) and Wilms tumor (18.3 years), it must be noted that at least 10 reported cases of renal neoplasia were reported to occur synchronously with Wilms tumor, compared to only 1 case of neuroblastoma.
Although 2 patients with Wilms tumor in our study had benign tumors that may have been detected due to more stringent imaging protocols, at least 5 of 14 patients had evidence of metastatic RCC and 2 of these patients died of disease-related complications. In summary, our results suggest that renal neoplasia occurring as a second tumor following childhood cancer or chemotherapy or radiation for other indications have a varied morphologic spectrum and rare cases may have an underlying genetic predisposition. The primary risk factor for the majority of these patients is likely early cytotoxic chemotherapy or radiation. Furthermore, the interval to the development of a second renal neoplasm extended beyond the fourth decade for several patients and this therefore supports extended clinical follow-up into adulthood.
Highlights.
Post-Wilms Tumor: 6 patients; post-neuroblastoma: 5 patients; others: 3 patients.
Most common tumor type: clear cell renal cell carcinoma, 9/19 cases.
Second most common: metanephric adenoma and TFE3/TFEB rearranged renal cell carcinoma, 3/19 cases.
Potential syndromal associations: hyperparathyroidism-jaw tumor syndrome and HLRCC.
History of chemotherapy and/or radiation: 14 of 14 cases.
Average time to subsequent renal neoplasia: 31 years.
6.0. Acknowledgement
The authors have no conflicts of interest or funding to disclose. SG, CMV, LHH, AR, WRS, KAR, CML, RHT, BCL, VER, REJ and JCC performed the research. SG and JCC designed the research study, analyzed the data and wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors of this article have no relevant financial relationships with commercial interests to disclose.
7.0 References
- 1.Al-Mashaikhi N, Yang J, Terry J, Barr R. Renal cell carcinoma with Xp 11.2 translocation as a second tumor in a long-term survivor of advanced neuroblastoma. Pediatr Hematol Oncol 2015; 32, 215–222. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DN, Henderson TO. Late Effects and Survivorship Issues in Patients with Neuroblastoma. Children (Basel) 2018; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rich BS, McEvoy MP, La Quaglia MP. A case of renal cell carcinoma after successful treatment of Wilms tumor. J Pediatr Surg 2010; 45, 1883–1886. [DOI] [PubMed] [Google Scholar]
- 4.Wilson CL, Ness KK, Neglia JP, Hammond S, Shnorhavorian M, Leisenring WL, Stovall M, Robison LL, Armstrong GT. Renal carcinoma after childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 2013; 105, 504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argani P, Lae M, Ballard ET, Amin M, Manivel C, Hutchinson B, Reuter VE, Ladanyi M. Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol 2006; 24, 1529–1534. [DOI] [PubMed] [Google Scholar]
- 6.Falzarano SM, McKenney JK, Montironi R, Eble JN, Osunkoya AO, Guo J, Zhou S, Xiao H, Dhanasekaran SM, Shukla S, Mehra R, Magi-Galluzzi C. Renal Cell Carcinoma Occurring in Patients With Prior Neuroblastoma: A Heterogenous Group of Neoplasms. Am J Surg Pathol 2016; 40, 989–997. [DOI] [PubMed] [Google Scholar]
- 7.Schafernak KT, Yang XJ, Hsueh W, Leestma JL, Stagl J, Goldman S. Pediatric renal cell carcinoma as second malignancy: reports of two cases and a review of the literature. Can J Urol 2007; 14, 3739–3744. [PubMed] [Google Scholar]
- 8.Fairchild RS, Kyner JL, Hermreck A, Schimke RN. Neuroblastoma, pheochromocytoma, and renal cell carcinoma. Occurrence in a single patient. JAMA 1979; 242, 2210–2211. [PubMed] [Google Scholar]
- 9.Schimke RN, Collins DL, Stolle CA. Paraganglioma, neuroblastoma, and a SDHB mutation: Resolution of a 30-year-old mystery. Am J Med Genet A 2010; 152A, 1531–1535. [DOI] [PubMed] [Google Scholar]
- 10.Applebaum MA, Vaksman Z, Lee SM, Hungate EA, Henderson TO, London WB, Pinto N, Volchenboum SL, Park JR, Naranjo A, Hero B, Pearson AD, Stranger BE, Cohn SL, Diskin SJ. Neuroblastoma survivors are at increased risk for second malignancies: A report from the International Neuroblastoma Risk Group Project. Eur J Cancer 2017; 72, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016; 70, 93–105. [DOI] [PubMed] [Google Scholar]
- 12.Zou XP, Jiang YY, Liao Y, Dang YW, Chen G, Feng ZB, Ma Y. The coexistence of a Wilms' tumor and renal cell carcinoma in children: a case report and review of the literature. Onco Targets Ther 2019; 12, 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argani P, Lae M, Hutchinson B, Reuter VE, Collins MH, Perentesis J, Tomaszewski JE, Brooks JS, Acs G, Bridge JA, Vargas SO, Davis IJ, Fisher DE, Ladanyi M. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol 2005; 29, 230–240. [DOI] [PubMed] [Google Scholar]
- 14.Rais-Bahrami S, Drabick JJ, De Marzo AM, Hicks J, Ho C, Caroe AE, Argani P. Xp11 translocation renal cell carcinoma: delayed but massive and lethal metastases of a chemotherapy-associated secondary malignancy. Urology 2007; 70, 178 e173–176. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Cheville JC, Jungbluth AA, Zhang Y, Zhang L, Chen YB, Tickoo SK, Fine SW, Gopalan A, Al-Ahmadie HA, Sirintrapun SJ, Blum KA, Lohse CM, Hakimi AA, Thompson RH, Leibovich BC, Berger MF, Arcila ME, Ross DS, Ladanyi M, Antonescu CR, Reuter VE. JAK2/PD-L1/PD-L2 (9p24.1) amplifications in renal cell carcinomas with sarcomatoid transformation: implications for clinical management. Mod Pathol 2019; 32, 1344–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ, Montironi R, Srigley JR, Members of the IRTP. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013; 37, 1490–1504. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Argani P, Jungbluth AA, Chen YB, Tickoo SK, Fine SW, Gopalan A, Al-Ahmadie HA, Sirintrapun SJ, Sanchez A, Hakimi AA, McFarlane T, Salazar PA, Williamson SR, Skala SL, Mehra R, Hes O, Antonescu CR, Ladanyi M, Arcila ME, Reuter VE. TFEB Expression Profiling in Renal Cell Carcinomas: Clinicopathologic Correlations. Am J Surg Pathol 2019; 43, 1445–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, Gillanders EM, Kennedy AM, Chen JD, Agarwal SK, Sood R, Jones MP, Moses TY, Haven C, Petillo D, Leotlela PD, Harding B, Cameron D, Pannett AA, Hoog A, Heath H 3rd, James-Newton LA, Robinson B, Zarbo RJ, Cavaco BM, Wassif W, Perrier ND, Rosen IB, Kristoffersson U, Turnpenny PD, Farnebo LO, Besser GM, Jackson CE, Morreau H, Trent JM, Thakker RV, Marx SJ, Teh BT, Larsson C, Hobbs MR. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 2002; 32, 676–680. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs MR, Pole AR, Pidwirny GN, Rosen IB, Zarbo RJ, Coon H, Heath H 3rd, Leppert M, Jackson CE. Hyperparathyroidism-jaw tumor syndrome: the HRPT2 locus is within a 0.7-cM region on chromosome 1q. Am J Hum Genet 1999; 64, 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Tuin K, Tops CMJ, Adank MA, Cobben JM, Hamdy NAT, Jongmans MC, Menko FH, van Nesselrooij BPM, Netea-Maier RT, Oosterwijk JC, Valk GD, Wolffenbuttel BHR, Hes FJ, Morreau H. CDC73-Related Disorders: Clinical Manifestations and Case Detection in Primary Hyperparathyroidism. J Clin Endocrinol Metab 2017; 102, 4534–4540. [DOI] [PubMed] [Google Scholar]
- 21.Lau HD, Chan E, Fan AC, Kunder CA, Williamson SR, Zhou M, Idrees MT, Maclean FM, Gill AJ, Kao CS. A Clinicopathologic and Molecular Analysis of Fumarate Hydratase-deficient Renal Cell Carcinoma in 32 Patients. Am J Surg Pathol 2020; 44, 98–110. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Swanson AA, Chen YB, Lopez T, Milosevic D, Kipp BR, Leibovich BC, Thompson RH, Herrera-Hernandez L, Cheville JC, Jimenez RE. Incidence of succinate dehydrogenase and fumarate hydratase-deficient renal cell carcinoma based on immunohistochemical screening with SDHA/SDHB and FH/2SC. Hum Pathol 2019; 91, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allsbrook WC Jr., Boswell WC Jr., Takahashi H, Pantazis CG, Howell CG Jr,, Martinez JE, Beck JR. Recurrent renal cell carcinoma arising in Wilms' tumor. Cancer 1991; 67, 690–695. [DOI] [PubMed] [Google Scholar]
- 24.Anderson BW, Halverstadt DB, Culkin DJ, Johnson SF, Parkhurst JB, Kropp BP. Wilms' tumor with renal cell carcinoma overgrowth in a 3-year-old child. Urology 1998; 52, 128–130. [DOI] [PubMed] [Google Scholar]
- 25.Banner MP, Bleshman MH, Novicki DE. Renal cell carcinoma in a patient successfully treated for Wilms's tumor. AJR Am J Roentgenol 1977; 128, 77–80. [DOI] [PubMed] [Google Scholar]
- 26.Beckwith JB. Wilms' tumor and other renal tumors of childhood: a selective review from the National Wilms' Tumor Study Pathology Center. Hum Pathol 1983; 14, 481–492. [DOI] [PubMed] [Google Scholar]
- 27.Breatnach F, Androulakakis PA. Renal papillary adenocarcinoma following treatment for Wilms' tumor. Cancer 1983; 52, 520–523. [DOI] [PubMed] [Google Scholar]
- 28.Breslow NE, Lange JM, Friedman DL, Green DM, Hawkins MM, Murphy MF, Neglia JP, Olsen JH, Peterson SM, Stiller CA, Robison LL. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer 2010; 127, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherullo EE, Ross JH, Kay R, Novick AC. Renal neoplasms in adult survivors of childhood Wilms tumor. J Urol 2001; 165, 2013–2016; discussion 2016-2017. [DOI] [PubMed] [Google Scholar]
- 30.Hartley AL, Birch JM, Blair V, Jones PM, Gattamaneni HR, Kelsey AM. Second primary neoplasms in a population-based series of patients diagnosed with renal tumours in childhood. Med Pediatr Oncol 1994; 22, 318–324. [DOI] [PubMed] [Google Scholar]
- 31.Kodet R, Marsden HB. Papillary Wilms' tumour with carcinoma-like foci and renal cell carcinoma in childhood. Histopathology 1985; 9, 1091–1102. [DOI] [PubMed] [Google Scholar]
- 32.Kraushaar G, Wiebe S. Renal cell carcinoma as a second malignant neoplasm in a patient with non-syndromic hemihypertrophy and previous Wilms tumor. Pediatr Radiol 2005; 35, 1208–1211. [DOI] [PubMed] [Google Scholar]
- 33.Kushner BH, Meyers PA, Gerald WL, Healey JH, La Quaglia MP, Boland P, Wollner N, Casper ES, Aledo A, Heller G, et al. Very-high-dose short-term chemotherapy for poor-risk peripheral primitive neuroectodermal tumors, including Ewing's sarcoma, in children and young adults. J Clin Oncol 1995; 13, 2796–2804. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus J, Moolman C. Renal cell carcinoma as second malignancy in patient with previous Wilms tumor. Urology 2009; 74, 598–600. [DOI] [PubMed] [Google Scholar]
- 35.Abdulfatah E, Kennedy JM, Hafez K, Davenport MS, Xiao H, Weizer AZ, Palapattu GS, Morgan TM, Mannan R, Wang XM, Dhanasekaran SM, Kaffenberger SD, Spratt DE, Kunju L, Wu A, Lew M, Udager AM, Chinnaiyan AM, Mehra R. Clinicopathological characterisation of renal cell carcinoma in young adults: a contemporary update and review of literature. Histopathology 2020; 76, 875–887. [DOI] [PubMed] [Google Scholar]
- 36.Altinok G, Kattar MM, Mohamed A, Poulik J, Grignon D, Rabah R. Pediatric renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions and clinicopathologic associations. Pediatr Dev Pathol 2005; 8, 168–180. [DOI] [PubMed] [Google Scholar]
- 37.Fenton DS, Taub JW, Amundson GM, Padiyar NP, Cushing B. Renal cell carcinoma occurring in a child 2 years after chemotherapy for neuroblastoma. AJR Am J Roentgenol 1993; 161, 165–166. [DOI] [PubMed] [Google Scholar]
- 38.Flaig TW, Kondo KL, La Rosa FG, Kavanagh B, Crawford ED. A young woman with multiple kidney lesions. Oncology (Williston Park) 2010; 24, 282–286. [PubMed] [Google Scholar]
- 39.Fleitz JM, Wootton-Gorges SL, Wyatt-Ashmead J, McGavran L, Koyle M, West DC, Kurzrock EA, Martin KW, Odom LF. Renal cell carcinoma in long-term survivors of advanced stage neuroblastoma in early childhood. Pediatr Radiol 2003; 33, 540–545. [DOI] [PubMed] [Google Scholar]
- 40.Koyle MA, Hatch DA, Furness PD 3rd, Lovell MA, Odom LF, Kurzrock EA. Long-term urological complications in survivors younger than 15 months of advanced stage abdominal neuroblastoma. J Urol 2001; 166, 1455–1458. [PubMed] [Google Scholar]
- 41.Krigman HR, Bentley RC, Strickland DK, Miller CR, Dehner LP, Washington K. Anaplastic renal cell carcinoma following neuroblastoma. Med Pediatr Oncol 1995; 25, 52–59. [DOI] [PubMed] [Google Scholar]
- 42.Lack EE, Cassady JR, Sallan SE. Renal cell carcinoma in childhood and adolescence: a clinical and pathological study of 17 cases. J Urol 1985; 133, 822–828. [DOI] [PubMed] [Google Scholar]
- 43.Li FP, Cassady JR, Jaffe N. Risk of second tumors in survivors of childhood cancer. Cancer 1975; 35, 1230–1235. [DOI] [PubMed] [Google Scholar]
- 44.Medeiros LJ, Palmedo G, Krigman HR, Kovacs G, Beckwith JB. Oncocytoid renal cell carcinoma after neuroblastoma: a report of four cases of a distinct clinicopathologic entity. Am J Surg Pathol 1999; 23, 772–780. [DOI] [PubMed] [Google Scholar]
- 45.Tefft M, Vawter GF, Mitus A. Second primary neoplasms in children. Am J Roentgenol Radium Ther Nucl Med 1968; 103, 800–822. [DOI] [PubMed] [Google Scholar]
- 46.Wallace B, Organ M, Bagnell S, Rendon R, Merrimen J. Renal cell carcinoma after neuroblastoma: A case study and review of the literature. Can Urol Assoc J 2015; 9, E316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook A, Lorenzo AJ, Salle JL, Bakhshi M, Cartwright LM, Bagi D, Farhat W, Khoury A. Pediatric renal cell carcinoma: single institution 25-year case series and initial experience with partial nephrectomy. J Urol 2006; 175, 1456–1460; discussion 1460. [DOI] [PubMed] [Google Scholar]
- 48.Hedgepeth RC, Zhou M, Ross J. Rapid development of metastatic Xp11 translocation renal cell carcinoma in a girl treated for neuroblastoma. J Pediatr Hematol Oncol 2009; 31, 602–604. [DOI] [PubMed] [Google Scholar]
- 49.Donnelly LF, Rencken IO, Shardell K, Matthay KK, Miller CR, Vartanian RK, Gooding CA. Renal cell carcinoma after therapy for neuroblastoma. AJR Am J Roentgenol 1996; 167, 915–917. [DOI] [PubMed] [Google Scholar]
- 50.Manion S, Hayani A, Husain A, Rink R, Hatch D. Partial nephrectomy for pediatric renal cell carcinoma: an unusual case presentation. Urology 1997; 49, 465–468. [DOI] [PubMed] [Google Scholar]
- 51.Mehra R, Vats P, Cao X, Su F, Lee ND, Lonigro R, Premkumar K, Trpkov K, McKenney JK, Dhanasekaran SM, Chinnaiyan AM. Somatic Bi-allelic Loss of TSC Genes in Eosinophilic Solid and Cystic Renal Cell Carcinoma. Eur Urol 2018; 74, 483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trpkov K, Hes O, Bonert M, Lopez JI, Bonsib SM, Nesi G, Comperat E, Sibony M, Berney DM, Martinek P, Bulimbasic S, Suster S, Sangoi A, Yilmaz A, Higgins JP, Zhou M, Gill AJ, Przybycin CG, Magi-Galluzzi C, McKenney JK. Eosinophilic, Solid, and Cystic Renal Cell Carcinoma: Clinicopathologic Study of 16 Unique, Sporadic Neoplasms Occurring in Women. Am J Surg Pathol 2016; 40, 60–71. [DOI] [PubMed] [Google Scholar]
- 53.Guo J, Tretiakova MS, Troxell ML, Osunkoya AO, Fadare O, Sangoi AR, Shen SS, Lopez-Beltran A, Mehra R, Heider A, Higgins JP, Harik LR, Leroy X, Gill AJ, Trpkov K, Campbell SC, Przybycin C, Magi-Galluzzi C, McKenney JK. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am J Surg Pathol 2014; 38, 1457–1467. [DOI] [PubMed] [Google Scholar]
- 54.Andrici J, Gill AJ, Hornick JL. Next generation immunohistochemistry: Emerging substitutes to genetic testing? Semin Diagn Pathol 2018; 35, 161–169. [DOI] [PubMed] [Google Scholar]