Abstract
Background
For biomechanical investigations on bone or bone implants, bone quality represents an important potential bias. Several techniques for assessing bone quality have been described in the literature. This study aims to systematically summarize the methods currently available for assessing bone quality in human bone tissue, and to discuss the advantages and limitations of these techniques.
Methods
A systematic review of the literature was carried out by searching the PubMed and Web of Science databases from January 2000 to April 2021. References will be screened and evaluated for eligibility by two independent reviewers as per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Studies must apply to bone quality assessment with imaging techniques, mechanical testing modalities, and compositional characterization. The terms used for the systematic search were: “(bone quality”. Ti,ab.) AND “(human bone specimens)”.
Results
The systematic review identified 502 relevant articles in total. Sixty-eight articles met the inclusion criteria. Among them, forty-seven articles investigated several imaging modalities, including radiography, dual-energy X-ray absorptiometry (DEXA), CT-based techniques, and MRI-based methods. Nineteen articles dealt with mechanical testing approaches, including traditional testing modalities and novel indentation techniques. Nine articles reported the correlation between bone quality and compositional characterization, such as degree of bone mineralization (DBM) and organic composition. A total of 2898 human cadaveric bone specimens were included.
Conclusions
Advanced techniques are playing an increasingly important role due to their multiple advantages, focusing on the assessment of bone morphology and microarchitecture. Non-invasive imaging modalities and mechanical testing techniques, as well as the assessment of bone composition, need to complement each other to provide comprehensive and ideal information on the bone quality of human bone specimens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-022-03041-4.
Keywords: Bone quality, Imaging, Mechanical testing, Bone composition
Introduction
As humans age, the rate of bone resorption by osteoclast cells outpaces the rate of bone formation. The mineral content of aged bones declines, eventually resulting in osteoporosis—a condition in which bones become more fragile and prone to fractures [1]. In accordance with World Health Organization (WHO) criteria, 10% of US women older than 50 years had osteoporosis and another 49% had osteopenia at the femur neck in 2005–2006 [2]. In 2010, osteoporosis affected roughly 22 million women and 5.5 million men in the European Union. In view of the variety of fragility fractures, including hip fractures, vertebral fractures, forearm fractures, the estimated economic burden is €37 billion per year [3].
Hence, research on osteoporotic fractures has increased over the past decades. Although bone mineral density (BMD) is considered to be the gold standard for the evaluation of bone strength and fracture risk [4], bone strength is determined by many other factors as bone microstructure, and bone components [4].
Besides methods for bone quality assessment that have been established in the clinical context, there are methods available to directly analyse the mechanical strength of bone tissue, such as micro-indentation, or nano-indentation tests [5, 6].
The aim of this study was to systematically summarize the current techniques commonly used to assess bone quality in human bone specimens, as well as the advantages and limitations of these methods.
Methods
The PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) checklist and algorithm [7] was used to conduct a systematic review of the literature to find all studies concerning the bone quality assessment of human bone specimens. Since data collection has already been completed at the time of PROSPERO registration, this review could not be registered with PROSPERO.
No primary personal data were collected; therefore, no additional ethical approval needed to be obtained.
Information source
A systematic review of the literature was searched by PubMed and Web of Science databases from January 2000 to April 2021. The language of the journal was limited to English, and the searched species was selected to be “human”. To further extend the search, the “similar articles” option of PubMed was employed in each paper.
Search strategy
A search was performed independently by two reviews (F.W. and L.Z.), and terms were used for the systematic search: “(bone quality”. Ti,ab.) AND “(human bone specimens). After removing duplicates, the reviewers scanned the search results by titles and abstracts. After identifying potentially pertinent articles, full-text articles were sourced and checked for suitability according to the inclusion and exclusion criteria (Fig. 1). Any controversy between the two authors was sent and discussed with a third independent author.
Fig. 1.
Preferred reporting item systematic reviews and meta-analysis (PRISMA) flow diagram of study selection
Eligibility criteria
Studies were selected on the basis of the following inclusion criteria: (a) in vitro experiments regarding the bone quality assessment of human bone specimens; (b) research on bone composition (DBM, organic composition); (c) articles published in English. Exclusion criteria were: (a) no access to full text; (b) case reports and review papers; (c) studies on bone implants or screws, bone histological analysis, and clinical patients; (d) non-English language publications; (e) studies on animal bone specimens; (f) studies on finite element analysis (FEA) models.
Data extraction and analysis
Two authors (F.W. and L.Z.) independently performed data extraction and recorded this data using standard spreadsheet software (Excel for Mac 2016, version 16.2.9, Microsoft, Redmond, WA, USA). This included testing methods, authors and year of publication, journal of publication, study design, number of bone specimens, age, the site of specimens, main findings or summaries.
Assessment of study quality
The Newcastle–Ottawa Scale (NOS) [8] which contains three primary components: selection, comparability, and exposure/outcome, is being used to evaluate the quality of non-randomized trials. For this review, the quality of all studies, including bias, was assessed using the adapted Newcastle–Ottawa Quality Assessment Scale (Additional file 1). According to the total quality score, studies were evaluated with the highest score of eleven, as unsatisfactory (0–5), satisfactory (6–8), and good (9–11), which refers to a published article [9]. Two authors (F.W., and L.Z.) assessed all the included articles independently. Disagreements were recorded by discussion.
Statistical analysis
Continuous variables were described by the mean and standard deviation or median and range. Categorical variables were expressed with absolute and relative frequencies. Statistical significance is defined with P < 0.05. The large heterogeneity and lack of randomized controlled trials made it impossible to perform a meta-analysis. Furthermore, since the distributions of some indicators were only ranges, no other statistical analysis was possible.
Results
Study description and quality assessment
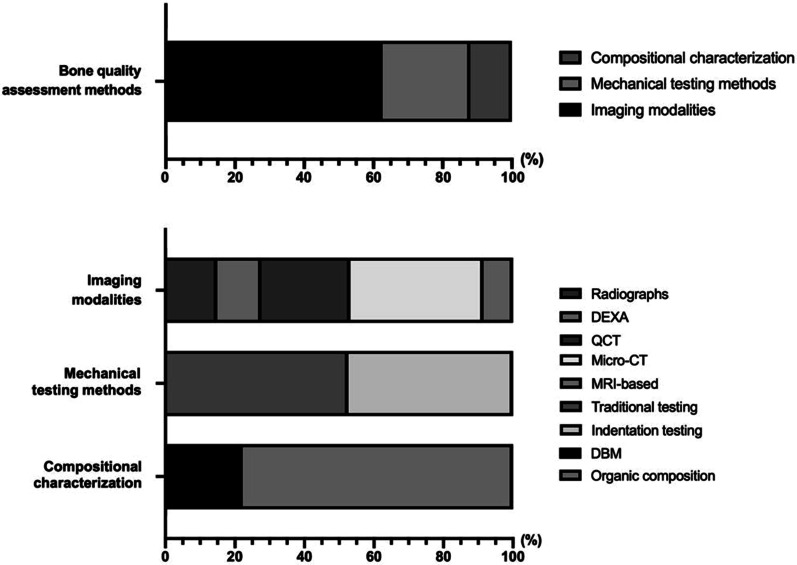
After scrutinizing the titles and abstracts, as well as examining the full texts, the remaining sixty-eight studies were included in the systematic review. Among them, forty-seven articles investigated several imaging modalities, including radiography, dual-energy X-ray absorptiometry (DEXA), CT-based techniques, and MRI-based modalities. Nineteen articles dealt with mechanical testing approaches, including traditional testing methods and novel indentation techniques. Nine articles reported the correlation between bone quality and bone composition, such as DBM, organic composition (Figs. 2 and 3).
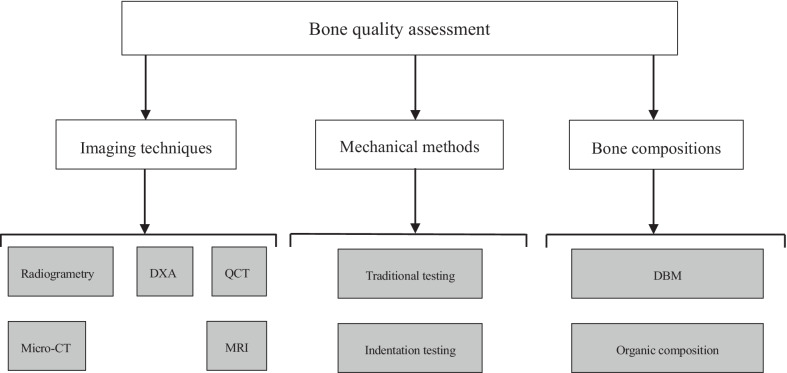
Fig. 2.
Different testing methods of bone quality, as well as the intrinsic bone composition that affects bone quality
Fig. 3.
The percentage of different modalities in assessing bone quality
A total of 2898 human cadaveric bone specimens were included (Table 1). The number of specimens ranged from 4 to 189 with a mean of 42.62 ± 42.39. Each bone quality assessment method has its advantages and limitations, the details see Table 2. The individual scores of each study are recorded in Table 3. Overall, 4, 52, and 12 of the included studies were rated as “unsatisfactory”, “satisfactory”, and “good”, respectively.
Table 1 .
Summary of methods of bone quality with important outcomes, advantages, and limitations
| Testing methods | Authors and year of publication | Journal of publication | Study design | Number of specimens | Age (years) | The site of specimens | Main findings or summaries |
|---|---|---|---|---|---|---|---|
| Radiographs | Tingart, M. J. et al. 2003 | The Journal of Bone and Joint Surgery | CTI | 19 | 72 ± 11 | Humeri | The cortical thickness of the proximal diaphysis is a reliable predictor of the bone quality of the proximal humerus |
| Radiographs | Ebraheim N. et al. 2000 | Spine | Internal architecture | 7 | 57–78 | Sacrum | The strongest part of the sacrum is the anterior cortex above the foramina in S1 and S2. The weakest point of the sacrum was found to lie at the level of the junction of S2 and S3 |
| Radiographs | Huber, M. B. et al. 2009 | Medical Physics | BMD, texture information | 14 | 70.8 (66.1–73.2) | Femoral specimens | Texture information contained in trabecular bone structure visualized on radiographs may predict whether an implant anchorage can be used and may determine the local bone quality from preoperative radiographs |
| Radiographs | Thevenot, J. et al. 2013 | Journal of Bone and Mineral Research | BMD, THI | 178 | 79.3 ± 10.4 | Femoral bone | Conventional radiography is a low‐cost method for evaluating geometry, structure, and fracture risk of bone |
| Plain radiographs, DEXA, pQCT | Clavert, P. et al. 2016 | Surgical Radiologic Anatomy | BMD, CMI | 21 | NR | Distal humerus | More than a direct evaluation of the bone density with a CT-scan, the cortio-medullar index (CMI) of the distal humerus diaphysis is a predictor of the bone quality of the distal humerus |
| DEXA | Tan, J. S. et al. 2010 | The Spine Journal | BMD | 189 | NR | Lumbar specimens | In vitro BMD scan on explanted specimens measured lower DEXA values than in situ BMD scans on full cadavers. A correction factor when used resulted in more accurate measure of the in situ BMD |
| DEXA | Hua Y. et al. 2009 | Clinical Oral Implants Research | Fractal analysis, morphometry | 19 | NR | Mandibular bone | They investigated the accuracy of fractal analysis and morphometry for bone quality assessment as measured with DEXA |
| DEXA | Choel, L. et al. 2003 | Oral Surgery Oral Medicine Oral Pathology, and Oral Radiology | BMD, BMC | 63 | 80.8 ± 10, 82.7 ± 7.3 | Mandibular bone | The intra-alveolar trabecular bone of these 21 mandibles is affected by the same local and systemic influences as cortical bone, whereas the infra-alveolar trabecular bone is mostly sensitive to dental status |
| DEXA | Yang, J. et al. 2012 | Journal of Biomechanical Engineering | BMD, BMC | 9 | NR | Femurs | The proposed technique is capable of detecting differences in bone quality. The ability to measure site-specific properties without exposure to radiation has the potential to be further developed for clinical applications |
| DEXA, QCT | Johannesdottir, F. et al. 2017 | Bone | BMD, microstructures | 76 | 74 ± 8.8 | Proximal femurs | Both cortical and trabecular bone contribute to femoral strength, the contribution of cortical bone being higher in femurs with lower trabecular bone density |
| pQCT | Chaplais E et al. 2014 | BMC Musculoskelet Disord | Material properties of bone | 11 | 75 (59–93) | Leg | This protocol extends the capabilities of pQCT to evaluate bone quality in people who may be at an increased risk of metatarsal insufficiency fractures |
| HR-pQCT | Kirchhoff C. et al. 2012 | BMC Musculoskelet Disord | General morphology | 64 | 72.3 ± 17.4 | Humeral head | The presented microarchitectural data measured by HR-pQCT allow for future subtle biomechanical testing comprising knowledge on age- and sex-related changes of the tuberosities of the humeral head |
| HR-pQCT | de Jong, J. J. et al. 2016 | The Journal of Bone and Joint Surgery | Bone parameters | 15 | 62–90 | Distal radial | HR-pQCT can be used a promising tool to assess the fracture-healing process in patients with fiberglass cast |
| HR-pQCT; micro-CT | Liu X.S., Sekhon K.K. et al. 2010 | J Bone and Mineral Research | Microstructural of human distal tibia | 19 | 70.6 (55–84) | Tibia | Microstructural measurements and mechanical parameters of distal tibia can be efficiently derived from HR-pQCT images and provide additional information regarding bone fragility |
| HR-pQCT; micro-CT | Jorgenson, B. L. et al. 2015 | Bone | Cortical porosity and density | 23 | 66.3 (55–85) | Mid-shaft region of tibiae | The accuracy of the threshold-based method will improve as new HR-pQCT systems emerge and provide a robust quantitative approach to measure cortical porosity |
| pQCT | Diederichs, G. et al. 2006 | Archives of Orthopaedic and Trauma Surgery | Regional BMD | 88 | 75.8 ± 13.5 | Humeri | Bone quality at the humeral head is best predicted by BMD measurements at the contralateral location rather than the ipsilateral distal site |
| HR-pQCT | Manske, S. L. et al. 2015 | Bone | Bone microarchitecture | 20 | 70 (49–95) | Radii | These data support the application of analysis techniques in HR-pQCT that are analogous to those traditionally used for micro-CT to assess trabecular microarchitecture |
| QCT | Mann, C. et al. 2018 | Scientific Reports | BMD | 10 | 80 (59–92) | Lumbar spine | A well-established alternative to DXA is QCT, a three-dimensional method which measures trabecular BMD in milligrams per cubic centimeter by indirectly quantifying hydroxyapatite in comparison with a reference phantom |
| QCT | Zheng Y et al. 2000 | Spine | BMD | 13 | 31 (24–36) | Sacrum | This report detailed BMD variations of the S1 body and ala in a young male group of specimens |
| pQCT | Lu WW. et al. 2000 | Clinical Orthopaedics and Related Research | BMD | 13 | 31 (24–36) | Sacrum | The highest bone mineral density in the lumbosacral spine is found at the pedicles and regions closest to pedicle bases |
| Micro-CT | Lee, J. H. et al. 2017 | Journal of Periodontal &Implant Science | 3D-microstructure | 60 | 75.7 (67.3–96) | Hemimaxillae | Bone quality depended on trabecular separation (Tb.Sp) and number—that is, endosteal space architecture—rather than bone surface and trabecular thickness (Tb.Th). Regardless of bone quality, Tb.Th showed little variation |
| Micro-CT | Chen, R. E. et al. 2019 | Clinical Orthopaedics and Related Research | BMD, CTI | 10 | 63 (59–67) | Distal clavicular regions | In the distal clavicle, BMD and cortical thickness are greatest in the conoid tubercle and intertubercle space |
| Micro-CT | Xie, F. et al. 2018 | Archives of Osteoporosis | Microstructural properties | 67 | NR | Spinous processes | Post-menopausal women and older men with osteoporosis have worse bone quality in autografts than non-osteoporotic men and women. Postmenopausal women with osteoporosis presented serious microarchitectural deterioration in older population |
| Micro-CT | Ding, M. et al. 2012 | Bone | microarchitectural, mechanical, collagen and mineral properties of normal adolescent cancellous bone | 23 | NR | Left proximal tibiae | Micro-CT can be used to measure various parameters, such as 3D microarchitecture, mechanical properties, collagen and mineral properties of adolescent cancellous bone |
| Micro-CT, radiography | Rupprecht, M. et al. 2006 | Journal of Orthopaedic Research | Bone microarchitecture | 60 | NR | Calcanei | Bone mass and structure are risk factors in respect to the occurrence and severity of calcaneal fractures, and indicate that calcaneal fractures are at least in part osteoporotic fractures |
| Micro-CT | Greenwood, C. et al. 2018 | Aging and Disease | Bone microarchitecture | 164 | 21–93 | Femoral heads | Micro-computed tomography was utilised to investigate the microarchitecture of femoral head trabecular bone from a relatively large cohort of non-fracture and fracture human donors |
| Micro-CT | Kuhn, G. et al. 2007 | Journal Homo of Comparative Human Biology | Bone surface structures, microarchitecture | 5 | NR | Postcranial | Micro-CT is a tool of high value for the examination of postcranial bone disorders. It cannot replace histological examinations completely because it cannot assess the bone quality (woven or lamellar) |
| Micro-CT | Arnold, E. L. et al. 2020 | Journal of the mechanical behaviour of biomechanical materials | BMD, TMD, microarchitectural parameters | 100 | 20–93 | Femoral heads | Properties which are not age dependent are significantly different between age-matched non-fracture and fracture specimens, indicating osteoporosis is a disease, and not just an accelerated aging process |
| Micro-CT | Ding, M. et al. 2003 | The Journal of Bone and Joint Surgery | 3D microstructural properties | 120 | 73 (63–81); 72 (58–85) | Proximal tibiae | Using unbiased 3-D methods, we have demonstrated microstructural changes in subchondral cancellous bone in human tibial early OA |
| Micro-CT | Marinozzi, F. et al. 2012 | Ann Ist Super Sanita | 3D-structure, morphometric parameters | 6 | NR | femoral heads | Micro-CT is a promising technique for trabecular bone analysis. Bone morphometric parameters obtained by microtomographic processing allows to completely characterize human bone |
| Micro-CT | Kim, Y. J. et al. 2015 | Clinical Implant Density and Related Research | BMD, 3D-microarchitecture | 34 | NR | Jaw | Two aspects of bone density using micro-CT, the BV/TV and BMD, are highly correlated with 3D micro-architecture parameters, which represent the quality of trabecular bone. This noninvasive method may adequately enhance evaluation of the alveolar bone |
| Micro-CT | Kamal, M. et al. 2018 | The Journal of Craniofacial Surgery | BMD, structural morphometric | 60 | 69.5 (57.3–81.2) | Calvarium, maxillary tuberosity, mandibular ramus, mandibular symphysis, anterior iliac crest, and tibia | The results show great variation in bone densities and 3D morphometric values across different donor sites |
| Micro-CT | Thomsen J.S. et al. 2013 | Bone | BV/TV, Tb.Th, Tb.N, SMI, CD, DA | 79 | 21.7–96.4; 22.6–94.6 | Second lumbar vertebral (L2) | Vertical and horizontal oriented bone density decreases with age in both women and men, and that vertical oriented bone is lost more quickly in women than in men, |
| NMR | Ni, Q.W. et al. 2007 | Measurement Science and Technology | Bound and mobile water | 10 | 65.9 (51–87) | Femurs | Bound to mobile water may be used as a measure of bone quality describing both porosity and water content, both of which may be important determinants of bone strength and fracture resistance |
| HR-MRI | Link, T. M. et al. 2003 | European Radiology | Trabecular bone structure | 39 | 76.9 ± 7.2 | Distal radius | High-resolution MR-derived structure parameters, however, performed better in the prediction of trabecular bone structure |
| MRI (HR-MRI) | Vieth V. et al. 2001 | Investigative Radiology | Trabecular bone structure parameters | 30 | 68.5 ± 8.2 | Calcaneus | Trabecular bone structure depicted by HR-MRI is significantly correlated with that shown in macro-sections |
| Micro-MRI | Liu, X. S., Rajapakse C. S. et al. 2010 | Journal of Bone and Mineral Research | 3D model-independent microstructural measurements | 25 | 70.6 (55–84) | Distal tibias | Most microstructural and mechanical properties of the distal tibia can be derived efficiently from micro-MR images and can provide additional information regarding bone quality |
| Cyclic compressive loading | Goff, M. G.et al. 2015 | Bone | Bone microdamage | 32 | 78 ± 8.8 | Vertebral cancellous bone | Microdamage accumulation in fatigue is likely dominated by heterogeneity in tissue material properties rather than stress concentrations caused by micro-scale geometry |
| Compression-tension loading | Bevill, G. et al. 2006 | Bone | Bone volume fraction and architecture, bone strength | 54 | 70 ± 11 | Femoral neck, greater trochanter, proximal tibia, vertebral body | Within very low-density bone, the potentially important biomechanical effect of large-deformation failure mechanisms on trabecular bone strength is highly heterogeneous and is not well explained by standard architectural metrics |
| Compression test | Ding M et al. 2001 | Acta Orthop Scand | Mechanical and compositional properties | 10 | 73 (63–81) | Proximal tibiae | Cancellous bone quality is reflected by the amount of bone tissue present, the mechanical properties of the tissue, and its trabecular architecture |
| Compression test | Kalouche, I. et al. 2010 | Clinical biomechanics | Mechanical properties | 82 | 88.9 (76–96) | Cadaveric shoulders | Good correlation between apparent density and elastic modulus was found only in the sagittal planes but not in the coronal and axial plane |
| Compression test | Bayraktar, H. H. et al. 2004 | Journal of Biomechanics | Elastic and yield properties | 94 | 65.5 ± 9.1; 71.8 ± 8.8 | Femoral neck | The elastic modulus and yield strains for trabecular tissue are just slightly lower than those of cortical tissue, because of the cumulative effect of these differences, tissue strength is about 25% greater for cortical bone |
| Micro-indentation | Dall'Ara, E. et al. 2012 | Bone | Bone microdamage | 35 | 44–82 | Thoracolumbar vertebral bodies (T12-L5) | Micro-indentation was found to discriminate between highly damaged and intact tissue in both trabecular and cortical bone tested in vitro. It remains to be investigated whether this technique would be able to detect also the damage |
| RPI, bending test | Granke, M. et al. 2014 | Journal of the mechanical behaviour of biomechanical materials | Tissue anisotropy, mechanical behaviour | 26 | 25–101 | Femoral mid-shaft | With a transverse isotropic behaviour akin to tissue hardness and modulus as determined by micro- and nanoindentation and a significant association with toughness, RPI properties are likely influenced by both elastic and plastic behaviour of bone tissue |
| RPI | Jenkins, T. et al. 2015 | Journal of the mechanical behaviour of biomechanical materials | Maximum load, sample orientation, mode of use, sample preparation and measurement spacing | 5 | 67–89 | Femoral heads | RPI users can minimize the potential confounding effects associated with the variables investigated here and reduce the coefficient of variation, hence achieving more consistent testing |
| Nanoindentation | Albert, C. et al. 2013 | Clinical biomechanics | Bone tissue elastic modulus and hardness | 11 | 5–18 | Lower extremity long bones | Nanoindentation can be used to measure bone material properties, providing valuable data |
| Cyclic fatigue loading; Micro-CT | Lambers, F. M.et al. 2013 | PLoS One | Mechanical properties, microdamage and bone microarchitecture | 32 | 76 ± 8.8 | The third lumbar vertebral bodies | Even small amounts of microscopic tissue damage in human vertebral cancellous bone may have large effects on subsequent biomechanical performance |
| Micro-CT, compression testing | Charlebois, M. et al. 2010 | Journal of Biomechanics Engineering | Volume fraction, compressive behaviour | 148 | 53–100 | T12 vertebrae, distal radii, femoral head, calcanei | Reasonable predictions of their compressive mechanical behaviour can be made using the volume fraction and fabric over a broad range of strains |
| Radiographs, Micro-CT, compressive loading | Yeni, Y. N., Wu B., et al. 2013 | Journal of Biomechanics Engineering | Microstructure at various levels of compressive deformation | 7 | NR | Femoral and tibial cancellous bone cylinders | The heterogeneity of the microstructure is especially sensitive to deformation and these could be good parameters to use to estimate strain history in the tissue |
| QCT, uniaxial compression test | Wachter NJ. et al. 2001 | Clinical Biomechanics | Singh index, mechanical competence | 31 | 68.3 ± 11.7 | Femurs | Assessment of bone mineral density by QCT is a reliable and precise method for the estimation of cancellous bone material properties |
| NMR, three-point bending testing | Nyman, J S. et al. 2008 | Bone | Mobile and bound water; Bone strength and toughness | 18 | 66.3 (47–87) | Femurs | Quantifying mobile and bound water with magnetic resonance techniques could potentially serve as indicators of bone quality |
| Bending test, CT scanner | Lettry, S. et al. 2003 | Bone | Mechanical properties, CT numbers | 5 | 85.8 (53–106) | Mandible | A weak correlation was found between the modulus values and the CT number of the mandible. This would not be sufficient for accurate predictions of the bone properties from CT scans |
| Micro-CT, compression test | Karim, L. et al. 2011 | Journal of Orthopaedic Research | Bone microdamage | 26 | 18–97 | Tibial plateaus | Low bone volume fraction and increased structure model index have strong influences on microdamage accumulation in bone through altered initiation |
| Micro-CT, micro-indentation, and bending test | Merlo, K. et al. 2020 | Journal of Orthopaedic Research | Microarchitecture, Mechanical Properties, and AGEs | 40 | 73.1 ± 10.9 | Tibias | The accumulation of AGEs would cause lower elastic modulus and lower fracture toughness in human cortical bone |
| Micro-CT, RPI | Beutel, B. G. et al. 2015 | Bone | BV/TV, Porosity, Mechanical outputs | 6 | 79 (76–88) | Tibiae | RPI parameters will help to further facilitate its use as a clinical diagnostic tool |
| RPI, bending test | Krege J.B. et al. 2016 | Bone | IDI, TID, bone toughness | 4 | 76–85 | Femora | RPI measurements alone, as compared to bending tests, are insufficient to reach conclusions regarding mechanical properties of bone |
| Indentation testing, CT scanner | Zumstein, V. et al. 2012 | Journal of Shoulder and Elbow Surgery | Mechanical strength, subchondral mineralization | 32 | 80.5 (59–95) | Shoulder | Mechanical strength and subchondral mineralization in the humeral head are significantly associated |
| X-ray radiograms, tensile fracture toughness | Yeni, Y. N. Brown C. U.et al. 2013 | Journal of the mechanical behaviour of biomechanical materials | Femoral cortex geometry, tissue mechanical properties | 25 | 53.3 ± 19.7 | femurs | Fracture toughness of the tissue was significantly related to radiogram metric indices and that some of these indices explained a greater variability in toughness than porosity, age or gender |
| Micro-CT, nanoindentation, compressive loading | Li, Z. C. et al. 2012 | Arthritis Rheum | Fatigue strength, microarchitecture, mineralization degree, and biomechanical properties | 60 | 53–86; 59–87 | Femoral head | The difference in mechanical properties between osteoarthritis and osteoporosis cancellous bone is attributed to different bone mass and bone structure |
| Compressive loading, microscopic analysis | Hernandez, C. J. et al. 2014 | Bone | Mechanical properties, BV/TV and microdamage | 47 | 64–92 | Vertebral cancellous bone | Small amounts of microdamage do not necessarily indicate impaired mechanical performance, the presence of modest amounts of microdamage is always indicative of large reductions in cancellous bone stiffness and strength |
| Bending test, RPI, nanoindentation | Katsamenis, O. L. et al. 2015 | Bone | Fracture toughness, crack growth resistance | 4 | 63.25 (43–83) | Femurs | RPI is an emerging technique with the clinical potential for the direct assessment of the mechanical properties of the bone |
| Bone composition; Compression test | Follet, H. et al. 2004 | Bone | DBM, mechanical properties | 20 | 78 ± 8 | Calcaneus | The increase in bone strength when DBM is modified in a physiological range without necessary changes of bone matrix volume and bone microarchitecture |
| Bone composition | Saito, M. et al. 2006 | Calcified Tissue International | DBM, collagen crosslinking | 50 |
78 ± 6 77 ± 6 |
Hip | Detrimental crosslinking in both low and high mineralized bone result in impaired bone quality in osteoporotic patients |
| Bone composition | Karim, L. et al. 2012 | PLoS One | Heterogeneous glycation | 42 | 59.3 ± 22.1 | tibial plateaus | The extent of NEG in tibial cancellous bone was the dominant predictor of bone fragility and was associated with changes in microarchitecture and microdamage |
| Bone composition | Willett, T. L.et al. 2019 | Bone | bone collagen integrity parameters, fracture toughness | 54 | 64.4 ± 21.3 | Femurs or femur mid-shafts | Bone collagen integrity as measured by thermomechanical methods is a key factor in cortical bone fracture toughness |
| Bone composition | Poundarik, A. A. et al. 2015 | Journal of the mechanical behaviour of biomechanical materials | Glycated collagen | 9 | 34–85 | Tibiae | Advanced glycation end-products (AGEs) are predictive of bone quality in aging humans and have diagnostic applications in fracture risk |
| Bone composition | Ural, A. et al. 2015 | Osteoporosis international | NEG | 96 | 60.6 ± 21.0 | Proximal end of tibiae | AGEs alter the resorption process and/or accumulate in the tissue as a result of reduced resorption and may lead to bone fragility by adversely affecting fracture resistance through altered bone matrix properties |
| Bone composition | Wang X et al. 2002 | Bone | Collagen molecular structures, mechanical integrity of the collagen network, mechanical properties of bone | 30 | 19–89 | Femurs | The adverse changes in the collagen network occur as people age and such changes may lead to the decreased toughness of bone. Also, the results suggest that nonenzymatic glycation may be an important contributing factor causing changes in collagen and, consequently, leading to the age-related deterioration of bone quality |
CTI: Cortical thickness; 2D: Two-dimensional; 3D: Three-dimensional; DEXA: Dual-energy X-ray absorptiometry; BMD: Bone mineral density; BMC: Bone mineral content; Micro-CT: Micro-computed tomography; μMRI: Micro-magnetic resonance imaging; BV/TV: Bone volume fraction; Tb.Th: Trabecular thickness; Tb.Sp: Trabecular spacing; Tb.N: Trabecular number; BS/TV: Bone surface density; SMI: Structure model index; Conn.D.: Connectivity density; CD: Connectivity density; DA: Degree of anisotropy; HR-MRI: High-resolution magnetic resonance imaging; RPI: Reference point indentation; HR-pQCT: High-resolution peripheral quantitative computed tomography; CMI: Cortical-medullar index; THI: Trabecular homogeneity index; NMR: Nuclear magnetic resonance; IDI: Indentation distance increase; TID: Total indentation distance; DBM: Degree of bone mineralization; AGEs: Advanced glycation end products; NEG: Non-enzymatic glycation; TMD: Tissue mineral density
Table 2 .
Summary of studies characteristics, patient or specimen demographic details and main findings or summaries
| Category | Methods | Main indicators | Advantages | Limitations | |
|---|---|---|---|---|---|
| Imaging modalities | X-ray-based modalities | Radiography | CTI, CMI, THI | Simplicity, low-cost, low radiation dose | Insufficient precision, 2D imaging |
| DEXA | BMC, BMD | Low radiation dose, accuracy, simplicity | 2D imaging, cannot capture the 3D micro-architecture | ||
| CT-based modalities | QCT, pQCT, HR-pQCT | 3D-morphology, BMC, BMD | High spatial resolution,, reproducible, 3D imaging, non-invasiveness | Larger radiation does, expensive equipment | |
| Micro-CT | 3D-microstructure, BV/TV, Tb.Th, Tb.Sp; Tb.N, BS/TV, BS/BV, SMI, Conn.D |
Comprehensive, high spatial resolution, 3D bone structure, non-invasiveness |
Larger radiation does, expensive equipment | ||
| MR-based modalities | NMR, HR-MRI, μMRI | 3D bone geometry, trabecular morphology |
High accuracy, no radiation, high-resolution 3D imaging, non-invasiveness |
Expensive equipment, professional operation, more susceptible to image post-processing | |
| Mechanical testing | Traditional testing | Compression, tension, bending, and torsion tests |
Elastic modulus, Ultimate strength, Yield strength |
Directness, accuracy, simplicity | Destructive testing, cannot be repeated |
| Indentation testing | Macro-indentation, RPI, nano-indentation | Hardness, Brittleness | Directness, simplicity, minimally invasiveness | Its outcomes are relatively sole, limited to superficial sites, reliability and significance of parameters need to be validated further | |
| Bone composition | – | Computerized quantitative contact microradiography method, HPLC, et al | DBM, Organic phases | An intrinsic effect on bone stiffness and strength | Not comprehensive enough |
Table 3.
Quality Assessment of the Studies by the Newcastle–Ottawa Scale
| Study | Selection comparability outcome | Total (11/11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | |||||||||
| Representativeness of anatomical sites or factors | Representativeness of parametric data | Sample size | Comparability of test/controls on the basis of the analysis | Assessment of outcome | Assessment method | Outcome description | Specimen information (age) | Amount of specimens large enough | Statistical test | ||
| Wang X. et al. 2002 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Tang JS et al. 2010 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | 0 | ★ | ★ | 7/11 |
| Ebraheim N et al. 2000 | ★ | 0 | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 6/11 |
| Tingart MJ et al. 2003 | ★ | ★ | ★ | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Link TM et al. 2003 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Kim YJ et al. 2015 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | 0 | 0 | ★ | 6/11 |
| Xie F et al. 2018 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | 0 | ★ | ★ | 7/11 |
| Chen RE et al. 2019 | ★ | ★ | 0 | ★ | 0 | ★ | 0 | ★ | 0 | ★ | 6/11 |
| Greenwood C et al. 2018 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Hua Y. et al. 2019 | ★ | 0 | 0 | ★ | ★ | ★ | ★ | 0 | 0 | ★ | 6/11 |
| Choel L et al. 2003 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Huber, M. B. et al. 2009 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Johannesdottir, F. et al. 2017 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | ★ | ★ | 9/11 |
| Yang J et al. 2012 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | 0 | 0 | ★ | 6/11 |
| Thevenot, J. et al. 2013 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Kuhn, G. et al. 2007 | ★ | ★ | 0 | ★ | 0 | ★ | 0 | 0 | 0 | 0 | 4/11 |
| Arnold, E. L. et al. 2020 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Lee JH et al. 2017 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Kamal M et al. 2018 | ★ | ★ | 0 | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 7/11 |
| Lu WW et al. 2000 | ★ | 0 | 0 | ★ | 0 | ★ | 0 | ★ | 0 | ★ | 5/11 |
| Zheng Y et al. 2000 | ★ | ★ | 0 | ★ | 0 | 0 | 0 | ★ | 0 | ★ | 5/11 |
| Mann, C. et al. 2018 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Manske, S. L. et al. 2015 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Diederichs, G. et al. 2006 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Kirchhoff C. et al. 2012 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Rupprecht, M. et al. 2006 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | 0 | 0 | ★ | 7/11 |
| Albert, C. et al. 2013 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Ding, M. et al. 2012 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | 0 | 0 | ★ | 6/11 |
| Jenkins, T. et al. 2015 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Clavert, P. et al. 2016 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | 0 | 0 | ★ | 7/11 |
| Katsamenis, O. L. et al. 2015 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Hernandez, C. J. et al. 2014 | ★ | ★ | ★ | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 9/11 |
| Liu, X. S. et al. 2010 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| de Jong, J. J. et al. 2016 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Chaplais E et al. 2014 | ★ | 0 | 0 | ★★ | 0 | ★ | 0 | ★ | 0 | ★ | 6/11 |
| Li, Z. C. et al. 2012 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | ★ | ★ | 9/11 |
| Yeni, Y. N. et al. 2013 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | 0 | 0 | ★ | 7/10 |
| Zumstein, V. et al. 2012 | ★ | ★ | ★ | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 9/11 |
| Krege, J.B. et al. 2016 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Beutel, B. G. et al. 2015 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Merlo, K. et al. 2020 | ★ | ★ | ★ | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 9/11 |
| Karim, L. et al. 2011 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Ni, Q.W. et al. 2007 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | 0 | 6/11 |
| Bayraktar, H. H. et al. 2004 | ★ | ★ | 0 | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 7/11 |
| Lettry, S. et al. 2003 | ★ | 0 | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Lambers, F. M.et al. 2013 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Kalouche, I. et al. 2010 | ★ | ★ | ★ | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 9/11 |
| Ding M et al. 2001 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Bevill, G. et al. 2006 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Goff, M. G.et al. 2015 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Dall'Ara, E. et al. 2012 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
|
Granke, M. et al 2014 |
★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Follet, H. et al. 2004 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Ural, A. et al. 2015 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Poundarik, A. A. et al. 2015 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | 0 | 6/11 |
| Nyman JS. et al. 2006 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | 0 | ★ | 7/11 |
| Willett, T. L.et al. 2019 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Karim, L. et al. 2012 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Saito, M. et al. 2006 | ★ | ★ | 0 | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 8/11 |
| Yeni, Y. N. et al. 2013 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | 0 | ★ | 8/11 |
| Charlebois, M. et al. 2010 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | ★ | ★ | 9/11 |
| Thomsen, J S. et al. 2013 | ★ | ★ | 0 | ★★ | 0 | ★ | ★ | ★ | ★ | ★ | 9/11 |
| Wachter NJ. et al. 2001 | ★ | ★ | 0 | ★★ | ★ | ★ | ★ | ★ | 0 | ★ | 9/11 |
| Jorgenson, B. L. et al. 2015 | ★ | ★ | 0 | ★★ | ★ | ★ | ★ | ★ | 0 | ★ | 9/11 |
| Marinozzi, F. et al. 2012 | ★ | ★ | 0 | ★ | 0 | ★ | 0 | 0 | 0 | 0 | 4/11 |
|
Ding, M. et al 2003 |
★ | ★ | 0 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 9/11 |
|
Liu XS. et al 2010 |
★ | ★ | 0 | ★★ | ★ | ★ | ★ | ★ | 0 | ★ | 9/11 |
|
Vieth V. et al 2001 |
★ | ★ | 0 | ★ | ★ | ★ | ★ | ★ | 0 | ★ | 8/11 |
Imaging modalities
Imaging modalities for assessing bone quality have various advantages, including non-invasiveness, multiple measurements. Especially, the development of advanced imaging techniques allows the assessment of bone quality at the three-dimensional (3D) microstructure level, such as QCT, micro-CT, high-resolution magnetic resonance imaging (HR-MRI).
X-ray-based modalities
Radiography
Traditional radiography is a cost-effective, widely available method for examining bone geometry, structure, and fracture risk that has been utilized in a wide range of studies [10–16]. The fracture toughness of bone tissue is highly connected to the bone shape defined by parameters based on plane X-ray radiogrammetry [11]. Furthermore, cortical thickness (CTI) and cortical-medullar index (CMI), as predictors of bone quality, can be obtained from anteroposterior radiographs [10, 15]. Tingart et al. [15] used anteroposterior radiographs to measure the CTI of 19 human cadaver humeri. The results indicated that the CTI of the proximal diaphysis can be a reliable indicator of the bone quality of the proximal humerus. Moreover, the CTI measured by radiographs has a significant positive correlation with BMD evaluated by DEXA. Clavert et al. [10] tested the CMI of 21 cadaveric distal humeri by plain radiographs showed that it is a predictor of the bone quality of the distal humerus and has a significant positive correlation with BMD measured by DEXA and CT-scan (pQCT). Aside from CTI and CMI, the trabecular homogeneity index (THI) was also used to assess bone quality using a plain radiograph, and it shows a strong connection with DEXA and CT-derived data [12]. However, radiography has its limitations, such as low sensitivity, unable to further visualize the microstructure of bone specimens, and that only 2D images are available.
Dual-energy X-ray absorptiometry (DEXA)
DEXA can provide an integrated examination of cortical and trabecular bone, which is frequently practiced in routine practice [10, 17–21]. Choel et al. [17] used 63 mandibular bone specimens to investigate the potential utilization of DEXA for the assessment of bone mineral content (BMC) and BMD prior to implant placement. Furthermore, Hua et al. [18] used 19 mandibular bone samples to evaluate the accuracy of fractal analysis and morphometry measured by DEXA. However, the limitations of the DEXA technique also need to be considered. Yang et al. [19] suggested that BMD measured by DEXA is only one aspect of the complex understanding of bone quality. Tan et al. [20] used 189 human lumbar specimens to verify and quantify the difference in DEXA-BMD between unexplained (in situ) and explanted (in vitro) scans. They found that the in vitro BMDs of the specimens were lower than those of in situ scans. This implied that several factors can affect the accuracy of the DEXA technique, such as the process of preparation, the surrounding soft tissue and their composition, and the scanning conditions. Additionally, Johannesdottir F [21] found that the aBMD of the femoral neck by DEXA (R2 = 0.69) was significantly lower than bone strength measured by QCT-based FEA in predicting femoral failure load.
CT-based modalities
Quantitative computed tomography (QCT)
As a reliable and accurate technique, QCT and HR-pQCT scans have been used in the laboratory and provide us with valuable and comprehensive insight into bone quality [10, 22–32]. A study by Wachter et al. [30] concluded that QCT is a better predictor for the mechanical strength of the intertrochanteric region with objectivity and high precision. Liu et al. [28] reported that microstructural measurements and mechanical characteristics of the distal tibia can be efficiently derived from HR-pQCT images. Also, HR-pQCT is a promising tool for assessing the fracture healing process at the microscale [23].
Briefly, pQCT has emerged as an accurate technique for measuring bone quality with multiple advantages, including measured density-independent of overlying tissue, less susceptible to interference from bone size, relatively safe, higher accuracy, and 3D visualization [26, 31, 32]. Compared to the first-generation HR-pQCT with a nominal isotropic voxel size of 82 μm, the second-generation HR-pQCT has been improved to 61 μm, which allows a more accurate assessment of trabecular thickness (Tb.Th) [24].
Micro-computed tomography (Micro-CT, μCT)
Micro-CT is an advanced imaging modality for quantifying bone quality with high resolution. Currently, it has been gradually applied to assess the bone quality of human bone specimens, with a range of isotropic voxel resolution from 9 to 37 μm [14, 25, 28, 33–47]. Micro-CT imaging technique has higher accuracy compared to HR-pQCT, and it is considered as the “gold standard” in bone quality assessment, which allows objective and quantitative evaluation of trabecular bone structure [28, 38, 46]. Moreover, the combination of micro-CT images and mechanical tests can provide valuable and comprehensive information about the microarchitecture and microdamage of human cancellous bone specimens [41, 43]. To explore the influences of osteoporosis and gender on the microstructure of bone grafts, Xie et al. [35] used micro-CT to measure several important microstructure parameters, including bone volume fraction (BV/TV), bone surface density (BS/TV), specific bone surface (BS/BV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), structure model index (SMI), and connectivity density (Conn.D.). This non-invasive technique may be sufficient to enhance the evaluation of the bone quality of human bone tissue.
MR-based modalities
The MR-based modalities are a promising tool for evaluating bone morphometry due to their non-invasiveness, and high innate contrast between bone and soft tissue [46, 48–50]. Applications of MR-based scanning include nuclear magnetic resonance (NMR), high-resolution MRI (HR-MRI), and micro-MRI (μMRI).
Link et al. [49] compared parameters of the trabecular bone structure obtained from HR-MRI and multi-slice computed tomography (MSCT) with 39 distal radius bone specimens. Their data indicated that structure parameters derived from HR-MRI performed better in the prediction of trabecular bone structure, although this technology is more susceptible to image post-processing. In addition, the distribution and changes of water within bone tissue in relation to bone quality (i.e. bone strength and toughness) were also investigated by nuclear magnetic resonance (NMR) [48]. The findings demonstrated that quantification of mobile and bound water by MR imaging techniques could potentially serve as an indicator of bone quality. Correspondingly, Ni et al. [48] reported that the distribution of bound and free water measured by NMR could be considered as an important factor to determine bone quality. For microstructure analysis of micro-MRI images, Liu et al. [46] validated the 3D model-independent microstructure measurements by micro-MRI and micro-CT. They concluded that the microstructural and mechanical properties of most bone specimens could be efficiently derived from micro-MRI, as well as provide additional information on bone quality.
Mechanical testing methods
Traditional testing
In addition to indirect imaging modalities, traditional mechanical methods can provide an accurate and direct assessment of bone quality at the tissue level, such as structural stiffness, bone strength, elastic modulus, and ultimate stress [51–55]. Several studies quantitatively investigate the changes in microstructure and morphology of human bone specimens using a combination of mechanical testing methods and micro-CT [56–60].
A uniaxial compression test was employed by Kalouche et al. [55] to determine the mechanical characteristics of glenoid cancellous bone in the three planes (axial, coronal, and sagittal). Bayraktar et al. [51] compared the mechanical properties at the tissue level by compression and tensile tests using human trabecular and cortical bone specimens. Charlebois et al. [56] studied 148 bone specimens from different anatomical regions using unconfined and confined compression methods. The data on the behaviour of human trabecular bone at large strain under compression indicated that the influence of tissue fabric would decrease with strain and plays a significant role in the softening behaviour of bone tissue. Furthermore, the application of mechanical loading also allows the microdamage of bone specimens to be studied, which is an aspect of bone quality [59, 60]. Lambers et al. [57] suggested that microdamage has a greater impact on the bone quality of human cancellous bone. Hence, the application of these traditional testing methods can provide more direct data on bone quality at both the tissue and micro-levels when combined with micro-CT.
Indentation testing
Currently, micro-indentation testing can measure bone properties at the millimeter level, and nano-indentation testing has the potential to measure the mechanical properties of bone at the level of trabeculae or osteons. These novel techniques are being used in vitro to evaluate bone quality at various anatomical sites [5, 6, 61–67].
A study by Dall'Ara et al. [5] concluded that micro-indentation has the ability to distinguish between severely damaged and intact tissue for human vertebral bone tissue. Jenkins et al. [63] claimed that reference point micro-indentation (RPI) can be used as a useful tool for evaluating the mechanical properties of bone in the laboratory. Two studies directly compared RPI with traditional mechanical tests (bending test) [66, 67]. Granke et al. [66] claimed that RPI properties are likely to be influenced by both elastic and plastic behaviour of bone tissue. However, Krege et al. [67] reported that the RPI technique alone is not sufficient to evaluate the mechanical properties of bone.
For nanoindentation, it provides a novel perspective that has been applied to the research on bone materials, especially for volumes as small as lamellae [6, 65]. Albert et al. [6] used nanoindentation to investigate the effects of disease severity (osteogenesis imperfecta) on the local elastic modulus and hardness of bone tissue. The nanoindentation technique makes it possible to investigate the characterization of bone material properties and evaluate modulus and hardness at a smaller scale.
Compositional characterization
As mentioned above, water within bone tissue has a certain effect on bone quality (i.e. bone strength and toughness), which can be measured by NMR. But additionally, compositional characterization (i.e. DBM and organic compositions) is generally acknowledged as being important.
Degree of bone mineralization
The mineralization process consists of a primary deposition of mineral substance on the calcification front, followed by a slow and progressive increase in mineral deposition named secondary mineralization [68]. According to Follet et al. [68], the more mineralized the cancellous bone, the greater the stiffness and compressive strength. Although the increase in DBM may make bone stiffer and more resistant to mechanical loading, too high a mineral to matrix ratio would result in increased brittleness (i.e. higher tendency to crack propagation), and decreased toughness (i.e. the ability to deform without fracturing). Contrastly, this ratio that is too low can lead to bone softening, reduced stiffness and strength. Saito et al. [69] reported that DBM is related to distinct patterns of enzymatic and non-enzymatic cross-links in human bones and is an important element in assessing bone quality.
Organic composition
The ability of bone strength is not only determined by DBM, but also by organic composition (i.e. collagen glycation, collagen cross-links), which has been explored in various studies [47, 70–75]. As the major organic interagent, type I collagen is vulnerable to enzymatic and non-enzymatic biomechanical alterations that impact bone quality in numerous ways [47, 73]. The testing data by Poundarik et al. [72] indicated that advanced glycation end products (advanced glycation end products, AGEs) created by non-enzymatic glycation could be used for diagnostic applications in fracture risk assessment. Ural et al. [70] measured total fluorescent AGEs from 96 human cortical bone specimens indicated that AEGs may contribute to bone fragility by altering bone matrix properties. Furthermore, the extent of non-enzymatic glycation (NEG) is linked to alterations in the microarchitecture and microdamage of cancellous bone [73]. Willett et al. [71] used hydrothermal isometric tension (HIT) to measure the collagen’s thermal stability and network connectivity in order to observe the correlation between bone collagen integrity and fracture toughness of cortical bone. They found that the integrity of bone collagen is a critical factor for the fracture toughness of cortical bone. Therefore, the investigation of the bone matrix at the microstructure, and in particular collagen, plays a fundamental role in the mechanical properties of bone tissue at the macroscopic level.
Discussion
The application of each method is closely related to the study design and the outcomes of interest. The X-ray-based imaging methods, including radiography and DEXA, have the advantages of low-cost, low-radiation. However, their limitations make it impossible to provide comprehensive and accurate information on bone quality. For CT-based techniques, including QCT, HR-pQCT, and micro-CT, they can perform 3D image reconstruction and microstructure analysis of human bone specimens, which enables more accurate and comprehensive bone quality information. Many studies have taken micro-CT imaging analysis as the “gold standard” of bone quality assessment [28, 38, 46]. The micro-CT technique can be considered as a comprehensive, high-resolution, three-dimensional, non-invasive technique for the assessment of bone microstructure. Moreover, the development of advanced MRI-based techniques, such as NMR, HR-MRI, micro-MRI, has shown promising results in the assessment of bone structure and water composition, providing additional information [46, 50]. However, most of the advanced imaging techniques described in this review are limited to a minority of laboratories due to expensive equipment and professional operation. Related research and technological breakthroughs need to be explored in order to make these novel imaging techniques to in vivo research and eventually to the clinic.
Compared to indirect imaging techniques, conventional mechanical methods can directly provide the performance of whole bone or bulk tissue specimens. Nevertheless, they are used only for ex vivo bone specimens due to the nature of destruction. However, with the development of indentation techniques, it is possible to directly test bone quality in a minimally invasive manner [5, 62, 66]. This technique has the advantages of being direct, simple, minimally invasive, as well as allowing in vivo testing. The shortcomings are that its results are relatively sole (only tissue hardness and brittleness) [66] and are limited to superficial sites, such as the tibial midshaft. Also, the reliability and significance of the parameters need to be validated further [76]. From our perspective, the combination of imaging modalities and mechanical testing methods would be a good choice for the assessment of bone quality ideally and comprehensively at both micro- and tissue levels.
Furthermore, compositional characterization, both DBM and organic composition, plays an essential role in assessing the mechanical properties of bone tissue and can provide more fundamental information that yields mechanistic insights into affecting bone quality [68, 69, 71]. Especially for bone collagen, there is a significant association with clinically relevant bone diseases, such as osteoporosis, osteogenesis imperfecta, and diabetes-related diseases [77, 78].
Previous studies have reported that collagen content in human bones reaches a maximum during adolescence and gradually decreases thereafter with aging [77, 79]. Compared with age-matched healthy subjects, osteoporotic bone indicated that reductions in the enzymatic cross-links and an increase in AGEs cross-links in bone [69, 80]. In diabetic bone tissue, BMD may be normal, but bone strength has decreased, which correlates with the increased formation of AGEs [77, 81]. For osteogenesis imperfecta, it is also a disease closely associated with collagen, and studies have shown that the orientation of collagen is highly disordered and that the collagen-mineral particle network is profoundly altered [78]. Therefore, the alteration of bone quality and biomechanical performances is the macroscopic result of a sequence of composition and microstructural events.
There are several limitations to our systematic review. First, not all test methods and studies were summarized in our review, which is a limitation of all systematic reviews. In this article, “bone quality” and “human bone specimens” were used as search terms. Actually, “bone quality” is not universally defined, and there are several other interchangeable phrases used, including “bone material quality”, “bone matrix quality”, etc. Similarly, the search term “human bone specimens” is interchangeable with “human bone samples”. Due to the limitation of content, it is difficult, or even almost impossible, to use all possible search terms in a review.
In order to further expand the search, the “similar articles” option of PubMed and the references for main articles were used in this review. Second, there is the risk of selection bias since the presence of heterogeneous. Finally, this review focuses only on imaging techniques, mechanical testing methods, and the effects of compositional characterization, computational techniques such as FEA are not included.
Conclusions
Advanced techniques are playing an increasingly important role due to their multiple advantages, focusing on the assessment of bone morphology and microarchitecture. Non-invasive imaging modalities and mechanical testing techniques, as well as the assessment of bone composition, need to complement each other in order to provide comprehensive and ideal information on the bone quality of human bone specimens.
Supplementary Information
Additional file 1. The adapted Newcastle-Ottawa Quality Assessment Scale was used for quality assessment of the included studies.
Acknowledgements
Not applicable.
Abbreviations
- CTI
Cortical thickness
- 2D
Two-dimensional
- 3D
Three-dimensional
- DEXA
Dual-energy X-ray absorptiometry
- BMD
Bone mineral density
- BMC
Bone mineral content
- μCT
Micro-computed tomography
- μMRI
Micro-magnetic resonance imaging
- BV/TV
Bone volume fraction
- Tb.Th
Trabecular thickness
- Tb.Sp
Trabecular spacing
- Tb.N
Trabecular number
- BS/TV
Bone surface density
- SMI
Structure model index
- Conn.D.
Connectivity density
- HR-MRI
High-resolution magnetic resonance imaging
- RPI
Reference point indentation
- HR-pQCT
High-resolution peripheral quantitative computed tomography
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- WHO
World Health Organization
- FEA
Finite element analysis
- NOS
Newcastle–Ottawa Scale
- CMI
Cortical-medullar index
- THI
Trabecular homogeneity index
- NMR
Nuclear magnetic resonance
- MSCT
Multi-slice computed tomography
- DBM
Degree of bone mineralization
- AGEs
Advanced glycation end products
- NEG
Non-enzymatic glycation
- HIT
Hydrothermal isometric tension
Authors’ contributions
GO and FXW performed study design. FXW and LYZ participated in the literature search and article writing; JT, SS, and CEH were in charge of quality assessment and manuscript review. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All the data of the manuscript are presented in the paper or additional supporting files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Patient and public involvement
No patient involved.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fangxing Wang and Leyu Zheng have contributed equally to this publication
Contributor Information
Fangxing Wang, Email: fw92xadu@studserv.uni-leipzig.de.
Leyu Zheng, Email: leyu.zheng@medizin.uni-leipzig.de.
Jan Theopold, Email: jan.theopold@medizin.uni-leipzig.de.
Stefan Schleifenbaum, Email: Stefan.Schleifenbaum@medizin.uni-leipzig.de.
Christoph-Eckhard Heyde, Email: Heyde@medizin.uni-leipzig.de.
Georg Osterhoff, Email: Georg.Osterhoff@medizin.uni-leipzig.de.
References
- 1.Osterhoff G, Morgan EF, Shefelbine SJ, et al. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:S11–S20. doi: 10.1016/S0020-1383(16)47003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker AC, Melton LJ, 3rd, Harris TB, et al. Prevalence and trends in low femur bone density among older US adults: NHANES 2005–2006 compared with NHANES III. J Bone Miner Res. 2010;25(1):64–71. doi: 10.1359/jbmr.090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svedbom A, Hernlund E, Ivergard M, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8:137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca H, Moreira-Goncalves D, Coriolano HJ, et al. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 5.Dall'Ara E, Schmidt R, Zysset P. Microindentation can discriminate between damaged and intact human bone tissue. Bone. 2012;50(4):925–929. doi: 10.1016/j.bone.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Albert C, Jameson J, Toth JM, et al. Bone properties by nanoindentation in mild and severe osteogenesis imperfecta. Clin Biomech (Bristol, Avon) 2013;28(1):110–116. doi: 10.1016/j.clinbiomech.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 7.LA Moher D, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses—the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA SB, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://www.ohrica/programs/clinical_epidemiology/oxfordasp.2014. Accessed 27 Aug 2014.
- 9.Schoeb M, Hamdy NAT, Malgo F, et al. Added value of impact microindentation in the evaluation of bone fragility: a systematic review of the literature. Front Endocrinol (Lausanne) 2020;11:15. doi: 10.3389/fendo.2020.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clavert P, Javier RM, Charrissoux JL, et al. How to determine the bone mineral density of the distal humerus with radiographic tools? Surg Radiol Anat. 2016;38(4):389–393. doi: 10.1007/s00276-015-1569-6. [DOI] [PubMed] [Google Scholar]
- 11.Yeni YN, Brown CU, Gruen TA, et al. The relationships between femoral cortex geometry and tissue mechanical properties. J Mech Behav Biomed Mater. 2013;21:9–16. doi: 10.1016/j.jmbbm.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Thevenot J, Hirvasniemi J, Finnila M, et al. Trabecular homogeneity index derived from plain radiograph to evaluate bone quality. J Bone Miner Res. 2013;28(12):2584–2591. doi: 10.1002/jbmr.1987. [DOI] [PubMed] [Google Scholar]
- 13.Huber MB, Carballido-Gamio J, Fritscher K, et al. Development and testing of texture discriminators for the analysis of trabecular bone in proximal femur radiographs. Med Phys. 2009;36(11):5089–5098. doi: 10.1118/1.3215535. [DOI] [PubMed] [Google Scholar]
- 14.Rupprecht M, Pogoda P, Mumme M, et al. Bone microarchitecture of the calcaneus and its changes in aging: a histomorphometric analysis of 60 human specimens. J Orthop Res. 2006;24(4):664–674. doi: 10.1002/jor.20099. [DOI] [PubMed] [Google Scholar]
- 15.Tingart MJ, Apreleva M, von Stechow D, et al. The cortical thickness of the proximal humeral diaphysis predicts bone mineral density of the proximal humerus. J Bone Joint Surg Br. 2003;85-B(4):611–617. doi: 10.1302/0301-620x.85b4.12843. [DOI] [PubMed] [Google Scholar]
- 16.Ebraheim NSF, Nadim Y, Xu R, Yeasting RA. Internal architecture of the sacrum in the elderly. Spine (Phila Pa 1976) 2000;25(3):292–297. doi: 10.1097/00007632-200002010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Choel L, Duboeuf F, Bourgeois D, et al. Trabecular alveolar bone in the human mandible: a dual-energy x-ray absorptiometry study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(3):364–370. doi: 10.1067/moe.2003.119. [DOI] [PubMed] [Google Scholar]
- 18.Hua YNO, Duyck J, Maes F, Jacobs R. Bone quality assessment based on cone beam computed tomography imaging. Clin Oral Implants Res. 2009;20(8):767–771. doi: 10.1111/j.1600-0501.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Sangiorgio SN, Borkowski SL, et al. Site-specific quantification of bone quality using highly nonlinear solitary waves. J Biomech Eng. 2012;134(10). [DOI] [PubMed]
- 20.Tan JS, Kayanja MM, St Clair SF. The difference in spine specimen dual-energy X-ray absorptiometry bone mineral density between in situ and in vitro scans. Spine J. 2010;10(9):784–788. doi: 10.1016/j.spinee.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Johannesdottir F, Thrall E, Muller J, et al. Comparison of non-invasive assessments of strength of the proximal femur. Bone. 2017;105:93–102. doi: 10.1016/j.bone.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Mann C, Ziegeler K, Mews J, et al. Bone mineral density assessment using iterative reconstruction compared with quantitative computed tomography as the standard of reference. Sci Rep. 2018;8(1):15095. doi: 10.1038/s41598-018-33444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jong JJ, Arts JJ, Meyer U, et al. Effect of a cast on short-term reproducibility and bone parameters obtained from HR-pQCT measurements at the distal end of the radius. J Bone Joint Surg Am. 2016;98(5):356–362. doi: 10.2106/JBJS.O.00127. [DOI] [PubMed] [Google Scholar]
- 24.Manske SL, Zhu Y, Sandino C, et al. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone. 2015;79:213–221. doi: 10.1016/j.bone.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Jorgenson BL, Buie HR, McErlain DD, et al. A comparison of methods for in vivo assessment of cortical porosity in the human appendicular skeleton. Bone. 2015;73:167–175. doi: 10.1016/j.bone.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Chaplais EGD, Hood A, Telfer S, du Toit V, Singh-Grewal D, Burns J, Rome K, Schiferl DJ, Hendry GJ. Reproducibility of a peripheral quantitative computed tomography scan protocol to measure the material properties of the second metatarsal. BMC Musculoskelet Disord. 2014;15:242. doi: 10.1186/1471-2474-15-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchhoff CBV, Milz S, Sprecher CM, Kirchhoff S, Graw M, Imhoff AB, Hinterwimmer S. Age and gender as determinants of the bone quality of the greater tuberosity- A HR-pQCT cadaver study. BMC Musculoskelet Disord. 2012;13:221. doi: 10.1186/1471-2474-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XS, Zhang XH, Sekhon KK, et al. High-resolution peripheral quantitative computed tomography can assess microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res. 2010;25(4):746–756. doi: 10.1359/jbmr.090822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diederichs G, Korner J, Goldhahn J, et al. Assessment of bone quality in the proximal humerus by measurement of the contralateral site: a cadaveric analyze. Arch Orthop Trauma Surg. 2006;126(2):93–100. doi: 10.1007/s00402-006-0103-z. [DOI] [PubMed] [Google Scholar]
- 30.Wachter NJAP, Hoellen IP, Krischak GD, Sarkar MR, Mentzel M, Kinzl L, Claes L. Predictive value of Singh index and bone mineral density measured by quantitative computed tomography in determining the local cancellous bone quality of the proximal femur. Clin Biomech (Bristol, Avon) 2001;16(3):257–262. doi: 10.1016/s0268-0033(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 31.Zheng YLW, Zhu Q, Qin L, Zhong S, Leong JC. Variation in bone mineral density of the sacrum in young adults and its significance for sacral fixation. Spine (Phila Pa 1976) 2000;25(3):353–357. doi: 10.1097/00007632-200002010-00016. [DOI] [PubMed] [Google Scholar]
- 32.Lu WWZY, Holmes A, Zhu Q, Luk KD, Zhong S, Leong JC. Bone mineral density variations along the lumbosacral spine. Clin Orthop Relat Res. 2000;378:255–263. doi: 10.1097/00003086-200009000-00036. [DOI] [PubMed] [Google Scholar]
- 33.Arnold EL, Clement J, Rogers KD, et al. The use of μCT and fractal dimension for fracture prediction in osteoporotic individuals. J Mech Behav Biomed Mater. 2020;103:103585. doi: 10.1016/j.jmbbm.2019.103585. [DOI] [PubMed] [Google Scholar]
- 34.Chen RE, Soin SP, El-Shaar R, et al. What regions of the distal clavicle have the greatest bone mineral density and cortical thickness? A cadaveric study. Clin Orthop Relat Res. 2019;477(12):2726–2732. doi: 10.1097/CORR.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie F, Zhou B, Wang J, et al. Microstructural properties of trabecular bone autografts: comparison of men and women with and without osteoporosis. Arch Osteoporos. 2018;13(1):18. doi: 10.1007/s11657-018-0422-z. [DOI] [PubMed] [Google Scholar]
- 36.Kamal M, Gremse F, Rosenhain S, et al. Comparison of bone grafts from various donor sites in human bone specimens. J Craniofac Surg. 2018;29(6):1661–1665. doi: 10.1097/SCS.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 37.Greenwood C, Clement J, Dicken A, et al. Age-related changes in femoral head trabecular microarchitecture. Aging Dis. 2018;9(6):976–987. doi: 10.14336/AD.2018.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Kim HJ, Yun JH. Three-dimensional microstructure of human alveolar trabecular bone: a micro-computed tomography study. J Periodontal Implant Sci. 2017;47(1):20–29. doi: 10.5051/jpis.2017.47.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YJ, Henkin J. Micro-computed tomography assessment of human alveolar bone: bone density and three-dimensional micro-architecture. Clin Implant Dent Relat Res. 2015;17(2):307–313. doi: 10.1111/cid.12109. [DOI] [PubMed] [Google Scholar]
- 40.Marinozzi F, Marinozzi A, Bini F, et al. Variability of morphometric parameters of human trabecular tissue from coxo-arthritis and osteoporotic samples. Ann Ist Super Sanita. 2012;48(1):19–25. doi: 10.4415/ANN_12_01_04. [DOI] [PubMed] [Google Scholar]
- 41.Li ZC, Dai LY, Jiang LS, et al. Difference in subchondral cancellous bone between postmenopausal women with hip osteoarthritis and osteoporotic fracture: implication for fatigue microdamage, bone microarchitecture, and biomechanical properties. Arthritis Rheum. 2012;64(12):3955–3962. doi: 10.1002/art.34670. [DOI] [PubMed] [Google Scholar]
- 42.Ding M, Danielsen CC, Hvid I, et al. Three-dimensional microarchitecture of adolescent cancellous bone. Bone. 2012;51(5):953–960. doi: 10.1016/j.bone.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Karim L, Vashishth D. Role of trabecular microarchitecture in the formation, accumulation, and morphology of microdamage in human cancellous bone. J Orthop Res. 2011;29(11):1739–1744. doi: 10.1002/jor.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhn G, Schultz M, Muller R, et al. Diagnostic value of micro-CT in comparison with histology in the qualitative assessment of historical human postcranial bone pathologies. Homo. 2007;58(2):97–115. doi: 10.1016/j.jchb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Ding M, Odgaard A, Hvid I, et al. Changes in the three-dimensional microstructure of human tibial cancellous bone in early osteoarthritis. J Bone Joint Surg Br Vol. 2003;85B(6):906–912. [PubMed] [Google Scholar]
- 46.Liu XS, Zhang XH, Rajapakse CS, et al. Accuracy of high-resolution in vivo micro magnetic resonance imaging for measurements of microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res. 2010;25(9):2039–2050. doi: 10.1002/jbmr.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomsen JS, Niklassen AS, Ebbesen EN, et al. Age-related changes of vertical and horizontal lumbar vertebral trabecular 3D bone microstructure is different in women and men. Bone. 2013;57(1):47–55. doi: 10.1016/j.bone.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Ni Q, Nyman JS, Wang X, et al. Assessment of water distribution changes in human cortical bone by nuclear magnetic resonance. Meas Sci Technol. 2007;18(3):715–723. [Google Scholar]
- 49.Link TM, Vieth V, Stehling C, et al. High-resolution MRI vs multislice spiral CT: which technique depicts the trabecular bone structure best? Eur Radiol. 2003;13(4):663–671. doi: 10.1007/s00330-002-1695-5. [DOI] [PubMed] [Google Scholar]
- 50.Vieth VLT, Lotter A, Persigehl T, Newitt D, Heindel W, Majumdar S. Does the trabecular bone structure depicted by high-resolution MRI of the calcaneus reflect the true bone structure? Invest Radiol. 2001;36(4):210–217. doi: 10.1097/00004424-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Bayraktar HH, Morgan EF, Niebur GL, et al. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech. 2004;37(1):27–35. doi: 10.1016/s0021-9290(03)00257-4. [DOI] [PubMed] [Google Scholar]
- 52.Lettry S, Seedhom BB, Berry E, et al. Quality assessment of the cortical bone of the human mandible. Bone. 2003;32(1):35–44. doi: 10.1016/s8756-3282(02)00921-3. [DOI] [PubMed] [Google Scholar]
- 53.Bevill G, Eswaran SK, Gupta A, et al. Influence of bone volume fraction and architecture on computed large-deformation failure mechanisms in human trabecular bone. Bone. 2006;39(6):1218–1225. doi: 10.1016/j.bone.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Ding MDC, Hvid I. Bone density does not reflect mechanical properties in early-stage arthrosis. Acta Orthop Scand. 2001;72(2):181–185. doi: 10.1080/000164701317323444. [DOI] [PubMed] [Google Scholar]
- 55.Kalouche I, Crepin J, Abdelmoumen S, et al. Mechanical properties of glenoid cancellous bone. Clin Biomech (Bristol, Avon) 2010;25(4):292–298. doi: 10.1016/j.clinbiomech.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Charlebois M, Pretterklieber M, Zysset PK. The role of fabric in the large strain compressive behavior of human trabecular bone. J Biomech Eng. 2010;132(12):121006. doi: 10.1115/1.4001361. [DOI] [PubMed] [Google Scholar]
- 57.Lambers FM, Bouman AR, Rimnac CM, et al. Microdamage caused by fatigue loading in human cancellous bone: relationship to reductions in bone biomechanical performance. PLoS ONE. 2013;8(12):e83662. doi: 10.1371/journal.pone.0083662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeni YN, Wu B, Huang L, et al. Mechanical loading causes detectable changes in morphometric measures of trabecular structure in human cancellous bone. J Biomech Eng. 2013;135(5):54505. doi: 10.1115/1.4024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goff MG, Lambers FM, Nguyen TM, et al. Fatigue-induced microdamage in cancellous bone occurs distant from resorption cavities and trabecular surfaces. Bone. 2015;79:8–14. doi: 10.1016/j.bone.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernandez CJ, Lambers FM, Widjaja J, et al. Quantitative relationships between microdamage and cancellous bone strength and stiffness. Bone. 2014;66:205–213. doi: 10.1016/j.bone.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merlo K, Aaronson J, Vaidya R, et al. In vitro-induced high sugar environments deteriorate human cortical bone elastic modulus and fracture toughness. J Orthop Res. 2020;38(5):972–983. doi: 10.1002/jor.24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zumstein V, Kraljevic M, Wirz D, et al. Correlation between mineralization and mechanical strength of the subchondral bone plate of the humeral head. J Shoulder Elbow Surg. 2012;21(7):887–893. doi: 10.1016/j.jse.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins T, Coutts LV, Dunlop DG, et al. Variability in reference point microindentation and recommendations for testing cortical bone: maximum load, sample orientation, mode of use, sample preparation and measurement spacing. J Mech Behav Biomed Mater. 2015;42:311–324. doi: 10.1016/j.jmbbm.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 64.Beutel BG, Kennedy OD. Characterization of damage mechanisms associated with reference point indentation in human bone. Bone. 2015;75:1–7. doi: 10.1016/j.bone.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 65.Katsamenis OL, Jenkins T, Thurner PJ. Toughness and damage susceptibility in human cortical bone is proportional to mechanical inhomogeneity at the osteonal-level. Bone. 2015;76:158–168. doi: 10.1016/j.bone.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 66.Granke M, Coulmier A, Uppuganti S, et al. Insights into reference point indentation involving human cortical bone: sensitivity to tissue anisotropy and mechanical behavior. J Mech Behav Biomed Mater. 2014;37:174–185. doi: 10.1016/j.jmbbm.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krege JB, Aref MW, McNerny E, et al. Reference point indentation is insufficient for detecting alterations in traditional mechanical properties of bone under common experimental conditions. Bone. 2016;87:97–101. doi: 10.1016/j.bone.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Follet H, Boivin G, Rumelhart C, et al. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34(5):783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006;79(3):160–168. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 70.Ural A, Janeiro C, Karim L, et al. Association between non-enzymatic glycation, resorption, and microdamage in human tibial cortices. Osteoporos Int. 2015;26(3):865–873. doi: 10.1007/s00198-014-2938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willett TL, Dapaah DY, Uppuganti S, et al. Bone collagen network integrity and transverse fracture toughness of human cortical bone. Bone. 2019;120:187–193. doi: 10.1016/j.bone.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poundarik AA, Wu PC, Evis Z, et al. A direct role of collagen glycation in bone fracture. J Mech Behav Biomed Mater. 2015;52:120–130. doi: 10.1016/j.jmbbm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE. 2012;7(4):e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang XSX, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 75.Nyman JSRA, Acuna RL, Gayle HJ, Reyes MJ, Tyler JH, Dean DD, Wang X. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone. 2006;39(6):1210–1217. doi: 10.1016/j.bone.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donnelly E. Methods for assessing bone quality: a review. Clin Orthopaed Related Res. 2010;469(8):2128–2138. doi: 10.1007/s11999-010-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 78.Mahr M, Blouin S, Misof BM, et al. Bone properties in osteogenesis imperfecta: what can we learn from a bone biopsy beyond histology? Wien Med Wochenschr. 2021;171(5–6):111–119. doi: 10.1007/s10354-021-00818-w. [DOI] [PubMed] [Google Scholar]
- 79.Zioupos PCJ, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45(2):108–116. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 80.Saito M, Fujii K, Soshi S, et al. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17(7):986–995. doi: 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 81.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The adapted Newcastle-Ottawa Quality Assessment Scale was used for quality assessment of the included studies.
Data Availability Statement
All the data of the manuscript are presented in the paper or additional supporting files.