Abstract
Background
Ambient air pollution poses a major risk for the development and aggravation of respiratory diseases. Evidence suggests that even in low-level air pollution environments there is a risk for an increase in adverse respiratory symptoms. We examined whether variations in daily air pollution levels of nitrogen dioxide, ozone, or particulate matter in Berlin, Germany were associated with hospital admissions of chronic obstructive pulmonary disease (COPD) and asthma patients in a time series analysis.
Methods
We calculated single and multi-pollutant models, investigated possible lags in effect, and analysed the influence of meteorological variables on the results. Data from January 2005 through December 2015 were used to quantify the concentration–response.
Results
The risk ratio for asthma patients to be hospitalised on the same day of NO2 exposure was 1.101 per 10 µg/m3 NO2 increase (95% CI: 1.013 to 1.195), for COPD patients 1.123 (95% CI: 1.081 to 1.168). Neither the exposure to ozone (95% CI: 0.904 to 1.020), PM10 (95% CI: 0.990 to 1.127), nor PM2.5 (95% CI: 0.981 to 1.148) was associated with an increased risk ratio for asthma patients to be hospitalised. Risk ratios for the hospital admission of COPD patients were also not increased due to ozone (95% CI: 0.981 to 1.033), PM10 (95% CI: 0.988 to 1.032), or PM2.5 (95% CI: 0.966 to 1.019) exposure. The presented risk ratios and confidence intervals relate to the day of exposure. We found no increased hospitalisation risks with a delayed occurrence on subsequent days.
Conclusions
A quantifiable, statistically significant increase in risk for asthma and COPD exacerbations owing to NO2 exposure at levels well below European regulatory limit values was observed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-01983-1.
Keywords: Nitrogen dioxide, Ozone, Particulate matter, Hospital admission, Morbidity, Limit values
Background
Ambient air pollution poses a major risk for the development and aggravation of respiratory diseases. Positive associations between short-term exposures to particulate matter (PM with an aerodynamic diameter of 2.5 µm or less, PM2.5), sulphur dioxide (SO2), nitrogen dioxide (NO2) and health endpoints related to chronic obstructive pulmonary disease (COPD) morbidity and mortality have been observed [1]. Adverse effects of short-term ozone (O3) exposure on respiratory health endpoints have also been reported [2, 3]. Air pollution was shown to be associated with the development of asthma and morbidity among children [4]. Evidence suggests that even in low-level air pollution environments, there is a risk for an increase in adverse respiratory symptoms in adults and children; exposure to traffic and related pollutants is associated with decreased lung function and the onset of asthma [5–9]. Relative risk estimates from air pollution studies vary, since they use different increment units and target groups for analysis. World Health Organization (WHO) Air Quality Guidelines suggested that relative risks for various respiratory health endpoints associated with nitrogen dioxide led to a 1.4–3.3% increase in hospitalisation or hospital visits per 10 µg/m3 (24 h) increase [10].
In Germany, one of the main sources of air pollutant emissions is the transportation sector [11]. Despite the policies implemented in recent years to ameliorate the air pollution situation in Berlin, NO2 and PM10 concentrations still exceed the limit values in certain areas of the city, posing health risks [11]. While European ambient air quality standards apply to NO2, emission limit values for vehicles are specified as emissions of NOx (the sum of NO and NO2). Estimates of the fraction of NOx emitted as NO2 vary between 15 and 25% [12]. Much of the NO2 measured at urban background locations is due to secondary production through reaction of NO with O3, a reversible process which simultaneously generates NO2 and removes O3 [13]. The reversible nature of this process often leads to higher observed ozone concentrations under conditions of low NOx in urban areas [14]. In short, the concentrations of NO2 and O3 are strongly linked.
Few studies have been conducted in Germany, examining the relation of air pollution and health. A study on behalf of the German Environment Agency demonstrated that in 2014, background concentrations of NO2 appeared to be detrimental for several non-communicable diseases and mortality in Germany [15]. However, a study in Hamburg found that respiratory emergency department visits were significantly associated with temporal variables, but environmental variables showed no direct associations [16]. Interactive effects between equivalent temperature and air pollution on mortality for Berlin and Lisbon have also been demonstrated [17]. Air pollution as a risk factor of respiratory morbidity has not been researched for Berlin. In our study, we aimed to identify and quantify associations between short-term exposure to air pollutant concentrations and respiratory health endpoints in Berlin. The objective of the study was to examine whether daily air pollution variations of the pollutants NO2, O3, PM10, and PM2.5 were associated with hospital admissions of COPD and asthma patients, and if so, to quantify the concentration–response. We analysed urban background pollutant concentrations that are characteristic of the urban area as a whole, independent of local hot-spots [11].
Methods
Study design
The study was designed as a retrospective time series analysis. The ethics committee of the Charité-Universitätsmedizin Berlin approved the project (EA2/147/17). We performed the study according to the principles of the Declaration of Helsinki.
The study population consisted of patients hospitalised due to an acute exacerbation of COPD or asthma. Trained physicians diagnosed the diseases according to the current guidelines: COPD diagnosis was based on post-bronchodilator spirometry results, as recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [18]. For the diagnosis of asthma, the guidelines of the Global Initiative for Asthma (GINA) were applied [19]. COPD patients with an age below 40 years and asthma patients younger than 18 years were excluded from the study.
Study setting
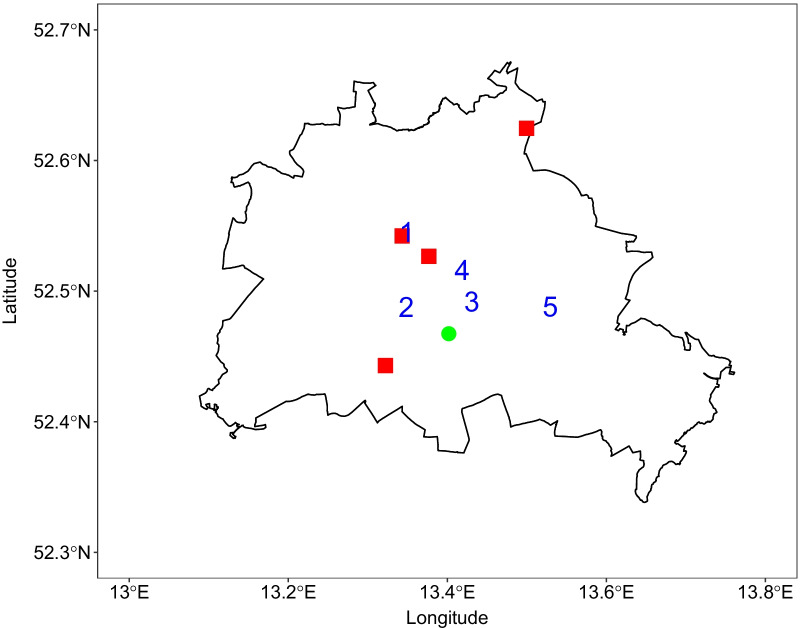
The patient data originated from the Charité-Universitätsmedizin Berlin, a university hospital with four campuses across Berlin, shown in Fig. 1. Within the Charité, 3,000 hospital beds are available for the treatment of about 150,000 inpatients and 690,000 outpatients per year.
Fig. 1.
City of Berlin boundaries (black line) with Charité campuses (red squares), meteorological station (green dot), and urban background air quality monitoring stations (blue numbers). NO2 data was
available at all five stations, PM at stations 1, 3, and 4, O3 at stations 1 and 3
Data collection and endpoints
Hospital admission data
The primary endpoint of the study was the daily number of hospital admissions for COPD or asthma exacerbation. We retrieved the admission data from the digital hospital information system. Admissions between January 1st, 2005 to December 31st, 2015 were included from all Charité hospitals in Berlin. To qualify for inclusion, cases had to be labelled with the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, German Modification (ICD-10-GM) codes J44 (COPD) or J45 to J46 (asthma) in the main or secondary diagnoses. If codes J44 to J46 were part of the secondary diagnoses, the main diagnosis had to be J09 to J11 (influenza), J12 to J18 (pneumonia), J20 or J22 (other acute infection of the lower respiratory tract), J40 to J43, or J47 (chronic disease of the lower respiratory tract). Smoking status was identified by the presence or absence of ICD code F17 (mental and behavioural disorders due to use of tobacco).
Air pollution data
Air pollution data including NO2, O3, PM10, and PM2.5 were retrieved from the Berlin city regulatory air quality monitoring network [20]. The network of measuring stations is run by Berlin’s Senate Department for the Environment, Transport, and Climate Protection. Within the network, NO2 was measured by chemiluminescence, O3 by ultra-violet absorption, and PM by sequential filter sampling, conforming to EU standards. The daily concentration values originated from five urban background stations for NO2, three stations for PM10 and PM2.5, and two stations for O3, marked in Fig. 1. The values from all available urban background stations were averaged to calculate one daily concentration value for each pollutant. The total oxidants (Ox) concentration was calculated as the sum of nitrogen dioxide and ozone concentrations.
Meteorological data
The daily mean temperature, average humidity and wind speed were obtained from a meteorological station at the former Berlin-Tempelhof airport, available online through the website of the German Meteorological Office (Deutscher Wetterdienst, www.dwd.de). The meteorological station is located in the urban area of Berlin at 48 m above sea level on 52.47°N and 13.40°E, see Fig. 1.
Statistical analysis
The statistical analysis was performed with IBM SPSS Statistics version 25 (IBM Corporation, Armonk, NY, USA) and R: A language and environment for statistical computing [21]. A common method in environmental epidemiology to analyse the effects of air pollutants or meteorological parameters on a population’s health is a time series regression [22]. For the time series analysis, we used the distributed lag non-linear model (dlnm) package created by Gasparrini [23]. The method serves to calculate risk ratios: the probability of an outcome (e.g. hospitalisation) after a certain exposure (e.g. air pollutant concentration). If the risk ratio (RR) is greater than 1, the probability of the outcome is increased by the exposure. Furthermore, the dlnm allows the consideration of lag days, the delayed occurrence of health effects after exposure. We calculated the risk ratios for the day of exposure and the following 7 (lag) days. All lag terms were modelled together in an unconstrained dlnm. For the generalised linear model, a quasi-Poisson distribution was assumed. When creating the cross-basis, which specifies the exposure-lag-response dependency, we chose a linear function to model the relationship for air pollutants and strata for the ambient temperature. The variable for lag days was defined as integer values. A natural cubic spline of time with 7 degrees of freedom per year (76 in total) was created to control for seasonal and long-term trends. First, single pollutant models were calculated, including the mean temperature. If the model showed statistical significance, the remaining meteorological variables wind speed and humidity were added one by one. The single pollutant models with statistical significance were then combined into multi-pollutant models. P-values less than 0.05 were considered statistically significant.
Sample size calculations
The precision of time series analyses increases with the length of the series and the number of events per day. For studies on the effects of air pollutants, several thousands of observation days are recommended [22]. We analysed a time series of 4,017 days.
Results
The characteristics of the asthma and COPD patients included into the study are reported in Table 1. The majority of the asthma patients were female (61.1%), while the COPD cohort consisted of more male patients (58.3%). Asthma and COPD patients had a mean age of 52 and 68 years, respectively. Only 4.9% of the asthma and 8.3% of the COPD patients were current smokers. A minor percentage of the admitted patients were infected with influenza.
Table 1.
Description of patient cohorts
| Parameter | Asthma | COPD |
|---|---|---|
| Admissions, n | 876 | 8645 |
| Male, n (%) | 341 (38.9%) | 5038 (58.3%) |
| Female, n (%) | 535 (61.1%) | 3607 (41.7%) |
| Age, years, mean ± SD | 52.2 ± 19.5 | 68.1 ± 10.2 |
| Current smoker, n (%) | 43 (4.9%) | 721 (8.3%) |
| Influenza, n (%) | 8 (0.9%) | 20 (0.2%) |
| Length of stay, days, mean ± SD | 7.5 ± 8.8 | 10.9 ± 14.2 |
| Died in hospital, n (%) | 15 (1.7%) | 392 (4.5%) |
Table 2 summarizes the range of air pollutant and meteorological variable values. The median pollutant concentrations were all below the limit values set by the WHO Air Quality Guidelines, as well as by the EU Air Quality Directive [10, 24].
Table 2.
Median and range of daily air pollutant concentrations and meteorological variables
| Parameter | Median (range) |
|---|---|
| NO2 [µg/m3] | 25.0 (6.0–87.0) |
| O3 [µg/m3] | 42.0 (1.0–135.0) |
| Ox [µg/m3] | 68.0 (20.5–157.0) |
| PM10 [µg/m3] | 21.7 (4.7–188.3) |
| PM2.5 [µg/m3] | 15.5 (3.8–168.8) |
| Daily mean temperature [°C] | 10.8 (− 15.6–30.5) |
| Humidity [%] | 76.38 (0.00–99.96) |
| Wind speed [m/s] | 35.0 (0.0–106.0) |
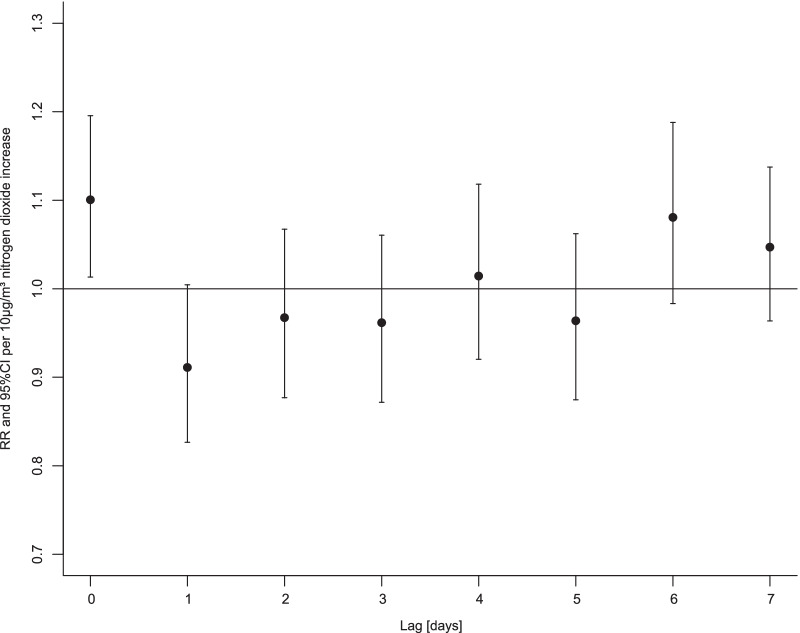
Plots of NO2, O3, PM10, and PM2.5 concentrations over time can be found in Additional file 1: Fig. S1–S4. In the single pollutant model, the risk ratio for asthma patients to be hospitalised on the same day of NO2 exposure was 1.101 per 10 µg/m3 NO2 increase (95% confidence interval, CI: 1.013 to 1.195). There were no lagged effects seven days after exposure, as shown in Fig. 2. Neither the exposure to ozone (95% CI: 0.904 to 1.020), total oxidants (95% CI: 0.962 to 1.117), PM10 (95% CI: 0.990 to 1.127), nor PM2.5 (95% CI: 0.981 to 1.148) was associated with an increased risk ratio for asthma patients to be hospitalised. The listed confidence intervals refer to the day of exposure. No multi-pollutant model showed statistical significance.
Fig. 2.
Hospitalisation risk ratios of asthma patients after NO2 exposure Displayed are risk ratios (RR, dots) and 95% confidence intervals (CI, whiskers) per 10 µg/m3 increase in nitrogen dioxide concentration
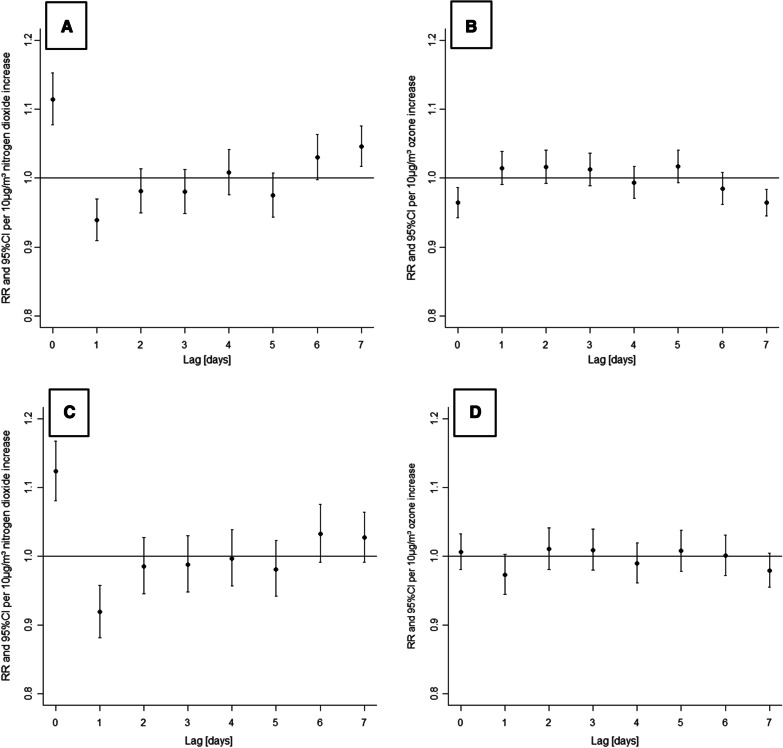
The single pollutant models for NO2 and ozone showed significantly changed risk ratios for COPD patients. In both models, wind speed had a significant risk increasing effect (NO2: p < 0.001, O3: p = 0.035). NO2 exposure was associated with an increased risk ratio for a disease exacerbation on the same day (Fig. 3A, 1.114 per 10 µg/m3 NO2 increase, 95% CI: 1.077 to 1.152), while exposure to ozone was linked to decreased risk ratios (see Fig. 3B). No change in risk ratios was observed due to PM10 exposure (95% CI: 0.988 to 1.032) or exposure to PM2.5 (95% CI: 0.966 to 1.019).
Fig. 3.
Hospitalisation risk ratios of COPD patients after exposure to NO2 and ozone (single and multi-pollutant modelling results). Panel A (NO2) and B (O3) show the single pollutant modelling results, including mean temperature and wind speed. The results of the multi-pollutant model combining exposure to NO2, ozone, mean temperature, and wind speed are given in panels C (NO2) and D (O3). Displayed are risk ratios (RR, dots) and 95% confidence intervals (CI, whiskers) per 10 µg/m3 increase in air pollutant concentration
When the models for NO2 and ozone were combined to one multi-pollutant model, there was no longer a decreased risk ratio for COPD patients to be hospitalised due to ozone exposure (95% CI: 0.981 to 1.033 on the day of exposure, Fig. 3D). The risk ratio for COPD patients to be hospitalised on the same day of NO2 exposure was 1.123 per 10 µg/m3 NO2 increase (95% CI: 1.081 to 1.168).
To confirm the results of the multi-pollutant model, we analysed a time series with the total oxidants (Ox) concentrations, consisting of the added NO2 and O3 values. Exposure to Ox also showed an increased risk ratio of 1.030 per 10 µg/m3 Ox increase for a hospitalisation on the day of exposure (95% CI: 1.005 to 1.056), as shown in Additional file 1: Fig. S5.
Discussion
Our results indicate that in Berlin, at relatively low levels of air pollutant concentrations there is an increased risk of COPD and asthma exacerbations leading to hospitalisation. Effects on hospitalisations of COPD patients were larger with higher wind speed. Compared to other pollutants (PM10, PM2.5, ozone), only NO2 was associated with an increased risk. The NO2 concentration increased the risk for asthma patients to be hospitalised on the day of exposure by 10% per 10 µg/m3 NO2 increase. Our multi-pollutant model showed that only NO2 contributed to an increased morbidity associated with COPD hospitalisation on the day of increase of pollutant concentration (by 12% per 10 μg/m3 NO2). The increased O3 concentrations did not offset the benefits of lower NO2 concentrations. We note that the analysis presented here is based on urban background measurements of NO2, which are lower than the European limit values for ambient concentration, and likely a lower bound for exposure.
Our analysis resulted in higher effect estimates per 10 µg/m3 increase of NO2 on asthma and COPD patients than described in other studies. Respiratory disease hospitalisations in relation to NO2 have been reported by studies in Iran [25] and China [26], however, with lower risk estimates compared to our results. Similar to this study, Gao et al. observed short-term effects of ambient air pollution on COPD hospitalisation, with NO2 showing the most pronounced effect, compared to other gaseous pollutants and particles [27]. The results of our study compared to some European studies are similar: relative to PM10, NO2 had a stronger effect on multiple respiratory events in six Italian cities. Effects were instantaneous (lag 0–1) and mostly expressed with regard to COPD [28]. Gaseous air pollutants were shown to be important factors for acute hospitalisations for respiratory health endpoints in a time series analysis from Rome [29]. Furthermore, a systematic review by Mills et al. reported associations between NO2 and adverse health outcomes that were independent of PM mass [30].
Conflicting results with regard to the delayed effects on respiratory hospitalisations have been reported in other studies: Szyszkowicz et al. found that NO2 was positively associated with COPD emergency visits for males 3–6 days after exposure and for females after 8 lag days [9]. Effects of air pollution on respiratory hospital admissions in Turkey showed that the relative risk was highest at lag day 4 [31]. These inconsistencies could be due to variations in study designs, sample sizes, disease prevalence, cohort characteristics, access to healthcare, air pollution concentrations, geographical differences, and availability of air pollution data. For respiratory patients in Berlin, an immediate hospital admission could be explained by the availability of hospitals in close proximity, with 24-h emergency departments.
The single and multi-pollutant models for the effects of NO2 exposure on COPD patients in our study both showed a decreased risk for hospital admission at lag day 1 (Fig. 3A and C). One explanation for this harvesting effect [22] is that on a day with high ambient concentrations of NO2, a disease exacerbation is triggered in many vulnerable COPD patients. On the following day, the number of vulnerable COPD patients not already in hospitals is reduced, which leads to fewer exacerbations and a decreased risk ratio for hospitalisation.
We also observed that wind speed played a role in increasing the risk of COPD exacerbations in the presence of air pollution. Environmental and chemical exposures are known to trigger COPD exacerbations [32] and many people with COPD often report difficulties with breathing during cold air and strong winds [33]. Few publications exist on the influence of meteorological factors on COPD and asthma patients in Germany. However, in North Bavaria, a 1% increase in daily ambulatory visits of COPD patients was associated with an increased wind speed. The authors stated that the exact mechanism how strong winds increase COPD morbidity is unclear, but could have complex reasons, as heterogeneous as the pathogenesis of COPD itself [34].
We analysed the effects of air pollution on two cohorts: asthma and COPD patients. Asthma is predominantly an airway disease, while COPD is a progressive disease including emphysema and chronic bronchitis, affecting different age groups (Table 1). Our study design was chosen to investigate the effects of air pollution on the respiratory health of adults of all ages.
Two possible confounding risk factors for exacerbation, smoking and influenza infection, were considered. The prevalence of smoking was low in both cohorts and few patients were admitted with influenza (Table 1). Both single and multi-pollutant models were applied to test the robustness of the NO2 results. The analysis of the total oxidants Ox (NO2 + O3) served as additional confirmation and demonstrated that high ozone concentrations do not outweigh the effects of NO2.
Our study had several limitations. We did not measure indoor air pollution or personal exposure to pollutants. Nevertheless, the location of the monitoring stations in the city of Berlin, as per EU guidelines, does ensure that the concentrations of air pollutants used in this study are broadly representative of the urban area. We used urban background concentrations in our model which are consistently lower than, for example, concentrations measured next to locations with high traffic. It should be taken into account that we only investigated exacerbations that led to a hospitalisation. COPD and asthma exacerbations not requiring admissions were not included, probably leading to an underestimation of the effects of air pollutants on respiratory outcomes. The applied time series model does not consider demographic, socio-economic, or behavioural variables of the individual participants. This could be an area of further research.
While the observed correlation of NO2 and hospital admissions is not definite proof for a causal relationship between NO2 exposure and physiological damage, NO2 should be seen as an indicator substance for a more complex mixture of pollutants, which is released e.g. by combustion processes in vehicles, airplanes, ships, and factories. Detrimental effects could be caused by other substances of this pollutant mixture that correlated with NO2. Chronic and sub-chronic exposure to low levels of NO2 is reported to be unfavourable with regard to lung metabolism, function, structure, and even patients’ susceptibility to pulmonary infections [10].
Our results have important policy implications as NO2 is a pollutant produced locally with a short lifetime in the atmosphere. In Berlin, NO2 emissions from traffic are estimated to be responsible for about 70–80% of the pollution in the inner city residential areas [11]. Acute exacerbations of COPD and asthma, and consequent hospitalisations generate significant costs for the healthcare system and are correlated with a decline in lung function and disease progression [35]. Addressing the risk factors for these adverse events plays an important role, given that some projections show that NO2 will be the pollutant most profoundly associated with respiratory hospital admissions in the coming years [36].
Conclusions
We demonstrated that NO2 is the pollutant with the largest risk ratios for hospital admissions of asthma and COPD patients in Berlin. Our models show that NO2 contributes to increased morbidity more significantly than PM, and that increased O3 typically associated with episodes of lower NO2 does not offset the benefits of lower NO2 concentrations. Furthermore, the analysis presented here is based on urban background measurements of NO2, which are consistently lower than the European limit values for ambient concentrations.
Supplementary Information
Additional file 1: Figure S1. Urban background concentrations of nitrogen dioxide over the study period. Figure S2. Urban background concentrations of ozone over the study period. Figure S3. Urban background concentrations of PM10 over the study period. Figure S4. Urban background concentrations of PM2.5 over the study period. Figure S5. Hospitalisation risk ratios of COPD patients after exposure to total oxidants (Ox).
Acknowledgements
We thank Stefan Barthel (Charité, corporate controlling) for his assistance with data retrieval from the hospital information system.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- NO2
Nitrogen dioxide
- CI
Confidence interval
- PM10
Particulate matter with an aerodynamic diameter of 10 µm or less
- PM2.5
Particulate matter with an aerodynamic diameter of 2.5 µm or less
- SO2
Sulphur dioxide
- O3
Ozone
- WHO
World Health Organization
- NOx
The sum of nitrogen oxide and nitrogen dioxide concentrations
- NO
Nitrogen oxide
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- GINA
Global Initiative for Asthma
- ICD-10-GM
International Statistical Classification of Diseases and Related Health Problems, 10th Revision, German Modification
- EU
European Union
- Ox
Total oxidants, the sum of nitrogen dioxide and ozone concentrations
- dlnm
Distributed lag non-linear model
- RR
Risk ratio
- SD
Standard deviation
Authors' contributions
Study conception and design: T.B., M.M., E.v.S., C.W., P.H., and C.H.; data acquisition: P.H., E.v.S., T.B., and M.M.; data analysis and interpretation: C.H., M.M, T.B., P.H., and E.v.S.; manuscript draft and revision: M.M., C.H., E.v.S., T.B., P.H., and C.W. All authors have read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Institute for Advanced Sustainability Studies (IASS) e.V., Potsdam, is funded by the German Federal Government’s Ministry of Education and Research as well as the Federal State of Brandenburg’s Ministry for Science, Research, and Culture.
Availability of data and materials
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was reviewed and approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA2/147/17). We performed the study according to the principles of the Declaration of Helsinki. Due to the retrospective time series design, the data protection commissioner of the Charité-Universitätsmedizin Berlin allowed the use of anonymized patient data for research purposes without obtaining individual consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christina Hoffmann and Mariam Maglakelidze are first authors, contributed equally to the manuscript
Peter Hoffmann and Tim Butler are last authors, contributed equally to the manuscript
Contributor Information
Christina Hoffmann, Email: Christina.Hoffmann2@charite.de.
Mariam Maglakelidze, Email: Maka.Bsha@gmail.com.
Erika von Schneidemesser, Email: Erika.vonSchneidemesser@iass-potsdam.de.
Christian Witt, Email: Christian.Witt@charite.de.
Peter Hoffmann, Email: Peter.Hoffmann@charite.de.
Tim Butler, Email: Tim.Butler@iass-potsdam.de.
References
- 1.DeVries R, Kriebel D, Sama S. Outdoor air pollution and COPD-related emergency department visits, hospital admissions, and mortality: a meta-analysis. COPD J Chronic Obstr Pulm Dis. 2017;14:113–121. doi: 10.1080/15412555.2016.1216956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Héroux M-E, Anderson HR, Atkinson R, Brunekreef B, Cohen A, Forastiere F, et al. Quantifying the health impacts of ambient air pollutants: recommendations of a WHO/Europe project. Int J Public Health. 2015;60:619–627. doi: 10.1007/s00038-015-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luong LMT, Phung D, Dang TN, Sly PD, Morawska L, Thai PK. Seasonal association between ambient ozone and hospital admission for respiratory diseases in Hanoi, Vietnam. PLoS ONE. 2018 doi: 10.1371/journal.pone.0203751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel MM, Miller RL. Air pollution and childhood asthma: recent advances and future directions. Curr Opin Pediatr. 2009;21:235–242. doi: 10.1097/MOP.0b013e3283267726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmes JR, Earnest G, Katz PP, Yelin EH, Eisner MD, Chen H, et al. Exposure to traffic: Lung function and health status in adults with asthma. J Allergy Clin Immunol. 2009;123:626–631. doi: 10.1016/j.jaci.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVries R, Kriebel D, Sama S. Low level air pollution and exacerbation of existing copd: a case crossover analysis. Environ Health. 2016 doi: 10.1186/s12940-016-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JJ, Huen K, Adams S, Smorodinsky S, Hoats A, Malig B, et al. Residential traffic and children’s respiratory health. Environ Health Perspect. 2008;116:1274–1279. doi: 10.1289/ehp.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szyszkowicz M, Kousha T, Castner J, Dales R. Air pollution and emergency department visits for respiratory diseases: a multi-city case crossover study. Environ Res. 2018;163:263–269. doi: 10.1016/j.envres.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization, editor. WHO Air quality guidelines for particulate matter, ozone, nitrogendioxide and sulfur dioxide. WHO Press; 2006. http://whqlibdoc.who.int/hq/2006/WHO_SDE_PHE_OEH_06.02_eng.pdf. Accessed 27 Apr 2021.
- 11.Senate Department for Urban Development and the Environment, editor. Air Quality Plan for Berlin 2011–2017. Medialis Offsetdruck GmbH; 2014. https://www.berlin.de/sen/uvk/umwelt/luft/luftreinhaltung/archiv/luftreinhalteplan-1-fortschreibung/. Accessed 27 Apr 2021.
- 12.Grange SK, Lewis AC, Moller SJ, Carslaw DC. Lower vehicular primary emissions of NO 2 in Europe than assumed in policy projections. Nat Geosci. 2017;10:914–918. doi: 10.1038/s41561-017-0009-0. [DOI] [Google Scholar]
- 13.Beekmann M, Vautard R. A modelling study of photochemical regimes over Europe: robustness and variability. Atmos Chem Phys. 2010;10:10067–10084. doi: 10.5194/acp-10-10067-2010. [DOI] [Google Scholar]
- 14.Sicard P, De Marco A, Agathokleous E, Feng Z, Xu X, Paoletti E, et al. Amplified ozone pollution in cities during the COVID-19 lockdown. Sci Total Environ. 2020;735:139542. doi: 10.1016/j.scitotenv.2020.139542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoll J. Quantifizierung von umweltbedingten Krankheitslasten aufgrund der Stickstoffdioxid-Exposition in Deutschland. Umweltbundesamt; 2018. https://www.umweltbundesamt.de/en/publikationen/quantifizierung-von-umweltbedingten. Accessed 28 Apr 2021.
- 16.Krefis AC, Fischereit J, Hoffmann P, Pinnschmidt H, Sorbe C, Augustin M, et al. Temporal analysis of determinants for respiratory emergency department visits in a large German hospital. BMJ Open Respir Res. 2018 doi: 10.1136/bmjresp-2018-000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkart K, Canário P, Breitner S, Schneider A, Scherber K, Andrade H, et al. Interactive short-term effects of equivalent temperature and air pollution on human mortality in Berlin and Lisbon. Environ Pollut. 2013;183:54–63. doi: 10.1016/j.envpol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 19.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 20.City of Berlin. Darstellung von Luftmessdaten | Berliner Luftgüte Messnetz (BLUME) | Luftqualität und Luftgüte in Berlin. https://luftdaten.berlin.de/pollution/overview. Accessed 27 Apr 2021.
- 21.The R Foundation for Statistical Computing. The R Project for Statistical Computing v.3.6.1. 1020 Vienna, Austria: The R Foundation for Statistical Computing; 2019.
- 22.Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. 2013;42:1187–1195. doi: 10.1093/ije/dyt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasparrini A. Distributed lag linear and non-linear models in R: the Package dlnm. J Stat Softw. 2011;43:1–20. doi: 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The European Parliament and the Council of the European Union, editor. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe; OJ L 152, 11.6.2008, p. 1–44. Official Journal of the European Union; 2008. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0050. Accessed 27 Apr 2021.
- 25.Vahedian M, Khanjani N, Mirzaee M, Koolivand A. Associations of short-term exposure to air pollution with respiratory hospital admissions in Arak, Iran. J Environ Health Sci Eng. 2017;15:17. doi: 10.1186/s40201-017-0277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang L, Cai Y, Barratt B, Lyu B, Chan Q, Hansell AL, et al. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013–17: an ecological analysis. Lancet Planet Health. 2019;3:e270–e279. doi: 10.1016/S2542-5196(19)30085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao N, Li C, Ji J, Yang Y, Wang S, Tian X, et al. Short-term effects of ambient air pollution on chronic obstructive pulmonary disease admissions in Beijing, China (2013–2017) Int J Chron Obstruct Pulmon Dis. 2019;14:297–309. doi: 10.2147/COPD.S188900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faustini A, Stafoggia M, Colais P, Berti G, Bisanti L, Cadum E, et al. Air pollution and multiple acute respiratory outcomes. Eur Respir J. 2013;42:304–313. doi: 10.1183/09031936.00128712. [DOI] [PubMed] [Google Scholar]
- 29.Fusco D, Forastiere F, Michelozzi P, Spadea T, Ostro B, Arcà M, et al. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J. 2001;17:1143–1150. doi: 10.1183/09031936.01.00005501. [DOI] [PubMed] [Google Scholar]
- 30.Mills IC, Atkinson RW, Anderson HR, Maynard RL, Strachan DP. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: a systematic review and meta-analysis. BMJ Open. 2016 doi: 10.1136/bmjopen-2015-010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Çapraz Ö, Deniz A, Doğan N. Effects of air pollution on respiratory hospital admissions in İstanbul, Turkey, 2013 to 2015. Chemosphere. 2017;181:544–550. doi: 10.1016/j.chemosphere.2017.04.105. [DOI] [PubMed] [Google Scholar]
- 32.Sama SR, Kriebel D, Gore RJ, DeVries R, Rosiello R. Environmental triggers of COPD symptoms: a case cross-over study. BMJ Open Respir Res. 2017 doi: 10.1136/bmjresp-2017-000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng C-M, Chen Y-T, Ou S-M, Hsiao Y-H, Li S-Y, Wang S-J, et al. The effect of cold temperature on increased exacerbation of chronic obstructive pulmonary disease: a nationwide study. PLoS ONE. 2013 doi: 10.1371/journal.pone.0057066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrari U, Exner T, Wanka ER, Bergemann C, Meyer-Arnek J, Hildenbrand B, et al. Influence of air pressure, humidity, solar radiation, temperature, and wind speed on ambulatory visits due to chronic obstructive pulmonary disease in Bavaria, Germany. Int J Biometeorol. 2012;56:137–143. doi: 10.1007/s00484-011-0405-x. [DOI] [PubMed] [Google Scholar]
- 35.Brzezińska-Pawłowska OE, Rydzewska AD, Łuczyńska M, Majkowska-Wojciechowska B, Kowalski ML, Makowska JS. Environmental factors affecting seasonality of ambulance emergency service visits for exacerbations of asthma and COPD. J Asthma. 2016;53:139–145. doi: 10.3109/02770903.2015.1075547. [DOI] [PubMed] [Google Scholar]
- 36.Pannullo F, Lee D, Neal L, Dalvi M, Agnew P, O’Connor FM, et al. Quantifying the impact of current and future concentrations of air pollutants on respiratory disease risk in England. Environ Health. 2017 doi: 10.1186/s12940-017-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Urban background concentrations of nitrogen dioxide over the study period. Figure S2. Urban background concentrations of ozone over the study period. Figure S3. Urban background concentrations of PM10 over the study period. Figure S4. Urban background concentrations of PM2.5 over the study period. Figure S5. Hospitalisation risk ratios of COPD patients after exposure to total oxidants (Ox).
Data Availability Statement
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.