Abstract
Skin fibrosis is a debilitating feature of several systemic and dermatologic diseases. While current treatment options carry significant risk of side effects and recurrence, high-fluence light emitting diode-generated red light (LED-RL) is an alternative therapeutic that is safe, non-invasive, and accessible. We previously demonstrated LED-RL decreases fibroblast proliferation, a key pathogenic component of fibrosis. However, the cellular mechanism by which high fluence LED-RL modulates fibroblast proliferation is unclear. Herein, we explored the effects of high fluence LED-RL on human dermal fibroblast cell cycle progression. We demonstrate that LED-RL at 640 J/cm2 induced significant arrest of cells in G0/G1 compared to temperature-matched control. This was accompanied by a corresponding increase in expression of checkpoint regulator p53 in irradiated cells. These data demonstrate high fluence LED-RL may exert its anti-proliferative effect on fibroblasts by inducing G0/G1 arrest. Further, this study provides insight into the molecular mechanism underlying LED-RL as an anti-fibrotic therapeutic.
Keywords: Skin fibrosis, light emitting diode, red light, fibroblast, photobiomodulation
Description:
Light-emitting diode red light (LED-RL) has demonstrated promising evidence as a potential treatment for skin fibrosis. We previously reported LED-RL significantly decreased cutaneous dermal fibroblasts without inducing apoptosis. Herein, we demonstrate that LED-RL is capable of inhibiting cell cycle progression in fibroblasts, further elucidating the mechanism by which LED-RL induces its anti-proliferative effects on fibroblasts.
Introduction
Skin fibrosis is a debilitating feature of several systemic and dermatologic diseases, including systemic sclerosis, mixed connective tissue disease, and hypertrophic scar. Skin fibrosis is estimated to affect 100 million people per year in the developed world, imparting a significant socioeconomic burden due to functional, psychosocial and aesthetic impairments [1, 2]. Skin fibrosis encompasses a group of disorders characterized by excessive fibroblast proliferation and collagen deposition [3]. Skin fibrosis may occur secondary to surgery or trauma, as seen in hypertrophic scars and keloids, or immune-mediated processes such as scleroderma and chronic graft vs. host disease [1, 3]. Current therapies are limited and carry significant side effect burden and risk of recurrence. Intralesional corticosteroid and 5-flurouracil injections are painful, require multiple rounds of injections and may cause skin discoloration, atrophy, and blistering [4, 5]. Ultraviolet (UV) light therapy may be used to treat fibrosis but UV exposure increases the risk of skin cancer and premature aging [6].
Light emitting diode red light (LED-RL) may be a safe alternative to other therapeutic approaches, as LED-RL is not associated with cancer or aging [7]. Light interacts with the skin via chromophores that absorb certain wavelengths of light, leading to downstream effects on the skin [8]. Cytochrome C oxidase in the electron transport chain is receptive to red and near-infrared light, and stimulate reactive oxygen species (ROS) generation. We have previously found that LED-RL at fluences of 320 J/cm2 and above decreases human dermal fibroblast (HDF) migration, collagen deposition, and proliferation [9]. From a mechanistic standpoint, we further demonstrated that ROS inhibition and modulation of the PI3K/Akt pathway restored HDF migration following LED-RL phototherapy [9, 10]. Lastly, we recently reported that high fluence LED-RL irradiation decreases the expression of pro-fibrotic proteins TGF-β1 and pSMAD2 [3, 11–13]. Taken together, our previous data provides insight into the cellular mechanisms by which LED-RL exerts its anti-fibrotic effects. However, we have not previously identified the specific mechanism and cellular pathways related to the anti-proliferative effect seen in HDFs treated with LED-RL. While LED-RL irradiation significantly decreased fibroblast cell counts, this was not accompanied by an increase in corresponding apoptosis levels, leading us to hypothesize that the changes observed were due to alterations in cell cycle progression [9]. The aim of this study is to further elucidate the mechanism by which high fluence LED-RL induces these anti-proliferative effects on HDFs. To accomplish this, we analyzed HDF cell cycle regulation using propidium iodide (PI) flow cytometry and Western blot.
Materials and methods
Materials
Tissue culture dishes (35mm) were obtained from Corning (Corning, NY). Dulbecco’s Modified Eagle’s Medium (DMEM), phosphate buffered saline (PBS), penicillin/streptomycin, PI, eBioscience™ Intracellular Fixation Buffer and eBioscience™ Permeabilization Buffer were obtained from Thermo Fisher Scientific (Waltham, MA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Flowery Branch, GA). HDFs were purchased from ATCC (CRL-2617; Manassas,VA) and Coriell Instititute (AG13145, Camden, NJ).
Cell culture and synchronization
AG13145 (Coriell Instititute, Camden, NJ). and CRL-2617 (ATCC, Manassas, VA) HDF cells were maintained in a humified incubator at 37°C in 5% CO2. Cells were cultured in 1 g/L glucose DMEM (Thermo Fisher Scientific) with 10% FBS (Atlanta Biologicals; Flowery Branch, GA) and 1% antibiotic-antimycotic (Thermo Fisher Scientific) at a density of 40,000 cells per 35 mm dish. All experiments were performed on cell cultures passage 12 or less. Experiments were performed in triplicate and repeated for reproducibility and accuracy.
For synchronization at G0/G1 phase, cells were first seeded using regular 10% FBS-enriched medium. After 24 hours, the cells were washed twice with PBS and starved for 24 hours in 0.625% FBS-enriched medium to sync cell cycle (Supp. Fig. 1A–B) [14]. Starvation medium was then replaced with regular 10% FBS-enriched media and the cells underwent irradiation.
High fluence LED-RL irradiation
Each cell dish was irradiated at a fluence of 640 J/cm2 (2 hours at a power density of 87 mW/cm2) by placing the LED array (Omnilux revive2; GlobalMed Technologies, CA) directly above the dishes. This device has a 6 by 14 cm array of LEDs that emit red light (633 ± 6 nm). At a distance of 10 mm from the bottom of the tissue culture dish, the light has a power density of approximately 87 mW/cm2. To limit the effects of thermal output of the LED array, a control group was placed on a heating block at 37°C for 2 hours to match irradiation times. Culture media temperatures for irradiated and control cells were measured to be 34±1.5°C after 2 hours. At 18 post-irradiation, cells were collected for cell cycle flow cytometry and Western blot analyses. To assess how long the cell-cycle effects of LED-RL persisted, we also performed cell cycle flow cytometry at 48 hours post-irradiation.
Cell cycle analysis
Cells were trypsinized, collected, and resuspended in fixation buffer for 15 minutes. Fixed cells were permeabilized with permeabilization buffer for 30 minutes according to manufacturer guidelines (Thermo Fisher Scientific). RNA was then degraded with RNAse-A incubation for 75 minutes. Cells were stained with PI and analyzed by flow cytometry with a BD LSRFortessa flow cytometer (BD BioSciences, San Jose, CA). FlowJo software was used to model G1, S, and G2/M phases using the Watson Pragmatic algorithm (BD BioSciences).
Western blot
Cells were collected using RIPA buffer with 1X protease inhibitor cocktail (Cell Signaling Technology, Danvers, MA) and 1X phenylmethylsulfonyl fluoride (Cell Signaling Technology, Danvers, MA). Protein concentration was determined by Bradford Assay (BioRad, Hercules, CA). Gel electrophoresis was performed with BioRad precast gels and transferred onto polyvinylidene difluoride (PVDF) membranes. PVDF membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences Lincoln, NE) and then incubated with primary antibodies (Cell Signaling Technology, Danvers, MA) overnight at 4°C. PVDF membranes were then washed and incubated with IRDye infrared secondary antibodies (LI-COR Biosciences) for 1 hour at room temperature. Protein bands on the blots were scanned with an Odyssey Fc Imager (LI-COR Biosciences) and quantified using the ImageStudio program (LI-COR Biosciences). Primary antibodies against p21, p53, phosphorylated-p53 (Serine-15), and GAPDH were used at 1:1,000 dilution. Secondary antibodies were used at 1:10,000 dilutions.
Statistical analysis
Statistical analyses with Student’s t-test was performed using GraphPad Prism (GraphPad Software, San Diego, CA) to compare LED-RL irradiated cells with matched controls. Significance level was set at p < 0.05.
Results
LED-RL induces cell cycle arrest at G0/G1
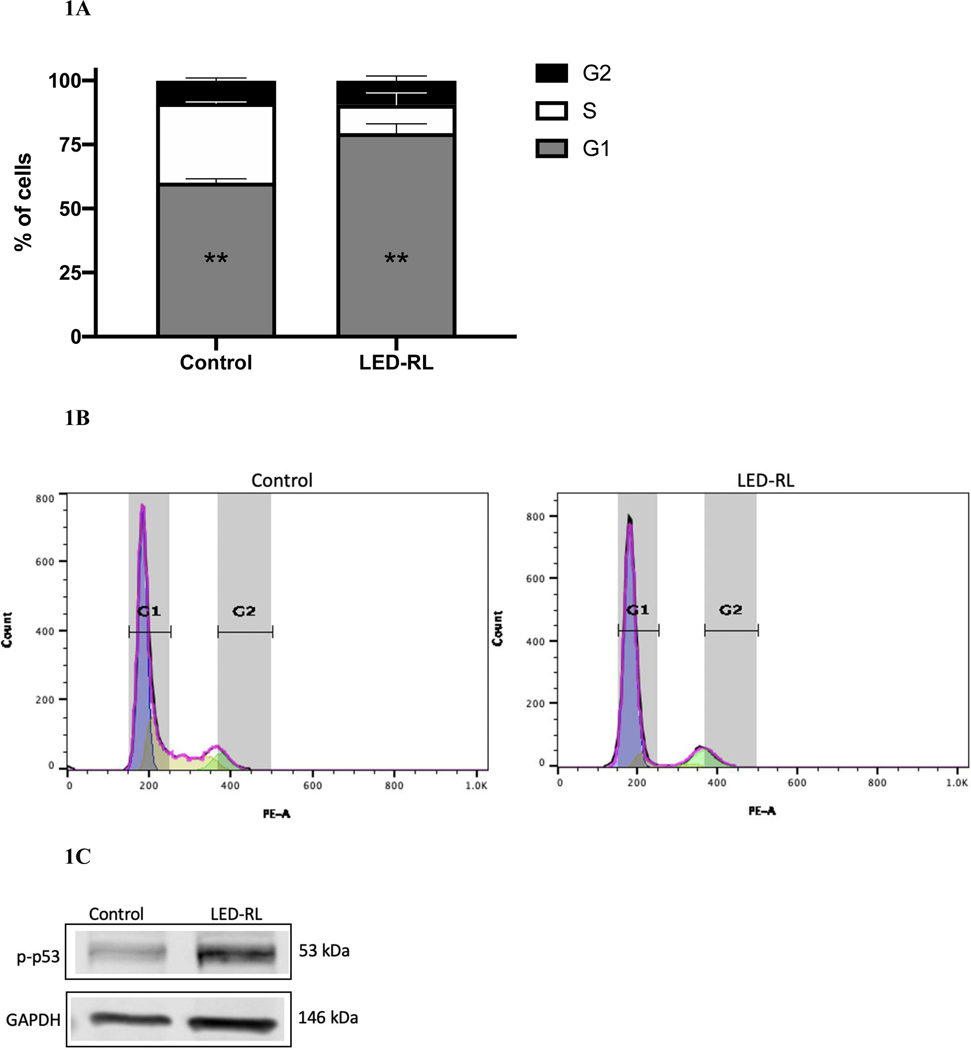
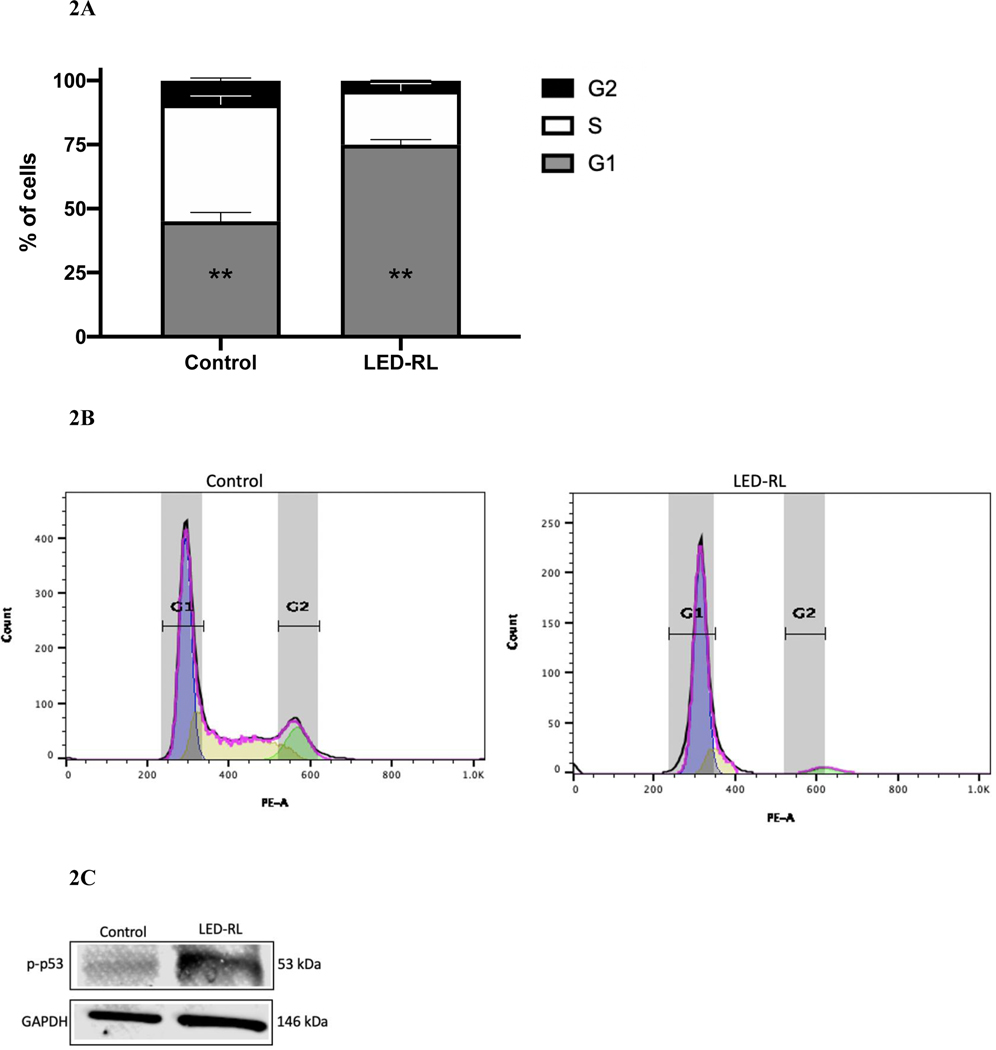
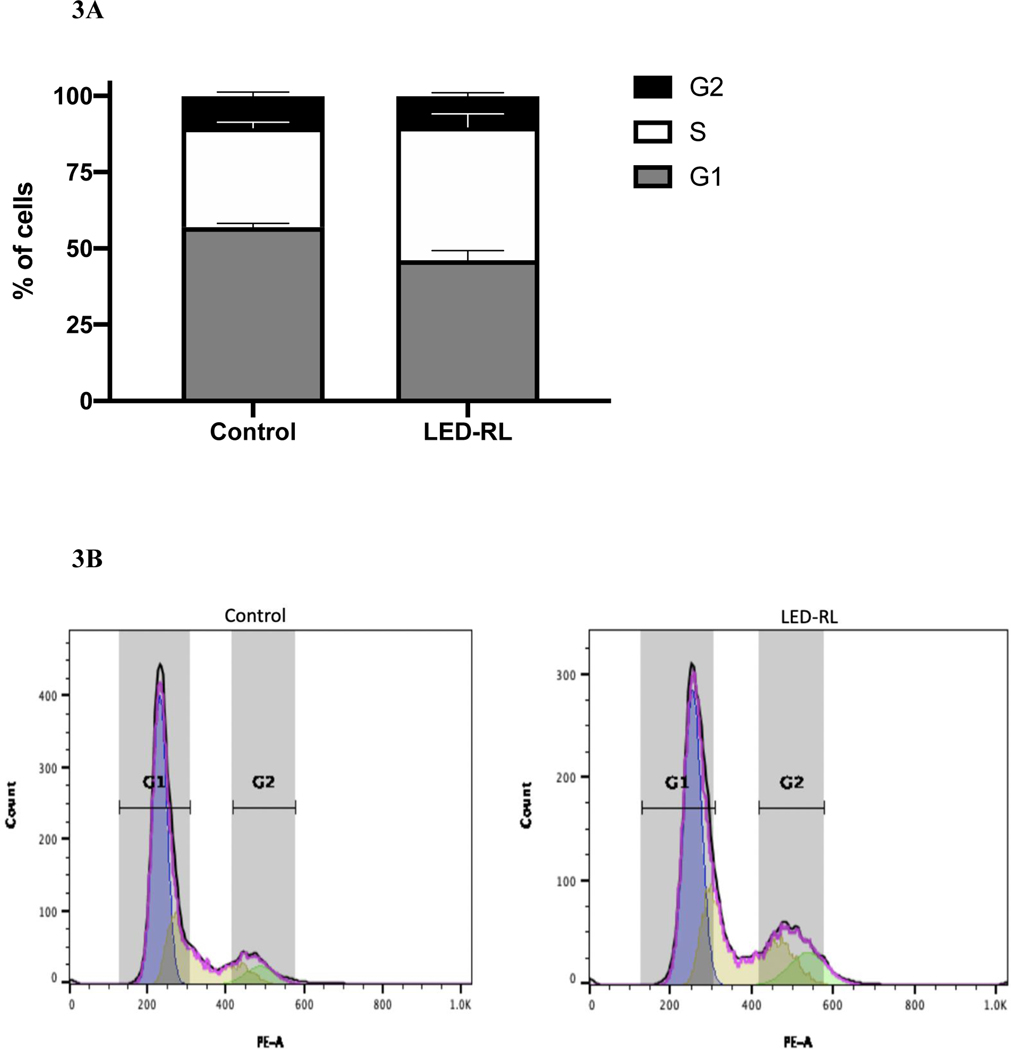
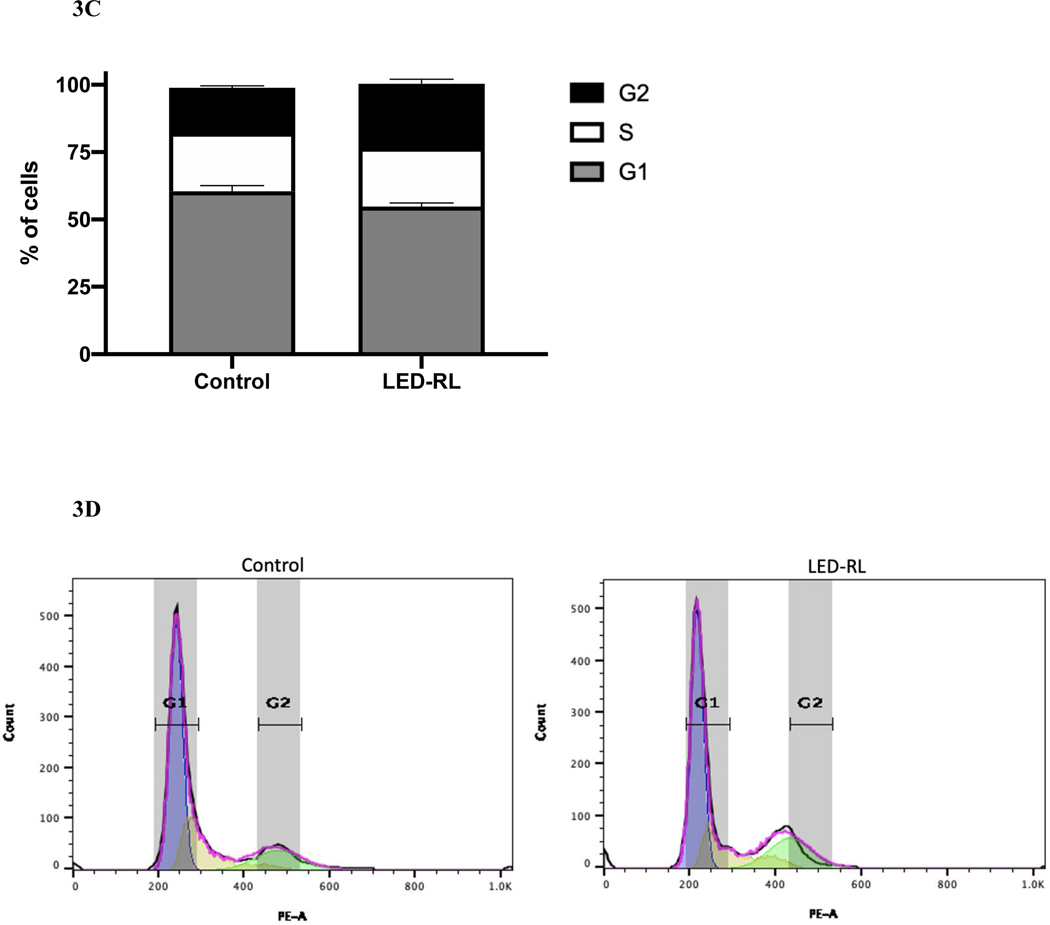
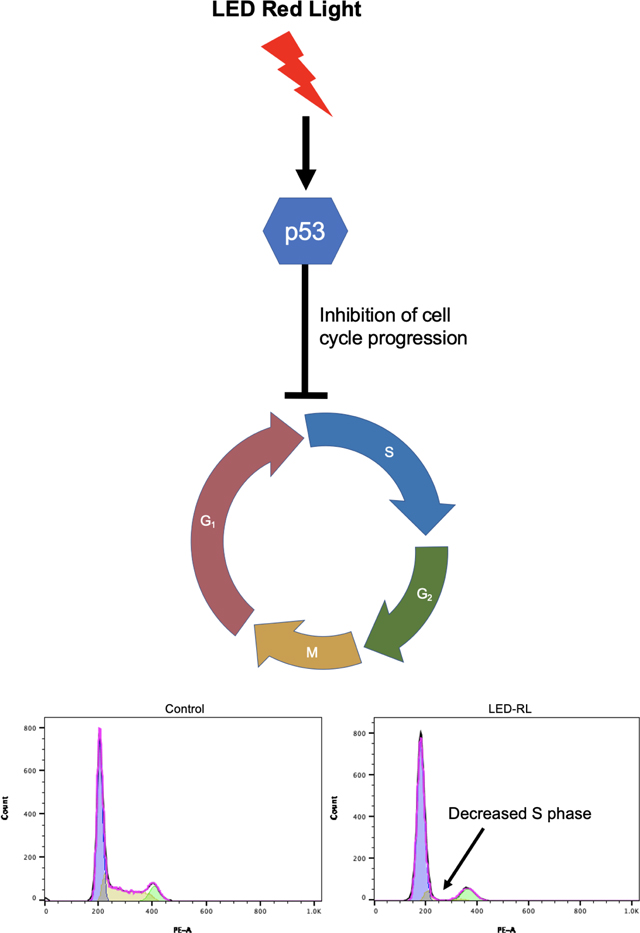
After LED-RL irradiation at a dose of 640 J/cm2, we found significant inhibition of cell cycle progression in irradiated cells compared to temperature-matched control at 18 hours post-irradiation in both CRL-2617 and AG13145. LED-RL irradiation significantly increased the percentage of cells in G1/G0 phase from 59% to 80% (p < 0.01) in CRL-2617 (Fig. 1A–B) and from 65% to 86% (p < 0.01) in AG13145 fibroblasts (Fig. 2A–B). This was accompanied by a corresponding decrease in the percentage of cells in G0/G1 phase. We also assessed cell cycle profile at 48 hours post-irradiation and found that the effect of LED-RL diminishes by this time point in both HDF cell lines (Fig. 3A–3D).
Figure 1A.
High fluence LED-RL prevents cell cycle progression and is associated with arrest at G0/G1 phase in CRL-2617 fibroblasts at 18 hours post-irradiation (** p < 0.01).
Figure 1B Corresponding flow diagram demonstrating high fluence LED-RL is associated with G0/G1arrest of CRL-2617 fibroblasts at 18 hours post-irradiation.
Figure 1C High fluence LED-RL was associated with increased phosphorylated p53 expression in CRL-2617 fibroblasts at 18 hours post-irradiation. GAPDH served as a loading control.
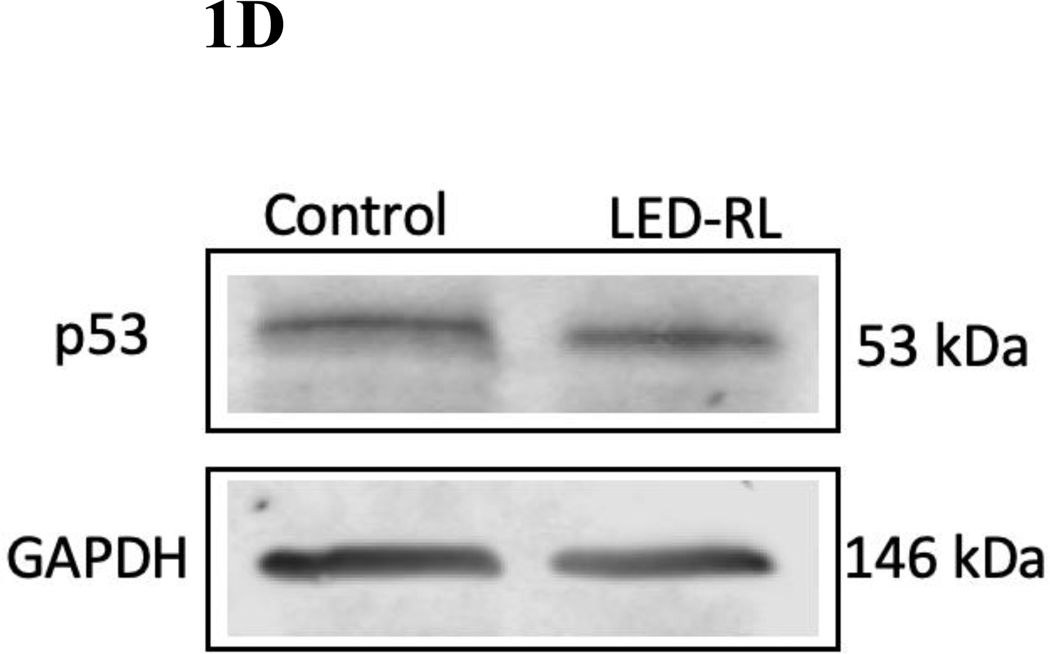
Figure 1D High fluence LED-RL did not change baseline p53 expression in CRL-2617 fibroblasts at 18 hours post-irradiation. (.88 AG13145, .83 CRL-2617) GAPDH served as a loading control.
Figure 2A.
High fluence LED-RL prevents cell cycle progression and is associated with arrest at G0/G1 phase in AG13134 fibroblasts at 18 hours post-irradiation (** p < 0.01).
Figure 2B Corresponding flow diagram demonstrating high fluence LED-RL is associated with G0/G1 arrest of AG13145 fibroblasts at 18 hours post-irradiation.
Figure 2C High fluence LED-RL was associated with increased phosphorylated p53 expression in AG13145 fibroblasts at 18 hours post-irradiation. GAPDH served as a loading control.
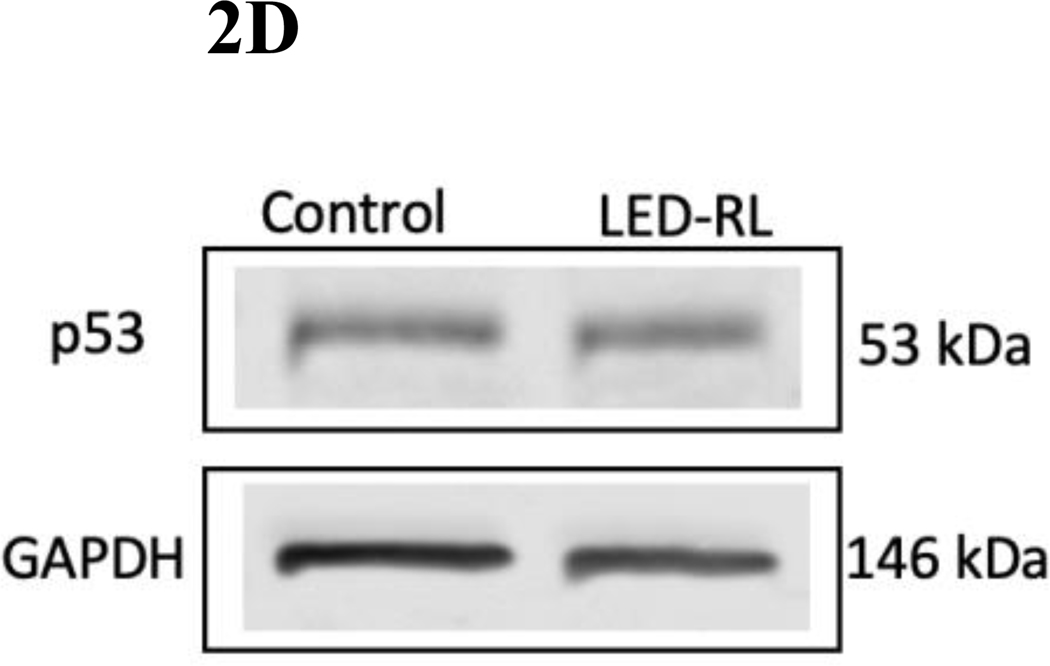
Figure 2D High fluence LED-RL did not change baseline p53 expression in AG13145 fibroblasts at 18 hours post-irradiation. GAPDH served as a loading control.
Figure 3A.
High fluence LED-RL did not demonstrate significant changes in cell cycle progression in CRL-2617 fibroblasts at 48 hours post-irradiation.
Figure 3B Corresponding flow diagram demonstrating high fluence LED-RL did not demonstrate significant changes in cell cycle progression in CRL-2617 fibroblasts at 48 hours post-irradiation.
Figure 3C High fluence LED-RL did not demonstrate significant changes in cell cycle progression in AG13145 fibroblasts at 48 hours post-irradiation.
Figure 3D Corresponding flow diagram demonstrating high fluence LED-RL did not demonstrate significant changes in cell cycle progression in AG13145 fibroblasts at 48 hours post-irradiation.
LED-RL was associated with increased expression of phosphorylated p53
We then assessed expression of G1 phase regulators p53 and p21 after LED-RL irradiation. p53 and p21 are important regulators of cell cycle progression and have demonstrated significant roles in hyperproliferative disease. Phosphorylated p53 increases the transcription of CDKN1A, the gene that codes for p21. LED-RL was associated with increased phosphorylated p53 expression in both HDF cell lines. Phosphorylated p53 expression increased 2.7-fold in AG13145 (Fig. 2C, 2D) and 1.6-fold in CRL-2617 (Fig. 1C, 1D) fibroblasts relative to temperature-matched control at 18 hours post-irradiation. However, LED-RL did not lead to a significant increase in p21 expression at 18 hours post-irradiation (Supp. Fig. 1).
Discussion
Skin fibrosis is a complex process involving several cellular processes including fibroblast proliferation, migration, and collagen production [9]. Fibroblast proliferation in particular is necessary for physiologic wound healing, but excessive proliferation leads to skin fibrosis [3]. Our group has previously demonstrated that LED-RL irradiation can significantly decrease fibroblast cell count without an accompanying increase in apoptosis levels [9]. To interrogate the mechanism of this decrease in cell count, we assessed changes in cell cycle progression. Accelerated cell cycle progression and prolonged periods in the G2/M phase have been implicated in the development of fibrotic phenotypes [15, 16]. Consequently, cell cycle arrest has been described as the cellular mechanism underlying numerous anti-fibrotic pharmacologic agents in pulmonary fibrosis and hepatic fibrosis [17, 18]. We now report that cell cycle arrest may be the underlying mechanism by which skin fibrosis can be targeted using LED-RL.
LED-RL irradiation at a fluence of 640 J/cm2 was associated with fibroblast cell cycle arrest in the G1 phase. This cell cycle arrest was accompanied by increased phosphorylation of p53. p53 has tumor-suppressor capabilities, and is crucial to a number of biological processes including cell cycle arrest, apoptosis, and DNA damage repair [19–21]. p53 mutation and inhibition is a hallmark of hyperproliferative disease, including cancer [22, 23]. p53-induced cell growth arrest is mediated by its ability to regulate multiple cell cycle check point regulators, including Gadd45 and p21 [20, 21]. Interestingly, we did not observe significant change in p21 expression at 18 hours post-irradiation. However, as Hafner et al have described, expression of p21 and p53 is pulsatile, resulting in heterogenous gene expression of downstream mRNA targets [24]. Additionally, current research suggests that the ratio of p21 to the pro-apoptotic protein p53-upregulated modulator of apoptosis (PUMA) is important in determining cellular fate [24]. One limitation of this study is that PUMA expression was not assessed; however, this can be the direction of future investigations into the cellular mechanism of LED-RL as an anti-fibrotic therapeutic.
A major challenge of in vitro photomedicine studies is addressing the independent effects of temperature and environmental conditions on experimental outcomes. We addressed this concern by pairing each irradiated culture plate with a temperature-matched control plate to ensure that the measured effects were the result of LED-RL treatment and not due to environment or temperature. Furthermore, another strength of this study is that the effects of LED-RL were demonstrated in different HDF cell lines of widely different donor characteristics including age, sex, Fitzpatrick skin type and anatomic location (CRL-2617: 42 year old African-American female, abdomen; AG13145: 57 year old white male, arm).
Cell cycle arrest is just one of several mechanisms by which LED-RL alters fibroblast activity to reduce skin fibrosis. As we reported previously, LED-RL has significant effects on HDF collagen deposition, migration speed, and TGF-β expression. Taken with this present study, our work collectively demonstrates that LED-RL affects several cellular processes including cell cycle progression to decrease fibrosis in vitro.
Conclusion
Current treatments for skin fibrosis are limited by clinical efficacy, utility, and cost. In addition to having beneficial effects for fibrosis, LED-RL is more affordable than traditional therapies, accessible by patients, and can be easily combined with other therapeutics. Our group has previously demonstrated that LED-RL may be a promising therapeutic for the treatment of fibrotic diseases. In this study, we further demonstrate that LED-RL is capable of arresting fibroblast cell cycle progression, and thus potentially alleviating the burden of fibrotic disease. Our collective results present a novel approach to modulating fibroblast function for the treatment of skin fibrosis. We are currently translating these efforts clinically through our ongoing Phase II trial (NCT03795116) investigating the clinical efficacy of LED-RL in post-surgical scarring [25]. Further characterization of the photobiomodulatory effects of LED-RL in human subjects may also reveal new insight into the utility of this therapy for the treatment of fibrotic disease.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. K23GM117309
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- [1].Bayat A, McGrouther DA, Ferguson MWJ BMJ 2003, 326, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lim HW, Collins SAB, Resneck JS Jr., Bolognia JL, Hodge JA, Rohrer TA, Van Beek MJ, Margolis DJ, Sober AJ, Weinstock MA, Nerenz DR, Smith Begolka W, Moyano JV J Am Acad Dermatol 2017, 76, 958. [DOI] [PubMed] [Google Scholar]
- [3].Wynn TA, Ramalingam TR Nature medicine 2012, 18, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poetschke J, Gauglitz GG J Dtsch Dermatol Ges 2016, 14, 467. [DOI] [PubMed] [Google Scholar]
- [5].Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Matrix Biol 2016, 51, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matsumura Y, Ananthaswamy HN Expert Rev Mol Med 2002, 4, 1. [DOI] [PubMed] [Google Scholar]
- [7].Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD J Invest Dermatol 2012, 132, 1901. [DOI] [PubMed] [Google Scholar]
- [8].Clement M, Daniel G, Trelles M. J Cosmet Laser Ther 2005, 7, 177. [DOI] [PubMed] [Google Scholar]
- [9].Mamalis A, Koo E, Garcha M, Murphy WJ, Isseroff RR, Jagdeo J. J Biophotonics 2016, 9, 1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mamalis A, Jagdeo J. Dermatol Surg 2017, 43, 81. [DOI] [PubMed] [Google Scholar]
- [11].Lichtman MK, Otero-Vinas M, Falanga V. Wound Repair Regen 2016, 24, 215. [DOI] [PubMed] [Google Scholar]
- [12].Mamalis A, Siegel D, Jagdeo J. Curr Dermatol Rep 2016, 5, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mamalis A, Jagdeo J. Dermatol Surg 2018, 44, 1317. [DOI] [PubMed] [Google Scholar]
- [14].Chen M, Huang J, Yang X, Liu B, Zhang W, Huang L, Deng F, Ma J, Bai Y, Lu R, Huang B, Gao Q, Zhuo Y, Ge J. PLoS One 2012, 7, e28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV Nat Med 2010, 16, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koyano T, Namba M, Kobayashi T, Nakakuni K, Nakano D, Fukushima M, Nishiyama A, Matsuyama M. Sci Rep 2019, 9, 12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Urushiyama H, Terasaki Y, Nagasaka S, Kokuho N, Endo Y, Terasaki M, Kunugi S, Makita K, Isago H, Hosoki K, Souma K, Ishii T, Matsuzaki H, Hiraishi Y, Mikami Y, Noguchi S, Tamiya H, Mitani A, Yamauchi Y, Shimizu A, Nagase T. J Cell Mol Med 2019, 23, 3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu H, Hong S, Yan Z, Zhao Q, Shi Y, Song N, Xie J, Jiang X. Life Sci 2019, 229, 200. [DOI] [PubMed] [Google Scholar]
- [19].Beckerman R, Prives C. Cold Spring Harb Perspect Biol 2010, 2, a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bieging KT, Mello SS, Attardi LD Nat Rev Cancer 2014, 14, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Joerger AC, Fersht AR Annu Rev Biochem 2016, 85, 375. [DOI] [PubMed] [Google Scholar]
- [22].Olivier M, Hollstein M, Hainaut P. Cold Spring Harb Perspect Biol 2010, 2, a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Richardson RB Cell Cycle 2013, 12, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hafner A, Bulyk ML, Jambhekar A, Lahav G. Nat Rev Mol Cell Biol 2019, 20, 199. [DOI] [PubMed] [Google Scholar]
- [25].Nguyen JK, Weedon J, Jakus J, Heilman E, Isseroff RR, Siegel DM, Jagdeo JR Trials 2019, 20, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.