Abstract
The recent increase of COVID-19-associated mucormycosis (CAM) has been commanding global attention. However, basic epidemiologic characteristics have not firmly been established. In this systematic review and meta-analysis, we sought to determine the clinical manifestations, potential risk factors, and outcomes of CAM. Observational studies reporting CAM were searched with PubMed and EMBASE databases in January 2022. We collected data on comorbidities and treatment for COVID-19, and performed a one-group meta-analysis on the frequency of orbital exenteration procedure and mortality of CAM using a random-effect model. Fifty-one observational studies, including a total of 2,312 patients with proven CAM, were identified. Among the 51 studies, 37 were conducted in India, 8 in Egypt, and 6 in other countries. The most common comorbidity was diabetes mellitus (82%). While 57% required oxygenation, 77% received systemic corticosteroids. Among CAM, 97% were rhino-orbital-cerebral (ROCM), and 2.7% were pulmonary mucormycosis. Usual presentations were headache (54%), periorbital swelling/pain (53%), facial swelling/pain (43%), ophthalmoplegia (42%), proptosis (41%), and nasal discharge/congestion (36%). Regarding the outcomes, orbital exenteration was performed in 17% (95% CI: 12–21%, I2 = 83%) of the COVID-19-associated ROCM patients. The mortality of CAM was 29% (95% CI; 22–36%, I2 = 92%). In conclusion, this systematic review and meta-analysis indicated that the most prevalent type of CAM was ROCM, and most CAM patients had diabetes mellitus and received systemic glucocorticoids. Clinicians in the endemic areas should have a high index of suspicion for this invasive fungal complication of COVID-19 when a diabetic patient who received high-dose systemic glucocorticoids developed rhino-orbital symptoms.
Keywords: COVID-19, Mucormycosis, Glucocorticoids, Diabetes mellitus, Meta-analysis
Introduction
The number of COVID-19 cases complicated by secondary fungal infections has been soaring [1]. This trend has been commanding global attention because secondary infections can result in worse outcomes, as several studies on COVID-19-associated pulmonary aspergillosis (CAPA) have reported higher mortality. Excessive treatments with glucocorticoids and anti-cytokine agents possibly increase the risk of invasive fungal infections [2, 3]. Since the RECOVERY trial demonstrated the mortality benefit of dexamethasone in July 2020, glucocorticoids have been the standard therapy for severe COVID-19 pneumonia [4], possibly leading to the recent noticeable increase of secondary invasive fungal infection [1, 5].
Compared to CAPA, less is known about COVID-19-associated mucormycosis (CAM). Mucormycosis is a fungal infection characterized by infarction and necrosis of host tissues because of its angio-invasive nature of the fungal hyphae [6]. The first case series of CAM in India collected the clinical data between August and December 2020 and indicated the probable relationship between COVID-19 and mucormycosis [7]. This relationship was supported by other reports which described the impact of glucocorticoid therapy for COVID-19 on the subsequent mucormycosis [8, 9]. In contrast, several case reports described the simultaneous diagnosis of mucormycosis with COVID-19 [10, 11]. These cases emphasized the influences of shared background risk factors for both diseases, such as immunodeficiency and diabetes mellitus (DM), rather than the causal relationship brought by glucocorticoid treatment for COVID-19.
Observational studies and reviews of case reports and case series on CAM reported the mortality of 14–30%. However, the actual impact of CAM has not been comprehensively explored as one of the largest observational studies included non-proven mucormycosis as well [12, 13]. Furthermore, whereas rhino-orbital-cerebral mucormycosis (ROCM) and pulmonary mucormycosis had been the major types of the disease before the current pandemic of COVID-19, the difference in clinical characteristics between non-COVID-19 mucormycosis and CAM remain under-investigated. In the present study, we conducted a systematic review and meta-analysis to elucidate the clinical presentations, potential risk factors, and outcomes of CAM, aiming to seize comprehensive pictures of this invasive fungal complication.
Materials and Methods
Data Sources and Search
All prospective, retrospective, and cross-sectional observational studies and case series reporting CAM were searched using a two-level search strategy. First, we conducted a comprehensive literature search of PubMed and Embase databases on January 20, 2022. The search terms included ("COVID-19" OR "SARS-CoV-2" OR "coronavirus") AND ("mucor" OR "mucormycosis" OR "zygomycosis"). Second, we performed an additional manual search of secondary sources, including references of initially identified articles, to maximize the completeness of the collection of relevant studies. The search was performed without language restriction.
Study Selection
A study meeting the following criteria was included in the meta-analysis; (1) the study was published in a peer-reviewed journal, (2) the study design was prospective, retrospective, cross-sectional observational study, or case series, (3) the study was reporting mucormycosis cases with past or concurrent COVID-19 infection, (4) the diagnosis of COVID-19 was confirmed with nucleic acid amplification tests (e.g., reverse transcription polymerase chain reaction [RT-PCR]), rapid antigen tests, or serum antibody tests, (5) the diagnosis of mucormycosis was confirmed by microscopic visualization, histopathologic examination, or culture, in accordance with guidelines [14, 15]. We excluded observational studies that did not clearly state the diagnostic criteria used for COVID-19 or mucormycosis, as well as case reports. When multiple studies from the same authors and the same institutes were detected, smaller (and earlier) studies were omitted because larger studies were assumed to include the cases reported in the smaller studies.
Data Extraction
Two investigators (AW and MS) reviewed the search results separately to select the studies based on the inclusion and exclusion criteria and assessed the eligibility of each study. The full texts of articles were retrieved for eligibility assessment and further analysis after the initial screening with title and abstract. Any discrepancies were resolved by discussion and consensus. The following data were extracted from each eligible study: authors’ names, study location, design, setting, observational period, and sample size (the number of CAM). We also collected the following patient characteristics and outcomes: co-morbidities, initial symptoms, the type and extension of mucormycosis, the interval between COVID-19 and the onset of mucormycosis, treatment for COVID-19, intervention for mucormycosis, and the number of deceased patients.
Statistical Analysis
The endpoints of this study were the frequency of endoscopic or surgical intervention, orbital exenteration, and mortality among patients with CAM. We performed a one-group meta-analysis with a random-effects model using the DerSimonian-Laird method. Statistical analyses were executed with OpenMetaAnalyst version 12.11.14 (available at http://www.cebm.brown.edu/openmeta/) [16]. Heterogeneity among studies was evaluated with I2, more than 50% indicating substantial heterogeneity. This meta-analysis was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [17].
Results
We identified a total of 512 articles through the initial database and subsequent manual searches. After removing duplicated items and screening based on title and abstract, 221 articles were assessed for eligibility. Among these articles, 168 were excluded, including 42 case reports and 14 retrospective studies without clearly stipulated diagnostic criteria for COVID-19 and mucormycosis. In particular, the largest observational study by Sen M et al.[12] (N = 2,826) was excluded because this study included probable/possible cases of CAM as well as microbiologically or histopathologically confirmed CAM; we were unable to extract data of proven CAM separately from their report. Of the remaining 53 studies, we excluded 2 case series [18, 19] because observational studies with larger sample sizes have been published by the same authors. Eventually, 36 observational studies and 15 case series with a total of 2,312 proven CAM cases were included in our meta-analysis [7, 9, 20–68](Fig. 1).
Fig. 1.
Flow diagram of study selection
The characteristics of the included studies are summarized in Table 1. Among the selected 51 studies, 37 were conducted in India, 8 in Egypt, 2 in Iran, and 1 in the United Kingdom, Germany, Spain, and the Czech Republic. The observational period of each study varied between January 2020 and August 2021. The median age of the patients ranged from 36 to 63. The percentage of males ranged from 20 to 100%. 2.1% (6/284) were fully vaccinated against SARS-CoV-2. The most common co-morbidity was diabetes 82% (1,853/2,251, [382 patients were newly detected at the time of COVID-19 diagnosis, 61 patients presented with DKA]), followed by hypertension 42% (250/596), chronic kidney diseases 15% (113/735), immunosuppression (i.e., solid organ transplantation, acquired immunodeficiency syndrome, the use of immunosuppressive medications) 14% (97/675), and malignancy 2.6% (10/391). 57% (807/1,420) required oxygen supplementation because of COVID-19 pneumonia, and 12% (89/766) were mechanically ventilated. 77% (1,503/1,949) and 4.7% (23/489) received corticosteroids and tocilizumab, respectively, and 27% (191/699) were admitted to intensive care units. The median interval between COVID-19 and the onset of initial symptoms of mucormycosis ranged from 10 to 31 days. Concurrent infections with other fungi were observed in 6.1% (24/393) of CAM.
Table 1.
Study and patients characteristics
| Author | Country | Design | Setting | Confirmed CAM, N | Observational period | Age (Mean, SD) [range] | Male, % (N) | Diabetes, % (N) |
|---|---|---|---|---|---|---|---|---|
| Moorthy A. [20] | India | Retrospective | Multi-center | 17 | May 2020–Dec 2020 | 55 [35–73] | 83 (14) | 88 (15) |
| El-Kholy NA. [21] | Egypt | Prospective | Single-center | 28 | Aug 2020–Dec 2020 | 53 ± 11 | 53 (15) | – |
| Ravani SA. [22] | India | Retrospective | Single-center | 31 | Sep 2020–Mar 2021 | 56 | 65 (20) | 94 (30) |
| Ramaswami A. [23] | India | Retrospective | Single-center | 70 | Apr 2021–Jun 2021 | 45 [38–56] | 60 (42) | 70 (49) |
| Gupta NK. [24] | India | Retrospective | Single-center | 74 | Mar 2021–May 2021 | 52 | 65 (48) | 97 (72) |
| Mishra Y. [25] | India | Retrospective | Single-center | 32 | Apr 2021–May 2021 | 58 ± 9 | 47 (15) | 88 (28) |
| Kumari A. [26] | India | Retrospective | Single-center | 20 | Mar 2021–May 2021 | 54 [35–67] | 55 (11) | 80 (16) |
| Gupta DP. [27] | India | Prospective | Single-center | 70 | Mar 2020–Dec 2020 | [20–75] | 67 (47) | 100 (70) |
| Patel A. [28] | India | Retrospective | Multi-center | 187 | Sep 2020–Dec 2020 | 57 ± 13 | 80 (150) | 60 (113) |
| Dubey S. [29] | India | Retrospective | Single-center | 46 | Apr 2021–Jun 2021 | 53 ± 10 | 64 (29) | 100 (46) |
| Bhanuprasad K. [30] | India | Prospective | Single-center | 132 | Jun 2020–Jul 2021 | 51 ± 12 | 77 (101) | 98 (129) |
| Selarka L. [31] | India | Prospective | Multi-center | 47 | Jan 2021–Mar 2021 | 55 ± 13 | 75 (35) | 77 (36) |
| Muthu V. [32] | India | Retrospective | Single-center | 31 | – | 53 [48–58] | 76 (23) | 75 (21) |
| Pakdel F. [33] | Iran | Cross-sectional | Multi-center | 15 | Apr 2020–Sep 2020 | 52 [14–71] | 66 (10) | 87 (13) |
| Meawed TE. [34] | India | Cross-sectional | Single-center, ICU | 11 | Oct 2020–Apr 2021 | – | – | – |
| Zirpe K. [35] | India | Retrospective | Single-center | 84 | Feb 2021–Jun 2021 | 49 ± 12 | 83 (70) | 64 (54) |
| Pal P. [36] | India | Prospective | Single-center | 31 | May 2021–Jun 2021 | 53 [20–78] | 68 (21) | 100 (31) |
| Pippal SK. [37] | India | Prospective | Single-center | 80 | May 2021–Aug 2021 | – | 69 (55) | 90 (72) |
| Ismaiel WF. [38] | Egypt | Retrospective | Single-center | 10 | – | – | – | – |
| Avatef Fazeli M. [39] | Iran | Retrospective | Single-center | 9 | Oct 2020–Nov 2020 | 59 | 33 (3) | 78 (7) |
| Goddanti N. [40] | India | Retrospective | Single-center | 300 | Apr 2021–Jun 2021 | 49 ± 12 | 74 (222) | 96 (287) |
| Kulkarni R. [41] | India | Retrospective | Multi-center | 49 | Dec 2020–Jun 2021 | 53 ± 11 | 71 (35) | 82 (40) |
| Dokania V. [42] | India | Prospective | Single-center | 21 | Apr 2021–Jun 2021 | 50 ± 14 | 71 (15) | 90 (19) |
| Nair AG. [43] | India | Retrospective | Multi-center | 13 | – | 36 [20–51] | 69 (9) | 0 (0) |
| Kumar S. [44] | India | Retrospective | Single-center | 287 | May 2021–Jul 2021 | – | 79 (227) | 80 (229) |
| Joshi AR. [45] | India | Retrospective | Single-center | 25 | Mar 2021–Apr 2021 | 55 ± 13 | – | 88 (22) |
| Choksi T. [46] | India | Retrospective | Single-center | 73 | Mar 2021–May 2021 | 54 ± 13 | 66 (48) | 74 (48) |
| Bansai SB. [47] | India | Retrospective | Single-center | 11 | Feb 2020–May 2021 | 47 ± 11 | 100 (11) | 64 (7) |
| Fouad YA. [48] | Egypt | Retrospective | Single-center | 26 | Apr 2021–Aug 2021 | 63 [46–70] | 54 (14) | 96 (25) |
| Meher R. [49] | Iran | Prospective | Single-center | 111 | May 2021–Jun 2021 | 53 ± 12 | 70 (78) | 94 (104) |
| Guzman-Castro S. [50] | UK | Retrospective | Single-center | 6 | May 2021 | 52 [45–57] | 83 (5) | 83 (5) |
| Kumar H. [51] | India | Retrospective | Single-center | 28 | Apr 2021–May 2021 | 54 [48–59] | 79 (22) | 75 (21) |
| Yadav T. [52] | India | Retrospective | Single-center | 50 | Dec 2020–Jun 2021 | 50 [28–70] | 62 (31) | 86 (43) |
| Meshram HS. [53] | India | Retrospective | Multi-center | 61 | Nov 2020–Jul 2021 | 45 [38–54] | 89 (54) | 49 (30) |
| Popli H. [54] | India | Retrospective | Single-center | 23 | Jul 2020–Jun 2021 | 48 ± 12 | 57 (13) | 78 (18) |
| Patel DD. [55] | India | Cross-sectional | Single-center | 96 | Mar 2021–May 2021 | 49 [21–76] | 73 (70) | 72 (69) |
| Sen M. [7] | India | Case series | Single-center | 5 | Aug 2020–Dec 2020 | 58 | 100 (5) | 100 (5) |
| Saidha PK. [9] | India | Case series | Single-center | 6 | – | [29–68] | 66 (4) | 100 (5) |
| Roushdy T. [56] | Egypt | Case series | Single-center | 2 | Jan 2021–Apr 2021 | [59–73] | 100 (2) | 100 (2) |
| Sai Krishna D. [57] | India | Case series | Single-center | 2 | – | [34–50] | 100 (2) | 100 (2) |
| Nehara HR. [58] | India | Case series | Single-center | 5 | – | [33–70] | 20 (1) | 100 (5) |
| Singh Y. [59] | India | Case series | Single-center | 13 | Nov 2020–Jan 2021 | [5–75] | 85 (11) | 62 (8) |
| Garg R. [60] | India | Case series | Single-center | 7 | May 2021 | [38–70] | 71 (5) | 100 (7) |
| Arana C. [61] | Spain | Case series | Single-center | 2 | – | [48–62] | 100 (2) | 50 (1) |
| Barman Roy D. [62] | India | Case series | Single-center | 2 | – | [39–50] | 50 (1) | 50 (1) |
| Ashour MM. [63] | Egypt | Case series | Single-center | 6 | – | [41–67] | 50 (3) | 100 (6) |
| Pal P. [64] | India | Case series | Single-center | 10 | May 2021–Jun 2021 | [28–66] | 60 (6) | 70 (7) |
| Riad A. [65] | Czech | Case series | Multi-center | 6 | Apr 2021–May 2021 | [47–68] | 71 (5) | 83 (5) |
| Said Ahmed WM. [66] | Egypt | Case series | Single-center | 14 | – | [29–77] | 71 (10) | 100 (14) |
| Alloush TK. [67] | Egypt | Case series | Single-center | 14 | May 2021–Jun 2021 | [49–82] | 64 (9) | 93 (!3) |
| Seidel D. [68] | Germany | Case series | Multi-center | 13 | Feb 2020–Jun 2021 | [30–75] | 62 (8) | 23 (3) |
| Author | HTN, % (N) | CKD, % (N) | Immunosuppression, % (N) | Malignancy, % (N) | Hypoxia, % (N) | Mechanical ventilation, % (N) | Corticosteroids treatment, % (N) | ICU admission, % (N) |
|---|---|---|---|---|---|---|---|---|
| Moorthy A. [20] | – | – | – | – | – | – | 88 (15) | – |
| El-Kholy NA. [21] | – | – | – | – | – | – | – | – |
| Ravani SA. [22] | 53 (17) | 6.3 (2) | – | – | – | – | 100 (19) | – |
| Ramaswami A. [23] | 24 (17) | 8.6 (6) | 2.9 (2) | – | – | – | 70 (49) | – |
| Gupta NK. [24] | – | 6.8 (5) | – | 1.4 (1) | 69 (51) | – | 91 (67) | – |
| Mishra Y. [25] | 50 (16) | – | 0 (0) | – | 69 (22) | – | 94 (30) | – |
| Kumari A. [26] | – | 15 (3) | – | – | – | – | 80 (16) | – |
| Gupta DP. [27] | – | – | 7.1 (5) | 2.9 (2) | – | – | – | – |
| Patel A. [28] | – | – | 1.6 (3) | 1.1 (2) | 56 (74) | – | 78 (146) | 31 (58) |
| Dubey S. [29] | – | – | – | – | 72 (33) | 0 (0) | 52 (24) | – |
| Bhanuprasad K. [30] | – | 5.3 (7) | 1.5 (2) | – | 14 (19) | 2.3 (3) | 55 (73) | – |
| Selarka L. [31] | – | – | – | – | 81 (38) | 43 (20) | 100 (47) | – |
| Muthu V. [32] | 32 (9) | – | – | – | 55 (16) | 24 (7) | 71 (20) | – |
| Pakdel F. [33] | 47 (7) | – | – | 13 (2) | 60 (9) | 6.7 (1) | 47 (7) | – |
| Meawed TE. [34] | – | – | – | – | – | – | – | – |
| Zirpe K. [35] | 37 (31) | 3.6 (3) | – | – | 68 (57) | – | 83 (70) | – |
| Pal P. [36] | – | – | – | – | – | – | – | – |
| Pippal SK. [37] | 75 (60) | 1.3 (1) | – | – | – | – | – | – |
| Ismaiel WF. [38] | – | – | – | – | – | – | – | – |
| Avatef Fazeli M. [39] | 44 (4) | 22 (2) | 11 (1) | – | – | 13 (1) | 100 (7) | 25 (2) |
| Goddanti N. [40] | – | – | – | – | 60 (180) | – | 79 (237) | – |
| Kulkarni R. [41] | 37 (18) | – | – | – | – | – | – | – |
| Dokania V. [42] | 0.5 (1) | – | – | – | – | 4.8 (1) | 90 (19) | 19 (4) |
| Nair AG. [43] | – | – | – | – | 38 (5) | – | 54 (7) | – |
| Kumar S. [44] | – | – | – | – | – | – | 90 (257) | 23 (66) |
| Joshi AR. [45] | – | – | 8.0 (2) | – | 100 (25) | 48 (12) | 100 (25) | 100 (25) |
| Choksi T. [46] | – | – | – | – | 82 (60) | 4.1 (3) | 98 (58) | – |
| Bansai SB. [47] | 45 (5) | 100 (11) | 100 (11) | – | 36 (4) | 0 (0) | 36 (4) | – |
| Fouad YA. [48] | 27 (7) | 12 (3) | – | – | – | – | 77 (20) | – |
| Meher R. [49] | – | – | – | – | 27 (30) | 5.4 (6) | 60 (67) | 5.4 (6) |
| Guzman-Castro S. [50] | – | – | – | – | 50 (3) | 33 (2) | 100 (6) | – |
| Kumar H. [51] | – | – | – | – | 57 (16) | 21 (6) | 68 (19) | – |
| Yadav T. [52] | – | – | 2.0 (1) | – | 38 (19) | – | 44 (22) | – |
| Meshram HS. [53] | – | 100 (61) | 100 (61) | – | 67 (41) | 0 (0) | 44 (27) | – |
| Popli H. [54] | – | – | – | – | – | – | – | – |
| Patel DD. [55] | 40 (38) | 2.1 (2) | – | – | 73 (70) | 6.3 (6) | 82 (79) | – |
| Sen M. [7] | – | – | – | – | – | – | 80 (4) | – |
| Saidha PK. [9] | – | – | – | – | – | – | 17 (1) | – |
| Roushdy T. [56] | 100 (2) | – | – | – | – | – | 50 (1) | – |
| Sai Krishna D. [57] | 50 (1) | – | – | – | – | – | – | – |
| Nehara HR. [58] | 40 (2) | – | – | – | – | 20 (1) | 40 (2) | – |
| Singh Y. [59] | 46 (6) | – | 15 (2) | 23 (3) | 85 (11) | 69 (9) | 77 (10) | 77 (10) |
| Garg R. [60] | – | – | – | 29 (2) | 0 (0) | 100 (7) | 0 (0) | |
| Arana C. [61] | 100 (2) | 100 (2) | 100 (2) | 100 (2) | 0 (0) | 100 (2) | 50 (1) | |
| Barman Roy D. [62] | – | – | – | 0 (0) | 0(0) | 50 (1) | – | |
| Ashour MM. [63] | – | 17 (1) | – | – | – | – | – | |
| Pal P. [64] | – | 10 (1) | – | 30 (3) | – | 80 (8) | – | |
| Riad A. [65] | 17 (1) | – | – | 50 (3) | – | 100 (6) | – | |
| Said Ahmed WM. [66] | – | – | – | – | – | – | 21 (3) | |
| Alloush TK. [67] | 43 (6) | 14 (2) | – | 100 (14) | 0 (0) | 93 (13) | – | |
| Seidel D. [68] | – | 7.7 (1) | 38 (5) | – | 79 (11) | 79 (11) | 79 (11) |
Value is shown as mean ± SD or median [range]. The columns of hypoxia, mechanical ventilation, corticosteroids treatment, and ICU admission was the data regarding COVID-19 severity and management. Abbreviations: SD, Standard deviation; HTN, Hypertension; CKD, Chronic kidney disease; ICU, Intensive care units;
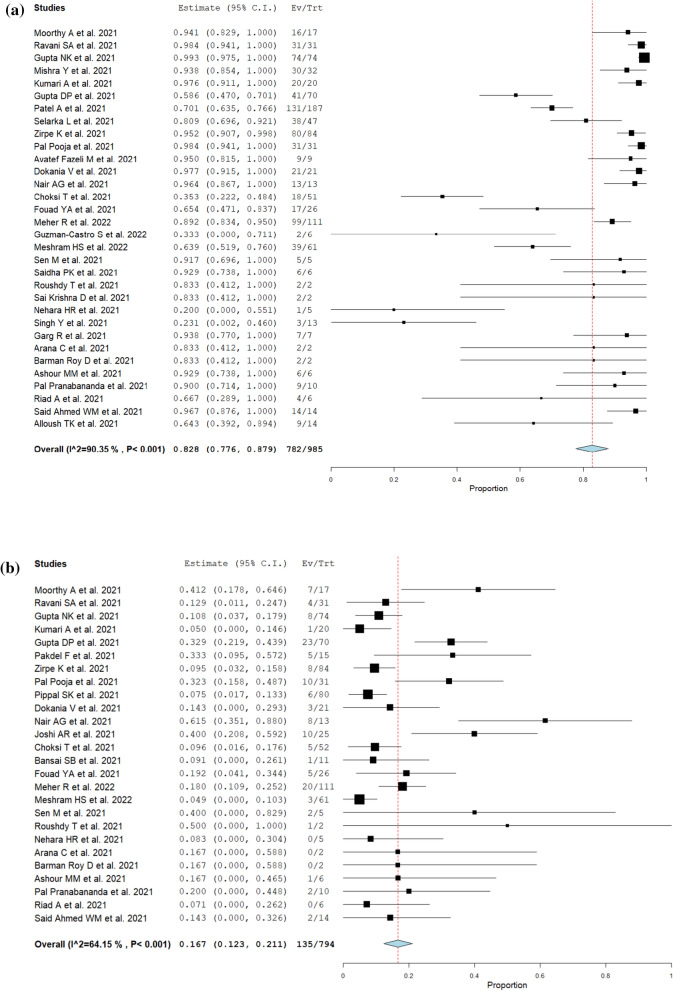
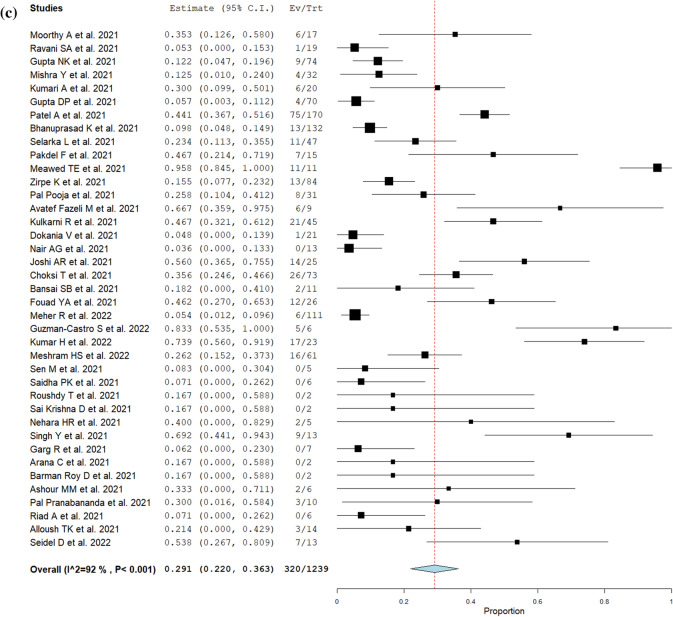
The type of CAM, symptoms, treatments, and outcomes are summarized in Table 2. The most common type of CAM was ROCM 97% (2,242/2,312), followed by pulmonary 2.7% (63/2,312), and others (cutaneous, renal, gastrointestinal, disseminated). Among COVID-19-associated ROCM patients, orbital and intracranial involvement was observed in 50% (731/1,451) and 25% (404/1,638), respectively. The most common presentation was headache 54% (254/474), followed by periorbital swelling or pain 53% (242/460), facial swelling or pain 43% (230/532), ophthalmoplegia 42% (98/236), proptosis 41% (182/447), nasal congestion or discharge 36% (184/515), decreased or loss of vision 31% (181/584), ptosis 28% (78/276), dental pain or loosened teeth 25% (31/124), and palatal discoloration or ulcers 22% (90/404). 83% (95% CI; 78–88%, I2 = 90%) of CAM patients were endoscopically or surgically treated (Fig. 2a). Orbital exenteration was performed in 17% (95% CI; 12–21%, I2 = 63%) of ROCM patients (Fig. 2b). The mortality of CAM was 29% (95% CI; 22–36%, I2 = 92%) (Fig. 2c).
Table 2.
Symptoms, treatments, and outcomes of the patients with COVID-19-associated mucormycosis
| Author | Days from COVID-19 diagnosis, median [range] | Type of CAM, % (N) | Symptoms, % (N) | Orbital involvement, % (N) | Cranial involvement, % (N) | Endoscopic or surgical procedures, % (N) | Orbital exenteration, % (N) | Mortality, % (N) |
|---|---|---|---|---|---|---|---|---|
| Moorthy A | – | ROCM, 100 (17) | Loss of vision, 65 (11) | – | 47 (8) | 94 (16) | 41 (7) | 35 (6) |
| El-Kholy NA | 18 ± 3 | ROCM, 100 (28) | – | – | – | – | – | – |
| Ravani SA | – | ROCM, 100 (31) | Ophthalmoplegia, 77 (24), Decreased vision, 94 (29), Proptosis, 26 (8), Eyelid swelling, 29 (9) | – | 100 (31) | 13 (4) | 5.3 (19) | |
| Ramaswami A | 20 [13–25] | ROCM, 100 (70) | Orbital pain, 81 (57), Loss of vision, 37 (26), Proptosis, 34 (24), Ptosis, 20 (14), Nasal blockage, 39 (27), Facial pain, 34 (24), Headache, 29 (20), Facial ulcer, 4.3 (3), Facial deviation, 2.9 (2), Oral ulcer, 5.7 (4) | 44 (31) | 24 (17) | – | – | – |
| Gupta NK | 18 | ROCM, 100 (74) | Headache, 45 (33), Dental pain, 27 (20), Visual symptoms, 16 (12), Facial swelling, 11 (8) | 20 (15) | 4.1 (3) | 100 (74) | 11 (8) | 12 (9) |
| Mishra Y | 11 | ROCM, 97 (31), Cutaneous, 3 (1) | Headache, 94 (30), Nasal symptoms, 94 (30), Eye symptoms, 59 (19), Palatal discoloration, 9.4 (3), Cutaneous symptoms, 0.3 (1) | 59 (19) | 0 (0) | 94 (30) | – | 13 (4) |
| Kumari A | [7–15] | ROCM, 100 (20) | Nasal obstruction, 75 (15), Eye swelling, 40 (8), Facial pain, 35 (7), Headache, 35 (7), Proptosis, 50 (10), Ptosis, 10 (2), Vision loss, 5 (1), Ophthalmoplegia, 5 (1), Palatal discolorations, 5 (1) | 55 (11) | 20 (4) | 100 (20) | 5 (1) | 30 (6) |
| Gupta DP | – | ROCM, 100 (70) | Periorbital swelling, 53 (37), Facial swelling, 46 (32), Loss of vision, 39 (27), Palatal ulcer, 16 (11), Ptosis, 19 (13), Diplopia, 4.3 (3), Nasal blockage, 7.1 (5) | – | – | 59 (41) | 33 (23) | 5.7 (4) |
| Patel A | 18 [11–27] | ROCM, 86 (167), Pulmonary, 8.6 (16), Renal, 0.5 (1), Disseminated, 2.1 (4) | – | – | 24 (44) | 70 (131) | – | 44 (75) |
| Dubey S | [7–56] | ROCM, 100 (46) | – | 72 (33) | 17 (8) | – | – | – |
| Bhanuprasad K | – | ROCM, 100 (132) | – | 70 (93) | 30 (39) | – | – | 9.8 (13) |
| Selarka L | 12 ± 5 | ROCM, 100 (47) | Nasal congestion, 100 (47), Headache, 74 (35), Loss of vision, 26 (12), Facial weakness, 17 (8), Ophthalmoplegia, 19 (9), Proptosis, 2.1 (1) | 40 (19) | 19 (9) | 81 (38) | – | 23 (11) |
| Muthu V | 11 | ROCM, 83 (24), Pulmonary, 17 (5) | – | – | 52 (15) | – | – | – |
| Pakdel F | 7 [1–37] | ROCM, 100 (15) | Periorbital pain, 73 (11), Ptosis, 73 (11), Vision loss, 73 (11), Proptosis, 67 (10), Facial edema, 60 (9), Headache, 33 (5), Fever, 27 (4), Nasal blockage, 13 (2), Ear pain, 6.7 (1) | 93 (14) | 67 (10) | – | 33 (5) | 47 (7) |
| Meawed TE | – | Pulmonary, 100 (11) | – | – | – | – | – | 100 (11) |
| Zirpe K | 11 | ROCM, 100 (84) | Facial swellings, 21 (18), Eye pain, 33 (28), Headache, 27 (23), Blurred vision, 9.5 (8), Nasal discharge, 14 (12), Proptosis, 3.6 (3), Diplopia, 2.4 (2), Facial palsy, 2.4 (2) | 30 (25) | 24 (20) | 95 (80) | 9.5 (8) | 15 (13) |
| Pal P | – | ROCM, 100 (31) | Facial symptoms, 71 (22), Palatal symptoms, 58 (18), Orbital symptoms, 71 (22) | 32 (10) | – | 100 (31) | 32 (10) | 26 (8) |
| Pippal SK | – | ROCM, 100 (80) | – | 38 (30) | 13 (10) | – | 7.5 (6) | – |
| Ismaiel WF | – | ROCM, 100 (10) | – | – | – | – | – | – |
| Avatef Fazeli M | [8–50 | ROCM, 100 (9) | – | 78 (7) | – | 100 (9) | – | 67 (6) |
| Goddanti N | – | ROCM, 100 (300) | – | – | – | – | – | – |
| Kulkarni R | 18 [13–25] | ROCM, 85 (48), Pulmonary, 15 (8) | Motor weakness, 76 (34), Altered mental status, 11 (5), Aphasia, 20 (9), Hemianopia, 4.4 (2) | – | 100 (49) | – | – | 47 (21) |
| Dokania V | – | ROCM, 100 (21) | Headache/orbital pain, 86 (18), Periorbital swellings, 62 (13), Blurred vision, 24 (5), Ophthalmoplegia, 19 (4), Nasal blockage, 19 (4), Facial numbness, 14 (3), Chemosis, 14 (3), Toothache, 9.5 (2), Palatal ulcer, 9.5 (2) | 48 (10) | 24 (5) | 100 (21) | 14 (3) | 4.8 (1) |
| Nair AG | 14 [9–22] | ROCM, 100 (13) | – | 57 (8) | – | 100 (13) | 57 (8) | 0 (0) |
| Kumar S | – | ROCM, 100 (287) | – | 36 (102) | 21 (60) | – | – | – |
| Joshi AR | – | ROCM, 100 (25) | – | 92 (23) | 28 (7) | – | 40 (10) | 56 (14) |
| Choksi T | 31 ± 20 | ROCM, 100 (73) | Ophthalmoplegia, 38 (28), Proptosis, 66 (48), Ptosis, 18 (13), Decreased vision, 16 (12) | 93 (68) | 8.2 (6) | 35 (18) | 9.6 (5) | 36 (26) |
| Bansai SB | 17 [10–30] | ROCM, 91 (!0), Pulmonary, 9 (1) | Headache, 73 (8), Facial numbness, 36 (4), Facial swelling, 18 (2), Eye pain, 27 (3), Vision loss, 9.1 (1), Hemoptysis, 9.1 (1) | 18 (2) | – | – | 9.1 (1) | 18 (2) |
| Fouad YA | 21 [15–30] | ROCM, 100 (“6) | Facial swelling, 50 (13), Lid swelling, 27 (7), Skin discoloration, 12 (3), Nasal obstruction, 7.7 (2), Decreased vision, 3.8 (1) | – | – | 65 (17) | 19 (5) | 46 (12) |
| Meher R | 19 [10–28] | ROCM, 100 (111) | – | 66 (73) | 3.6 (4) | 89 (99) | 18 (20) | 5.4 (6) |
| Guzman-Castro S | 15 [8–21] | ROCM, 83 (5), Pulmonary, 17 (1) | Orbital edema, 83 (5), Palatal ulcers, 67 (4), Facial edema, 67 (4), Proptosis, 50 (3), Headache, 50 (3), Dyspnea, 17 (1) | – | – | 33 (2) | – | 83 (5) |
| Kumar H | – | ROCM, 86 (24), Pulmonary 14 (4) | – | – | 54 (15) | – | – | 74 (17) |
| Yadav T | 14 ± 7 | ROCM, 100 (50) | – | 36 (18) | 50 (25) | – | – | – |
| Meshram HS | – | ROCM, 92 (56), Pulmonary, 8 (5) | Facial swelling, 80 (49), Skin necrosis, 9.8 (6), Paresthesia, 34 (21), Nasal discharge, 30 (18), Epistaxis, 23 (14), Chemosis, 61 (37), Proptosis, 74 (45), Headache, 82 (50), Fever, 28 (17) | 36 (15) | 14 (6) | 64 (39) | 4.9 (3) | 26 (16) |
| Popli H | – | ROCM, 100 (23) | – | – | – | – | – | – |
| Patel DD | – | ROCM, 100 (96) | – | 59 (57) | 22 (21) | – | – | – |
| Sen M | 14 [0–42] | ROCM, 100 (5) | Ophthalmoplegia, 80 (4), Ptosis, 80 (4), Proptosis, 80 (4), Nasal signs, 60 (3), Palatal eschar, 60 (3) | 100 (5) | 80 (4) | 100 (5) | 40 (2) | 0 (0) |
| Saidha PK | [2–3 months] | ROCM, 100 (6) | Facial pain, 83 (5), Loosened teeth, 17 (1), Nasal discharge, 17 (1), Headache, 33 (2) | – | – | 100 (6) | – | 0 (0) |
| Roushdy T | [10–14] | ROCM, 100 (2) | Ophthalmoplegia, 100 (2), Chemosis, 100 (2), Ptosis, 100 (2), Proptosis, 100 (2) | 100 (2) | 100 (2) | 100 (2) | 50 (1) | 0 (0) |
| Sai Krishna D | – | ROCM, 100 (2) | Facial pain, 100 (2), Toothache, 100 (2) | – | – | 100 (2) | – | 0 (0) |
| Nehara HR | [5 days–1 month] | ROCM, 100 (5) | – | 80 (4) | 0 (0) | 20 (1) | 0 (0) | 40 (2) |
| Singh Y | [0–16] | ROCM, 92 (12), Pulmonary, 8 (1) | Fever, 38 (5), Epistaxis, 23 (3), Facial pain, 38 (5), Orbital pain, 15 (2) | 62 (8) | 15 (2) | 23 (3) | – | 69 (9) |
| Garg R | – | ROCM, 100 (7) | Facial pain, 100 (7) | 29 (2) | 0 (0) | 100 (7) | – | 0 (0) |
| Arana C | [7–21] | ROCM, 50 (1), Musculoskeletal, 50 (1) | – | – | – | 100 (2) | 0 (0) | 0 (0) |
| Barman Roy D | – | ROCM, 100 (2) | Facial pain, 50 (1), Decreased vision, 50 (1), Headache, 50 (1) | – | 0 (0) | 100 (2) | 0 (0) | 0 (0) |
| Ashour MM | [2 weeks–2 months] | ROCM, 100 (6) | Chemosis, 17 (1), Ophthalmoplegia, 67 (4), Decreased vision, 67 (4), Proptosis, 33 (2), Facial edema, 17 (1), Palatal ulcers, 33 (2) | 100 (6) | 33 (2) | 100 (6) | 17 (1) | 33 (2) |
| Pal P | [5–40] | ROCM, 100 (10) | Headache, 20 (2), Eye swelling. 60 (6), Ophthalmoplegia, 30 (3), Nasal discharge, 10 (1), Skin discoloration, 20 (2), Loss of vision, 40 (4), Loosened teeth, 20 (2) | 50 (5) | – | 90 (9) | 20 (2) | 30 (3) |
| Riad A | [3 days–4 weeks] | ROCM, 100 (7) | Palatal ulcers, 29 (2), Fever, 43 (3), Facial pain, 43 (3) | 0 (0) | 0 (0) | 67 (4) | 0 (0) | 0 (0) |
| Said Ahmed WM | – | ROCM, 100 (14) | Palatal ulcers, 100 (14), Loss of vision, 14 (2), Loosened teeth, 14 (2) | 21 (3) | – | 100 (14) | 14 (2) | – |
| Alloush TK | 13 [7–30] | ROCM, 100 (14) | Ptosis, 93 (13), Chemosis, 93 (13), Proptosis, 93 (13), Decreased vision, 100 (14), Ophthalmoplegia, 93 (13), Nasal discharge, 79 (11), Facial pain, 86 (12), Periorbital edema, 71 (10), Palatal ulcers, 64 (9), Headache, 100 (14) | 93 (13) | 64 (9) | 64 (9) | – | 21 (3) |
| Seidel D | 10 [0–62] | Pulmonary, 85 (11), ROCM, 8 (1), Gastrointestinal, 8 (1) | – | – | – | – | – | 54 (7) |
Value is shown as mean ± SD or median [range]. Abbreviations: SD, Standard deviation; CAM, COVID-19-associated mucormycosis; ROCM, Rhino-orbital-cerebral mucormycosis
Fig. 2.
Forrest plots for management and outcomes (random-effect model). a Frequency of endoscopic or surgical procedure performed in patients with CAM. b Frequency of orbital exenteration performed in patients with COVID-19-associated ROCM. c Overall mortality of CAM
Discussion
This systematic review and meta-analysis identified 2,312 confirmed cases of CAM and provided a comprehensive picture of this disease. ROCM comprised more than 90% of CAM. Diabetes was the most prevalent comorbid condition found in CAM patients. While 57% of the CAM patients had required oxygen therapy for COVID-19 pneumonia, 77% had received corticosteroids. Most mucormycosis was treated endoscopically or surgically, and orbital exenteration was performed on 17% of ROCM patients. The overall mortality of CAM was estimated to be 29%.
The most usual clinical presentation of CAM was ROCM. As for non-COVID-19-associated mucormycosis, pulmonary mucormycosis has frequently been observed in patients with hematologic malignancies or histories of solid organ transplantation, while ROCM has been found primarily in diabetic patients [69, 70]. As more than 80% of the CAM patients had diabetes, the high prevalence of ROCM in CAM may be plausible. Although the reason why ROCM is more prevalent than other types of mucormycosis in diabetic patients is unknown, this interlink appears to be true in CAM as well.
The most common underlying comorbidity in patients with CAM was diabetes. Diabetes has been the most frequent risk factor for non-COVID-19-associated mucormycosis before the COVID-19 outbreak [69, 71]. Due to the lack of a comparison group in this study, we cannot prove that diabetes caused mucormycosis in COVID-19 patients. Nevertheless, the proportion of diabetes in CAM patients was noteworthy. Interestingly, in our meta-analysis, 17% of the patients with hyperglycemia had never been diagnosed with diabetes until the onset of COVID-19. These newly-diagnosed diabetic patients might have developed hyperglycemia as an adverse effect of the glucocorticoids for COVID-19 treatment [72]. Although our data were insufficient to discuss the hypothesis because detailed information on long-term glycemic control such as hemoglobin A1c or changes in blood glucose level after hospitalization was unavailable, further investigations about the relationship between steroid-induced hyperglycemia and mucormycosis are warranted.
More than three-fourths of CAM patients had received corticosteroids for COVID-19. Since the RECOVERY trial showed the mortality benefit of glucocorticoid treatment for severe COVID-19 pneumonia, it has been the standard care for COVID-19 patients requiring oxygen supplementation. As a randomized trial has shown that 12 mg dexamethasone did not improve the outcomes compared to 6 mg [73], the National Institutes of Health guidelines recommend 6 mg of dexamethasone up to 10 days or until hospital discharge for hospitalized adults with COVID-19 who require supplemental oxygen [74]. In our meta-analysis, however, whereas 57% of the patients required oxygen therapy, corticosteroids were administered to 77%. Given the theoretical concern for increased risk of CAM due to hyperglycemia and immunosuppression brought by glucocorticoid therapy, the corticosteroids should be administered with caution.
The majority of CAM patients were endoscopically or surgically treated. While systemic antifungal therapy plays a vital role in treating mucormycosis, endoscopic or surgical debridement is crucial to improving outcomes as well [75]. In particular, 17% of ROCM patients underwent orbital exenteration in this meta-analysis. This invasive procedure may be helpful even for the cases with intracranial spread and should be considered for actively infected orbit with a blind, immobile eye [76]. However, all efforts should be made to preserve the eye because whether orbital exenteration reduces mortality is controversial, and the physical psycho-social consequences are significant [77, 78]. The mortality of the hospitalized patients with COVID-19 varies but seems to be decreasing as the pandemic progresses. According to large observational studies, the mortality of hospitalized COVID-19 patients declined from 30% in early-2020 to 15% in mid-2020 [79]. Observational studies in India, where many CAM studies were conducted, reported the mortality of hospitalized COVID-19 patients of 10% in mid-2020 [80]. Based on our meta-analysis, patients who developed CAM may present worse outcomes than the COVID-19 patients without CAM, although the causal relationship remains unclear. Since early surgical intervention combined with antifungal therapy has been shown to reduce mortality of mucormycosis, healthcare providers should be vigilant with possible clinical manifestations and take swift moves once patients with a history of COVID-19 develop suspicious symptoms.
Several limitations should be noted in our meta-analysis. Firstly, the number of participants in each study was small. Moreover, the study settings varied from ambulatory ophthalmologic clinics to ICUs. These two factors contributed to the substantial heterogeneity of the meta-analysis. Initial presentations and mortality could vastly differ from case to case. More focused analysis (e.g., mortality of CAM in ICU) may improve the prediction performance in specific settings. Secondly, because most of the included studies reported on ROCM, applying the results to other types of mucormycosis can be misleading. However, this systematic review revealed that most of the CAM were ROCM. Meticulous observations of those symptoms will be essential to detect the disease early and ameliorate the outcomes. Thirdly, most of the included studies were conducted in India. In western countries, the trend of mucormycosis has been shifting towards malignancy- or transplantation-associated pulmonary mucormycosis [81, 82]. Because diagnosing pulmonary or gastrointestinal mucormycosis is challenging by symptoms, those subtypes might have been under-reported compared to ROCM. Due to such epidemiological differences, our findings may not be generalizable worldwide. Lastly, some data points were unobtainable, such as details on time from glucocorticoid initiation to CAM onset and the glycemic control during hospitalization. Therefore, the relationship between hyperglycemia and immunosuppression due to glucocorticoids on CAM was not conclusive. Extensive, prospective studies will be of help to understand these relationships.
In conclusion, diabetes and glucocorticoid treatment were frequently observed in patients with CAM. CAM may be associated with higher mortality of COVID-19, and around one-fifth of the patients may lose their eyes. Physicians should be aware of those potential risk factors and the typical clinical presentations of CAM. Once CAM is suspected, multidisciplinary approaches with antifungal therapy combined with surgical intervention should be encouraged to improve outcomes.
Footnotes
Handling Editor: Vishnu Chaturvedi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Atsuyuki Watanabe and Matsuo So are the co-first author of this article.
References
- 1.Nucci M, Barreiros G, Guimarães LF, Deriquehem VAS, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64:152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitaka H, Kuno T, Takagi H, Patrawalla P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021;64:993–1001. doi: 10.1111/myc.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajmal S, Mahmood M, Abu Saleh O, Larson J, Sohail MR. Invasive fungal infections associated with prior respiratory viral infections in immunocompromised hosts. Infection. 2018;46:555–558. doi: 10.1007/s15010-018-1138-0. [DOI] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54:S23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 7.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saidha PK, Kapoor S, Das P, Gupta A, Kakkar V, Kumar A, et al. Mucormycosis of paranasal sinuses of odontogenic origin post COVID19 infection: a case series. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:e40–80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC) report 1. Indian J Ophthalmol. 2021;69:1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal R, Singh B, Bhadada SK, Banerjee M, Bhogal RS, Hage N, et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64:1452–1459. doi: 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. doi: 10.18637/jss.v049.i05. [DOI] [Google Scholar]
- 17.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouad YA, Abdelaziz TT, Askoura A, Saleh MI, Mahmoud MS, Ashour DM, et al. Spike in rhino-orbital-cerebral mucormycosis cases presenting to a tertiary care center during the COVID-19 pandemic. Front Med. 2021;8:645270. doi: 10.3389/fmed.2021.645270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meshram HS, Kute VB, Chauhan S, Dave R, Patel H, Banerjee S, et al. Mucormycosis as SARS-CoV2 sequelae in kidney transplant recipients: a single-center experience from India. Int Urol Nephrol. 2021 doi: 10.1007/s11255-021-03057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. SARS-CoV-2, Uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective. Multi-Centric Anal J Maxillofac Oral Surg. 2021 doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Kholy NA, El-Fattah AMA, Khafagy YW. Invasive fungal sinusitis in post COVID-19 patients: a new clinical entity. Laryngoscope. 2021 doi: 10.1002/lary.29632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswami A, Sahu AK, Kumar A, Suresh S, Nair A, Gupta D, et al. COVID-19 associated mucormycosis presenting to the emergency department - an observational study of 70 patients. QJM. 2021 doi: 10.1093/qjmed/hcab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta NK, Kapre M, Gupta H, Vaidya GK, Jani S, Meshram S, et al. Risk based decision algorithms for management of COVID-19 associated rhino-orbital mucormycosis. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra Y, Prashar M, Sharma D, Kumar VP, Tilak TVSVGK. Diabetes, COVID 19 and mucormycosis: clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab Syndr. 2021;15:102196. doi: 10.1016/j.dsx.2021.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari A, Rao NP, Patnaik U, Malik V, Tevatia MS, Thakur S, et al. Management outcomes of mucormycosis in COVID-19 patients: a preliminary report from a tertiary care hospital. Armed Forces Med J India. 2021;77:S289–S295. doi: 10.1016/j.mjafi.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta DP, Gupta S, Shah CK, Sreevidya SR. Clinical study of surge of mucormycosis in COVID-19 pandemic: a tertiary care center study. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubey S, Mukherjee D, Sarkar P, Mukhopadhyay P, Barman D, Bandopadhyay M, et al. COVID-19 associated rhino-orbital-cerebral mucormycosis: An observational study from Eastern India, with special emphasis on neurological spectrum. Diabetes Metab Syndr. 2021;15:102267. doi: 10.1016/j.dsx.2021.102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhanuprasad K, Manesh A, Devasagayam E, Varghese L, Cherian LM, Kurien R, et al. Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int J Infect Dis. 2021;111:267–270. doi: 10.1016/j.ijid.2021.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selarka L, Sharma S, Saini D, Sharma S, Batra A, Waghmare VT, et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses. 2021;64:1253–1260. doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthu V, Kumar M, Paul RA, Zohmangaihi D, Choudhary H, Rudramurthy SM, et al. Is there an association between zinc and COVID-19-associated mucormycosis? Results of an experimental and clinical study. Mycoses. 2021;64:1291–1297. doi: 10.1111/myc.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, Mehrparvar G, et al. Mucormycosis in patients with COVID-19: a cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64:1238–1252. doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meawed TE, Ahmed SM, Mowafy SMS, Samir GM, Anis RH. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J Infect Public Health. 2021;14:1375–1380. doi: 10.1016/j.jiph.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zirpe K, Pote P, Deshmukh A, Gurav SK, Tiwari AM, Suryawanshi P. A retrospective analysis of risk factors of COVID-19 associated mucormycosis and mortality predictors: a single-center study. Cureus. 2021;13:e18718. doi: 10.7759/cureus.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pal P, Singh B, Singla S, Kaur R. Mucormycosis in COVID-19 pandemic and its neurovascular spread. Eur Arch Otorhinolaryngol. 2021 doi: 10.1007/s00405-021-07106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pippal SK, Kumar D, Ukawat L. Management challenge of rhino-orbito-cerebral mucormycosis in Covid 19 era: a prospective observational study. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismaiel WF, Abdelazim MH, Eldsoky I, Ibrahim AA, Alsobky ME, Zafan E, et al. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am J Otolaryngol. 2021;42:103080. doi: 10.1016/j.amjoto.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avatef Fazeli M, Rezaei L, Javadirad E, Iranfar K, Khosravi A, Amini Saman J, et al. Increased incidence of rhino-orbital mucormycosis in an educational therapeutic hospital during the COVID-19 pandemic in western Iran: an observational study. Mycoses. 2021;64:1366–1377. doi: 10.1111/myc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goddanti N, Reddy YM, Kumar MK, Rajesh M, Reddy LS. Role of COVID 19 inflammatory markers in rhino-orbito-cerebral mucormycosis: a case study in predisposed patients at a designated nodal centre. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulkarni R, Pujari SS, Gupta D, Ojha P, Dhamne M, Bolegave V, et al. Cerebrovascular involvement in mucormycosis in COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2021;31:106231. doi: 10.1016/j.jstrokecerebrovasdis.2021.106231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dokania V, Gaikwad NS, Gite V, Mhashal S, Shetty N, Shinde P, et al. Emergence of invasive fungal rhinosinusitis in recently recovered COVID-19 patients. Ann Otol Rhinol Laryngol. 2021 doi: 10.1177/00034894211060923. [DOI] [PubMed] [Google Scholar]
- 43.Nair AG, Adulkar NG, D’Cunha L, Rao PR, Bradoo RA, Bapaye MM, et al. Rhino-orbital mucormycosis following COVID-19 in previously non-diabetic, immunocompetent patients. Orbit. 2021;40:499–504. doi: 10.1080/01676830.2021.1960382. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, Choudhary R, Pandey VP. “MuCovid-21” study: Mucormycosis at an Indian tertiary care centre during the COVID-19 pandemic. J R Coll Physicians Edinb. 2021;51:352–358. doi: 10.4997/JRCPE.2021.407. [DOI] [PubMed] [Google Scholar]
- 45.Joshi AR, Muthe MM, Patankar SH, Athawale A, Achhapalia Y. CT and MRI findings of invasive mucormycosis in the setting of COVID-19: experience from a single center in India. AJR Am J Roentgenol. 2021;217:1431–1432. doi: 10.2214/AJR.21.26205. [DOI] [PubMed] [Google Scholar]
- 46.Choksi T, Agrawal A, Date P, Rathod D, Gharat A, Ingole A, et al. Cumulative mortality and factors associated with outcomes of mucormycosis after COVID-19 at a multispecialty tertiary care center in India. JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2021.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal SB, Rana A, Babras M, Yadav D, Jha P, Jain M, et al. Risk factors and outcomes of COVID associated mucormycosis in kidney transplant recipients. Transpl Infect Dis. 2021 doi: 10.1111/tid.13777. [DOI] [PubMed] [Google Scholar]
- 48.Fouad YA, Bakre HM, Nassar MA, Gad MOA, Shaat AAK. Characteristics and outcomes of a series of COVID-associated mucormycosis patients in two different settings in egypt through the third pandemic wave. Clin Ophthalmol. 2021;15:4795–4800. doi: 10.2147/OPTH.S344937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meher R, Wadhwa V, Kumar V, Shisha Phanbuh D, Sharma R, Singh I, et al. COVID associated mucormycosis: a preliminary study from a dedicated COVID Hospital in Delhi. Am J Otolaryngol. 2022;43:103220. doi: 10.1016/j.amjoto.2021.103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzmán-Castro S, Chora-Hernandez LD, Trujillo-Alonso G, Calvo-Villalobos I, Sanchez-Rangel A, Ferrer-Alpuin E, et al. COVID-19-associated mucormycosis, diabetes and steroid therapy: experience in a single centre in Western Mexico. Mycoses. 2022;65:65–70. doi: 10.1111/myc.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar HM, Sharma P, Rudramurthy SM, Sehgal IS, Prasad KT, Pannu AK, et al. Serum iron indices in COVID-19-associated mucormycosis: a case-control study. Mycoses. 2022;65:120–127. doi: 10.1111/myc.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav T, Tiwari S, Gupta A, Garg PK, Khera PS, Rajagopal R, et al. Magnetic resonance imaging in coronavirus disease—2019 associated rhino-orbital-cerebral mucormycosis (CA-ROCM)—imaging analysis of 50 consecutive patients. Curr Probl Diagn Radiol. 2022;51:112–120. doi: 10.1067/j.cpradiol.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meshram HS, Kute VB, Yadav DK, Godara S, Dalal S, Guleria S, et al. Impact of COVID-19-associated mucormycosis in kidney transplant recipients: a multicenter cohort study. Transpl Direct. 2022;8:e1255. doi: 10.1097/TXD.0000000000001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popli H, Gupta A, Singh V, Agarwal V, Akilan R, Kumar A. Are low serum vitamin D levels a risk factor for advent of COVID-19 associated rhinocerebral mucormycosis: a preliminary case control study. Indian J Otolaryngol Head Neck Surg. 2022 doi: 10.1007/s12070-022-03080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel DD, Adke S, Badhe PV, Lamture S, Marfatia H, Mhatre P. COVID-19 associated rhino-orbito-cerebral mucormycosis: imaging spectrum and clinico-radiological correlation- a single centre experience. Clin Imaging. 2022;82:172–178. doi: 10.1016/j.clinimag.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roushdy T, Hamid E. A case series of post COVID-19 mucormycosis-a neurological prospective. Egypt J Neurol Psychiatr Neurosurg. 2021;57:100. doi: 10.1186/s41983-021-00355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sai Krishna D, Raj H, Kurup P, Juneja M. Maxillofacial infections in Covid-19 era-actuality or the unforeseen: 2 case reports. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nehara HR, Puri I, Singhal V, Ih S, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: case series from the north-western part of India. Indian J Med Microbiol. 2021;39:380–383. doi: 10.1016/j.ijmmb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh Y, Ganesh V, Kumar S, Patel N, Aggarwala R, Soni KD, et al. Coronavirus disease-associated mucormycosis from a tertiary care hospital in India: a case series. Cureus. 2021;13:e16152. doi: 10.7759/cureus.16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg R, Bharangar S, Gupta S, Bhardwaj S. Post Covid-19 infection presenting as rhino-orbital mycosis. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arana C, Cuevas Ramírez RE, Xipell M, Casals J, Moreno A, Herrera S, et al. Mucormycosis associated with COVID-19 in two kidney transplant patients. Transpl Infect Dis. 2021;23:e13652. doi: 10.1111/tid.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barman Roy D, Gupta V, Biswas A, Verma M. Early surgical intervention followed by antifungals in rhino-orbital mucormycosis in patients with COVID-19 favors clinical outcome: a case series. Cureus. 2021;13:e17178. doi: 10.7759/cureus.17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashour MM, Abdelaziz TT, Ashour DM, Askoura A, Saleh MI, Mahmoud MS. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: a case series and a review of literature. J Neuroradiol. 2021;48:319–324. doi: 10.1016/j.neurad.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pal P, Chatterjee N, Ghosh S, Ray BK, Mukhopadhyay P, Bhunia K, et al. COVID associated mucormycosis: a study on the spectrum of clinical, biochemical and radiological findings in a series of ten patients. J Assoc Phys India. 2021;69:11–12. [PubMed] [Google Scholar]
- 65.Riad A, Shabaan AA, Issa J, Ibrahim S, Amer H, Mansy Y, et al. COVID-19-associated mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi (Basel). 2021 doi: 10.3390/jof7100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Said Ahmed WM, Elsherbini AM, Elsherbiny NM, El-Sherbiny M, Ramzy NI, Arafa AF. Maxillary mucormycosis osteomyelitis in post COVID-19 patients: a series of fourteen cases. Diagnostics (Basel). 2021 doi: 10.3390/diagnostics11112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alloush TK, Mansour O, Alloush AT, Roushdy T, Hamid E, El-Shamy M, et al. Rhino-orbito-cerebral mucormycosis during the COVID-19 third wave in 2021: an Egyptian preliminary report from a single tertiary hospital. Neurol Sci. 2021 doi: 10.1007/s10072-021-05740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seidel D, Simon M, Sprute R, Lubnow M, Evert K, Speer C, et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses. 2022;65:103–109. doi: 10.1111/myc.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26:944.e9–944.e15. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 70.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 72.Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68:1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 73.COVID STEROID 2 Trial Group. Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.NIH. Corticosteroids [Internet]. COVID-19 Treatment Guidelines. 2021 [cited 2022 Feb 4]. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/
- 75.Vaughan C, Bartolo A, Vallabh N, Leong SC. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years? Clin Otolaryngol. 2018;43:1454–1464. doi: 10.1111/coa.13175. [DOI] [PubMed] [Google Scholar]
- 76.Rapidis AD. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;15:98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 77.Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope. 2013;123:1112–1118. doi: 10.1002/lary.23912. [DOI] [PubMed] [Google Scholar]
- 78.Shah K, Dave V, Bradoo R, Shinde C, Prathibha M. Orbital exenteration in rhino-orbito-cerebral mucormycosis: a prospective analytical study with scoring system. Indian J Otolaryngol Head Neck Surg. 2019;71:259–265. doi: 10.1007/s12070-018-1293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doidge JC, Gould DW, Ferrando-Vivas P, Mouncey PR, Thomas K, Shankar-Hari M, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med. 2021;203:565–574. doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmad S, Kumar P, Shekhar S, Saha R, Ranjan A, Pandey S. Epidemiological, clinical, and laboratory predictors of in-hospital mortality among COVID-19 patients admitted in a tertiary COVID dedicated hospital, Northern India: a retrospective observational study. J Prim Care Commun Health. 2021 doi: 10.1177/21501327211041486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol [Internet]. 2018;56:93–101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect [Internet]. 2011;17:1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]