Coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), is an ongoing global pandemic with high morbidity and mortality.1 Patients with preexisting conditions have increased morbidity and mortality when infected with COVID-19.1 There is no published data on the outcomes of patients with severe valvular heart disease (VHD) and COVID-19. Our aim was to assess the clinical outcomes of severe VHD patients infected with COVID-19 from sites participating in a large multicenter collaboration.
The COVID-19 Valve Disease (CVD) Registry is an international multicenter collaboration founded by the Institute of Valvular Research (Redmond, WA, United States) in March 2020, in response to the urgent public health crisis of COVID-19. Patients with severe VHD and either confirmed COVID-19 by real-time polymerase chain reaction (RT-PCR) test or strongly suspected COVID-19 by clear clinical presentation (typical symptoms after direct contact with an identified patient) and/or imaging were included. Patients were included between February 2020 and May 2020 from 26 centers in 9 countries (Spain, Italy, United States, France, Brazil, Denmark, Poland, United Kingdom and Israel). The study was also centrally approved by an institutional review board of Hebrew University. Anonymized data were collected in a dedicated electronic case report form after local institutional review board approval. Due to the observational nature of the study informed consent was waived. The primary endpoint of the present analysis is all-cause mortality from the start of the hospitalization to 30 days. Student’s t test or one-way analysis of variance (ANOVA) were used to compare means of normally distributed continuous data between groups. The Mann-Whitney U-test and the Kruskal-Wallis one-way ANOVA were used to compare distributions of non-normally distributed continuous data. χ2 and Fisher’s exact tests were used to compare proportions of categorical variables. Time-to-first event rates were estimated using the Kaplan-Meier method and compared with the log-rank test. A two-tailed p-value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 23 (IBM Corporation, Armonk, NY, USA).
A total of 136 patients with severe VHD were included, among whom a COVID-19 RT-PCR test was positive in 123 (90.4%). All but one patient (99.3%) were hospitalized. Only nine patients (6.6%) did not have any COVID-19 symptoms (i.e. fever, tachycardia, altered mental status, anosmia, cough, fatigue, shortness of breath, diarrhea or nausea). Severe AS was the most frequent type of VHD (54.4%), followed by severe mitral regurgitation (MR; 20.6%), severe tricuspid regurgitation (TR; 10.3%), severe aortic regurgitation (AR; 8.1%) and severe mitral stenosis (MS; 6.6%). Overall, patients were elderly (mean age 80.0 ± 9.7 years) and frequently had comorbidities including hypertension (77.2%), chronic kidney disease (36.0%), and diabetes (34.6%). Many patients had preexisting symptoms related to their VHD, such as dyspnea (83.1%), chest pain (19.1%), and syncope (8.1%) before COVID-19 infection. Most patients presented with shortness of breath (64.9%) and cough (57.5%), although fever (41.3%) was also prevalent.
Left ventricular ejection fraction was preserved in the majority of cases (mean 52.9% ± 14.3) and only approximately 20.0% of patients had evidence of right ventricular dysfunction. Five patients had bioprosthetic valve failure, all in the aortic position. Mixed VHD was present in 25 (18.4%) patients, most commonly severe AS plus another valve condition. Among those with severe AS patients, 27.4% also had ≥moderate MR and 12.4% had ≥moderate TR. The mean pulmonary artery systolic pressure was moderately elevated (43.8 mmHg ± 14.0) and was similar across the different VHDs (p = 0.59).
Regarding clinical management, most patients were treated with antibiotics (84.3%) and hydroxychloroquine (74.6%); both were used in 66.2% of patients. Inotropic support was most commonly utilized in MR patients (25.0% vs. 3.8% in the other valve diseases, p = 0.001). Anticoagulation was used in 55.1% of patients, most commonly using low molecular weight heparin.
Most patients (84.6%) were treated conservatively for their VHD, with medications alone. Valve replacement was infrequent among patients with AS (17.6%) and AR (18.2%). Transcatheter valve repair with the MitraClip (Abbott, Santa Clara, CA) was performed in 7.1% of MR patients. Balloon valvuloplasty was performed in 22.2% of MS patients and 2.7% of AS patients. Patients treated invasively with other non-AS VHD were all symptomatic for COVID-19.
Transcatheter (n = 11) or surgical (n = 2) aortic valve replacement or aortic valvuloplasty (n = 2) was performed in 15 (20.3%) of the 74 patients with severe AS. Of the AS patients treated invasively, only one patient (6.7%) did not have clinical symptoms of COVID-19. Baseline characteristics of AS patients treated invasively were similar to those not treated, with the exception of more severe baseline symptoms and a higher incidence of chronic obstructive pulmonary disease in patients ≥ 80 years old that were invasively treated (50.0% vs. 4.8%, p = 0.001). Median PSI-PORT score at admission was 99 [91.3–110.5] in the invasively treated AS group and 109 [96– 141] in the non-invasively treated group (p = 0.22). Almost all of these procedures were performed urgently, at median 3 days [interquartile range 2–7 days] from hospital admission to intervention. Transcatheter heart valves utilized were Evolut (Medtronic Inc., Minneapolis, MN) (54.5%), Acurate Neo (Boston Scientific, Marlborough, MA) (27.3%), SAPIEN 3 (Edwards Lifesciences, Irvine, CA) (9.1%) and Myval (Meril Life Sciences, Vapi, India) (9.1%). Invasive management of AS was associated with significant clinical improvement within 48 hours in 12/15 (80.0%) patients. In 57.6% of the 59 AS patients managed conservatively, the treating heart team cited expected lack of clinical benefit as the rationale, while in 42.4% of cases the procedure was deferred for a later date pending patient recovery.
Regarding clinical outcomes, respiratory failure was common (51.9%). Sepsis was diagnosed in 26.7% of patients. Heart failure developed more frequently in patients with MR and TR than in other valve diseases (50.0% vs. 50.0% vs. 21.4% respectively; p = 0.005). Shock developed in 18.6% of patients and was more common in those with MR than other valve diseases (33.3% vs. 15.0%; p = 0.05). Overall mortality of all patients at 30 days was 41.8% ( Figure 1). Mortality tended to be higher in patients with severe MR (54.4%) and severe AS (42.6%) compared to other valvular diseases (29.7%), although these differences were not significant (p = 0.09) (Figure 1). However, among AS patients, valve interventions were associated with lower 30-day mortality irrespective of age (Figure 1).
Figure 1.
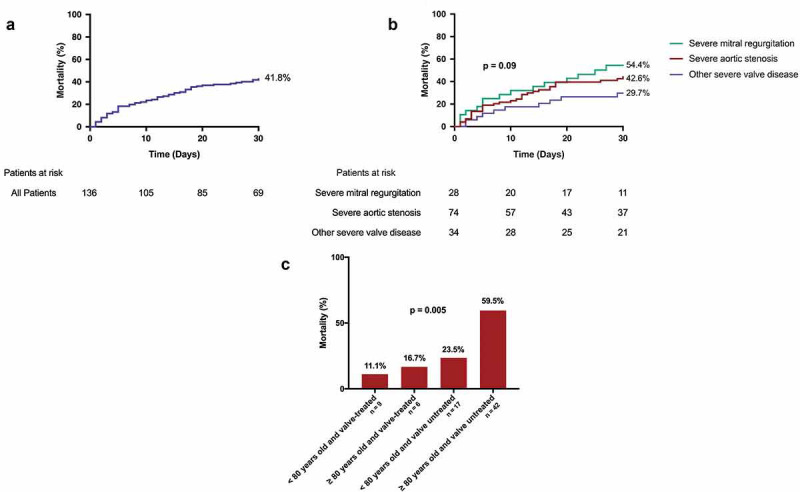
(a) Kaplan-Meier curve of overall 30-day mortality in COVID-19 patients with severe valvular heart disease. (b) Kaplan-Meier curve of 30-day mortality stratified by type of valvular heart disease. (c) Thirty-day mortality of aortic stenosis patients stratified by age and valve treatment (invasive [transcatheter or surgical aortic valve replacement or valvuloplasty] vs. conservative).
The present report is the first to examine the outcomes of patients with severe VHD and COVID-19. Patients with severe VHD and COVID-19 had poor short-term clinical outcomes, with 30-day mortality approaching 50.0%. Valve interventions were infrequent among these patients despite high rates of shock and heart failure to which the valve disease may have been contributing. Among patients with severe AS, those who had aortic valve intervention early after the infection had lower 30-day mortality than those who were treated conservatively, a finding that was present in both younger and older patients. Limitations include the relatively small number of cases, the retrospective and observational nature of the study, and the lack of a control group, although as the outcomes in the present cohort are substantially worse than have been previously reported in age-matched infected patients without valve disease.2 , 3 We also did not have information on specific treatment protocols for valvular disease used in each reporting hospital.
Patients with COVID-19 and severe VHD have poor clinical outcomes, with elevated mortality rates. In addition to strict conventional measures to prevent infection in high-risk patients with valvular disease, valve repair or replacement in appropriate patients should be considered before possible infection and even during the infection. Further studies are warranted to confirm these results.
Acknowledgments
Funding
Institute of Valvular Research. A nonprofit organization.
Data sharing
Data sharing with qualified researchers may be considered after submission of a proposal to the board of the Institute of Valvular Research.
Disclosure statement
Dr. Dvir reports consulting for Edwards Lifesciences, Medtronic, Abbott. Dr. Estévez-Loureiro reports consulting for Abbott and Boston Scientific. Dr. Wojakowski reports having received speaker’s honoraria from Edwards Lifesciences, Abbott and Boston Scientific, as well as advisory board participation for Medtronic Inc. Dr. Stone has received speaker or other honoraria from Cook, Terumo, QOOL Therapeutics and Orchestra Biomed; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme, Cardiomech; and has equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, Valfix. No other conflicts of interest were reported.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [Internet]. February 28, http://www.ncbi.nlm.nih.gov/pubmed/32109013. Accessed March 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. American Medical Association. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. doi:10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [Internet] JAMA. American Medical Association. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. https://www.who.int/docs/default-. Accessed July 4, 2020. [DOI] [PubMed] [Google Scholar]


