Abstract
Background:
Discontinuation of daily oral PrEP is frequent among adolescent girls and young women (AGYW) in African settings. We explored factors influencing early PrEP discontinuation and persistence among Kenyan AGYW who accepted PrEP within a programmatic setting.
Methods:
We conducted in-depth interviews (IDI) with AGYW (aged 15–24 years) who accepted PrEP from 4 maternal child health (MCH) and family planning (FP) clinics. AGYW were identified by nurses at routine clinic visits and purposively sampled based on 4 categories: 1) accepted PrEP pills, but never initiated PrEP use (e.g., never swallowed PrEP pills), 2) discontinued PrEP <1 month after initiation, 3) discontinued PrEP within 1–3 months, and 4) persisted with PrEP use >3 months. Informed by the Stages of Change Model, thematic analysis characterized key influences on PrEP discontinuation/persistence.
Results:
We conducted 93 IDIs with AGYW who accepted pills. Median age was 22 years, 71% were married; 89% were from MCH and 11% were from FP clinics. Early PrEP use was positively influenced by encouragement from close confidants and effective concealment of PrEP pill-taking when necessary to avoid stigma or negative reactions from partners. Pregnancy helped conceal PrEP use because pill-taking is normalized during pregnancy, but concealment became more difficult postpartum. AGYW found keeping up with daily PrEP pill-taking challenging and many noted only episodic periods of HIV risk. Frequently testing HIV-negative reassured AGYW that PrEP was working and motivated persistence.
Discussion:
As PrEP programs scale-up in MCH/FP, it is increasingly important to enhance protection-effective PrEP use through approaches tailored to AGYW, with special considerations during pregnancy and postpartum.
Keywords: pre-exposure prophylaxis, PrEP, HIV prevention, women, adolescents, Africa
INTRODUCTION
HIV incidence rates remain unacceptably high among adolescent girls and young women (AGYW).[1] Globally, 6,000 AGYW aged 15–24 years are newly infected with HIV weekly, with the majority of those infections occurring in sub-Saharan Africa.[1] In July 2016, the Kenya Ministry of Health (MOH) released guidelines recommending daily oral tenofovir-based pre-exposure prophylaxis (PrEP) for all persons at substantial risk of HIV infection, including AGYW as a priority population.[2] PrEP implementation is progressing in HIV high-burden regions in Kenya, with >55,000 individuals initiating PrEP as of January 2020.[3] However, an ongoing challenge is early and frequent discontinuation of PrEP use. In most studies, a substantial proportion of AGYW starting daily oral PrEP do not return for a medication refill 1 month after initiation, and most discontinue within the first 6 months.[4]
In the PrEP Implementation for Young Women and Adolescents (PrIYA) Program, we previously demonstrated feasibility of integrated PrEP delivery for AGYW within routine maternal and child health (MCH) and family planning (FP) clinics in Kenya.[5–7] The PrIYA program found that 16% of AGYW with HIV risk factors accepted PrEP pills for daily oral use when offered during routine MCH and FP services,[5, 6] though other studies among Kenyan AGYW report more modest PrEP uptake (<5%).[8, 9] Only one-third of AGYW who initiated PrEP in the PrIYA Program persisted with use after 1 month.[5, 6] Elucidating reasons for PrEP discontinuation and understanding barriers to effective continued use of daily oral PrEP could inform ongoing PrEP programs and guide future introduction of novel PrEP options when they become available.[4]
Understanding real-world AGYW experiences with and beliefs about daily oral PrEP use can inform programmatic PrEP implementation tailored to AGYW. Several qualitative studies have evaluated PrEP acceptability[10–12] and motivations for PrEP persistence among AGYW within the context of clinical trials and demonstration studies[13–16], yet few data are available that explore discontinuation and persistence of daily oral PrEP use within programmatic settings. We explored the personal experiences of AGYW who initiated PrEP within routine MCH and FP settings at four PrIYA sites and subsequently discontinued or persisted with PrEP use. We aimed to evaluate modifiable factors that impede PrEP use among women receiving PrEP within real-world MCH and FP clinics and recommend potential solutions within this setting.
METHODS
Study Design and Population
From October to December 2018, we conducted individual in-depth interviews (IDIs) with AGYW ages 15 to 24 who were participants in the PrIYA Program and accepted PrEP pills through MCH and FP clinics at four facilities in Kisumu County, Kenya. [5–7]
Recruitment and Data Collection
A subset of AGYW across four categories were purposively recruited to capture a range of perspectives, including AGYW who: 1) accepted PrEP pills, but never initiated PrEP use (e.g., never swallowed PrEP pills), 2) discontinued PrEP <1 month after initiation, 3) discontinued PrEP within 1–3 months, and 4) persisted with PrEP use >3 months. Potential AGYW were identified by clinic staff during routine MCH or FP visits via self-reported PrEP use and referred to study staff if interested. The interview team included six female Kenyan social scientists. IDIs were conducted on the same day as FP or MCH visits or scheduled for a later date depending the participant’s preference.
IDIs were conducted using a semi-structured interview guide, which was developed collaboratively by the study team based on literature reviews and prior PrEP research experience among Kenyan women. The guide was informed by the Stages of Change Model (or Transtheoretical Model)13 and explored participant experiences and decisions regarding whether to accept/decline PrEP, continue/discontinue PrEP use, relationship dynamics with partner(s), and whether AGYW would recommend PrEP to friends.
IDIs averaged 30–40 minutes in length and were conducted in English, Dholuo, or Kiswahili based on participant preference. All interviews were recorded, transcribed verbatim, and translated into English as needed. Following each IDI, interviewers summarized their subjective impression of the interview and briefly captured the participants accounts related to key themes in a structured debrief report.[17]
Data Analysis
Data were analyzed using a combination of thematic network and directed content analysis methods.[18, 19] Directed content analysis, based on the Stages of Change Model, was used to capture the stages AGYW move through as they make decisions about PrEP.13 Our current analysis focused on the following Stages of Change Model phases: action (initiating PrEP after accepting PrEP at clinic and taking PrEP daily 1–3 months after initiation) and maintenance (persisting with daily PrEP use beyond the first three months of use).
Thematic network analysis was used to identify specific influences motivating PrEP decisions.[19] We used open coding to derive codes that captured key concepts from the data and iteratively refined our codebook. First, factors influencing PrEP decisions were compiled from the targeted debrief report summaries of each IDI. Identified factors were further refined and expanded after reviewing a subset of full-length transcripts from each AGYW recruitment category. Additional transcripts from each category were reviewed until no new factors influencing PrEP decisions were identified. Influencing factors were grouped into categories and subcategories, and the study team established and revised definitions for each through a collaborative, iterative process of reviewing transcripts against the developing codebook and group discussion. Transcripts were imported into Dedoose software (version 7.0.23, Los Angeles, CA, USA: Sociocultural Research Consultants, LLC) for coding.
The coding team consisted of 6 Kenya- and US-based female study personnel with training in qualitative research methods. Two Kenyan coders identified as young women. All transcripts were independently coded using a final version of the codebook by members of the study team. Code application was reviewed by another member of the team. Disagreements in code application were resolved through group discussion until consensus was reached. Key themes were identified by running queries to compare key factors influencing PrEP decisions among AGYW and different stages of change. Thematic memos were drafted throughout the analysis and used to identify similarities across AGYW experiences. Thematic network analysis was used to categorize individual themes into related networks and overall patterns were synthesized.[19]
Ethical considerations
All study procedures received approval from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee, University of Washington Institutional Review Board, Kisumu County Department of Health, and administrators in respective health facilities. All interview participants provided informed consent. Interviews were independent of the clinic’s services provided and no clinic staff involved in the participants’ routine care were present during IDIs.
RESULTS
Overall, 93 AGYW participated in IDIs. Median age was 22 years, 89% of AGYW were recruited from MCH clinics, and 85% had living children. One-quarter (26%) of AGYW were currently enrolled in school and over two-thirds (69%) were unemployed. The majority of AGYW were married, with 58% in monogamous marriages and 13% in polygamous marriages; 23% had steady boyfriends. Among the 87 AGYW who currently had male partners, 28% reported knowing their partner was living with HIV, 48% reported that their partner did not have HIV, and 24% had partners of unknown HIV status. Demographic characteristics varied by PrEP use status (Table 1). Among AGYW who persisted with PrEP use >3 months (n=24), 84% reported consistent PrEP use for ≥6 months.
Table 1.
Demographics characteristics of AGYW IDI participants who previously accepted PrEP (n=93)
| |
Median (IQR) or N (%) |
|||||
|---|---|---|---|---|---|---|
| PrEP use status after accepting PrEP pills |
||||||
| Characteristic | Overall (n=93) | Never initiated PrEP (n=21) | Discontinued <1 mo. (n=24) | Discontinued within 1–3 mos. (n=24) | Persisted >3 mos. (n=24) | |
| Age, years | 22 (20–23) | 22 (19–23) | 22 (20–23) | 22 (20–23) | 22 (20–24) | |
| Recruitment clinic | ||||||
| FP | 10 (11%) | 6 (29%) | 1 (4%) | 1 (4%) | 2 (8%) | |
| MCH | 83 (89%) | 15 (71%) | 23 (96%) | 23 (96%) | 22 (92%) | |
| Currently has children | 79 (85%) | 14 (67%) | 21 (88%) | 20 (83%) | 24 (100%) | |
| Number of living children (n=79) | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–2) | |
| Current school enrollment status | ||||||
| Primary | 5 (5%) | 1 (5%) | 1 (4%) | 1 (4%) | 2 (8%) | |
| Secondary | 7 (8%) | 2 (10%) | - | 3 (13%) | 2 (8%) | |
| Tertiary/University | 12 (13%) | 7 (33%) | 3 (13%) | - | 2 (8%) | |
| Not enrolled in school | 69 (74%) | 11 (52%) | 20 (83%) | 20 (83%) | 18 (75%) | |
| Currently unemployed | 65 (69%) | 16 (76%) | 16 (67%) | 18 (75%) | 15 (63%) | |
| Relationship status | ||||||
| Single | 4 (4%) | 2 (9%) | - | 1 (4%) | 1 (4%) | |
| Steady boyfriend | 21 (23%) | 9 (43%) | 6 (25%) | 1 (4%) | 5 (21%) | |
| Married, monogamous | 54 (58%) | 9 (43%) | 15 (63%) | 14 (59%) | 16 (67%) | |
| Married, polygamous | 12 (13%) | 1 (5%) | 2 (8%) | 7 (29%) | 2 (8%) | |
| Divorced/widow | 2 (2%) | - | 1 (4%) | 1 (4%) | - | |
| Primary partner HIV status (n=87) | ||||||
| Negative | 42 (48%) | 13 (68%) | 13 (57%) | 10 (45%) | 6 (26%) | |
| Positive | 24 (28%) | 2 (11%) | 3 (13%) | 7 (32%) | 12 (52%) | |
| Unknown | 21 (24%) | 4 (21%) | 7 (30%) | 5 (23%) | 5 (22%) | |
PrEP=pre-exposure prophylaxis; FP=family planning; MCH=maternal child health
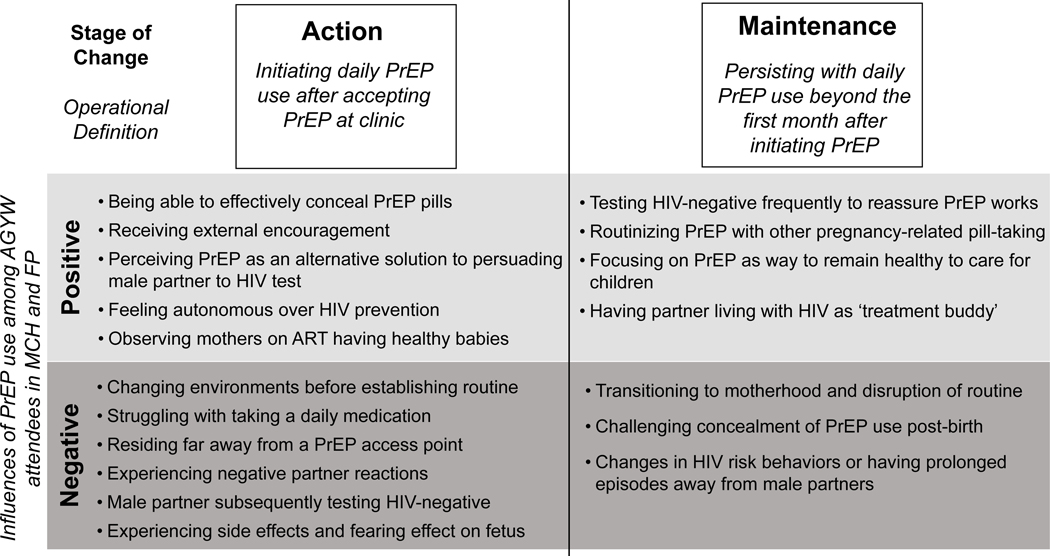
After AGYW accepted PrEP pills within MCH or FP clinics, a range of individual, interpersonal, clinic-level, and societal-level factors influenced decisions to initially initiate PrEP and either discontinue or persist with early PrEP use. We organized emerging positive and negative influences according to the action and maintenance phases of the Stages of Change Model (Figure 1).
Figure 1. Key influencers of PrEP use among AGYW organized by stages of change.
Positive Influences of Action
The ability to discreetly take PrEP was a key influence on initiation. AGYW who feared negative reactions from partners or others often found effective strategies to conceal pill-taking. Concealment took many forms, from physically hiding or repackaging pills to avoid conflation with HIV treatment drugs, to convincing partners that PrEP pills were related to pregnancy and not HIV.
“When I reach home, I transfer the medication [to a plastic bag] and throw away the bottles because when friends come to see you and find [the bottles] they will not believe that those are PrEP and not HIV drugs.” Age 21, MCH
“My husband doesn’t know that I am taking those drugs [PrEP] because if he knows…he will chase me away. He will just be convinced that I am taking HIV medication. For now, he thinks they [PrEP pills] are for pregnancy.” Age 24, MCH
Receiving external encouragement positively influenced early PrEP use, especially when encouragement came from a close relative or friend. Some AGYW were encouraged to use PrEP to preserve the family unit while mitigating HIV risk brought on by male partner behavior.
“I actually shared [my PrEP use] with my mum. …I had a lot of quarrels with my husband and I had to run back to my mum’s house…So my mum sat me down and told me that there is no need to keep running away all the time. That I should stay put because it is men’s nature to wander away [have outside partners] when they have cash. She advised me that I should look for a way to protect myself [against HIV].” Age 20, MCH
Many AGYW reported that taking PrEP was a simpler solution for preventing HIV than persuading hesitant male partners to test for HIV or convincing partners to consistently use condoms. AGYW felt that PrEP afforded them autonomy to protect themselves against HIV, regardless of partner decisions, which positively influenced early PrEP use and motivated plans to continue PrEP.
“It [PrEP] makes me feel happy because I’m now strong enough and I can’t get HIV even if I engage in sexual intercourse with my partner without a condom.” Age 23, MCH
Negative Influences of Action
Despite initially accepting PrEP and perceiving themselves to be at risk for HIV, some AGYW struggled with finding internal motivation to consistently take daily pills. Forgetfulness, often triggered by a change of environment or routine, also limited persistence with PrEP. AGYW recognized that longer-acting PrEP could help overcome these challenges.
“Taking PrEP [pills daily], in fact it is a burden, it is not easy. I prefer you inject me instead of giving me pills”. Age 23, MCH
Some AGYW reported that logistical barriers, including limited accessibility, challenged PrEP persistence. Experiencing side effects that did not subside and issues with swallowing PrEP pills due to their size were frequently reported as reasons for early PrEP discontinuation:
“My blood pressure would shoot up after swallowing [PrEP] and then I used to feel dizzy. That made me suspect that all that I was experiencing could be because of PrEP. So that was the reason why I decided not to continue using it.” Age 22, FP
“I found it very hard to take it because the pill was so big so I had to divide it into half before taking it. And I would then go to sleep immediately after taking it.” Age 22, FP
Male partners frequently influenced early PrEP pill-taking decisions, sometimes sharing misinformation about PrEP that instilled fear about taking PrEP on a long-term basis.
“He [boyfriend] told me that if you use PrEP for a long time, like three to six months, if you ever contract HIV and use ARVs, [HIV] will be resistant to them. Meaning that ARVs won’t be of value to you. … I got scared and I panicked, I said let me not use them.” Age 18, MCH
Negative partner reactions to PrEP use also included confiscating PrEP pills. PrEP catalyzed conflicts in some instances, even in partnerships without a history of discord.
“My husband saw the drugs [PrEP]. His conclusion was that those are ARVs and I’m already on [HIV] care…I tried to explain to him, he was furious…He was thinking like maybe I could be having some other partners outside and that is why I would opt for PrEP.” Age 24, FP
In some cases, decisions to not initiate PrEP or to discontinue use were informed by male partners subsequently testing HIV-negative. This included both couples testing at the clinic and the use of HIV self-tests that were co-dispensed with PrEP for at-home couples testing.
“When I went home, I discussed [HIV testing] with my boyfriend and he was like, ‘fine, let’s go to the clinic, let’s test ourselves.’ When we went to the clinic, he tested negative and I tested negative. …So, I stopped using PrEP.” Age 23, FP
A small minority of AGYW reported fears about harmful PrEP effects on infants, which influenced their decisions to delay PrEP use until after breastfeeding cessation. However, most reported that they did not worry about PrEP leading to adverse effects, because of the counseling they received from health workers or their own observations of mothers living with HIV using ART during pregnancy.
“I have seen those who are on ART get pregnant and continue to take medication throughout the period and have healthy babies…so I was sure that it won’t affect my baby in any way.” Age 22, MCH
Positive Influences of Maintenance
For many AGYW, frequently testing HIV-negative during PrEP follow-up visits assured them that PrEP was working and motivated PrEP continued use.
“HIV testing is so good, it assures me that [PrEP] is working because any time I come for testing, I turn negative. That makes me like testing because I get to know my status.” Age 23, MCH
“I used to think that even though I am on PrEP, I was eventually going to contract HIV…Now, I am not worried. I got tested for HIV when almost giving birth, then I waited another three months after that and tested again. I was HIV negative…This has really given me the courage to continue with PrEP drugs.” Age 23, MCH
AGYW with children focused on PrEP as a way to remain alive and healthy to care for them. This motivation was especially pronounced among AGYW who knew their partner’s behaviors placed them at risk of acquiring HIV. PrEP helped alleviate their worries about the longer-term security of their relationships and families.
“The way I see it, I should just take [PrEP] until one day this man changes, that is when I can stop taking it. But, if he is still continuing with these behaviors I feel I should just continue taking it so that at least I am protected and I can live longer to take care of my children.” Age 22, MCH
Among AGYW with partners on ART, having a ‘treatment buddy’ reinforced PrEP pill-taking behavior through tangible support, such as collection of pills and reminders.
“My husband is also taking his medicine [ARVs] at the same time with me, so I have not seen any difficulty [in remembering to take PrEP]. Sometimes if I forget, he reminds me. Sometimes my phone alarm might go off and he reminds that it is time [to take PrEP].” Age 24, MCH
AGYW who initiated PrEP during pregnancy reported that routinizing PrEP along with other pregnancy-specific pill-taking, such as malaria prophylaxis, iron supplementation, and multivitamins, helped facilitate long-term pill-taking habits.
“I take PrEP at the same time that I take the iron tablets…I had been told [by nurses] that I should take PrEP with the iron tablets just when I was about to go to bed, so that is what I do.” Age 17, MCH
Negative Influencers of Maintenance
New motherhood was a key barrier to maintained PrEP use. Among AGYW who initiated PrEP during pregnancy, navigating PrEP refills and persistence during the postnatal period was challenging. The unpredictability of childbirth and disruption of routines that follow made persisting with PrEP use difficult during this transition period.
“I used PrEP diligently until the time I was due. My labor coincided with the day I was to come back for my [PrEP] refill. I could not make it on that day. When I left the maternity ward, I stayed home for one month and two weeks. So, I couldn’t come for the drugs.” Age 20, MCH
For AGYW who reported that pregnancy helped conceal PrEP use from male partners, concealment became challenging after delivery since postnatal pill-taking is less common. This led some AGYW to later disclose their PrEP use to male partners.
“I feared him questioning me if he’d spot them [PrEP pills] or see me taking them, but I found a way around it. I decided to tell him that these were part of the pregnancy drugs I was issued at the clinic. But now that I have delivered my baby, I planned to tell the truth about the PrEP drugs because I truly do not know his HIV status.” Age 20, MCH
Other AGYW reconsidered their daily PrEP use after realizing they were not regularly at high risk for HIV. Some decided to discontinue or pause PrEP use due to lack of sexual activity following delivery or time apart from male partners.
“…I only have one sex partner who does not live around here. He works away from town…He comes for one month after a very long period of time and I don’t have another partner around here. So, I don’t think I am at risk at the moment.” Age 23, MCH
DISCUSSION
In this qualitative evaluation among AGYW who accepted PrEP at public sector MCH and FP clinics in Kenya, several emerging factors influenced decisions to initiate PrEP use and either discontinue or persist with daily oral PrEP. As PrEP programs scale-up in MCH and FP settings, it will become increasingly important to enhance protection-effective PrEP use through approaches tailored to AGYW with special considerations for PrEP use during pregnancy and the postpartum period.[6, 20, 21]
AGYW reported that bundling PrEP pill-taking with other pregnancy-specific pills helped habit formation. Male partners were also less likely to question PrEP use or react negatively when they surmised that PrEP pills were just another pregnancy-related medication. However, childbirth and the transition to motherhood disrupted routines and PrEP pill-taking concealment became harder during the postpartum period. Interventions that facilitate PrEP use during the transition from pregnancy to postpartum are needed, such as dispensing multiple months of PrEP pills or health providers coaching the navigation of PrEP services via SMS during this transitional period.[22, 23] Additionally, PrEP persistence may be reduced postpartum if perception of the infant’s risk of HIV is reduced, similar to patterns of ART use in the postpartum period.[24–26] Although some AGYW reported fearing effects of PrEP on infant safety, others were reassured because they observed women living with HIV using ART while pregnant and having healthy babies. These findings could inform future messaging for PrEP demand creation and counseling. Efforts to rebrand PrEP as a health-affirming medication may help promote daily pill-taking into the postpartum period.[27–29]
Pill burden and difficulty concealing pill-taking have been identified as reasons for declining and discontinuing PrEP in previous studies, including in the parent PrIYA Program.[6, 30–32] Though AGYW who accepted PrEP pills recognized their HIV risk and saw PrEP as beneficial, some still struggled with daily pill-taking. Anticipated new PrEP-delivery tools, including implants and injectable PrEP, will address concerns related to daily pill-taking and concealment.[33] Some AGYW in our study expressed a preference for injectable PrEP options unprompted. Future research on novel PrEP agents that offer flexibility, choice, and convenience should prioritize AGYW in African settings and include pregnant and postpartum mothers to expedite implementation in these groups.
HIV testing after acceptance of PrEP pills influenced PrEP persistence among AGYW in our study. In the PrIYA program, some sites co-dispensed PrEP and HIV self-test kits for at-home couples testing.[34] AGYW reported that frequently testing HIV-negative at PrEP follow-up visits was reassuring and motivated persisting with PrEP use, acting as a positive reinforcer. In the PrIYA program, PrEP users were tested for HIV at all PrEP refill visits (every 1–3 months). On the other hand, some AGYW decided to not initiate PrEP or to discontinue PrEP after their male partner subsequently self-tested HIV negative or agreed to HIV test at clinic. This finding supports ongoing efforts to dispense HIV self-tests within MCH settings for male partner HIV testing to foster appropriate PrEP decision-making.[35] Future implementation studies could consider HIV self-testing approaches to motivate continued PrEP use and streamline PrEP follow-up visits while reducing health system burdens associated with clinic-based HIV testing.
PrEP use among AGYW in our study was positively influenced by encouragement from close confidants (e.g., peers and family members), while partners sharing misinformation discouraged use. Approaches that provide psychosocial support such as SMS counseling tools or peer counseling groups or the ‘treatment buddy’ model for serodifferent couples,[36–39] as well as provide PrEP education, could encourage PrEP use among AGYW. PrEP services in Africa are often provided free of charge; however, transportation and time costs may be a hindrance to uptake and continued use.[40] Ongoing work to deliver PrEP within community-based models, including retail pharmacies, peer-led distribution, and disseminated care approaches could be particularly attractive for AGYW who are frequently unemployed and may lack resources to travel to urban centers for PrEP refills.[21]
Our study has limitations. We sampled AGYW seeking MCH and FP services from public facilities, thus our results may not reflect experiences among AGYW initiating PrEP from other locations. Participants were not recruited based on pregnancy/postpartum status and this information was not systematically captured for all participants, though issues regarding pregnancy and the postpartum period were reported by participants. Our study did not quantify HIV risk or confirm PrEP adherence with objective biomarkers. The use of self-reported information about HIV risk factors and PrEP use may have introduced reporting bias. Future studies could use sample participants based on objective PrEP exposure confirmed with laboratory testing for purposive sampling based on adherence.
CONCLUSION
In this qualitative evaluation among AGYW who accepted PrEP pills within routine MCH and FP settings, we identified several factors that positively and negatively influenced decisions to discontinue or persist with PrEP use. As PrEP programs scale-up with AGYW as a priority population, it will become increasingly important to enhance protection-effective PrEP use through approaches tailored to AGYW with special considerations for PrEP use during pregnancy and the postpartum period. Interventions that promote PrEP use during the transition from pregnancy to postpartum, rebrand PrEP as a health-affirming medication, facilitate discreet PrEP use, and continue positive enforcement could enhance PrEP persistence among AGYW within MCH and FP settings.
Acknowledgements:
We thank the PrIYA study team and clients for their time and contributions. We thank the Kenyan Ministry of Health nationally and the Kisumu County Department of health, as well as the facility heads and in-charges for their collaboration.
Disclaimer: This work was funded by a grant from the United States Department of State as part of PEPFAR’s DREAMS Partnership, managed by JSI Research & Training Institute, Inc. (JSI). The opinions, findings, and conclusions stated herein are those of the authors and do not necessarily reflect those of the United States Department of State or JSI.
Funding: This study was funded by the US National Institutes of Health (R01HD094630, R01HD100201, and R01AI125498). The PrEP Implementation for Young Women and Adolescents (PrIYA) Program was funded by the United States Department of State as part of the DREAMS Innovation Challenge (Grant # 37188-1088 MOD01), managed by JSI Research & Training Institute, Inc. The PrIYA Team was supported by the University of Washington’s Center for AIDS Research Behavioral Sciences Core (CFAR BSC) (P30 AI027757) and the Global Center for Integrated Health of Women, Adolescents, and Children (Global WACh).
References
- 1.UNAIDS. Fact Sheet – Global AIDS Update 2019. In; 2019.
- 2.Kenya Ministry of Health. Guidelines on the use of antiretroviral drugs for treating and preventing HIV infection in Kenya. In; 2016.
- 3.AVAC. Global PrEP Tracker. In; October 23, 2018.
- 4.Irungu EM, Baeten JM. PrEP rollout in Africa: status and opportunity. Nat Med 2020; 26(5):655–664. [DOI] [PubMed] [Google Scholar]
- 5.Kinuthia J, Pintye J, Abuna F, Mugwanya KK, Lagat H, Onyango D, et al. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: results from an implementation programme. Lancet HIV 2020; 7(1):e38–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugwanya KK, Pintye J, Kinuthia J, Abuna F, Lagat H, Begnel ER, et al. Integrating preexposure prophylaxis delivery in routine family planning clinics: A feasibility programmatic evaluation in Kenya. PLoS Med 2019; 16(9):e1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pintye J, Kinuthia J, Roberts DA, Wagner AD, Mugwanya K, Abuna F, et al. Integration of PrEP Services Into Routine Antenatal and Postnatal care: Experiences from an Implementation Program in Western Kenya. J Acquir Immune Defic Syndr 2018. [DOI] [PMC free article] [PubMed]
- 8.Oluoch L, Mugo N, Roxby A, Wald A, Selke S, Magaret A, et al. LOW UPTAKE OF PREEXPOSURE PROPHYLAXIS AMONG KENYAN ADOLESCENT GIRLS AT RISK OF HIV | CROI Conference. CROI 2019.
- 9.Dunbar M, Kripke K, Haberer J, Castor D, Dalal S, Mukoma WM, Saiqa, et al. Understanding and measuring uptake and coverage of oral pre-exposure prophylaxis delivery among adolescent girls and young women in sub-Saharan Africa. Sexual Health 2019; 15:513 – 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govender E, Mansoor L, MacQueen K, Abdool Karim Q. Secrecy, empowerment and protection: positioning PrEP in KwaZulu-Natal, South Africa. Cult Health Sex 2017; 19(11):1268–1285. [DOI] [PubMed] [Google Scholar]
- 11.Pintye J, Beima-Sofie KM, Makabong’O PA, Njoroge A, Trinidad SB, Heffron RA, et al. HIV-Uninfected Kenyan Adolescent and Young Women Share Perspectives on Using Preexposure Prophylaxis During Pregnancy. AIDS Patient Care STDS 2018. [DOI] [PMC free article] [PubMed]
- 12.Mack N, Evens EM, Tolley EE, Brelsford K, Mackenzie C, Milford C, et al. The importance of choice in the rollout of ARV-based prevention to user groups in Kenya and South Africa: a qualitative study. J Int AIDS Soc 2014; 17(3 Suppl 2):19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camlin CS, Koss CA, Getahun M, Owino L, Itiakorit H, Akatukwasa C, et al. Understanding Demand for PrEP and Early Experiences of PrEP Use Among Young Adults in Rural Kenya and Uganda: A Qualitative Study. AIDS Behav 2020. [DOI] [PMC free article] [PubMed]
- 14.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One 2014; 9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. J Acquir Immune Defic Syndr 2015; 68(5):578–584. [DOI] [PubMed] [Google Scholar]
- 17.Simoni JM, Beima-Sofie K, Amico KR, Hosek SG, Johnson MO, Mensch BS. Debrief Reports to Expedite the Impact of Qualitative Research: Do They Accurately Capture Data from In-depth Interviews? AIDS Behav 2019; 23(8):2185–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005; 15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 19.Attride-Stirling J Thematic networks: an analytic tool for qualitative research. Qualitative Research 2001; 1(3):385–405. [Google Scholar]
- 20.Decker MR, Miller E, McCauley HL, Tancredi DJ, Anderson H, Levenson RR, et al. Recent partner violence and sexual and drug-related STI/HIV risk among adolescent and young adult women attending family planning clinics. 2014. [DOI] [PMC free article] [PubMed]
- 21.Celum CL, Delany‐Moretlwe S, Baeten JM, van der Straten A, Hosek S, Bukusi EA, et al. HIV pre‐exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc 2019; 22(Suppl Suppl 4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pintye J, Rogers Z, Kinuthia J, Mugwanya KK, Abuna F, Lagat H, et al. Two-Way Short Message Service (SMS) Communication May Increase Pre-Exposure Prophylaxis Continuation and Adherence Among Pregnant and Postpartum Women in Kenya. Glob Health Sci Pract 2020. [DOI] [PMC free article] [PubMed]
- 23.Pintye J, Davey DLJ, Wagner AD, John-Stewart G, Baggaley R, Bekker LG, et al. Defining gaps in pre-exposure prophylaxis delivery for pregnant and post-partum women in high-burden settings using an implementation science framework. Lancet HIV 2020; 7(8):e582–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fayorsey RN, Chege D, Wang C, Reidy W, Peters Z, Syengo M, et al. Mother Infant Retention for Health (MIR4Health): Study Design, Adaptations, and Challenges With PMTCT Implementation Science Research. J Acquir Immune Defic Syndr 2016; 72 Suppl 2:S137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnack A, Rempis E, Decker S, Braun V, Rubaihayo J, Busingye P, et al. Prevention of Mother-to-Child Transmission of HIV in Option B+ Era: Uptake and Adherence During Pregnancy in Western Uganda. AIDS Patient Care STDS 2016; 30(3):110–118. [DOI] [PubMed] [Google Scholar]
- 26.Elwell K. Facilitators and barriers to treatment adherence within PMTCT programs in Malawi. AIDS Care 2016; 28(8):971–975. [DOI] [PubMed] [Google Scholar]
- 27.Celum CL, Delany-Moretlwe S, McConnell M, van Rooyen H, Bekker LG, Kurth A, et al. Rethinking HIV prevention to prepare for oral PrEP implementation for young African women. J Int AIDS Soc 2015; 18(4 Suppl 3):20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golub SA. PrEP Stigma: Implicit and Explicit Drivers of Disparity. Curr HIV/AIDS Rep 2018; 15(2):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivet Amico K, Bekker LG. Global PrEP roll-out: recommendations for programmatic success. Lancet HIV 2019; 6(2):e137–e140. [DOI] [PubMed] [Google Scholar]
- 30.Kinuthia J, Pintye J, Abuna F, Mugwanya KK, Lagat H, Onyango D, et al. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: results from an implementation programme. Lancet HIV 2019. [DOI] [PMC free article] [PubMed]
- 31.Kesler MA, Kaul R, Myers T, Liu J, Loutfy M, Remis RS, et al. Perceived HIV risk, actual sexual HIV risk and willingness to take pre-exposure prophylaxis among men who have sex with men in Toronto, Canada. AIDS care 2016; 28(11):1378–1385. [DOI] [PubMed] [Google Scholar]
- 32.Meyers K, Wu Y, Brill A, Sandfort T, Golub SA. To switch or not to switch: Intentions to switch to injectable PrEP among gay and bisexual men with at least twelve months oral PrEP experience. PLoS One 2018; 13(7):e0200296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coelho LE, Torres TS, Veloso VG, Landovitz RJ, Grinsztejn B. Pre-exposure prophylaxis 2.0: new drugs and technologies in the pipeline. Lancet HIV 2019; 6(11):e788–e799. [DOI] [PubMed] [Google Scholar]
- 34.Pintye J, Drake AL, Begnel E, Kinuthia J, Abuna F, Lagat H, et al. Acceptability and outcomes of distributing HIV self-tests for male partner testing in Kenyan maternal and child health and family planning clinics. AIDS 2019; 33(8):1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dettinger JC, Kinuthia J, Pintye J, Mwongeli N, Gómez L, Richardson BA, et al. PrEP Implementation for Mothers in Antenatal Care (PrIMA): study protocol of a cluster randomised trial. BMJ Open 2019; 9(3):e025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10(9):e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muwonge TR, Ngure K, Katabira E, Mugo N, Kimemia G, Burns BFO, et al. Short Message Service (SMS) Surveys Assessing Pre-exposure Prophylaxis (PrEP) Adherence and Sexual Behavior are Highly Acceptable Among HIV-Uninfected Members of Serodiscordant Couples in East Africa: A Mixed Methods Study. AIDS and behavior 2019; 23(5):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psaros C, Haberer JE, Katabira E, Ronald A, Tumwesigye E, Campbell JD, et al. An intervention to support HIV preexposure prophylaxis adherence in HIV-serodiscordant couples in Uganda. Journal of acquired immune deficiency syndromes (1999) 2014; 66(5):522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware NC, Pisarski EE, Nakku-Joloba E, Wyatt MA, Muwonge TR, Turyameeba B, et al. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1 serodiscordant couples in East Africa: a qualitative evaluation study in Uganda. J Int AIDS Soc 2018; 21(5):e25113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer CM, Owaraganise A, Kabami J, Kwarisiima D, Koss CA, Charlebois ED, et al. Distance to clinic is a barrier to PrEP uptake and visit attendance in a community in rural Uganda. J Int AIDS Soc 2019; 22(4):e25276. [DOI] [PMC free article] [PubMed] [Google Scholar]