Abstract
Background.
Sleep and circadian timing shifts later during adolescence, conflicting with early school start times, and resulting in circadian misalignment. Although circadian misalignment has been linked to depression, substance use, and altered reward function, a paucity of experimental studies precludes determination of causality. Here we tested, for the first time, whether experimentally-imposed circadian misalignment alters the neural response to monetary reward and/or response inhibition.
Methods.
Healthy adolescents (n=25, ages 13–17) completed two in-lab sleep schedules in counterbalanced order: An ‘Aligned’ condition based on typical summer sleep-wake times (0000–0930) and a ‘Misaligned’ condition mimicking earlier school year sleep-wake times (2000–0530). Participants completed morning and afternoon functional magnetic resonance imaging scans during each condition, including monetary reward (morning only) and response inhibition (morning and afternoon) tasks. Total sleep time and circadian phase were assessed via actigraphy and salivary melatonin, respectively.
Results.
Bilateral ventral striatal (VS) activation during reward outcome was lower during the Misaligned condition after accounting for the prior night’s total sleep time. Bilateral VS activation during reward anticipation was lower during the Misaligned condition, including after accounting for covariates, but did not survive correction for multiple comparisons. Right inferior frontal gyrus activation during response inhibition was lower during the Misaligned condition, before and after accounting for total sleep time and vigilant attention, but only during the morning scan.
Conclusions.
Our findings provide novel experimental evidence that circadian misalignment analogous to that resulting from school schedules may have measurable impacts on healthy adolescents’ reward processing and inhibition of prepotent responses.
Introduction
The timing of sleep and circadian rhythms shifts progressively later during adolescence, conflicting with the early schedules imposed by high school, and resulting in circadian misalignment (Crowley, Wolfson, Tarokh, & Carskadon, 2018). Circadian misalignment--i.e., mismatch between the behavioral sleep-wake schedule and the timing of the circadian clock--results in insomnia, sleep loss, daytime sleepiness, and other daytime impairments. Specifically, misalignment may increase risk for mood and substance use problems, perhaps via effects on reward function and impulse control.
Evidence linking circadian misalignment to mood disturbance and substance abuse is mostly observational and cross-sectional. Circadian alignment is typically operationalized as the phase angle, or interval, between a circadian marker (e.g., the dim light melatonin onset, DLMO) and the timing of sleep (e.g., the midpoint of the sleep period). In adults, both short and long DLMO-sleep phase angles correlate with depression severity (Emens et al., 2009; Hasler, Buysse, Kupfer, & Germain, 2010; Swanson et al., 2017). In adolescents, shorter phase angles correlate with greater symptoms of substance use disorder (SUD) (Hasler, Bootzin, Cousins, Fridel, & Wenk, 2008) and greater weekend alcohol use (Hasler, Bruce, Scharf, Ngari, & Clark, 2019). Individuals with later circadian phase (i.e., evening “chronotypes”), in particular, have shorter DLMO-sleep phase angles due to early sleep-wake schedules imposed by school or work. Insufficient sleep on school/work nights and large weekday-weekend differences in sleep-wake timing (i.e., social jet lag) often result (Mongrain, Lavoie, Selmaoui, Paquet, & Dumont, 2004; Paine & Gander, 2016; Wittmann, Dinich, Merrow, & Roenneberg, 2006).
Associations between circadian misalignment and mood and substance use problems may occur via altered function of neural circuitry underlying reward processing and impulse control (Hasler & Clark, 2013; Logan et al., 2018). Evidence from animal models and human neuroimaging studies shows circadian modulation of reward circuitry (Byrne, Hughes, Rossell, Johnson, & Murray, 2017; Logan et al., 2018), supporting this hypothesis. Indeed, our two prior studies suggest altered neural response to reward among adolescents with evening chronotype (Hasler, Sitnick, Shaw, & Forbes, 2013) or social let lag (Hasler et al., 2012), consistently indicating reduced medial prefrontal cortex (mPFC) activation in response to monetary reward. We found divergent evidence for higher or lower ventral striatal (VS) activation in response to monetary reward, which we speculate may reflect developmental differences in the two samples. However, a paucity of experimental studies precludes determination of causality.
Circadian misalignment may also worsen impulse control. A preference for “eveningness” (Caci et al., 2005; Kang et al., 2015; Russo, Leone, Penolazzi, & Natale, 2012) and later circadian phase (Bullock et al., 2017) are associated with greater self-reported impulsivity. Also, sleep loss broadly impairs executive function (Horne, 1993; Nilsson et al., 2005). However, no published studies have experimentally probed whether circadian misalignment impacts impulse control or its neural correlates. Furthermore, prior studies examining circadian factors have primarily employed self-report measures of impulse control rather than behavioral or neuroimaging tasks.
We tested whether experimentally-imposed circadian misalignment alters the neural response to monetary reward and/or response inhibition. We hypothesized that circadian misalignment would lead to reduced response to monetary reward across the mPFC and VS, consistent with our prior findings on circadian misalignment (social jet lag) in a healthy adolescent sample (Hasler et al., 2012). We also hypothesized that circadian misalignment would lead to reduced activation of the right inferior frontal gyrus during response inhibition, reflecting impaired impulse control (Bari & Robbins, 2013). We accounted for individual differences in circadian phase that could confound interpretation. Given that effects of circadian misalignment likely stem from both adverse circadian phase and sleep loss/disturbances, we also conducted secondary analyses accounting for: (1) total sleep during the manipulation night preceding the fMRI scans, and (2) waking vigilant attention at times proximal to the fMRI scans.
Methods
Participants
We analyzed data from 25 healthy adolescents (13 females). Participants were 13–17 years old (mean=15.39±1.17); right-handed; free of major medical, psychiatric, and/or sleep disorders; and reported habitual sleep duration of 6–12 hours and habitual bedtime of 8 PM - 2 AM. Participants were excluded for alcohol use (≥1 standard drink per month), any illicit drug use, and/or daily tobacco use. Finally, participants were excluded for any history of head injury with loss of consciousness and for MRI-incompatible conditions including claustrophobia; current pregnancy; and metal-containing medical or cosmetic implants/devices. Inclusion/exclusion criteria were assessed with self-reported screening questionnaires and in-person interviews, including the Kiddie SADS-Present and Lifetime Version (K-SADS-PL: Kaufman, Birmaher, Brent, Rao, & Ryan, 1996), a locally-developed structured interview for sleep disorders based on DSM-IV and the International Classification of Sleep Disorders, 3rd edition (AASM, 2014) and the Lifetime Drinking and Drug Use History (Koenig, Jacob, & Haber, 2009). A breathalyzer and urine drug screen were administered at the start of each overnight visit to confirm that participants were alcohol- and drug-free. A pregnancy test was administered prior to each fMRI scan to confirm that female participants were not pregnant.
The protocol was approved by the University of Pittsburgh Institutional Review Board. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Participants were recruited via fliers and Internet postings, referrals from other ongoing studies, and the research registry of the University of Pittsburgh Clinical and Translational Science Institute. Written informed consent was obtained from a parent of each participant, and participants provided assent. All participants were compensated.
Thirty-six adolescents provided consent/assent. Of these, 5 were unable to participate due to scheduling difficulties, 4 withdrew at baseline, and 1 was excluded due to a past substance use disorder. One of the 26 participants who completed the main study protocol was excluded from analyses for excessive movement during fMRI scans (i.e., >20% of volumes with movement >3 standard deviation (SD) from the participant’s mean, >0.5 mm scan-to-scan translation, or >0.01 degrees of scan-to-scan rotation.) All of the remaining 25 participants had at least one good quality scan.
Protocol (Figure 1)
Figure 1.
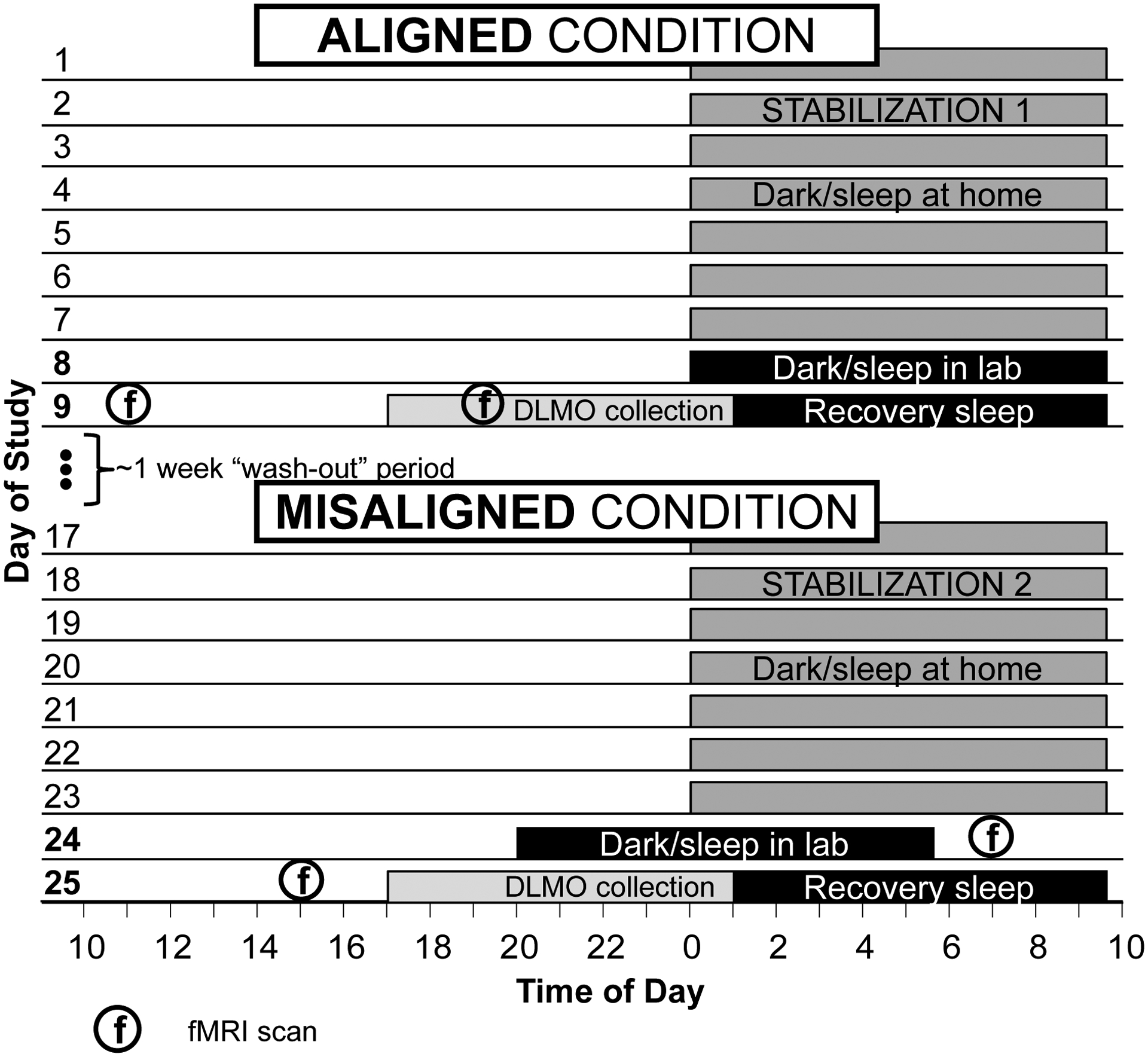
Overall study design. The protocol employed within-person cross-over design that randomized participants to one of two condition orders: Aligned first and Misaligned second, or Misaligned first and Aligned second. The order of the Aligned and Misaligned conditions was counterbalanced across participants, with one week to “wash out” in between conditions. The Card Guessing Task (monetary reward) was administered only at the morning fMRI scans, while the Go/No-Go task (response inhibition) was administered at all four scans.
Data collection was conducted solely during the summer to avoid conflicts with school schedules. Participants were randomized to one of two condition orders using a within-person cross-over design: Aligned-Misaligned, or Misaligned-Aligned. Order was counterbalanced across participants, with one week to “wash out” in between conditions. Each condition began with a 7-day stabilized sleep-wake schedule at home, with bedtime of 0000, wake time of 0930, and lights off during this period. This schedule minimizes between-participant differences in sleep/circadian history by aligning with typical adolescent sleep timing preferences (Crowley et al., 2018), and provides a 9.5-hour sleep opportunity consistent with estimated sleep need in this age group (Crowley et al., 2018; Short, Weber, Reynolds, Coussens, & Carskadon, 2018). Adherence to sleep-wake and light-dark schedules during the stabilization period was monitored via wrist actigraphs with light sensors (Philips Spectrum Classics). Participants were informed that compensation was contingent upon maintaining sleep-wake times within 1 hour of the prescribed schedule.
Following each 7-day stabilization period, participants had a 2-night lab visit. The sleep-wake manipulation occurred during Night 1 of each visit. For the Aligned condition, participants remained on the 0000–0930 schedule. For the Misaligned condition, the dark and sleep schedule was advanced by 4 hours to 2000–0530. The Misaligned schedule is intended to simulate the mismatch between adolescents’ (presumably delayed) circadian timing and the early start times of secondary education during the week.
A circadian phase assessment was conducted during the evening of Night 2, followed by an overnight “recovery” sleep opportunity. Across both nights in both conditions, light levels were maintained at <5 lux during sleep periods (Sper Scientific Light Meter 840006). Participants were not permitted to use their phones or other devices once in bed.
Participants completed four fMRI scans: one in the morning (AM) and one in the afternoon/evening (PM) of each of the two sleep lab visits. The AM and PM scans occurred 1.5- and 9.5-hours post-waking, corresponding to 1100 and 1900 in the Aligned condition and 0700 and 1500 in the Misaligned condition. Scan timing was selected based on prior evidence of daily and circadian rhythms in reward function (Hasler, Mehl, Bootzin, & Vazire, 2008; Murray et al., 2009): the AM scan was scheduled near the nadir, and the PM scan scheduled near the peak of the predicted rhythm in reward motivation. By scheduling fMRI scans at consistent times since awakening, we also partially controlled for homeostatic sleep drive, which also contributes to time-of-day differences in reward function (Murray et al., 2009).
Measures
Depression and anxiety
Baseline depression and anxiety symptoms were assessed with the Mood and Feelings Questionnaire-Long Version (MFQ-Long; Ancold & Stephen, 1995) and Screen for Child Anxiety Related Disorders-Child Version (SCARED-Child; Birmaher et al., 1999). Scores >27 on the MFQ-Long indicate likely depression. Scores ≥25 on the SCARED-Child may indicate presence of an anxiety disorder.
Sleep-wake behavior
Sleep diaries and wrist actigraphy were used to assess sleep-wake behavior throughout the protocol. Participants completed sleep diaries each morning via smartphone, with items assessing naps, sleep onset latency, number and duration of awakenings, total sleep time (TST), total time in bed (TIB), and quality of sleep, consistent with published recommendations (Carney et al., 2012)
Participants wore wrist actigraphs (Spectrum “Classic”; Philips-Respironics) on their non-dominant wrist throughout the study protocol. They were instructed to press an event marker on the actigraph each night at “lights out” and each morning at “out-of-bed” times. Event markers were used to set the rest interval for analyses, supplemented by diary data. Data were collected in 1-minute epochs and analyzed using the Medium sensitivity setting in the Actiware 6.0 software.
Diary and actigraphy outcomes included sleep onset and offset, TIB, TST, and sleep efficiency (% of time in bed spent asleep; TIB/TST × 100).
Circadian phase
Circadian phase was assessed via salivary Dim Light Melatonin Onset (DLMO; Benloucif et al., 2008) during the second evening of each Aligned and Misaligned study visit. Saliva samples were collected in Salivettes (Sarstedt, Newton, NC) under dim light conditions (<15 lux at any angle of gaze) every half-hour from 1800–0100. Dim light conditions began at 1730 and were confirmed using a light meter. DLMO was calculated as the interpolated clock time when salivary melatonin levels (pg/mL) exceeded the mean of three consecutive baseline samples plus twice the standard deviation of those samples (Voultsios, Kennaway, & Dawson, 1997). Expanded details regarding DLMO collection are provided in the Supplemental section.
Affect
Momentary affect was assessed with the Positive Affect and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), administered electronically via participants’ smartphones six times daily, beginning at scheduled rise time (0930 and 0530 for Aligned and Misaligned conditions) and ending 10 minutes prior to scheduled bedtime (1950 and 2350 for Aligned and Misaligned conditions). The remaining four assessments were distributed semi-randomly in between (mean interval 3.2 hours). We averaged the first two assessments of positive affect during each visit, which bookended the fMRI reward task. Mean (±SD) affect assessment times were 1045 (±57 minutes) and 0715 (±54 minutes) for Aligned and Misaligned visits.
Alertness
Alertness was evaluated using a variant of the Psychomotor Vigilance Task (PVT), the gold-standard in assessing vigilant attention in the context of sleep loss (Dorrian, Rogers, & Dinges, 2005). We shortened the standard 10-minute version of the PVT to 5 minutes (PVT-5) to reduce participant fatigue and burden; the 10-minute PVT and PVT-5 provide comparable outcomes (Roach, Dawson, & Lamond, 2006). The PVT-5 was delivered via E-Prime (Psychology Software Tools, Pittsburgh, PA) and consisted of 48 trials of a simple visual reaction time task. Responses were recorded via a Serial Response Box (Psychology Software Tools, Pittsburgh, PA) to ensure accurate and precise assessment of reaction times. The primary outcome is number of lapses, defined as reaction times >500 msec or a failure to respond. We administered the PVT-5 four times during each condition, at waketime and 3-hour intervals afterward. We averaged the first two PVT-5 assessments (which bookended the morning scan) as an objective measure of morning alertness (mean time: 1130 Aligned; 0630 Misaligned), and we used the fourth PVT-5 assessment as a measure of afternoon alertness (1830 Aligned; 1430 Misaligned).
fMRI Tasks
Given concerns over habituation to reward tasks during test-retest intervals of less than 2 weeks (Hasler, Forbes, & Franzen, 2014; Plichta et al., 2012), we only administered the Card Guessing Task during the AM scans.
fMRI Card Guessing Task.
We employed a slow event-related fMRI card-guessing paradigm designed to examine neural reactivity to anticipation and receipt of monetary reward and loss (Hasler et al., 2013). Trials were presented in pseudorandom order with predetermined outcomes. Each 20-second trial consisted of a 4-second decision phase; a 6-second anticipation period; a 1–second outcome period; and a 9-second interstimulus interval. In reward trials, participants were told they would win $5 if their guess was correct and there would be no change in earnings if their guess was incorrect. In loss trials, participants were told they would lose $1 if their guess was incorrect and there would be no change in earnings if their guess was correct. Twenty-four trials were presented in one run, with 12 reward-anticipation and 12 loss-anticipation trials. A balanced number of win-outcome and no-change outcome trials were included within reward-anticipation trials. Outcome probabilities were fixed trial-wise to ensure an identical win/loss time series modeling and pattern of outcome experiences for every participant. Participants were unaware of the fixed outcome probabilities in the paradigm and were led to believe their performance would determine net monetary gain. Each participant was given $25 in earnings.
Data analyses focused on anticipation and outcome phases of the reward trials, based on evidence that circadian preference is more strongly related to reward, appetitive motivation, and positive affect than to negative affect processes (Hasler, Allen, Sbarra, Bootzin, & Bernert, 2010). Consistent with our prior work (e.g., Hasler et al., 2012; Hasler et al., 2013), we focused on Reward Anticipation>Baseline and Reward Win>Baseline contrasts; baseline consisted of the last 3 seconds of the interstimulus interval.
fMRI Go/No-Go Task.
A variant of the Go/No-Go paradigm (Casey et al., 1997) was used to probe response inhibition. Participants were shown a continuous series of 120 letters and instructed to respond to any letter except “V,” which occurred in 25% of trials overall. The task included two conditions: Block A (“Go” condition) had 20 targets, and Block B (“NoGo” condition) had 10 targets and 10 nontargets. Within each 30-second block, targets are presented in pseudo-randomized order, and a 20-second rest block followed each round. Stimulus presentation duration was 0.5 seconds and inter-stimulus interval was 1.5 seconds. Participants were not given feedback on their performance. The contrast of interest was No-Go>Rest, which captures the neural correlates of active response inhibition. Behavioral outcomes included reaction time during the Go condition and accuracy during the No-Go condition.
Neuroimaging preprocessing and analysis
Neuroimaging data were preprocessed and analyzed using SPM12 (Ashburner et al., 2014) and the Artifact Detection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/). We used the rex toolbox (https://web.mit.edu/swg/software.htm) to extract mean activations (p=1.0) across all voxels from a priori regions of interest (ROIs): bilateral VS and mPRC for the Card Guessing Task, and the right inferior frontal gyrus (rIFG) for the Go/No-Go Task (Figure S1). We converted all extracted mean activations to z-scores in order to enhance interpretability.
Full details about task presentation, neuroimaging preprocessing, ROI creation/extraction, and analysis are provided in the Supplemental section.
Statistical analyses
All analyses were conducted in SPSS 25 (IBM Corp., 2017). In preliminary analyses, we examined the effects of the circadian alignment manipulation on actigraphy-based sleep during the first night in the lab using paired t-tests.
We tested primary hypotheses regarding circadian alignment effects on fMRI measures of monetary reward (2 AM scans) and response inhibition (2 AM scans, 2 PM scans) using linear mixed effects models. In Model 1 (primary hypotheses), we regressed each repeatedly-measured fMRI outcome on fixed effects of condition (Aligned versus Misaligned), scan visit (first or second), scan order (Aligned-Misaligned or Misaligned-Aligned), gender, scanner, and DLMO. In Model 1 for response inhibition, we also included time-of-day (AM or PM). In Model 2, we added actigraphy-based TST from the night before the scan to account for differences in TST due to Aligned vs. Misaligned condition; these analyses better isolate the circadian effects of misalignment. In Model 3, we added the number of lapses on the PVT near the time of scanning to account for variations in alertness.
All models included participant ID as a random effect to account for nesting within participants, used restricted maximum likelihood (REML), and based degrees of freedom on Satterthwaite to reduce Type-I error (Luke, 2017). We also calculated estimated marginal means within each model to plot effects of condition (Figures 2–4). Go/No-Go behavioral outcomes, self-reported positive affect, and PVT-5 scores were analyzed using similar models.
We provide adjusted p-values to account for multiple comparisons of primary analyses (Cao & Zhang, 2014), using a Bonferroni adjustment (padj) for four primary outcomes within each task (i.e., Reward: 2 conditions (Anticipation, Win) × 2 ROIs = 4 outcomes; Go/No-Go: 4 conditions × 1 ROI = 4 outcomes). However, given the innovative nature of the study and relatively small sample sizes, we focused on effect sizes with 95% confidence intervals, contextualized based on our expected detectable effect sizes (d=0.60 to 0.70 before and after correction for multiple comparisons; see power analysis in Supplement).
Results
Sample descriptives (Table 1)
Table 1.
Demographics and clinical summary
| Measure | M ± SD | range |
|---|---|---|
| Age (Years) | 15.39 ± 1.17 | 13–17 |
| Sex | 12 males/13 females | |
| Race | 15 white, 9 black, 1 biracial (white/black) | |
| Pubertal status | Boys: M=3.7 | 3–4 |
| Girls: M=4.2 | 3–5 | |
| MFQ-Long | 3.92 ± 3.66 | 0–12 |
| SCARED-Child | 14.92 ± 11.88 | 3–56 |
| Weekday | ||
| Bedtime | 22:11± 1:13 | 21:00–01:30 |
| Risetime | 6:46 ± 1:41 | 05:00–12:30 |
| Weekend | ||
| Bedtime | 23:59 ± 1:22 | 22:00–03:30 |
| Risetime | 9:08 ± 1:12 | 06:30–12:30 |
Note: MFQ-Long=Mood and Feelings Questionnaire-Long Version (Ancold & Stephen, 1995); SCARED-Child=Screen for Child Anxiety Related Disorders – Child version (Birmaher et al., 1999)
The baseline data were consistent with a healthy sample without clinically-significant symptoms of depressive or anxiety. The mean self-reported sleep schedule at baseline was approximately 0000–0900.
Manipulation checks of sleep and alertness (Tables S1 and S2)
We found no mean differences in actigraphy-based sleep parameters across each Stabilization week preceding the Aligned and Misaligned visits. In contrast, sleep significantly differed on Aligned and Misaligned nights in the lab. During the Misaligned night, sleep timing was earlier (lights out, sleep onset, sleep offset, out-of-bed), sleep was more disturbed (longer wake after sleep onset, lower sleep efficiency), and total sleep time was shorter.
For vigilance (mean lapses on the PVT-5), we did not observe an effect of condition (Aligned vs Misaligned), time-of-day (AM vs PM), or interaction between time-of-day and condition when all four timepoints were included. However, for the morning assessments alone, more mean lapses were observed during the Misaligned condition (B=−2.13, p=0.020) relative to the Aligned condition.
Effects of misalignment on neural response to monetary reward (Tables S3–S6, Figure 2A&B)
Figures 2A&B.
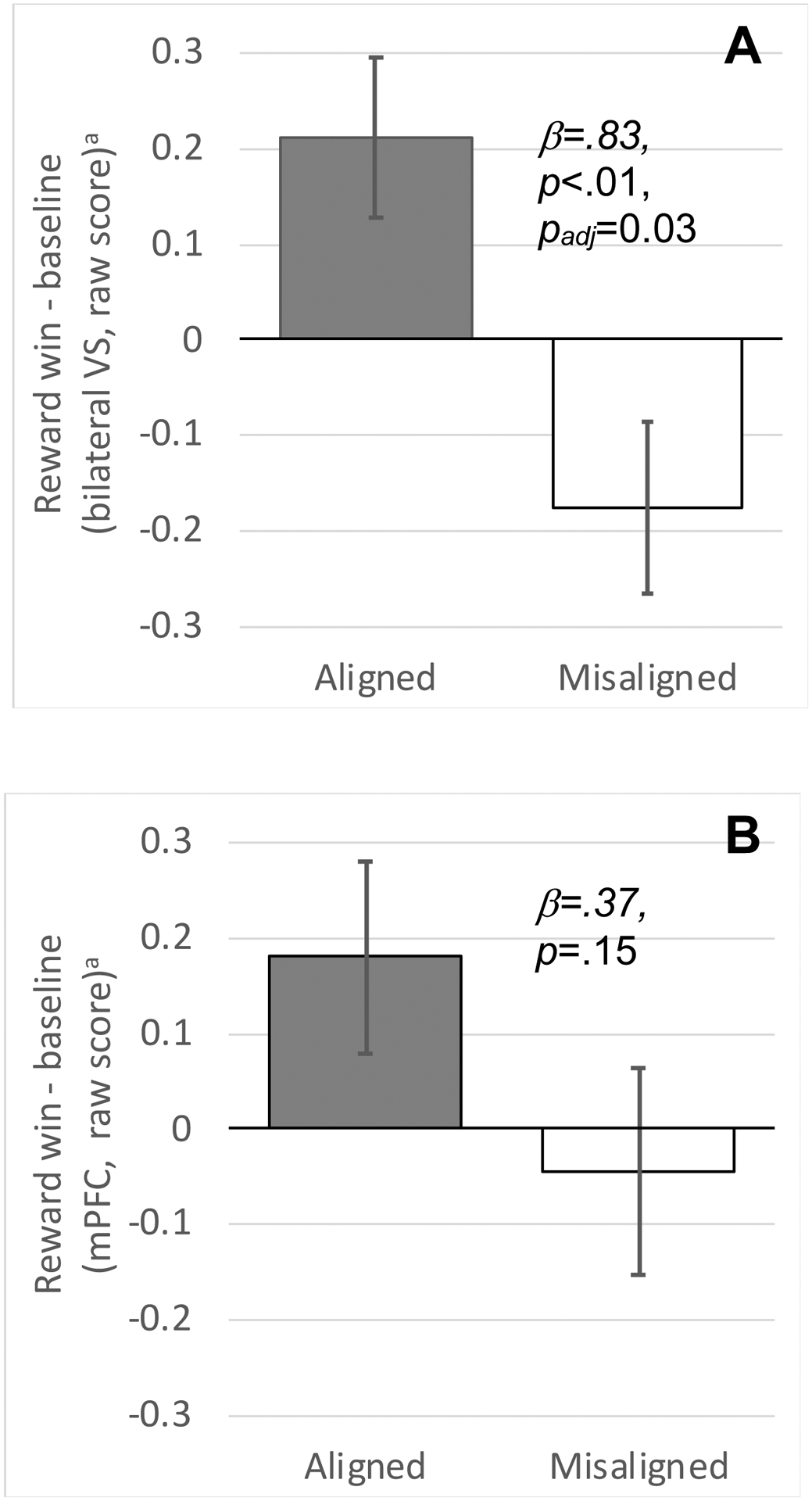
Greater activation in the bilateral ventral striatum (VS) during reward win (vs baseline) in the Aligned (A) morning scan compared to the Misaligned (M) morning scan (A). Activation in the medial prefrontal cortex (mPFC) showed a similar direction of effect but was not statistically significant (B). Linear mixed-effects model also accounted for sex, scanner, scan visit (first vs second), scan order (Aligned first vs Misaligned first), DLMO, and prior night’s total sleep time. Graphs depict estimated marginal means that account for these covariates, plotted as raw scores with error bars based on standard errors.
We observed lower bilateral VS responses to reward anticipation during the Misaligned relative to Aligned visit (β=0.69; 95%CI=0.09,1.28; p=0.026, padj=0.104; Table S3). This effect remained after accounting for the prior night’s TST (β=0.79; 95%CI=0.15,1.44; p=0.018; padj=0.072) or current vigilance (PVT; (β=0.87; 95%CI=0.15,1.58; p=0.021; padj=0.084). However, these moderate-to-large effects did not survive multiple comparison correction.
The mPFC response to reward anticipation was not different during Misaligned and Aligned schedules (β=0.43; 95%CI=−0.08,0.93; p=0.093; padj=0.372; Table S4), either in Model 1, or after accounting for the prior night’s TST (β=0.40; 95%CI=−0.22,1.02; p=0.192) or current vigilance (PVT; β=0.17; 95%CI=−0.39,0.74; p=0.519). These small effects were not detectable with our sample size.
Bilateral VS response to reward win was not different during Aligned relative to Misaligned schedules (β=0.54; 95%CI=−0.08,1.16; p = 0.086; padj=0.344; Table S5) in Model 1. We observed a large effect after accounting for the prior night’s TST (β=0.83; 95%CI=0.25,1.41; p =0.008; padj=0.032; Figure 2A). The same effect after accounting for current vigilance was moderate (PVT; β=0.73; 95%CI=0.01,1.45; p =0.048; padj=0.192) but did not survive multiple comparison correction.
We did not observe statistically significant effects of misalignment on the mPFC response to reward win, and effect sizes were small, regardless of covariates (Table S6). Self-reported positive affect on the morning of the lab visit also did not differ as a function of Aligned vs Misaligned condition (B=−0.05, p=0.968; estimated marginal means of PA: 19.48 (Aligned) and 20.72 (Misaligned).
Effects of misalignment on neural response to response inhibition (Tables S7&S8, Figures 3&4)
Figure 3.
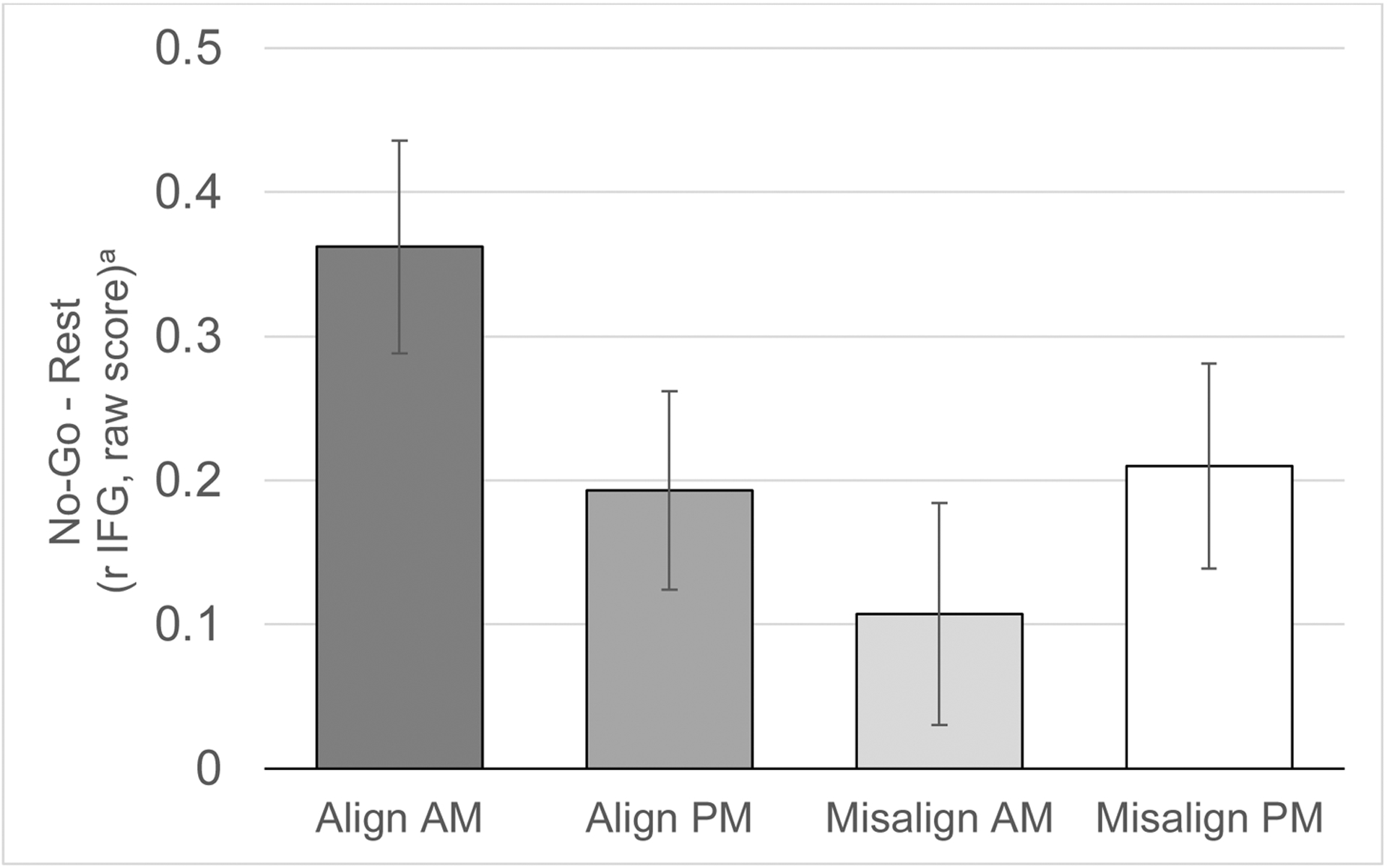
Activation in the right inferior frontal gyrus (rIFG) during no-go blocks (vs rest block) in the four scans (Aligned morning, Aligned afternoon, Misaligned morning, and Misaligned afternoon). While no overall differences were found based on condition (Aligned vs Misaligned) or time-of-day (morning vs afternoon), a condition X time-of-day interaction was significant in a linear mixed-effects model that also accounted for sex, scanner, scan visit (first vs second), scan order (Aligned first vs Misaligned first), and DLMO. Graphs depict estimated marginal means that account for these covariates, plotted as raw scores with error bars based on standard errors.
Figure 4.
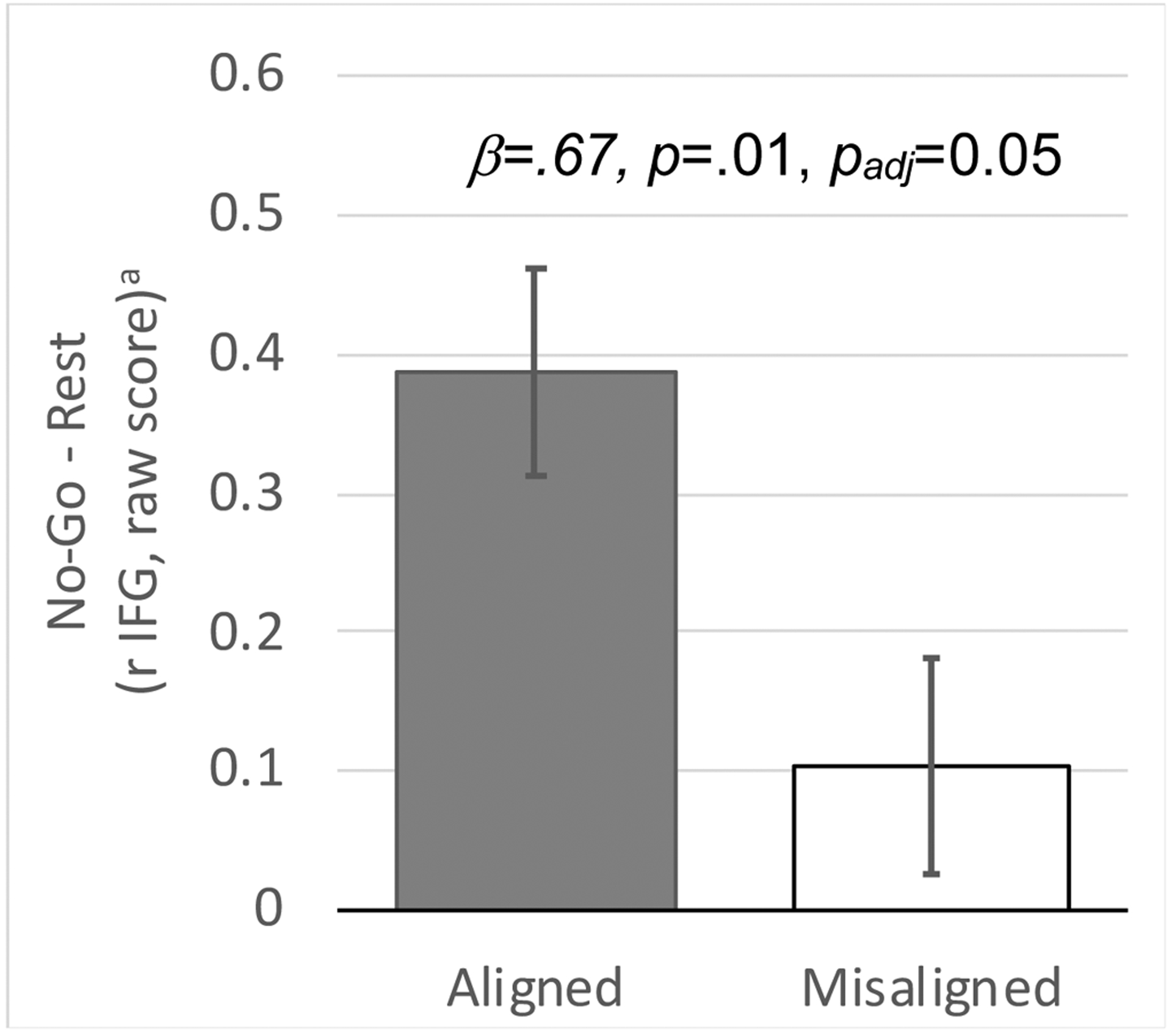
Greater predicted activation in the right inferior frontal gyrus (IFG) during no-go blocks (vs rest block) in the Aligned morning scan compared to the Misaligned morning scan (A). Linear mixed-effects model also accounted for sex, scanner, scan visit (first vs second), scan order (Aligned first vs Misaligned first), and DLMO. Graphs depict estimated marginal means that account for these covariates, plotted as raw scores with error bars based on standard errors.
We did not observe an overall effect of misalignment or time-of-day on the rIFG response during response inhibition, but we did observe an interaction between condition (Aligned vs Misaligned) and time-of-day (AM vs PM) (Table S7; β=0.64; 95%CI=0.11,1.18; p = 0.019; padj=0.076). This interaction reflects higher rIFG response during the Aligned-AM scan than during the Misaligned-AM scan (Figure 3).
To further interpret this interaction, we restricted analyses to AM scans only and found higher rIFG activation during response inhibition for the Aligned-AM scan compared to the Misaligned-AM scan (Figure 4, Table S8; β=0.67; 95%CI=0.16,1.18; p = 0.013; padj=0.052). These effects were large after accounting for the prior night’s TST (β=0.86; 95%CI=0.22,1.50; p=0.012; padj=0.048) and current vigilance (PVT; β=0.87; 95%CI=0.21,1.52; p = 0.012; padj=0.048), and survived multiple comparisons correction.
There were no apparent effects of misalignment or time-of-day on reaction time or accuracy for the Go/No-Go Task.
Discussion
Consistent with predictions, we found preliminary evidence that imposing circadian misalignment on healthy adolescents altered their neural response during receipt of rewards and during response inhibition in the morning. Specifically, we observed reduced bilateral VS responses to reward outcome and reduced right IFG response during response inhibition following experimentally-imposed circadian misalignment, in both cases only after accounting for confounding effects of the prior night’s sleep or vigilance proximal to the scan. Similar findings were observed for bilateral VS responses to reward anticipation but did not survive correction for multiple comparison. We did not find effects of misalignment on mPFC responses to monetary reward, neural responses during response inhibition, self-reported positive affect, or behavioral indicators related to response inhibition. These findings provide the first experimental evidence that circadian misalignment analogous to that resulting from school schedules has measurable impacts on healthy adolescents’ reward processing and inhibition of prepotent responses.
The effect of misalignment on reward-related brain function was strongest for bilateral VS during the reward outcome phase after accounting for confounding effects of total sleep time on the prior night. A moderate-to-large effect was also observed for bilateral VS during reward anticipation. This effect was undiminished by less sleep on the prior night or lower vigilance during testing but did not survive multiple comparisons correction. The effects of early school start times are often conceptualized as being driven by insufficient sleep (e.g., (Minges & Redeker, 2016), but our findings suggest that adverse circadian phase may independently contribute to impact on reward-related processes. The effects of misalignment on mPFC response to reward were small, suggesting that acute misalignment may have relatively larger impact on bottom-up versus top-down reward processing. The effects on VS response during anticipation and receipt were of similar magnitude, contrasting with prior suggestions of relatively greater circadian modulation of wanting or motivational processes versus liking or consummatory processes (Byrne & Murray, 2017). Our findings are broadly consistent with animal studies showing that that striatal dopamine transmission is directly modulated by sleep-wake state (Alonso, Pino, Kortagere, Torres, & Espana, 2020), and that genetic circadian disruption in the mesolimbic system alters dopamine neurotransmission and reward processing (Parekh & McClung, 2016).
Previously, we reported that greater social jet lag (a proxy for circadian misalignment) was associated with lower mPFC and striatal reactivity to reward anticipation and outcome, with the strongest effects for mPFC response during outcome in healthy 10–13 year-olds (Hasler et al., 2012). The decreased neural response to reward associated with misalignment parallels the present findings, although here we found stronger effects in the striatum. Similarly, we previously reported that evening-type late adolescent (18–22 year-old) males exhibited lower mPFC responses (during reward anticipation), but higher striatal responses (during reward win), compared to morning-types (Hasler et al., 2013). Presumably, the evening-types were more subject to misalignment; consistent with this explanation, evening-type individuals also reported worse sleep quality and longer sleep onset latency. We speculate that the divergent responses (higher versus lower VS) may be explained by developmental effects and differences between an experimental manipulation versus self-reported chronotype. The lower VS response to reward that we observed may help bridge findings linking circadian misalignment to depression (Emens et al., 2009; Hasler, Buysse, et al., 2010; Swanson et al., 2017), and findings linking a lower VS response to reward with increased incidence of major depressive disorder (Forbes & Dahl, 2012). Furthermore, in rodent models, disrupting rhythms specifically in the VS, environmental circadian disruptions (repeated phase advances or delays), and genetic disruptions to the circadian system all lead to increased depressive-like behaviors (Landgraf, Long, Proulx, et al., 2016; Landgraf, Long, & Welsh, 2016; Prendergast, Onishi, Patel, & Stevenson, 2014).
We also observed an effect of circadian misalignment on the rIFG activation during response inhibition, but only during the morning scans. Although there is some debate about the mechanistic role of the rIFG (Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010), an intact rIFG appears to be necessary for response inhibition (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Chikazoe, Konishi, Asari, Jimura, & Miyashita, 2007). The effect size of misalignment’s impact on the rIFG response was higher after accounting for prior nights’ sleep and concurrent vigilance. A lower rIFG response during No-Go in healthy early-mid adolescents was associated with a transition to heavy alcohol use (Norman et al., 2011). Furthermore, although we tried to separate the effects of sleep deprivation from those of adverse circadian phase, abundant evidence indicates that the two factors interact, including with respect to reward-related processes (Murray et al., 2009). Most germane to the present findings, individual differences in chronotype may influence both sleep deprivation and time-of-day effects on the neural response during response inhibition (Song et al., 2019; Song et al., 2018). Follow-up studies using larger samples could test whether chronotype moderates circadian misalignment and time-of-day effects on neural responses to reward and response inhibition. Notably, while the impact of circadian rhythms on response inhibition is understudied in the animal literature, circadian disruption is associated with impulsivity in rodent models (Balachandran et al., 2020).
We found limited evidence of misalignment effects on self-report and behavioral measures. Our sample of very healthy adolescents may be relatively resilient to circadian misalignment despite repeated exposure during the school year. Likewise, sleep stabilization during the week prior to sleep manipulations may have minimized pre-existing sleep debt or misalignment, and thereby, any effects of the misalignment manipulation.
The present study has several limitations. Based on our power analysis, the sample size precluded us from detecting small-to-moderate effect sizes; thus with a larger sample we may have observed more findings to be statistically significant, especially after multiple comparison correction. Although the acute single-night manipulation is not representative of the chronic misalignment experienced by many teens, effects observed with such a manipulation may systematically underestimate the real-world effects of chronic misalignment, including long-term sleep restriction. Next, test-retest reliability of fMRI is of increasing concern, with limited information for go/no-go tasks (Elliott et al., 2020), although it may be somewhat better for reward tasks repeated over shorter intervals (Kragel, Han, Kraynak, Gianaros, & Wager, 2020; Plichta et al., 2012). Finally, although our healthy sample reduced the risk of confounds related to substance use or chronic mental illness, it also limits generalizability. An important next step would be to study the effects of circadian misalignment in at-risk populations to see if it exacerbates neural vulnerability to mood and substance use disorder, consistent with observational studies (Carlisi, Hilbert, Guyer, & Ernst, 2017; Soehner et al., 2016).
In conclusion, imposing circadian misalignment resulted in altered neural responses to reward and response inhibition, and suggests that the outcomes were not simply driven by insufficient sleep. Further, the direction of these alterations in neural response are consistent with patterns observed in depressed youth and those who develop heavy drinking. Preventing circadian misalignment, whether via individual-level interventions (e.g., using bright light to advance their circadian timing) or policy-level prevention such as delaying school start times, may reduce patterns of neural activation related to risk for mood and substance use disorders.
Supplementary Material
Funding Statement:
This work was supported by the National Institutes of Health (K01 DA032557 (Hasler); R01 DA044143 (Hasler); K01MH111953 (Soehner))
Footnotes
No conflicts of interest to disclose.
References Cited
- Alonso I, Pino J, Kortagere S, Torres G, & Espana R (2021). Dopamine transporter function fluctuates across sleep/wake state: Potential impact for addiction. Neuropsychopharmacology, 46, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine. (2014). International Classification of Sleep Disorders–Third Edition (ICSD-3). American Academy of Sleep Medicine. [Google Scholar]
- Ancold A, & Stephen C (1995). Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research, 5, 237–249. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, & Robbins TW (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6, 115–116. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K, … Moran R (2014). SPM12 manual. Wellcome Trust Centre for Neuroimaging. [Google Scholar]
- Balachandran RC, Hatcher K, Sieg ML, Sullivan EK, Molina LM, Mahoney MM, & Eubig PA (2020). Pharmacological challenges examining the underlying mechanism of altered response inhibition and attention due to circadian disruption in adult Long-Evans rats. Pharmacology Biochemistry and Behavior, 193, 172915. [DOI] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, … Revell VL (2008). Measuring melatonin in humans. Journal of Clinical Sleep Medicine, 4, 66–69. [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child & Adolescent Psychiatry, 38(10), 1230–1236. [DOI] [PubMed] [Google Scholar]
- Bullock B, Murray G, Anderson J, Cooper-O’Neill T, Gooley J, Cain S, & Lockley S (2017). Constraint is associated with earlier circadian phase and morningness: Confirmation of relationships between personality and circadian phase using a constant routine protocol. Personality and Individual Differences, 104, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JE, & Murray G (2017). Diurnal rhythms in psychological reward functioning in healthy young men: ‘Wanting’, liking, and learning. Chronobiology International, 34(2), 287–295. [DOI] [PubMed] [Google Scholar]
- Byrne JEM, Hughes ME, Rossell SL, Johnson SL, & Murray G (2017). Time of day differences in neural reward functioning in healthy young men. Journal of Neuroscience, 37, 8895–8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caci H, Mattei V, Baylé FJ, Nadalet L, Dossios C, Robert P, & Boyer P (2005). Impulsivity but not venturesomeness is related to morningness. Psychiatry Research, 134(3), 259–265. [DOI] [PubMed] [Google Scholar]
- Cao J, & Zhang S (2014). Multiple comparison procedures. JAMA, 312(5), 543–544. [DOI] [PubMed] [Google Scholar]
- Carlisi CO, Hilbert K, Guyer AE, & Ernst M (2017). Sleep-amount differentially affects fear-processing neural circuitry in pediatric anxiety: A preliminary fMRI investigation. Cognitive, Affective, & Behavioral Neuroscience, 17(6), 1098–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep, 35(2), 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, … Cohen JD (1997). A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience, 9(6), 835–847. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, & Miyashita Y (2007). Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of Cognitive Neuroscience, 19(1), 69–80. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Wolfson AR, Tarokh L, & Carskadon MA (2018). An update on adolescent sleep: New evidence informing the perfect storm model. Journal of Adolescence, 67, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrian J, Rogers NL, & Dinges DF (2005). Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida C (Ed.). Sleep deprivation (pp. 39–70). Marcel Dekker. [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, … Hariri AR (2020). What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Science, 31, 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D, & Lewy AJ (2009). Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiology International, 26(3), 474–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2012). Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, & Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage, 50(3), 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, & Bernert RA (2010). Morningness-eveningness and depression: Preliminary evidence for the role of BAS and positive affect. Psychiatry Research, 176(2–3), 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Bootzin RR, Cousins JC, Fridel K, & Wenk GL (2008). Circadian phase in sleep-disturbed adolescents with a history of substance abuse: A pilot study. Behavioral Sleep Medicine, 6(1), 55–73. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Bruce S, Scharf D, Ngari W, & Clark DB (2019). Circadian misalignment and weekend alcohol use in late adolescent drinkers: preliminary evidence. Chronobiology International, 36(6), 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Buysse DJ, Kupfer DJ, & Germain A (2010). Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: Further evidence for circadian misalignment in non-seasonal depression. Psychiatry Research, 178(1), 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, & Clark DB (2013). Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism: Clinical and Experimental Research, 37(4), 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, … Forbes EE (2012). Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biological Psychology, 91(3), 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Forbes EE, & Franzen PL (2014). Time-of-day differences and short-term stability of the neural response to monetary reward: A pilot study. Psychiatry Research: Neuroimaging, 224(1), 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, & Vazire S (2008). Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. Journal of Research in Personality, 42, 1537–1546. [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, & Forbes EE (2013). An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging, 214(3), 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA (1993). Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. British Journal of Psychiatry, 162, 413–419. [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- Kang JI, Park CI, Sohn SY, Kim HW, Namkoong K, & Kim SJ (2015). Circadian preference and trait impulsivity, sensation-seeking and response inhibition in healthy young adults. Chronobiology International, 32(2), 235–241. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, & Ryan N (1996). Kiddie SADS– Present and Lifetime Version (K–SADS–PL). Unpublished instrument, Western Psychiatric Institute and Clinics, University of Pittburgh School of Medicine, PA. [Google Scholar]
- Koenig LB, Jacob T, & Haber JR (2009). Validity of the Lifetime Drinking History: A comparison of retrospective and prospective quantity-frequency measures. Journal of Studies on Alcohol and Drugs, 70(2), 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel P, Han X, Kraynak T, Gianaros PJ, & Wager T (2020). fMRI can be highly reliable, but it depends on what you measure. 10.31234/osf.io/9eaxk [DOI] [PMC free article] [PubMed]
- Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, & Welsh DK (2016). Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biological Psychiatry, 80(11), 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long JE, & Welsh DK (2016). Depression‐like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. European Journal of Neuroscience, 43(10), 1309–1320. [DOI] [PubMed] [Google Scholar]
- Logan RW, Hasler BP, Forbes EE, Franzen PL, Torregrossa MM, Huang YH, … McClung CA (2018). Impact of sleep and circadian rhythms on addiction vulnerability in adolescents. Biological Psychiatry, 83(12), 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke SG (2017). Evaluating significance in linear mixed-effects models in R. Behavior Research Methods, 49(4), 1494–1502. [DOI] [PubMed] [Google Scholar]
- Minges KE, & Redeker NS (2016). Delayed school start times and adolescent sleep: A systematic review of the experimental evidence. Sleep Medicine Reviews, 28, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, & Dumont M (2004). Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. Journal of Biological Rhythms, 19(3), 248–257. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, & Trinder J (2009). Nature’s clocks and human mood: The circadian system modulates reward motivation. Emotion, 9(5), 705–716. [DOI] [PubMed] [Google Scholar]
- Nilsson JP, Söderström M, Karlsson AU, Lekander M, Åkerstedt T, Lindroth NE, & Axelsson J (2005). Less effective executive functioning after one night’s sleep deprivation. Journal of Sleep Research, 14(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, & Tapert SF (2011). Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence, 119(3), 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine SJ, & Gander PH (2016). Differences in circadian phase and weekday/weekend sleep patterns in a sample of middle-aged morning types and evening types. Chronobiology International, 33(8), 1009–1017. [DOI] [PubMed] [Google Scholar]
- Parekh PK, & McClung CA (2016). Circadian mechanisms underlying reward-related neurophysiology and synaptic plasticity. Frontiers in Psychiatry, 6, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, … Meyer-Lindenberg A (2012). Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage, 60(3), 1746–1758. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Patel PN, & Stevenson TJ (2014). Circadian arrhythmia dysregulates emotional behaviors in aged Siberian hamsters. Behavioural Brain Research, 261, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach GD, Dawson D, & Lamond N (2006). Can a shorter psychomotor vigilance task be usedas a reasonable substitute for the ten‐minute psychomotor vigilance task? Chronobiology International, 23(6), 1379–1387. [DOI] [PubMed] [Google Scholar]
- Russo PM, Leone L, Penolazzi B, & Natale V (2012). Circadian preference and the big five: The role of impulsivity and sensation seeking. Chronobiology International, 29(8), 1121–1126. [DOI] [PubMed] [Google Scholar]
- Short MA, Weber N, Reynolds C, Coussens S, & Carskadon MA (2018). Estimating adolescent sleep need using dose-response modeling. Sleep, 41(4), zsy011. [DOI] [PubMed] [Google Scholar]
- Soehner AM, Bertocci MA, Manelis A, Bebko G, Ladouceur CD, Graur S, … Axelson D (2016). Preliminary investigation of the relationships between sleep duration, reward circuitry function, and mood dysregulation in youth offspring of parents with bipolar disorder. Journal of Affective Disorders, 205, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Feng P, Wu X, Li B, Su Y, Liu Y, & Zheng Y (2019). Individual differences in the neural basis of response inhibition after sleep deprivation are mediated by chronotype. Frontiers in Neurology, 10, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Feng P, Zhao X, Xu W, Xiao L, Zhou J, & Zheng Y (2018). Chronotype regulates the neural basis of response inhibition during the daytime. Chronobiology International, 35(2), 208–218. [DOI] [PubMed] [Google Scholar]
- Swanson LM, Burgess HJ, Huntley ED, Bertram H, Mooney A, Zollars J, … Arnedt JT (2017). Relationships between circadian measures, depression, and response to antidepressant treatment: A preliminary investigation. Psychiatry Research, 252, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, & Dawson D (1997). Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. Journal of Biological Rhythms, 12(5), 457–466. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive affect and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, & Roenneberg T (2006). Social jetlag: Misalignment of biological and social time. Chronobiology International, 23(1&2), 497–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


