Abstract
Since its outbreak in late 2019, the novel coronavirus disease 2019 (COVID-19) has spread to every continent on the planet. The global pandemic has affected human health and socioeconomic status around the world. At first, the global response to the pandemic was to isolate afflicted individuals to prevent the virus from spreading, while vaccine development was ongoing. The genome sequence was first presented in early January 2020, and the phase I clinical trial of the vaccine started in March 2020 in the United States using novel lipid-based nanoparticle (LNP), encapsulated with mRNA termed as mRNA-1273. Till now, various mRNA-based vaccines are in development, while one mRNA-based vaccine got market approval from US-FDA for the prevention of COVID-19. Previously, mRNA-based vaccines were thought to be difficult to develop, but the current development is a significant accomplishment. However, widespread production and global availability of mRNA-based vaccinations to combat the COVID-19 pandemic remains a major challenge, especially when the mutations continually occur on the virus (e.g., the recent outbreaks of Omicron variant). This review elaborately discusses the COVID-19 pandemic, the biology of SARS-CoV-2 and the progress of mRNA-based vaccines. Moreover, the review also highlighted a detailed description of mRNA delivery technologies and the application potential in controlling other life-threatening diseases. Therefore, it provides a comprehensive view and multidisciplinary insights into mRNA therapy for broader audiences.
Keywords: SARS-CoV-2, COVID-19, mRNA vaccine, mRNA therapeutics, Lipid nanoparticles, Spike-protein
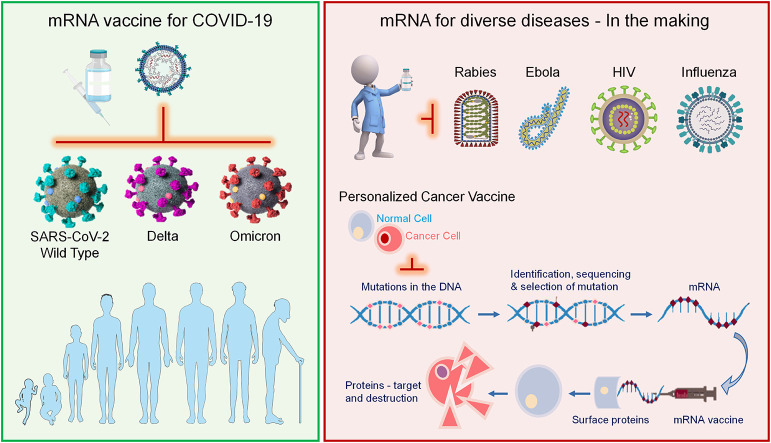
Graphical abstract
1. Introduction
The continuous spread of Novel Coronavirus Disease 2019 (COVID-19) poses a serious threat to human health and the global economy. The strategy of isolating infected individuals and tracing the close contacts-quarantining seems to be working in some parts of the world. However, the strategy seems difficult to implement in various countries [[1], [2], [3]]. The COVID-19 pandemic affected the economic condition of the people, and the individuals are in a state of the puzzle [[4], [5], [6], [7]]. The difficulty in containing the virus and the lack of appropriate therapy and vaccines have led to this situation. At first, the COVID-19 pandemic caused serious concerns and confusion about the future, and people thought it would end the same way as the Severe Acute Respiratory Syndrome (SARS) caused by Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) in 2003 [[8], [9], [10], [11], [12]]. The virus has spread to all countries and continents around the globe having 453 million confirmed cases and 6.03 million deaths and still counting [13]. Initially, social distancing, wearing masks and isolation/quarantine strategies were employed to curb the spread [[14], [15], [16]]. These actions limited the spread of the disease but did not provide immunity against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in the general population [17,18]. However, scientists suggested that the pandemic might persist, and vaccines are urgently needed if no proper treatment is available [[19], [20], [21], [22]].
Soon after the initial outbreak, the coronavirus Ribonucleic acid (RNA) sequence was reported by Chinese scientists in early January 2020. The scientists studying genetic-based vaccines turned their efforts to the novel and extremely contagious pathogen causing COVID-19 [[23], [24], [25], [26], [27]]. Generally, the mRNA is used by the living cells to make proteins from the gene sequence inside Deoxyribonucleic acid (DNA) [[28], [29], [30]]. These proteins then serve as a building block of all the essential structures. Generally, the DNA segments are transcribed into small mRNA, which is then read by the cell tools and hence proteins are synthesized [31]. In the case of mRNA vaccines for COVID-19, the message is copied from the coronavirus itself and used to produce protein such as spike protein (S protein). The coronavirus uses the S protein to enter the human cell and causes COVID-19 [[32], [33], [34], [35], [36]]. In general, the protein alone does not cause the disease. Instead, it stimulates an immune response for future recognition of the virus [[37], [38], [39]].
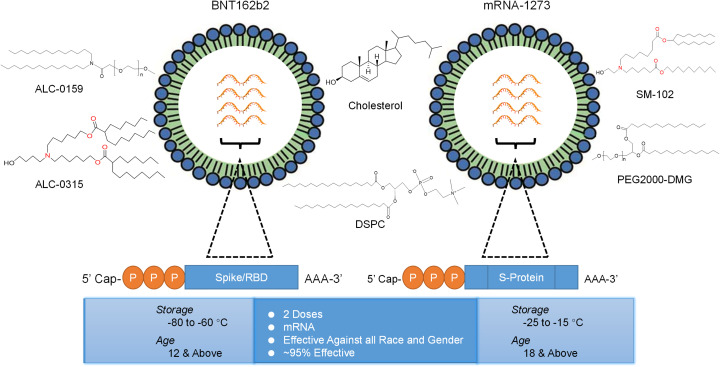
Right after 66 days of the outbreak, the first clinical trial for mRNA vaccine (mRNA-1273) began in the United States (US) against SARS-CoV-2 [40]. The mRNA-1273 was co-developed by Moderna, Inc., and the National Institute of Allergy and Infectious Diseases (NIAID). Another mRNA-based vaccine developed by BioNTech and Pfizer also got impressive results in phase I and II clinical trials in the United States. After encouraging results in phase I and II clinical trials, both the mRNA-1273 and BNT162b2 started phase III trials in late July 2020. Around 60,000 volunteers were enrolled for phase III clinical trials. This was the first time in history that a vaccine was developed in such a short period of time. According to the Director of National Institutes of Health (NIH) of the United States, “The milestone came at a remarkably rapid pace compared to the usual pace for vaccine preparation” [41]. At the end of the year 2020, both the mRNA-based vaccines developed by Pfizer and Moderna respectively got Emergency Use Authorization (EUA) in the United States (Fig. 6.). After the success in clinical trials and effectiveness of approximately ~95%, both vaccines were inoculated in the general population. Various other vaccine platforms and strategies were also encouraged for the development of the COVID-19 vaccine including protein subunit, virus-vectored, live attenuated virus and inactivated virus.
Fig. 6.
Schematic representation of two mRNA vaccines for human. The composition of BNT162b2 and mRNA-1273. Both containing mRNA encoding spike protein surrounded by lipid materials.
According to clinical trials and subsequent data, the two most widely used mRNA vaccines have been found to be safe and highly effective against SARS-CoV-2 and its various mutations. The mRNA vaccines retain distinctive advantages such as; flexibility, efficient delivery, use of the protein translational machinery of the host, and short developmental time. Therefore, in this review, we discussed the biology of SARS-CoV-2 and its mutations and the progress of mRNA-based vaccines. Moreover, a detailed description of the potential of mRNA vaccines for other life-threatening diseases is also provided. Various potential delivery technologies for mRNA are also described in detail. In addition, we also summarized updated clinical trials data on mRNA vaccines for cancer and other life-threatening diseases. Therefore, this review provides a broader view and multidisciplinary insights of mRNA vaccines for immunization against COVID-19 and its variant of concern and other infectious diseases in the future.
2. Biochemical and molecular roadmap of SARS-CoV-2
The international committee on Taxonomy of virus established the terminology RNA virus as SARS-CoV-2 due to its general homology to SARS coronavirus [[42], [43], [44], [45]]. The SARS-CoV-2 coronavirus belongs to the sub-family of Coronavirinae, having the lengthy genome among all the RNA viruses and comprised of six Open Reading Frames (ORFs) [46]. The SARS-CoV-2 shows the genomic structure (+)ss-RNA of 30 kb in length comprising a 5′-cap structure and 3’poly-A tail. Polyprotein 1a.1ab (ppla/pplab) is synthesized from the viral RNA in the host, and 16 non-structural proteins are formed, which arranges the Replication Transcription Complex (RTC) in double-membrane vesicles. Finally, minus-stranded sub-genomic RNAs (sgRNA) are synthesized discontinuously by resultant n-RTC. During the ORFs, the transcription dismisses, and ultimately the attainment of a lead of RNA occurs [47,48]. In the above process, the sgRNAs are needed by the sub-genomic mRNAs as the pattern and/or templates. Approximately six ORFs exist for a typical coronavirus, including SARS-CoV-2 [[49], [50], [51], [52]].
There are at least 16 non-structural proteins (nsps) encoded in the first ORFs, which is over 65% of the whole genome length. The main structural protein, including S protein, membrane protein, envelope protein and nucleoside protein are encoded in the ORF (35% of the genome length). All the above structural as well as non-structural proteins are translated from sub-genomic RNA [52,53]. Till the end of January 2022, there are 7,711,789 genomic sequences of SARS-CoV-2, including complete and partial, have been decoded and deposited in the Global Initiative on Sharing All Influenza Data (GISAID) database [54].
According to the phylogenetic studies, the SARS-CoV-2 closely resembles bat-SL-CoVZC45 and bat-SL-CoVZXC21 (two SARS-like viruses in bats). Moreover, the SARS-CoV-2 is 88% identical and 79% homologous to Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and 50% with Middle East Respiratory Syndrome (MERS-CoV) [55]. Interestingly, the virus also has a high genome sequence identity with pangolin coronavirus, especially in the receptor-binding domain (RBD), which shows some differences to Bat coronavirus (RaTG13). The homology analysis also revealed that the SARS-CoV and SARS-CoV-2 have a similar RBD structure [56]. This sequence identity at the RBD is a key factor for some groups postulating a recombination event that might occurred in pangolins, or other animal species as an intermediate, before infecting humans. However, the biochemical and molecular roadmap of the SARS-CoV-2 suggested that the virus causing COVID-19 emerged from a single animal source.
3. SARS-CoV-2 structure
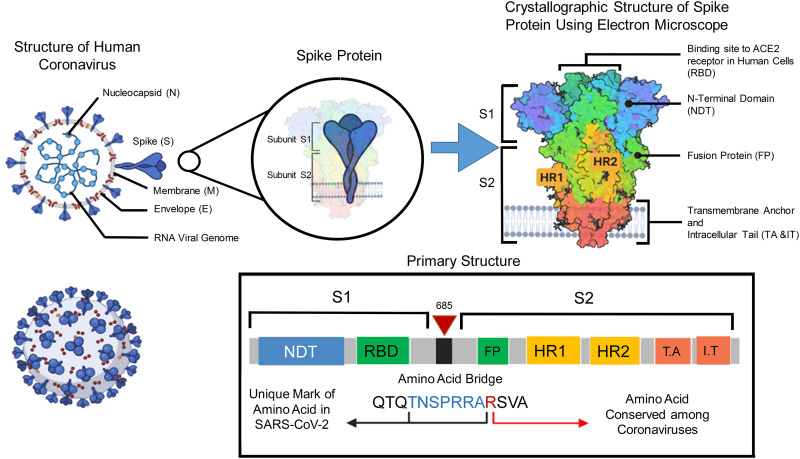
The SARS-CoV-2 is spherical in shape, positive sense and enclosed single-stranded RNA virus. There are mainly four structural proteins such as spike (S), envelope (E), membrane (M), and nucleocapsid (N) [57], as shown in Fig. 2. The role of the E protein is not clear, and it is expressed during replication [46].
Fig. 2.
The S glycoprotein of the virus causing COVID-19 is comprised of S1 and S2 (Subunits). These subunits are generally characterized as a sword-like spike. Using crystallography, the exact structure of this protein can be observed. The Protein Data Bank (PDB) model suggested that, the subunits of this glycoprotein are composed of various regions that are essential to the infection process. The essential bridge, which linked S1 and S2 together is termed as poly-basic amino acid bridge. This bridge is very important to understand viral targeting. “Created with BioRender.com”.
As E protein is not present in recombinant viruses, it exhibits condensed viral titers and retracted viral maturation. This suggests that the E protein is essential for viral replication as well as maturation [49]. Similarly, the M protein is the amplest structural protein, covering the membrane bilayer three times. The M protein presents both the NH2-terminal domain and COOH-terminus from outside and inside the virus. The N protein is responsible for RNA packaging as well as viral release following infection [58]. The S protein exhibits immense importance as it facilitates the entry of virus to the host cell followed by pathogenesis. The S1 domain of the S protein containing the RBD is mostly needed for receptor binding, whereas cell membrane fusion is the responsibility of the S2 domain [59]. Moreover, the host uses furin and transmembrane protease serine 2 (TMPRSS2) to further cleave the S protein. The S protein and the RBD of the SARS-CoV-2 and SARS-CoV are extremely similar, revealing that the cell entry mechanism of both shall be the same.
3.1. SARS-CoV-2 spike protein, a target for mRNA-based vaccine development
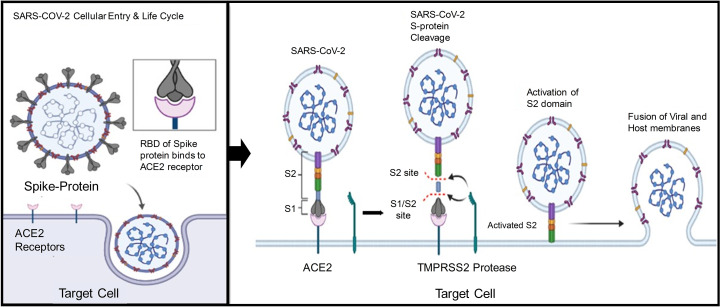
As discussed above, the SARS-CoV-2 makes its entry into the cell using spike protein, leading to cell membrane fusion and finally releasing RNA inside the cytoplasm (Fig. 1 ). The development of mRNA-based vaccines needs a suitable target, and therefore the S protein is considered the best target. Briefly, the S1 subunit separates from S2 due to trimeric instability when the S1 binds to the host cell receptor. The separation of S1 from S2 subunit makes a highly stable structure and is perfect for cell membrane fusion. The high homology of RBD of SARS-CoV-2 and SARS-CoV suggested that the virus uses angiotensin-converting enzyme (ACE2) as a binding site. Thus the S protein helps the virus to target the ACE2 and then enters the cell. Therefore, the vaccine development involved targeting the S protein leading to the inhibition of membrane fusion [[58], [59], [60]]. Accordingly, the vaccine targets the S protein and thereby prevents S-protein activation, resulting in the blockade of its binding with ACE2. Moreover, the SARS-CoV-2 cell entry is also blocked by TMPRSS2 inhibitors in cell lines, which are expressed with TMPRSS2.
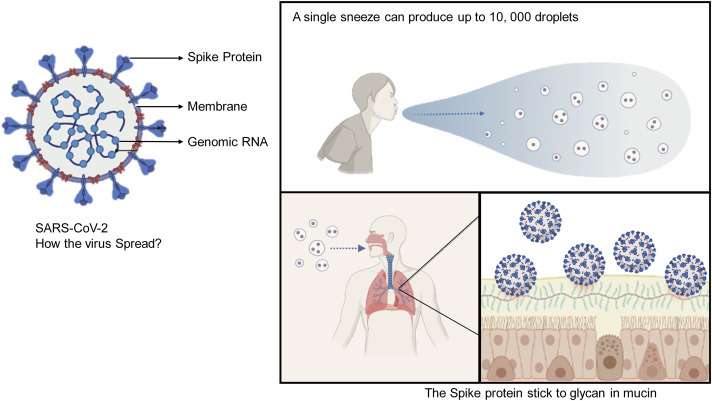
Fig. 1.
Spreading of COVID-19 in general population. Generation of thousands of small droplets containing SARS-CoV-2 after an infected person sneezes or coughs. The spike protein of SARS-CoV-2 play an important role in binding with high affinity to the mucins of the mucus that line the airways. “Created with BioRender.com”.
3.2. Viral Mutation and effect of mRNA vaccines on the variants of concern
Since the mutation is a natural phenomenon associated with viruses and sometimes, the mutation makes the virus weaker and less infective. However, the mutation also benefits the virus and makes it more infectious and highly transmissible. Mutation occurs in the genetic materials (RNA or DNA) of the viruses at a different rate, depending on the virus type. The mutation rate of an RNA virus is higher than DNA virus. For example, the Human immunodeficiency virus (HIV) and influenza virus (flu) are the two RNA viruses with high mutation rates [61,62]. Generally, once the virus enters the host cell, it uses polymerase to copy the genetic material, and these copies are used to infect new cells. Since, without perfection, the polymerase mistakes the copying process and result in mutation. Mostly, a mutation either harms the virus or does nothing. However, sometimes a mutation gives a newly produced virus an advantage. It provides the virus to evade the immune system or bind tightly to a host cell, which makes them highly transmissible. Since the COVID-19 pandemic has progressed for a long time, many mutations have occurred in SARS-CoV-2, and now different variants are infecting people in various parts of the world. However, some variants are considered “Variants of Concern” because they are believed to be highly transmissible and can evade the immune response of vaccinated individuals [63].
The variants of concern are discussed below:
3.2.1. Alpha (lineage B.1.1.7)
The B.1.1.7 variant, first detected in the United Kingdom (UK), was highly transmissible compared to the original variant first detected in Wuhan, China. According to a study conducted in the UK, a high viral load has been linked with B.1.1.7 variant; therefore, a high risk of death is associated with this variant. The World Health Organization (WHO) preliminary studies revealed that the mRNA vaccine candidate maintained efficacy against the B.1.1.7 variant. The mRNA vaccines have retained antibody neutralization against B.1.1.7 variant [64]. Early reports also suggested that the BNT162b2 & mRNA-1273 vaccines protect against this variant [65,66].
3.2.2. Beta (lineage B.1.351)
In October 2020 in South Africa, the B.1.351, known as Nextstrain clade 20H, was first detected and believed to be 50% more transmissible than the original variant. Early studies revealed that due to the presence of E484K mutation in the spike protein, the antibodies produced from vaccination or past infection might be less effective against this variant. One of the main concerns about this variant is the ability of immune evasion. Preliminary studies reviewed by the WHO indicated that the Pfizer–BioNTech vaccine demonstrates reduced efficacy/effectiveness against the infection; yet, there is no data available for other vaccines [67]. Moreover, early data from Pfizer revealed that the neutralizing activity for this version had been lowered by two-thirds [68]. Several studies eventually verified that sera from patients inoculated with the Moderna and Pfizer-BioNTech vaccines against B.1.351 variant had lower neutralizing activity [69]. A recent trial of the Pfizer/BioNTech vaccine against the B.1.351 variant revealing that the vaccine had been 100% successful thus far. (i.e., there was no infection in vaccinated participants), Beta variants were found in six of nine infections in the placebo control group [70]. Recently, data from Qatar and Israel authorities revealed that the two mRNA vaccines prevent severe cases from COVID-19, including severe pneumonia leading to death caused by B.1.351 [71].
3.2.3. Gamma (lineage P.1)
This variant was first identified in Brazil and Japan. Like Beta variants, it appeared to be able to evade the immune system at first. The P.1 variant contains 17 unique mutations, including some of the key S protein mutations as B.1.1.7, B.1.351 variants, and some other key mutations. As with the other two variants, P.1 showed low transmissibility. Multiple exploratory trials assessed by the WHO have shown slightly reduced antibody neutralization with Pfizer–BioNTech and Moderna (minimal to moderate reduction) [72].
3.2.4. Delta (lineage B.1.617)
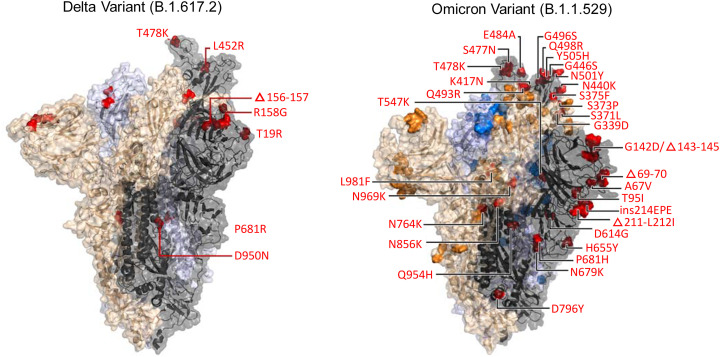
This variant emerged during the surge of COVID-19 cases in India and has since spread globally. It is nearly twice as infectious as previous variants. Several studies examined by the WHO indicated that the Pfizer–BioNTech and Moderna vaccines have remained effective against the Delta variant. They have also shown reduced antibody neutralization against Delta with Pfizer–BioNTech. Spike protein mutations D111D (synonymous), G142D, P681R, E484Q, and L452R, are among the 15 characteristic mutations (Fig. 3), whereas the later two mutations are responsible for evading antibodies up to some extent [73,74].
Fig. 3.
Comparative analysis of highly mutated Omicron with Delta variant. There are 32 mutations in the spike protein. Copyrights bioRxiv preprint [190].
3.2.5. Lambda (C.37 lineage)
A study has been conducted in the United States revealed that the vaccines used for COVID-19 are effective against the C.37 lineage of SARS-CoV-2. The lambda variant was first found in Peru in August 2020 and is currently prevalent in the South American region and various other countries.
The study found that the pseudotyped virus expressing the C.37 S protein was less susceptible to vaccine-provoked neutralizing antibodies. The study also revealed that the neutralizing antibodies reduction is very less. The results further suggested that the vaccines in current use against lambda variant remain protective [71].
3.2.6. Omicron (B.1.1.529)
Recently, a new variant of SARS-CoV-2 named Omicron (B.1.1.529) was identified in Africa. The Omicron variant contains at least 32 mutations in the S protein alone compared to 16 mutations in the already highly infectious delta variant (Fig. 3) and other proteins such as NSP12 and NSP14 that are crucial for viral replication [75]. The Omicron variant is thought to be at least three times more infectious than the original SARS-COV-2 and possibly more contagious than the Delta variant. More importantly, it may be partly resistant to existing vaccines [76]. Patients infected with Omicron was continually reported all over the world. The global prevalence of the Omicron variant remains to be closely monitored and controlled. Several research institutes and/or companies have announced that they have started developing new vaccines against the Omicron variant. The most recent study revealed that a booster dose of BNT162b2 & mRNA-1273 vaccines are effective against the variant. This study at Ragon Institute of MGH, MIT and Harvard involved the construction of a harmless version of Omicron called pseudovirus [77]. They evaluate the effectiveness of mRNA COVID-19 vaccines using this harmless version of the Omicron variant. The study also suggested that the fast and rapid spread of the Omicron variant is partial because of these mutations. During the study, blood samples were collected from 239 people vaccinated from either BNT162b2 or mRNA-1273. Among these 239 participants, there were 70 individuals got booster doses of mRNA vaccines. They found that individuals vaccinated with mRNA vaccines have low neutralization of the pseudovirus. However, significant neutralization was found against the Omicron variant in individuals who received a booster dose of the mRNA vaccine. The findings suggested that a possibility exists that the booster might induce antibodies to bind firmly to the S protein. Moreover, this additional dose of either of the mRNA vaccine produce antibodies targeting common regions in the S protein of all the variants of SARS-CoV-2 [77].
4. SARS-CoV-2 cellular entry and life cycle
Recent reports revealed that the S protein facilitates SARS-CoV-2 to recognize the ACE2 receptor that enables the virus to enter the cells. The reports also suggested that once the virus engages with the host cell membrane, the complex ACE2/SARS-CoV-2 or the viral RNA alone enters the cytosol (Fig. 4) [78,79]. In the case of ACE2/SARS-CoV-2, the entire virus is endocytosed, and the virus's membrane is fused with the luminal side of the endosome. This allows the viral RNA to enter the host cells' cytosol. Some reports suggested that the SARS-CoV-2 infects cells with ACE2 expression more effectively than those deficient in ACE2 expression. Besides ACE2, the TMPRSS2 also act as an important protein [80,81].
Fig. 4.
The cell entry mechanism of SARS-CoV-2. Using the spike protein, the virus gets its entry to the human cell by attachment to the Angiotensin-converting enzyme (ACE) 2 receptors. After development of protein by the host cell, the antibodies are produced against ACE2, which helps the body's immune system to recognize real infection. “Created with BioRender.com”.
Similarly, other reports suggested that the TMPRSS2 protein also has a key role in the viral entry into the host cell. The S protein is primed by the TMPRSS2 (serine protease) crucial to the pathogenesis of the virus (Fig. 4) [82]. The ACE2 is located mostly in the tissues of major organs, including the kidney, heart, lung, heart, testes, and brain. That is the reason that the infection of SARS-CoV-2 created problems in these major organs of the human body [83].
A comparative study was conducted to analyze the presence of ACE2 expression in a mouse model, non-human and human. The study results revealed that both the TMPRSS2 and ACE2 are highly expressed in nasal cellular goblets, gut enterocytes and specifically in the lung type II pneumocytes [84]. This suggested that as the S protein binds with the ACE2, it facilitates the conformational alteration, leading to the stimulation of viral envelope fusion with the host cell membrane. Once inside, the virus releases the RNA into the cell, and replication occurs.
Inside the cell, the RNA genome is translated into important viral replica polyproteins called pp1a and pp1b, which are then cleaved by viral proteinases into tiny products. As a result, a series of sub-genomic mRNAs are produced through discontinuous transcription via polymerase activity and finally translated into key viral proteins. Consequently, the viral protein and genomic RNA get assembled into virions in the endoplasmic reticulum. The resultant virions are then transported in the form of vesicles and released into the cytoplasm [25].
5. mRNA-based vaccines
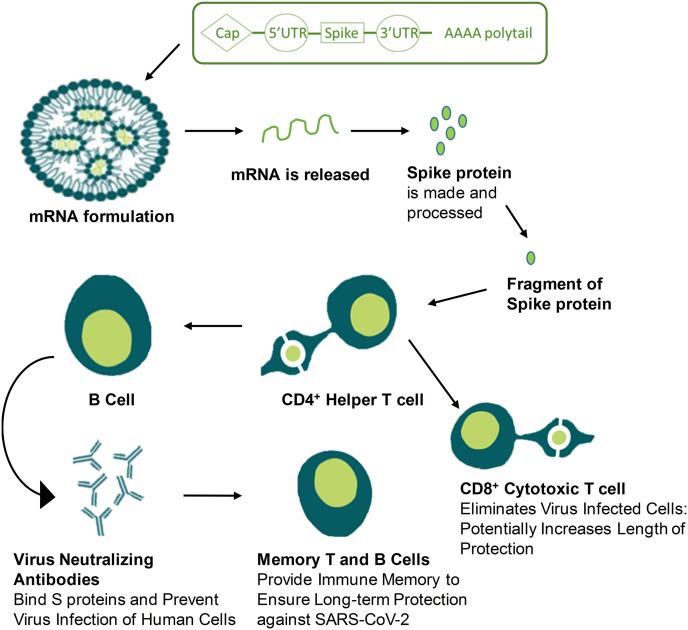
The mRNA does not intermingle with the host genome, as it is a safe, nominal and transient information carrier. Interestingly, the mRNA can be designed and manufactured rapidly as compared to other conventional vaccines. This rapid preparation potential of mRNA offers broad flexibility to develop a broad range of vaccines for other major infectious diseases. In simple words, the immunogens of interest are translated from input vaccine transcript by a conventional mRNA vaccine [85]. The mRNA shall be encapsulated in a specific liposome as well as a complexing agent. The suitable liposome and complexing agent in turn enhance the cellular uptake, and the higher cellular internalization brings about higher therapeutic delivery into the cytoplasm translation machinery. The liposomes also help to minimize the degradation of mRNA before it reaches the cytoplasm of the cell [85].
The clinical translation of mRNA-based vaccines is taking momentum as it is more advantageous in terms of efficacy, safety, economic production, and great perspectives in large-scale production. These advantages made mRNA-based vaccines a promising alternative to conventional vaccines. Moreover, the chimeric mRNAs that contain ORF viral sequences can be expressed in the cytoplasm and hence possess great potential in blocking chromosomal integration. Briefly, the immune cells process the injected mRNA to produce targeted protein via direct translation and further activate the immune system to detect and recognize viral protein to produce antibodies [86]. The first and important step of reaching the cytoplasm is to cross or penetrate the lipid membrane (Fig. 7) [[87], [88], [89], [90]]. However, the lipid nanoparticle encapsulation made the mRNA-based vaccines as a promising alternative to traditional-based vaccines due to better penetration and internalization. As reported, the SARS-CoV-2 is a (+)ss-RNA virus that has high replication inside cytosol. Furthermore, the virus's transmissibility is extremely high, which needs prompt prevention. Therefore, the development of mRNA-based vaccines played a vital role in protection against COVID-19. However, mRNA-based vaccines have to prove safety and efficacy in human beings at large since the early results of two mRNA-based vaccines developed for COVID-19 showed promising results in terms of safety and efficacy [91,92].
Fig. 7.
How mRNA vaccines work- Training the immune system for a real infection.
5.1. Advantages of mRNA-based Vaccines
Various prophylactic and therapeutic fields have been bridged by mRNA due to its versatility from the first-ever breakthrough in 1990 when the exogenous proteins were expressed in mice using in vitro transcribed (IVT) mRNA [[93], [94], [95], [96]]. To date, different strategies were employed and developed to deliver mRNA to treat infectious diseases and cancer [97].
Some of the advantages of in-vitro transcribed mRNA are given below;
-
a)
During the recent pandemic of COVID-19, the first-ever mRNA vaccine was inoculated in humans after the presentation of the viral genome before ten weeks. This shows that the time required to develop mRNA vaccines is very short [98].
-
b)
Most importantly, the IVT reaction is easy and quick, and a high yield can be obtained for scale-up manufacturing [97]. Having an advanced manufacturing setup, the manufacturing can be as large as kilograms [99].
-
c)
Due to the in-situ synthesis of antigen protein's stability, the mRNA vaccine excludes the need for protein purification. Moreover, it excludes the need for long-term stabilization, which is challenging for some antigens.
-
d)
If the protection of mRNA against RNases is ensured, then the transportation and storage of mRNA might become easier than protein-based vaccines because of low degradation chances compared to protein [100,101].
The above advantages certainly help to manufacture, store and transport mRNA vaccines in response to outbreaks and infectious disease outbreaks.
5.2. Disadvantages of mRNA-based Vaccines
Despite various advantages, the field of mRNA delivery faces some challenges such as:
-
a)
There is still concern regarding the instability of mRNA due to the enzymatic degradation by the natural RNases in the body [27].
-
b)
There are hundreds to thousands of nucleotides in mRNA, and these nucleotides must reach in full length to the cytosol for active translation. Therefore, due to degradation by RNases, the in vivo delivery of mRNA is a major concern.
6. mRNA Delivery technologies
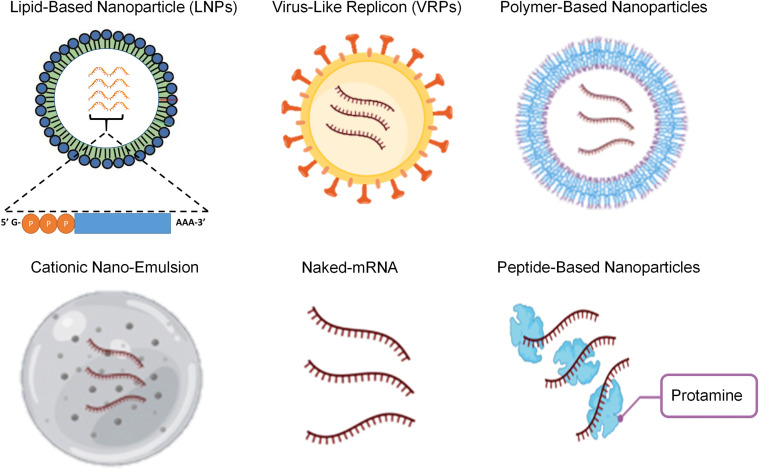
Due to the instability of mRNA, the introduction of mRNA vaccines at large further needs some delivery strategies such as carrier molecules. Therefore, scientists have made efforts to develop various kinds of carrier molecules to prevent the degradation of mRNA from RNases as well for better transportation of mRNA to the target site in the body. Examples of such carrier molecules are; Lipid-based delivery, polymer-based delivery, peptide-based delivery, virus-like replicon particle delivery, cationic nano-emulsion delivery and inoculation of naked mRNA [102]. This review section highlighted different delivery technologies associated with mRNA (Fig. 5 and Table 1 ).
Fig. 5.
Various delivery technologies for mRNA vaccines are shown: lipid-based particles, virus-like replicon particle, polymer-based delivery, cationic nanoemulsion, naked mRNAs, and peptide-based delivery.
Table 1.
Various delivery technologies for mRNA delivery.
| System | RNA | Disease/Condition | Composition | Ref |
|---|---|---|---|---|
| Lipids | HxB-2 HIV-1 Gag antigen mRNA |
HIV | DOTAP/DOPE | [192] |
| eGFP mRNA | N/A | DOPE/DC-Cholesterol [2:1] | [193] | |
| HSV I Thymidine kinase mRNA |
Cancer | DOTAP/Cholesterol [1:1] liposome with DSPE-PEG and DSPE-PEG-AA | [194] | |
| EPO mRNA | N/A | C12-200:Cholesterol: DOPE:C14-PEG2000 | [111] | |
| Ovalbumin mRNA | Malenoma | A18 | [195] | |
| HER2 antibody mRNA | Cancer | cKK-E12 | [196] | |
| Human erythropoietin | N/A | MC3, DSPC, cholesterol, and PEG2000-DMG | [197] | |
| HIV-1 antigen Gag mRNA | HIV | DOTAP/DOPE [1:1] | [192] | |
| eGFP mRNA | N/A | DC-Cholesterol)/DOPE (1:2) | [193] | |
| Polymers | Erythropoietin (EPO) mRNA | Anemia and myelodysplasia | Poly(glycoamidoamine) | [122] |
| HIV-1 gag mRNA | HIV | Polyethyleneimine | [198] | |
| eGFP mRNA | N/A | Poly(β-amino ester) (PBAE) | [199] | |
| eGFP and ovalbumin | N/A | Triblock copolymer | [200] | |
| Luciferase-encoding mRNA | N/A | DEAE-Dextran | [201] | |
| Peptides and peptide-polymer hybrids | eGFP mRNA | Ovarian Cancer | PepFect14 | [202] |
| eGFP mRNA& OVA mRNA | N/A | RALA | [132] | |
| eGFPmRNA & FLuc mRNA | N/A | RALA-PLA | [203] | |
| Lipid polymer hybrid NPs | Firefly luciferase (FLuc) mRNA and eGFP mRNA |
N/A | TT3:DOPE:Cholesterol:DMG-PEG2000 with PLGA core | [204] |
| FLuc mRNA | N/A | PBAE:C14-PEG2000 | [205] | |
| Ovalbumin mRNA | N/A | PBAE:EDOPC/DOPE/DSPE-PEG | [206] | |
| eGFP mRNA | N/A | PBAE: DOPC, DOTAP, and DSPE-PEG | [207] |
6.1. Lipid-based delivery
Lipid nanoparticles (LNP) are negatively charged mRNA delivery vehicles, categorized as ionizable amino lipids, phospholipids, polyethylene glycol (PEG) and cholesterol (chol). The LNPs have been extensively studied as delivery vehicles for mRNA and emerged as the first-ever clinically tested platform to date [[103], [104], [105], [106]]. The PEG facilitates prolong circulation during systemic circulation and hinders the binding of plasma proteins to mRNA. While the essential ionizable lipids facilitate the endosomal escape of mRNA, the phospholipids and cholesterol bring stability to the LNP structure [107,108]. Moreover, as an mRNA vaccine vector, the LNP has two advantages: protecting the mRNA from degradation by endosomal enzymes and better biocompatibility, which helps efficient delivery of mRNAs for expression of proteins [109].
Multiple lipid components and modifications in lipids decides the efficient delivery of LNPs [110]. Cationic or ionizable lipids are the primary substance to deliver RNA such as (i) 1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine (DOPE) [111], (ii) N-[1-(2,3-dioleoyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTMA) [112], (iii) N1,N3,N5-tris(3-(didodecylamino)-propyl)benzene-1,3,5-tricarboxamide (TT3) [113], (iv) dilinoleylmethyl-4-dimethylaminobutyrate (Dlin-MC3-DMA), (v) N,N-Dimethyl-2,3-bis[(9Z,12Z)-octadeca-9,12-dienyloxy]propan-1-amine (DLinDMA) [114] and (vi) 1,2-dioleoyloxy-3-trimethylammonium propane chloride (DOTAP) [115]. At certain pH, these lipids are positively charged and thus feasible for the mRNA to interact electrostatically, and easily fused with cellular membrane [116]. After endocytosis, the ionizable cationic lipid becomes more positively charged due to proton-pump mediated pH reduction [117]. Once inside, the cationic lipids are removed by the endogenous anionic lipids and resulting in the release of mRNA in to the cytoplasm [118]. Currently, the LNP is a prospective mRNA delivering vector due to better biocompatibility and high delivery efficiency [119,120].
6.2. Polymer-based delivery
Clinically, the polymeric-based materials are less explored as compared to ionizable LNPs. However, the mRNA has been successfully coated by polymeric-based materials to prevent its degradation leading to the expression of proteins of interest. In order to address the disadvantages associated with the polymeric materials, various researchers added lipid chains, enlarged branch structures, and built biodegradation enhancing domains to improve polydispersity and enhance clearance of large molecules [[121], [122], [123]].
Various cationic polymers such as polyamidoamine (PAMAM) dendrimer, polyethylenimine (PEI) and polymerand polysaccharide are used [[124], [125], [126], [127]]. For example, PEIpolyplex was used to coat mRNA encoding hemagglutinin of influenza virus and nucleocapsid. The cytosolic composite enables mRNA translation and encourages both humoral and cellular responses [128]. Moreover, the dendrimer is considered a possible delivery vehicle due to the presence of multiple functional groups with high tolerability [129]. For example, the PAMAM dendritic polymers containing NH2 and OH functionalities enter A549 human lung epithelial carcinoma cells faster than the hyperbranched polymers [130]. The polymer-based delivery material is a hopeful platform for the delivery of mRNA. However, it is in the early pre-clinical stage of investigation and needs further research.
6.3. Peptide-based delivery
Due to the electrostatic interaction, the negatively charged mRNA can be carried via cationic peptide. The presence of amino groups, such as arginine and lysine, makes the peptide positively charged. This positive charge enables the negatively charged mRNA to adsorb onto the cationic peptide [131]. However, the negative and positive ratios determine the loaded mRNA amount [132]. Two aspects make protamines one of the prospective cationic peptides to deliver mRNA, such as it protects mRNA from the degradation of RNase in the serum [132]. On the other hand, the protamine acts as an adjuvant. The latter one is evident from a study, which suggested that a formulation containing protamine delivering mRNA activated both dendritic cells (DCs) and monocytes, resulting in TNF-α and IFN-α secretion. Moreover, the formulation also activated immune cells by recognizing protamine and mRNA [133]. Another study was conducted in the glioma animal model, which revealed that the protamine and mRNA formulation had a higher antitumor effect than naked nucleic acid adjuvants [134]. As a result, only the protamine is undergoing clinical trials among all the peptide-based delivery carriers [135,136].
Similarly, cationic cell-penetrating peptides (CPPs) are another kind of small peptide, comprised of approximately 8 to 30 amino acids, and are considered to be an excellent carrier. These CPPs are furnished with low charged densities as well as capable of disrupting membrane during the endosomal escape. The ability to disrupt membranes is valuable for protein synthesis [137]. Additionally, the anionic peptides became efficient once conjugated with a positively charged substance [138].
6.4. Virus-like replicon particles (VRPs)
The VRPs are like a virus-infecting method where the antigen-encoding saRNA is encapsulated for efficient delivery into the cytosol. Generally, the in-vitro synthesis of viral structural protein is performed first, and then antigen-encoding saRNA is encapsulated [139]. The lipid inorganic nanoparticles (LIONs) are recently prepared to encapsulate the alphavirus-derived replicon RNA encoding the SARS-CoV-2 S protein. The resultant LIONs were administered to primates and mice. As a result, a high amount of anti-SARS-CoV-2 S protein IgG antibody was produced [140]. Similarly, in another study, the VRPs were loaded with mRNA derived from HIV encoding clade C envelope glycoprotein. As a result, the formulation triggered a complex immune response in the rhesus [141]. A concise summary is presented by Lundstrom comprising VRPs-saRNA against viral diseases, bacterial diseases, and cancer. Although the VRPs demonstrated excellent therapeutic effect against most viral diseases, bacterial diseases, and cancer, there are still few limitations which hiners its clinical translation. Moreover, the combination involving VRPs are believed to endorse anti-vector antibodies, which hamper the ongoing clinical trials [142].
6.5. Cationic Nano-Emulsion (CNE)
CNE is a non-viral delivery platform, which enhances the efficiency of mRNA vaccines by binding to saRNAs. The cationic lipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOTAP) is an essential component of CNE. Comparatively, the cellular-immune response induced by CNE derived from saRNA is believed to be robust compared to saRNA-derived VRPs. Similarly, studies also demonstrated robust protective immunogenicity against the Zika virus and Venezuelan equine encephalitis virus, followed by the saRNA vaccine delivered by CNE [143]. CNE showed great potential to be evaluated clinically based on the above preclinical studies.
6.6. Naked mRNA vaccines
The naked-mRNAs are delivered directly by injecting mRNA using Ringer's and lactated Ringer's solutions. Since the naked-mRNA is unable to cross the membrane, scientists hypothesized the cell uptake mechanism, such as the involvement of DC-mediated macro-pinocytosis. They further suggested that it diminishes the mRNA as the DCs mature [144]. Some researchers suggested that the uptake of mRNA can be brought by the disruption of the membrane, such as the direct penetration and permeability, including microinjection and mechanical membrane disruption or electroporation, respectively [145,146]. However, the degradation of mRNA in the serum by RNase hinders its further clinical progress and needs extensive evaluation.
7. mRNA vaccines for COVID-19
mRNA therapeutics is currently an emerging technology for the prevention of infectious diseases. In the mid of 2019, fifteen mRNA-based vaccines were developed against infectious diseases, but unfortunately, none entered phase III trials. Therefore, it was believed that the mRNA-based technology might take another 5 to 6 years to reach the market. Fortunately, the COVID-19 pave the way for mRNA-based vaccines to be the ultimate test for the first time in humans at large. Till the end of 2021, there were around 200 vaccine candidates in pre-clinical trials. In addition, more than 100 vaccine candidates are already in the clinical trials, of which around 20 candidates are mRNA-based vaccines. Only two of them (Fig. 6) got emergency approval from the United States Food & Drug Administration (US FDA). The two mRNA-based vaccines, BNT162b2 & mRNA-1273, are approved for large inoculation in humans worldwide.
The BNT162b2 mRNA-based COVID-19 vaccine is co-developed by Pfizer and BioNTech [147]. They started to develop five candidates encoding the S protein of SARS-CoV-2. Two of their lead candidates named BNT162b1 and BNT162b2 were encapsulated in LNPs. The ionizable lipid, ALC-0315, and a nucleoside-modified mRNA in which N1-methylpseudouridine was used as a replacement of uridines for enhanced mRNA translation [148]. A secreted type of the S protein's RBD, trimerized, was encoded in the BNT162b1, whereas a full-length of SARS-CoV-2 S protein along with proline replacements in the S2 subunit was encoded in the BNT162b2 type. During pre-clinical studies, two doses of BNT162b2 (100 μg each) were given to rhesus macaques 21 days apart. The results were impressive as the two doses triggered neutralizing antibodies 10.2 to 18.0 times more compared to a convalescent patient serum. Furthermore, the doses also elicited CD4+ and CD8+ T cell responses [149].
Moreover, during the phase I trials, both neutralizing antibodies and robust CD4+ and CD8+ were recorded with two doses of 30 μg at 21 days apart with mild to moderate adverse effects [[150], [151], [152], [153]]. As both the lead candidates showed promising outcomes during phase I trials, the BNT162b2 was further tested for phase II/III trials because of the mild adverse effects both locally and systemically compared to BNT162b1. Overall, 95% efficacy (Table 3) was shown by BNT162b2 in preventing COVID-19, while 90–100% was recorded across subgroups defined by ethnicity, BMI, age, sex as well as co-existing conditions [154]. Furthermore, in a trial conducted in Israel, recruiting 3,159,136 individuals was inoculated with BNT162b2 with 94% efficacy in preventing COVID-19 and preventing hospitalization at a rate of 87% as 92% effective to prevent severe symptoms of COVID-19 [155].
Table 3.
General characteristics of mRNA-based vaccines for COVID-19.
| BNT162b2 | mRNA-1273 | |
|---|---|---|
| Efficacy | 95% | 94.1% |
| Technology | mRNA | mRNA |
| Required doses | 2 | 2 |
| Days between doses | 21 | 1 month |
| Recommended age group as per US FDA | 12 years of age and older (purple cap, gray cap), 5–11 years of age (orange cap) |
18 years and older |
| Common adverse reactions | acute anaphylactic reaction, myocarditis and pericarditis, fainting, fatigue, headache, muscle pain, chills, joint pain, swelling at the injection site, fever, redness at the injection site | acute allergic reactions, myocarditis and pericarditis, syncope, pain at the injection site, fatigue, headache, myalgia, arthralgia, chills, nausea/vomiting, axillary swelling/tenderness, fever, erythema at the injection site, and rash |
| Storage temperature | −80 to −60 °C | −25 °C to −15 °C |
| Duration of stability at fridge temperature | 5 days | 30 days |
Similarly, another mRNA-based COVID-19 vaccine, known as mRNA-1273, was developed by Moderna in collaboration with the National Institute for Allergy and Infectious Diseases at the National Institutes of Health and the Biomedical Advanced Research and Development Authority. The mRNA-1273 also uses LNPs comprising an ionizable lipid, SM-102, in which N1- methylpseudouridine-modified mRNA was encapsulated [156].
During the pre-clinical trial, mice were injected with two doses (1 μg, primer dose and booster dose), resulting in both neutralizing the virus and a potent response of CD4+ and CD8+ T cell [156]. Moreover, it was also noted that the mRNA-1273 prevents airway infections for up to 3 months. A potent humoral and cellular immune response was also noted after two doses (100 μg) in rhesus macaques [157]. In a comparative study, the serum from vaccinated macaque demonstrated 15-folds more neutralizing antibodies, potent spike-ACE2 inhibition activity of 348-folds and virus-neutralizing activity of 12-folds as compared to the serum from COVID-19 recovered patients [157]. These promising results paved the way for mRNA-1273 in clinical trials where it showed acceptable tolerability and exceptional efficacy. In phase III trials, the mRNA-1273 showed 94.1% efficacy (Table 3) against COVID-19 infection after two doses (100 μg). There were no obvious side effects recorded except the local pain at the injection site. However, fatigue, muscle pain and joint pain were noticed in volunteers after the second dose of mRNA-1273, but these side effects were subsided after 48 h [158]. Some severe adverse reaction following the two mRNA vaccines are discussed in the next section. Other mRNA based COVID-19 vaccines candidates are in different stages of development, summarized in Table 2 .
Table 2.
In-progress clinical trials of mRNA vaccines for COVID-19.
| Type of vaccine | Developers | Route of administration | Schedule | Ref |
|---|---|---|---|---|
| Phase IV | ||||
| mRNA-1273 | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | IM | Day 0 + 28 | [208] |
| BNT162b2 (LNP-mRNAs) | Pfizer/BioNTech + Fosun Pharma | IM | Day 0 + 21 | [209] |
| Phase III | ||||
| SARS-CoV-2 (ARCoV) | Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | IM | Day 0 + 14 or Day 0 + 28 | [210] |
| CVnCoV Vaccine | CureVac AG | IM | Day 0 + 28 | [211] |
| Phase II/III | ||||
| ARCT-021 | Arcturus Therapeutics | IM | ND | [212] |
| MRT5500 | Sanofi Pasteur and Translate Bio | IM | Day 0 + 21 | [213] |
| mRNA-1273.211 | ModernaTX, Inc. | IM | Day 0 | [214] |
| Phase I/II | ||||
| DS-5670a | Daiichi Sankyo Co., Ltd. | IM | ND | [215] |
| EXG-5003 | Elixirgen Therapeutics, Inc | ID | Day 0 | [216] |
| mRNA-1283 | ModernaTX, Inc. | IM | Day 0 + 28 | [217] |
| ChulaCov19 | Chulalongkorn University | IM | Day 0 + 21 | [218] |
| PTX-COVID19-B | Providence Therapeutics | IM | Day 0 + 28 | [219] |
| Phase I | ||||
| HDT-301 | SENAI CIMATEC | IM | Day 0 + 28 | [220] |
| mRNA-1273.351 | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | IM | Day 0 or Day 0 + 28 or Day 56 | [221] |
| LNP-nCOV saRNA-02 vaccine | MRC/UVRI and LSHTM Uganda Research Unit | IM | Day 0 + 28 | [222] |
| CoV2 SAM (LNP) | GlaxoSmithKline | IM | Day 0 + 30 | [223] |
| LNP-nCoVsaRNA | Imperial College London | IM | ND | [222] |
8. COVID-19 mRNA vaccines and adverse effects
Millions of mRNA COVID-19 vaccines have been inoculated globally, and plenty of safety evaluations have been conducted. The data showed that the vaccines are safe and effective against COVID-19. However, rare adverse reactions, such as myocarditis and pericarditis, are commonly reported in males under the age of 30 within one week of their second vaccination dose [159]. These cases have been observed especially in Ontario, Canada and the United States after the administration of mRNA vaccines manufactured by Moderna Spikevax®, while less pragmatically after administering vaccines manufactured by PfizerBioNTech Comirnaty®. Taking precautions, the National Advisory Committee on Immunization (NACI) recommended halting the second dose if an adverse effect was experienced to the first dose of vaccine until more information is available. Individuals who suffer myocarditis and pericarditis after administering the vaccine experienced common symptoms including; shortness of breath, pain in the chest, palpitations [160]. Furthermore, a recently published report in American Heart Association revealed that the adverse reaction of myocarditis in young people under the age of 21 is mild and improved quickly [161]. Cases of suspected vaccine-associated myocarditis were categorized as “probable” or “confirmed” using Center for Disease Control and Prevention (CDC) definitions.
Similarly, analysis of observational data of adverse events following mRNA vaccination from the largest health care organization in Israel was compared with unvaccinated individuals. The results suggested that vaccination is most strongly associated with a high risk of myocarditis (risk ratio, 3.24; 95% CI, 1.55 to 12.44; risk difference, 2.7 events per 100,000 persons; 95% CI, 1.0 to 4.6) [162].
Myocarditis and pericarditis are more typically reported in children who develop multisystem inflammatory syndrome (MIS-C) following COVID-19 infection. MIS-C is a condition that causes different parts of the body to become inflamed, including the heart, lungs, kidneys, brain, and gastrointestinal organs [163]. Children who develop MIS-C are much more likely to be admitted to the intensive care unit (ICU) and need medication. The CDC reports at least 5900 cases of MIS-C causing 52 deaths among children in the US. Similarly, a study in the US and UK that included adults found that myocarditis is more likely common after COVID-19 infection instead after vaccination [164,165].
Myocarditis and pericarditis are conditions that have occurred following COVID-19 vaccination in a very small number of people. Most cases are mild, and individuals often recover on their own or with minimal treatment. The risks to the heart from COVID-19 infection can be more severe. Conclusively, the NACI reported that the benefits of COVID-19 mRNA vaccines continue to outweigh the risks of COVID-19 illness in the authorized population as mRNA vaccines are effective in reducing COVID-19 infections, hospitalizations, and deaths.
9. Future of mRNA-based therapeutics to treat other major disease
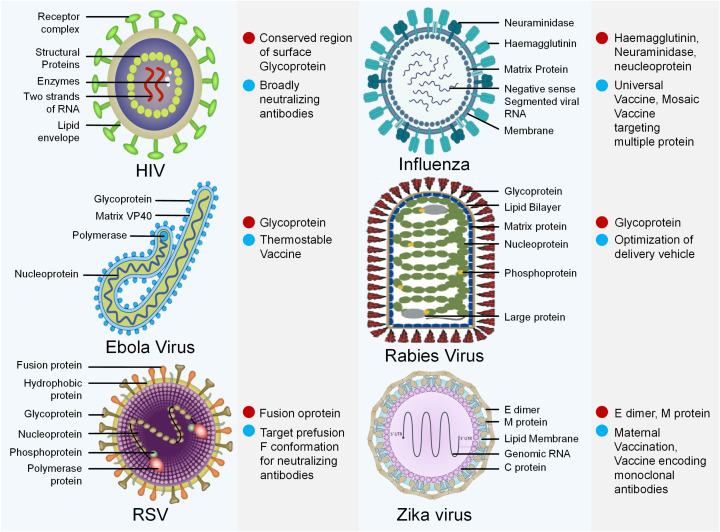
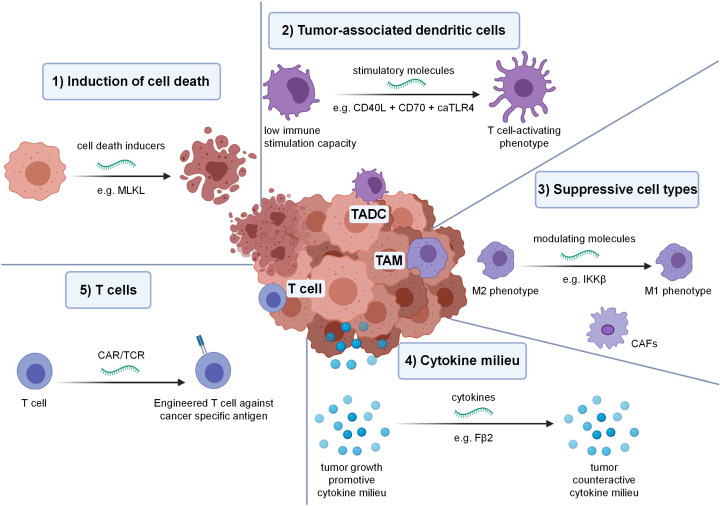
Since the mRNA-based vaccines have proven effective against COVID-19 and its emerging variants, the implications and role of mRNA are now expanding far beyond COVID-19. This ultimate success will pave the way for extensive use in emerging and recognized pathogens. In making mRNA-based vaccines, these great strides have changed the future approach for vaccine development. This attracted various researchers to the field of mRNA vaccines against cancer and other infectious diseases, which are in the early stages of clinical trials. Crucial advantages such as quick designing, rapid generation, and ease in manufacturing made the mRNA vaccines the best choice against various life-threatening diseases in the near future [166]. Some of the examples of mRNA vaccines in the developmental stages against various infectious diseases (Fig. 8) and cancer (Fig. 9) are highlighted below:
Fig. 8.
Viruses causing diverse diseases. Possible targets (Red dot) and strategies (Sky blue dot) for mRNA vaccines. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
In vivo tumor modulation by mRNA: various startegies includes, (1) Induction of cell death e.g. mRNA encoding mixed lineage kinase domain like pseudokinase induce cells death in an immunogenic way. (2) Tumor-associated dendritic cells (TADC) modulation e.g. mRNA encoding for cluster of differentiation 40 ligand, cluster of differentiation 70 ligand & constitutive active toll like receptor 4 (TriMix) can be combined to stimulate immature TADC to mature TADC. (3) Suppressive cell types e.g. the tumor-associated macrophages can be genetically reprogrammed by mRNA encoding inhibitor of nuclear factor kappa-β kinase. (4) Cytokine milieu e.g. mRNA encoding IFN-β fused to the ectodomain of the TGF-β receptor II (Fβ2). (5) Induction of cancer-specific T cells e.g. mRNA can be used to genetic engineer T cells to express cancer-specific T cell receptors. Reprinted with permission from, Copyright Springer Nature [191].
Comparable to SARS-CoV-2, the influenza pandemic in 1918 caused 40 M deaths around the globe. Several vaccines, including live attenuated, inactivated, and recombinant haemagglutinin (HA) have been developed, targeting the haemagglutinin (HA) protein responsible for viral entry into the host. However, due to the rapid and constant mutations, the virus is still causing severe respiratory illness, especially in winters [167,168]. Various mRNA vaccines are undergoing clinical trials, such as modified mRNA VAL-506440 encoding full-length, a membrane-bound form of the HA glycoprotein isolated from either of the two prominent strains, H10N8 influenza strain and H7N9 influenza strain (Table 5) [169] [170]. For the H10N8 study, a total of 201 participants were recruited and given 100 μg intramuscularly. The administered dose induced humoral immunization inhibition and microneutralization titers of 1:40 in 100% & 1:20 in 87.0% of the participants. Compared to 34.5% of the participants receiving the intramuscular dose, the 25 μg intradermal dose generated humoral immunization inhibition titers of more than 1:40 in 64.7% of the participants [170]. Similarly, 10, 25 and 50 μg doses were administered to 156 participants intramuscularly for the H7N9 study. These administered doses generated humoral immunization inhibition titers of 1:40 in 36.0%, 96.3%, and 89.7% of the participants, respectively. For 10 and 25 μg administered groups, the microneutralization titers around were 1:20 by 100% participants, while 96.6% in the 50 μg group [170]. The above clinical data revealed high neutralization antibody titers, suggesting that the mRNA vaccine candidates possess great promise in protecting against influenza infection.
Table 5.
Clinical trials of mRNA vaccines against infectious diseases except COVID-19.
| Funding source | Name | Target Infection | Type | Phase | |
|---|---|---|---|---|---|
| Moderna | mRNA-1647 | CMV | Nucleoside-modified mRNA–LNP | Phase II (NCT04232280) Phase III (NCT05085366) |
[224,225] |
| Moderna | mRNA-1443 | CMV | Nucleoside-modified mRNA–LNP | Phase I (NCT03382405), Phase II (NCT04917861) |
[226,227] |
| Moderna | mRNA-1893 | Zika | Nucleoside-modified mRNA–LNP | Phase I (NCT04064905) Phase II (NCT04917861) |
[228,229] [227] |
| Moderna | mRNA-1325 | Zika | Nucleoside-modified mRNA–LNP | Phase I (NCT03014089) | [230] |
| Moderna | mRNA-1653 | hMPV/PIV3 | Nucleoside-modified mRNA–LNP | Phase I (NCT04144348, NCT03392389) | [231] |
| Moderna | mRNA-1345 | RSV | Nucleoside-modified mRNA–LNP | Phase I (NCT04528719), Phase II & III (NCT05127434) |
[232,233] |
| Moderna | mRNA-1851 (VAL-339851) | Influenza A (H7N9) | Nucleoside-modified mRNA–LNP | Phase I (NCT03345043) | [170] |
| Moderna | mRNA-1440 (VAL-506440) | Influenza A (H10N8) | Nucleoside-modified mRNA–LNP | Phase I (NCT03076385) | [234] |
| Moderna | mRNA-1944 | Chikungunya | Nucleoside-modified mRNA–LNP | Phase I (NCT03829384) | [235] |
| Moderna | mRNA-1388 (VAL-181388) | Chikungunya | Nucleoside-modified mRNA–LNP | Phase I (NCT03325075) | [234] |
| CureVac | CV7201 | Rabies | Unmodified mRNA complexed in RNActive | Phase I (NCT02241135) | [236] |
| CureVac | CV7202 | Rabies | Unmodified mRNA–LNP | Phase I (NCT03713086) | [237] |
| GSK | GSK3903133A | Rabies | Self-amplifying mRNA in cationic nanoemulsion | Phase I (NCT04062669) | [238] |
The Rabies virus is transmitted from an infected dog or cat and causes Rabies infection in the central nervous system. During the infection, flu-like symptoms develop at first, and due to progressive encephalomyelitis, the patient finally develops severe neurotropic symptoms [171]. Over the years, different conventional vaccines have been approved to treat rabies, but the mortality is still very high [172]. To address this, two mRNA-based vaccines, namely CV7201 and CV7202, have been developed and tested against rabies in clinical trials (Table 5). The lyophilized CV7201 and CV7202 are temperature-stable vaccines comprising mRNA encoding free form glycoprotein (RABV-G) from rabies virus itself, and cationic protein protamine is used for complexation [173]. During the phase I clinical trials in Germany, a total of 306 doses of CV7201 were administered to the participants. The vaccine administered intradermally prompted neutralizing antibody titers of 0.5 IU/mL against the virus with 32 (71%) of 45 participants, while 6 (46%) of 13 participants were administered intramuscularly. Moreover, 8 (57%) out of 14 participants were given a booster dose of CV7201 intradermally, which induces neutralizing antibody titers of more than 0.5 IU/mL. Similarly, CV7202 is also under phase I clinical trials with a total of 53 participants. The phase I clinical trials are expected to complete by next year [[173], [174], [175]].
Similarly, endemic to central Africa in 2014, the Ebola virus affects the central nervous and gastrointestinal systems at first, leading to multi-organ failure, coma, and death in severe cases [176,177]. In 2019, the US FDA approved a recombinant vesicular stomatitis virus (VSV) Ebola vaccine. However, certain safety concerns were also noticed at higher doses, such as skin rashes and acute arthritis [178]. Later on, two mRNA vaccines were developed that encodes the Ebola virus envelope glycoprotein (EBOV GP). Lipid nanoparticles are used to encapsulate mRNA to facilitate efficient delivery. The vaccine was tested on two groups of guinea pigs (vaccine A, B) to test the neutralizing antibodies. The results revealed that increased EBOV GP titers were induced after 21 and 42 days post-treatment [179].
The transmission of Epstein–Barr Virus (EBV) from saliva, blood or other body fluid causes fever, sore throat, fatigue, lack of appetite, rash, swollen glands in the neck and sometimes Guillain-Barre syndrome and cancer of the nose and throat as well. The mRNA-1189 candidate developed against EBV targeting the glycoprotein 350 (gp350) showed promising results in preclinical studies [180]. The mRNA-1189 induced antibody titers in Balb/c mice after two doses against viral protein responsible for epithelial cell entry. These promising results bring hope for viable immunity and defense against EBV-related infections and complications.
Similarly, the Respiratory Syncytial Virus (RSV) causes infection in children, leading to acute bronchiolitis with a high morbidity and mortality rate. Previously, certain RSV vaccine candidates were developed, specifically targeting the conserved F protein. But during the clinical trials, most of the vaccines were failed due to low neutralizing antibody titers against the virus. In recent times, an mRNA-based vaccine candidate (mRNA-1345) has been developed by optimizing the codon of the previous mRNA-1777 candidate to enhance translation and immunogenicity (Table 5) [181]. The ongoing phase I clinical trials for mRNA-1345 recruited 160 participants, where several doses will be given to evaluate the tolerability and reactogenicity in adults and children. The study is expected to be completed in 2023, and then further evaluation will be conducted [182].
Zika Virus (ZIKV) causes influenza-like illness in milder cases, while the severe cases include multi-organ failure, meningitis, and encephalitis [183]. In order to prevent viral fusion, the membrane and envelope protein is excellent targets for mRNA vaccines against ZIKV. A study showed that the mRNA vaccine generated neutralizing antibody titers against ZIKV were 50–100 times higher than other vaccine candidates [184,185]. Another mRNA-based vaccine (mRNA-1893) is undergoing phase I clinical trials to evaluate safety, tolerability, and immunogenicity. The study is expected to be completed at the end of 2021. The results will be available soon (NCT04917861).
Likewise, the pandemic of Human Immunodeficiency Virus (HIV) has infected around 17.5 M people worldwide and is caused by the direct transmission of humans to humans [186]. Due to the antigenic multiplicity of the protein envelope and the compact glycan shield, still, there are no effective vaccines against HIV. Recently, a Self-amplifying mRNA vaccine was developed, which encodes the clade C envelope glycoprotein and viral replicon particle. The vaccine was tested in rhesus macaques, and the results showed a higher generation of antibody titers against the virus's envelope protein [187]. Some other mRNA-based vaccines are also in the developmental stages against various infections, including Human Metapneumovirus (HMPV) and Parainfluenza Virus Type 3 (PIV3), Human Cytomegalovirus (HCMV). Moreover, Moderna is set to have various therapeutic targets in its sights, such as heart failure, more effective influenza shots and mosquito-borne viral disease chikungunya. Similarly, the world would probably see the first malaria vaccine developed by BioNTech at the end of 2022.
In late 2021, the first dose of BNT111, an mRNA-based therapy developed by BioNTech, was administered to a patient in a phase II cancer vaccine trial. The BNT111 was given in combination with Libtayo, co-developed by Sanofi and Regeneron. The patient was suffering anti-PD1-refractory/relapsed un-resectable Stage III or IV melanoma. A total of 120 patients will be recruited to evaluate the safety, efficacy and tolerability of the combination of BNT111 and Libtayo. The development of BNT111 is the leading candidate of BioNTech, comprising a fixed combination of tumor-associated antigens with mRNA encode. The aim of the combination is to activate a specific and strong response to the tumor. The phase II clinical trials have been approved in the US, UK, Australia and some other European countries, including Spain, Germany, Italy and others. The Phase II trials were launched after the success in preliminary results in early clinical evaluation and Phase I clinical trial. The phase I clinical trials results revealed promising safety profile in 89 patients with advanced melanoma. The efficacy data also showed that the BNT111 is highly effective in 42 patients both as a single agent and in combination with anti-PD-1 antibodies [188].
Similarly, mRNA-4157 developed by ModernaTX, Inc., is a personalized vaccine encoding multiple neoantigens and encapsulated in lipid. The selection of multiple neoantigens is based on a proprietary algorithm, designed to prompt neoantigen specific T-cells and accompanying anti-tumor responses [189]. In Phase I clinical trials the safety profile of mRNA-4157 was evealuated using adjuvant monotherapy in patients with resected solid tumors. On other hand, the mRNA-4157-pembrolizumab combination was also used in patients with advanced or metastatic cancer. The results of 13 patients received mRNA-4157 as monotherapy, and 20 patients received mRNA-4157-pembrolizumab combination showed no clinically significant adverse events, however reversible and low-grade adverse events were noticed. Moroever, 12 patients among the total 13 on adjuvant monotherapy were noticed disease free till 8 months during the study. Whereas the combination of mRNA-4157-pembrolizumab results revealed that 12 patients progressed on prior checkpoint inhibitor, 1 complete response and 16 have been restaged. However, one patient is observed to be non-evaluable for response but remains on study. The study further revealed that neoantigen specific T cell responses were also noticed via IFN-γ ELISpot from cryopreserved peripheral blood mononuclear cells. The phase I clinical trials results showed that all the doses of mRNA-4157 are safe and mRNA-4157-pembrolizumab showed desired clinical responses inducing neoantigen specific T cells. These promising results made the mRNA-4157 to progress to phase II clinical trials. Similarly, BioNTech also developed BNT113 and BNT122 for human papillomavirus 16, head, neckcancer and adjuvant colorectal cancer respectively. The phase II clinical trials for BNT113 & BNT122 are undergoing after promising results in Phase I clinical trials.
10. Conclusion and prospectives
The mRNA-based technology has been explored over the past three decades. Efforts are made, and billions of dollars are already spent to make them functional for human use. The mRNA-based treatment and vaccines with the potential of quick production are essential in coping with viral diseases and infections. As the viruses are highly mutating pathogens, the mRNA-based technology can be easily modified and manufactured in large quantities quickly. Furthermore, the technology transfer is extremely easy compared to conventional vaccines technology because the shipping and transport might take weeks and months. But in the case of mRNA, the genetic sequence can be sent via a computer. The COVID-19 opened the door for mRNA-based vaccines and therapies for other major diseases. The impact and implication of mRNA-based technology could be far beyond COVID-19.
Moreover, mRNA gained much attention in the field of bio-pharmaceuticals. The reason for interest in this new kind of vaccine arises from their safety, precision and flexibility compared to conventional approaches. The increasing number of clinical trials in cancer therapies (Table 4) and infectious diseases (Table 5) other than COVID-19 have recently revealed significant interest from pharmaceutical industries. The mRNA vaccines can be manufactured on a large scale for clinical-grade applications, which brings a quick platform to address sudden outbreaks when a quick response is needed.
Table 4.
In-Progress Clinical trials using mRNA vaccines for the treatment of various cancer types.
| Target disease | Status | Phase | NCT number |
|---|---|---|---|
| Non-small cell lung cancer (NSCLC) | Completed | I/II | NCT03164772 |
| Not yet recruiting | – | NCT03908671 | |
| Unknown | I/II | NCT02688686 | |
| Ovarian cancer | Recruiting | I | NCT04163094 |
| Unknown | I | NCT01456065 | |
| Melanoma | Completed | I/II | NCT00204607 |
| Completed | I | NCT00978913 | |
| Completed | I/II | NCT00940004 | |
| Completed | I | NCT01066390 | |
| Completed | II | NCT02285413 | |
| Completed | I/II | NCT00204516 | |
| Completed | I/II | NCT01278940 | |
| Completed | I/II | NCT01530698 | |
| Completed | I/II | NCT00243529 | |
| Active, not recruiting | II | NCT03897881 | |
| Active, not recruiting | I | NCT01456104 | |
| Active, not recruiting | I | NCT02410733 | |
| Terminated | I/II | NCT00961844 | |
| Terminated | I | NCT03480152 | |
| Terminated | I/II | NCT00929019 | |
| Brain cancer | Completed | I/II | NCT00846456 |
| Completed | I | NCT00626483 | |
| Completed | II/III | NCT03548571 | |
| Suspended | II | NCT03927222 | |
| Recruiting | I/II | NCT02649582 | |
| Recruiting | II | NCT03688178 | |
| Recruiting | II | NCT02465268 | |
| Unknown | I | NCT02808416 | |
| Unknown | I | NCT02709616 | |
| Active, not recruiting | I | NCT00639639 | |
| Unknown | II | NCT02366728 | |
| Unknown | I/II | NCT01291420 | |
| Completed | I | NCT00890032 | |
| Prostate cancer | Completed | I/II | NCT01278914 |
| Completed | II | NCT01446731 | |
| Completed | II | NCT02692976 | |
| Active, not recruiting | I/II | NCT01197625 | |
| Withdrawn | I/II | NCT01153113 | |
| Terminated | II | NCT02140138 | |
| Unknown | I/II | NCT02452307 | |
| Blood cancer (leukemia) | Completed | I | NCT00834002 |
| Completed | I/II | NCT01734304 | |
| Completed | II | NCT00510133 | |
| Completed | I/II | NCT02528682 | |
| Recruiting | II | NCT01686334 | |
| Active, not recruiting | I | NCT01995708 | |
| Active, not recruiting | I/II | NCT03083054 | |
| Terminated | I | NCT00514189 | |
| Unknown | NCT00965224 | ||
| Cancer related to digestive tract | Completed | II | NCT00228189 |
| Unknown | – | NCT03468244 | |
| Unknown | I/II | NCT02693236 |
However, to further establish itself more, the technology must address sustainability and costly manufacturing processes in large scale. Compared with conventional established vaccines, the IVT reaction of mRNA is much safer and fast, but the use of expensive and limited materials made the scientists to research the manufacturing process. Furthermore, the downstream processing of the vaccine needs improvement to make it easy for scalability. In order to maintain integrity and develop deep roots, the technology needs to address some key issues such as the design of mRNA sequence in the first place. The IVT reaction is acceptable for small scale production however, major efforts are required to enhance the production in larger scale especially when the demand is of universal need. Such implementation of advanced research technologies are required to maintain less amount mRNA needed for anticipated therapeutic effect while maintaining safety profile. Furthermore, next-generation delivery technology are required to ensure safety as well as increase long-term stability of the final product within a more reasonable and feasible temperature range. Finally, proper regulatory guidelines shall be implemented for studying the safety and efficacy of novel carriers of mRNA vaccines.
Authorship contribution statement
Abid Hussain: Conceptualization, Data curation, Investigation, Software, Methodology, Formal analysis, Writing - original draft, Writing-revise...editing. Haiyin Yang: Formal analysis, Investigation. Mengjie Zhang: Formal analysis, Investigation. Qing Liu: Formal analysis, Investigation, Conceptualization, Methodology. Ghallab Alotaibi: Formal analysis, Investigation. Muhammad Irfan: Formal analysis, Investigation. Huining He: Formal analysis, Investigation. Jin Chang: Formal Analysis, Investigation. Xing-Jie Liang: Formal Analysis, Supervision, Investigation . Yuhua Weng: Formal analysis, Investigation, Conceptulization, Methodology. Yuanyu Huang: Conceptualization, Methodology, Formal analysis, Data curation, Writing - review ... editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by the Beijing-Tianjin-Hebei Basic Research Cooperation Project (19JCZDJC64100), the Beijing Nova Program from Beijing Municipal Science & Technology Commission (Z201100006820005), the National Key R&D Program of China (2021YFE0106900), the National Natural Science Foundation of China (31871003, 31901053, 32001008, 32171394), the Young Elite Scientist Sponsorship Program of Beijing Association for Science and Technology (2020-2022), the Natural Science Foundation of Guangdong Province (2019A1515010776), and the Postdoctoral Science Foundation of China (2020M670169). They thank Biological & Medical Engineering Core Facilities (Beijing Institute of Technology) for providing advanced equipment.
References
- 1.Contreras S., Dehning J., Loidolt M., Zierenberg J., Spitzner F.P., Urrea-Quintero J.H., Mohr S.B., Wilczek M., Wibral M., Priesemann V. The challenges of containing SARS-CoV-2 via test-trace-and-isolate. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-020-20699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girum T., Lentiro K., Geremew M., Migora B., Shewamare S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop. Med. Health. 2020;48:1–15. doi: 10.1186/s41182-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kucharski A.J., Klepac P., Conlan A.J., Kissler S.M., Tang M.L., Fry H., Gog J.R., Edmunds W.J., Emery J.C., Medley G. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect. Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedrosa A.L., Bitencourt L., Fróes A.C.F., Cazumbá M.L.B., Campos R.G.B., de Brito S.B.C.S., e Silva A.C.S. Emotional, behavioral, and psychological impact of the COVID-19 pandemic. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.566212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry C.R., Fowler A., Glazer T., Handel-Meyer S., MacMillen A. Evaluating the effects of shelter-in-place policies during the COVID-19 pandemic. Proc. Natl. Acad. Sci. 2021;118 doi: 10.1073/pnas.2019706118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aristodemou K., Buchhass L., Claringbould D. The COVID-19 crisis in the EU: the resilience of healthcare systems, government responses and their socio-economic effects. Eur. Econ. Rev. 2021:1–31. [Google Scholar]
- 7.Mallapaty S. India’s massive COVID surge puzzles scientists. Nature. 2021;592:667–668. doi: 10.1038/d41586-021-01059-y. [DOI] [PubMed] [Google Scholar]
- 8.Wilder Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect. Dis. 2020;20:102–107. doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes E.A., O’Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Silver R.C., Everall I., Ford Tamsin, John Ann, Kabir Thomas, King Kate, Madan Ira, Michie Susan, Przybylski Andrew K., Shafran Roz, Sweeney Angela, Worthman Carol M., Yardley Lucy, Cowan Katherine, Cope Claire, Hotopf Matthew, Bullmore Ed. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfefferbaum B., North C.S. Mental health and the Covid-19 pandemic. N. Engl. J. Med. 2020;383:510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 11.Dubey S., Biswas P., Ghosh R., Chatterjee S., Dubey M.J., Chatterjee S., Lahiri D., Lavie C.J. Psychosocial impact of COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Z.Q., Ma J., Hao Y.N., Shen X.L., Liu F., Gao Y., Zhang L. The social psychological impact of the COVID-19 pandemic on medical staff in China: a cross-sectional study. Eur. Psychiatry. 2020;63 doi: 10.1192/j.eurpsy.2020.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nande A., Adlam B., Sheen J., Levy M.Z., Hill A.L. Dynamics of COVID-19 under social distancing measures are driven by transmission network structure. PLoS Comput. Biol. 2021;17:1008684. doi: 10.1371/journal.pcbi.1008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19 doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 16.Li F., Li Y.Y., Liu M.J., Fang L.Q., Dean N.E., Wong G.W., Yang X.B., Longini I., Halloran M.E., Wang H.J., Liu Pu Lin, Pang Yan Hui, Qiong Y. Ya, Liu Su, Xia Wei, Lu Xiao Xia, Liu Qi, Yang Yang, Shun Q.X. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect. Dis. 2021;21:617–628. doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R.P. Niu Faxian, Zhan Xuejun, Ma Dayan, Wang Wenbo, Xu Guizhen, Wu George, Gao Wenjie, Tan, a novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382(2020):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Hui Dong C., Jing C., Yun L., Hua G., Di J. Ren, Mei Qin L., Ying C., Rui S. Xu, Xi W., Shuang Xiao, Zheng Z. Kai, Jiao C. Quan, Fei D., Lin L. Lin, Bing Y., Xian Z. Fa, Yi W. Yan, Fu X. Geng, Li S. Zheng. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forni G., Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., Zhengshan C., Yingying G., Jinlong Z., Yaning L., Xiaohong S., Yi C., Lu X., Ling F., Lihua H., Junjie X., Changming Y., Jianmin L., Qiang Z., Wei C. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., Ge G., Xue H., Yanan Z., Zhou T., Weijin H., William Jun L., Guizhen W., Bo Z., Lan W., Jianxun Q., Hui F., Fu Sheng W., Qihui W., George Fu G., Zhiming Y., Jinghua Y. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 22.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Chinese researchers reveal draft genome of virus implicated in Wuhan pneumonia outbreak. Science. 2020;11 [Google Scholar]
- 24.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: What we know so far. Pathogens. 2020;9:231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S.K., Haigh B.J., Griffin F.J., Wheeler T.T. The mammalian secreted RNases: mechanisms of action in host defence. Innate Immun. 2013;19:86–97. doi: 10.1177/1753425912446955. [DOI] [PubMed] [Google Scholar]
- 28.Weber S.C., Brangwynne C.P. Getting RNA and protein in phase. Cell. 2012;149:1188–1191. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Denison C., Kodadek T. Small-molecule-based strategies for controlling gene expression. Chem. Biol. 1998;5:129–145. doi: 10.1016/s1074-5521(98)90167-3. [DOI] [PubMed] [Google Scholar]
- 30.Clayton C. Regulation of gene expression in trypanosomatids: living with polycistronic transcription. Open Biol. 2019;9 doi: 10.1098/rsob.190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buccitelli C., Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020;21:630–644. doi: 10.1038/s41576-020-0258-4. [DOI] [PubMed] [Google Scholar]
- 32.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J., Thiago O., Zhi Y., Morgan A., Kathryn T., Arlene H., Martina T., Kamille W., Kristie G., Katrina M., Victor R., Justin Da S., Jianliang X., Robert C., Roshni P., Juan D., Cecille O.B., Irina S., Anna G., Marina C., Pamela B., Rafael C., Theodora H., Paul B., N. Michel, mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat. Rev. Immunol. 2021:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 36.Bettini E., Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines. 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie M. Vol. 368. 2020. T cells found in coronavirus patients ‘bode well’for long-term immunity; pp. 809–810. [DOI] [PubMed] [Google Scholar]
- 38.Hussain A., Hasan A., Babadaei M.M.N., Bloukh S.H., Chowdhury M.E., Sharifi M., Haghighat S., Falahati M. Targeting SARS-CoV2 spike protein receptor binding domain by therapeutic antibodies. Biomed. Pharmacother. 2020;110559 doi: 10.1016/j.biopha.2020.110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andres C., Garcia C.D., Gregori J., Piñana M., Rodriguez F.F., Guerrero M.M., Esperalba J., Rando A., Goterris L., Codina M.G., Susanna Q., Maria C.M., Magda C., Ricard F., Benito A.J., Ignacio Esteban P., Tomas A., Andres Q. Josep. Naturally occurring SARS-CoV-2 gene deletions close to the spike S1/S2 cleavage site in the viral quasispecies of COVID19 patients. Emerg. Microb. Infect. 2020;9:1900–1911. doi: 10.1080/22221751.2020.1806735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Andrea P., Adrian M. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbasi J. COVID-19 and mRNA vaccines—first large test for a new approach. Jama. 2020;324:1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- 42.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.C.S.G. of the International The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irfan M., Shahid A.L., Ahmad M., Iqbal W., Elavarasan R.M., Ren S., Hussain A. Assessment of public intention to get vaccination against COVID-19: evidence from a developing country. J. Eval. Clin. Pract. 2021;28:63–73. doi: 10.1111/jep.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Gayle A.A., Wilder-Smith A., Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020;54:159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng W., Liu G., Ma H., Zhao D., Yang Y., Liu M., Mohammed A., Zhao C., Yang Y., Xie J., Chengchao D., Xiaoling M., Jianping W., Yong G., Hongliang H., Tengchuan J. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem. Biophys. Res. Commun. 2020;527:618–623. doi: 10.1016/j.bbrc.2020.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Yuhai B., Xuejun M., Faxian Z., Liang W., Tao H., Hong Z., Zhenhong H., Weimin Z., Li Z., Jing C., Yao M., Ji W., Yang L., Jianying Y., Zhihao X., Jinmin M., William J.L., Dayan W., Wenbo X., Edward C.H., George F.G., Guizhen W., Weijun C., Weifeng S., Wenjie T. Genomic characterisation and epidemiology of 2019 Novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z., Jie C., Jian L. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020;7:1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Kathryn M.H., Matthew D.P.D., Partridge G., Cariad M.E., Timothy M.F., Thushan D.S., Charlene M., Lautaro G.P., Haili T., Alex M., Sean P.W., Celia C.L., Erica O.S., David C.M. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarghampoor F., Azarpira N., Khatami S.R., Behzad-Behbahani A., Foroughmand A.M. Improved translation efficiency of therapeutic mRNA. Gene. 2019;707:231–238. doi: 10.1016/j.gene.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Alexander E.G., Susan C.B., Ralph S.B., Raoul J.G., Christian D., Anastasia A.G., Bart L.H., Chris L., Andrey M.L., Benjamin W.N., Dmitry P., Stanley P., Leo M.P., Dmitry V.S., Igor A.S., Isabel S., John Z., Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.GISAID . 2022. Genomic Epidemiology of hCoV-19, in. [Google Scholar]
- 55.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharya M., Sharma A.R., Ghosh P., Patra P., Patra B.C., Lee S.S., Chakraborty C. Bioengineering of novel non-replicating mRNA (NRM) and self-amplifying mRNA (SAM) vaccine candidates against SARS-CoV-2 using immunoinformatics approach. Mol. Biotechnol. 2022:1–16. doi: 10.1007/s12033-021-00432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q., Yan Y., Changsheng Z., Hong S., Shoudeng C. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020;10:1228–1238. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao X., Qin B., Chen P., Zhu K., Hou P., Wojdyla J.A., Wang M., Cui S. Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharm. Sin. B. 2021;11:237–245. doi: 10.1016/j.apsb.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16:3000003. doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanjuán R., Domingo-Calap P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016;73:4433–4448. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta R.K. Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat. Rev. Immunol. 2021;21:340–341. doi: 10.1038/s41577-021-00556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.W.H. Organization . 2021. COVID-19 Weekly Epidemiological Update, Edition 45, 22 June 2021. [Google Scholar]
- 65.Muik A., Wallisch A.-K., Sanger B., Swanson K.A., Muhl J., Chen W., Cai H., Maurus D., Sarkar R., Tureci O., Philip R.D., Ugur S. Neutralization of SARS-CoV-2 lineage B. 1.1. 7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371:1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Barney S.G., John R.M., Jennifer Y.C., Michael T.Y., Magdalena S., Christos A.K., Lawrence S., Zizhang S., Yaoxing H., David D.H. Antibody resistance of SARS-CoV-2 variants B. 1.351 and B. 1.1. 7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 67.Tanne J.H. Covid-19: Moderna plans booster doses to counter variants. bmj. 2021;372:232. doi: 10.1136/bmj.n232. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y., Liu J., Xia H., Zhang X., Fontes G.C.R., Swanson K.A., Cai H., Sarkar R., Chen W., Cutler M., David C., Scott C.W., Alexander M., Ugur S., Kathrin U.J., Xuping X., Philip R.D., Pei Y.S. Neutralizing activity of BNT162b2-elicited serum. N. Engl. J. Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann M., Arora P., Grob R., Seidel A., Hörnich B.F., Hahn A.S., Kruger N., Graichen L., Hofmann-Winkler H., Kempf A., Martin S.W., Sebastian S., Hans M.J., Bernd J., Hubert S., Martin M., Alexander K., Jan M., Stefan P. SARS-CoV-2 variants B. 1.351 and P. 1 escape from neutralizing antibodies. Cell. 2021;184:2384–2393. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doshi P. Covid-19 vaccines: in the rush for regulatory approval, do we need more data? bmj. 2021;373:1244. doi: 10.1136/bmj.n1244. [DOI] [PubMed] [Google Scholar]
- 71.AbuRaddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 vaccine against the B. 1.1. 7 and B. 1.351 variants. N. Engl. J. Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.W.H. Organization . 2021. Weekly Epidemiological Update on COVID-19-1 June 2021, in. [Google Scholar]
- 73.Ives A.R., Bozzuto C. Estimating and explaining the spread of COVID-19 at the county level in the USA. Commun. Biol. 2021;4:1–9. doi: 10.1038/s42003-020-01609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Padma T. India's COVID-vaccine woes–by the numbers. Nature. 2021;592:500–501. doi: 10.1038/d41586-021-00996-y. [DOI] [PubMed] [Google Scholar]
- 75.Gao S.J., Guo H., Luo G. Wiley Online Library; 2021. Omicron variant (B. 1.1. 529) of SARS-CoV-2, a global urgent public health alert!, in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torjesen I. Covid-19: omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:2943. doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Beltran W.F., Denis K.J.S., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., Jared F., David J.G., Mark C.P., Aaron G.S., John I., Vivek N., Alejandro B.B. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell. 2022;12:21267755. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2020;296 doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X., Wu Y., Chen C., Gu Y., Zhu C., Wang S., Chen J., Zhang L., Lv L., Zhang G., Yongfang Y., Yifeng C., Mingshe Z., Caisheng W. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm. Sin. B. 2021;11:222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bian J., Li Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm. Sin. B. 2020;11:1–12. doi: 10.1016/j.apsb.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashimoto R., Sakamoto A., Deguchi S., Yi R., Sano E., Hotta A., Takahashi K., Yamanaka S., Takayama K. Dual inhibition of TMPRSS2 and Cathepsin B prevents SARS-CoV-2 infection in iPS cells. Mol. Therapy-Nucleic Acids. 2021;26:1107–1114. doi: 10.1016/j.omtn.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Bryan E.T., Charles A.G., Zhang Y., Jonathan L.T., Gregory J.G., Phillip L.B., Ryan N.P., Aaron F.G., Logan L., Jared F., Xin Y., Yuan P., Blake M.H., Timothy M.C., Benjamin P.K., Cameron M., Philip L.S.M.G., Sumit K.C., Aaron G.S., Kamil G., Sandra L.L., Joyce J., Kevin D.C., Andrew B.W., Aaron F.C., Jeffrey D.E. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziegler C.G., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Jared F., Christoph M., Marc H.W., Samuel W.K., Travis K.H., Benjamin D., James G., Marko V., Faith T., Benjamin E.M., Zhiru G., Jennifer P.W., Delphine G., Magali P., Meshal A., Ilias A., Heiko A., Jennifer M.S.S., Chase J.T., Brian L., Avinash W., Vanessa M., Daniel F.D., Kathleen M.B., Joshua A.B., Nora A.B., Tanya M.L., Shaina L.C., Lucrezia C., Victor T., Christopher W.P., Alison Y., Hengqi B., Zheng H.P.G., Caylin G.W., Philana L.L., Colin D.B., Scott B.S., Jonathan A.K., Fabian J.T., Herbert B.S., LaureEmmanuelle Z., Pascal B.A.L., Hans P.K., JoAnne L.F., Sarah M.F., Bonnie B., Robert W.F., Leslie S.K., Manuel G., Aaron G.S., Daniel L., Alex K.S., Jose O.M. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schlake T., Thess A., Fotin M.M., Kallen K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9:1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iavarone C., O’hagan D.T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of mRNA-based vaccines. Exp. Rev. Vacc. 2017;16:871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 88.Pardi N., Weissman D. RNA Vaccines. Springer, Methods in Molecular Biology; 2017. Nucleoside modified mRNA vaccines for infectious diseases; pp. 109–121. [DOI] [PubMed] [Google Scholar]
- 89.Midoux P., Pichon C. Lipid-based mRNA vaccine delivery systems. Exp. Rev. Vacc. 2015;14:221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 90.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:1–5. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; 2022. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 92.Agrawal S., Goel A.D., Gupta N. Emerging prophylaxis strategies against COVID-19. Monaldi Arch. Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1289. [DOI] [PubMed] [Google Scholar]
- 93.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:1–17. [Google Scholar]
- 94.Xiong Q., Lee G.Y., Ding J., Li W., Shi J. Biomedical applications of mRNA nanomedicine. Nano Res. 2018;11:5281–5309. doi: 10.1007/s12274-018-2146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li B., Zhang X., Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip. Rev. 2019;11:1530. doi: 10.1002/wnan.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo P. The emerging field of RNA nanotechnology. Nat. Nanotechnol. 2010;5:833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 99.Versteeg L., Almutairi M.M., Hotez P.J., Pollet J. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines. 2019;7:122. doi: 10.3390/vaccines7040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stitz L., Vogel A., Schnee M., Voss D., Rauch S., Mutzke T., Ketterer T., Kramps T., Petsch B. A thermostable messenger RNA based vaccine against rabies. PLoS Negl. Trop. Dis. 2017;11:0006108. doi: 10.1371/journal.pntd.0006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chakraborty C., Sharma A.R., Bhattacharya M., Lee S.S. From COVID-19 to cancer mRNA vaccines: moving from bench to clinic in the vaccine landscape. Front. Immunol. 2021;12:2648. doi: 10.3389/fimmu.2021.679344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J., Chang J., Jiang Y., Meng X., Sun T., Mao L., Xu Q., Wang M. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv. Mater. 2019;31:1902575. doi: 10.1002/adma.201902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lokugamage M.P., Gan Z., Zurla C., Levin J., Islam F.Z., Kalathoor S., Sato M., Sago C.D., Santangelo P.J., Dahlman J.E. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv. Mater. 2020;32:1904905. doi: 10.1002/adma.201904905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Nicole F.S. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 106.Tenchov R., Bird R., Curtze A.E., Zhou Q. Lipid nanoparticles— from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 107.Ramishetti S., Hazan H.I., Palakuri R., Chatterjee S., Naidu Gonna S., Dammes N., Freilich I., Kolik Shmuel L., Danino D., Peer D. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv. Mater. 2020;32:1906128. doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 108.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv. Drug Deliv. Rev. 2020;154:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 109.Lou G., Anderluzzi G., Schmidt S.T., Woods S., Gallorini S., Brazzoli M., Giusti F., Ferlenghi I., Johnson R.N., Roberts C.W., Derek T.O.H., Barbara C.B., Yvonne P. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: the impact of cationic lipid selection. J. Control. Release. 2020;325:370–379. doi: 10.1016/j.jconrel.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 110.Jasinski D., Haque F., Binzel D.W., Guo P. Advancement of the emerging field of RNA nanotechnology. ACS Nano. 2017;11:1142–1164. doi: 10.1021/acsnano.6b05737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 112.Felgner P.L., Ringold G. Cationic liposome-mediated transfection. Nature. 1989;337:387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 113.Li B., Luo X., Deng B., Wang J., McComb D.W., Shi Y., Gaensler K.M., Tan X., Dunn A.L., Kerlin B.A., Yizhou D. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 2015;15:8099–8107. doi: 10.1021/acs.nanolett.5b03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hajj K.A., Melamed J.R., Chaudhary N., Lamson N.G., Ball R.L., Yerneni S.S., Whitehead K.A. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 2020;20:5167–5175. doi: 10.1021/acs.nanolett.0c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang G., Guo B., Wu H., Tang T., Zhang B.-T., Zheng L., He Y., Yang Z., Pan X., Chow H., Kinwah T., Yaping L., Dahu L., Xinluan W., Yixiang W., Kwongman L., Zhibo H., Nan D., Gang L., Kwoksui L., Leungkim H., Fuchu H., Lingqiang Z., Ling Q. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012;18:307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 116.Li C., Yang T., Weng Y., Zhang M., Zhao D., Guo S., Hu B., Shao W., Wang X., Hussain A., Xing-Jie L., Yuanyu H. Ionizable lipid-assisted efficient hepatic delivery of gene editing elements for oncotherapy. Bioactive Mater. 2021;9:590–601. doi: 10.1016/j.bioactmat.2021.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li C., Zhou J., Wu Y., Dong Y., Du L., Yang T., Wang Y., Guo S., Zhang M., Hussain A., Haihua X., Yuhua W., Yong H., Xiaoxia W., Zicai L., Huiqing C., Yongxiang Z., Xing-Jie L., Anjie D., Yuanyu H. Core role of hydrophobic core of polymeric nanomicelle in endosomal escape of siRNA. Nano Lett. 2021;21:3680–3689. doi: 10.1021/acs.nanolett.0c04468. [DOI] [PubMed] [Google Scholar]
- 118.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li K., Zhang Y., Hussain A., Weng Y., Huang Y. Progress of photodynamic and RNAi combination therapy in cancer treatment. ACS Biomater. Sci. Eng. 2021;7:4420–4429. doi: 10.1021/acsbiomaterials.1c00765. [DOI] [PubMed] [Google Scholar]
- 120.Ho W., Gao M., Li F., Li Z., Zhang X.Q., Xu X. Next generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv. Healthcare Mater. 2021;10:2001812. doi: 10.1002/adhm.202001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dong Y., Dorkin J.R., Wang W., Chang P.H., Webber M.J., Tang B.C., Yang J., Abutbul-Ionita I., Danino D., DeRosa F., Michael H., Robert L., Daniel G.A. Poly (glycoamidoamine) brushes formulated nanomaterials for systemic siRNA and mRNA delivery in vivo. Nano Lett. 2016;16:842–848. doi: 10.1021/acs.nanolett.5b02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patel A.K., Kaczmarek J.C., Bose S., Kauffman K.J., Mir F., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31:1805116. doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lei C., Cui Y., Zheng L., Chow P.K.H., Wang C.H. Development of a gene/drug dual delivery system for brain tumor therapy: potent inhibition via RNA interference and synergistic effects. Biomaterials. 2013;34:7483–7494. doi: 10.1016/j.biomaterials.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 125.Vaidyanathan S., Orr B.G., Banaszak Holl M.M. Role of cell membrane–vector interactions in successful gene delivery. Acc. Chem. Res. 2016;49:1486–1493. doi: 10.1021/acs.accounts.6b00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stefan J., Kus K., Wisniewska A., Lorkowska-Zawicka B., Kaminski K., Szczubialka K., Nowakowska M., Korbut R. The antiatherogenic effect of new biocompatible cationically modified polysaccharides: chitosan and pullulan-the comparison study. J. Physiol. Pharmacol. 2018;69:26402. doi: 10.26402/jpp.2018.6.15. [DOI] [PubMed] [Google Scholar]
- 127.Ullah A., Chen G., Hussain A., Khan H., Abbas A., Zhou Z., Shafiq M., Ahmad S., Ali U., Usman M., Faisal R., Abrar A., Zijie Q., Maochao Z., Daojun L. Cyclam-modified polyethyleneimine for simultaneous TGFβ siRNA delivery and CXCR4 inhibition for the treatment of CCl4-induced liver fibrosis. Int. J. Nanomedicine. 2021;16:4451–4470. doi: 10.2147/IJN.S314367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Démoulins T., Milona P., Englezou P.C., Ebensen T., Schulze K., Suter R., Pichon C., Midoux P., Guzmán C.A., Ruggli N., Kenneth C.M. Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine. 2016;12:711–722. doi: 10.1016/j.nano.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 129.Guo P. RNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapy. J. Nanosci. Nanotechnol. 2005;5:1964–1982. doi: 10.1166/jnn.2005.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kannan S., Kolhe P., Raykova V., Glibatec M., Kannan R.M., Lieh-Lai M., Bassett D. Dynamics of cellular entry and drug delivery by dendritic polymers into human lung epithelial carcinoma cells. J. Biomater. Sci. Polym. Ed. 2004;15:311–330. doi: 10.1163/156856204322977201. [DOI] [PubMed] [Google Scholar]
- 131.Qiu Y., Man R.C., Liao Q., Kung K.L., Chow M.Y., Lam J.K. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 132.Udhayakumar V.K., De Beuckelaer A., McCaffrey J., McCrudden C.M., Kirschman J.L., Vanover D., Van Hoecke L., Roose K., Deswarte K., De Geest B.G., Stefan L., Philip J.S., Johan G., Helen O.M., Stefaan D.K. Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic T cell immunity dependent on the amphipathic organization of the peptide. Adv. Healthcare Mater. 2017;6:1601412. doi: 10.1002/adhm.201601412. [DOI] [PubMed] [Google Scholar]
- 133.Scheel B., Teufel R., Probst J., Carralot J.P., Geginat J., Radsak M., Jarrossay D., Wagner H., Jung G., Rammensee H.G., Ingmar H., Steve P. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur. J. Immunol. 2005;35:1557–1566. doi: 10.1002/eji.200425656. [DOI] [PubMed] [Google Scholar]
- 134.Scheel B., Aulwurm S., Probst J., Stitz L., Hoerr I., Rammensee H.G., Weller M., Pascolo S. Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur. J. Immunol. 2006;36:2807–2816. doi: 10.1002/eji.200635910. [DOI] [PubMed] [Google Scholar]
- 135.Feyerabend S., Stevanovic S., Gouttefangeas C., Wernet D., Hennenlotter J., Bedke J., Dietz K., Pascolo S., Kuczyk M., Rammensee H.G., Arnulf S. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate. 2009;69:917–927. doi: 10.1002/pros.20941. [DOI] [PubMed] [Google Scholar]
- 136.Westdorp H., Creemers J.H., van Oort I.M., Schreibelt G., Gorris M.A., Mehra N., Simons M., de Goede A.L., van Rossum M.M., Croockewit A.J., Carl G.F.A.W., Erik H.J.G.A., Roel D.M.M., Mareke B., Katja P., Martin G., Jelle O.B., Jolanda M.D.V., Winald R.G. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J. Immunotherapy Cancer. 2019;7:1–15. doi: 10.1186/s40425-019-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pollard C., De Koker S., Saelens X., Vanham G., Grooten J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol. Med. 2013;19:705–713. doi: 10.1016/j.molmed.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 138.Lou B., De Koker S., Lau C.Y.J., Hennink W.E., Mastrobattista E. mRNA polyplexes with post-conjugated GALA peptides efficiently target, transfect, and activate antigen presenting cells. Bioconjug. Chem. 2018;30:461–475. doi: 10.1021/acs.bioconjchem.8b00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li W., Ma L., Guo L.-P., Wang X.-L., Zhang J.-W., Bu Z.-G., Hua R.-H. West Nile virus infectious replicon particles generated using a packaging-restricted cell line is a safe reporter system. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-03670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Erasmus J.H., Khandhar A.P., O’Connor M.A., Walls A.C., Hemann E.A., Murapa P., Archer J., Leventhal S., Fuller J.T., Lewis T.B., Kevin E.D., Samantha R., Kathryn A.G., Malcolm S.D., Darrick C., Steven G.R., David W.H., Heinz F., Michael G.J., David V., Peter B., Deborah H.F. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020;12:11–26. doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bogers W.M., Oostermeijer H., Mooij P., Koopman G., Verschoor E.J., Davis D., Ulmer J.B., Brito L.A., Cu Y., Banerjee K., Gillis R.O., Brian B., Antu D., Jonathan L.H., Xiaoying S., Georgia D.T., Celia L., David C.M., HuaXin L., Barton H., Andrew J.G., Susan W.B. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J. Infect. Dis. 2015;211:947–955. doi: 10.1093/infdis/jiu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fuchs J.D., Frank I., Elizaga M.L., Allen M., Frahm N., Kochar N., Li S., Edupuganti S., Kalams S.A., Tomaras G.D., Rebecca S., Michael P., Marc A.T., Terry J.H., Theresa L., Michael A.E., David K.C., John H.E., Mark M., Nadine R., Scharla E., Kyle R., Deb D., Susan B., Theresa W., Reese I., Victoria C., Jin B., Gina E., Jenny T., Ramey F., Shelly R., Gail B., Liz B., Adi F. Open Forum Infectious Diseases. Oxford University Press; 2015. First-in-human evaluation of the safety and immunogenicity of a recombinant vesicular stomatitis virus human immunodeficiency virus-1 gag vaccine (HVTN 090) p. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Samsa M.M., Dupuy L.C., Beard C.W., Six C.M., Schmaljohn C.S., Mason P.W., Geall A.J., Ulmer J.B., Yu D. Self-amplifying RNA vaccines for Venezuelan equine encephalitis virus induce robust protective immunogenicity in mice. Mol. Ther. 2019;27:850–865. doi: 10.1016/j.ymthe.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Selmi A., Vascotto F., Kautz-Neu K., Türeci Ö., Sahin U., von Stebut E., Diken M., Kreiter S. Uptake of synthetic naked RNA by skin-resident dendritic cells via macropinocytosis allows antigen expression and induction of T-cell responses in mice. Cancer Immunol. Immunother. 2016;65:1075–1083. doi: 10.1007/s00262-016-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brachet J., Huez G., Hubert E. Microinjection of rabbit hemoglobin messenger RNA into amphibian oocytes and embryos. Proc. Natl. Acad. Sci. 1973;70:543–547. doi: 10.1073/pnas.70.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gallie D.R. The cap and poly (A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- 147.Knezevic I., Liu M.A., Peden K., Zhou T., Kang H.N. Development of mRNA vaccines: scientific and regulatory issues. Vaccines. 2021;9:81. doi: 10.3390/vaccines9020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., Kranz L.M., Walzer K.C., Hein S., Güler A., Jakob L., Mohan S.M., Ayuko O.S., Kristin T., Journey C., Bonny G.L., Thomas Z., Arianne P., David E., Sarah C.D., Stephanie F., Stephanie E., Ferdia B., Diana S., Bernadette J., Bianca S., AnnKathrin W., Yvonne F., Hanna J., Stefanie A.K., Andre P.H., Petra A.Q., Julia S., Stefan S., Christoph K., Ramon D.L.C.G.G., Thomas H., Leyla F., Rani S.S., Shambhunath C., Olga G., Fulvia V., Matthew R.G., Jane A.F., Shannan H.-U., Kathleen B., Matthew C.G., Seungil H., Andreas A.H.S., Joshua A.L., Nicole L.N., Ellene H.M., Parag V.S., Charles Y.T., Danka P., Guy S., Camila F.G., Michael P., Ingrid L.S., Tara C., Jennifer O., Michal G., Ricardo C.J., Kendra J.A., Warren V.K., Deepak K., Pei Y.S., Thorsten K., Corinna R., Andreas N.K., Ozlem T., Philip R.D., Kathrin U.J., Ugur S. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 150.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Sebastian B., Verena L., Julian S., Rolf H., Dirk B., Ann K.E., Jan G., Carsten B., Corinna R., Marie C.K., Ulrich L., Alexandra K.B., David L., Martin B., Stefanie B., Katalin K., Tania P., Boris F., Armin S., PeiYong S., Camila F.G., John L.P., Kena A.S., Jakob L., Ingrid L.S., Mark C., Warren K., Christos A.K., David C., Philip R.D., Kathrin U.J., Tureci O. COVID-19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 151.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Kristen E.P., Daniel M., Sebastian B., Verena L., Julian S., Peter K., Rolf H., Dirk B., AnnKathrin E., Jan G., Manuel T., Carsten B., Corinna R., Ludwig H., Marie C.K., Asaf P., Jesse Z.D., Ulrich L., Alexandra K.B., David L., Martin B., Stefanie B., Tania P., Armin S., Sybille B., Azita J.M., Boros G., Jonas R., Gabor T.S., Katalin K., Pei Y.S., Camila F.G., John L.P., Mark C., David C., Christos A.K., Philip R.D., Kathrin U.J., Tureci O. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 152.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Ping L., Kenneth K., Warren K., David C., Camila F.G., Pei Y.S., Tureci O., Kristin R.T., Edward E.W., Robert F., Ann R.F., Philip R.D., William C.G., Sahin U., Kathrin U.J. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 153.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Kena A.S., Ping L., Kenneth K., Warren K., David C., Camila F.G., Pei Y.S., Ozlem T., Kristin R.T., Kirsten E.L., Vanessa R., Philip R.D., Kathrin U.J., Sahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Ruth B., Kena A.S., Satrajit R., Kenneth K., Ping L., Warren V.K., David C., Robert W.F.J., Laura L.H., Ozlem T., Haylene N., Axel S., Serhat U., Dina B.T., Susan M., Philip R.D., Sahin U., Kathrin U.J., William C.G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., Kenneth H.D., Sayda M.E., Christine A.S., Angela W., Ethan J.F., David R.M., Kevin W.B., Mahnaz M., Bianca M.N., Geoffrey B.H., Kai W., Carole H., Kapil B., Dario G.D., LingZhi M., Isabella R., Wing P.K., Stephen D.S., Lingshu W., Yi Z., Emily P., Lauren A.C., Rebecca J.L., Nedim E.A., Elisabeth N., Mihir M., Vlad P., Cuiping L., Mark K.L., Wei S., Kwanyee L., Eun S.Y., Ande W., Kendra L.G., Laura J.S., Nianshuang W., Daniel W., Nicole A.D.R., Guillaume S.J., Hamilton B., Gabriela S.A., Martha C.N., Tracy J.R., Jason S.M., Mark R.D., James D.C., Ian N.M., Kaitlyn M.M., John R.M., Ralph S.B., Andrea C., Barney S.G. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M., Bianca M.N., Hanne A., David R.M., Amy T.N., Naomi D., Mitzi M.D., Nadesh N.N., Gabriela S.A., Darin K.E., Dillon R.F., Evan L., Nicole A.D.R., Bob C.L., Mark K.L., Sijy O.D., Stephen D.S., Emily P., Lauren A.C., Christina Y., John P.M.T., Laurent P., Alex V.R., Shanai B., Jack G., Tammy P.T., Amanda S., Tracey A.C., Anthony C., Alan D., Katelyn S., Wei S., Yi Z., Olubukola M.A., Lingshu W., Amarendra P., Eun S.Y., Kwanyee L., Tongqing Z., Ting T., Alicia W., Ingelise G., Laura N., Rebecca A.G., Rebecca J.L., Juan I.M., Guillaume S.J., Sunny H., Wing P.K., Martha C.N., Kaitlyn M.M., Tracy J.R., Julie E.L., Martin R.G., Peter D.K., John R.M., Andrea C., Mark G.L., Ralph S.B., Adrian M., Ian N.M., Nancy J.S., Mario R., Robert A.S., Barney S.G. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., John M., Shishir K., Nathan S., Joel S., Adam B., Carlos F., Howard S., Kathleen N., Larry C., Peter G., Holly J., Dean F., Mary M., John M., Laura P., Julie L., Barney S.G., Hamilton B., Rolando P., Conor K., Brett L., Weiping D., Honghong Z., Shu H., Melanie I., Jacqueline M., Tal Z. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Julia M.W., Gargano W., Hadler Stephen C., Langley Gayle, Su John R., Oster Matthew E., Broder Karen R., Gee Julianne, Weintraub Eric, Shimabukuro Tom, Scobie Heather M., Moulia Danielle, Markowitz Lauri E., Wharton Melinda, McNally Veronica V., Romero Jose R., Talbot H. Keipp, Lee Grace M., Daley Matthew F., Oliver Sara E. Morbidity and Mortality Weekly Report. 2021. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021, in; pp. 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario: December 13, 2020 to January 16, 2022., in. 2022. [Google Scholar]
- 161.Post-Vaccine Myocarditis in Young People is Rare and Usually Mild, Study Confirms, in. 2021. [Google Scholar]
- 162.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., Hernán M.A., Lipsitch M., Kohane I., Netzer D., Ben Y.R., Ran D.B. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McMurray J.C., May J.W., Cunningham M.W., Jones O.Y. Multisystem inflammatory syndrome in children (MIS-C), a post-viral myocarditis and systemic vasculitis—a critical review of its pathogenesis and treatment. Front. Pediatr. 2020;8:871. doi: 10.3389/fped.2020.626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., Watkinson P., Khunti K., Harnden A., Coupland C.A., Keith M.C., Nicholas L.M., Aziz S., Hippisley C. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2021:1–13. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., Romano S.D., Gundlapalli A.V., Oster M.E., Harris A.M. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morb. Mortal. Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weng Y., Li C., Yang T., Hu B., Zhang M., Guo S., Xiao H., Liang X.J., Huang Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020;40 doi: 10.1016/j.biotechadv.2020.107534. [DOI] [PubMed] [Google Scholar]
- 167.Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., García-Sastre A. Influenza. Nat. Rev. Dis. Prim. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taubenberger J.K., Morens D.M. The 1918 influenza pandemic and its legacy. Cold Spring Harbor Perspect. Med. 2020;10 doi: 10.1101/cshperspect.a038695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 170.Feldman R.A., Fuhr R., Smolenov I., Ribeiro A.M., Panther L., Watson M., Senn J.J., Smith M., Almarsson Ӧ., Pujar H.S., Michael E.L., James T., Tal Z., Giuseppe C. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 171.Schnee M., Vogel A.B., Voss D., Petsch B., Baumhof P., Kramps T., Stitz L. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and Newborn pigs. PLoS Negl. Trop. Dis. 2016;10:0004746. doi: 10.1371/journal.pntd.0004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., Barrat J., Blanton J.D., Briggs D.J., Cleaveland S., Costa P., Freuling C.M., Hiby E., Knopf L., Leanes F., Meslin F.X., Metlin A., Miranda M.E., Müller T., Nel L.H., Recuenco S., Rupprecht C.E., Schumacher C., Taylor L., Vigilato M.A., Zinsstag J., Dushoff J. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9:0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., Fotin-Mleczek M., Hoerr I., Clemens R., von Sonnenburg F. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 174.Aldrich C., Leroux-Roels I., Huang K.B., Bica M.A., Loeliger E., Schoenborn-Kellenberger O., Walz L., Leroux-Roels G., von Sonnenburg F., Oostvogels L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine. 2021;39:1310–1318. doi: 10.1016/j.vaccine.2020.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Armbruster N., Jasny E., Petsch B. Advances in RNA vaccines for preventive indications: a case study of a vaccine against rabies. Vaccines (Basel) 2019;7 doi: 10.3390/vaccines7040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kourtis A.P., Appelgren K., Chevalier M.S., McElroy A. Ebola virus disease: focus on children. Pediatr. Infect. Dis. J. 2015;34:893–897. doi: 10.1097/INF.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matua G.A., Van der Wal D.M., Locsin R.C. Ebolavirus and haemorrhagic syndrome. Sultan Qaboos Univ. Med. J. 2015;15:171–176. [PMC free article] [PubMed] [Google Scholar]
- 178.Agnandji S.T., Huttner A., Zinser M.E., Njuguna P., Dahlke C., Fernandes J.F., Yerly S., Dayer J.A., Kraehling V., Kasonta R., Adegnika A.A., Altfeld M., Auderset F., Bache E.B., Biedenkopf N., Borregaard S., Brosnahan J.S., Burrow R., Combescure C., Desmeules J., Eickmann M., Fehling S.K., Finckh A., Goncalves A.R., Grobusch M.P., Hooper J., Jambrecina A., Kabwende A.L., Kaya G., Kimani D., Lell B., Lemaître B., Lohse A.W., Massinga-Loembe M., Matthey A., Mordmüller B., Nolting A., Ogwang C., Ramharter M., Schmidt-Chanasit J., Schmiedel S., Silvera P., Stahl F.R., Staines H.M., Strecker T., Stubbe H.C., Tsofa B., Zaki S., Fast P., Moorthy V., Kaiser L., Krishna S., Becker S., Kieny M.P., Bejon P., Kremsner P.G., Addo M.M., Siegrist C.A. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N. Engl. J. Med. 2016;374:1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meyer M., Huang E., Yuzhakov O., Ramanathan P., Ciaramella G., Bukreyev A. Modified mRNA-based vaccines elicit robust immune responses and protect Guinea pigs from Ebola virus disease. J. Infect. Dis. 2018;217:451–455. doi: 10.1093/infdis/jix592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fugl A., Andersen C.L. Epstein-Barr virus and its association with disease - a review of relevance to general practice. BMC Fam. Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piedimonte G., Perez M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 2014;35:519–530. doi: 10.1542/pir.35-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aliprantis A.O., Shaw C.A., Griffin P., Farinola N., Railkar R.A., Cao X., Liu W., Sachs J.R., Swenson C.J., Lee H., Cox K.S., Spellman D.S., Winstead C.J., Smolenov I., Lai E., Zaks T., Espeseth A.S., Panther L. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Human Vacc. Immunotherap. 2021;17:1248–1261. doi: 10.1080/21645515.2020.1829899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Noorbakhsh F., Abdolmohammadi K., Fatahi Y., Dalili H., Rasoolinejad M., Rezaei F., Salehi-Vaziri M., Shafiei-Jandaghi N.Z., Gooshki E.S., Zaim M., Nicknam M.H. Zika virus infection, basic and clinical aspects: A review article. Iran. J. Public Health. 2019;48:20–31. [PMC free article] [PubMed] [Google Scholar]
- 184.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., Julander J.G., Tang W.W., Shresta S., Pierson T.C., Ciaramella G., Diamond M.S. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168:1114–1125. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., Dowd K.A., Sutherland L.L., Scearce R.M., Parks R., Wagner W., Granados A., Greenhouse J., Walker M., Willis E., Yu J.S., McGee C.E., Sempowski G.D., Mui B.L., Tam Y.K., Huang Y.J., Vanlandingham D., Holmes V.M., Balachandran H., Sahu S., Lifton M., Higgs S., Hensley S.E., Madden T.D., Hope M.J., Karikó K., Santra S., Graham B.S., Lewis M.G., Pierson T.C., Haynes B.F., Weissman D. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Human Immunodeficiency Virus (HIV) Transfusion medicine and hemotherapy: offizielles organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2016;43:203–222. doi: 10.1159/000445852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Emmer K.L., Wieczorek L., Tuyishime S., Molnar S., Polonis V.R., Ertl H.C. Antibody responses to prime-boost vaccination with an HIV-1 gp145 envelope protein and chimpanzee adenovirus vectors expressing HIV-1 gp140. AIDS (London, England) 2016;30:2405–2414. doi: 10.1097/QAD.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T., Lena M.K., Mustafa D., Sebastian K., Heinrich H., Sebastian A., Richard R., Katarina C., Alexandra K.B., Andrea B., Claudia T., Janina C., Juliane Q., Lisa H., Malte S., Alexander H., Isabel V., Inga L., Stephanie R., Julian S., Melanie L., Verena M., Heidrun M.R., Matthias M., Christoph H., Stephan G., Jochen U., Andreas P., Roland K., Jessica C.H., Carmen L., Tureci O. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 189.Burris H.A., Patel M.R., Cho D.C., Clarke J.M., Gutierrez M., Zaks T.Z., Frederick J., Hopson K., Mody K., Binanti-Berube A., Celine R., Bree G., Ben B., Jing S., Shan Z., Scott P., Karen K., Robert M., Justin G. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019;37:2523. [Google Scholar]
- 190.Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J. Med. Virol. 2021;94:1641–1649. doi: 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- 191.Van Hoecke L., Verbeke R., Dewitte H., Lentacker I., Vermaelen K., Breckpot K., Van Lint S. mRNA in cancer immunotherapy: beyond a source of antigen. Mol. Cancer. 2021;20:1–14. doi: 10.1186/s12943-021-01329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pollard C., Rejman J., De Haes W., Verrier B., Van Gulck E., Naessens T., De Smedt S., Bogaert P., Grooten J., Vanham G., Stefaan D.K. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013;21:251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Michel T., Luft D., Abraham M.-K., Reinhardt S., Medina M.L.S., Kurz J., Schaller M., Avci-Adali M., Schlensak C., Peter K., Hans P.W., Xiaowei W., Stefanie K. Cationic nanoliposomes meet mRNA: efficient delivery of modified mRNA using hemocompatible and stable vectors for therapeutic applications. Mol. Therapy-Nucleic Acids. 2017;8:459–468. doi: 10.1016/j.omtn.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Y., Su H.H., Yang Y., Hu Y., Zhang L., Blancafort P., Huang L. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol. Ther. 2013;21:358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miao L., Li L., Huang Y., Delcassian D., Chahal J., Han J., Shi Y., Sadtler K., Gao W., Lin J., Joshua C.D., Robert L., Daniel G.A. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 196.Rybakova Y., Kowalski P.S., Huang Y., Gonzalez J.T., Heartlein M.W., DeRosa F., Delcassian D., Anderson D.G. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol. Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sedic M., Senn J.J., Lynn A., Laska M., Smith M., Platz S.J., Bolen J., Hoge S., Bulychev A., Jacquinet E., Victoria B., Peter F.S. Safety evaluation of lipid nanoparticle–formulated modified mRNA in the Sprague-dawley rat and cynomolgus monkey. Vet. Pathol. 2018;55:341–354. doi: 10.1177/0300985817738095. [DOI] [PubMed] [Google Scholar]
- 198.Zhao M., Li M., Zhang Z., Gong T., Sun X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 2016;23:2596–2607. doi: 10.3109/10717544.2015.1038856. [DOI] [PubMed] [Google Scholar]
- 199.Sunshine J.C., Sunshine S.B., Bhutto I., Handa J.T., Green J.J. Poly (β-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS One. 2012;7:37543. doi: 10.1371/journal.pone.0037543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Islam M.A., Reesor E.K., Xu Y., Zope H.R., Zetter B.R., Shi J. Biomaterials for mRNA delivery. Biomater. Sci. 2015;3:1519–1533. doi: 10.1039/c5bm00198f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Siewert C., Haas H., Nawroth T., Ziller A., Nogueira S., Schroer M., Blanchet C., Svergun D., Radulescu A., Bates F., Huesemann Y., Radsak M.P., Sahin U., Langguth P. Investigation of charge ratio variation in mRNA–DEAE-dextran polyplex delivery systems. Biomaterials. 2019;192:612–620. doi: 10.1016/j.biomaterials.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 202.van den Brand D., Gorris M.A., van Asbeck A.H., Palmen E., Ebisch I., Dolstra H., Hällbrink M., Massuger L.F., Brock R. Peptide-mediated delivery of therapeutic mRNA in ovarian cancer. Eur. J. Pharm. Biopharm. 2019;141:180–190. doi: 10.1016/j.ejpb.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 203.Lacroix C., Humanes A., Coiffier C., Gigmes D., Verrier B., Trimaille T. Polylactide-based reactive micelles as a robust platform for mRNA delivery. Pharm. Res. 2020;37:1–12. doi: 10.1007/s11095-019-2749-6. [DOI] [PubMed] [Google Scholar]
- 204.Zhao W., Zhang C., Li B., Zhang X., Luo X., Zeng C., Li W., Gao M., Dong Y. Lipid polymer hybrid nanomaterials for mRNA delivery. Cell. Mol. Bioeng. 2018;11:397–406. doi: 10.1007/s12195-018-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kaczmarek J.C., Patel A.K., Kauffman K.J., Fenton O.S., Webber M.J., Heartlein M.W., DeRosa F., Anderson D.G. Polymer–lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew. Chem. 2016;128:14012–14016. doi: 10.1002/anie.201608450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Persano S., Guevara M.L., Li Z., Mai J., Ferrari M., Pompa P.P., Shen H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials. 2017;125:81–89. doi: 10.1016/j.biomaterials.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Su X., Fricke J., Kavanagh D.G., Irvine D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 2011;8:774–787. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Efficacy and Safety of the BNT162b2, mRNA-1273, and ChAdOx1-S COVID-19 Vaccine in Patients who have received allogeneic hematopoietic stem cell transplantation for hematological diseases: An observational study., in. 2021. [Google Scholar]
- 209.National Cohort Study of Effectiveness and Safety of SARS-CoV-2/COVID-19 Vaccines, in. 2021. [Google Scholar]
- 210.A Phase III Clinical Study of a SARS-CoV-2 Messenger Ribonucleic Acid (mRNA) Vaccine Candidate Against COVID-19 in Population Aged 18 Years and Above, in. 2021. [Google Scholar]
- 211.A Study to Determine the Safety and Efficacy of SARS-CoV-2 mRNA Vaccine CVnCoV in Adults for COVID-19, in. 2021. [Google Scholar]
- 212.A Trial Evaluating the Safety and Immunogenicity of 3 COVID-19 SARS-CoV-2 RNA Vaccines in Healthy Adults, in. 2021. [Google Scholar]
- 213.Sanofi and Translate Bio initiate Phase 1/2 clinical trial of mRNA COVID-19 vaccine candidate, in. 2021. [Google Scholar]
- 214.Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O’Dell S., McDanal C., Feng W., Tong J., Eaton A., Maglinao M., Tang H., Manning K.E., Edara V.-V., Lai L., Ellis M., Moore K.M., Floyd K., Foster S.L., Posavad C.M., Atmar R.L., Lyke K.E., Zhou T., Wang L., Zhang Y., Gaudinski M.R., Black W.P., Gordon I., Guech M., Ledgerwood J.E., Misasi J.N., Widge A., Sullivan N.J., Roberts P.C., Beigel J.H., Korber B., Baden L.R., El Sahly H., Chalkias S., Zhou H., Feng J., Girard B., Das R., Aunins A., Edwards D.K., Suthar M.S., Mascola J.R., Montefiori D.C. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Study of DS-5670a (COVID-19 Vaccine) in Japanese Healthy Adults and Elderly Subjects, in. 2021. [Google Scholar]
- 216.Safety and Immunogenicity of EXG-5003, in. 2021. [Google Scholar]
- 217.A Study to Evaluate the Immunogenicity and Safety of mRNA-1283 COVID-19 Vaccine Boosters, in. 2022. [Google Scholar]
- 218.ChulaCov19 Vaccine in Healthy Adults, in. 2021. [Google Scholar]
- 219.PTX-COVID19-B, an mRNA Humoral Vaccine, Intended for Prevention of COVID-19 in a General Population . 2022. This Study is Designed to Demonstrate the Safety, Tolerability, and Immunogenicity of PTX-COVID19-B in Comparison to the Pfizer-BioNTech COVID-19 Vaccine. [Google Scholar]
- 220.Safety And Immunogenicity Of HDT-301 Targeting A SARS-CoV-2 Variant Spike Protein, in. 2022. [Google Scholar]
- 221.Safety and Immunogenicity Study of a SARS-CoV-2 (COVID-19) Variant Vaccine (mRNA-1273.351) in Naïve and Previously Vaccinated Adults, in. 2022. [Google Scholar]
- 222.Safety and Immunogenicity of LNP-nCOV saRNA-02 Vaccine Against SARS-CoV-2, the Causative Agent of COVID-19 (COVAC-Uganda), in. 2021. [Google Scholar]
- 223.A Study of the Safety of and Immune Response to Varying Doses of a Vaccine Against COVID-19 in Healthy Adults, in. 2021. [Google Scholar]
- 224.Dose-Finding Trial to Evaluate the Safety and Immunogenicity of Cytomegalovirus (CMV) Vaccine mRNA-1647 in Healthy Adults, in. 2021. [Google Scholar]
- 225.A Study to Evaluate the Efficacy, Safety, and Immunogenicity of mRNA-1647 Cytomegalovirus (CMV) Vaccine in Healthy Participants 16 to 40 Years of Age, in. 2021. [Google Scholar]
- 226.Safety, Tolerability, and Immunogenicity of Zika Vaccine mRNA-1893 in Healthy Flavivirus Seropositive and Seronegative Adults. 2021. [Google Scholar]
- 227.A Study of Zika Vaccine mRNA-1893 in Adult Participants Living in Endemic and Non-Endemic Flavivirus Areas, in. 2021. [Google Scholar]
- 228.Scarpini S., Morigi F., Betti L., Dondi A., Biagi C., Lanari M. Development of a vaccine against human cytomegalovirus: advances, barriers, and implications for the clinical practice. Vaccines. 2021;9:551. doi: 10.3390/vaccines9060551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jagger B.W., Dowd K.A., Chen R.E., Desai P., Foreman B., Burgomaster K.E., Himansu S., Kong W.-P., Graham B.S., Pierson T.C. Protective efficacy of nucleic acid vaccines against transmission of zika virus during pregnancy in mice. J. Infect. Dis. 2019;220:1577–1588. doi: 10.1093/infdis/jiz338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Safety, Tolerability, and Immunogenicity of mRNA-1325 in Healthy Adult Subjects, in. 2019. [Google Scholar]
- 231.Phase M.A.P.I. 2019. Data for First Combination Vaccine Against the Respiratory Viruses hMPV and PIV3. [Google Scholar]
- 232.A Dose Escalation Study to Evaluate Safety, Reactogenicity, and Immunogenicity of mRNA-1345 in Healthy Adults and in Children Who Are Respiratory Syncytial Virus (RSV)-Seropositive. 2021. [Google Scholar]
- 233.A Study to Evaluate the Safety and Efficacy of mRNA-1345 Vaccine Targeting Respiratory Syncytial Virus (RSV) in Adults ≥60 Years of Age. 2022. [Google Scholar]
- 234.Safety, Tolerability, and Immunogenicity of VAL-181388 in Healthy Subjects, (2020)., in. 2020. [Google Scholar]
- 235.Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of mRNA-1944 in Healthy Adults, in. 2021. [Google Scholar]
- 236.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., Mariola F.M., Ingmar H., Ralf C., Frank V.S. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 237.CureVac Announces Positive Results in Low Dose −1 μg – Rabies Vaccine Clinical Phase 1 Study Curevac reported pipeline. 2020. [Google Scholar]
- 238.A Study to Evaluate the Safety and Immunogenicity of GlaxoSmithKline (GSK) Biologicals’ Experimental Rabies Vaccine in Healthy Adults, in. 2021. [Google Scholar]