Abstract
Background
Tiotropium and long‐acting beta2‐agonists (LABAs) are both accepted in the routine management for people with stable chronic obstructive pulmonary disease (COPD). There are new studies which have compared tiotropium with LABAs, including some that have evaluated recently introduced LABAs.
Objectives
To compare the relative clinical effects of tiotropium bromide alone versus LABA alone, upon measures of quality of life, exacerbations, lung function and serious adverse events, in people with stable COPD.
To critically appraise and summarise current evidence on the costs and cost‐effectiveness associated with tiotropium compared to LABA in people with COPD.
Search methods
We identified randomised controlled trials (RCTs) from the Cochrane Airways Group Specialised Register of trials and economic evaluations from searching NHS EED and HEED (date of last search February 2012). We found additional trials from web‐based clinical trial registers.
Selection criteria
We included RCTs and full economic evaluations if they compared effects of tiotropium alone with LABAs alone in people with COPD. We allowed co‐administration of standard COPD therapy.
Data collection and analysis
Two review authors independently assessed studies for inclusion, then extracted data on study quality and outcomes. We contacted study authors and trial sponsors for additional information. We analysed data using the Cochrane Review Manager(RevMan 5.1) software.
Main results
Seven clinical studies totalling 12,223 participants with COPD were included in the review. The studies used similar designs and were generally of good methodological quality. Inclusion criteria for RCTs were similar across the included studies, although studies varied in terms of smoking history and COPD severity of participants. They compared tiotropium (which was delivered by HandiHaler in all studies) with salmeterol (four studies, 8936 participants), formoterol (one study, 431 participants) and indacaterol (two studies, 2856 participants). All participants were instructed to discontinue anticholinergic or long‐acting beta2‐agonist bronchodilators during treatment, but could receive inhaled corticosteroids (ICS) at a stable dose. Study duration ranged from 3 to 12 months. We extracted data for 11,223 participants. In general, the treatment groups were well matched at baseline. Overall, the risk of bias across the included RCTs was low.
In the analysis of the primary outcomes in this review, a high level of heterogeneity amongst studies meant that we did not pool data for St George's Respiratory Questionnaire quality of life score. Subgroup analyses based on the type of LABA found statistically significant differences among effects on quality of life depending on whether tiotropium was compared with salmeterol, formoterol or indacaterol. Tiotropium reduced the number of participants experiencing one or more exacerbations compared with LABA (odds ratio (OR) 0.86; 95% confidence interval (CI) 0.79 to 0.93). For this outcome, there was no difference seen among the different types of LABA. There was no statistical difference in mortality observed between the treatment groups.
For secondary outcomes, tiotropium was associated with a reduction in the number of COPD exacerbations leading to hospitalisation compared with LABA treatment (OR 0.87; 95% 0.77 to 0.99), but not in the overall rate of all‐cause hospitalisations. There was no statistically significant difference in forced expiratory volume in one second (FEV1) or symptom score between tiotropium and LABA‐treated participants. There was a lower rate of non‐fatal serious adverse events recorded with tiotropium compared with LABA (OR 0.88; 95% CI 0.78 to 0.99). The tiotropium group was also associated with a lower rate of study withdrawals (OR 0.89; 95% CI 0.81 to 0.99).
We identified six full economic evaluations assessing the cost and cost‐effectiveness of tiotropium and salmeterol. The studies were based on an economic model or empirical analysis of clinical data from RCTs. They all looked at maintenance costs and the costs for COPD exacerbations, including respiratory medications and hospitalisations. The setting for the evaluations was primary and secondary care in the UK, Greece, Netherlands, Spain and USA. All the studies estimated tiotropium to be superior to salmeterol based on better clinical outcomes (exacerbations or quality of life) and/or lower total costs. However, the authors of all evaluations reported there was substantial uncertainty around the results.
Authors' conclusions
In people with COPD, the evidence is equivocal as to whether or not tiotropium offers greater benefit than LABAs in improving quality of life; however, this is complicated by differences in effect among the LABA types. Tiotropium was more effective than LABAs as a group in preventing COPD exacerbations and disease‐related hospitalisations, although there were no statistical differences between groups in overall hospitalisation rates or mortality during the study periods. There were fewer serious adverse events and study withdrawals recorded with tiotropium compared with LABAs. Symptom improvement and changes in lung function were similar between the treatment groups. Given the small number of studies to date, with high levels of heterogeneity among them, one approach may be to give a COPD patient a substantial trial of tiotropium, followed by a LABA (or vice versa), then to continue prescribing the long‐acting bronchodilator that the patient prefers. Further studies are needed to compare tiotropium with different LABAs, which are currently ongoing. The available economic evidence indicates that tiotropium may be cost‐effective compared with salmeterol in several specific settings, but there is considerable uncertainty around this finding.
Plain language summary
Tiotropium versus long‐acting beta2‐agonists (LABAs) in the management of COPD
Tiotropium is an inhaled medication that helps open the airways (bronchodilator) and is used to manage persistent symptoms of COPD. We found seven studies including 12,223 participants that compared tiotropium with long‐acting beta2‐agonists (LABAs), which are another type of bronchodilator. This systematic review found that currently there is insufficient evidence to suggest which of these treatments provides greater long‐term benefit in quality of life. Furthermore, both treatments had similar effects on symptoms, lung function and death rates.
Tiotropium appears better than LABAs in preventing COPD exacerbations (worsening of COPD symptoms) and reducing the number of COPD‐related hospitalisations. Furthermore, there were fewer participants during the study period with serious adverse events or who withdrew early from the studies with tiotropium compared with LABA treatment. However, there was no difference in the total number of people who were hospitalised.
We found six economic evaluations looking at the cost and effectiveness of tiotropium and the LABA salmeterol that were conducted in the UK, Greece, Netherlands, Spain, or USA. All the studies estimated tiotropium to be better than salmeterol based on medical outcomes (exacerbations or quality of life) and/or lower total costs, including respiratory medications and hospitalisations. However, these results were very uncertain.
Summary of findings
Summary of findings for the main comparison. Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease.
| Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease | ||||||
|
Patient or population: patients with stable chronic obstructive pulmonary disease and > 10 pack years smoking history
Settings: community
Intervention: tiotropium Comparison: LABA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| LABA | Tiotropium | |||||
|
Quality of life (SGRQ) Follow‐up: 3 to 12 months |
See comment | See comment | Not estimable | 4935 (4 studies) | See comment | The results were not pooled because of substantial heterogeneity between the studies |
|
Patients with 1 or more exacerbations Follow‐up: 3 to 12 months |
29 per 100 | 26 per 100 (25 to 28) | OR 0.86 (0.79 to 0.93) | 12,123 (6 studies) | ⊕⊕⊕⊝ moderate1 | |
| Mortality (all‐cause) Follow‐up: 3 to 12 months | 14 per 1000 | 11 per 1000 (8 to 15) | OR 0.82 (0.60 to 1.13) | 12,123 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
|
Cost‐effectiveness Follow‐up: 1 to 5 years |
See comment | See comment | See comment | (6 economic evaluations) | ⊕⊕⊝⊝ low4,5 | In all 6 studies tiotropium was estimated to be superior to salmeterol based on better clinical outcomes (exacerbations or quality of life), lower total costs6 or both |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LABA: long‐acting beta2‐agonist; OR: odds ratio; SGRQ: St George's Respiratory Questionnaire | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 (‐1) In two out of six studies tiotropium treatment was not blinded. 2 (‐1) There was moderate heterogeneity between the studies (I2 = 51%). 3 (‐1) There were very few events leading to wide confidence intervals.
4 (‐1) There was substantial uncertainty around the results in all of the studies.
5 (‐1) Two studies drew conclusions about the cost‐effectiveness of tiotropium compared to salmeterol through indirect comparisons with placebo.
6 Total costs included maintenance costs and the costs for COPD exacerbations, including respiratory medications and hospitalisations.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterised by chronic and progressive breathlessness, cough, sputum production and airflow obstruction, which leads to restricted activity and poor quality of life (GOLD). The World Health Organization (WHO) has estimated that COPD is the fourth or fifth most common single cause of death worldwide and the treatment and management costs present a significant burden to public health. Furthermore, because of the slow onset and the under‐recognition of the disease, it is heavily under‐diagnosed (GOLD). COPD comprises a combination of bronchitis and emphysema and involves chronic inflammation and structural changes in the lung. Cigarette smoking is the most important risk factor, however air pollution and occupational dust and chemicals are also recognised risk factors. COPD is a progressive disease leading to decreased lung function over time, even with the best available care. There is currently no cure for COPD, though it is both a preventable and treatable disease. As yet, apart from smoking cessation and non‐pharmacological treatments such as long‐term oxygen therapy in hypoxic patients, no intervention has been shown to reduce mortality (GOLD). Management of the disease is multi‐faceted and includes reducing risk factors (van der Meer 2001), pharmacological treatments (GOLD; NICE), education (Effing 2007) and pulmonary rehabilitation (Lacasse 2006). Pharmacological therapy is aimed at relieving symptoms, improving exercise tolerance and quality of life, slowing decline and even improving lung function, or preventing and treating exacerbations. COPD exacerbations impair patients' quality of life (GOLD; NICE). Furthermore, a large part of the economic burden of COPD is attributed to the cost of managing exacerbations, particularly those resulting in use of acute care services or hospitalisations (Hutchinson 2010). Appropriate pharmacological management of the disease is therefore important to reduce and prevent exacerbations.
Description of the intervention
COPD pharmacological management tends to begin with one treatment, with additional therapies introduced as necessary to control symptoms (GOLD). The first step is often a short‐acting bronchodilator for control of breathlessness when needed: either a short‐acting beta2‐agonist (SABA) or the short‐acting anticholinergic, ipratropium. For persistent or worsening breathlessness associated with lung function decline long‐acting bronchodilators may be introduced (GOLD). Long‐acting bronchodilators include long‐acting beta2‐agonists (LABA), such as salmeterol, formoterol or indacaterol; and the long‐acting anticholinergic agent tiotropium. Regular treatment with long‐acting bronchodilators may be more efficient and convenient than treatment with regular short‐acting bronchodilators (Beeh 2010). However, the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guidelines do not specify a preference between the two drug classes. For symptomatic patients with severe or very severe COPD (forced expiratory volume in one second (FEV1) < 50% predicted) and with repeated exacerbations, GOLD recommends the addition of inhaled corticosteroids (ICS) to bronchodilator treatment.
How the intervention might work
Tiotropium
Tiotropium bromide (tiotropium) is an anticholinergic agent which blocks the action of the neurotransmitter acetylcholine. It has an antagonistic effect on muscarinic acetylcholine receptors. Tiotropium has similar affinity for the five different subtypes of muscarinic receptors (M1‐M5), however airway smooth muscle expresses only the M2 and M3 subtypes (Proskocil 2005). Activation of the M3 receptor stimulates a number of intracellular signalling cascades, leading to changes in intracellular Ca2+ homeostasis and contraction. Tiotropium dissociates slowly from M3 receptors giving a bronchodilator effect lasting over 24 hours, but dissociates rapidly from M2 receptors, which appear to be feedback inhibitory receptors (Barr 2005).
Tiotropium has gained widespread acceptance as a once‐daily maintenance therapy in stable COPD for its effects on symptoms and exacerbations (GOLD). In a recent Cochrane review (Karner 2012a), tiotropium was shown to improve quality of life (mean difference (MD) ‐3.19; 95% confidence interval (CI) ‐3.74 to ‐2.64) and COPD exacerbations compared to placebo (odds ratio (OR) 0.78; 95% CI 0.70 to 0.87). Tiotropium was also associated with a significant benefit over placebo in lung function and a reduction in exacerbations requiring hospitalisation (Karner 2012a). Anticholinergic side effects that may occur with tiotropium include dry mouth, constipation and tachycardia (Tashkin 2008). Different devices are available for tiotropium and these may have different efficacy and associated risks (Boehringer Ingelheim 2010).
Long‐acting beta2‐agonists
Inhaled beta2‐agonists activate beta2‐receptors in the smooth muscle of the airway leading to a cascade of reactions resulting in bronchodilation. Beta2‐agonists may also act through other mechanisms such as respiratory muscle function or mucociliary clearance, because patients have shown improvement in symptoms whilst showing no improvement in lung function tests. Beta2‐agonists are particularly useful bronchodilators because they reverse bronchoconstriction regardless of the initial cause. The commonly‐used LABAs, salmeterol and formoterol, and the ultra long‐acting beta2‐agonist indacaterol, all have a higher selectivity for beta2‐receptors than beta1‐receptors. Beta2‐receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1‐receptors are the predominant receptors in the heart, although 10% to 50% of the total beta‐receptors in the heart are comprised of beta2‐receptors (Wallukat 2002). The presence of beta2‐receptors in the heart raises the possibility that even highly selective beta2‐agonists may have cardiac effects. The duration of action for salmeterol and formoterol is approximately 12 hours, and therefore are usually taken twice daily. Indacaterol has a duration of action of 24 hours and can, therefore, be taken once daily. The mechanism for activating beta2‐receptors differs between the LABAs. Formoterol is taken up into a membrane depot from where it gradually leaks out to interact with the receptor, whilst salmeterol binds near the receptor, allowing it to remain at the receptor site, continually binding and releasing (Johnson 1998). Indacaterol has a higher affinity to lipid domains within the membrane than salmeterol, which may potentially explain its prolonged duration of action (Beier 2011). Independent of LABA type, stimulation of the beta2‐receptors leads to changes in intracellular Ca2+ homeostasis and bronchodilation (Tanaka 2005). As with tiotropium, LABAs are commonly used to control symptoms and reduce exacerbations in stable COPD. A prior Cochrane review found that salmeterol improves lung function compared to placebo (Appleton 2006). A more recent, large (3045 participants), long‐term (three‐year) randomised controlled trial (RCT) also compared salmeterol to placebo (TORCH) (Calverley 2007). In this trial salmeterol use was associated with an increase in lung function, and a significant reduction in moderate or severe exacerbations compared with placebo (OR 0.85, P < 0.001). A systematic review, which included the TORCH study and another 13 trials with a total of 6453 participants, showed that treatment with a salmeterol or formoterol reduced the rate of exacerbations, and improved lung function and quality of life compared to placebo, but had no significant effect on mortality (Rodrigo 2008). Studies on indacaterol have shown that it produced statistically significant improvement in FEV1 lung function when compared to salmeterol (Kornmann 2011) and formoterol (Dahl 2010), and has a similar safety profile and tolerability compared with other LABAs (Donohue 2011a). Possible side effects of LABAs include cardiac effects such as arrhythmia and palpitations, muscle tremors, headache and dry mouth (Berger 2008).
Why it is important to do this review
Both tiotropium and LABAs are recommended for treatment of stable COPD (GOLD). However, it is unclear what clinical advantage treatment with tiotropium has over LABA treatment for people with COPD. This review is necessary to specify and quantify the potential benefits from treatment with tiotropium compared to LABAs.
Comparisons between tiotropium and other bronchodilators in patients with COPD suggest a greater benefit in symptom control and lung function with tiotropium than with either short‐acting anticholinergics (ipratropium), or LABAs (Sears 2008). A systematic review of RCTs comparing tiotropium with LABAs found that the incidence of exacerbations was lower in patients on tiotropium than on a LABA, and tiotropium led to a greater improvement in lung function than LABA (Rodrigo 2008). Furthermore, while tiotropium has a higher average price than LABAs, once daily dosing may be more convenient than twice daily, thus increasing the likelihood of patient compliance.
This review forms part of a suite of reviews on the various combinations of tiotropium, LABAs and inhaled corticosteroids for the treatment of COPD. These reviews will ultimately be summarised in an overview. The reviews cover the following comparisons: tiotropium versus placebo (Karner 2012a); tiotropium versus ipratropium (Cheyne 2012); tiotropium plus LABA versus tiotropium or LABA (Karner 2012); tiotropium versus combination of inhaled corticosteroids (ICS) and LABAs (Welsh 2010); triple therapy of tiotropium plus ICS/LABA combination inhaler versus tiotropium or combination inhaler (Karner 2011a); and triple therapy versus tiotropium plus LABA (Karner 2011b).
Objectives
To compare the relative clinical effects of tiotropium bromide alone versus LABA alone, upon measures of quality of life, exacerbations, lung function and serious adverse events, in people with stable COPD.
To critically appraise and summarise current evidence on the costs and cost‐effectiveness associated with tiotropium compared to LABA in people with COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs with a parallel‐group design conducted for at least 12 weeks duration. We did not exclude studies on the basis of blinding. Cross‐over trials were not included because the condition of patients with COPD gradually deteriorates over time and therefore may affect comparisons.
We also included full economic evaluation studies (cost‐effectiveness analyses, cost‐utility analyses and cost‐benefit analyses), cost analyses and comparative resource utilisation studies. To be included, any type of economic evaluation (full economic evaluation, cost analysis, comparative resource utilisation study) had to be conducted alongside a RCT. Full economic evaluations could also utilise effect data generated using either a meta‐analysis of RCTs or a single RCT.
Types of participants
Study participants had a diagnosis of stable COPD as judged by a set of criteria equivalent to e.g. GOLD, ATS, BTS, TSANZ.
Types of interventions
In each study, participants were randomised to receive either inhaled tiotropium bromide or a LABA. Tiotropium bromide and LABAs were allowed in any formulation. Participants were allowed inhaled corticosteroids and other concomitant COPD medication provided they were not part of the randomised treatment.
Types of outcome measures
Primary outcomes
Quality of life (measured with a validated scale for COPD, e.g. St George's Respiratory Questionnaire, Chronic Respiratory Disease Questionnaire)
Exacerbations; requiring short‐burst oral corticosteroids and/or antibiotic
Mortality; all‐cause
Secondary outcomes
Hospital admissions; all‐cause and due to exacerbations
Disease‐specific mortality, if independently adjudicated
Forced expiratory volume in one second (FEV1)
All‐cause, non‐fatal serious adverse events
Withdrawals
Cost and cost‐effectiveness
Search methods for identification of studies
Electronic searches
Randomised controlled trials
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). All records in the CAGR coded as 'COPD' were searched using the following terms:
(tiotropium or spiriva) AND (*formoterol or salmeterol or bambuterol or indacaterol or clenbuterol or Serevent or Foradil or Oxis or (beta* and agonist*))
We also conducted a search of ClinicalTrials.gov and the relevant manufacturers' registers of clinical trials for additional trials (please see Appendix 2 for further details). We searched all databases from their inception to the present, and there was no restriction on language of publication.
Economic evaluations
We identified economic evaluations through searching the NHS Economic Evaluation Database (NHS EED) and the Health Economic Evaluations Database (HEED). We searched the databases using the term 'tiotropium'.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references and contacted manufacturers requesting information on any other published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (JC and CK) screened the titles and abstracts of citations retrieved through literature searches and obtained those deemed to be potentially relevant. We then assigned each reference to a study identifier and assessed them against the inclusion criteria for studies in this review.
Data extraction and management
We extracted information from each study for the characteristics listed below. Two review authors extracted data from the studies into data collection forms, discussing any discrepancies in the data, and consulted a third party where necessary. We then transferred information from data collection forms into Review Manager 5.
Randomised controlled trials
Design (design, total duration study and run‐in period, number of study centres and location, withdrawals, date of study)
Participants (N, mean age, age range, gender, COPD severity, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria)
Interventions (run‐in, intervention A (tiotropium) and inhaler type, intervention B (LABA) and inhaler type, and concomitant medications)
Outcomes (primary and secondary outcomes specified and collected, time points reported)
Economic evaluations
Design (type of economic evaluation, country of study, currency, price year, length of follow‐up, perspective, setting, participants/population, intervention and control(s))
Data (resources used, source of unit cost data, clinical outcomes measured, source of clinical data including utilities, where appropriate)
Outcomes and results (quality‐adjusted life years (QALY), exacerbations, costs, incremental cost‐effectiveness ratios (ICERs))
Analysis (cost data handled appropriately, subgroup analysis, statistical analysis for patient‐level stochastic data and appropriateness, uncertainty around cost‐effectiveness and appropriateness, sensitivity analysis and appropriateness)
Funding
Assessment of risk of bias in included studies
Randomised controlled trials
We assessed the risk of bias according to recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) for the following items:
allocation sequence generation;
concealment of allocation;
blinding of participants and investigators;
incomplete outcome data;
selective outcome reporting.
We graded each potential source of bias low, high or unclear risk of bias. We also noted other potential sources of bias.
Economic evaluations
Assessment of the quality of economic evaluations was informed by application of either a checklist for quality assessment in economic decision analytic models (Philips 2004) or for non‐model based evaluations by Drummond 1996. Two review authors completed checklists independently by and resolved disagreements through discussion.
Measures of treatment effect
Dichotomous data
We analysed dichotomous data variables (such as mortality and withdrawals) with Mantel‐Haenzsel odds ratios using a fixed‐effect model with 95% confidence intervals. When events were rare we employed the Peto odds ratio (OR) since this does not require a continuity correction for zero cells. If count data were not available as the number of participants experiencing an event, we planned to analyse this as continuous, time‐to‐event or rate ratios, depending on how it was reported. This included the outcomes: hospital admissions, exacerbations and serious adverse events.
Continuous data
We analysed continuous outcome data (such as FEV1 and quality of life) as fixed‐effect mean differences with 95% confidence intervals (CI). All continuous outcomes used the same scale and therefore no standardised mean difference analyses were needed. Where treatment effects were reported as a mean difference with standard deviations or an exact P value, we calculated the standard error and entered it with the mean difference and combined the results using a fixed‐effect generic inverse variance model in Review Manager 5.
We used results from intention‐to‐treat (ITT) analyses wherever possible. The time of analysis was the end of the study in all cases.
We planned to calculate numbers needed to treat (NNT) from the pooled OR and its CI, and applied to appropriate levels of risk in the comparison group, LABA.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis (rather than events) to avoid counting the same participant more than once. Thus, we divided participants into whether or not they had an exacerbation during the study period. This was a specific strategy to circumvent the statistical issues that arise with repeated events such as exacerbations. For continuous data, the mean difference based on change from baseline was preferred over mean difference based on end of study measurements.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible. We also considered the impact of the unknown status of participants who withdrew from the trials as part of the sensitivity analysis.
Assessment of heterogeneity
We assessed the amount of statistical variation among the studies with the I2 statistic.
Assessment of reporting biases
We assessed outcome reporting bias by recording which outcomes were described in the study methods and correlating these with the published results. Individual study protocols were not sought. We minimised outcome reporting bias by contacting study authors asking for additional information when needed.
We minimised reporting bias from non‐publication of studies by using a broad search strategy and checking references of included studies. In addition, we planned to visually inspected funnel plots for evidence of reporting bias, however we did not have a sufficient number of included studies to do so.
Data synthesis
We presented the findings of our primary outcomes in a 'Summary of findings' table generated using GradePro software and based on recommendations in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008).
Subgroup analysis and investigation of heterogeneity
For the primary outcomes, we analysed studies within subgroups where data were available, according to:
type of LABA (salmeterol, formoterol and indacaterol);
severity of disease at baseline (GOLD staging);
concomitant medication (participants receiving inhaled corticosteroid (ICS) treatment during the study period versus no ICS);
study duration (< 6 months, ≥ 6 months)
Sensitivity analysis
We assessed the robustness of our primary analysis by performing sensitivity analysis, comparing the overall result with that exclusively from trials of a double‐blind study design.
Results
Description of studies
Results of the search
Randomised controlled trials
Studies for this review were sought from a systematic search of the Cochrane Airways Group trial register in Feburary 2012 which identified a total of 173 references. A search of the Clinicaltrials.gov database in February 2012 generated a list of 67 registered trials. After cross‐checking these results with our initial search, the two review authors (JC and CK) identified 59 references that were appropriate for further appraisal. We sought full texts of these to determine eligibility for this review. Two further abstracts were identified from searching through the reference lists of identified references.
Of these references, 33 met the inclusion criteria. For study flow diagram see Figure 1. By comparing the official trial numbers, trial duration and timeline, together with other identifiable study features, we identified where there were multiple reports of the same study. We then collated the published journal articles and conference abstracts and proceeded to extraction of the data.
1.
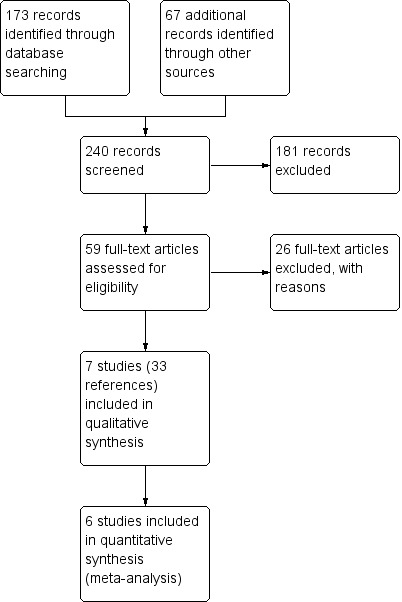
Study flow diagram.
Economic evaluations
We searched the Health Economic Evaluations Database (HEED) and NHS Economic Evaluation Database (EED) databases for economic evaluations of tiotropium in February 2012. The searches returned 29 references from HEED and 13 references from NHS EED. Of these we identified six as relevant for inclusion in the review. We identified another five potential references from other sources, which are awaiting classification (see Characteristics of studies awaiting classification).
Included studies
Randomised controlled trials
Seven separate studies (total of 12,223 participants) studying the effects of tiotropium compared with salmeterol (four studies with 8936 participants), formoterol (one study with 431 participants) and indacaterol (two studies with 2856 participants) met the inclusion criteria. All studies used tiotropium 18 µg once daily via the HandiHaler device.
Inclusion criteria were similar across the included studies, with participants required to be aged 40 years or older, with a cigarette smoking history of equal or greater than 10 pack years, except for the Donohue 2010 study, which specified a smoking history of 20 pack years or more. Across the various studies, mean smoking pack year history ranged from 35 years (Vogelmeier 2008) to 56 years (Briggs 2005). Criteria for a clinical diagnosis of COPD were not specified in three of the seven included studies (Briggs 2005; Brusasco 2003; Mahmud 2007). The remaining studies used the definitions outlined in the GOLD guidelines (Burl 2011; Donohue 2010; Vogelmeier 2008) or American Thoracic Society classification (Vogelmeier 2011). COPD severity was described as severe (Briggs 2005; Brusasco 2003; mean % predicted FEV1 between 35% to 40%), moderate to severe (Burl 2011; Donohue 2010; Vogelmeier 2008; Vogelmeier 2011; mean % predicted FEV1 between 49% to 57%), and was not described in Mahmud 2007. The study participants had a mean age of between 60 and 65 years and were predominately male. All participants could receive inhaled corticosteroids (ICS) at a stable daily dose (Briggs 2005; Burl 2011; Donohue 2010; Vogelmeier 2011: % of participants using ICS at baseline varying from 35% to 56%) but were instructed to discontinue treatment with anticholinergic bronchodilators or with other long‐acting beta2‐agonist. All studies had an appropriate washout period. Ongoing methylxanthine use was allowed in two studies (Briggs 2005; Mahmud 2007). In all of the included studies, participants with a history of asthma or atopic disease, or any significant medical condition that could preclude participation for the full duration of the study, were excluded. For more details see Characteristics of included studies.
Salmeterol trials
Three studies evaluated the comparative effects of tiotropium versus salmeterol 50 µg in participants with moderate to severe COPD using a double‐dummy study design. Study duration varied from 12 weeks (Briggs 2005) to six months (two RCTs reported in Brusasco 2003), or 12 months (Vogelmeier 2011).
Another six‐month study (Mahmud 2007) was undertaken in a group of Bangladeshi participants; however, we were unable to obtain any more information about the study, nor include any data in the analyses.
Formoterol trials
Vogelmeier 2008 was the only study in this review to compare tiotropium with formoterol, which was given as 10 µg twice daily over a six‐month period.
Indacaterol trials
Donohue 2010 was a three‐phase study. The first phase comprised an indacaterol dose‐finding phase; then a second phase evaluated the efficacy and safety of two doses of indacaterol compared to tiotropium and placebo; followed by a third phase where only indacaterol or placebo was continued. Data from the second phase of the study only were included in this review. In this phase, two indacaterol doses ‐ 150 and 300 µg once daily ‐ were used, with treatment continued to 26 weeks. The tiotropium treatment was not blinded. Additional participants were recruited and randomised. Where possible, we pooled data for the two indacaterol groups according to the recommendations outlined in the Cochrane Handbook, section 16.5 (Higgins 2008). A further study (Burl 2011) was a 12‐week double‐dummy study of tiotropium versus indacaterol 150 µg given once daily via a single‐dose dry powder inhaler (SDDPI).
Economic evaluations
The six included economic studies were all full economic evaluations (cost‐utility analysis or cost‐effectiveness analysis) of tiotropium 18 µg once daily compared to salmeterol 50 µg twice daily for patients with COPD. Three of these (Gani 2010; Maniadakis 2006; Rutten‐van Molken 2007) were based on a Markov model first described in another of the included economic studies (Oostenbrink 2005). The Markov model included three health states describing COPD severity (moderate, severe and very severe) and three health states describing exacerbation status (no exacerbation, mild exacerbation and severe exacerbation). A health state of death was included only in the five‐year model (Rutten‐van Molken 2007), but not in the other one‐year models. These four economic evaluations were sponsored by Boehringer Ingelheim, the manufacturer of tiotropium. Naik 2010 also used a Markov model with a one‐year time horizon based on RCT data. The model comprised pairs of health states; 'on treatment' and 'maintenance therapy', 'response' and inadequate response', and exacerbations of different severity. For all five model‐based economic evaluations probabilities for transitions between states were based on pooled data from RCTs. Oba 2007 used empirical analysis based on systematic literature review of clinical trial data. The time horizon for this study was one year.
The setting for all the studies was primary and secondary care. The analytical perspective differed between second‐ and third‐party payer depending on the country of the study. The countries of study were the UK (Gani 2010), Greece (Maniadakis 2006), USA (Naik 2010; Oba 2007), Netherlands and Canada (Oostenbrink 2005), and Spain (Rutten‐van Molken 2007). The reported price year and currency differed between the studies (see Table 2).
1. Cost‐effectiveness.
| Study | Design | Country | Perspective | Price year | Model |
Additional QALM/Y (/patient /year) |
Exac free time or exac avoided (/patient /year) | Incremental cost | ICER | Uncertainty assessment | Uncertainty around cost‐effectiveness |
| Gani 2010 | CUA | England | NHS, second‐party payer | 2009 | 3‐state Markov model | QALY 0.014 | GBP ‐169 | PSA and OSA based on either severity of COPD or exacerbation rate | At GBP 0/QALY tio was 86% likely to be cost‐effective against sal, and at GBP 20,000/QALY ≥ 97% | ||
| Scotland, Wales, N Ireland | GBP ‐136 | At GBP 0/QALY tio was 84% likely to be cost‐effective against sal, and at GBP 20,000/QALY ≥ 97% | |||||||||
| Maniadakis 2006 | CUA | Greek NHS | NHS, second‐party payer | 2005 | 3‐state Markov model | QALM 0.26 (95% CI ‐0.93 to 1.50) | EXA 0.17 (95% CI ‐0.02 to 0.37) | EUR ‐151 (95% CI ‐926 to 580) | PSA and OSA based on either severity of COPD or exacerbation rate | At EUR 0 tio was 65% likely to be cost‐effective against sal. At EUR 1000 tio was 77% likely to be cost‐effective against sal. | |
| Naik 2010 | CEA | USA | Third‐party payer | 2006 | Markov model | EXA mean (SD) Tio 1.13 (0.46) Sal 1.05 (0.52) |
Direct cost Mean (SD) Tio USD 1409 (312) Sal USD 1269 (310) |
Compared with no treatment, the incremental cost per EXA was USD 1817.37 with tio USD 2454.48 with sal |
OSA based on either probability of exacerbation, hospitalisation, or severe exacerbation, and compliance rate | ||
| Oba 2007 | CUA | USA | Third‐party payer | 2005 | Empirical analysis | Compared to no treatment Tio QALY 0.032 (95% CI 0.050 to 0.014) Sal QALY 0.026 (95% CI 0.040 to 0.012) |
Compared to no treatment Tio USD 835 Sal USD 1066 |
Compared with no treatment, the incremental cost per additional QALY was USD 26,094 (11,780 to 77,214) for tio USD 41,000 (23,650 to 98,750) for sal |
MUSA and OSA on lowest costs and greatest incremental QALYs were used for best‐case scenarios, and highest costs and smallest incremental QALYs were used for worst‐case scenarios | At USD 50,000, the probability that tio or sal is cost‐effective compared to no treatment is 93% or 67%, respectively | |
| Oostenbrink 2005 | CUA | Netherlands | Healthcare system, second‐ and third‐party payer? | 2001 | 3‐state Markov model | QALM 0.25 (95% CI ‐0.90 to 1.47) | EXA 0.17 (95% CI ‐0.02 to 0.37) | EUR ‐42 (95% CI ‐484 to 353) | PSA and OSA based on either severity of COPD, exacerbation rate, utility values, or oxygen therapy | At EUR 0/ EXA tio was 43% likely to be cost‐effective against sal, and at EUR 500/EXA 60%. The cost‐effectiveness acceptability frontier of exacs showed that tio was associated with the maximum expected net benefit for all values of the ceiling ratio above EUR 0. | |
| Canada | Healthcare system, second‐party payer? | EUR 3 (95% CI ‐227 to 203) | Above a threshold per EXA of EUR 160 tio had the highest probability of being cost‐effective, and above a cost of EUR 120 per QALM | ||||||||
| Rutten‐van Molken 2007 | CEA, CUA | Spain | NHS and societal | 2005 | 3‐state Markov model | QALY 0.14 (95% CI ‐0.16 to 0.49) | EFM 1.54 (95% CI ‐2.50 to 6.81) | EUR 555 (95% CI ‐647 to 1651) | Incremental costs per EFM was EUR 360 Incremental costs per QALY was EUR 4118 |
PSA based on severity of COPD, and discount rate | Above a threshold per additional EFM of EUR 1050 tio had the highest probability of being cost‐effective, and above a cost of EUR 11,000 per QALY |
Tiotropium versus salmeterol. The settings for all the studies were primary and secondary care, and they were all comparing 18 µg of tiotropium once daily with 50 µg of salmeterol twice daily.
CUA: cost utility analysis; CEA: cost‐effectiveness analysis; NHS: national health service; QALM: quality‐adjusted life month; QALY: quality‐adjusted life year; EFM: exacerbation free month; EXA: exacerbation avoided; ICER: incremental cost‐effectiveness ratio; CI: confidence interval; exac: exacerbation; tio: tiotropium; sal: salmeterol; PSA: probabilistic sensitivity analysis; OSA: one‐way sensitivity analysis; MUSA: multivariate sensitivity analysis
All the economic evaluations looked at maintenance costs and the costs for exacerbations. This generally included respiratory medications, hospitalisations, physician visits (inpatient or outpatient), visits to general practitioners, visits to emergency departments and laboratory tests.
The effectiveness data on exacerbations were based on data from RCTs comparing tiotropium to salmeterol, or tiotropium or salmeterol to placebo. Utility data were based either on RCTs (Naik 2010; Oba 2007; Rutten‐van Molken 2007) or observational study data (Gani 2010; Maniadakis 2006; Oostenbrink 2005).
Excluded studies
See Characteristics of excluded studies.
Excluded were data from the phase I (two‐week dose selection) and phase III (comparison arm changing from placebo to tiotropium/indacaterol during treatment) periods of the Donohue 2010 trial, as well as abstracts which belonged to the same study but did not report data on tiotropium use (Barnes 2010). Six studies were excluded because of cross‐over design (Golubev 2006; Meyer 2008; ten Hacken 2007; van Noord 2003; van Noord 2005; van Noord 2006), and three studies as they had a study duration of less than 12 weeks (Di Marco 2006; Gross 2003; Tashkin 2009).
Risk of bias in included studies
Economic evaluations
The results of the assessments of quality of the economic evaluations appear in Appendix 3.
The reliability of full economic evaluations partly depends on the use of reliable clinical data. The economic evaluations included in this review were all based on clinical data from RCTs. Of these, a full assessment of the risk of bias can be found in the Characteristics of included studies table for the RCTs comparing tiotropium with salmeterol which are included in this review (Brusasco 2003 and Donohue 2002 which is one of the trials included in Brusasco 2003).
Both Naik 2010 and Oba 2007 based their cost‐effectiveness calculations on RCTs comparing tiotropium or salmeterol to placebo. As directly comparing the clinical data from these trials for tiotropium and salmeterol with each other would suffer from lack of randomisation, they presented all data as tiotropium versus placebo and salmeterol versus placebo. However, from these data sets they inappropriately drew conclusions about the relative cost‐effectiveness of tiotropium compared to salmeterol. The other studies presented more appropriate statistical analyses.
All the studies reported costs and resource use separately, though Gani 2010 and Oba 2007 did not present measures of uncertainty for estimates of mean costs. All the studies also reported different sensitivity analyses. Oostenbrink 2005, and the studies based on its model also included a probabilistic sensitivity analysis. Oba 2007 also included a multivariate sensitivity analysis.
Randomised controlled trials
Full details of our 'Risk of bias' judgements may be found in Characteristics of included studies and in the summary graphic in Figure 2. We were unable to contact the author and gain further information about study design related to the risk of bias for the Mahmud 2007 study.
2.
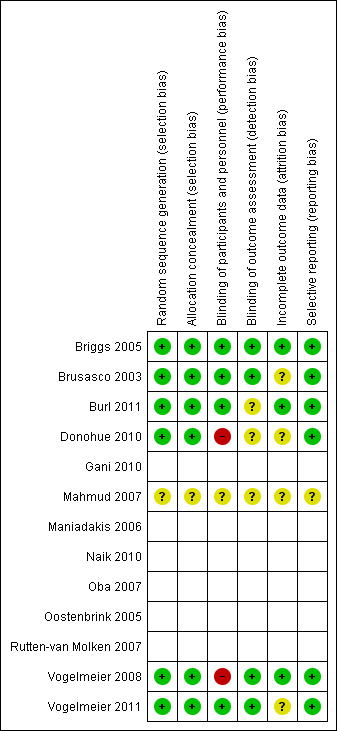
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation and allocation concealment was sufficiently described within the main texts or online supplements for three of the seven studies identified (Briggs 2005; Vogelmeier 2008; Vogelmeier 2011). Further information was provided by Norvartis on request for two studies (Burl 2011; Donohue 2010), and by Boehringer Ingelehim for one study (Brusasco 2003). Each of these used a central automated system to conceal medication status from the study investigators.
Two studies did not report using accepted external criteria for diagnosing COPD (Briggs 2005; Brusasco 2003). However, in these studies the criteria used were similar to those in common use, and efforts were made to exclude anyone with asthma.
Blinding
Double‐blinding of participants and study personnel was apparent in all studies except for Donohue 2010 and Vogelmeier 2008, where a blinded form of tiotropium was not available.
Four studies described blinding of outcome assessors (detection bias) (Briggs 2005; Brusasco 2003; Vogelmeier 2008; Vogelmeier 2011).
Incomplete outcome data
Withdrawal rates ranged from 9% to 21% for tiotropium and from 8% to 21% in the LABA treated groups. When studies were combined, this resulted in a small, but statistically significant difference, with fewer number of withdrawals in the tiotropium group compared to the LABA group (see secondary outcome below, and Analysis 1.21).
1.21. Analysis.
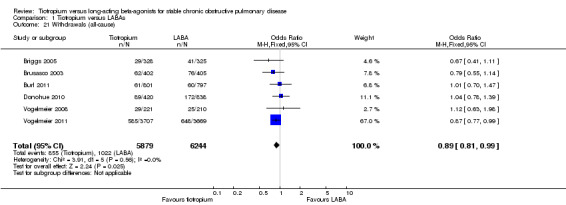
Comparison 1 Tiotropium versus LABAs, Outcome 21 Withdrawals (all‐cause).
Selective reporting
In the studies included in the analyses data were reported for all primary and secondary outcomes specified in published references and trial reports, and safety and adverse events.
Effects of interventions
See: Table 1
Primary outcomes
Quality of life
Four of the included studies (Brusasco 2003; Burl 2011; Donohue 2010; Vogelmeier 2008; 3605 participants) looked at changes in quality of life using the St George's Respiratory Questionnaire (SGRQ) (Figure 3). Two studies showed an improvement in quality of life with long‐acting beta2‐agonist (LABA) which was significantly larger than with tiotropium. In both of these studies, the LABA used was indacaterol (Burl 2011; Donohue 2010). The other studies (Brusasco 2003; Vogelmeier 2008), which used salmeterol and formoterol respectively, showed a non‐significant difference, but had means which were in different directions. For the Vogelmeier 2008 study, although direct comparisons between the tiotropium and formoterol treatment groups for change in SGRQ score were not reported, this was estimated by subtracting the mean difference between LABA versus placebo from tiotropium versus placebo. The variance of the difference between tiotropium and LABA was calculated using the variances of all the trial arms in the study (Spencer 2011). Owing to the high level of heterogeneity (I2 = 68%) and the limited number of studies, we felt it was not appropriate to pool the data.
3.
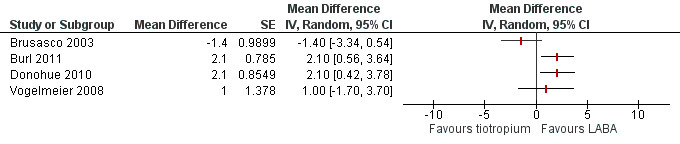
Forest plot of comparison: 1 Tiotropium versus long‐acting beta2‐agonists, outcome: 1.1 HRQoL (SGRQ).
Subgroup analysis showed that the difference among the effects of the different types of LABA was statistically significant (test for subgroup difference: P = 0.009; Analysis 1.2). This result appeared to be independent of study duration (test for subgroup difference: P = 0.28, Analysis 1.3).
1.2. Analysis.
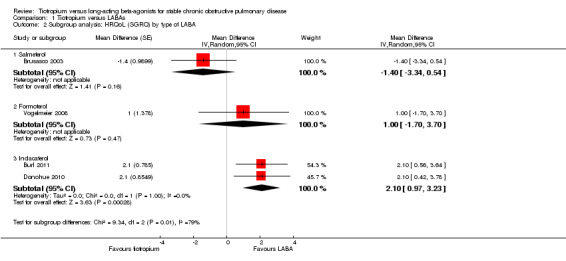
Comparison 1 Tiotropium versus LABAs, Outcome 2 Subgroup analysis: HRQoL (SGRQ) by type of LABA.
1.3. Analysis.
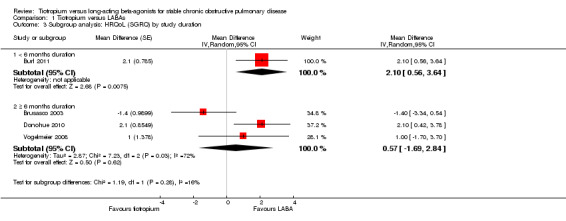
Comparison 1 Tiotropium versus LABAs, Outcome 3 Subgroup analysis: HRQoL (SGRQ) by study duration.
Two studies reported data on the number of participants who had a clinically significant improvement (a fall of at least four units) or worsening (an increase of at least four units) in quality of life. We analysed these data using risk difference to make it easier to compare participants with improvements (Analysis 1.4) to patients with deteriorations in quality of life (Analysis 1.5). In Brusasco 2003, there was no statistically significant difference between tiotropium and salmeterol in the number of participants with a clinically significant improvement in quality of life; while in Burl 2011, there was a small statistically significant benefit observed in favour of indacaterol over tiotropium. Brusasco 2003 was the only study to report the number of participants with clinically significant worsening in SGRQ (data obtained on request); fewer participants with a worsening received tiotropium than received salmeterol.
1.4. Analysis.
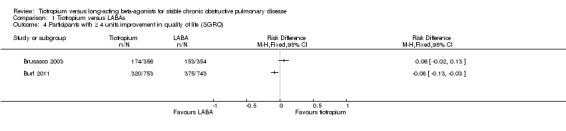
Comparison 1 Tiotropium versus LABAs, Outcome 4 Participants with ≥ 4 units improvement in quality of life (SGRQ).
1.5. Analysis.

Comparison 1 Tiotropium versus LABAs, Outcome 5 Participants with ≥ 4 units worsening in quality of life (SGRQ).
Chronic obstructive pulmonary disease (COPD) exacerbations
Most studies used a similar definition of a COPD exacerbation; being the onset of one or more respiratory symptoms lasting for three or more consecutive days and requiring additional treatment (Briggs 2005; Brusasco 2003; Donohue 2010; Vogelmeier 2011). This was not formally defined in Burl 2011 or Vogelmeier 2008.
Tiotropium treatment was associated with fewer participants experiencing one or more COPD exacerbations compared with LABA (six studies including 12,123 participants, Briggs 2005; Brusasco 2003; Burl 2011; Donohue 2010; Vogelmeier 2008; Vogelmeier 2011) (odds ratio (OR) 0.86; 95% confidence interval (CI) 0.79 to 0.93) (Figure 4). The largest and longest study, Vogelmeier 2011, showed a statistically significant difference favouring tiotropium over LABA (salmeterol), whereas all the other studies showed no statistically significant difference and wider confidence intervals (Figure 5). Vogelmeier 2011 contributed the most weight to the overall result in this analysis. It is estimated that one additional person on tiotropium will stay exacerbation‐free for every 29 people treated with tiotropium instead of LABA for a year (number needed to treat (NNT) 29; 95% confidence interval (CI) 19 to 59). This was based on comparisons with the event rate from the LABA group in Vogelmeier 2011 as this was by far the largest and longest study, and was of good methodological quality.
4.
In the LABA group 29 people out of 100 had one or more exacerbations, compared to 26 (95% CI 25 to 28) out of 100 for the tiotropium (treatment) group.
5.
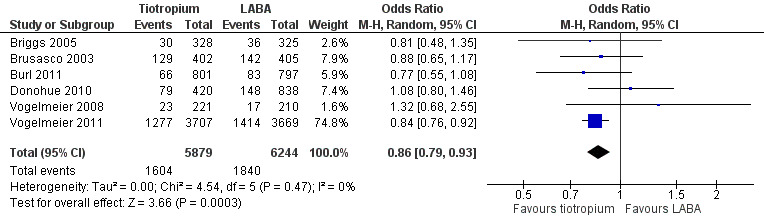
Forest plot of comparison: 1 Tiotropium versus LABAs, outcome: 1.6 Patients with 1 or more exacerbations.
Based on subgroup analyses, there was no significant difference in results among the different types of LABA (test for all subgroup differences: P > 0.05, Analysis 1.7), or for different study durations (test for subgroup difference: P = 0.39, Analysis 1.11). Vogelmeier 2011 was the only study to report data according to disease severity (Analysis 1.8). In this study, it was found that in participants with more severe disease (i.e. with a diagnosis of GOLD stage IV), tiotropium treatment was associated with a greater proportion of participants remaining exacerbation‐free compared to salmeterol, than for GOLD stages II (P = 0.02) and III (P = 0.03). Two studies (Brusasco 2003; Vogelmeier 2011) presented subgroup data on participants who were, or were not, taking inhaled corticosteroids (ICS) during the treatment period. The data from Brusasco 2003 were presented as an odds ratio and Vogelmeier 2011 as a hazard ratio. The data therefore could not be pooled. Both studies showed a larger favourable effect with tiotropium in participants who did not take ICS compared to participants who did, but again, the difference among the subgroups was not statistically significant (test for subgroup difference: Brusasco 2003 P = 0.13; Vogelmeier 2011 P = 0.15)
1.7. Analysis.
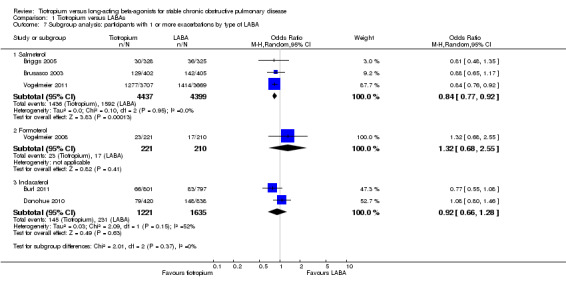
Comparison 1 Tiotropium versus LABAs, Outcome 7 Subgroup analysis: participants with 1 or more exacerbations by type of LABA.
1.11. Analysis.
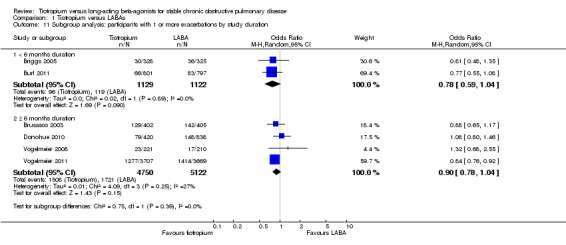
Comparison 1 Tiotropium versus LABAs, Outcome 11 Subgroup analysis: participants with 1 or more exacerbations by study duration.
1.8. Analysis.
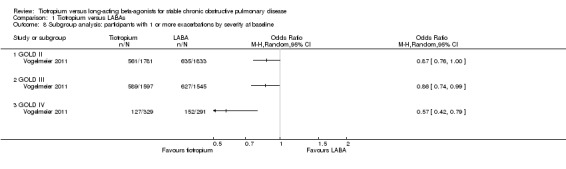
Comparison 1 Tiotropium versus LABAs, Outcome 8 Subgroup analysis: participants with 1 or more exacerbations by severity at baseline.
Mortality
While there were fewer deaths in the tiotropium (70 deaths out of 5879 people) compared with the LABA (85/6244) treatment groups, this was not statistically significant. There was some heterogeneity among studies (I² = 31%) and the pooled result was therefore calculated using a random‐effects model (odds ratio (OR) 0.82; 95% CI 0.60 to 1.13; Figure 6). The total number of events in each group was too few to consider subgroup differences for LABA type, disease severity or study duration.
6.
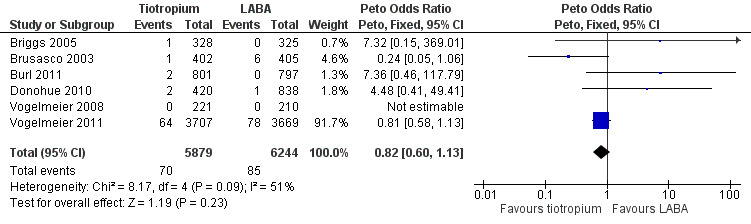
Forest plot of comparison: 1 Tiotropium versus long‐acting beta2‐agonists, outcome: 1.4 Mortality (all‐cause).
Sensitivity analysis
There were two studies which did not use a blinded form of tiotropium (Donohue 2010; Vogelmeier 2008). Exclusion of these studies did not explain the high heterogeneity in quality of life data, nor did it significantly affect the pooled result in COPD exacerbation or mortality data.
Secondary outcomes
Hospital admission due to COPD exacerbations and all causes
The number of participants requiring hospitalisation for a COPD exacerbation was significantly lower among participants who received tiotropium compared to participants receiving LABA (OR 0.87; 95% CI 0.77 to 0.99; Analysis 1.15) in the four studies which reported this as an outcome (9267 participants, Briggs 2005; Brusasco 2003; Vogelmeier 2008; Vogelmeier 2011).
1.15. Analysis.
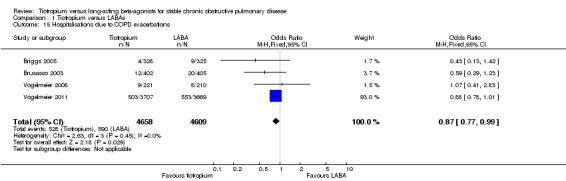
Comparison 1 Tiotropium versus LABAs, Outcome 15 Hospitalisations due to COPD exacerbations.
Data from three studies (Briggs 2005; Burl 2011; Donohue 2010, 3509 participants) were available to compare the rate of hospitalisation due to all causes. In this analysis, there was no statistical difference in hospitalisations between tiotropium and LABAs (OR 0.93; 95% CI 0.57 to 1.54, Analysis 1.16) using a random‐effects model.
1.16. Analysis.
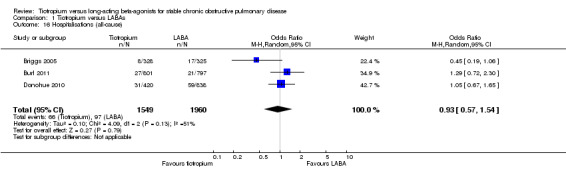
Comparison 1 Tiotropium versus LABAs, Outcome 16 Hospitalisations (all‐cause).
Forced expiratory volume in one second (FEV1)
Four studies with 4600 participants (Briggs 2005; Burl 2011; Donohue 2010; Vogelmeier 2008) measured the difference in trough FEV1 at the end of the study (duration three or six months). This showed no statistically significant difference in the effect on lung function (mean difference (MD) 10.52 mL; 95% CI ‐11.47 to 32.51, Analysis 1.17) between tiotropium and LABAs using a random‐effects model, with heterogeneity observed among the studies (I2 = 48%).
1.17. Analysis.
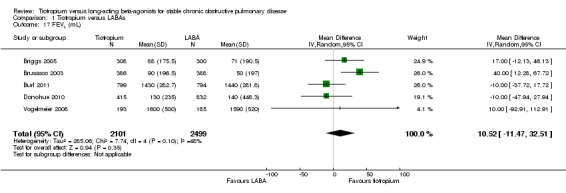
Comparison 1 Tiotropium versus LABAs, Outcome 17 FEV1 (mL).
Symptom score
There was no significant difference between tiotropium and LABA treatments in the Transitional Dyspnoea Index (TDI) score, based on the results of three studies (MD ‐0.22; 95% CI ‐0.63 to 0.19, Brusasco 2003; Burl 2011; Donohue 2010, 3307 participants) (Analysis 1.18). There was moderate heterogeneity among the three studies (I² = 55%) and the result was therefore analysed using a random‐effects model. Vogelmeier 2008 showed no statistically significant difference between tiotropium and formoterol in daily total symptom score (MD ‐0.12; 95% CI ‐0.67 to 0.43, Analysis 1.19).
1.18. Analysis.
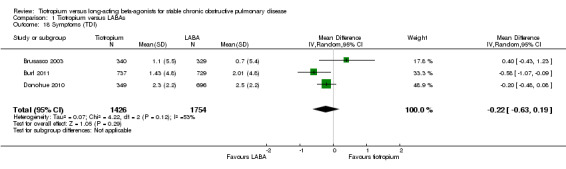
Comparison 1 Tiotropium versus LABAs, Outcome 18 Symptoms (TDI).
1.19. Analysis.
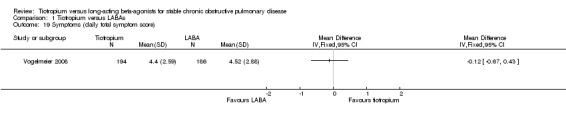
Comparison 1 Tiotropium versus LABAs, Outcome 19 Symptoms (daily total symptom score).
Non‐fatal serious adverse events
There were 10.1% and 11.1% of participants recorded as having a non‐fatal serious adverse event in the tiotropium and LABA treatment groups respectively (Briggs 2005; Burl 2011; Donohue 2010; Vogelmeier 2008; Vogelmeier 2011) (OR 0.88; 95% CI 0.78 to 0.99, Analysis 1.20). The Vogelmeier 2011 study contributed the greatest weight to this outcome, as it had the largest number of participants, and was 12 months in duration.
1.20. Analysis.
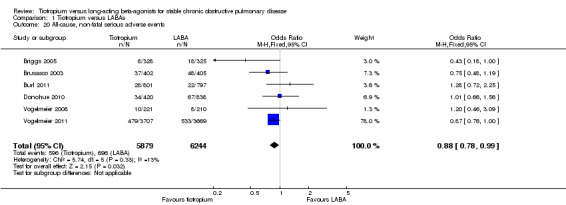
Comparison 1 Tiotropium versus LABAs, Outcome 20 All‐cause, non‐fatal serious adverse events.
Withdrawals
Those treated with tiotropium had a significantly lower rate of study withdrawal (14.5% versus 16.3%) compared to those receiving a LABA (OR 0.89; 95% CI 0.81 to 0.99, Analysis 1.21). Again, the Vogelmeier 2011 study contributed the highest number of events to this outcome.
Cost and cost‐effectiveness
For a summary table see Table 2.
All the economic evaluations looked at maintenance costs and the costs for exacerbations. This generally included respiratory medications, hospitalisations, physician visits (inpatient or outpatient), visits to general practitioners, visits to emergency departments and laboratory tests.
Cost
The annual maintenance cost and cost for exacerbations per patient was lower with tiotropium compared to salmeterol in England (GBP ‐169), Scotland, Wales and Northern Ireland (GBP ‐136) (Gani 2010, price year 2009, 95% CI not reported); in Greece (EUR ‐151, 95% uncertainty interval (UI) ‐926 to 580, Maniadakis 2006, price year 2005); and in the Netherlands (EUR ‐42, 95% UI ‐484 to 353, Oostenbrink 2005, price year 2001). In Spain, the cost per patient per year was higher with tiotropium than with salmeterol (EUR 555, 95% CI ‐647 to 1651, Rutten‐van Molken 2007, price year 2005), and in Canada the cost was similar for both treatments (EUR 3, 95% UI ‐227 to 203, Oostenbrink 2005, price year 2001). Naik 2010 and Oba 2007, which looked at data from the USA, did not present a direct comparison of the costs associated with tiotropium and salmeterol.
Cost‐effectiveness
Incremental cost‐effectiveness data, which is the ratio of the difference in costs to the difference in effects between the two treatments, were reported by three studies (Naik 2010; Oba 2007; Rutten‐van Molken 2007). However, Naik 2010 and Oba 2007 did not present direct comparisons of the cost‐effectiveness between tiotropium and salmeterol. In Spain, the incremental cost per exacerbation‐free month was EUR 360 when comparing tiotropium with salmeterol (Rutten‐van Molken 2007, price year 2005, 95% CI not reported). The same study also showed that the incremental cost per quality‐adjusted life year (QALY) was EUR 4120 for the same comparison. Cost‐effectiveness acceptability curves showed that tiotropium had the highest probability of being cost‐effective above a threshold per additional exacerbation‐free month of EUR 1050 and above a cost of EUR 11,000 per QALY. A sensitivity analysis showed that the threshold value above which tiotropium had the highest expected net benefit increased with the severity of COPD. The threshold values for the costs per QALY above which tiotropium became the preferred option were EUR 7600 for moderate COPD, EUR 8800 for severe COPD and EUR 12,500 for very severe COPD.
Discussion
Summary of main results
There have been four studies completed to date that have included data comparing quality of life in participants receiving tiotropium versus a long‐acting beta2‐agonist (LABA). The first finding was that there was a significant amount of heterogeneity among these studies, so no meaningful conclusion can be drawn at this time. Some of this heterogeneity may be explained by the type of LABA used in the study (salmeterol, formoterol or indacaterol): a statistically significant effect was observed in two recently published studies using indacaterol (Burl 2011; Donohue 2010) over tiotropium; however the mean data did not reach the threshold of four units regarded as a clinically significant change in quality of life (Jones 2005). There was no statistically significant difference observed between tiotropium and salmeterol, or formoterol. Unfortunately, the largest study comparing tiotropium versus salmeterol (Vogelmeier 2011) did not include quality of life as an outcome.
There was no statistically significant difference detected in the number of participants with a clinically significant improvement in quality of life (improvement of ≥ 4 St George's Respiratory Questionnaire (SGRQ) units) in one study that compared tiotropium with salmeterol; however, in this study it was found that tiotropium may have benefit over salmeterol in reducing the number of participants who experience a clinically significant deterioration in quality of life. Furthermore, there was a statistically greater number of participants who achieved a clinically significant improvement in SGRQ with indacaterol versus tiotropium.
The second major finding was that tiotropium, compared with LABAs, significantly reduced the number of participants experiencing a chronic obstructive pulmonary disease (COPD) exacerbation during the study period. This corresponded to exacerbation risks in the two groups of around 27% and 29% respectively (14% relative difference). There was no statistically significant difference in the number of participants with one or more exacerbations according to LABA subgroup or study duration. It is important to note that Vogelmeier 2011 (comparison between tiotropium and salmeterol) contributed the most weight to the overall result in this analysis as it had the largest number of participants by far. Moreover, data from Vogelmeier 2011 suggest that tiotropium may have a greater benefit over LABA in participants with very severe disease (GOLD stage IV) than in moderate (GOLD stage II) or severe (GOLD stage III) COPD.
Overall, there were few deaths reported in the studies, with no statistically significant differences in the number of events between the treatment groups. Again, the Vogelmeier 2011 study contributed the highest number of events to this outcome.
FIndings relating to secondary outcomes were:
a slightly lower number of hospitalisations due to COPD exacerbations in the tiotropium group compared with those receiving LABA, but no statistically significant difference in the rates of hospital admission for all causes;
a small but significant difference in rate of serious adverse events favouring tiotropium treatment over LABA;
the proportion of participants from each group who withdrew from the study being slightly lower in the tiotropium group than with LABAs;
no statistically significant difference between tiotropium and LABAs in the effect on forced expiratory volume in one second (FEV1) lung function;
no statistically significant difference in symptom score as measured by the Transitional Dyspnoea Index (TDI) and daily symptom score.
There is an ongoing debate on the cardiovascular safety of tiotropium and therefore underlying comorbidity might guide the treating physician in choosing the optimal treatment. In particular, there is evidence that there exists an elevated mortality risk when using tiotropium administered with the Respimat, but not with the HandiHaler (Karner 2012). Analysis of specific cardiovascular adverse events was beyond the scope of this review, although this will be important to look at in future updates if more data are available.
All six studies evaluating the cost and cost‐effectiveness of tiotropium and salmeterol estimated tiotropium to be superior to salmeterol based on better clinical outcomes (exacerbations or quality of life), lower total costs or both. Three of the studies found tiotropium treatment to be associated with lower annual total cost than salmeterol (UK, Greece, Netherlands). The only study with a time horizon longer than one year found tiotropium treatment to lead to higher costs but also the highest expected net benefit within acceptable values for the willingness‐to‐pay (Spain). Two studies did not compare tiotropium directly to salmeterol but drew conclusions about the difference in cost indirectly by comparing either treatment to no treatment (USA). However, there was substantial uncertainty around all of the results based on variable findings of sensitivity analyses in different studies.
Overall completeness and applicability of evidence
This systematic review suggests that more research is needed in order to conclude which of tiotropium or any of the different LABAs leads to the best improvement in quality of life. At present, the high level of heterogeneity across the studies cannot be explained by study duration or the use of concomitant medications. There may be some difference among various LABAs in how much they improve quality of life; for example the current review raises the possibility that the new LABA indacaterol compares more favourably with tiotropium in terms of quality of life measures than either salmeterol and formoterol. However, pointedly none of the differences within each study exceeded the quoted minimal change of four units necessary to detect a clinically significant improvement (with indacaterol the difference was 2.10 (95% confidence interval (CI) 0.97 to 3.23)). It may be helpful to present results in future updates in the form of a responders analysis (i.e. % patients with a clinically significant change). The value of responders analysis (as demonstrated in Karner 2012) shows that even if the mean data and its 95% CI is below the four unit threshold, there may be a significantly greater number of patients with a clinically significant improvement favouring one treatment over another, which is statistically significant. Indacaterol did not appear to exert its benefit through fewer adverse effects than the other LABAs when compared with tiotropium. These findings need to be confirmed with further studies that compare tiotropium and the different LABAs.
On the other hand, there appears to be evidence for clinical benefit in favour of tiotropium over LABAs in prevention of COPD exacerbations. Furthermore, in one study (Vogelmeier 2011), analysis stratified by disease severity demonstrated that participants with very severe disease (GOLD IV) had lower exacerbation rates when receiving tiotropium versus salmeterol than in those with moderate or severe COPD, where the difference between the treatment groups was much smaller. This result may be a true finding that requires pathophysiological explanation, or else may be contributed to by a sampling artefact of lower event rates in milder COPD participants.
We performed a subgroup analysis to determine whether or not the use of concomitant inhaled ICS had any bearing on the relative effectiveness of tiotropium and LABAs on exacerbations (Analysis 1.9; Analysis 1.10). It might be expected that as patients with more severe COPD are more likely to be on inhaled ICS, these patients might benefit the most from tiotropium compared with LABA. However, this was not the case. If anything, the data from two separate studies (not able to be combined), suggest that concomitant inhaled corticosteroid (ICS) use may be associated with a smaller, not greater, difference between the two treatments. Statistically, in neither study did the subgroup difference reach significance. One possibility is that participants on inhaled ICS may have fewer exacerbations and be more likely to be included in, and remain in a study; yet they behave more like participants with mild or moderate disease. Given the uncertainty of subgroup analysis, this observation requires further confirmation and clarification.
1.9. Analysis.
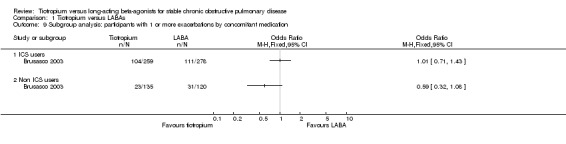
Comparison 1 Tiotropium versus LABAs, Outcome 9 Subgroup analysis: participants with 1 or more exacerbations by concomitant medication.
1.10. Analysis.
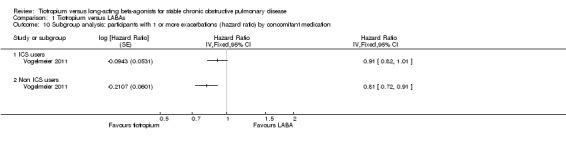
Comparison 1 Tiotropium versus LABAs, Outcome 10 Subgroup analysis: participants with 1 or more exacerbations (hazard ratio) by concomitant medication.
Quality of the evidence
This systematic review evaluated seven studies (including 12,223 participants) that compared the effects of tiotropium bromide with a LABA in the treatment of people with COPD. Seven studies were included in the review and quality assessed to determine risk of bias, and six studies were used in pooled analysis of data. The only reference for Mahmud 2007 was a conference abstract. No further study information or primary outcome data were obtained for this study and therefore it could not be included in any meta‐analysis.
The primary outcomes measured in this review were the effects on measures of quality of life, exacerbations and mortality. There was only one study which presented data according to subgroups for disease severity measuring exacerbations, but not for quality of life or mortality. We also assessed other outcomes including lung function, symptom scores and treatment safety.
Randomisation and participant allocation were adequately described in most studies, and double‐blinding was used except for two studies where a placebo form of the tiotropium HandiHaler device was not available. Sensitivity analyses found that there was no significant reduction in heterogeneity when these two studies were excluded from meta‐analysis of the quality of life outcome. There was no identified issue in the selective reporting of outcomes. Baseline predicted lung function and smoking varied across the studies, but in general the groups were well matched at baseline. In all studies, participants who were receiving a stable dose of ICS prior to the study commencement were permitted to continue using these. There were no other sources of potential bias that were identified. As there were significantly fewer withdrawals in the tiotropium treatment group compared with LABA, this may have led to an underestimation of the differences in treatment effects.
Potential biases in the review process
The strength of this review process was that studies were identified, and data extracted, by two review authors independently. Inclusion and exclusion criteria were clearly specified to minimise the number of trials which were missed. This included a search of both published reports from international journals as well as unpublished information available on pharmaceutical company websites. We also searched relevant trial registers and cross‐checked these search results with the corresponding study name and characteristics to obtain all available data and avoid double‐counting. We contacted study authors and pharmaceutical companies if data or study information required had not already been published.
A limitation of this systematic review is that we only included studies that followed participants over a minimum of 12 weeks and therefore found a limited number of eligible studies for comparison. On the other hand, as COPD exacerbation and mortality rates, as well as quality of life, take time to determine, we felt it inappropriate to look at shorter trials. Owing to a high level of heterogeneity across studies for some outcomes (quality of life and exacerbations), it was agreed that the results were not pooled because of the risk of other factors, apart from the type of treatment under study, influencing results. Once more studies are conducted, the conclusions will be firmer.
Agreements and disagreements with other studies or reviews
The current systematic review includes several randomised controlled trials (RCTs) that examined the efficacy and safety of tiotropium versus LABA treatments in longer‐term trials which had not been identified in a previous systematic review (Yohannes 2011). Our search identified one further abstract presented at an international conference during this period (Mahmud 2007); one large 12‐month study comparing tiotropium to salmeterol which had only recently been published (Vogelmeier 2011); as well as two studies using the newest LABA, indacaterol (Burl 2011; Donohue 2010). However, because of the high level of unexplained heterogeneity, we did not think it appropriate to pool the data for quality of life. Some of this heterogeneity is likely to be related to differences between the various LABA formulations.
Consistent with the subgroup analysis findings in the present review, there was no statistically significant difference in a study that compared indacaterol and formoterol for change in quality of life and exacerbation rate (Dahl 2010). While a previous study highlighted that indacaterol was more effective than salmeterol in improving SGRQ total score and the percentage of participants who achieved clinically significant improvement in quality of life (Kornmann 2011), but did not report data on exacerbations. Both of these studies were conducted in patients with moderate to severe COPD. A review of the safety of indacaterol, tiotropium and other bronchodilators showed statistically significant decreases in exacerbation rates in all the different bronchodilators compared to placebo, but with no significant difference among the bronchodilators (Donohue 2011a).
Authors' conclusions
Implications for practice.
Based on the small amount of studies published to date, the difference between tiotropium and long‐acting beta2‐agonists (LABAs) on the outcome of quality of life is relatively small and may vary depending of the type of LABA with which tiotropium is being compared.
Notwithstanding this, we found tiotropium is significantly better at preventing exacerbations than LABAs as a group. Consistent with this finding is that tiotropium use is associated with fewer chronic obstructive pulmonary disease (COPD)‐related hospitalisations than LABAs; along with fewer serious adverse events and withdrawals during treatment. The number needed to treat (NNT) to prevent one exacerbation is 29 (95% CI 19 to 59). However, the results for all these outcomes were heavily dependent on the largest study in the review comparing tiotropium to salmeterol (Vogelmeier 2011).
Neither tiotropium nor any LABA showed any significant benefit over another in effect on lung function, symptoms of breathlessness, mortality and the overall number of hospitalisations. Until further information is available, and given such small differences in effect between tiotropium and LABAs, plus the relatively large NNT for benefit on exacerbations, one approach may be to give a COPD patient a substantial trial of tiotropium, followed by a LABA (or vice versa), then to continue prescribing the long‐acting bronchodilator that the patient prefers. To guide treatment decisions about combination therapy, end users are directed to the reviews of tiotropium plus LABA versus tiotropium or LABA (Karner 2012).
The available economic evidence suggests that tiotropium may be cost‐effective compared with salmeterol in several specific settings, but there was considerable uncertainty around this finding. End users of this review will need to assess the extent to which the results of identified economic evaluations may be applicable or transferable to their own setting. Furthermore, there are no studies of how tiotropium compares to formoterol or indacaterol in terms of cost‐effectiveness.
Implications for research.
More head‐to‐head studies of tiotropium and several different types of LABA would be helpful to confirm the main findings of this review. This review hints at differences among LABAs in their effect on the outcome of quality of life. This outcome should be reported routinely, given that both tiotropium and LABAs may cause adverse effects. Any future studies should stratify recruitment of participants and report results by COPD severity and use of concomitant inhaled corticosteroids (ICS) to explore what was suggested in subgroup analysis; namely that those with greater disease severity may respond differently to tiotropium compared with LABA, when the outcome is exacerbations. It will also be important to compare any differences in device use for both tiotropium and LABA (e.g. HandiHaler versus Respimat for tiotropium) that may impact on drug bioavailability. Similarly, more studies on different doses of indacaterol might find differences in efficacy and risk of adverse events. In addition, whether a drug is administered once daily versus twice daily might result in differences in compliance. For this reason, it is important to compare indacaterol to tiotropium – drugs which are both administered once daily. Further studies that compare tiotropium with different LABAs are currently ongoing (clinicaltrials.gov).
In terms of outcomes, it is clear that it is insufficient to look only at the average response, as this may disguise subgroups of responders. The proportion of participants who achieved a clinically significant benefit (e.g. ≥ 4 units in SGRQ score) should not be interpreted alone as an outcome unless the number who had a clinically significant deterioration is also reported. This is because without this value we cannot be certain whether the intervention has had an effect of shifting the mean or has merely widened the distribution of results
Future studies might quantify the time to first COPD exacerbation, as this figure can help with planning of health services and patient counselling.
In practice, patients may not just take a LABA or tiotropium. Often these medicines are used together, and/or in conjunction with ICS. More randomised controlled trials are needed that compare combinations of these medicines, as well as testing add‐on strategies, to see how much there is to gain by changing or adding another treatment.
Economic evaluations comparing the cost‐effectiveness of tiotropium and indacaterol in different settings are needed. Indacaterol is a once‐daily medicine, like tiotropium, and that may be important.
Feedback
Feedback about reporting of uncertainty and bias, 27 October 2016
Summary
Thank you for your review into tiotropium vs. LABA for the prevention of COPD exacerbations.
Your conclusion states that tiotropium is more effective than long‐acting beta‐agonists (LABA) as a group in the prevention of COPD exacerbations, and related hospitalizations, and that tiotropium use is associated with a reduction in withdrawal during treatment. These statements may be misguided due to the degree of uncertainty within the literature included in your review.
The risk of bias assessment for incomplete outcome reporting, or attrition bias, was generous in rating the Vogelmeier 2011 trial as “uncertain” (1,2). Vogelmeier 2011, the largest and most heavily weighted study included in this review, lists 1,233 of 7,376 patients as withdrawing prematurely for a variety of reasons (1). This accounts for 16.7% of the study population, and over 10% of the entire population included in your meta‐analysis. We are concerned by the number of events which may not have been recorded due to the high rate of attrition. Your summary of findings suggests that treating 100 patients with tiotropium prevents 3 individuals from having at least one exacerbation compared to LABA (2). However, the number of patients lost to attrition in the meta‐analysis, 1,877, is five times greater than the estimated number of patients who would benefit. Such a large rate of attrition compared to treatment effect could alter the estimated effect of tiotropium. Imputation may improve the robustness of your meta‐analysis (3). Imputation may overestimate the number of events by effectively double counting patients who had an event counted prior to their withdrawal; however it would act as a robust comparator for the effect of attrition bias. Without imputation to compare with your result, the large amount of missing data from these trials impairs the reader’s ability to draw definitive conclusions despite your well‐executed review.
Considering the small number of patients exposed to formoterol and indacaterol in your meta‐analysis, and that the difference seen is driven by a salmeterol trial there is insufficient evidence to suggest that the difference is between tiotropium and LABA, as a group. Rather, we believe the data presented reflects potential differences between tiotropium and salmeterol only.
Finally, as mentioned in your review, COPD‐related hospitalizations appeared to decrease, without a decrease in all‐cause hospitalization. This suggests that there is an increase in the rate of non‐COPD related hospitalizations. The conclusion should weigh more heavily on the fact that all‐cause hospitalization is not reduced.
References
Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten‐van Mölken MPMH, Beeh KM, et al. 2011 Tiotropium versus Salmeterol for the Prevention of Exacerbations of COPD. New England Journal of Medicine, 364(12), 1093–1103. http://doi.org/10.1056/NEJMoa1008378
Chong J, Karner C, Poole P, 2012 Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews, (9), CD009157–128. http://doi.org/10.1002/14651858.CD009157.pub2
Akl EA, Kahale LA, Agoritsas T, Brignardello‐Petersen R, Busse JW, Carrasco‐Labra A, et al. 2015 Handling trial participants with missing outcome data when conducting a meta‐analysis: a systematic survey of proposed approaches. Systematic Reviews, 4(1), 1–7. http://doi.org/10.1186/s13643‐015‐0083‐6
Reply
Thank you for your interest in our review and for your feedback. Responses to your points are made below. While we have not made any changes to the review, your comments will be helpful at the time of the next update.
This review analysed the results of six studies comparing tiotropium with LABA (12,123 participants). The largest and longest study, Vogelmeier 2011, showed a statistically significant difference favouring tiotropium over LABA (salmeterol) in terms of COPD exacerbations, whereas all the other studies showed no statistically significant difference and wider confidence intervals (Figure 5). The combined odd ratio for this outcome was OR 0.86 (95% CI 0.79 to 0.93). There was no significant heterogeneity noted in these results. The number of participants requiring hospitalisation for a COPD exacerbation was significantly lower among participants who received tiotropium compared to participants receiving LABA (OR 0.87; 95% CI 0.77 to 0.99; Analysis 1.15) in the four studies which reported this as an outcome (9267 participants). Those treated with tiotropium had a significantly lower rate of study withdrawal (14.5% versus 16.3%) compared to those receiving a LABA (OR 0.89; 95% CI 0.81 to 0.99, Analysis 1.21).
The risk of bias assessment for incomplete outcome reporting for this study was rated as “uncertain” as it is unclear as to the direction and extent of possible attrition bias in this analysis. The authors agree that in future updates of this review an imputation analysis could be conducted for comparison. Upon this, reconsideration of upgrading this risk of bias heading to “high risk” can be made. Unfortunately, a high level of participant drop outs are not an uncommon occurrence in long term COPD trials. However, the rate of withdrawals from treatment was significantly reduced in the tiotropium group and was thus highlighted as an important finding in this review.
Research is still emerging looking into how newer LABAs compare with other formulations. With only a handful of studies, it goes beyond the scope of the review to suggest that there are differences when comparing tiotropium between the types of LABA based on current evidence. Furthermore, as the trials were not conducted in the same populations or conditions, metaanalysis can only hypothesise there may be a difference among LABAs. To highlight this, the authors have raised this issue in the 'implications for research' section, where more head‐to‐head comparative studies would be helpful to confirm the main findings and consider possible differences among LABA types.
We agree it would be concerning if the treatment increased hospitalisations for other causes. However, some of the difficulty in assessing hospitalisation data is (a) the relative infrequency of this outcome, (b) the variability in how this is collected and reported across the different studies (triallists may be more focussed on the outcomes of interest, than others), (c) the patients in these trials are older and have comorbidities that may require hospitalisation that have nothing to do with COPD. All three of these may impact on the accuracy of this outcome. Although the absence of a decrease in all‐cause hospitalisations may show (as pointed out by this reviewer) an increase in non‐COPD related hospitalisations, it is useful to point out that these separate analyses each involve different studies and are not a direct comparison. Where possible, COPD‐related hospitalisations were preferred for inclusion in this review.
Contributors
Wright RC, Beach J, Tejani AM
Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, Canada.
What's new
| Date | Event | Description |
|---|---|---|
| 17 November 2016 | Feedback has been incorporated | Feedback recieved. The authors responded to the feedback and identified possible ares for consideration in a future update of this review. No change made to the review. |
History
Protocol first published: Issue 6, 2011 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 11 June 2013 | Amended | Typo in summary of findings table corrected |
| 12 April 2013 | Amended | Funder acknowledgement added |
Acknowledgements
We are grateful to Elizabeth Stovold for help designing the search strategy, and to Steve Edwards for feedback and guidance regarding all aspects of the inclusion of economic evaluations in the review.
Boehringer Ingelheim and Novartis provided further information on request.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (T he Cochrane Library) | Monthly |
| PSYCINFO (Ovid) | Monthly |
| CINAHL (Ebsco) | Monthly |
| AMED (Ebsco) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy for Clinicaltrials.gov
| Keyword | Search results* |
| COPD + tiotropium + salmeterol | 33 |
| COPD + tiotropium + formoterol | 21 |
| COPD + tiotropium + indacaterol | 13 |
*Search conduction July 2011.
Appendix 3. Methodological quality checklists for economic evaluations
| Gani 2010 | ||||||
| Item | Dimension of quality | Question for critical appraisal | Yes | No | N/A | Comment |
| Structure | ||||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | x | |||
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | x | |||||
| Is the primary decision‐maker specified? | x | |||||
| S2 | Statement of scope/perspective | Is the perspective of the model stated clearly? | x | |||
| Are the model inputs consistent with the stated perspective? | x | |||||
| Has the scope of the model been stated and justified? | x | |||||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | x |
|||||
| S3 | Rationale for structure | Has the evidence regarding the model structure been described? | x | Referencing original study; Oostenbrink 2005 | ||
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | x | The model does not include a health state of death | ||||
| Have any competing theories regarding model structure been considered? | x | |||||
| Are the sources of data used to develop the structure of the model specified? | x | Referencing original study; Oostenbrink 2005 | ||||
| Are the causal relationships described by the model structure justified appropriately? | x | |||||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | x | |||
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | x | |||||
| S5 | Strategies/ comparators | Is there a clear definition of the options under evaluation? | x | |||
| Have all feasible and practical options been evaluated? | x | |||||
| Is there justification for the exclusion of feasible options? | x | |||||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | x | |||
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | x | |||
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | x | |||||
| Has a lifetime horizon been used? If not, has a shorter time horizon been justified? | x | |||||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | x |
|||
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | x | |||
| Data | ||||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | x | |||
| Where choices have been made between data sources, are these justified appropriately? | x | Not described | ||||
| Has particular attention been paid to identifying data for the important parameters in the model? | x | |||||
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | x | |||||
| Has the quality of the data been assessed appropriately? | x | Referencing original study; Oostenbrink 2005 | ||||
| Where expert opinion has been used, are the methods described and justified? | x | |||||
| D2 | Pre‐model data analysis | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | x | |||
| D2a | Baseline data | Is the choice of baseline data described and justified? | x | Described but not justified | ||
| Are transition probabilities calculated appropriately? | x | |||||
| Has a half‐cycle correction been applied to both cost and outcome? | x | |||||
| If not, has this omission been justified? | x | |||||
| D2b | Treatment effects | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | x | For each comparison data were derived from a single trial | ||
| Have the methods and assumptions used to extrapolate short‐term results to final outcomes been documented and justified? | x | |||||
| Have alternative assumptions used to extrapolate short‐term results to final outcomes been explored through sensitivity analysis? | x | |||||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | x | |||||
| Have alternative assumptions regarding the continuing effect of treatment once treatment is complete been explored through sensitivity analysis? | x | |||||
| D2c | Costs | Are the costs incorporated into the model justified? | x | |||
| Has the source for all costs been described? | x | |||||
| Have discount rates been described and justified given the target decision‐maker? | x | |||||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | x | |||
| Is the source for the utility weights referenced? | x | |||||
| Are the methods of derivation for the utility weights justified? | x | Referencing original study; Oostenbrink 2005 | ||||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | x | |||
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | x | |||||
| Is the process of data incorporation transparent? | x | |||||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | x | Referencing original study; Oostenbrink 2005 | ||||
| If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | x | |||||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty (D4a ‐ D4d below) been addressed? | x | |||
| If not, has the omission of particular forms of uncertainty been justified? | x | |||||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | x | |||
| D4b | Structural | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | x | Referencing original study; Oostenbrink 2005 | ||
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | x | |||
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | x | |||
| Has probabilistic sensitivity analysis been done? If, not has this been justified? | x | |||||
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | x | |||||
| Consistency | ||||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | x | |||
| C2 | External consistency | Are the conclusions valid given the data presented? | x | |||
| Are any counterintuitive results from the model explained and justified? | x | |||||
| If the model has been calibrated against independent data, have any differences been explained and justified? | x | |||||
| Have the results of the model been compared with those of previous models and any differences in results explained? | x | |||||
| Maniadakis 2006 | ||||||
| Item | Dimension of quality | Question for critical appraisal | Yes | No | N/A | Comment |
| Structure | ||||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | x | |||
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | x | |||||
| Is the primary decision‐maker specified? | x | |||||
| S2 | Statement of scope/perspective | Is the perspective of the model stated clearly? | x | |||
| Are the model inputs consistent with the stated perspective? | x | |||||
| Has the scope of the model been stated and justified? | x | |||||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | x | |||||
| S3 | Rationale for structure | Has the evidence regarding the model structure been described? | x | Referencing original study; Oostenbrink 2005 | ||
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | x | The model does not include a health state of death | ||||
| Have any competing theories regarding model structure been considered? | x | |||||
| Are the sources of data used to develop the structure of the model specified? | x | Referencing original study; Oostenbrink 2005 | ||||
| Are the causal relationships described by the model structure justified appropriately? | x | |||||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | x | |||
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | x | |||||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | x | |||
| Have all feasible and practical options been evaluated? | x | |||||
| Is there justification for the exclusion of feasible options? | x | |||||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | x | |||
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | x | |||
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | x | |||||
| Has a lifetime horizon been used? If not, has a shorter time horizon been justified? | x | |||||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | x | |||
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | x | |||
| Data | ||||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | x | |||
| Where choices have been made between data sources, are these justified appropriately? | x | Not described | ||||
| Has particular attention been paid to identifying data for the important parameters in the model? | x | |||||
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | x | |||||
| Has the quality of the data been assessed appropriately? | x | Referencing original study; Oostenbrink 2005 | ||||
| Where expert opinion has been used, are the methods described and justified? | x | |||||
| D2 | Pre‐model data analysis | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | x | |||
| D2a | Baseline data | Is the choice of baseline data described and justified? | x | Described but not justified | ||
| Are transition probabilities calculated appropriately? | x | |||||
| Has a half‐cycle correction been applied to both cost and outcome? | x | |||||
| If not, has this omission been justified? | x | |||||
| D2b | Treatment effects | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | x | For each comparison data were derived from a single trial | ||
| Have the methods and assumptions used to extrapolate short‐term results to final outcomes been documented and justified? | x | |||||
| Have alternative assumptions used to extrapolate short‐term results to final outcomes been explored through sensitivity analysis? | x | |||||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | x | |||||
| Have alternative assumptions regarding the continuing effect of treatment once treatment is complete been explored through sensitivity analysis? | x | |||||
| D2c | Costs | Are the costs incorporated into the model justified? | x | |||
| Has the source for all costs been described? | x | |||||
| Have discount rates been described and justified given the target decision‐maker? | x | |||||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | x | |||
| Is the source for the utility weights referenced? | x | |||||
| Are the methods of derivation for the utility weights justified? | x | Referencing original study; Oostenbrink 2005 | ||||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | x | |||
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | x | |||||
| Is the process of data incorporation transparent? | x | |||||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | x | Referencing original study; Oostenbrink 2005 | ||||
| If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | x | |||||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty (D4a ‐ D4d below) been addressed? | x | |||
| If not, has the omission of particular forms of uncertainty been justified? | x | |||||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | x | |||
| D4b | Structural | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | x | Referencing original study; Oostenbrink 2005 | ||
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | x | |||
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | x | |||
| Has probabilistic sensitivity analysis been done? If, not has this been justified? | x | |||||
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | x | |||||
| Consistency | ||||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | x | |||
| C2 | External consistency | Are the conclusions valid given the data presented? | x | |||
| Are any counterintuitive results from the model explained and justified? | x | |||||
| If the model has been calibrated against independent data, have any differences been explained and justified? | x | |||||
| Have the results of the model been compared with those of previous models and any differences in results explained? | x | |||||
| Naik 2010 | ||||||
| Item | Dimension of quality | Question for critical appraisal | Yes | No | N/A | Comment |
| Structure | ||||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | x | |||
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | x | |||||
| Is the primary decision‐maker specified? | x | |||||
| S2 | Statement of scope/perspective | Is the perspective of the model stated clearly? | x | |||
| Are the model inputs consistent with the stated perspective? | x | |||||
| Has the scope of the model been stated and justified? | x | |||||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | x |
|||||
| S3 | Rationale for structure | Has the evidence regarding the model structure been described? | x | |||
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | x | The model does not include a health state of death | ||||
| Have any competing theories regarding model structure been considered? | x | |||||
| Are the sources of data used to develop the structure of the model specified? | x | |||||
| Are the causal relationships described by the model structure justified appropriately? | x | |||||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | x | Transparent but not justified | ||
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | x | |||||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | x | |||
| Have all feasible and practical options been evaluated? | x | |||||
| Is there justification for the exclusion of feasible options? | x | |||||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | x | |||
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | x | |||
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | x | |||||
| Has a lifetime horizon been used? If not, has a shorter time horizon been justified? | x | |||||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | x |
|||
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | x | Described but not justified | ||
| Data | ||||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | x | |||
| Where choices have been made between data sources, are these justified appropriately? | x | |||||
| Has particular attention been paid to identifying data for the important parameters in the model? | x | |||||
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | x | |||||
| Has the quality of the data been assessed appropriately? | x | |||||
| Where expert opinion has been used, are the methods described and justified? | x | |||||
| D2 | Pre‐model data analysis | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | x | |||
| D2a | Baseline data | Is the choice of baseline data described and justified? | x | Described but not justified | ||
| Are transition probabilities calculated appropriately? | x | |||||
| Has a half‐cycle correction been applied to both cost and outcome? | x | |||||
| If not, has this omission been justified? | x | |||||
| D2b | Treatment effects | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | x | |||
| Have the methods and assumptions used to extrapolate short‐term results to final outcomes been documented and justified? | x | |||||
| Have alternative assumptions used to extrapolate short‐term results to final outcomes been explored through sensitivity analysis? | x | |||||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | x | |||||
| Have alternative assumptions regarding the continuing effect of treatment once treatment is complete been explored through sensitivity analysis? | x | |||||
| D2c | Costs | Are the costs incorporated into the model justified? | x | |||
| Has the source for all costs been described? | x | |||||
| Have discount rates been described and justified given the target decision‐maker? | x | |||||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | x | |||
| Is the source for the utility weights referenced? | x | |||||
| Are the methods of derivation for the utility weights justified? | x | |||||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | x | |||
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | x | |||||
| Is the process of data incorporation transparent? | x | |||||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | x | |||||
| If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | x | |||||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty (D4a ‐ D4d below) been addressed? | x | |||
| If not, has the omission of particular forms of uncertainty been justified? | x | |||||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | x | |||
| D4b | Structural | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | x | |||
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | x | |||
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | x | |||
| Has probabilistic sensitivity analysis been done? If, not has this been justified? | x | |||||
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | x | |||||
| Consistency | ||||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | x | |||
| C2 | External consistency | Are the conclusions valid given the data presented? | x | |||
| Are any counterintuitive results from the model explained and justified? | x | |||||
| If the model has been calibrated against independent data, have any differences been explained and justified? | x | |||||
| Have the results of the model been compared with those of previous models and any differences in results explained? | x | |||||
| Oba 2007 | ||||
| Yes | No | N/A | Comment | |
| Study design | ||||
| (I) The research question is stated | x | |||
| (2) The economic importance of the research question is stated | x | |||
| (3) The viewpoint(s) of the analysis are clearly stated and justified | x | |||
| (4) The rationale for choosing the alternative programmes or interventions compared is stated | x | |||
| (5) The alternatives being compared are clearly described | x | |||
| (6) The form of economic evaluation used is stated | x | |||
| (7) The choice of form of economic evaluation is justified in relation to the questions addressed | x | |||
| Data collection | ||||
| (8) The source(s) of effectiveness estimates used are stated | x | |||
| (9) Details of the design and results of effectiveness study are given (if based on a single study) | x | |||
| (10) Details of the method of synthesis or meta‐analysis of estimates are given (if based on an overview of a number of effectiveness studies) | x | |||
| (I I) The primary outcome measure(s) for the economic evaluation are clearly stated | x | |||
| (12) Methods to value health states and other benefits are stated | x | |||
| (13) Details of the subjects from whom valuations were obtained are given | x | |||
| (14) Productivity changes (if included) are reported separately | x | |||
| (15) The relevance of productivity changes to the study question is discussed | x | |||
| (6) Quantities of resources are reported separately from their unit costs | x | |||
| (17) Methods for the estimation of quantities and unit costs are described | x | |||
| (18) Currency and price data are recorded | x | |||
| (19) Details of currency of price adjustments for inflation or currency conversion are given | x | |||
| (20) Details of any model used are given | x | |||
| (21) The choice of model used and the key parameters on which it is based are justified | x | |||
| Analysis and interpretation of results | ||||
| (22) Time horizon of costs and benefits is stated | x | |||
| (23) The discount rate(s) is stated | x | |||
| (24) The choice of rate(s) is justified | x | |||
| (25) An explanation is given if costs or benefits are not discounted | x | |||
| (26) Details of statistical tests and confidence intervals are given for stochastic data | x | |||
| (27) The approach to sensitivity analysis is given | x | |||
| (28) The choice of variables for sensitivity analysis is justified | x | |||
| (29) The ranges over which the variables are varied are stated | x | |||
| (30) Relevant alternatives are compared | x | |||
| (31) Incremental analysis is reported | x | |||
| (32) Major outcomes are presented in a disaggregated as well as aggregated form | x | |||
| (33) The answer to the study question is given | x | |||
| (34) Conclusions follow from the data reported | x | |||
| (35) Conclusions are accompanied by the appropriate caveats | x | |||
| Oostenbrink 2005 | ||||||
| Item | Dimension of quality | Question for critical appraisal | Yes | No | N/A | Comment |
| Structure | ||||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | x | |||
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | x | |||||
| Is the primary decision‐maker specified? | x | |||||
| S2 | Statement of scope/perspective | Is the perspective of the model stated clearly? | x | |||
| Are the model inputs consistent with the stated perspective? | x | |||||
| Has the scope of the model been stated and justified? | x | |||||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | x | |||||
| S3 | Rationale for structure | Has the evidence regarding the model structure been described? | x | |||
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | x | The model does not include a health state of death | ||||
| Have any competing theories regarding model structure been considered? | x | |||||
| Are the sources of data used to develop the structure of the model specified? | x | |||||
| Are the causal relationships described by the model structure justified appropriately? | x | |||||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | x | |||
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | x | |||||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | x | |||
| Have all feasible and practical options been evaluated? | x | |||||
| Is there justification for the exclusion of feasible options? | x | |||||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | x | |||
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | x | |||
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | x | |||||
| Has a lifetime horizon been used? If not, has a shorter time horizon been justified? | x | |||||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | x | |||
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | x | |||
| Data | ||||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | x | |||
| Where choices have been made between data sources, are these justified appropriately? | x | Not described | ||||
| Has particular attention been paid to identifying data for the important parameters in the model? | x | |||||
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | x | |||||
| Has the quality of the data been assessed appropriately? | x | |||||
| Where expert opinion has been used, are the methods described and justified? | x | |||||
| D2 | Pre‐model data analysis | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | x | |||
| D2a | Baseline data | Is the choice of baseline data described and justified? | x | |||
| Are transition probabilities calculated appropriately? | x | |||||
| Has a half‐cycle correction been applied to both cost and outcome? | x | |||||
| If not, has this omission been justified? | x | |||||
| D2b | Treatment effects | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | x | For each comparison data were derived from a single trial | ||
| Have the methods and assumptions used to extrapolate short‐term results to final outcomes been documented and justified? | x | |||||
| Have alternative assumptions used to extrapolate short‐term results to final outcomes been explored through sensitivity analysis? | x | |||||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | x | |||||
| Have alternative assumptions regarding the continuing effect of treatment once treatment is complete been explored through sensitivity analysis? | x | |||||
| D2c | Costs | Are the costs incorporated into the model justified? | x | |||
| Has the source for all costs been described? | x | |||||
| Have discount rates been described and justified given the target decision‐maker? | x | |||||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | x | |||
| Is the source for the utility weights referenced? | x | |||||
| Are the methods of derivation for the utility weights justified? | x | |||||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | x | |||
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | x | |||||
| Is the process of data incorporation transparent? | x | |||||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | x | |||||
| If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | x | |||||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty (D4a ‐ D4d below) been addressed? | x | |||
| If not, has the omission of particular forms of uncertainty been justified? | x | |||||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | x | |||
| D4b | Structural | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | x | |||
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | x | |||
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | x | |||
| Has probabilistic sensitivity analysis been done? If, not has this been justified? | x | |||||
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | x | |||||
| Consistency | ||||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | x | |||
| C2 | External consistency | Are the conclusions valid given the data presented? | x | |||
| Are any counterintuitive results from the model explained and justified? | x | |||||
| If the model has been calibrated against independent data, have any differences been explained and justified? | x | |||||
| Have the results of the model been compared with those of previous models and any differences in results explained? | x | |||||
| Rutten‐van Molken 2007 | ||||||
| Item | Dimension of quality | Question for critical appraisal | Yes | No | N/A | Comment |
| Structure | ||||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | x | |||
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | x | |||||
| Is the primary decision‐maker specified? | x | |||||
| S2 | Statement of scope/perspective | Is the perspective of the model stated clearly? | x | |||
| Are the model inputs consistent with the stated perspective? | x | |||||
| Has the scope of the model been stated and justified? | x | |||||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | x | |||||
| S3 | Rationale for structure | Has the evidence regarding the model structure been described? | x | Referencing original study; Oostenbrink 2005 | ||
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | x | |||||
| Have any competing theories regarding model structure been considered? | x | |||||
| Are the sources of data used to develop the structure of the model specified? | x | Referencing original study; Oostenbrink 2005 | ||||
| Are the causal relationships described by the model structure justified appropriately? | x | |||||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | x | |||
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | x | |||||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | x | |||
| Have all feasible and practical options been evaluated? | x | |||||
| Is there justification for the exclusion of feasible options? | x | |||||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | x | |||
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | x | |||
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | x | |||||
| Has a lifetime horizon been used? If not, has a shorter time horizon been justified? | x | |||||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | x | |||
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | x | |||
| Data | ||||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | x | |||
| Where choices have been made between data sources, are these justified appropriately? | x | Not described | ||||
| Has particular attention been paid to identifying data for the important parameters in the model? | x | |||||
| Has the process of selecting key parameters been justified and systematic methods used to identify the most appropriate data? | x | |||||
| Has the quality of the data been assessed appropriately? | x | Referencing original study; Oostenbrink 2005 | ||||
| Where expert opinion has been used, are the methods described and justified? | x | |||||
| D2 | Pre‐model data analysis | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | x | |||
| D2a | Baseline data | Is the choice of baseline data described and justified? | x | Described but not justified | ||
| Are transition probabilities calculated appropriately? | x | |||||
| Has a half‐cycle correction been applied to both cost and outcome? | x | |||||
| If not, has this omission been justified? | x | |||||
| D2b | Treatment effects | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | x | For each comparison data were derived from a single trial | ||
| Have the methods and assumptions used to extrapolate short‐term results to final outcomes been documented and justified? | x | |||||
| Have alternative assumptions used to extrapolate short‐term results to final outcomes been explored through sensitivity analysis? | x | |||||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | x | |||||
| Have alternative assumptions regarding the continuing effect of treatment once treatment is complete been explored through sensitivity analysis? | x | |||||
| D2c | Costs | Are the costs incorporated into the model justified? | x | |||
| Has the source for all costs been described? | x | |||||
| Have discount rates been described and justified given the target decision‐maker? | x | |||||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | x | |||
| Is the source for the utility weights referenced? | x | |||||
| Are the methods of derivation for the utility weights justified? | x | Referencing original study; Oostenbrink 2005 | ||||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | x | |||
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | x | |||||
| Is the process of data incorporation transparent? | x | |||||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | x | Referencing original study; Oostenbrink 2005 | ||||
| If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | x | |||||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty (D4a ‐ D4d below) been addressed? | x | |||
| If not, has the omission of particular forms of uncertainty been justified? | x | |||||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | x | |||
| D4b | Structural | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | x | Referencing original study; Oostenbrink 2005 | ||
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | x | |||
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | x | |||
| Has probabilistic sensitivity analysis been done? If, not has this been justified? | x | |||||
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | x | |||||
| Consistency | ||||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | x | |||
| C2 | External consistency | Are the conclusions valid given the data presented? | x | |||
| Are any counterintuitive results from the model explained and justified? | x | |||||
| If the model has been calibrated against independent data, have any differences been explained and justified? | x | |||||
| Have the results of the model been compared with those of previous models and any differences in results explained? | x | |||||
Data and analyses
Comparison 1. Tiotropium versus LABAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HRQoL (SGRQ) | 4 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 2 Subgroup analysis: HRQoL (SGRQ) by type of LABA | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Salmeterol | 1 | Mean Difference (Random, 95% CI) | ‐1.4 [‐3.34, 0.54] | |
| 2.2 Formoterol | 1 | Mean Difference (Random, 95% CI) | 1.0 [‐1.70, 3.70] | |
| 2.3 Indacaterol | 2 | Mean Difference (Random, 95% CI) | 2.1 [0.97, 3.23] | |
| 3 Subgroup analysis: HRQoL (SGRQ) by study duration | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 < 6 months duration | 1 | Mean Difference (Random, 95% CI) | 2.1 [0.56, 3.64] | |
| 3.2 ≥ 6 months duration | 3 | Mean Difference (Random, 95% CI) | 0.57 [‐1.69, 2.84] | |
| 4 Participants with ≥ 4 units improvement in quality of life (SGRQ) | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Participants with ≥ 4 units worsening in quality of life (SGRQ) | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Patients with 1 or more exacerbations | 6 | 12123 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.79, 0.93] |
| 7 Subgroup analysis: participants with 1 or more exacerbations by type of LABA | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Salmeterol | 3 | 8836 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.77, 0.92] |
| 7.2 Formoterol | 1 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.68, 2.55] |
| 7.3 Indacaterol | 2 | 2856 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.28] |
| 8 Subgroup analysis: participants with 1 or more exacerbations by severity at baseline | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 GOLD II | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 GOLD III | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 GOLD IV | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Subgroup analysis: participants with 1 or more exacerbations by concomitant medication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 ICS users | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Non ICS users | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Subgroup analysis: participants with 1 or more exacerbations (hazard ratio) by concomitant medication | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 10.1 ICS users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Non ICS users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Subgroup analysis: participants with 1 or more exacerbations by study duration | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 < 6 months duration | 2 | 2251 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.59, 1.04] |
| 11.2 ≥ 6 months duration | 4 | 9872 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.04] |
| 12 Mortality (all‐cause) | 6 | 12123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.60, 1.13] |
| 13 Subgroup analysis: mortality (all cause) by type of LABA | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Salmeterol | 3 | 8836 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.25, 1.87] |
| 13.2 Formoterol | 1 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Indacaterol | 2 | 2856 | Odds Ratio (M‐H, Random, 95% CI) | 4.36 [0.66, 28.70] |
| 14 Subgroup analysis: mortality (all cause) by study duration | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 < 6 months duration | 2 | 2251 | Odds Ratio (M‐H, Random, 95% CI) | 3.91 [0.43, 35.45] |
| 14.2 ≥ 6 months duration | 4 | 9872 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.22, 2.63] |
| 15 Hospitalisations due to COPD exacerbations | 4 | 9267 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.77, 0.99] |
| 16 Hospitalisations (all‐cause) | 3 | 3509 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.54] |
| 17 FEV1 (mL) | 5 | 4600 | Mean Difference (IV, Random, 95% CI) | 10.52 [‐11.47, 32.51] |
| 18 Symptoms (TDI) | 3 | 3180 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.63, 0.19] |
| 19 Symptoms (daily total symptom score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20 All‐cause, non‐fatal serious adverse events | 6 | 12123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.99] |
| 21 Withdrawals (all‐cause) | 6 | 12123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.99] |
1.1. Analysis.
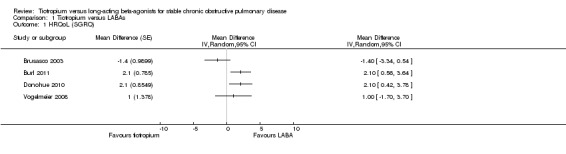
Comparison 1 Tiotropium versus LABAs, Outcome 1 HRQoL (SGRQ).
1.6. Analysis.
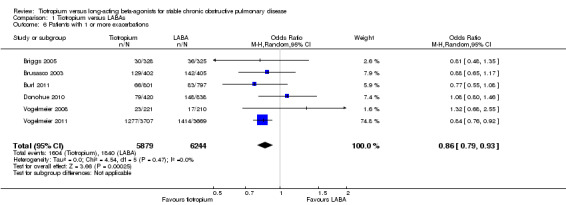
Comparison 1 Tiotropium versus LABAs, Outcome 6 Patients with 1 or more exacerbations.
1.12. Analysis.
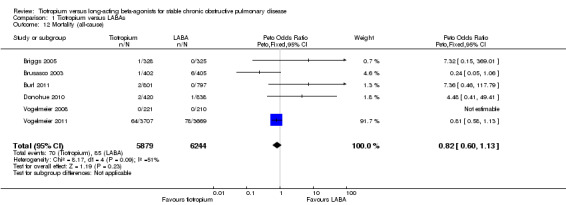
Comparison 1 Tiotropium versus LABAs, Outcome 12 Mortality (all‐cause).
1.13. Analysis.
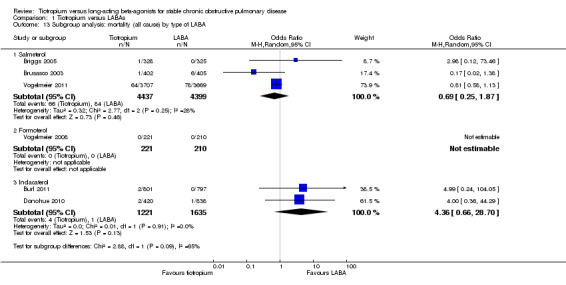
Comparison 1 Tiotropium versus LABAs, Outcome 13 Subgroup analysis: mortality (all cause) by type of LABA.
1.14. Analysis.
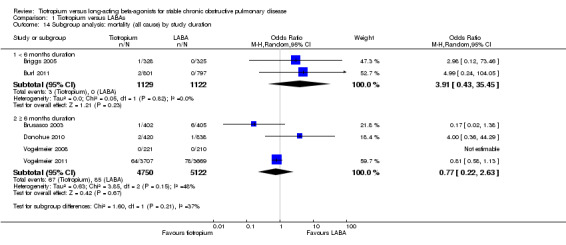
Comparison 1 Tiotropium versus LABAs, Outcome 14 Subgroup analysis: mortality (all cause) by study duration.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Briggs 2005.
| Methods |
Design: 12‐week, randomised, double‐blind, double‐dummy, parallel‐group study Setting: the study was conducted in 50 centres located in 8 countries, including Finland, Greece, Italy, Portugal, Sweden, Turkey, the United Kingdom and the United States Date of study: May 2002 to March 2003 |
|
| Participants |
Participants: n = 653 (tiotropium: 328, salmeterol: 325) Baseline characteristics: mean age (tiotropium: 64.2 years, salmeterol 64.6 years); gender (tiotropium 65% male, salmeterol 68% male); mean % predicted FEV1 (tiotropium 37.7, salmeterol 37.7%); mean smoking pack year history (tiotropium 55.6 years, salmeterol 56.1 years) Diagnostic criteria: not specified COPD severity: severe Inclusion criteria: patients who were ≥ 40 years of age, with a cigarette smoking history of ≥ 10 pack years, and a clinical diagnosis of COPD, were eligible for inclusion in the study if they had a FEV1 % predicted ≤ 60% and FVC ≤ 70% Exclusion criteria: patients with a history of asthma, allergic rhinitis, atopy or a total (absolute) blood eosinophil count ≥ 600 mm were excluded from the study, as were those with any significant medical condition that could preclude participation for the full duration of the trial or interfere with the interpretation of the study results. Patients were also excluded from the study if they took systemic corticosteroids at unstable doses or in daily doses of ≥ 10 mg (or its equivalent), if they were using beta‐blockers, cromones, or anti‐leukotrienes prior to enrolment in the trial, or if they had experienced a respiratory tract infection or a COPD exacerbation within 30 days of randomisation. Patients using oxygen for more than 1 h per day and who were unable to refrain from its use during pulmonary function testing were also excluded. Additionally, patients were excluded who were actively participating in a rehabilitation programme or had completed such a programme during the previous 30 days. |
|
| Interventions |
Run‐in period: a 2‐week screening period during which baseline use of rescue salbutamol (albuterol) use was recorded on a diary card. During the screening period, patients who were taking fixed combination respiratory medications (i.e. combinations of ICS plus LABA, or anticholinergics plus short‐acting beta‐agonists) prior to study enrolment were switched to the component monoproducts. Patients taking LABAs were required to stop this medication 24 h prior to randomisation. Interventions: 1. Tiotropium, 18 µg once daily via the HandiHaler device; or 2. Salmeterol, 2 actuations of 25 µg each, twice daily via a metered dose inhaler Concomitant medication
"Patients were not permitted to take anticholinergic agents or LABAs other than study medication during the treatment period. Patients otherwise received usual medical care, and were permitted to use rescue salbutamol, which was provided during the study, as well as previously prescribed theophylline compounds, inhaled steroids, and modest doses of oral steroids". |
|
| Outcomes |
Primary outcome(s): the co‐primary efficacy outcomes were average post‐dose FEV1 over 12 h and peak FEV1 after 12 weeks of treatment. Average FEV1 was estimated from the area under the curve from 0 to 12 h (AUC 0–12). Secondary outcome(s): secondary outcomes including morning pre‐dose FEV1, FEV1 at each time point over 12 h, corresponding FVC parameters, incidence and frequency of COPD exacerbations (the number or percentage of patients with at least one COPD exacerbation, time to first exacerbation, number of exacerbations, and exacerbation days), rescue medication use, and incidence of serious adverse events |
|
| Notes |
Funding: this study was funded by Boehringer Ingelheim and Pfizer Boehringer Ingelheim trial number 205.264 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively even between the groups and relatively small (tiotropium 8.8%, salmeterol 12.6%) |
| Selective reporting (reporting bias) | Low risk | All collected data reported |
Brusasco 2003.
| Methods |
Design: 2 studies of 6 months, randomised, double‐blind, double‐dummy, parallel‐group Setting: the studies was conducted in 18 countries Date of study: January 2008 to April 2009 |
|
| Participants |
Participants: n = 1207 (tiotropium: 402, salmeterol: 405) Baseline characteristics: mean age (tiotropium: 63.8 years, salmeterol 64.1 years); gender (tiotropium 77% male, salmeterol 75% male); mean % predicted FEV1 (tiotropium 39.2%, salmeterol 37.7%); mean smoking pack year history (tiotropium 44.1 years, salmeterol 44.8 years) Diagnostic criteria: unspecified COPD severity: severe Inclusion criteria: patients were required to have relatively stable airway obstruction with FEV1 < 65% of predicted normal and < 70% of FVC, > 40 years of age, with a smoking history of > 10 pack years Exclusion criteria: patients with a history of asthma, allergic rhinitis, atopy or with an increased total eosinophil count were excluded. Other exclusion criteria included use of supplemental oxygen or an upper respiratory tract infection in the 6 weeks before screening. Those patients with a significant disease other than COPD were not enrolled. |
|
| Interventions |
Run‐in period: a 2‐week baseline period followed an initial screening visit Interventions: 1. 18 µg of tiotropium once daily, delivered through the HandiHaler inhalation device, plus MDI placebo; or 2. 50 µg of salmeterol twice daily, delivered through a pressurised, metered‐dose inhaler, plus HandiHaler placebo Concomitant medication
|
|
| Outcomes | FEV1, FVC, dyspnoea (evaluated using the baseline dyspnoea index (BDI) and the transition dyspnoea index (TDI)), health‐related quality of life (determined using the St George’s Respiratory Questionnaire (SGRQ)), exacerbations of COPD (number of exacerbations, number of exacerbation days, percentage of patients with at least one COPD exacerbation, time to first COPD exacerbation), hospital admissions (hospital admissions for any reason, number of hospital admissions for an exacerbation, days hospitalised, percentage of patients with at least one hospital admission for a COPD exacerbation, time to first hospital admission due to a COPD exacerbation), concomitant medications, non‐scheduled contacts with physicians and other healthcare providers (use of the intensive care unit (ICU)), disability days (days unable to perform daily activities) and employment status | |
| Notes |
Funding: these studies were funded by Boehringer Ingelheim Additional notes: this reports the results of 2 studies: Boehringer Ingelheim trial number 205.130 and 205.137 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock. Double‐dummy technique was used to blind different application devices. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively even between the groups but moderately high (tiotropium 15.4%, salmeterol 18.8%) |
| Selective reporting (reporting bias) | Low risk | All collected data reported |
Burl 2011.
| Methods |
Design: 12 week, multi‐centre, randomised, parallel‐group, blinded, double‐dummy Setting: 223 centres in 22 countries: Austria, Belgium, Canada, Colombia, Denmark, Finland, France, Germany, Greece, Hungary, Israel, Italy, Mexico, Norway, Poland, Russia, Slovakia, Spain, Switzerland, Turkey, UK and USA Date of study: June 2009 to March 2010 |
|
| Participants |
Participants: n = 1598 (tiotropium: 797, indacaterol: 801) Baseline characteristics: mean age (tiotropium: 63.6 years, indacaterol 63.4 years); gender (tiotropium 70% male, indacaterol 67%); mean % predicted FEV1 (tiotropium 54.3%, indacaterol 54.6%); mean smoking pack year history (tiotropium 41.8 years, indacaterol 43.2 years) Diagnostic criteria: GOLD guideline definition COPD severity: moderate to severe Inclusion criteria: patients with a diagnosis of COPD, smoking history of at least 10 pack years, post‐bronchodilator FEV1 < 80% and ≥ 30% of the predicted normal value, post‐bronchodilator FEV1/FVC < 70% Exclusion criteria: patients who have received systemic corticosteroids or antibiotics and/or was hospitalised for a COPD exacerbation in the 6 weeks prior to screening, respiratory tract infection within 6 weeks prior to screening, concomitant pulmonary disease, history of asthma, diabetes Type I or uncontrolled diabetes Type II, lung cancer or history of lung cancer, history of certain cardiovascular comorbid conditions |
|
| Interventions |
Run‐in period: unclear Interventions: 1. Tiotropium, 18 µg once daily via the HandiHaler device or 2. Indacaterol 150 µg delivered via a SDDPI (single‐dose dry powder inhaler) Concomitant medication
|
|
| Outcomes |
Primary outcome(s): trough FEV1 24h post‐dose after 12 weeks of treatment Secondary outcome(s): FEV1 AUC 5 min to 4 hours post‐dose on day 1, week 4 and week 12. Rescue medication use over 12 weeks. Safety and tolerability. |
|
| Notes |
Funding: this study was funded by Novartis Pharma AG Novartis study code CQAB149B2350 Clinicaltrials.gov study code NCT00900731 Supplementary data on non‐fatal serious adverse events were obtained on request from Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in a 1:1 ratio, and stratified by smoking status (current/ex‐smoker) The order of use of the inhalers was randomly assigned. The randomisation list was produced by the IVRS. |
| Allocation concealment (selection bias) | Low risk | The IVRS assigned a randomisation number to the patient (not identified to the caller). Only the medication pack number was communicated to the caller. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients receiving indacaterol also took placebo via the inhaler used for tiotropium, and patients receiving tiotropium took placebo via the inhaler used for indacaterol |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates were low and even (tiotropium 7.6%, indacaterol 7.5%) |
| Selective reporting (reporting bias) | Low risk | Data for all specified outcomes were reported |
Donohue 2010.
| Methods |
Design: 26‐week, randomised, partly‐blind, placebo‐controlled, parallel‐group study Setting: unclear Date of study: April 2007 to August 2008 |
|
| Participants |
Participants: n = 1683 (tiotropium: 420, indacaterol 150 µg: 420, indacaterol 300 µg: 418) Baseline characteristics: mean age (tiotropium: 64.0 years, indacaterol 150 µg: 63.4 years, indacaterol 300 µg: 63.3 years); gender (tiotropium 65% male, indacaterol 150 µg 62% male, indacaterol 300 µg 63%); mean % predicted FEV1 (tiotropium 53.9%, indacaterol 150 µg 56.1%, indacaterol 300 µg 56.3%); mean smoking pack year history (tiotropium 50.0 years, indacaterol 150 µg 48.3 years, indacaterol 300 µg 50.8 years) Diagnostic criteria: GOLD guideline definition COPD severity: moderate to severe Inclusion criteria: patients who were ≥ 40 years of age at COPD onset, with a cigarette smoking history of ≥ 20 pack years, and a clinical diagnosis of moderate‐to‐severe COPD. Entry criteria included post‐bronchodilator (within 30 min of inhaling albuterol 360 μg) FEV1 < 80% and ≥ 30% predicted and FEV1/FVC < 70%. Exclusion criteria: patients with a history of asthma were excluded |
|
| Interventions |
Run‐in period: 14‐day run‐in to check eligibility and record baseline assessments Interventions: 1. Tiotropium, 18 µg once daily via the HandiHaler device; or 2a. Indacaterol 150 µg via single‐dose dry powder inhaler taken once daily 2b. Indacaterol 300 µg via single‐dose dry powder inhaler taken once daily Concomitant medication
“Patients could continue inhaled corticosteroid (ICS) monotherapy if stable for 1 month before screening; dose and regimen were to remain stable throughout the study. Before the start of the run‐in period, treatment with anticholinergic bronchodilators or with beta2‐agonists was discontinued with appropriate washout, and patients receiving fixed‐combination beta2‐agonist /ICS were switched to ICS monotherapy at an equivalent dose. All patients were supplied with albuterol for use as needed". |
|
| Outcomes |
Primary outcome(s): 24 hours post‐dose (trough) FEV1 (mean of 23 h 10 min and 23 h 45 min post‐dose measurements) at Week 12 Secondary outcome(s): to demonstrate non‐inferiority of at least one indacaterol dose to tiotropium for trough FEV1 at week 12 (and, if met, to demonstrate superiority) |
|
| Notes |
Funding: this study was funded by Novartis Pharma AG Data pooled for continuous and dichotomous data for indacaterol 150 µg and indacaterol 300 µg subgroups Novartis study code CQAB149B2335s Clinicaltrials.gov study code NCT00463567 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using an automated interactive voice response system (IVRS), and was stratified by smoking status (current or ex‐smoker) |
| Allocation concealment (selection bias) | Low risk | The randomisation numbers were linked to different treatment groups, which in turn were linked to medication numbers. A separate medication list was produced using a validated system that automated the random assignment of medication numbers to medication packs. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinded tiotropium was not available, but personnel involved in the continuing clinical study (stage 2) remained blinded for the remainder of the study, as to whom were on indacaterol and placebo. The blinding of indacaterol and placebo continued until the study database was locked at the end of stage 2. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively even between the treatment groups but they were also relatively large (tiotropium 21%, indacaterol 150 µg 23% and indacaterol 300 µg 18%) |
| Selective reporting (reporting bias) | Low risk | All collected data reported |
Gani 2010.
| Methods |
Study design: cost‐utility analysis, Markov model based on Oostenbrink 2005 Time horizon: 1 year Currency used, year of study: pounds (GBP), 2009 |
|
| Participants |
Analytic perspective: National Health Service UK (second‐party payer) Setting, country of study: primary and secondary care, UK Population: patients with COPD Effectiveness data: exacerbations from 6 multi‐centre, double‐blind, double‐dummy, parallel‐group RCTs (Brusasco 2003; Casaburi 2002; Vincken 2002) Utility scores: for health states obtained from an observational study (references original model, Oostenbrink 2005) Resource use and costs: treatments costs, hospitalisations, healthcare visits, physician visits, etc. Cost data via Delphi panel for England, Scotland, Wales and Northern Ireland |
|
| Interventions |
Intervention: tiotropium 18 μg once daily via a HandiHaler Control 1: salmeterol 50 μg twice daily via a metered dose inhaler (MDI) Control 2: ipratropium 40 μg 4 times daily via a MDI |
|
| Outcomes | QALY, exacerbations, costs and utilities for patients with COPD | |
| Notes |
Sensitivity analysis: probability sensitivity analysis and one‐way sensitivity analysis based on either severity of COPD or exacerbation rate Funded by: Boehringer Ingelheim and Pfizer (manufacturer and co‐promoter of tiotropium) |
|
Mahmud 2007.
| Methods |
Design: 6‐month, randomised, parallel‐group study Setting: the study was conducted in Bangladesh Date of study: not described |
|
| Participants |
Participants: n = 90 (tiotropium: 47, salmeterol: 43) Baseline characteristics: not described Diagnostic criteria: not described COPD severity: not described Inclusion criteria: not described Exclusion criteria: not described |
|
| Interventions |
Run‐in period: not described 1. Tiotropium, 18 µg once daily; or 2. Salmeterol 50 µg via twice daily Concomitant medication
“Both groups received beclomethasone 500 μg twice daily + methylxanthines. Patients were allowed to use salbutamol as per need basis". |
|
| Outcomes | Outcome parameters being FEV1, health‐related quality of life (HRQoL), baseline dyspnoea index and frequency of COPD exacerbations | |
| Notes | Unable to contact author to obtain additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
Maniadakis 2006.
| Methods |
Study design: cost‐utility analysis, Markov model based on Oostenbrink 2005 Time horizon: 1 year Currency used, year of study: Euros (EUR), 2005 |
|
| Participants |
Analytic perspective: National Health Service Greece (second‐party payer) Setting, country of study: primary and secondary care, Greece Population: patients with COPD Effectiveness data: exacerbations from RCTs (Brusasco 2003; Casaburi 2002) Utility scores: for health states obtained from an observational study (references original model, Oostenbrink 2005) Resource use and costs: treatments costs, hospitalisations, healthcare visits, physician visits, etc. from medical records at the University General Hospital of Heraklion, Greece |
|
| Interventions |
Intervention: tiotropium 18 μg once daily via a HandiHaler Control 1: salmeterol 50 μg twice daily via a metered dose inhaler (MDI) |
|
| Outcomes | QALY, exacerbations, costs and utilities for patients with COPD | |
| Notes |
Sensitivity analysis: probability sensitivity analysis and one‐way sensitivity analysis based on either severity of COPD or exacerbation rate Funded by: Boehringer Ingelheim |
|
Naik 2010.
| Methods |
Study design: cost‐effectiveness analysis, Markov model Time horizon: 1 year Currency used, year of study: US dollars (USD), price year 2006 |
|
| Participants |
Analytic perspective: third‐party payer Setting, country of study: primary and secondary care, USA Population: patients with moderate COPD Effectiveness data: exacerbations; tiotropium data from 3 RCTs (Casaburi 2002; Donohue 2002; Vincken 2002), salmeterol data from 2 RCTs (Donohue 2002; Rennard 2001), no treatment (placebo) data from 3 RCTs (Casaburi 2002; Donohue 2002; Rennard 2001) Utility scores: from published RCTs of various treatments. No reference given. Resource use and costs: costs of drugs, hospitalisations, monitoring (laboratory tests) and physician visits. The drug costs and cost of maintenance therapy were based on average wholesale prices. All other costs were from Medicare sources. |
|
| Interventions |
Intervention: tiotropium 18 μg once daily Control 1: salmeterol 50 μg twice daily Control 2: no treatment |
|
| Outcomes | Cost per exacerbation avoided per patient per year. ICERs were calculated as additional cost per patient to prevent one exacerbation, compared with the next most expensive option | |
| Notes |
Sensitivity analysis: one‐way sensitivity analysis; the probability of exacerbation, the probability of hospitalisation, the probability of severe exacerbation, and the compliance rate Funding: not stated |
|
Oba 2007.
| Methods |
Study design: cost‐utility analysis (CUA) Time horizon: 1 year Currency used, year of study: US dollar (USD), 2005 |
|
| Participants |
Analytic perspective: third‐party payer’s Setting, country of study: primary and secondary care, USA Population: patients with moderate to severe COPD Effectiveness data and utility scores: data from 4 tiotropium versus placebo RCTs (Brusasco 2003; Casaburi 2002; Dusser 2006; Niewoehner 2005), 1 tiotropium versus salmeterol (Brusasco 2003) and 4 salmeterol versus placebo trials (Brusasco 2003; Chapman 2002; Jones 1997; Stockley 2006) Resource use and costs: treatment costs, hospital admission costs, ED visits, physician visits and unscheduled office visits. Costs for medications were based on the average wholesale price. Cost of hospitalisations from Solucient’s Medicare Database. Costs for inpatient physician visits and emergency department visits for COPD exacerbations were based on data from a study by Wilson 2000. The cost of antibiotic treatment was based on data from a study by Sin 2004. |
|
| Interventions |
Intervention: tiotropium 18 μg once daily Control 1: salmeterol 50 μg twice daily Control 2: placebo |
|
| Outcomes | HRQL and hospitalisation rates, incremental QALY | |
| Notes |
Sensitivity analysis: worst case and best case using 95% CIs (for costs and benefits) were used as sensitivity analyses compared to placebo Funded by: not stated |
|
Oostenbrink 2005.
| Methods |
Study design: cost‐utility analysis (CUA), 3‐state Markov model Time horizon: 1 year Currency used, year of study: Euro (EUR), 2004 |
|
| Participants |
Analytic perspective: healthcare system in Netherlands or Canada Setting, country of study: primary and secondary care, Netherlands and Canada Population: patients with COPD Effectiveness data: data from 6 RCTs (Brusasco 2003; Casaburi 2002; Vincken 2002) Utility scores: utility values per disease state were based on empiric data from an observational study in patients with COPD classified into the GOLD stages (Borg 2004) Resource use and costs: treatments costs, hospitalisations, healthcare visits, physician visits, etc. Resource utilisation captured from 2 ipratropium‐controlled RCTs in the Netherlands with list prices used for drug costs (Oostenbrink 2004; Oostenbrink 2004a). For Canada this was collected from a prospective multi‐centre observational study (no reference stated) with drug costs from the Ontario Drug Benefit Formulary (Canada) |
|
| Interventions |
Intervention: tiotropium 18 μg once daily Control 1: salmeterol 50 μg twice daily Control 2: ipratropium 40 μg 4 times daily |
|
| Outcomes | Exacerbations, QALM | |
| Notes |
Sensitivity analysis: Monte Carlo simulation, probabilistic sensitivity analysis and one‐way sensitivity analysis based on either severity of COPD, exacerbation rate, utility values, oxygen therapy Funded by: Boehringer Ingelheim (manufacturer of tiotropium) |
|
Rutten‐van Molken 2007.
| Methods |
Study design: cost‐effectiveness analysis (CEA) and cost‐utility analysis (CUA), Markov model based on Oostenbrink 2005 Time horizon: 5 years with a 1‐year cycle duration Currency used, year of study: Euro, 2005 |
|
| Participants |
Analytic perspective: Spanish National Health System (NHS) and societal perspective Setting, country of study: primary and secondary care in Spain Population: patients with stable moderate‐to‐severe COPD Effectiveness data: exacerbations, hospitalisations from 6 RCTs (Brusasco 2003; Casaburi 2002; Vincken 2002) Utility scores: utilities were obtained from a subset of patients in the UPLIFT trial (Tashkin 2008) Resource use and costs: visits to respiratory physicians inside and outside of the hospital, visits to the general practitioner, pulmonary function tests, blood tests, imaging tests and respiratory medications, hospital admissions and visits to the emergency room (ER), and cost of absence from work due to illness were primarily derived from 2 studies performed in Spain (Miravitlles 2002; Miravitlles 2003). Unit costs of healthcare resources from SOIKOS health database, costs of pulmonary drugs based on public prices. |
|
| Interventions |
Intervention: tiotropium 18 μg once daily Control 1: salmeterol 50 μg twice daily Control 2: ipratropium 40 μg 4 times daily |
|
| Outcomes | Exacerbations, exacerbation‐free months and quality‐adjusted life‐years (QALYs) | |
| Notes |
Sensitivity analysis: probabilistic sensitivity analysis based on severity of COPD, discount rate Funded by: Boehringer Ingelheim and Pfizer (manufacturer and co‐promoter of tiotropium) |
|
Vogelmeier 2008.
| Methods |
Design: 6‐month, randomised, partly‐blind and partly placebo‐controlled, parallel‐group study Setting: the study was conducted in 86 centres in Germany, Italy, Netherlands, Russian federation, Poland, Czech Republic, Spain and Hungary Date of study: October 2004 to November 2005 |
|
| Participants |
Participants: n = 847 (tiotropium (and placebo): 221, formoterol: 210) Baseline characteristics: mean age (tiotropium: 63.4 years, formoterol 61.8 years); gender (tiotropium 79% male, formoterol 76% male); mean % predicted FEV1 (tiotropium 51.6%, formoterol 51.6%); mean smoking pack year history (tiotropium 38.6 years, salmeterol 35.4 years) Diagnostic criteria: GOLD guideline definition COPD severity: moderate to severe Inclusion criteria: patients who were ≥ 40 years of age at COPD onset, with a cigarette smoking history of ≥ 10 pack years, and a clinical diagnosis of stable COPD, were eligible for inclusion in the study if they had a FEV1 % predicted ≤ 70% and FVC ≤ 70% Exclusion criteria: the study excluded patients who had a respiratory tract infection or had been hospitalised for an acute exacerbation of COPD within the month prior to screening. Patients with a clinically significant condition such as ischaemic heart disease that might compromise patient safety or compliance were also excluded. |
|
| Interventions |
Run‐in period: a screening period of up to 4 weeks included 2 weeks for washout of disallowed medications and 2 weeks for eligibility assessment and baseline evaluations Interventions: 1. Tiotropium, 18 µg once daily via the HandiHaler device + placebo bid via MDDPI; or 2. Formoterol 10 µg twice daily (bid) via multi‐dose dry powder inhaler Concomitant medication
“Salbutamol pMDI (2 × 100 µg/puff) was permitted as rescue medication. Patients were asked not to use salbutamol in the 8 h before a study visit. Patients could receive inhaled corticosteroids (ICS) at a stable daily dose (any patients receiving fixed combinations of ICS and beta2‐agonists were switched to receive the same dose of ICS and on demand salbutamol)” |
|
| Outcomes |
Primary outcome(s): FEV1 measured 2 h post‐dose after 24 weeks of treatment Secondary outcome(s): FEV1 and FVC at other time points during the study (5 min, 2 and 3 h post‐dose following the first dose of treatment, and after 12 and 24 weeks of treatment); COPD exacerbations ((1) ‘bad days’, (2) ‘COPD exacerbation days’, (3) ‘COPD exacerbations requiring additional therapy’, (4) ‘COPD‐related hospitalisations’); symptom scores, rescue medication use and PEF; quality of life, and 6‐minute walking distance |
|
| Notes |
Funding: this study was funded by Novartis Pharma AG Novartis study code CFOR258F2402 Clinicaltrials.gov study code NCT00134979 Supplementary data for FEV1 and non‐fatal serious adverse events were obtained on request from Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was produced using a validated system that automates the random assignment of treatment groups to randomisation numbers in the specified ratio. The randomisation scheme will be reviewed by a Biostatistics Quality Assurance Group and locked by them after approval. |
| Allocation concealment (selection bias) | Low risk | Randomisation data were kept strictly confidential until the time of unblinding, and were not accessible by anyone else involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The study was partially blinded. The study was double‐blind for treatment comparisons formoterol versus placebo and tiotropium + formoterol versus tiotropium + placebo (MDDPI only), but not for other comparisons as tiotropium was administered open‐label. Randomization was not stratified” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Persons performing the assessments and data analysts were blinded to the identity of the treatment from the time of randomisation until database lock. Study was partly blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of withdrawals in the different groups were relatively low and similar in both groups (formoterol 11.9% and tiotropium + placebo 13.1%) |
| Selective reporting (reporting bias) | Low risk | All collected data reported |
Vogelmeier 2011.
| Methods |
Design: 12‐month, randomised, double‐blind, double‐dummy, parallel‐group study Setting: the study was conducted in 725 centres in 25 countries Date of study: January 2008 to April 2009 |
|
| Participants |
Participants: n = 7384 (tiotropium: 3711, salmeterol: 3673) Baseline characteristics: mean age (tiotropium: 62.9 years, salmeterol 62.8 years); gender (tiotropium 74% male, salmeterol 75% male); mean % predicted FEV1 (tiotropium 49.2%, salmeterol 49.4%); mean smoking pack year history (tiotropium 38.8 years, salmeterol 37.8 years) Diagnostic criteria: American Thoracic Society classification COPD severity: moderate to severe Inclusion criteria: patients who were ≥ 40 years of age, with a cigarette smoking history of ≥ 10 pack years, and a clinical diagnosis of COPD, were eligible for inclusion in the study if they had a FEV1 % predicted ≤ 70% and FVC ≤ 70%, and a documented history of at least 1 exacerbation leading to treatment with systemic glucocorticoids or antibiotics or hospitalisation within the previous year Exclusion criteria: significant diseases other than COPD (particular chronic lung disease), diagnosis of asthma, bladder neck obstruction, narrow angle glaucoma, past cardiac event or severe cardiovascular disorder or recent hospitalisation. Renal or thyroid disease, untreated diabetes, drug insensitivities, unable systemic corticosteroid use, previous participation in a clinical study. Recent infection or exacerbation in the 4 weeks prior to participation. |
|
| Interventions |
Run‐in period: during the 2‐week run‐in period, patients who were receiving tiotropium were required to switch to 40 µg of ipratropium 4 times a day, and this therapy was discontinued at the time of randomisation. Patients who were receiving a long‐acting beta2‐agonist were permitted to continue the use of that medication during the run‐in period. Interventions: 1. 18 µg of tiotropium once daily, delivered through the HandiHaler inhalation device, plus placebo twice daily, delivered through a pressurised, metered‐dose inhaler; or 2. 50 µg of salmeterol twice daily, delivered through a pressurised, metered‐dose inhaler, plus placebo once daily, delivered through the HandiHaler device Concomitant medication
"Patients receiving fixed‐dose combinations of long‐acting beta2‐agonists and inhaled glucocorticoids were instructed to switch to inhaled glucocorticoid monotherapy at the start of the treatment phase of the study. Patients were allowed to continue their usual medications for COPD, except for anticholinergic drugs and long‐acting beta2‐agonists, during the double‐blind treatment phase. Concomitant medication at baseline was defined as the therapy the patients were receiving at the time of the screening visit". |
|
| Outcomes |
Primary outcome(s): time to first exacerbation Secondary outcome(s): (1) occurrence of at least one exacerbation, (2) number of COPD exacerbations, (3) time to first hospitalisation due to COPD exacerbation, (4) occurrence of at least 1 hospitalisation due to COPD exacerbations, (5) number of hospitalisations due to COPD exacerbations, (6) time to premature discontinuation of trial medication, (7) occurrence of premature discontinuation of trial medication, (8) pre‐dose morning PEFR measured by patients at home during the first 4 months of randomised treatment (weekly means will be calculated), (9) time to first COPD exacerbation or time to discontinuation of study medication because of worsening of underlying disease, whichever comes first |
|
| Notes |
Funding: this study was funded by Boehringer Ingelheim and Pfizer Boehringer Ingelheim trial number 205.389 Clinicaltrials.gov study code NCT00563381 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was generated by the sponsor using a validated system involving a pseudo‐random number generator. Patients were randomised to treatment via an Interactive Voice Response System (IVRS, Perceptive Informatics Inc., Berlin, Germany). Patients were randomised in a 1:1 ratio in blocks of 4, with equal allocation of treatment within each block per country site. |
| Allocation concealment (selection bias) | Low risk | The access to the randomisation code was supervised by Clinical Trial Support (Medical Data Services). Blinding of the study medications was such that the treatments were indistinguishable. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was maintained by allocation of a dummy placebo MDI to those randomised to the tiotropium arm and a dummy placebo HandiHaler to those in the salmeterol arm. Tiotropium and placebo capsules were identical in size and colour and were therefore indistinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mortality Adjudication Committee: provided consistent, systematic and independent assessment of the primary cause of death (blinded to treatment group) by reviewing the information provided in the Council for International Organisation of Medical Sciences (CIOMS) form for each patient |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients who prematurely discontinued treatment were followed for vital status (i.e. whether they were alive and, if they had died, the primary cause of death) until the end of the planned treatment period of 360 days. Information on vital status was considered to be complete for patients who attended all trial visits through day 360 and for those who prematurely discontinued study medication but whose vital status was confirmed at day 360. Exacerbations were not systematically followed up after a patient’s premature discontinuation of the trial medication. Patients who withdrew from the trial prematurely without having had an exacerbation were considered as having had no exacerbation, and in the time‐to‐event analysis, their data were censored at the time of withdrawal. There were a statistically significant higher number of withdrawals in the salmeterol group (17.7%, 648/3669), compared with the tiotropium group (15.8%, 585/3707) |
| Selective reporting (reporting bias) | Low risk | All outcome data reported |
AUC: area under the curve; bid: twice a day; CEA: cost‐effectiveness analysis; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CUA: cost utility analysis; ED: emergency department; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Pulmonary Disease; h: hour; HRQL: health‐related quality of life; ICER: incremental cost‐effectiveness ratio; ICS: inhaled corticosteroids; IVRS: interactive voice response system; LABA: long‐acting beta2‐agonist; MDDPI; metered dose dry powder inhaler;MDI: metered dose inhaler; QALM: quality‐adjusted life month; QALY: quality‐adjusted life year; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire; TDI: Transitional Dyspnoea Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnes 2010 | These studies cover phase I (2‐week dose selection) and III (comparison arm changing from placebo to tiotropium/indacaterol during treatment period) of the Donohue 2010 trial, or did not report data with tiotropium |
| Chapman 2010 | Cross‐over study design |
| Di Marco 2006 | Single dose of treatment only |
| Donohue 2011 | Cross‐over study design |
| Golubev 2006 | Cross‐over study design |
| Gross 2003 | Study duration ‐ 4 weeks |
| Meyer 2008 | Cross‐over study design |
| Meyer 2011 | Study duration ‐ 2 weeks |
| Reisner 2011 | Cross‐over study design; study duration ‐ 1 week |
| Rossi 2012 | Cross‐over study design |
| Tashkin 2009 | Study duration ‐ 2 weeks |
| ten Hacken 2007 | Cross‐over study design |
| van der Vaart 2011 | Cross‐over study design |
| van Noord 2003 | Cross‐over study design |
| van Noord 2005 | Cross‐over study design |
| van Noord 2006 | Cross‐over study design |
| Vogelmeier 2010 | Cross‐over study design |
Characteristics of studies awaiting assessment [ordered by study ID]
Garcia Ruiz 2005.
| Methods | A cost‐effectiveness analysis in the setting of the Spanish National Health System |
| Participants | Patients with COPD |
| Interventions | Tiotropium versus ipratropium or salmeterol |
| Outcomes | FEV1, quality of life (SGRQ), dyspnoea (TDI), mean stay in hospital, exacerbations and direct healthcare costs associated with hospital treatment |
| Notes |
Geitona 2011.
| Methods | A cost‐utility and a cost‐effectiveness analysis based on a Markov model, for the Greek NHS |
| Participants | Patients with COPD |
| Interventions | Indacaterol versus tiotropium or formoterol |
| Outcomes | QALYs, life years gained (LYG) and exacerbation rates, cost |
| Notes |
Price 2011.
| Methods | A cost‐utility analysis based on a Markov model, from a German healthcare provider perspective |
| Participants | Patients with moderate to severe COPD |
| Interventions | indacaterol versus tiotropium or salmeterol |
| Outcomes | FEV1, mortality, exacerbations, cost, ICER |
| Notes |
Sanz‐Martinez 2004.
| Methods | A cost‐efficacy analysis within the framework of the Spanish National Health System |
| Participants | Patients with COPD |
| Interventions | Tiotropium versus ipratropium or salmeterol |
| Outcomes | |
| Notes | Pending access to full article and translation |
Schramm 2005.
| Methods | A cost‐effectiveness study for the Swiss public health insurance system |
| Participants | Patients with moderate to severe COPD |
| Interventions | Tiotropium compared to ipratropium, salmeterol or standard care |
| Outcomes | Exacerbations, hospitalisations, direct yearly total cost for COPD therapy |
| Notes | Pending access to full article and translation |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; QALY: quality‐adjusted life year; SGRQ: St George's Respiratory Questionnaire; TDI: Transitional Dyspnoea Index
Differences between protocol and review
We expanded the objective of the review to encompass evidence on the cost and cost‐effectiveness associated with tiotropium compared to LABA. We added specific methods for economic evaluations regarding type of studies to include, outcomes, search strategy, data extraction and management, and 'Risk of bias' assessment.
Contributions of authors
Charlotta Karner contributed to protocol development, went through the searches and identified RCTs and economic evaluations for inclusion, extracted data, wrote the parts on economic evaluations and assisted in results interpretation, and checking and write‐up of the review.
Jimmy Chong went through the searches and identified RCTs for inclusion, extracted data and wrote the review.
Phillippa Poole assisted in refinement of the protocol, results interpretation, and checking and write‐up of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
Programme grant funding
Declarations of interest
None known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Briggs 2005 {published data only}
- Briggs Jr D, Covelli H, Lapidus R, Bhattacharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared to salmeterol in COPD patients [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:C22 Poster 511.
- Briggs Jr DD, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulmonary Pharmacology & Therapeutics 2005;18(6):397‐404. [DOI] [PubMed] [Google Scholar]
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. Proceedings of the American Thoracic Society. 2006:A110 [Poster J10].
- Langley J, Briggs Jr D, Bhattacharya S, Kesten S, Cassino C. Spirometric outcomes of COPD patients with tiotropium compared with salmeterol according to previous maintenance long acting beta‐agonist use [Abstract]. European Respiratory Journal 2004;24(Suppl 48):289s. [Google Scholar]
Brusasco 2003 {published data only}
- Bateman ED, Hodder R, Miravitlles M, Lee A, Towse L, Serby C. A comparative trial of tiotropium, salmeterol and placebo: health‐related quality of life. European Respiratory Journal 2001;18(Suppl 33):26s. [Google Scholar]
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58(5):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders following treatment with tiotropium and salmeterol in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 420.
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. Proceedings of the American Thoracic Society. 2006:A110 [Poster J10].
- Brusasco V, Thompson P, Vincken W, Lee A, Towse L, Witek TJ. Improvement of dyspnea following of six months treatment with tiotropium but not with salmeterol in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):26s. [Google Scholar]
- Donohue JF. Alterations in bronchodilator effectiveness over six months with tiotropium and salmeterol. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227. [Google Scholar]
- Donohue JF, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respiratory Medicine 2003;97(9):1014‐20. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, et al. A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122(1):47‐55. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Noord JA, Langley SJ, Lee A, Kesten S, Towse LJ. Superior bronchodilation of once daily tiotropium compared to twice daily salmeterol in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):26s. [Google Scholar]
- Gunther K, Lee A, Kesten S, Towse L. Superior bronchodilation of once daily tiotropium compared with twice daily salmeterol in patients with COPD [Abstract]. International Primary Care Respiratory Group Congress: Amsterdam, June 2002. 2002:112.
- Hodder R, Kesten S, Menjoge S, Viel K. Outcomes in COPD patients receiving tiotropium or salmeterol plus treatment with inhaled corticosteroids. International Journal of COPD 2007;2(2):157‐67. [PMC free article] [PubMed] [Google Scholar]
- Langley J, Briggs Jr D, Bhattacharya S, Kesten S, Cassino C. Spirometric outcomes of COPD patients with tiotropium compared with salmeterol according to previous maintenance long acting beta‐agonist use [Abstract]. European Respiratory Journal 2004;24(Suppl 48):289s. [Google Scholar]
- Rutten‐van Mölken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5‐year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. The European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 2007;8(2):123‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burl 2011 {published and unpublished data}
- Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M, et al. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. European Respiratory Journal 2011;38:797‐803. [DOI] [PubMed] [Google Scholar]
- Dunn LJ, Buhl R, Lassen C, Henley M, Kramer B. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD [Abstract]. Chest. 2010; Vol. 138:719A. [DOI] [PubMed]
Donohue 2010 {published data only}
- Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. American Journal of Respiratory & Critical Care Medicine 2010;182(2):155‐62. [DOI] [PubMed] [Google Scholar]
- Fogarty C, Hebert J, Iqbal A, Owen R, Higgins M, Kramer B. Indacaterol once‐daily provides effective 24‐h bronchodilation in COPD patients: a 26‐week evaluation vs placebo and tiotropium [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2025].
- Jones PW, Mahler DA, Gale R, Owen R, Kramer B. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respiratory Medicine 2011;105(6):892‐9. [DOI] [PubMed] [Google Scholar]
- Lotvall J, Cosio BG, Iqbal A, Swales J, Owen R, Kramer B, et al. Indacaterol once‐daily improves day and night‐time symptom control in COPD patients: a 26‐week study versus placebo and tiotropium [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2029].
- Mahler D, Palange P, Iqbal A, Owen R, Higgins M, Kramer B. Indacaterol once‐daily improves dyspnoea in COPD patients: a 26‐week placebo‐controlled study with open‐label tiotropium comparison [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[E4360].
- Worth H, Kleerup E, Iqbal A, Owen R, Kramer B, Higgins M. Safety and tolerability of indacaterol once‐daily in COPD patients versus placebo and tiotropium: a 26‐week study [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2030].
- Yorgancioglu A, Mahler D, Iqbal A, Owen R, Higgins M, Kramer B. Indacaterol once‐daily improves health‐related quality of life in COPD patients: a 26‐week comparison with placebo and tiotropium [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2028].
Gani 2010 {published data only}
- Gani R, Griffin J, Kelly S, Rutten‐van Molken M. Economic analyses comparing tiotropium with ipratropium or salmeterol in UK patients with COPD. Primary Care Respiratory Journal: Journal of the General Practice Airways Group 2010;19(1):68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmud 2007 {published data only}
- Mahmud AM, Gupta DK, Khan AS, Hassan R, Hossain A, Rahman M, et al. Comparison of once daily tiotropium with twice daily salmeterol in Bangladeshi patients with moderate COPD [Abstract]. Respirology 2007;12(Suppl 4):A211. [Google Scholar]
Maniadakis 2006 {published data only}
- Maniadakis N, Tzanakis N, Fragoulakis V, Hatzikou M, Siafakas N. Economic evaluation of tiotropium and salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) in Greece. Current Medical Research and Opinion 2006;22(8):1599‐607. [DOI] [PubMed] [Google Scholar]
Naik 2010 {published data only}
- Naik S, Kamal KM, Keys PA, Mattei TJ. Evaluating the cost‐effectiveness of tiotropium versus salmeterol in the treatment of chronic obstructive pulmonary disease. ClinicoEconomics and Outcomes Research: CEOR 2010;2:25‐36. [PMC free article] [PubMed] [Google Scholar]
Oba 2007 {published data only}
- Oba Y. Cost‐effectiveness of long‐acting bronchodilators for chronic obstructive pulmonary disease. Mayo Clinic Proceedings 2007;82(5):575‐82. [DOI] [PubMed] [Google Scholar]
Oostenbrink 2005 {published data only}
- Oostenbrink JB, Rutten‐van Molken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost‐effectiveness of bronchodilator therapy in COPD patients in different countries. Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research 2005;8(1):32‐46. [DOI] [PubMed] [Google Scholar]
Rutten‐van Molken 2007 {published data only}
- Rutten‐van Molken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5‐year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. The European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 2007;8(2):123‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogelmeier 2008 {published data only}
- Arievich H, Potena A, Fonay K, Vogelmeier CF, Overend T, Smith J, et al. Formoterol given either alone or together with tiotropium reduces the rate of exacerbations in stable COPD patients [Abstract]. European Respiratory Journal 2006;28(Suppl 50):440s [P2514]. [Google Scholar]
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono‐ and combination therapy with tiotropium in patients with COPD: a 6‐month study. Respiratory Medicine 2008;102(11):1511‐20. [DOI] [PubMed] [Google Scholar]
- Vogelmeier CF, Harari SA, Fonay K, Beier J, Overend T, Till D, et al. Formoterol and tiotropium both improve lung function in stable COPD patients with some additional benefit when given together [Abstract]. European Respiratory Journal 2006;28(Suppl 50):429s [P2506]. [Google Scholar]
Vogelmeier 2011 {published data only}
- Beeh KM, Hederer B, Glaab T, Muller A, Rutten‐van Moelken M, Kesten S, et al. Study design considerations in a large COPD trial comparing effects of tiotropium with salmeterol on exacerbations. International Journal of COPD 2009;4(1):119‐25. [PMC free article] [PubMed] [Google Scholar]
- Beeh KM, Vogelmeier C, Rutten‐van Mölken M, Rabe KF, Glaab T, et al. Tiotropium vs salmeterol in GOLD II and maintenance‐naive COPD patients: subgroup analyses of POET‐COPD™ trial [Abstract]. European Respiratory Society Annual Congress, Amsterdam, The Netherlands, September 24‐28 2011; Vol. 38, issue 55:20s [P251].
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten‐van Mölken MPMH, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. New England Journal of Medicine 2011;364(12):1093‐103. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Rabe K, Beeh KM, Glaab T, Schmidt H, Rutten‐van Mölken M, et al. Tiotropium reduces exacerbations versus salmeterol irrespective of baseline ICS treatment in the Poet‐COPD; Study [Abstract]. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A1600.
- Vogelmeier CF, Beeh KM, Rutten‐van Molken M, Glaab T, Schmidt H, Hederer B. Baseline characteristics according to gender in a large exacerbations trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4495. [Google Scholar]
References to studies excluded from this review
Barnes 2010 {published data only}
- Barnes P, Pocock S, Magnussen H, Iqbal A, Lawrence D, Kramer B, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. American Journal of Respiratory and Critical Care Medicine. 2009; Vol. 179, issue Meeting Abstracts:A4543. [DOI] [PubMed]
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulmonary Pharmacology and Therapeutics 2010;23(3):165‐71. [DOI] [PubMed] [Google Scholar]
- Fogarty C, Worth H, Hebert J, Iqbal A, Owen R, Higgins M, et al. Sustained 24‐h bronchodilation with indacaterol once‐daily in COPD: a 26‐week efficacy and safety study. American Journal of Respiratory and Critical Care Medicine. 2009; Vol. 179, issue 1 Meeting Abstracts:A4547.
- Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, D'Urzo A, et al. A dose‐ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respiratory Medicine 2008;102(7):1033‐44. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Chapman KR, Luthra A, Swales J, Lassen C, Owen R, et al. Once‐daily indacaterol provides effective bronchodilation over 1 year of treatment in patients with chronic obstructive pulmonary disease (COPD). Chest 2009;136(4):4S‐f. [Google Scholar]
Chapman 2010 {published data only}
- Chapman KR, Fogarty CM, Peckitt C, Lassen C, Kramer B. Patient handling and preference for the single‐dose dry‐powder inhalers used with indacaterol and tiotropium [Abstract]. Chest. 2010; Vol. 138:470A.
Di Marco 2006 {published data only}
- Marco F, Carlucci P, Ccarlucci P, Verga M, Mondoni M, Pistone A, et al. A single dose of formoterol (FOR) and tiotropium (TIO) reduce hyperinflation in COPD patients [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: [P1844]. [Google Scholar]
- Marco F, Verga M, Santus P, Cazzola M, Mondoni M, Belloi E, et al. Formoterol (Modulite ®), tiotropium and their combination in patients with acute exacerbations of chronic bronchitis: preliminary data [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1905. [Google Scholar]
- Marco F, Verga M, Santus P, Morelli N, Cazzola M, Centanni S. Effect of formoterol, tiotropium, and their combination in patients with acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Respiratory Medicine 2006;100(11):1925‐32. [DOI] [PubMed] [Google Scholar]
Donohue 2011 {published data only}
- Donohue J, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. Dose‐related efficacy of GSK573719: a long‐acting muscarinic receptor antagonist (LAMA) with sustained 24‐hour activity in COPD [Abstract]. Chest 2011; Vol. 140, issue 4:1043A.
Golubev 2006 {published data only}
- Golubev L, Gorbunova M, Babak S, Tatarskiy. Sleep architecture in COPD patients during salmeterol versus tiotropium bromide treatment [Abstract]. European Respiratory Journal 2006;28(Suppl 50):199s [P1197]. [Google Scholar]
Gross 2003 {published data only}
- Gross NJ, Paulson D, Kennedy D, Korducki L, Kesten S. A comparison of maintenance tiotropium and salmeterol on arterial blood gas tensions in patients with COPD [Abstract]. Respiratory Care 2003;48(11):1081. [Google Scholar]
Meyer 2008 {published data only}
- Meyer T, Brand P, Reitmeir P, Scheuch G. Effect of formoterol, salbutamol and tiotropium bromide on the efficacy of lung clearance in patients with COPD [Abstract]. American Thoracic Society International Conference, May 16‐21, 2008, Toronto. 2008:A646[#F1].
Meyer 2011 {published data only}
Reisner 2011 {published data only}
- Reisner C, Fogarty C, Spangenthal S, Dunn L, Kerwin EM, Quinn D, et al. Novel combination of glycopyrrolate and formoterol MDI (GFF‐MDI) provides superior bronchodilation compared to its components administered alone, tiotropium DPI, and formoterol DPI in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011; Vol. 183, issue Meeting Abstracts:A6435.
- Reisner C, St. Rose E, Strom S, Fischer T, Golden M, Thomas M, et al. Fixed combination of glycopyrrolate and formoterol MDI (GFF‐MDI) demonstrates superior inspiratory capacity (IC) compared to tiotropium DPI (Tio) following 7 days dosing, in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. European Respiratory Society Annual Congress, Amsterdam, The Netherlands, September 24‐28 2011; Vol. 38, issue 55:150s [P879].
Rossi 2012 {published data only}
Tashkin 2009 {published data only}
- Tashkin D, Donohue J, Mahler D, Huang H, Schaefer K, Hanrahan J, et al. Effect of arformoterol twice daily, tiotropium once daily, and their combination in subjects with COPD [Abstract]. Chest 2008;134(4):104003s. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Donohue JF, Mahler DA, Huang H, Goodwin E, Schaefer K, et al. Effects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPD. Respiratory Medicine 2009;103(4):516‐24. [DOI] [PubMed] [Google Scholar]
ten Hacken 2007 {published data only}
- Hacken NH, Haart H, Grevink R, Thompson S, Roemer W, Postma DS. Bronchodilation improves endurance but not muscular efficiency in COPD [Abstract]. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. 2007:Poster #M72.
van der Vaart 2011 {published data only}
- Vaart H, Postma DS, Grevink R, Roemer W, Hacken N. Bronchodilation improves endurance but not muscular efficiency in chronic obstructive pulmonary disease. International Journal of COPD 2011;6:229‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Noord 2003 {published data only}
- Noord JA, Aumann J, Janssens E, Folgering H, Mueller A, Cornelissen JPG. Tiotropium maintenance therapy in patients with COPD and the 24‐h spirometric benefit of adding once or twice daily formoterol during 2‐week treatment periods [abstract]. American Thoracic Society 99th International Conference. 2003:A035 Poster D82.
van Noord 2005 {published data only}
- Noord JA, Aumann J, Janssens E, Mueller A, Cornelissen PJ. Comparison of once daily tiotropium, twice daily formoterol and the free combination, once daily, in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024 Poster 421.
- Noord JA, Aumann J, Janssens E, Mueller A, Cornelissen PJG. A comparison of the 24 hour bronchodilator effect of tiotropium QD [T10] salmeterol BID [SALM] or their combination in COPD [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:[B93] [Poster: 311].
- Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. European Respiratory Journal 2005;26(2):214‐22. [DOI] [PubMed] [Google Scholar]
van Noord 2006 {published data only}
- Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006;129(3):509‐17. [DOI] [PubMed] [Google Scholar]
- Noord JA, Smeets JJ, Otte A, Bindels H, Mueller A, Cornellissen PJG. The effect of tiotropium, salmeterol and it's combination on dynamic hyperinflation in COPD [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:[B93] [Poster: 310].
Vogelmeier 2010 {published data only}
- Vogelmeier C, Ramos‐Barbon D, Jack D, Piggott S, Owen R, Higgins M, et al. Indacaterol provides 24‐hour bronchodilation in COPD: a placebo‐controlled blinded comparison with tiotropium. Respiratory Research. 2010; Vol. 11:135. [DOI] [PMC free article] [PubMed]
References to studies awaiting assessment
Garcia Ruiz 2005 {published data only}
- Garcia Ruiz AJ, Leiva Fernandez F, Martos Crespo F. Cost‐effectivenessanalysis of tiotropium compared to ipratropium and salmeterol. Archivos de Bronconeumología 2005;41:242‐8. [DOI] [PubMed] [Google Scholar]
Geitona 2011 {published data only}
- Geitona M, Hatzikou M, Bania E. Economic evaluation of indacaterol versus tiotropium or formoterol for patients with moderate to severe COPD in Greece. Value in Health. 2011; Vol. ISPOR 14th Annual European Congress, Madrid, Spain.
Price 2011 {published data only}
- Price D, Gray A, Gale R, Asukai Y, Mungapen L, Lloyd A, et al. Cost‐utility analysis of indacaterol in Germany: a once‐daily maintenance bronchodilator for patients with COPD. Respiratory Medicine 2011;105(11):1635‐47. [DOI] [PubMed] [Google Scholar]
Sanz‐Martinez 2004 {published data only}
- Sanz‐Martinez H, Perez‐Maroto MT. Cost‐efficacy analysis of tiotropium versus ipratropium and salmeterol in the treatment of chronic obstructive pulmonary disease. Farmacia Aten Primaria 2004;2:72‐82. [Google Scholar]
Schramm 2005 {published data only}
- Schramm W, Haake D, Brandt A. Economic value of tiotropium in the treatment of chronic obstructive pulmonary disease. Praxis 2005;94(46):1803‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Appleton 2006
- Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan MMK, et al. Long‐acting beta2‐agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001104.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr 2005
- Barr RG, Bourbeau J, Camargo Carlos A. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beeh 2010
- Beeh KM, Beier J. The short, the long and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐9. [DOI] [PubMed] [Google Scholar]
Beier 2011
- Beier J, Beeh KM. Long‐acting beta‐adrenoceptor agonists in the management of COPD: focus on indacaterol. International Journal of Chronic Obstructive Pulmonary Disease. 2011/08/05 2011; Vol. 6:237‐43. [1178‐2005: (Electronic)] [DOI] [PMC free article] [PubMed]
Berger 2008
- Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2008;102(2):173‐88. [DOI] [PubMed] [Google Scholar]
Boehringer Ingelheim 2010
- Boehringer Ingelheim. Tiotropium (SPIRIVA®) RESPIMAT®: Evaluation of fatal events. www.trials.boehringer‐ingelheim.com/res/trial/data/pdf/Pooled_Analysis_U10‐3255‐01.pdf 2010.
Borg 2004
- Borg S, Ericsson A, Wedzicha J, Gulsvik A, Lundback B, Donaldson GC, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value in Health 2004;7(2):153‐67. [DOI] [PubMed] [Google Scholar]
Calverley 2007
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. [DOI] [PubMed] [Google Scholar]
Casaburi 2002
- Casaburi R, Mahler DA, Jones PW, Wanner A, San Pedro G, ZuWallack RL, et al. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal 2002;19(2):217‐24. [DOI] [PubMed] [Google Scholar]
Chapman 2002
- Chapman KR, Arvidsson P, Chuchalin AG, Dhillon DP, Faurschou P, Goldstein RS, et al. The addition of salmeterol 50 microg bid to anticholinergic treatment in patients with COPD: a randomized, placebo controlled trial. Chronic obstructive pulmonary disease. Canadian Respiratory Journal 2002;9(3):178‐85. [DOI] [PubMed] [Google Scholar]
Cheyne 2012
- Cheyne L, Irvin‐Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009552] [DOI] [Google Scholar]
clinicaltrials.gov
- National Institutes of Health. Comparison of indacaterol and tiotropium on lung function and related outcomes in patients with chronic obstructive pulmonary disease (COPD) (INVIGORATE). http://clinicaltrials.gov/ct2/show/NCT00845728?term=tiotropium+indacaterol+copd&rank=6 2012.
Dahl 2010
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled β2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65(6):473. [DOI] [PubMed] [Google Scholar]
Donohue 2002
- Donohue JF, Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, et al. A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122(1):47‐55. [DOI] [PubMed] [Google Scholar]
Donohue 2011a
- Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2011; Vol. 6:477. [DOI] [PMC free article] [PubMed]
Drummond 1996
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313(7052):275‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dusser 2006
Effing 2007
- Effing T, Monninkhof Evelyn EM, Valk Paul PDLPM, Zielhuis Gerhard GA, Walters EH, Palen Job J, et al. Self‐management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
GOLD
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD. http://www.goldcopd.com 2009.
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008], The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2008.
Hutchinson 2010
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medicine Journal 2010;40(5):364‐71. [DOI] [PubMed] [Google Scholar]
Johnson 1998
- Johnson M. The beta‐adrenoceptor. American Journal of Respiratory and Critical Care Medicine 1998;158(5 Pt 3):S146‐53. [DOI] [PubMed] [Google Scholar]
Jones 1997
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1283‐9. [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones P. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2:75‐9. [DOI] [PubMed] [Google Scholar]
Karner 2011a
- Karner C, Cates CJ. Combination inhaled steroid and long‐acting beta2‐agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD008532.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2011b
- Karner C, Cates CJ. The effect of adding inhaled corticosteroids to tiotropium and long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD009039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2012
- Karner C, Cates CJ. Long‐acting beta2‐agonist in addition to tiotropium versus either tiotropium or long‐acting beta2‐agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008989.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2012a
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009285] [DOI] [PubMed] [Google Scholar]
Kornmann 2011
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once‐daily indacaterol versus twice‐daily salmeterol for COPD: a placebo‐controlled comparison. European Respiratory Journal 2011;37(2):273‐9. [DOI] [PubMed] [Google Scholar]
Lacasse 2006
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
Miravitlles 2002
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 2002;121(5):1449‐55. [DOI] [PubMed] [Google Scholar]
Miravitlles 2003
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1‐year follow‐up study. Chest 2003;123(3):784‐91. [DOI] [PubMed] [Google Scholar]
NICE
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease (updated). http://www.nice.org.uk/guidance/CG101 2012.
Niewoehner 2005
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper Jr JAD, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Annals of Internal Medicine 2005; Vol. 143, issue 5:317‐26. [CTG: NCT00274547] [DOI] [PubMed]
Oostenbrink 2004
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98(9):883‐91. [DOI] [PubMed] [Google Scholar]
Oostenbrink 2004a
- Oostenbrink JB, Rutten‐van Molken MP, Al MJ, Noord JA, Vincken W. One‐year cost‐effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. European Respiratory Journal 2004;23(2):241‐9. [DOI] [PubMed] [Google Scholar]
Philips 2004
Proskocil 2005
- Proskocil BJ, Fryer AD. Beta2‐agonist and anticholinergic drugs in the treatment of lung disease. Proceedings of the American Thoracic Society 2005;2(4):305‐10; discussion 311‐2. [DOI] [PubMed] [Google Scholar]
Rennard 2001
- Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long‐acting inhaled beta2‐adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(5):1087‐92. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rodrigo 2008
- Rodrigo GJ, Nannini LJ, Rodríguez‐Roisin R. Safety of long‐acting β‐agonists in stable COPD. Chest 2008;133(5):1079‐87. [DOI] [PubMed] [Google Scholar]
Sears 2008
- Sears MR. Long‐acting bronchodilators in COPD. Chest 2008;133(5):1057‐8. [DOI] [PubMed] [Google Scholar]
Sin 2004
- Sin DD, Golmohammadi K, Jacobs P. Cost‐effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. American Journal of Medicine 2004;116(5):325‐31. [DOI] [PubMed] [Google Scholar]
Spencer 2011
- Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockley 2006
- Stockley RA, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax 2006;61(2):122‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2005
- Tanaka Y, Horinouchi T, Koike K. New insights into beta‐adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clinical and Experimental Pharmacology and Physiology 2005;32(7):503‐14. [DOI] [PubMed] [Google Scholar]
Tashkin 2008
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. New England Journal Medicine 2008;359(15):1543‐54. [DOI] [PubMed] [Google Scholar]
van der Meer 2001
- Meer RM, Wagena E, Ostelo RWJG, Jacobs AJE, Schayck OP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vincken 2002
- Vincken W, Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. European Respiratory Journal 2002;19(2):209‐16. [DOI] [PubMed] [Google Scholar]
Wallukat 2002
- Wallukat G. The beta‐adrenergic receptors. Herz 2002;27(7):683‐90. [DOI] [PubMed] [Google Scholar]
Welsh 2010
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007891.pub2] [DOI] [PubMed] [Google Scholar]
WHO
- World Health Organization. Chronic Respiratory Diseases. http://www.who.int.
Wilson 2000
- Wilson L, Devine EB, So K. Direct medical costs of chronic obstructive pulmonary disease: chronic bronchitis and emphysema. Respiratory Medicine 2000;94(3):204‐13. [DOI] [PubMed] [Google Scholar]
Yohannes 2011
- Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta‐analysis of clinically relevant outcomes. Respiratory Care 2011;56(4):477‐87. [DOI] [PubMed] [Google Scholar]


