Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by SARS-CoV-2, continues to cause substantial morbidity and mortality. While most infections are mild, some patients experience severe and potentially fatal systemic inflammation, tissue damage, cytokine storm and acute respiratory distress syndrome. The innate immune system acts as the first line of defense, sensing the virus through pattern recognition receptors and activating inflammatory pathways that promote viral clearance. Here, we discuss the innate immune processes involved in virus recognition and the resultant inflammation, and how this insight has enabled the development of therapeutic interventions against COVID-19. An improved understanding of how the innate immune system detects and responds to SARS-CoV-2 will help identify targeted therapeutic modalities that mitigate severe disease and improve patient outcomes.
Keywords: SARS-CoV-2, COVID-19, coronavirus, innate immunity, pattern recognition receptors, TLRs, RLRs, NLRs, interferons, pro-inflammatory cytokines, TNF-α, IFN-γ, cell death, PANoptosis, PANoptosome, cytokine storm, IFN-α, IFN-β, IFN-λ
In 2019, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged as a novel pathogen that resulted in the coronavirus disease 2019 (COVID-19) pandemic. Initially identified in Wuhan, China in December 20191,2, SARS-CoV-2 rapidly spread throughout the world, leading to an ongoing public health crisis, and as of November 2, 2021, there have been over 247 million infections and 5 million deaths3. SARS-CoV-2 causes upper and lower respiratory tract infections that often are associated with fever, cough and loss of smell and taste. Most infections remain mild, and up to 20–40% of patients are asymptomatic. However, some patients experience more severe disease and develop systemic inflammation, tissue damage, acute respiratory distress syndrome (ARDS), thromboembolic complications, cardiac injury and/or cytokine storm, which can be fatal1,4 (Figure 1). The risk of COVID-19 disease severity depends on co-morbidities (e.g., diabetes, hypertension and obesity), age, ethnicity, genetic factors, vaccination status and other conditions5, making understanding the underlying disease mechanisms important for risk stratification and clinical triage.
Figure 1. Clinical manifestations of COVID-19.
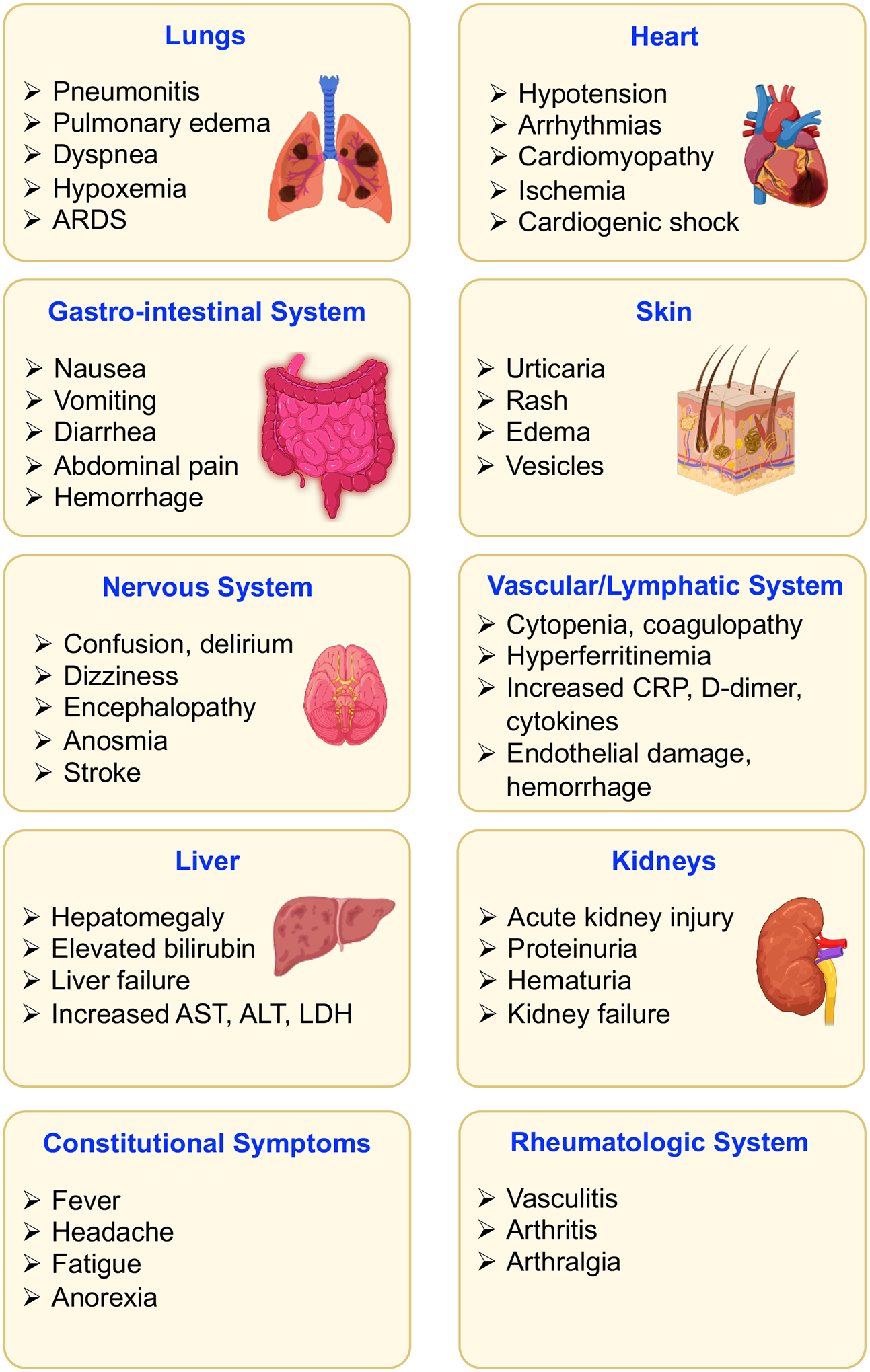
SARS-CoV-2 infection affects several body systems, including the cardiovascular, gastro-intestinal, nervous, vascular/lymphatic, rheumatological systems and others.
The extensive morbidity and mortality associated with the COVID-19 pandemic made the development of SARS-CoV-2 vaccines a global health priority. In less than one year, several effective vaccines targeting the SARS-CoV-2 spike protein from multiple platforms (lipid nanoparticle encapsulated mRNA, inactivated virion or viral-vectored vaccine platforms6) gained emergency use authorization (EUA) or approval and were deployed to billions of people worldwide. In countries with high rates of vaccination, markedly reduced numbers of infections, hospitalizations and deaths have been observed, although the success of vaccination has been jeopardized by the emergence of variants including B.1.351 (Beta), B.1.1.28 (Gamma) and B.1.617.2 (Delta) and vaccine hesitancy7. Compared to vaccine countermeasures, specific treatment options have remained more limited. Remdesivir, a viral polymerase inhibitor, has been FDA approved for hospitalized patients, and EUA has been granted to the antivirals molnupiravir and paxlovid (combination of nirmatrelvir and ritonavir) as well as baricitinib, a janus kinase inhibitor (in combination with remdesivir); tocilizumab, an anti–IL-6 receptor monoclonal antibody (mAb); and sotrovimab and mAb cocktails (casirivimab and imdevimab, as well as bamlanivimab and etesevimab), which are all neutralizing mAbs that bind the viral spike protein. Among these, baricitinib and tocilizumab represent immunomodulatory strategies and have been used along with systemic administration of steroids to control SARS-CoV-2–induced inflammation8. Notwithstanding these EUA and approvals, many of these drugs have limited therapeutic indications, and most cases of severe disease worldwide remain untreated with specific drugs.
The innate immune system functions as the first line of host defense against pathogens, including SARS-CoV-2. Innate immune responses limit viral entry, translation, replication and assembly, help identify and remove infected cells, and coordinate and accelerate the development of adaptive immunity. Cell surface, endosomal and cytosolic pattern recognition receptors (PRRs) respond to pathogen-associated molecular patterns (PAMPs) to trigger inflammatory responses and programmed cell death that limit viral infection and promote clearance9. However, excessive immune activation can lead to systemic inflammation and severe disease, highlighting the balance that must be achieved. In response to innate immune-dependent viral clearance mechanisms, coronaviruses (CoVs) have evolved evasion strategies to limit host control and enhance replication and transmission10–13. Here, we review the innate immune detection and signaling pathways that the host uses against SARS-CoV-2, highlighting the role of viral entry, the critical PRRs and signaling pathways, cytokine production and cell death, as well as viral immune evasion strategies. An improved understanding of these processes may increase our ability to treat and prevent CoV infections in this pandemic and beyond.
SARS-CoV-2 viral entry to facilitate pattern recognition receptor sensing
SARS-CoV-2 is a member of the Betacoronavirus genus in the Coronaviridae family and is related closely to SARS-CoV and Middle East respiratory syndrome (MERS)-CoV14,15. The CoV virion contains the spike (S), envelope (E) and membrane (M) proteins in the viral membrane, with the genomic RNA complexed with the nucleocapsid (N) protein to create a helical capsid. The S protein is a type I glycoprotein that forms peplomers on the virion surface. The E protein is hydrophobic, and the M protein contains a short N-terminal ectodomain with a cytoplasmic tail14. The virus also produces several open reading frames (ORFs) that encode accessory proteins with diverse roles in viral pathogenesis10,11.
For infection of most host cells, the SARS-CoV-2 S protein binds to its principal cellular receptor, angiotensin-converting enzyme 2 (ACE2)2 (Figure 2). Additionally, the host serine protease TMPRSS2 is important for proteolytic priming of the S protein for receptor interactions and entry16. Other host proteins, such as neuropilin-1, heparin sulfate proteoglycans, C-type lectins or furin, also can act as co-factors for viral entry17–20. Upon S protein binding to cells, the viral and host membranes can fuse16, releasing the viral genomic RNA directly into the cytoplasm. Alternatively, in some cells, SARS-CoV-2 is internalized into endosomes, and after low pH-triggered cathepsin-mediated cleavage, the viral membranes fuse with the endosomal membrane to facilitate nucleocapsid entry into the cytoplasm21 (Figure 2). Once in the cytoplasm, CoV genomic RNA is translated into two large polyproteins, pp1a and pp1ab, that encode 16 nonstructural proteins (NSPs). These proteins facilitate formation of the viral replicase-transcriptase complex22,23, which generates an antisense negative-strand template from the viral RNA. The NSPs then reorganize membranes derived from the endoplasmic reticulum (ER) and Golgi to form double-membrane vesicles to compartmentalize viral replication and transcription away from host sensor detection24. When new viral structural proteins are synthesized, they traffic to the ER or Golgi membranes and combine with the genomic RNA and N proteins to create nascent viral particles. Assembly at the ER-Golgi intermediate compartment (ERGIC) leads to the creation of virion-containing vesicles that can fuse with the plasma membrane during exocytosis and release virus into the extracellular space25,26 (Figure 2). The steps in viral entry and replication present several opportunities for the innate immune system to sense viral components. For example, the virion’s S, E and M proteins are exposed to host cell surface sensors during the binding step, and host cytoplasmic sensors could detect viral proteins and nucleic acids prior to their compartmentalization by the NSPs. These detection steps facilitate the activation of inflammatory signaling pathways, cytokine production and cell death.
Figure 2. SARS-CoV-2 viral entry.
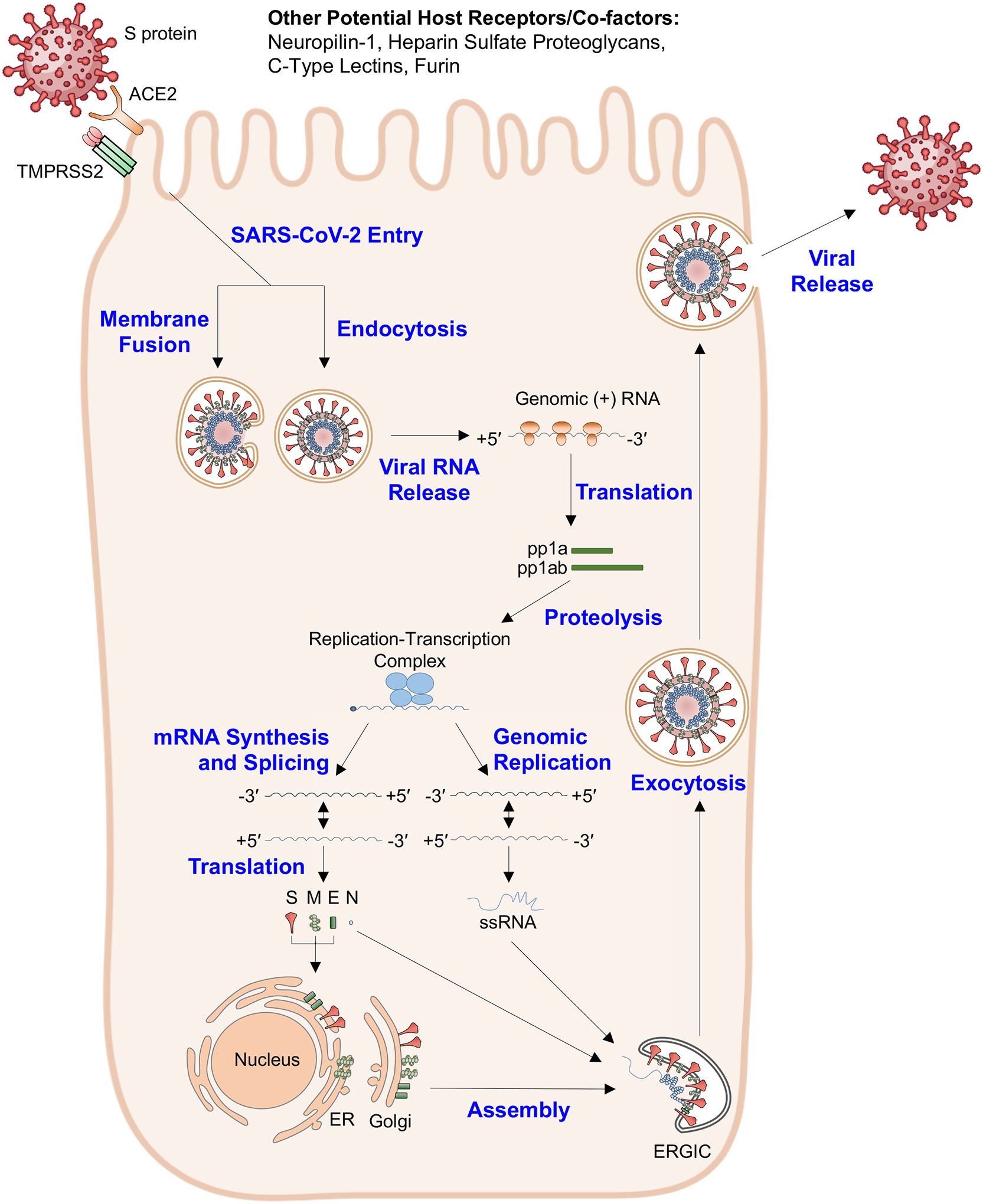
On most cells, SARS-CoV-2 spike protein (S) binds to the cell surface and its cognate receptor angiotensin-converting enzyme 2 (ACE2). The host transmembrane protease serine 2 (TMPRSS2) helps mediate entry by cleaving S. Other potential host receptors and co-factors have been implicated in this process, including neuropilin-1, heparin sulfate proteoglycans, C-type lectins and/or furin. The virion enters through membrane fusion or endocytosis. After entry, the viral RNA is released and translated into the viral polyproteins pp1a and pp1ab. These polyproteins are processed by virus-encoded proteases to facilitate replication and produce the full-length negative-strand RNA and subgenomic RNA. The subgenomic RNA is translated into the structural and accessory proteins, including S, membrane (M), envelope (E) and nucleocapsid (N). The structural proteins are inserted into the endoplasmic reticulum (ER) and Golgi membranes and transverse to the ER-Golgi intermediate compartment (ERGIC), where the virions can assemble. Fully formed virions are exocytosed.
Innate immune cells, including macrophages, monocytes, dendritic cells, neutrophils and innate lymphoid cells (ILCs) such as natural killer (NK) cells, are armed with an arsenal of PRRs that recognize PAMPs or damage-associated molecular patterns (DAMPs) to induce inflammatory signaling pathways and immune responses. The five primary PRR families include Toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs) and the absent in melanoma 2 (AIM2)-like receptors (ALRs)9. Signaling through these receptors in innate immune cells in response to PAMPs or DAMPs leads to the production of inflammatory cytokines and chemokines as well as the induction of cell death to clear infected cells. To date, several PRRs, in particular TLRs, RLRs and NLRs, have been shown to activate their signaling pathways in response to SARS-CoV-2 and induce the production of cytokines.
TLRs and SARS-CoV-2
Many viruses activate the innate immune system through TLR signaling (Figure 3). The TLRs are expressed throughout the human respiratory tract but show heterogeneous expression across innate immune cell populations; for example, TLR3 is more abundant in NK cells, whereas TLR4 is more common in macrophages27. TLRs generally transduce signals via two key adaptor molecules, MyD88 and TRIF. Most TLRs utilize MyD88 to trigger inflammatory cytokine production; TLR3 is the exception and signals exclusively through TRIF. TLR4 is unique in that it can bind and signal through either MyD88 or TRIF28. Downstream of MyD88, NF-κB, MAPKs and interferon (IFN) regulatory factors (IRFs) are activated. Nuclear translocation of these molecules results in the transcriptional activation of several pro-inflammatory cytokines including TNF, IL-6 and IL-1 along with transcription of other innate immune sensors, such as NLRP3, and production of type I IFNs and IFN-stimulated genes (ISGs) (Figure 3). Signaling through TRIF also activates type I IFN production and several TLR4- and TLR3-dependent transcription factors, some of which have direct antiviral activity28.
Figure 3. Putative activation of PRR signaling during SARS-CoV-2 infection based on current data.
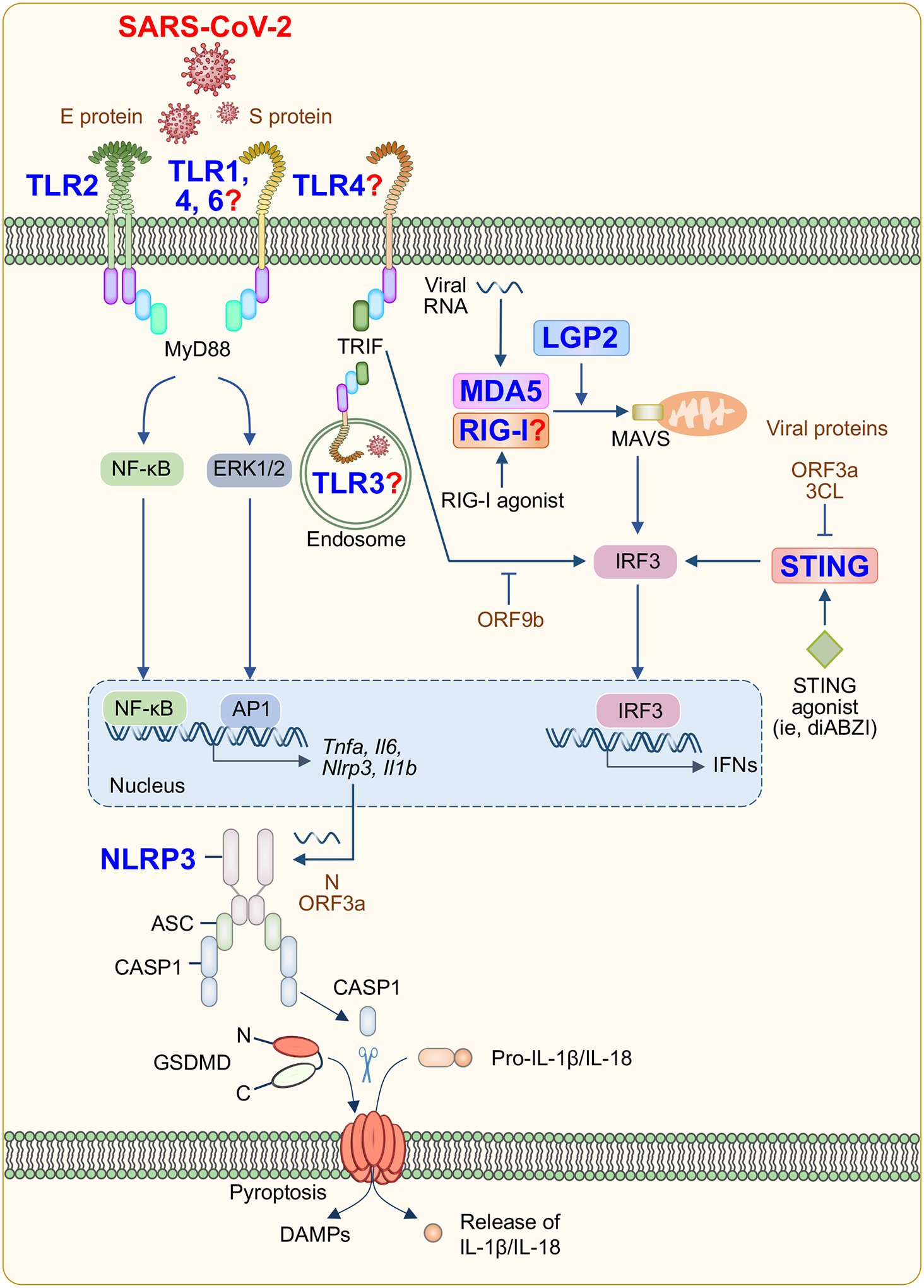
Pattern recognition receptors (PRRs) on the cell surface and endosomal membranes and in the cytosol may respond to SARS-CoV-2 pathogen-associated molecular patterns (PAMPs) to activate innate immune signaling pathways. Toll-like receptors (TLRs) 1, 2, 4 and 6 can signal through MyD88 to activate the NF-κB and MAPK signaling pathways to induce the transcription of pro-inflammatory cytokines and other sensors. TLR3 and 4 can signal through TRIF to activate IRF3 and induce the expression of type I and type III IFNs. Strong experimental evidence supports SARS-CoV-2–mediated activation of TLR2, while activation of TLR1, 3, 4 and 6 has been suggested bioinformatically and through associative studies. Signaling through RIG-I, MDA5 and STING also activates IRF3 and type I and type III IFN production. Data regarding the activation of RIG-I during SARS-CoV-2 infection remain mixed. NLRP3 inflammasome formation also can occur in response to SARS-CoV-2 infection, leading to cleavage of gasdermin D (GSDMD) to form membrane pores and release IL-1β and IL-18 and induce pyroptosis. Red question marks indicate sensors that are expected to be involved in SARS-CoV-2 sensing but that have not been experimentally validated to date.
SARS-CoV-2–mediated induction of pro-inflammatory signaling pathways and cytokine production are attenuated in TLR2-deficient murine macrophages and in human macrophages treated with a TLR2 inhibitor following stimulation with the SARS-CoV-2 E protein, suggesting that TLR2 senses the E protein to mount inflammatory responses29. Furthermore, the SARS-CoV-2 E protein induces TLR2-dependent inflammation in vivo, as serum levels of IL-6 are reduced in Tlr2–/– mice upon E protein administration. An independent single-cell computational analysis aimed at predicting targets that could be modulated to reduce the dysregulated innate immune response during SARS-CoV-2 infection also shows that TLR2 is a potential target30. Consistent with these findings, treatment of K18-hACE2 transgenic mice with a TLR2 inhibitor reduces the levels of inflammatory cytokines in the blood and improves survival following SARS-CoV-2 infection29. However, additional work is required to determine whether TLR2 directly binds the E protein or other SARS-CoV-2 ligands.
Beyond TLR2, the role of other TLRs in SARS-CoV-2 infection has not been as conclusively studied in vitro and in vivo. In the context of SARS-CoV, the TLR3 signaling pathway has a protective role in vivo26,31–33; Tlr3–/– mice have increased viral burden and impaired pulmonary function compared to control mice during infection with mouse-adapted SARS-CoV (MA15)33. Additionally, stimulation with a TLR3 agonist reduces SARS-CoV burden in human alveolar epithelial cells31. However, no studies to date have linked TLR3 with SARS-CoV-2 sensing specifically. While one genomic analysis of patients with severe COVID-19 found an association between inborn errors in TLR3 and disease severity34, a follow-up study failed to validate these associations35. Therefore, the role of TLR3 in SARS-CoV-2 infection remains unclear.
In silico studies suggest that the SARS-CoV-2 S protein may bind to TLR1, TLR4 and TLR6, with TLR4 having the highest affinity36. TLR4 activation in response to the S protein is supported by the reduced gene expression of Il1b in S protein-stimulated Tlr4–/– murine macrophages compared with expression in wild-type cells in vitro37. However, computational modeling and in vitro analyses show that the S protein has a high binding affinity to lipopolysaccharide (LPS)38, raising concerns that LPS may contaminate purified S protein preparations and contribute to the release of cytokines in a TLR4-dependent manner. Therefore, whether TLR1, TLR4 or TLR6 can directly sense the S protein requires further confirmation.
Additionally, TLR7/8 recognize antiphospholipid antibodies39,40, which are upregulated in patients with severe COVID-1941,42. X-chromosomal TLR7 genetic anomalies have been linked to severe COVID-19 disease in young individuals43,44, suggesting that TLR7 has a protective role during SARS-CoV-2 infection. More investigations are needed to corroborate the roles of these and other TLRs in response to SARS-CoV-2 infection.
SARS-CoV-2 with RIG-I-like receptors and IFN signaling
Single-stranded RNA derived from genomic, subgenomic or replicative intermediates of SARS-CoV-2 also can be sensed by RLRs intracellularly, which include MDA5, RIG-I and LGP245–47 (Figure 3). RIG-I and MDA5 are the most well-studied RLRs and provide key regulation of the IFN pathways. Following post-translational modifications and activation, RIG-I and MDA5 translocate to the mitochondria, where they interact with the adaptor protein mitochondria antiviral signaling (MAVS) to form a MAVS signalosome. This complex formation activates TRAF3, TBK1 and IKK to induce phosphorylation of IRF348,49, which facilitates its nuclear translocation and the transcription of type I and III IFNs (Figure 3). The production and subsequent release of IFNs stimulate downstream signaling through the IFN receptors (IFNAR1/IFNAR2 for type I IFNs; IFNLR1/IL10Rβ for type III IFNs) in an autocrine and paracrine manner to produce hundreds of ISGs with various antiviral functions50,51. For example, the ISG Ly6E can prevent SARS-CoV-2 entry52, members of the IFIT family IFIT1, IFIT3 and IFIT5 inhibit viral replication and BST2 can block viral egress53 in cell lines that ectopically or stably express these ISGs. Circulating autoantibodies that target type I IFN have also been identified; patients with these have reduced IFN responses after SARS-CoV-2 infection54 and are at increased risk of severe COVID-19 and death55–57. However, the fine-tuning of a type I IFN response is critical because both over and under activation of IFN signaling can be deleterious to the host58.
A screen for RNA sensors involved in the restriction of SARS-CoV-2 infection identified MDA5 and LGP2 as key regulators of antiviral type I IFN induction45. Silencing or deleting MDA5, LGP2 or the adaptor MAVS in human primary lung airway epithelial or Calu-3 cells results in reduced type I IFN expression during SARS-CoV-2 infection45–47. In contrast, there are conflicting results with RIG-I. One study found that siRNA silencing of RIG-I failed to reduce IFN-β production in response to SARS-CoV-2 infection in Calu-3 cells45, and its genetic deletion by CRISPR/Cas9 targeting in Calu-3 cells provided similar results47. However, another study reported that siRNA silencing of RIG-I in Calu-3 cells significantly reduced IFN-β expression during SARS-CoV-2 infection59. Instead of engaging the C-terminal domain of RIG-I that normally senses viral RNA, the SARS-CoV-2 genomic RNA 3’ untranslated region is recognized by the helicase domain, resulting in impaired ATPase activation in RIG-I60. This unconventional RIG-I engagement may limit its ability to activate the downstream MAVS signaling pathway for type I IFN production60, though additional confirmatory studies are required.
SARS-CoV-2, NLRs and inflammasome sensors
NLRs also are reported to respond to SARS-CoV-2 infection and induce the production of type I IFNs and pro-inflammatory cytokines. NLRP3, one of the best characterized inflammasome sensors, is triggered in response to PAMPs and DAMPs, leading to the activation of caspase-1, production and release of bioactive IL-1β and IL-18 and cleavage of gasdermin D (GSDMD), which forms pores in the plasma membrane to drive membrane rupture, mediated by ninjurin 1, and pyroptotic cell death61,62. Increased levels of IL-1β and IL-18 in plasma correlate with disease severity and mortality in patients with COVID-1963,64, and several reports have suggested that NLRP3 senses CoV infections26,65–69.
NLRP3 deficiency abolishes murine hepatitis virus (MHV)-induced caspase-1 and GSDMD activation in murine macrophages65, indicating that CoVs may induce NLRP3 inflammasome assembly. Several PAMPs from SARS-CoV, including those derived from ORF3a66, ORF8b67, the E protein68 and viral RNA69, can activate the NLRP3 inflammasome. In the case of SARS-CoV-2 infection, monocytes and lung tissues from patients with COVID-19 contain NLRP3 and apoptosis-associated speck-like protein containing a caspase activating and recruitment domain (ASC) puncta70, suggesting that the NLRP3 inflammasome forms in these patients. Additionally, human primary monocytes infected with SARS-CoV-2 show NLRP3-dependent caspase-1 and GSDMD cleavage and IL-1β maturation70,71.
Several PAMPs from SARS-CoV-2 are implicated in NLRP3 inflammasome assembly and subsequent cytokine release (Figure 3). The GU-rich single-stranded RNA derived from the SARS-CoV-2 genome activates the NLRP3 inflammasome and cytokine release in macrophages69. SARS-CoV-2 ORF3a, also known as viroporin, and the N protein also trigger NLRP3 inflammasome activation in human cell lines, HEK293 with or without inflammasome machinery transfection and A54972,73. Despite its ability to induce NLRP3 inflammasome activation, the N protein has also been associated with inhibition of GSDMD to block pyroptosis and IL-1β release74, and the direct role of the N protein in IL-1β release remains to be clarified. Upstream, the SARS-CoV-2 E protein triggers TLR2 signaling, which upregulates expression of Nlrp3 and Il1b mRNA in macrophages29. Additionally, the S protein upregulates NLRP3 protein expression and induces release of IL-1β in macrophages obtained from patients with COVID-19 but not in those from healthy volunteers75. Mechanistically, it has been proposed that SARS-CoV-2 infection causes an imbalance in the intracellular potassium efflux to drive NLRP3 inflammasome activation and IL-1β and IL-18 release71. Nonetheless, the role of specific SARS-CoV-2 proteins in triggering this imbalance remains to be characterized.
Beyond NLRP3, microscopy studies also have shown co-localization of the AIM2 inflammasome sensor with ASC specks in monocytes from patients with COVID-1976, although the functional role of AIM2, a DNA sensor, in this context remains unclear. The intracellular sensor of bacterial peptidoglycans NOD1 (NLRC1) also contributes to SARS-CoV-2 responses and cytokine production. Silencing of NLRC1 in lung epithelial cells reduces IFN-β expression during SARS-CoV-2 infection45. Additional studies are needed to establish the importance of other NLRs and inflammasome sensors in sensing of SARS-CoV-2 and production of cytokines.
cGAS, STING and SARS-CoV-2 infection
Beyond the TLRs, RLRs and NLRs, there are additional cytosolic sensors that can detect viruses and activate pro-inflammatory signaling pathways. The cGAS-STING signaling pathway, which is activated upon detection of cytoplasmic DNA, is critical for limiting the replication of both DNA and RNA viruses following infection44,77–82. SARS-CoV-2 infection induces mitochondrial damage83, which may release mitochondrial DNA into the cytoplasm to activate cGAS, contributing to innate immune responses. However, SARS-CoV-2 accessory proteins ORF3a and 3CL can antagonize cGAS-STING signaling, thereby suppressing antiviral immune responses84 (Figure 3). Indeed, the restoration of cGAS-STING activation and signaling by administering the exogenous STING agonist diABZI inhibits SARS-CoV-2 replication and improves survival rates in infected human ACE2-expressing transgenic mice85,86, emphasizing the importance of a robust signaling response to prevent viral spread.
Cytokine signaling, cell death and cytokine storm
The PRR signaling engaged by SARS-CoV-2 induces concurrent release of both IFNs and other pro-inflammatory cytokines87. The expression of numerous pro-inflammatory cytokines and IFNs is elevated in patients with COVID-19, including IL-1β, IL-6, TNF, IL-12, IFN-β, IFN-γ, IL-17 and others1,88,89. These cytokines aid in clearing infections, but also maintain cellular homeostasis. For instance, transient activation of inflammatory cytokine receptors, and in particular IL-1 signaling, stimulates insulin secretion from pancreatic β-cells, allowing the β-cell to adapt to increased insulin demand during stress90. However, the dysregulated release of pro-inflammatory cytokines contributes to cytokine storm, defined as a life-threatening condition caused by excessive production of cytokines mediated by inflammatory cell death, PANoptosis4. In the context of COVID-19, the combination of TNF and IFN-γ contributes to disease pathogenesis by signaling cooperatively and inducing inflammatory cell death, PANoptosis91 (Figure 4). PANoptosis is an inflammatory programmed cell death pathway regulated by the PANoptosome that shares molecular features with pyroptosis, apoptosis and/or necroptosis but cannot be accounted for by any of these three programmed cell death pathways alone65,91–108. PANoptosis induced by the synergism of TNF and IFN-γ depends on STAT1 and IRF1 signaling and leads to the activation of caspase-8 to drive cell death91,92 (Figure 4). The combination of TNF and IFN-γ signaling induces a lethal shock syndrome in mice91,109, which mirrors the cytokine storm seen in some patients with severe COVID-1991. Mice treated with blocking antibodies against TNF and IFN-γ have reduced mortality during SARS-CoV-2 infection, as well as in other models of cytokine storm91. A computational analysis of more than 300,000 single-cell transcriptomes concluded that the transcriptional programs of macrophages from patients with COVID-19 share many features with macrophages stimulated ex vivo with TNF and IFN-γ, including high levels of STAT1, IFNGR1, IFNGR2, NFKB1 and IL1B110. The abundance of these inflammatory macrophages is associated with disease severity in patients with COVID-19, as well as in patients with autoimmune disease (rheumatoid arthritis) and inflammatory disease (Crohn’s disease and ulcerative colitis)110. Overall, these studies suggest a positive feedback loop exists, whereby cytokine secretion causes PANoptosis that results in more cytokine release, culminating in a cytokine storm that causes life-threatening damage to host tissues and organs4,91 (Figure 4). PAMPs from pathogens and DAMPs from the host along with cytokines can all trigger PANoptosis65,91,93–97,99,104,108, which could initiate this feedback loop and promote inflammation and disease progression.
Figure 4. PANoptosis and cytokine storm during SARS-CoV-2 infection.
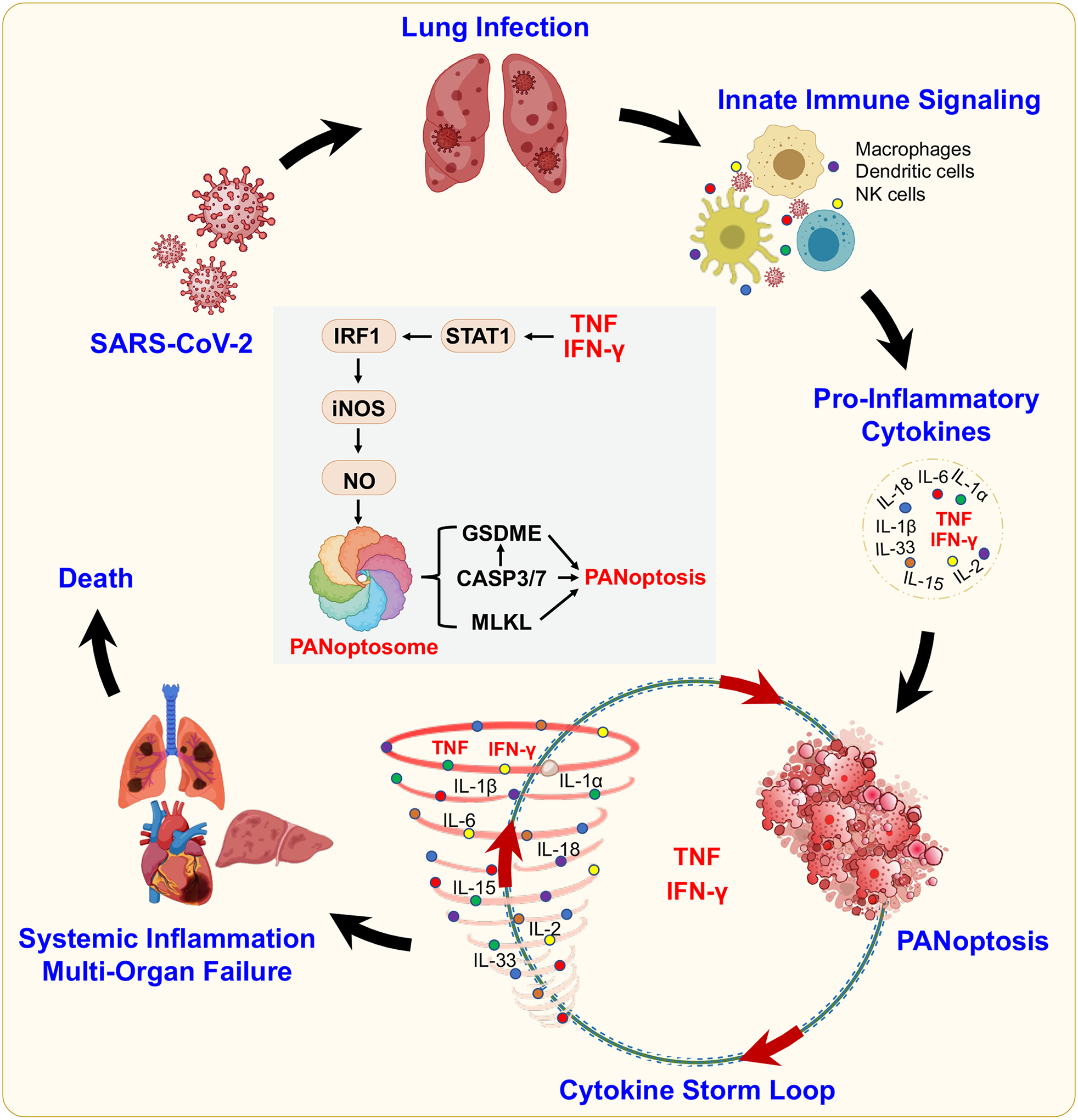
The induction of innate immune signaling during SARS-CoV-2 infection leads to the production of pro-inflammatory cytokines. TNF and IFN-γ induce a form of inflammatory cell death called PANoptosis, which is mediated through the formation of a multiprotein complex called the PANoptosome. TNF and IFN-γ–mediated PANoptosis can lead to a cytokine storm loop, featuring further pathogenic cytokine release that can perpetuate PANoptosis as well as systemic inflammation, multi-organ failure and lethality.
The link between the excessive cytokine signaling and cell death could explain the multiorgan damage observed in some patients with COVID-19. For example, lung damage and ARDS occur in many patients with severe COVID-19. Mounting evidence suggests that structural damage to endothelial cell membranes and ensuing vascular leakage contribute to the initiation and propagation of ARDS during SARS-CoV-2 infection111. Additionally, vascular damage, including damage to heart vessels, is a key feature of an unprecedented cluster of hyper-inflammatory shock syndromes in children with COVID-19 that is similar to Kawasaki disease and is termed multisystem inflammatory syndrome in children (MIS-C)112,113. MIS-C could be a result of endothelial cell damage and death triggered by cytokines, possibly due to the effects of TNF and IFN-γ. Moreover, endothelial cell-associated anticoagulant pathways can be impaired by pro-inflammatory cytokines114, which could explain some of the thromboembolic complications reported during severe COVID-19115,116, providing yet another pathogenic mechanism for cytokines in tissue damage.
Another feature of COVID-19 is a depletion of germinal centers in the spleen and lymph nodes117, which may be due to lymphocyte cell death promoted by TNF and IFN-γ signaling. TNF and IFN-γ shock can drive lymphopenia and immunosuppression91,118, and high amounts of TNF are found in the remaining germinal centers of patients with severe COVID-19, which could limit B cell affinity maturation, isotype-switching and production of mature antibodies117, leading to detrimental effects on patient outcomes119.
While accumulating evidence suggests that high levels of cytokines are associated with COVID-19 morbidity and mortality, some studies have questioned whether cytokine storm occurs in COVID-19120. For example, one group reported lower levels of IL-6 in patients with COVID-19 than those with sepsis or chimeric antigen receptor (CAR) T cell-induced cytokine storm syndromes121. RNAseq analysis has found reduced cytokine expression in the PBMCs of patients with COVID-19 compared to those with influenza virus infection122, whereas another group found increased expression of TNF and IL-1 in COVID-19123. However, the conclusions of these studies are based on comparisons among patients with cytokine storm-associated diseases, and not comparisons with healthy individuals121,122. Given that SARS-CoV-2 is a respiratory virus, the cytokine profile in the local tissue environment may be more informative than interrogation of peripheral blood samples, which has been used in most studies to date. Indeed, markedly increased levels of pro-inflammatory cytokines and chemokines were seen in lung homogenates from human ACE2-expressing transgenic mice after SARS-CoV-2 infection124. Additionally, a study examining the local immune response in nasal wash samples from ferrets infected with SARS-CoV-2 found increased levels of IL-6, IL-1 and other chemokines in SARS-CoV-2–infected cells compared to influenza virus-infected cells on day 7 post-infection12. Overall, while cytokines are critical for the innate immune response and successful clearance of viral infections, their release must be controlled to prevent systemic cytokine storm and pathogenic inflammation during SARS-CoV-2 infections.
Viral innate immune evasion strategies
One primary function of the innate immune system during viral infection is to induce an inflammatory response that limits viral replication. However, CoVs have evolved several evasion strategies to counteract this host defense. SARS-CoV-2 can specifically evade antiviral innate immune responses by reducing IFN levels; patients with mild and moderate COVID-19 have low type I and III IFN levels in their serum12. Indeed, SARS-CoV-2 infection limits type I and III IFN production at post-transcriptional levels by preventing the release of mRNA from sites of transcription and/or triggering transcript degradation in the nucleus13. SARS-CoV-2 also encodes several proteins that disrupt RLR sensing pathways and IFN induction, signaling or effector functions. For example, SARS-CoV-2 papain-like protease (PLpro) inhibits MDA5 activation by de-ISGylating MDA5, as ISG15 conjugation, or ISGylation, of the MDA5 CARD domain is essential for its activation following RNA virus infection125. Additionally, the SARS-CoV-2 ORF9b, N and M proteins can inhibit the expression of IFN-β and pro-inflammatory cytokines by interfering with the RIG-I and MDA5 pathways11,126–129. ORF9b also can block the TLR3-TRIF pathway128. ORF3b suppresses the induction of type I IFN more efficiently than its SARS-CoV ortholog10. SARS-CoV-2 ORF6 and ORF8 inhibit the expression of IFN-β and the activation of ISGs11. SARS-CoV-2 NSP1 and NSP14, and potentially other viral proteins, can inhibit translation and prevent the expression of components of the IFN signaling pathway130–132. Finally, SARS-CoV-2 N protein appears to prevent the aggregation of viral RNA with MAVS to block induction of the IFN pathway133. Gaining further insights into the SARS-CoV-2 immune evasion strategies and the PRRs and IFN and cytokine production pathways that can counteract them could provide further mechanistic targets for therapeutic development.
Innate immunity and therapeutic development
During the COVID-19 pandemic, many treatment strategies have been investigated. The rate of clinical trials for COVID-19 has been staggering, with nearly 7,000 registered through clinicaltrials.gov as of November 2, 2021. In addition to the vaccine strategies, which have been extensively covered elsewhere134, the therapeutic strategies can be divided into antiviral or immunomodulatory therapies (Table 1). Clinical trials of these treatments have led to the FDA approval of remdesivir and the EUA for the antivirals molnupiravir, paxlovid (combination of nirmatrelvir and ritonavir), sotrovimab and mAbs cocktails8. These compounds target the virus by blocking viral replication, preventing virus entry by binding the spike protein or promoting virus clearance through antibody Fc effector functions135–137. In addition, antiviral strategies that modulate immune cell activation and inflammation also have been investigated. In particular, treatment with type I IFNs has been tested in several clinical trials. IFN-α2b treatment reduced the time to viral clearance in the upper respiratory tract and the time to resolution of systemic inflammatory markers in an exploratory study138. A retrospective study found that administration of IFN-α2b within 5 days of hospitalization was associated with a decrease in mortality139. However, this study also found that later administration was associated with increased mortality139. Similarly, preclinical studies showed that exogenous type I IFN therapy reduces viral load when administered before SARS-CoV-2 infection, but this effect is limited once infection is established140. These results highlight the importance of understanding disease pathogenesis to identify therapeutic windows for treatments. Type I IFN has also been given in combination with other antivirals and shown to improve patient outcomes141. Additional evidence suggests that treatments that upregulate endogenous type I IFN production also may be beneficial. For instance, preclinical studies found that the IFN signaling triggered by treatment with the STING agonist limited SARS-CoV-2 infection85,86,142; similar results were observed with a RIG-I agonist143. In theory, STING or RIG-I agonists could be repurposed as prophylactic agents for vulnerable patient populations.
Table 1.
Approved and experimental treatment strategies for COVID-19
| Category | FDA approved/EUA | Under investigation |
|---|---|---|
| Antivirals | Remdesivir; molnupiravir; paxlovid (combination of nirmatrelvir and ritonavir); sotrovimab; anti-spike monoclonal antibody cocktails (REGEN-COV [casirivimab–imdevimab]; bamlanivimab–etesevimaba) | Chloroquine/ hydroxychloroquine; IFNα, IFNβ, IFNλ therapy; STING agonists; RIG-I agonists |
| Cytokine inhibition | Anti-IL-6 (Tocilizumab) | Anti-IL-6 (Sarilumab; siltuximab; levilimab) Anti-IL-1 (Canakinumab; anakinra; rilonacept) Anti-TNF (Infliximab; adalimumab; certolizumab; etanercept; golimumab) Anti-IFN-γ (Emapalumab) |
| Inflammatory signaling inhibition | Baricitinib | Dexamethasone |
On June 25, 2021, the FDA issued a pause on the distribution of bamlanivimab/etesevimab due to a lack of efficacy against the Beta and Gamma viral variants.
Treatment with type III IFN also might be advantageous during SARS-CoV-2 infection. While IFN-λ was found to induce lung epithelial barrier damage in response to viral infections in murine models144, increased IFN-λ1 and IFN-λ2 levels in the serum are associated with better prognosis for patients with COVID-19 in observational studies145,146. On a cellular level, pre-treatment of human primary airway epithelial cells, Calu-3 cells, intestinal epithelial cells and colon organoids with IFN-λ reduces levels of SARS-CoV-2 infection147–149, and loss of the type III IFN receptor increases the cellular susceptibility to infection148. In a murine model of SARS-CoV-2 infection, treatment with Peginterferon lambda 1a, a pegylated, recombinant IFN-λ1a, reduced SARS-CoV-2 replication in mice149. Based on these preclinical data, clinical trials have begun investigating Peginterferon lambda 1a in patients with COVID-19. Among patients with mild disease in a Phase II trial, there were no significant differences in time to resolution of symptoms or other clinical metrics between the Peginterferon lambda 1a and placebo groups150. However, a second Phase II trial found that treatment with Peginterferon lambda 1a improved the odds of achieving viral clearance by day 7151. Further studies are needed to determine whether IFN-λ treatment is advantageous for particular patient populations.
In addition to the antiviral therapies discussed above, immunomodulatory therapies have been extensively evaluated in clinical trials for COVID-19 due to the pathogenicity associated with excess cytokine production, cell death and cytokine storm. Corticosteroids, such as dexamethasone, can inhibit the production of pro-inflammatory cytokines and prevent systemic inflammation. However, the large randomized open-label Phase II/III RECOVERY trial evaluating treatment strategies in hospitalized patients showed that dexamethasone was effective only in patients requiring mechanical ventilation or supplemental oxygen152. Moreover, prolonged or over-use of corticosteroids can generally increase the risk of secondary infections, although studies in patients with COVID-19 have reported conflicting results to date153,154. A more targeted approach of modulating immune responses may be preferable. To this end, many anti-cytokine therapies have been evaluated, leading to the EUA for the anti–IL-6 receptor blocking antibody tocilizumab in hospitalized patients receiving systemic corticosteroids and requiring supplemental oxygen, ventilation or extracorporeal membrane oxygenation (ECMO)8. Elevated IL-6 levels are associated with disease severity1,88,89, as are increases in the downstream marker of inflammation C-reactive protein (CRP)155. CRP is functionally linked to complement activation and inflammatory processes156, and IL-6 is known to play both protective and pathological roles in the immune response to viral infections through its control of gene expression and signaling pathways157. However, clinical trial results with anti–IL-6 therapies in COVID-19 have been mixed, with the Phase III COVACTA and REMDACTA (tocilizumab) and Kevzara and SARTRE (sarilumab) trials failing to improve clinical status or reduce mortality158–161, and the Phase III EMPACTA trial of tocilizumab reducing the number of patients requiring ventilation but not improving mortality162. The Phase III REMAP-CAP trial did find tocilizumab and sarilumab to be superior to the control in increasing the number of organ support-free days for patients in the intensive care unit as well as showing an improvement in their 90-day survival163. Due to these conflicting findings, more studies are likely needed to identify the particular patient population or disease stage that will benefit the most from anti–IL-6 treatments.
Growing evidence suggests the importance of inflammasome-dependent cytokines in COVID-19 pathogenessis63,64,66–71, and anti–IL-1 therapies also have been evaluated. An observational study found that canakinumab, an anti–IL-1β antibody, can lead to clinical improvement for hospitalized patients with COVID-19164, but a more recent randomized Phase III study found no difference between the canakinumab and control groups for survival without ventilation165. The Phase III study only used a single canakinumab infusion, whereas the observational study used two, which may have impacted the findings. Anakinra, an IL-1R antagonist, also has been evaluated. While earlier studies failed to see an effect166, an open label trial reported a reduction in inflammatory markers in patients who received anakinra167, and a retrospective analysis found that early treatment with anakinra with or without glucocorticoids reduced mortality compared to standard of care treatment168.
Given the possible contribution of TNF to COVID-19 pathogenesis1,88,89, several anti-TNF antibodies are under evaluation in clinical trials. Case studies have suggested that anti-TNF therapies may confer protection as prophylaxis, as patients on anti-TNF treatment for rheumatic diseases who became infected with SARS-CoV-2 tended to have lower rates of hospitalization169. IFN-γ is another critical cytokine in COVID-19 pathogenesis1,88,89, and the FDA-approved anti–IFN-γ antibody emapalumab also is being evaluated as a COVID-19 treatment strategy. This therapeutic approach may be linked mechanistically to the preclinical data showing that combination of anti-TNF and anti–IFN-γ treatment can decrease clinical features of cytokine storm driven by PANoptosis and reduce SARS-CoV-2–associated death in mice91. Additionally, targeting the pro-inflammatory JAK/STAT signaling pathway in PANoptosis also is a potential strategy to mitigate disease91. Indeed, baricitinib, a JAK1/2 inhibitor, was granted EUA for treatment of COVID-19 based on the results of the Phase III ACTT-2 trial in hospitalized patients170. Baricitinib treatment combined with remdesivir was better than remdesivir alone in reducing recovery time in these patients170, highlighting the utility of blocking inflammatory signaling as a therapeutic strategy.
Beyond targeting specific cytokines, other strategies have focused on harnessing the power of ‘trained immunity’, which describes the long-term functional reprogramming of innate immune cells following activation that results in enhanced responses to subsequent infections171. This is seen most clearly in the Bacillus Calmette–Guérin (BCG) vaccination strategy, where patients are immunized with an anti-tuberculosis vaccine, and the non-specific protective effects can carry over to reduce the risk of respiratory infections172. In the context of COVID-19, correlative studies have suggested that countries with childhood BCG vaccination programs may have reduced rates of disease173,174, and a study of healthcare workers found decreased rates of SARS-CoV-2 infection in those who had previously received the BCG vaccine175. However, some results have been contradictory176, and randomized controlled trials are ongoing to confirm whether this type of trained immunity strategy should be pursued.
Overall, targeting of innate immunity has been a focus of COVID-19 disease interventions, although treatments to date have had limited success. Due to the various aspects of disease associated with SARS-CoV-2 infection and the ensuing host response, some therapeutics may be more beneficial in certain stages of disease. Identifying which therapies to use and when to implement them likely will be critical for successful treatment. Additionally, combination therapies (including with antiviral antibodies) or targeting multiple cytokines concurrently may have added benefits, and these strategies should continue to be evaluated.
Summary and Future Directions
In spite of rapid advances in basic and translational science in the past two years, SARS-CoV-2 and COVID-19 continue to pose a significant global health threat. Innate immune cells, including the innate lymphoid cells (ILCs) that reside in the mucosal epithelia, are an essential first line of defense against SARS-CoV-2 infection. Preliminary clinical studies have shown that worsened disease severity and increased risk of hospitalization are associated with reductions in ILC abundance177, and that expansion of the ILC2 population is linked to recovery from COVID-19178, highlighting the importance of innate immune cells to counteract the infection179. The innate immune system uses an array of sensors and effector molecules—including TLRs, RLRs, NLRs and inflammasome sensors, as well as cGAS and STING—to directly and indirectly sense the virus or viral components. Downstream of sensing, IFN signaling, cytokine production and cell death are features of the innate immune response that can reduce viral replication and eliminate infected cells to prevent viral spread. However, SARS-CoV-2 encodes proteins and mechanisms that counteract innate immune defenses. As a complicating factor, hyperactivation of the host innate immune response often is associated with cell death, cytokine storm, severe disease and mortality. For these reasons, host immunomodulatory drugs that temper inflammatory responses, including baricitinib and tocilizumab, have been evaluated and granted EUA for treatment of severe COVID-19, and many others are under investigation. A more detailed understanding of the host innate immune response to SARS-CoV-2 might help achieve the balance of inflammation and immunomodulation that optimizes antiviral responses without causing excessive pathological inflammation. One advantage of targeting innate immunity and host molecules is that this approach should be less vulnerable to viral evolution, variant emergence and resistance, which have jeopardized the efficacy of current COVID-19 vaccines and antiviral antibody-based therapies.
Beyond enhancing our understanding of the underlying mechanisms of disease in COVID-19, it will also be important to consider the differences in innate immune activation that occur based on co-morbidities, age, sex and other underlying factors. These likely contribute to the varying disease severities seen across patient groups5. Additionally, most studies to date have considered innate immune responses in adults. Given the growing number of pediatric infections180, some of which are severe and associated with inflammatory syndromes and MIS-C, there is a need to study immune responses in this population. A deeper understanding of innate immunity to SARS-CoV-2 and associated evasion strategies may help to generate new therapeutic approaches that mitigate severe disease, provide treatments for the ongoing pandemic and identify countermeasures to prevent the complications of future ones.
Acknowledgements
We apologize to our colleagues in the field whose work could not be cited due to space limitations. We thank members of the Kanneganti lab for their comments and suggestions. Additionally, we thank Dr. Min Zheng for scientific discussions and suggestions. Work from the Kanneganti laboratory is supported by the US National Institutes of Health (AI101935, AI124346, AI160179, AR056296, and CA253095 to T.-D.K.) and the American Lebanese Syrian Associated Charities (to T.-D.K.). Work on SARS-CoV-2 in the Diamond laboratory is supported by AI157155 and NIAID Centers of Excellence for Influenza Research and Response (CEIRR) contracts 75N93021C00014.
Conflict of interests
M.S.D. is a consultant for Inbios, Vir Biotechnology, and Carnival Corporation, and on the Scientific Advisory Boards of Moderna and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Vir Biotechnology, Kaleido, Moderna and Emergent BioSolutions.
References
- 1.Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506, doi: 10.1016/s0140-6736(20)30183-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273, doi: 10.1038/s41586-020-2012-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins University. COVID-19 Dashboard, <https://coronavirus.jhu.edu/map.html> (2021).
- 4.Karki R & Kanneganti TD The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol, doi: 10.1016/j.it.2021.06.001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth A et al. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS One 16, e0247461, doi: 10.1371/journal.pone.0247461 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS Rapid COVID-19 vaccine development. Science 368, 945–946, doi: 10.1126/science.abb8923 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Forman R, Shah S, Jeurissen P, Jit M & Mossialos E COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 125, 553–567, doi: 10.1016/j.healthpol.2021.03.013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food & Drug Administration. Emergency Use Authorization. (2021).
- 9.Kanneganti TD Intracellular innate immune receptors: Life inside the cell. Immunol Rev 297, 5–12, doi: 10.1111/imr.12912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konno Y et al. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep 32, 108185, doi: 10.1016/j.celrep.2020.108185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JY et al. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res 286, 198074, doi: 10.1016/j.virusres.2020.198074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Melo D et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181, 1036–1045.e1039, doi: 10.1016/j.cell.2020.04.026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JM, St Clair LA, Perera R & Parker R Rapid decay of host basal mRNAs during SARS-CoV-2 infection perturbs host antiviral mRNA biogenesis and export. bioRxiv, doi: 10.1101/2021.04.19.440452 (2021). [DOI] [Google Scholar]
- 14.Fehr AR & Perlman S Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol (Clifton, N.J.) 1282, 1–23, doi: 10.1007/978-1-4939-2438-7_1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D et al. The Architecture of SARS-CoV-2 Transcriptome. Cell 181, 914–921.e910, doi: 10.1016/j.cell.2020.04.011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271–280.e278, doi: 10.1016/j.cell.2020.02.052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou X et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11, 1620, doi: 10.1038/s41467-020-15562-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantuti-Castelvetri L et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860, doi: 10.1126/science.abd2985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey AL & Diamond MS A Crisp(r) New Perspective on SARS-CoV-2 Biology. Cell 184, 15–17, doi: 10.1016/j.cell.2020.12.003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lempp FA et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature 598, 342–347, doi: 10.1038/s41586-021-03925-1 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Bayati A, Kumar R, Francis V & McPherson PS SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem 296, 100306, doi: 10.1016/j.jbc.2021.100306 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawicki SG, Sawicki DL & Siddell SG A contemporary view of coronavirus transcription. J Virol 81, 20–29, doi: 10.1128/jvi.01358-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiel V et al. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol 84, 2305–2315, doi: 10.1099/vir.0.19424-0 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Knoops K et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol 6, e226, doi: 10.1371/journal.pbio.0060226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit E, van Doremalen N, Falzarano D & Munster VJ SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14, 523–534, doi: 10.1038/nrmicro.2016.81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Channappanavar R & Kanneganti TD Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol 41, 1083–1099, doi: 10.1016/j.it.2020.10.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G & Zhao Y Toll-like receptors and immune regulation: their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology 122, 149–156, doi: 10.1111/j.1365-2567.2007.02651.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S & Takeda K Toll-like receptor signalling. Nat Rev Immunol 4, 499–511, doi: 10.1038/nri1391 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Zheng M et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol, doi: 10.1038/s41590-021-00937-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung S, Potapov I, Chillara S & Del Sol A Leveraging systems biology for predicting modulators of inflammation in patients with COVID-19. Sci Adv 7, doi: 10.1126/sciadv.abe5735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J et al. Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections. J Virol 86, 11416–11424, doi: 10.1128/jvi.01410-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnard DL et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother 17, 275–284, doi: 10.1177/095632020601700505 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Totura AL et al. Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. mBio 6, e00638–00615, doi: 10.1128/mBio.00638-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science, doi: 10.1126/science.abd4570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Povysil G et al. Rare loss-of-function variants in type I IFN immunity genes are not associated with severe COVID-19. J Clin Invest 131, doi: 10.1172/jci147834 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhury A & Mukherjee S In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol 92, 2105–2113, doi: 10.1002/jmv.25987 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res 31, 818–820, doi: 10.1038/s41422-021-00495-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petruk G et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J Mol Cell Biol 12, 916–932, doi: 10.1093/jmcb/mjaa067 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurst J et al. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology 214, 683–691, doi: 10.1016/j.imbio.2008.12.003 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Döring Y et al. Human antiphospholipid antibodies induce TNFalpha in monocytes via Toll-like receptor 8. Immunobiology 215, 230–241, doi: 10.1016/j.imbio.2009.03.002 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Amezcua-Guerra LM et al. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann Rheum Dis, doi: 10.1136/annrheumdis-2020-218100 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Borghi MO et al. Anti-Phospholipid Antibodies in COVID-19 Are Different From Those Detectable in the Anti-Phospholipid Syndrome. Front Immunol 11, 584241, doi: 10.3389/fimmu.2020.584241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Made CI et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA 324, 663–673, doi: 10.1001/jama.2020.13719 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asano T et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol 6, doi: 10.1126/sciimmunol.abl4348 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin X et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep 34, 108628, doi: 10.1016/j.celrep.2020.108628 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang D, Geng T, Harrison AG & Wang P Differential roles of RIG-I-like receptors in SARS-CoV-2 infection. bioRxiv, doi: 10.1101/2021.02.10.430677 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebendenne A et al. SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J Virol 95, doi: 10.1128/jvi.02415-20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loo YM & Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity 34, 680–692, doi: 10.1016/j.immuni.2011.05.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horner SM, Liu HM, Park HS, Briley J & Gale M Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci U S A 108, 14590–14595, doi: 10.1073/pnas.1110133108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stark GR & Darnell JE Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514, doi: 10.1016/j.immuni.2012.03.013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wack A, Terczyńska-Dyla E & Hartmann R Guarding the frontiers: the biology of type III interferons. Nat Immunol 16, 802–809, doi: 10.1038/ni.3212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfaender S et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat Microbiol 5, 1330–1339, doi: 10.1038/s41564-020-0769-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin-Sancho L et al. Functional landscape of SARS-CoV-2 cellular restriction. Mol Cell 81, 2656–2668.e2658, doi: 10.1016/j.molcel.2021.04.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez J et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J Exp Med 218, doi: 10.1084/jem.20211211 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bastard P et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol 6, doi: 10.1126/sciimmunol.abl4340 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bastard P et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med 218, doi: 10.1084/jem.20210554 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bastard P et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, doi: 10.1126/science.abd4585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivashkiv LB & Donlin LT Regulation of type I interferon responses. Nat Rev Immunol 14, 36–49, doi: 10.1038/nri3581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorne LG et al. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J, e107826, doi: 10.15252/embj.2021107826 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada T et al. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat Immunol 22, 820–828, doi: 10.1038/s41590-021-00942-0 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Christgen S & Kanneganti TD Inflammasomes and the fine line between defense and disease. Curr Opin Immunol 62, 39–44, doi: 10.1016/j.coi.2019.11.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kayagaki N et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136, doi: 10.1038/s41586-021-03218-7 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Qin C et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71, 762–768, doi: 10.1093/cid/ciaa248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laing AG et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 26, 1623–1635, doi: 10.1038/s41591-020-1038-6 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Zheng M et al. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem, jbc.RA120.01503, doi: 10.1074/jbc.ra120.015036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siu KL et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J, fj201802418R, doi: 10.1096/fj.201802418R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi CS, Nabar NR, Huang NN & Kehrl JH SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov 5, 101, doi: 10.1038/s41420-019-0181-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nieto-Torres JL et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 485, 330–339, doi: 10.1016/j.virol.2015.08.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell GR, To RK, Hanna J & Spector SA SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience 24, 102295, doi: 10.1016/j.isci.2021.102295 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues TS et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 218, doi: 10.1084/jem.20201707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferreira AC et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov 7, 43, doi: 10.1038/s41420-021-00428-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu H et al. SARS-CoV-2 viroporin triggers the NLRP3 inflammatory pathway. bioRxiv, 2020.2010.2027.357731, doi: 10.1101/2020.10.27.357731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan P et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun 12, 4664, doi: 10.1038/s41467-021-25015-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma J et al. SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking Gasdermin D cleavage. EMBO J 40, e108249, doi: 10.15252/embj.2021108249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theobald SJ et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med, e14150, doi: 10.15252/emmm.202114150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Junqueira C et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv, doi: 10.1101/2021.03.06.21252796 (2021). [DOI] [Google Scholar]
- 77.Franz KM, Neidermyer WJ, Tan YJ, Whelan SPJ & Kagan JC STING-dependent translation inhibition restricts RNA virus replication. Proc Natl Acad Sci U S A 115, E2058–e2067, doi: 10.1073/pnas.1716937115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun B et al. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci Rep 7, 3594, doi: 10.1038/s41598-017-03932-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schoggins JW et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695, doi: 10.1038/nature12862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma Z et al. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A 112, E4306–4315, doi: 10.1073/pnas.1503831112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Briard B, Place DE & Kanneganti TD DNA Sensing in the Innate Immune Response. Physiology (Bethesda, Md.) 35, 112–124, doi: 10.1152/physiol.00022.2019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun L, Wu J, Du F, Chen X & Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791, doi: 10.1126/science.1232458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh KK, Chaubey G, Chen JY & Suravajhala P Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol 319, C258–c267, doi: 10.1152/ajpcell.00224.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rui Y et al. Unique and complementary suppression of cGAS-STING and RNA sensing- triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduct Target Ther 6, 123, doi: 10.1038/s41392-021-00515-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li M et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol 6, eabi9007, doi: 10.1126/sciimmunol.abi9007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Humphries F et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci Immunol 6, doi: 10.1126/sciimmunol.abi9002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schultze JL & Aschenbrenner AC COVID-19 and the human innate immune system. Cell 184, 1671–1692, doi: 10.1016/j.cell.2021.02.029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hadjadj J et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724, doi: 10.1126/science.abc6027 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucas C et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469, doi: 10.1038/s41586-020-2588-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burke SJ et al. Pancreatic deletion of the interleukin-1 receptor disrupts whole body glucose homeostasis and promotes islet beta-cell de-differentiation. Mol Metab 14, 95–107, doi: 10.1016/j.molmet.2018.06.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karki R et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 184, 149–168 e117, doi: 10.1016/j.cell.2020.11.025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malireddi RKS et al. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. Immunohorizons 5, 568–580, doi: 10.4049/immunohorizons.2100059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karki R et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep 37, 109858, doi: 10.1016/j.celrep.2021.109858 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuriakose T et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 1, doi: 10.1126/sciimmunol.aag2045 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kesavardhana S et al. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem 295, 8325–8330, doi: 10.1074/jbc.ra120.013752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banoth B et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem, jbc.RA120.01592, doi: 10.1074/jbc.ra120.015924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, Place DE, Briard B, Sharma BR, Tuladhar S, Samir P, Burton A, Kanneganti T-D Identification of the PANoptosome: A molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karki R et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI insight 5, doi: 10.1172/jci.insight.136720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gurung P, Burton A & Kanneganti T-D NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β–mediated osteomyelitis. Proc Natl Acad Sci U S A 113, 4452–4457, doi: 10.1073/pnas.1601636113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukens JR et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516, 246–249, doi: 10.1038/nature13788 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malireddi RK, Ippagunta S, Lamkanfi M & Kanneganti TD Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol 185, 3127–3130, doi: 10.4049/jimmunol.1001512 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malireddi RKS et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity–independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med 217, doi: 10.1084/jem.20191644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malireddi RKS et al. RIPK1 Distinctly Regulates Yersinia-Induced Inflammatory Cell Death, PANoptosis. Immunohorizons 4, 789–796, doi: 10.4049/immunohorizons.2000097 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng M, Karki R, Vogel P & Kanneganti TD Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell 181, 674–687.e613, doi: 10.1016/j.cell.2020.03.040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malireddi RKS et al. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med 215, 1023–1034, doi: 10.1084/jem.20171922 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lamkanfi M et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 7, 2350–2363, doi: 10.1074/mcp.M800132-MCP200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gurung P et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192, 1835–1846, doi: 10.4049/jimmunol.1302839 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee S et al. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature, doi: 10.1038/s41586-021-03875-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doherty GM et al. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J Immunol 149, 1666–1670 (1992). [PubMed] [Google Scholar]
- 110.Zhang F et al. IFN-γ and TNF-α drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 lungs and inflammatory diseases with tissue inflammation. Genome Med 13, 64, doi: 10.1186/s13073-021-00881-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ackermann M et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 383, 120–128, doi: 10.1056/nejmoa2015432 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belhadjer Z et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation, doi: 10.1161/circulationaha.120.048360 (2020). [DOI] [PubMed] [Google Scholar]
- 113.Rowley AH Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 20, 453–454, doi: 10.1038/s41577-020-0367-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esmon CT The interactions between inflammation and coagulation. Br J Haematol 131, 417–430, doi: 10.1111/j.1365-2141.2005.05753.x (2005). [DOI] [PubMed] [Google Scholar]
- 115.Poor HD Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest, doi: 10.1016/j.chest.2021.06.016 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leentjens J, van Haaps TF, Wessels PF, Schutgens REG & Middeldorp S COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol 8, e524–e533, doi: 10.1016/s2352-3026(21)00105-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaneko N et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 183, 143–157.e113, doi: 10.1016/j.cell.2020.08.025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhattacharjee S & Banerjee M Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr Clin Med, doi: 10.1007/s42399-020-00521-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zohar T et al. Compromised Humoral Functional Evolution Tracks with SARS-CoV-2 Mortality. Cell 183, 1508–1519.e1512, doi: 10.1016/j.cell.2020.10.052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fajgenbaum DC & June CH Cytokine Storm. Reply. N Engl J Med 384, e59, doi: 10.1056/NEJMc2036236 (2021). [DOI] [PubMed] [Google Scholar]
- 121.Leisman DE et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med, doi: 10.1016/s2213-2600(20)30404-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mudd PA et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv, doi: 10.1126/sciadv.abe3024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JS et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol 5, eabd1554, doi: 10.1126/sciimmunol.abd1554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Winkler ES et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21, 1327–1335, doi: 10.1038/s41590-020-0778-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu G et al. ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity. Nat Microbiol 6, 467–478, doi: 10.1038/s41564-021-00884-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen K et al. SARS-CoV-2 Nucleocapsid Protein Interacts with RIG-I and Represses RIG-Mediated IFN-β Production. Viruses 13, doi: 10.3390/v13010047 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu J et al. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep 34, 108761, doi: 10.1016/j.celrep.2021.108761 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han L et al. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J Med Virol 93, 5376–5389, doi: 10.1002/jmv.27050 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sui L et al. SARS-CoV-2 Membrane Protein Inhibits Type I Interferon Production Through Ubiquitin-Mediated Degradation of TBK1. Front Immunol 12, 662989, doi: 10.3389/fimmu.2021.662989 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thoms M et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 369, 1249–1255, doi: 10.1126/science.abc8665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hsu JC, Laurent-Rolle M, Pawlak JB, Wilen CB & Cresswell P Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2101161118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia H et al. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep 33, 108234, doi: 10.1016/j.celrep.2020.108234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S et al. Targeting liquid-liquid phase separation of SARS-CoV-2 nucleocapsid protein promotes innate antiviral immunity by elevating MAVS activity. Nat Cell Biol 23, 718–732, doi: 10.1038/s41556-021-00710-0 (2021). [DOI] [PubMed] [Google Scholar]
- 134.World Health Organization. COVID-19 vaccine tracker and landscape. (2021).
- 135.Gordon CJ et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 295, 6785–6797, doi: 10.1074/jbc.RA120.013679 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Regeneron Pharmaceuticals Inc. Fact sheet for health care providers: emergency use authorization (EUA) of casirivimab and imdevimab. (2020).
- 137.Schäfer A et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med 218, doi: 10.1084/jem.20201993 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou Q et al. Interferon-α2b Treatment for COVID-19. Front Immunol 11, 1061, doi: 10.3389/fimmu.2020.01061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang N et al. Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients. Cell Host Microbe 28, 455–464.e452, doi: 10.1016/j.chom.2020.07.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoagland DA et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity 54, 557–570.e555, doi: 10.1016/j.immuni.2021.01.017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hung IF et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704, doi: 10.1016/s0140-6736(20)31042-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu W et al. Activation of STING Signaling Pathway Effectively Blocks Human Coronavirus Infection. J Virol 95, doi: 10.1128/jvi.00490-21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mao T et al. A stem-loop RNA RIG-I agonist confers prophylactic and therapeutic protection against acute and chronic SARS-CoV-2 infection in mice. bioRxiv, doi: 10.1101/2021.06.16.448754 (2021). [DOI] [Google Scholar]
- 144.Broggi A et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 369, 706–712, doi: 10.1126/science.abc3545 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shahbazi M et al. Linkage of Lambda Interferons in Protection Against Severe COVID-19. J Interferon Cytokine Res 41, 149–152, doi: 10.1089/jir.2020.0187 (2021). [DOI] [PubMed] [Google Scholar]
- 146.Yosuke F et al. Downregulation of type III interferons in patients with severe COVID-19. J Med Virol 93, 4559–4563, doi: 10.1002/jmv.26993 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Felgenhauer U et al. Inhibition of SARS-CoV-2 by type I and type III interferons. J Biol Chem 295, 13958–13964, doi: 10.1074/jbc.AC120.013788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stanifer ML et al. Critical Role of Type III Interferon in Controlling SARS-CoV-2 Infection in Human Intestinal Epithelial Cells. Cell Rep 32, 107863, doi: 10.1016/j.celrep.2020.107863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dinnon KH 3rd et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586, 560–566, doi: 10.1038/s41586-020-2708-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jagannathan P et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nat Commun 12, 1967, doi: 10.1038/s41467-021-22177-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feld JJ et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 9, 498–510, doi: 10.1016/s2213-2600(20)30566-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horby P et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 384, 693–704, doi: 10.1056/NEJMoa2021436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Obata R, Maeda T, Rizk D & Kuno T Increased Secondary Infection in COVID-19 Patients Treated with Steroids in New York City. Jpn J Infect Dis 74, 307–315, doi: 10.7883/yoken.JJID.2020.884 (2021). [DOI] [PubMed] [Google Scholar]
- 154.Ritter LA et al. The Impact of Corticosteroids on Secondary Infection and Mortality in Critically Ill COVID-19 Patients. J Intensive Care Med, 8850666211032175, doi: 10.1177/08850666211032175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guan WJ et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382, 1708–1720, doi: 10.1056/NEJMoa2002032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sproston NR & Ashworth JJ Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol 9, 754, doi: 10.3389/fimmu.2018.00754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanaka T, Narazaki M & Kishimoto T IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6, a016295, doi: 10.1101/cshperspect.a016295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rosas IO et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med 384, 1503–1516, doi: 10.1056/NEJMoa2028700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rosas IO et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med, 1–13, doi: 10.1007/s00134-021-06507-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lescure FX et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 9, 522–532, doi: 10.1016/s2213-2600(21)00099-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sancho-López A et al. Efficacy and Safety of Sarilumab in patients with COVID19 Pneumonia: A Randomized, Phase III Clinical Trial (SARTRE Study). Infect Dis Ther, doi: 10.1007/s40121-021-00543-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salama C et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 384, 20–30, doi: 10.1056/NEJMoa2030340 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gordon AC et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med, doi: 10.1056/NEJMoa2100433 (2021). [DOI] [PubMed] [Google Scholar]
- 164.Generali D et al. Canakinumab as treatment for COVID-19-related pneumonia: A prospective case-control study. Int J Infect Dis 104, 433–440, doi: 10.1016/j.ijid.2020.12.073 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Caricchio R et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized With Severe COVID-19: A Randomized Clinical Trial. JAMA 326, 230–239, doi: 10.1001/jama.2021.9508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med 9, 295–304, doi: 10.1016/s2213-2600(20)30556-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kyriazopoulou E et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife 10, doi: 10.7554/eLife.66125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pontali E et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J Allergy Clin Immunol 147, 1217–1225, doi: 10.1016/j.jaci.2021.01.024 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gianfrancesco M et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79, 859–866, doi: 10.1136/annrheumdis-2020-217871 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kalil AC et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Eng J Med, doi: 10.1056/nejmoa2031994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Netea MG et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 20, 375–388, doi: 10.1038/s41577-020-0285-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Giamarellos-Bourboulis EJ et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 183, 315–323.e319, doi: 10.1016/j.cell.2020.08.051 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Escobar LE, Molina-Cruz A & Barillas-Mury C BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A 117, 17720–17726, doi: 10.1073/pnas.2008410117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gursel M & Gursel I Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic? Allergy 75, 1815–1819, doi: 10.1111/all.14345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rivas MN et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest 131, doi: 10.1172/jci145157 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hensel J et al. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep 10, 18377, doi: 10.1038/s41598-020-75491-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Silverstein NJ et al. Innate lymphoid cells and disease tolerance in SARS-CoV-2 infection. medRxiv, doi: 10.1101/2021.01.14.21249839 (2021). [DOI] [Google Scholar]
- 178.Gomes AM et al. SARS-CoV2 pneumonia recovery is linked to expansion of Innate Lymphoid Cells type 2 expressing CCR10. Eur J Immunol, doi: 10.1002/eji.202149311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kumar A et al. Innate lymphoid cells (ILC) in SARS-CoV-2 infection. Mol Aspects Med 80, 101008, doi: 10.1016/j.mam.2021.101008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.American Association of Pediatrics and the Children’s Hospital Association. Children and COVID-19: State Data Report. (2021).


