Abstract
Background:
The ultimate goal of furcation defect therapy is furcation closure via periodontal regeneration. However, the process of periodontal regeneration is affected by the regenerative environment of signaling molecules and growth factors due to which consistent findings of complete furcation closure could not be attained. In this study, we have evaluated the use of concentrated growth factor (CGF) which provides sustained release growth factors in conjunction with bovine-derived xenograft anorganic bovine bone (ABB) in guided tissue regeneration (GTR) of Degree II mandibular molar furcation defect.
Materials and Methods:
Twenty patients with Degree II mandibular molar furcation defects were selected for the study. Each group consisted of 10 patients and a total of 10 sites were treated in each group. The control sites were treated with GTR and ABB, while the experimental sites received CGF mixed with ABB along with GTR. Clinical parameters recorded were Plaque Index, Gingival Index, vertical probing depth, and horizontal probing depth measured at baseline and 6 months. Radiographic parameters such as the vertical height of defect, horizontal depth of defect, and percentage of vertical and horizontal bone fill were recorded at baseline and 6 months.
Results:
All the parameters recorded showed a significant reduction from baseline to 6 months in both the groups. Significantly higher vertical and horizontal bone fill was observed in the experimental group as compared to the control group.
Conclusion:
The use of CGF showed a positive additive efficacy in enhancing the events of periodontal regeneration in the treatment of Degree II mandibular molar furcation defect.
Keywords: Concentrated growth factor, Degree II mandibular molar furcation defect, periodontal regeneration
INTRODUCTION
The progression of periodontal disease into the furcal region of the multirooted teeth represents a clinical challenge with a higher prevalence of tooth loss compared to teeth that did not exhibit furcation defects.[1,2] The intricate anatomy and complex morphology of the furcation area complicate the detection and diagnosis of the lesion, access for conventional instrumentation, debridement, and home care maintenance, thereby limiting the prognosis of the furcation-involved tooth.[3,4,5]
Various treatment modalities have been proposed to improve the prognosis of furcation-involved teeth out of which guided tissue regeneration (GTR) along with bone graft proves to give better results with furcation closure and higher survival rates.[6,7,8] However, outcomes from short-term studies have shown that furcation closure of Degree II furcation defects could be attained but failed to observe a consistent finding.[9,10,11] The ultimate goal of complete closure of furcation defect is still affected by the regenerative environment provided by signaling molecules and growth factors that govern the events of periodontal regeneration.[12]
Various generations of bioactive autologous platelet concentrates, platelet-rich plasma (PRP),[13] and platelet-rich fibrin (PRF)[14] have been widely used as an adjunct to surgical regenerative therapy and its clinical utility is appreciated due to high concentrations of signaling growth factors.[15,16] Pradeep et al.,[17] Sharma and Pradeep,[18] and Lafzi et al.[19] have demonstrated the beneficial use of PRP, PRF as a regenerative material in the treatment of Degree II furcation defects. Since the introduction of third-generation autologous platelet concentrate, known as a concentrated growth factor (CGF) by Sacco in 2006,[20] regenerative properties of CGF have gained popularity in the treatment of intrabony defects, maxillary sinus lift, and osseointegration.[21,22,23] However, evidence-based studies on the efficacy of CGF for the regeneration therapy of furcation defects are relatively scarce.
With the boon of regenerative properties of CGF, the present study has been undertaken to evaluate and compare the bone fill and additive efficacy of CGF for treating Degree II mandibular molar furcation defect.
MATERIALS AND METHODS
The study design was a single-blinded, randomized controlled clinical trial to compare the clinical and radiographic outcomes at baseline and 6 months after the intervention. Ethical clearance for the study was obtained from the Institutional Ethics Committee. An informed written consent letter was signed by all the patients.
Inclusion criteria were cooperative, systemically healthy subjects aged between 30 and 55 years suffering from moderate periodontitis with probing pocket depth ≥5 mm and Degree II mandibular molar furcation defects after Phase I therapy with the presence of at least 2 mm of keratinized gingiva. Exclusion criteria were any root caries, internal or external root resorption, acute or chronic systemic disorders, hematologic disorders, current smokers and tobacco users, and pregnant and lactating female patients. Noncooperative patients with unacceptable oral hygiene and patients with postoperative exposure of membrane and graft were withdrawn from the study.
A total of 20 patients suffering from chronic periodontitis with periodontal pocket depth ≥5 mm and exhibiting Degree II furcation defects in mandibular molars were selected from the outpatient department of periodontology. The subjects were randomly divided into two groups. Each group consisted of ten patients with ten sites that were randomly allocated as the control group and the experimental group. The positive control sites were treated with GTR and anorganic bovine bone (ABB) (Bio-Oss®, Geistlich Switzerland), while the experimental sites received CGF mixed with ABB along with GTR. All the sites were treated with an ABB of particle size 0.25–1 mm and collagen membrane (Periocol®-GTR).
The following clinical and radiographic parameters were recorded at baseline and 6 months postoperatively for all the sites:
Plaque Index (PI) by Silness and Loe[24]
Gingival Index (GI) by Loe and Silness[25]
Vertical probing depth (VPD) and horizontal probing depth (HPD) were measured through furcal bone sounding according to the technique used by Mealey et al. 1994.[26]
Radiographic parameters were recorded through cone-beam computed tomography CBCT with furcation entrance as an anatomical landmark for measurements.[27] The scans were acquired at 120 kVp, 5 mA, 4 s, and 640/640 rows/column with a pixel spacing of (0.25/0.25). The scans were measured at 1 mm slicing thickness.
The vertical height of the furcation defect (VHF= the vertical height of the defect) was measured on a sagittal view from fornix to the crest of alveolar bone within the furcation where the slice showed the greatest amount of bone loss
The horizontal depth of the defect (HDF) was measured on an axial view by the distance drawn from the tangent to the deepest point where the slice showed the greatest amount of bone loss. The schematic diagram illustrates the CBCT measurement of HPD on an axial view [Figure 1]
-
Percentage vertical bone fill (%VBF) =

-
Percentage horizontal bone fill (%HBF) =
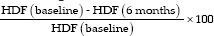
Figure 1.
The schematic diagram illustrates the cone-beam computed tomography measurement of horizontal probing depth on an axial view. On an axial view, an imaginary tangent line was drawn between the mesial root (M) and distal root (D) connecting the buccal or lingual surfaces of the two roots of mandibular molars, respectively. Horizontal depth of the defect is measured as the line drawn from the tangent to the deepest point where the slice showed the greatest amount of bone loss. Red and green arrows depict the furcation bone loss at buccal and lingual surface of the mandibular molar
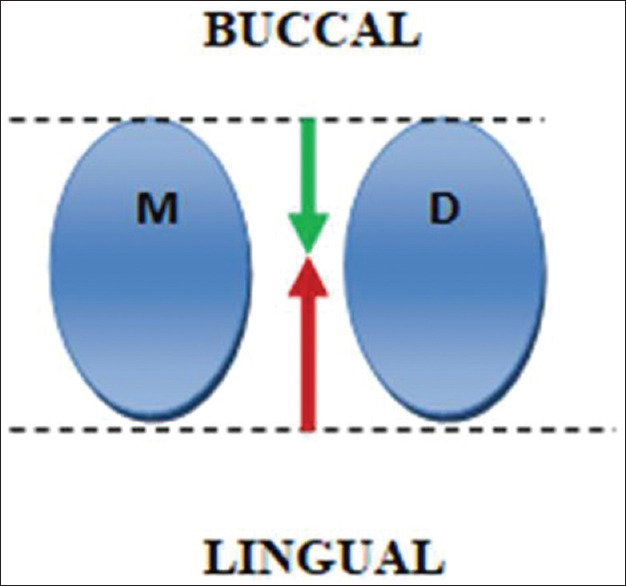
CGF was prepared using the standard protocol developed by Sacco in 2006 in which 5 ml blood was drawn from the patient's veins of antecubital fossa in a noncoated sterile vacutainer and centrifuged at 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 4 min, and then 3000 rpm for last 3 min in centrifugation machine (REMI™ centrifugation machine).[20] Three layers were obtained which consist of the upper layer, liquid phase platelet-poor plasma, the lower layer, free red blood cells, and the middle layer, the solid CGF consisting of three parts [Figure 2a-c]:
Figure 2.
Blood sample after concentrated growth factor protocol of centrifugation. (a) Three layers are obtained: platelet-poor plasma, upper layer; concentrated growth factor, middle layer; red blood cell, lower layer; (b) The prepared concentrated growth factor consists of three parts: The upper white part, the red part, and the middle buffy coat part; (c) Buffy coat of concentrated growth factor was cut into pieces and mixed with anorganic bovine bone graft in a 1:1 ratio
The upper white part
The bottom red part
Middle “buffy coat” (BC).[28]
The fibrin BC was cut into pieces of approximately 1–2 mm into glass dappen dish and mixed with ABB in the ratio of 1:1.
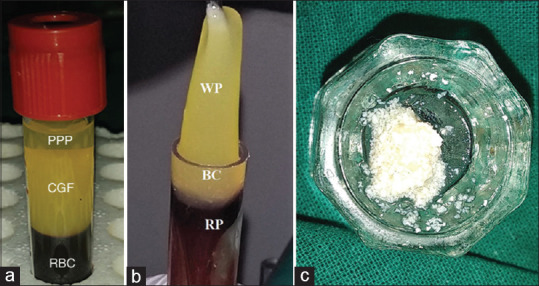
The patients were subjected to surgery after thorough scaling and root planing and occlusal adjustment was done, if necessary, to relieve occlusal traumatism. Sulcular incisions were made and mucoperiosteal envelop flaps were reflected under local anesthesia 2% lignocaine with 1:80,000 adrenaline. Meticulous defect debridement and root planing were carried out with the help of curettes. The direct examination after debridement confirmed the presence of a Degree II furcation defect. In Group A, the defects were filled with ABB followed by placement of collagen membrane over the defect [Figure 3a-c]. On the other hand, in Group B sites, the defects were tightly packed with the ABB/CGF mixture and collagen membrane was placed over the defect to ensure the stability of the graft [Figure 4a-c]. The flap was approximated with 3-0 mersilk suture and the periodontal dressing was given in both the groups.
Figure 3.
Treatment of Degree II furcation defect in mandibular molar in Group A (guided tissue regeneration + anorganic bovine bone). (a) Degree II furcation defect in mandibular molar; (b) Defect was filled with anorganic bovine bone of particle size 0.25–1 mm; (c) placement of guided tissue regeneration membrane over the defect
Figure 4.
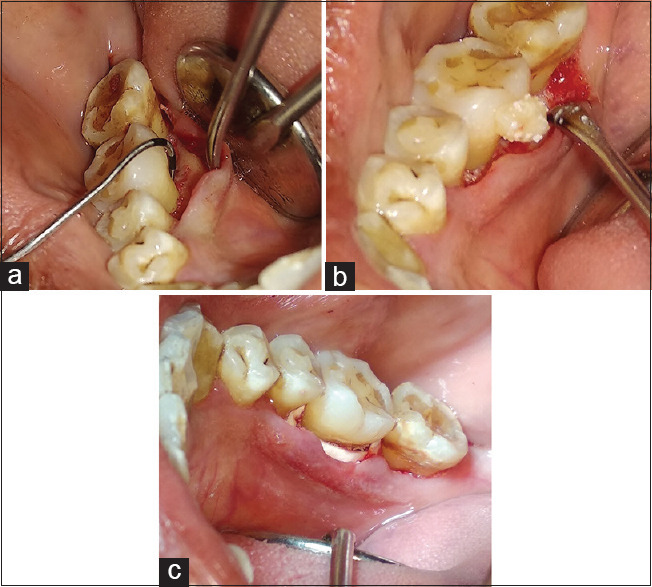
Treatment of Degree II furcation defect in mandibular molar in Group B (concentrated growth factor + guided tissue regeneration + anorganic bovine bone). (a) Degree II furcation defect in mandibular molar; (b) Defect was filled with concentrated growth factor/anorganic bovine bone mixed; (c) placement of guided tissue regeneration membrane over the defect
Postoperative instructions were given to all the patients and appropriate medications were prescribed (antibiotic – capsule amoxicillin 500 mg TDS; analgesic and anti-inflammatory drug – tablet diclofenac sodium 50 mg TDS and multivitamin capsule once daily for 5 days) along with 10 ml 0.2% chlorhexidine gluconate mouthwash twice daily for 2 weeks. Seven days after the surgery, dressings and sutures were removed.
All patients were recalled at 1, 3, and 6 months for evaluation and maintenance. The clinical and radiographic parameters were measured using the same probing techniques, standardized exposures, and slice thickness at 6 months.
RESULTS
Analyses were performed and data were summarized as mean ± standard error. pre and post values were compared by paired t-test and the changes in outcome measures of two independent groups were compared by independent Student's t-test. A two-tailed (α =2) P < 0.05 was considered statistically significant.
Table 1 shows the details of the comparison of clinical parameters from baseline to 6 months post surgery. The pre- to postmean PI and GI have shown a significant decrease in both groups (P < 0.001).
Table 1.
Comparison of clinical parameters at baseline and 6 months post surgery (mean±standard error) (n=10)
| Group A (GTR + ABB) | Group B (CGF + GTR + ABB) | P (baseline- 6 months)§ | |
|---|---|---|---|
| PI | |||
| Baseline | 2.70±0.10 | 2.48±0.14 | <0.001‡ |
| 6 months post-surgery | 0.60±0.08 | 0.48±0.10 | |
| GI | |||
| Baseline | 2.13±0.12 | 1.95±0.12 | <0.001‡ |
| 6 months post-surgery | 0.40±0.10 | 0.14±0.07 | |
| VPD | |||
| Baseline | 5.65±0.15 | 5.85±0.28 | <0.001‡ |
| 6 months post-surgery | 2.30±0.13 | 1.50±0.17 | |
| HPD | 7.00±0.25 | 7.05±0.30 | |
| Baseline | <0.001‡ | ||
| 6 months post-surgery | 2.70±0.20 | 2.15±0.11 |
§Paired t-test; ‡Significant difference (P<0.05). GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral; CGF + GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral mixed with concentrated growth factor; n – Sample size; P – Calculated probability; PI – Plaque Index; GI – Gingival Index; VPD – Vertical probing depth; HPD – Horizontal probing depth
The mean reduction of VPD from baseline to 6 months in Group A (3.35 ± 0.13) and Group B (4.35 ± 0.26) was statistically significant in both the groups (P < 0.001). Similarly, the mean reduction HPD from baseline to 6 months in Group A (4.30 ± 0.29) and Group B (4.90 ± 0.34) was statistically significant (P < 0.001).
Table 2 shows the comparison of mean difference (pre–post change) of clinical parameters at baseline and 6 months post surgery. The comparison of the net change in mean PI (P = 0.540) and GI (P = 0.701) in both groups has reported a similar decrease and is statistically insignificant.
Table 2.
Comparison of mean difference (pre-post-change) of clinical parameters at baseline and 6 months post surgery (n=10)
| Group A (GTR + ABB) | Group B (CGF + GTR + ABB) | P value of comparison of mean difference (pre-postchange)* | |
|---|---|---|---|
| PI | |||
| Mean difference (pre-postchange) | 2.10±0.14 | 2.00±0.09 | 0.540 |
| GI | |||
| Mean difference (pre-postchange) | 1.73±0.17 | 1.81±0.12 | 0.701 |
| VPD | |||
| Mean difference (pre-postchange) | 3.35±0.13 | 4.35±0.26 | 0.003‡ |
| HPD | |||
| Mean difference (pre-post) | 4.30±0.29 | 4.90±0.34 | 0.196 |
*(P<0.05) Students’s t-test; ‡Significant difference. n – Sample size; P – Calculated probability; GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral; CGF + GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral mixed with Concentrated growth factor; PI – Plaque Index; GI – Gingival Index; VPD – Vertical probing depth; HPD – Horizontal probing depth
The comparison of the net mean reduction in VPD has shown a statistically significant higher decrease (23.0%) in Group B to Group A (P = 0.003). However, the net mean reduction in HPD of the two groups has shown a similar decrease and statistically insignificant (P = 0.196) though it was 12.2% higher in Group B as compared to Group A.
Table 3 shows the details of the comparison of radiographic parameters from baseline to 6 months post surgery. A statistically significant reduction in the mean VHF and mean HDF from baseline to 6 months has been shown in both groups (P < 0.001).
Table 3.
Comparison of radiographic parameters at baseline and 6 months post surgery (mean±standard error) and pre- to postmean differences (n=10)
| Group A (GTR + ABB) | Group B (CGF + GTR + ABB) | P (baseline- 6 months)§ | |
|---|---|---|---|
| VHF | |||
| Baseline | 4.14±0.26 | 3.82±0.14 | <0.001‡ |
| 6 months post surgery | 0.61±0.11 | 0.04±0.03 | |
| Mean difference (pre-post) | 3.53±0.15 | 3.78±0.11 | |
| HDF | <0.001‡ | ||
| Baseline | 4.93±0.19 | 4.79±0.19 | |
| 6 months post surgery | 0.53±0.11 | 0.05±0.05 | |
| Mean difference (pre-post) | 4.40±0.08 | 4.74±0.14 |
§(P<0.05) Paired t-test; ‡Significant difference. n – Sample size; P – Calculated probability; GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral; CGF + GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral mixed with concentrated growth factor; VHF – Vertical height of the defect; HDF – Horizontal depth of the defect
However, the comparison of pre- to postmean differences has shown a statistically significant higher reduction in mean VHF of Group B (3.78 ± 0.11) than Group A (3.53 ± 0.15) (P < 0.001) [Figures 5 and 6]. Similarly, a significantly higher decrease in HDF was observed in Group B (4.74 ± 0.14) as compared to Group A (4.40 ± 0.08) (P < 0.001) [Figures 7a, b and 8a, b].
Figure 5.
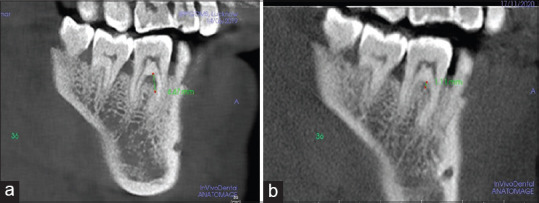
Vertical height of the defect (VHF) measured in Group A (GTR+ABB) (a) VHF at baseline (b) VHF at 6th month. GTR – Guided tissue regeneration; ABB – Anorganic bovine bone; VHF – Vertical height of the defect
Figure 6.
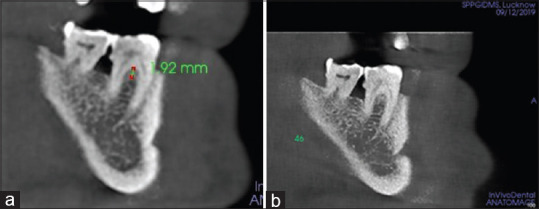
Vertical height of the defect (VHF) measured in Group B (CGF+GTR+ABB) (a) VHF at baseline; (b) VHF at 6th month. CGF – Concentrated growth factor; GTR – Guided tissue regeneration; ABB – Anorganic bovine bone; VHF – Vertical height of the defect
Figure 7.
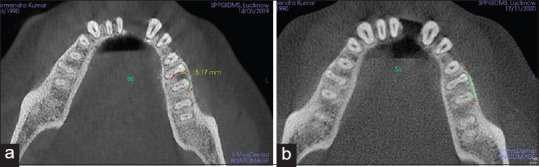
Horizontal depth of the defect (HDF) measured in group A (GTR+ABB) (a) HDF at baseline (b) HDF at 6th months. GTR – Guided tissue regeneration; ABB – Anorganic bovine bone
Figure 8.
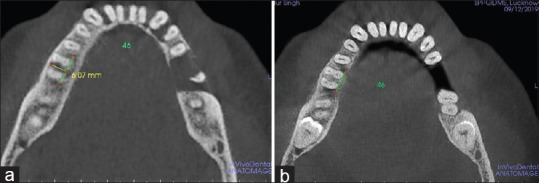
Horizontal depth of the defect (HDF) measured in Group B (CGF+GTR+ABB) (a) HDF at baseline (b) HDF at 6th month. CGF – Concentrated growth factor; GTR – Guided tissue regeneration; ABB – Anorganic bovine bone
Table 4 shows the comparison in %VBF and %HBF of the two groups. Student's t-test has shown statistically significant higher (14.2%) VBF of Group B (99.0%) to Group A (85.2%) (P < 0.001) and (9.3%) HBF of Group B (98.0%) as compared to Group A (89.2%) (P < 0.001).
Table 4.
Comparison of percentage bone fills (percentage vertical bone fill and percentage horizontal bone fill)
| Percentage bone fill | Group A (GTR + ABB) | Group B (CGF + GTR + ABB) | P value of comparison of percentage bone fill (%VBF and % HBF) |
|---|---|---|---|
| %VBF | 85.00±3.07 | 99.06±0.69 | <0.001‡ |
| %HBF | 89.59±1.99 | 98.74±1.26 | <0.001‡ |
‡(P<0.05) Significant difference. n – sample size; P – Calculated probability; GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral; CGF + GTR + ABB – Guided tissue regeneration with anorganic bovine bone mineral mixed with concentrated growth factor; %VBF – Percentage vertical bone fill; %HBF – Percentage horizontal bone fill
DISCUSSION
Regenerative approaches in Degree II furcation defect aimed at clinical furcation closure, complete bone fill which can be assessed by periodontal probing, radiographic analysis, and bone sounding.[7,8] The results of the present study demonstrated that the combination regenerative therapy with or without CGF was found to be effective in treating Degree II mandibular molar furcation defects with a significant reduction in PI, GI, VPD, HPD, and radiographic bone fill (%VBF and %HBF) from baseline to 6 months. Kinaia et al.,[29] Pajnigara et al.,[30] and Jepsen et al.[31] have reported that the treatment of Degree II furcation defects with a combination of bone grafts and resorbable membrane resulted in improvements in all the clinical parameters. Similar reductions in VPD and HPD were observed in the study, may have been attributed to collagen membrane application which enhances the augmentation of the particulate ABB graft.[32,33] However, a higher reduction of VPD (23.0%) was observed in (CGF + GTR + ABB) group as compared to (GTR + ABB) group which were significantly similar to results reported by Qaio J et al. in the treatment of intrabony defect and Degree II furcation defect in CGF + GTR + bone graft group which shows a positive role of CGF in combination regenerative therapy.[21,34]
Various long-term studies have shown that the extent and the defect morphology of the furcation defects influence the outcome of periodontal regeneration.[11,34] The resolution of the vertical defect component remains a critical aspect, regardless of complete furcation closure as this could influence the survival of the tooth.[8,29] Various signaling molecules govern the healing outcome following periodontal regenerative procedures in furcation defects.[15] Pradeep et al. and Sharma and Pradeep[17,18] reported the use of autologous PRP in the treatment of mandibular Degree II furcation defect as clinically less beneficial, while the role of PRF was highlighted with a complete closure of 66.7% of the defects, 83% were reduced to Degree I and greater vertical defect fill than the control group. The (CGF + GTR + ABB) group presented a more favorable radiographic bone fill with higher %VBF (14.2%) (3.53 ± 0.15 vs. 3.78 ± 0.11) and %HBF (9.3%) (4.40 ± 0.08 vs. 4.74 ± 0.14) than the GTR + ABB group which were in agreement with the outcomes of the study conducted by Qiao et al.[33,35] Apart from obtaining the furcation closure in both the groups with significant bone fill, the addition of CGF mixed with ABB could be the reason for greater resolution of vertical defect component with complete furcation closure in CGF + GTR + ABB group than the GTR + ABB group [Figure 5 and Figure 6]. This improvement in the vertical component could enhance the longevity of the treated tooth. CGF's positive activity in this respect may be due to its biologic characteristics and pattern of release growth factor when mixed with ABB.
CGF, a third-generation platelet concentrate, has an edge over PRP and PRF due to its complex 3-dimensional architecture fibrinogen containing platelets with a higher amount of cytokines, leukocytes, and growth factors.[36,37,38] The presence of growth factors such as transforming growth factor-beta1 (TGF-β1), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), and stem cells CD34 positive cells has been highlighted in various studies for stimulating cell proliferation, matrix remodeling, and angiogenesis during healing processes.[38,39,40]
Yu and Wang et al.,[40] Li et al.,[41] Chen et al.,[42] and Rochira et al.[43] studied the in vitro effects of CGF and observed the dose dependent activation of osteogenic stem cells by increasing the activity of alkaline phosphatase and promoting the proliferation of periodontal ligament stem cells and bone marrow stem cells. Moreover, the combination of CGF with biomaterials has been reported to be more beneficial than CGF alone as the cells are trapped without dissolving rapidly following the application. Bonazza et al.[44] reported that the addition of beta-tricalcium phosphate could enhance the release of bone morphogenetic protein 2 and 7, which have a pivotal role in bone regeneration. Thus, CGF activity of growth factor release is prolonged which is favorable for cell proliferation and osteogenic differentiation.
Another important characteristic of CGF accounts for the release of growth factors in a specific kinetic pattern. Yu et al.[45] in an in vitro study observed a unique dynamic pattern in growth factor release when CGF is mixed with scaffold biomaterials. The levels of growth factors released in CGF-DBBM (CGF mixed with deproteinised bovine bone mineral) increased significantly from 72 h with maximum peaks at 72 h (C5a), 7th day (IGF-1 and b FGF), 14th day (PDGF-BB, TGF-β1, and C3a), 21th day (VEGF), and bimodal pattern of 72 h, 14th day (TGF-β1), the trend continues with sustained release of growth factors for 28 days. However, the observations of the releasing pattern of the growth factors of CGF alone were reported to release till the 8th day with a maximum peak at the 8th day.[28]
In the present study, the CGF fibrin mixed with ABB to fill the furcation defect could have influenced the pattern as well as the dynamics of growth factor release. The ABB/CGF mix not only improves the wound stability, which is essential for the establishment of a new connective tissue attachment to the root surface, but also provides a scaffold supporting cytokine attachment and cellular migration enhancing the augmentation of the graft material. The experimental group (CGF + GTR + ABB) shows greater improvements in all the clinical and radiographic outcomes with complete closure of the Degree II mandibular molar furcation defect. The preparation of the ABB/CGF mix to fill the defect provides an additional scaffold and creates a regenerative environment with sustained release of growth factor, maximum peaks at 7th day, 21st day, and till 28th day which coincides with healing period of new bone formation.
CONCLUSION
It can be concluded from the study that the use of CGF provides a positive additive efficacy in enhancing the events of periodontal regeneration in the treatment of Degree II mandibular molar furcation defect. Nevertheless, the limitation of the present study was the sample size and observation time was limited. Further, no histological evaluation was performed to access the type of healing. Further studies for evaluation of the efficacy of CGF in combination regenerative therapy with larger sample size, histological analysis, and long-term follow-up duration are necessary to provide better and more reliable results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225–37. doi: 10.1902/jop.1978.49.5.225. [DOI] [PubMed] [Google Scholar]
- 2.McFall WT., Jr Tooth loss in 100 treated patients with periodontal disease.A long-term study. J Periodontol. 1982;53:539–49. doi: 10.1902/jop.1982.53.9.539. [DOI] [PubMed] [Google Scholar]
- 3.Bower RC. Furcation morphology relative to periodontal treatment.Furcation root surface anatomy. J Periodontol. 1979;50:366–74. doi: 10.1902/jop.1979.50.7.366. [DOI] [PubMed] [Google Scholar]
- 4.Nordland P, Garrett S, Kiger R, Vanooteghem R, Hutchens LH, Egelberg J. The effect of plaque control and root debridement in molar teeth. J Clin Periodontol. 1987;14:231–6. doi: 10.1111/j.1600-051x.1987.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer HC, Mellonig JT, Brayer WK, Gray JL, Barnett JD. Scaling and root planing efficacy in multirooted teeth. J Periodontol. 1989;60:402–9. doi: 10.1902/jop.1989.60.7.402. [DOI] [PubMed] [Google Scholar]
- 6.Avila-Ortiz G, De Buitrago JG, Reddy MS. Periodontal regeneration - furcation defects: A systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86:108–30. doi: 10.1902/jop.2015.130677. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, Jepsen K, Eickholz P, Jepsen S. Clinical concepts for regenerative therapy in furcations. Periodontol 2000. 2015;68:308–32. doi: 10.1111/prd.12081. [DOI] [PubMed] [Google Scholar]
- 8.Majzoub J, Barootchi S, Tavelli L, Wang CW, Travan S, Wang HL. Treatment effect of guided tissue regeneration on the horizontal and vertical components of furcation defects: A retrospective study. J Periodontol. 2020;91:1148–58. doi: 10.1002/JPER.19-0529. [DOI] [PubMed] [Google Scholar]
- 9.Jepsen S, Eberhard J, Herrera D, Needleman I. A systematic review of guided tissue regeneration for periodontal furcation defects.What is the effect of guided tissue regeneration compared with surgical debridement in the treatment of furcation defects? J Clin Periodontol. 2002;29(Suppl 3):103–16. doi: 10.1034/j.1600-051x.29.s3.6.x. [DOI] [PubMed] [Google Scholar]
- 10.Jepsen S, Heinz B, Jepsen K, Arjomand M, Hoffmann T, Richter S, et al. A randomized clinical trial comparing enamel matrix derivative and membrane treatment of buccal Class II furcation involvement in mandibular molars.Part I: Study design and results for primary outcomes. J Periodontol. 2004;75:1150–60. doi: 10.1902/jop.2004.75.8.1150. [DOI] [PubMed] [Google Scholar]
- 11.Novaes AB, Jr, Palioto DB, de Andrade PF, Marchesan JT. Regeneration of Class II furcation defects: Determinants of increased success. Braz Dent J. 2005;16:87–97. doi: 10.1590/s0103-64402005000200001. [DOI] [PubMed] [Google Scholar]
- 12.Kawase T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: Basic principles and concepts underlying recent advances. Odontology. 2015;103:126–35. doi: 10.1007/s10266-015-0209-2. [DOI] [PubMed] [Google Scholar]
- 13.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 14.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Panda S, Karanxha L, Goker F, Satpathy A, Taschieri S, Francetti L, et al. Autologous platelet concentrates in treatment of furcation defects – A systematic review and meta-analysis. Int J Mol Sci. 2019;20:E1347. doi: 10.3390/ijms20061347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal AA. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J Clin Cases. 2017;5:159–71. doi: 10.12998/wjcc.v5.i5.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradeep AR, Pai S, Garg G, Devi P, Shetty SK. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J Clin Periodontol. 2009;36:581–8. doi: 10.1111/j.1600-051X.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J Periodontol. 2011;82:1396–403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 19.Lafzi A, Shirmohammadi A, Faramarzi M, Jabali S, Shayan A. Clinical comparison of autogenous bone graft with and without plasma rich in growth factors in the treatment of grade II furcation involvement of mandibular molars. J Dent Res Dent Clin Dent Prospects. 2013;7:22–29. doi: 10.5681/joddd.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco L. International academy of implant prosthesis and osteoconnection. Lecture. 2006;12:4. [Google Scholar]
- 21.Qiao J, Duan J, Zhang Y, Chu Y, Sun C. The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Future Sci OA. 2016;2:FS136. doi: 10.4155/fsoa-2016-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn DS, Heo JU, Kwak DH, Kim DE, Kim JM, Moon JW, et al. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011;20:389–95. doi: 10.1097/ID.0b013e31822f7a70. [DOI] [PubMed] [Google Scholar]
- 23.Pirpir C, Yilmaz O, Candirli C, Balaban E. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int J Implant Dent. 2017;3:7. doi: 10.1186/s40729-017-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and perio- dontal condition. Acta Odontol Scand. 1964;22:112–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 25.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 26.Mealey BL, Neubauer MF, Butzin CA, Waldrop TC. Use of furcal bone sounding to improve accuracy of furcation diagnosis. J Periodontol. 1994;65:649–57. doi: 10.1902/jop.1994.65.7.649. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Foss K, Wang BY. A retrospective study on molar furcation assessment via clinical detection, intraoral radiography and cone beam computed tomography. BMC Oral Health. 2018;18:75. doi: 10.1186/s12903-018-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borsani E, Bonazza V, Buffoli B, Cocchi MA, Castrezzati S, Scari G, et al. Biological characterization and in vitro effects of human concentrated growth factor preparation: An innovative approach to tissue regeneration. Bio Med. 2015;7:1–10. [Google Scholar]
- 29.Kinaia BM, Steiger J, Neely AL, Shah M, Bhola M. Treatment of Class II molar furcation involvement: Meta-analyses of re-entry results. J Periodontol. 2011;82:413–28. doi: 10.1902/jop.2010.100306. [DOI] [PubMed] [Google Scholar]
- 30.Pajnigara NG, Kolte AP, Kolte RA, Pajnigara NG. Volumetric assessment of regenerative efficacy of demineralized freeze-dried bone allograft with or without amnion membrane in grade II furcation defects: A cone beam computed tomography study. Int J Periodontics Restorative Dent. 2017;37:255–62. doi: 10.11607/prd.2901. [DOI] [PubMed] [Google Scholar]
- 31.Jepsen S, Gennai S, Hirschfeld J, Kalemaj Z, Buti J, Graziani F. Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta-analysis of randomized clinical trials. J Clin Periodontol. 2020;47:352–74. doi: 10.1111/jcpe.13238. [DOI] [PubMed] [Google Scholar]
- 32.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in the human periodontium by guided tissue regeneration.Case reports. J Clin Periodontol. 1986;13:604–16. doi: 10.1111/j.1600-051x.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 33.Qiao J, Duan JY, Chu Y, Sun CZ. Effect of concentrated growth factors on the treatment of degree II furcation involvements of mandibular molars. Beijing Da Xue Xue Bao Yi Xue Ban. 2017;49:36–42. [PubMed] [Google Scholar]
- 34.Laugisch O, Cosgarea R, Nikou G, Nikolidakis D, Donos N, Salvi GE, et al. Histologic evidence of periodontal regeneration in furcation defects: A systematic review. Clin Oral Investig. 2019;23:2861–906. doi: 10.1007/s00784-019-02964-3. [DOI] [PubMed] [Google Scholar]
- 35.Li F, Qiao J, Duan JY, Zhang Y, Wang XJ. Effect of concentrated growth factors combined with guided tissue regeneration in treatment of Class II furcation involvements of mandibular molars. Beijing Da Xue Xue Bao Yi Xue Ban. 2020;52:346–52. doi: 10.19723/j.issn.1671-167X.2020.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardi S, Mummolo S, Tecco S, Continenza MA, Marzo G. Histological characterization of Sacco's concentrated growth factors membrane. Int J Morphol. 2017;35:114–19. [Google Scholar]
- 37.Park HC, Kim SG, Oh JS, You JS, Kim JS, Lim SC, et al. Early bone formation at a femur defect using CGF and PRF grafts in adult dogs: A comparative study. Implant Dent. 2016;25:387–93. doi: 10.1097/ID.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 38.Tabatabaei F, Aghamohammadi Z, Tayebi L. In vitro and in vivo effects of concentrated growth factor on cells and tissues. J Biomed Mater Res A. 2020;108:1338–50. doi: 10.1002/jbm.a.36906. [DOI] [PubMed] [Google Scholar]
- 39.Rodella LF, Favero G, Boninsegna R, Buffoli B, Labanca M, Sacco L, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74:772–7. doi: 10.1002/jemt.20968. [DOI] [PubMed] [Google Scholar]
- 40.Yu B, Wang Z. Effect of concentrated growth factors on beagle periodontal ligament stem cells in vitro. Mol Med Rep. 2014;9:235–42. doi: 10.3892/mmr.2013.1756. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Yang H, Zhang Z, Yan Z, Lv H, Zhang Y, et al. Concentrated growth factor exudate enhances the proliferation of human periodontal ligament cells in the presence of TNFα. Mol Med Rep. 2019;4:943–50. doi: 10.3892/mmr.2018.9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Wang J, Yu L, Zhou J, Zheng D, Zhang B. Effect of concentrated growth factor (CGF) on the promotion of osteogenesis in bone marrow stromal cells (BMSC) in vivo. Sci Rep. 2018;8:5876. doi: 10.1038/s41598-018-24364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochira A, Siculella L, Damiano F, Palermo A, Ferrante F, Carluccio MA, et al. Concentrated growth factors (CGF) induce osteogenic differentiation in human bone marrow stem cells. Biol. 2020;9:370–8. doi: 10.3390/biology9110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonazza V, Hajistilly C, Patel D, Patel J, Woo R, Cocchi MA, et al. Growth factors release from concentrated growth factors: Effect of beta-Tricalcium phosphate addition. J Craniofac Surg. 2018;29:2291–5. doi: 10.1097/SCS.0000000000004607. [DOI] [PubMed] [Google Scholar]
- 45.Yu M, Wang X, Liu Y, Qiao J. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin Oral Investig. 2019;23:1663–71. doi: 10.1007/s00784-018-2582-z. [DOI] [PubMed] [Google Scholar]


