Abstract
Context and Aims:
Calprotectin is a myeloid protein, exerting pro-inflammatory effects on various cells and are shown to be elevated in inflammatory diseases. Periodontal therapy has been shown to decrease the levels of calprotectin in body fluids. Hence, the present study was aimed at assessing salivary calprotectin levels in periodontitis associated with diabetes mellitus after low-level laser therapy (LLLT) as an adjunct to scaling and root planing (SRP).
Materials and Methods:
Sixty-four participants were divided into Groups A (periodontitis) and B (periodontitis associated with type 2 diabetes mellitus [T2DM]) based on probing depth of ≥5 mm, clinical attachment level (CAL) of >2 mm, and history of T2DM. Based on assigned treatments, the groups were subdivided into A1, A2, B1, and B2 where A1 and B1 were subjected to SRP alone and A2 and B2 received LLLT as an adjunct to SRP, respectively. The parameters assessed included probing pocket depth, CAL, bleeding on probing (BOP), plaque index (PI), and salivary calprotectin. All the values were subjected for comparison at baseline and 8 weeks.
Statistical Analysis Used:
Multiple group comparisons were done using analysis of variance; intragroup comparisons were made using t-test. The level of significance was assessed at P < 0.05 for all tests.
Results:
All parameters showed a significant difference within the groups from baseline to 8 weeks. Intergroup comparison of PI, BOP, and salivary calprotectin showed a significant difference (P < 0.05).
Conclusions:
Reduction in calprotectin levels was noticed with LLLT when used as an adjunct to SRP. Calprotectin may be used as a prognostic marker for periodontitis.
Keywords: Low-level laser therapy, periodontitis, salivary calprotectin, type 2 diabetes mellitus
INTRODUCTION
Periodontitis is an immunoinflammatory response induced by bacterial organisms in dental plaque that contributes to periodontal destruction and tooth loss.[1]
Periodontal tissues are constantly exposed to specific bacterial products that interplay with the cells of junctional epithelium and connective tissue resulting in the increased number of neutrophils into the pocket epithelium. At an initial stage, the leukocyte infiltrate is dominated by lymphocytes, including B- and T-cells. Subsequently, influencing more B-cells to appear, thereby reducing the efficiency of neutrophil migration. This is likely to stimulate the production of more neutrophils within the tissues.[2]
These activated neutrophils,[3] monocytes,[4] and macrophages,[5,6] participate in the release of myeloid-related proteins MRP8/14 also called calprotectin.
Calprotectin is a major immunogenic protein,[7] with 36.5 KDa,[8] released by myeloid cells, exerting its pro-inflammatory effects[9] on a wide range of cells and cell surface receptors,[10] that trigger signal transduction, recruitment of leukocytes,[11] and cytokine secretion in inflammatory regions.[12]
In its released extracellular form, calprotectin becomes a critical modulator of inflammation-promoting innate immune cell recruitment and effector functions via interacting with toll-like receptor 4 and potential receptors for advanced glycation end products.[13,14]
Calprotectin is a validated fecal marker of inflammation used in the diagnosis of various inflammatory diseases such as Crohn's disease,[15] cystic fibrosis,[15] rheumatoid arthritis,[16,17] psoriasis,[18] as well as diabetes.[19,20,21]
Recently calprotectin has been evaluated in serum,[20,22] Gingival crevicular Fluid (GCF),[21,20] saliva,[22,23] periodontitis, and periodontitis associated with diabetes mellitus which was shown to be elevated when compared with healthy controls, and the initial periodontal therapy can reduce the levels of calprotectin.[21]
The regular treatment of periodontal infections involves scaling and root planing (SRP), and maintenance therapy, that removes supra- and subgingival plaque and calculus and reduces colonization of bacteria.[24] In advanced treatment protocols, lasers have been widely used in the treatment of periodontal disease where biostimulatory effects on tissue cells were shown to promote faster tissue repair and wound healing.[25]
To our knowledge until now, there is scanty evidence on the effect of low-level laser therapy (LLLT) as an adjunct to SRP on the salivary calprotectin.
Hence, in the present study, an attempt was made to assess the effect of LLLT as an adjunct to SRP on salivary calprotectin in patients with periodontitis and periodontitis associated with diabetes mellitus.
SUBJECTS AND METHODS
A randomized, clinical trial was conducted to compare the levels of salivary calprotectin in individuals with periodontitis alone and periodontitis along with diabetes before and after LLLT as an adjunct to SRP. The institutional ethical committee approved the study protocol.
For allocation of the participants, a power analysis was performed with 95% confidence intervals, and a sample size of n = 64 was determined. Patients attending the department of periodontology from September 2019 to December 2020 were screened, and those fulfilling the inclusion and exclusion criteria were recruited into the study (n = 64). The study included males and females aged between 30 and 60 years, presence of periodontitis with probing pocket depth (PPD) ≥5 mm, clinical attachment level (CAL) ≥2 mm, involving minimum of 30% of the sites,[26] and patients with periodontitis associated with type 2 diabetes mellitus. Subjects excluded were, people who were on medication for the past 3 months other than anti-diabetic medication, pregnancy, lactating women,autoimmune disorders, oromucosal abnormalities, any form of tobacco or alcohol usage, and patients who underwent surgical or nonsurgical therapy within the past 6 months before the start of study, patients with any autoimmune disorders, oromucosal abnormalities, patients with the usage of tobacco, and alcohol in any form.
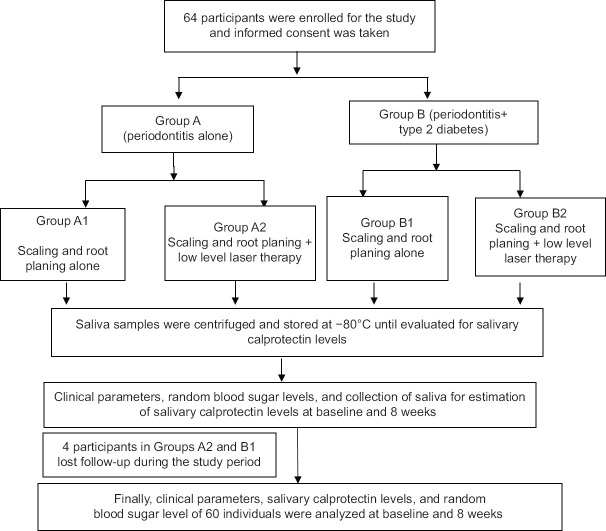
Participants were divided into Group A (periodontitis) and Group B (periodontitis associated with type 2 diabetes mellitus [T2DM]) based on probing depth of ≥5 mm, CAL of >2 mm, and history of T2DM. Based on assigned treatments, the groups were subdivided into A1, A2, B1, and B2 [Figure 1].
Figure 1.
Flowchart representing the study design
All the eligible participants were informed about the nature, potential risks, and benefits of their participation in the study, and informed consent was obtained. After taking details regarding general health, demographic data regarding age, gender, and body mass index (BMI)[27] were recorded, followed by full-mouth plaque (Silness and Loe, 1964)[28] and bleeding scores (Mombelli et al., 1987).[29] Pocket depths and CALs were recorded to the nearest millimeter at four sites per teeth at baseline and 8 weeks after treatment. Random blood glucose (RBS) levels were evaluated at baseline and 8 weeks posttreatment.
For Groups A1 and B1, SRP under local anesthesia in a full-mouth manner was completed in a single visit (approximately 80–90 min) with ultrasonic device and hand instruments (Gracey Curettes, Hu-Friedy).
For Groups A2 and B2 after SRP with appropriate laser safety precautions, the gingival margin to the base of the pocket was lased laterally and apically to remove the diseased pocket epithelium and decontaminate the pocket lining using a diode laser with power settings of 4.0 W and wavelength of 630–670 nm. Later digital compression of the tissues was done against the tooth for a closer approximation.
Three milliliters of unstimulated saliva was collected before SRP at baseline and 8 weeks posttreatment from all participants into sterile tubes.[30] These samples were centrifuged for 20 min at 2000–3000 rpm followed by the collection of the supernatant into an autoclaved 1.5-ml Eppendorf tube and were then stored at −80°C until the experiment. Calprotectin levels were determined by the enzyme-linked immunosorbent assay technique.
All the clinical and biochemical values were subjected to statistical analysis by using Jamovi (version 1.2.27). Basic descriptions included mean and standard deviations (SD). Multiple group comparisons were done by analysis of variance. For intragroup comparisons, t-test was used. The level of significance was considered at P < 0.05 for all tests.
RESULTS
In Groups A2 and B1, four participants lost the follow-up, therefore, the records of sixty participants were analyzed.
The demographic characteristics of participants (gender, age, and BMI) are represented in Table 1. Out of 60 individuals, 28 were male and 32 were female. The mean ± SD of age in Groups A1, A2, B1, and B2 was 47.5 ± 6.17, 43.8 ± 7.6, 46.4 ± 7.9, and 45 ± 9.1, respectively. The mean ± SD of BMI in Groups A1, A2, B1, and B2 was 23.3 ± 1.4, 22.5 ± 1.9, 22.2 ± 1.8, and 22.5 ± 1.7, respectively.
Table 1.
Demographic data representing age, gender, and body mass index in different groups
| Demographic variables | Groups | |||
|---|---|---|---|---|
|
| ||||
| A1 | A2 | B1 | B2 | |
| Gender | ||||
| Males | 7 | 5 | 9 | 7 |
| Females | 9 | 9 | 5 | 9 |
| Age, mean±SD | 47.56±6.17 | 43.86±7.63 | 46.43±7.93 | 45±9.15 |
| BMI, mean±SD | 23.3±1.46 | 22.5±1.90 | 22.2±1.86 | 22.5±1.72 |
A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving low-level laser therapy as an adjunct to scaling and root planing; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving low-level laser therapy as an adjunct to scaling and root planing; BMI – Body mass index; SD – Standard deviation
Intragroup comparison of clinical parameters (plaque index [PI], bleeding on probing [BOP], PPD, and CAL), salivary calprotectin, and RBS levels before and after treatment showed a significant difference (P < 0.05) from baseline to 8 weeks represented in Tables 2, 2a, 2b.
Table 2.
Intragroup comparison of clinical parameters from baseline and 8 weeks posttreatment
| Group | Parameters | Time interval | Mean±SD | Mean difference | 95% CI | t | df | P | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Lower | Upper | ||||||||
| A1 | PI | Baseline | 1.73±0.45 | 1.04 | 0.75 | 1.33 | 7.73 | 15.0 | <0.001* |
| 8 weeks | 0.69±0.21 | ||||||||
| BOP | Baseline | 1.90±0.63 | 1.46 | 1.14 | 1.77 | 9.86 | 15.0 | <0.001* | |
| 8 weeks | 0.44±0.17 | ||||||||
| PD | Baseline | 6.05±0.63 | 3.19 | 2.79 | 3.54 | 18.1 | 15.0 | <0.001* | |
| 8 weeks | 2.88±0.41 | ||||||||
| CAL | Baseline | 6.06±0.66 | 2.18 | 2.79 | 3.56 | 17.7 | 15.0 | <0.001* | |
| 8 weeks | 2.88±0.41 | ||||||||
| A2 | PI | Baseline | 1.71±0.51 | 1.11 | 0.81 | 1.41 | 8.04 | 13.0 | <0.001* |
| 8 weeks | 0.69±0.21 | ||||||||
| BOP | Baseline | 2.29±0.42 | 1.17 | 0.90 | 1.44 | 9.59 | 13.0 | <0.001* | |
| 8 weeks | 1.12±0.48 | ||||||||
| PD | Baseline | 5.98±0.59 | 2.99 | 2.57 | 3.54 | 15.3 | 13.0 | <0.001* | |
| 8 weeks | 2.99±0.43 | ||||||||
| CAL | Baseline | 2.99±0.43 | 3.03 | 2.58 | 3.49 | 14.4 | 13.0 | <0.001* | |
| 8 weeks | 2.99±0.43 | ||||||||
| B1 | PI | Baseline | 2.00±0.66 | 1.62 | 1.26 | 1.97 | 9.92 | 13.0 | <0.001* |
| 8 weeks | 0.38±0.14 | ||||||||
| BOP | Baseline | 1.96±0.62 | 1.54 | 1.53 | 1.92 | 8.66 | 13.0 | <0.001* | |
| 8 weeks | 0.43±0.17 | ||||||||
| PD | Baseline | 5.93±0.97 | 2.93 | 2.50 | 3.52 | 14.9 | 130 | <0.001* | |
| 8 weeks | 3.00±0.48 | ||||||||
| CAL | Baseline | 6.15±0.94 | 3.03 | 2.68 | 3.38 | 18.9 | 13.0 | <0.001* | |
| 8 weeks | 3.12±0.59 | ||||||||
| B2 | PI | Baseline | 2.14±0.57 | 1.83 | 1.55 | 2.10 | 14.30 | 15.0 | <0.001* |
| 8 weeks | 0.31±0.14 | ||||||||
| BOP | Baseline | 1.84±0.55 | 1.39 | 1.086 | 1.69 | 9.84 | 15.0 | <0.001* | |
| 8 weeks | 0.45±0.12 | ||||||||
| PD | Baseline | 5.87±0.94 | 3.01 | 2.50 | 3.52 | 12.6 | 15.0 | <0.001* | |
| 8 weeks | 2.86±0.44 | ||||||||
| CAL | Baseline | 5.97±1.00 | 3.04 | 2.55 | 3.53 | 13.3 | 15.0 | <0.001* | |
| 8 weeks | 2.93±0.49 | ||||||||
*Paired t-test; the value of P is considered statistically significant P<0.05. P – Probability; SD – Standard deviation; df – degrees of freedom; t – t statistics value; CI – Confidence interval; PI – Plaque index; BOP – Bleeding on probing; PD – Probing depth; CAL – Clinical attachment level; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Table 2a.
Intragroup comparison of salivary calprotectin levels at baseline and 8 weeks posttreatment
| Group | Timeline | n | Mean±SD | Mean difference | 95% CI of the difference | t | df | P | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Lower | Upper | ||||||||
| A1 | Baseline | 16 | 95.37±3.48 | 1.75 | 0.46 | 3.03 | 2.91 | 15.0 | 0.01* |
| 8 weeks | 16 | 93.62±4.33 | |||||||
| A2 | Baseline | 14 | 94.92±3.66 | 1.29 | 0.66 | 1.90 | 4.50 | 13.0 | <0.001* |
| 8 weeks | 14 | 93.64±3.69 | |||||||
| B1 | Baseline | 14 | 167.07±13.28 | 2.79 | 1.74 | 3.83 | 5.77 | 13.0 | <0.001* |
| 8 weeks | 14 | 164.28±11.97 | |||||||
| B2 | Baseline | 16 | 165.25±11.97 | 2.56 | 1.31 | 3.81 | 4.39 | 15.0 | <0.001* |
| 8 weeks | 16 | 162.68±11.17 | |||||||
*Paired t-test; value of P is considered statistically significant P<0.05. P – Probability; SD – Standard deviation; n – Number of participants; df – Degrees of freedom; t – t statistics value; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy; CI – Confidence interval
Table 2b.
Intragroup comparison of random blood sugar at baseline and 8 weeks posttreatment
| Group | Time line | n | Mean±SD | Mean difference | 95% CI of the difference | t | df | P | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Lower | Upper | ||||||||
| A1 | Baseline | 16 | 95.37±3.48 | 1.75 | 0.46 | 3.03 | 2.91 | 15.0 | 0.01* |
| 8 weeks | 16 | 93.62±4.33 | |||||||
| A2 | Baseline | 14 | 94.92±3.66 | 1.29 | 0.66 | 1.90 | 4.50 | 13.0 | <0.001* |
| 8 weeks | 14 | 93.64±3.69 | |||||||
| B1 | Baseline | 14 | 167.07±13.28 | 2.79 | 1.74 | 3.83 | 5.77 | 13.0 | <0.001* |
| 8 weeks | 14 | 164.28±11.97 | |||||||
| B2 | Baseline | 16 | 165.25±11.97 | 2.56 | 1.31 | 3.81 | 4.39 | 15.0 | <0.001* |
| 8 weeks | 16 | 162.68±11.17 | |||||||
*Paired t-test; value of P is considered statistically significant P<0.05. P – Probability; SD – Standard deviation; n – Number of participants; df – Degrees of freedom; t – t statistics value; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy; CI – Confidence interval
At baseline, intergroup comparison of salivary calprotectin and RBS showed a statistically significant difference (P < 0.05), whereas clinical parameters did not show any significant difference [Tables 3, 3a, 3b].
Table 3.
Intergroup comparison of clinical parameters at baseline
| Parameter | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| PI | Group A1 | 16 | 1.73±0.45 | 2.24 | 0.09 (NS) |
| Group A2 | 14 | 1.71±0.51 | |||
| Group B1 | 14 | 2.00±0.66 | |||
| Group B2 | 16 | 2.14±0.57 | |||
| BOP | Group A1 | 16 | 1.90±0.63 | 1.88 | 0.14 (NS) |
| Group A2 | 14 | 2.29±0.42 | |||
| Group B1 | 14 | 1.96±0.62 | |||
| Group B2 | 16 | 1.84±0.55 | |||
| PD | Group A1 | 16 | 6.05±0.63 | 0.14 | 0.94 (NS) |
| Group A2 | 14 | 5.98±0.59 | |||
| Group B1 | 14 | 5.93±0.97 | |||
| Group B2 | 16 | 5.87±0.94 | |||
| CAL | Group A1 | 16 | 6.06±0.66 | 0.13 | 0.94 (NS) |
| Group A2 | 14 | 6.02±0.62 | |||
| Group B1 | 14 | 6.15±0.94 | |||
| Group B2 | 16 | 5.97±1.00 | |||
Value of P, P>0.05 (NS). NS – Not significant; P – Probability; ANOVA – Analysis of variance; SD – Standard deviation; n – Number of participants; F – Fisher in ANOVA test; PI – Plaque index; BOP – Bleeding on probing; PD – Probing depth; CAL – Clinical attachment level; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to Low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Table 3a.
Intergroup comparison of salivary calprotectin at baseline
| Parameter | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| Calprotectin | Group A1 | 16 | 45.14±1.94 | 8.04 | <0.001* |
| Group A2 | 14 | 45.10±1.85 | |||
| Group B1 | 14 | 48.64±3.52 | |||
| Group B2 | 16 | 48.48±3.15 | |||
*Value of P is considered statistically significant P<0.05. ANOVA – Analysis of variance; P – Probability; SD – Standard deviation; n – Number of participants; F – Fisher in ANOVA test; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Table 3b.
Intergroup comparison of random blood sugar values at baseline
| Parameter | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| RBS | Group A1 | 16 | 95.62±3.14 | 282.2 | <0.001* |
| Group A2 | 14 | 96.52±3.66 | |||
| Group B1 | 14 | 167.07±13.68 | |||
| Group B2 | 16 | 165.25±11.176 | |||
*Value of P is considered statistically significant P<0.05. ANOVA – Analysis of variance; P – Probability; SD – Standard deviation; n – Number of participants; F – Fisher in ANOVA test; RBS – Random blood sugar; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Intergroup comparison of salivary calprotectin at 8 weeks posttreatment showed a statistically significant difference (P < 0.05) [Table 4].
Table 4.
Intergroup comparison of salivary calprotectin levels 8 weeks posttreatment
| Parameter | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| Calprotectin | Group A1 | 16 | 43.97±1.72 | 2.77 | <0.05 * |
| Group A2 | 14 | 43.48±1.86 | |||
| Group B1 | 14 | 42.09±3.00 | |||
| Group B2 | 16 | 40.49±6.14 | |||
*Value of P is considered statistically significant P<0.05. ANOVA – Analysis of variance; P – Probability; SD – Standard deviation; n – Number of participants; F – Fisher in ANOVA test; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Intergroup comparison of the change in PI, BOP, salivary calprotectin, and RBS showed a significant difference, whereas PPD and CAL did not show any significant change from baseline to 8 weeks [Table 5, 5a, 5b].
Table 5.
Intergroup comparison of the change in clinical parameters from baseline to 8 weeks posttreatment
| Parameters | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| PI | A1 | 16 | 1.04±0.54 | 7.68 | <0.001* |
| A2 | 14 | 1.11±0.52 | |||
| B1 | 14 | 1.62±0.61 | |||
| B2 | 16 | 1.83±0.51 | |||
| BOP | A1 | 16 | 1.46±0.59 | 7.05 | <0.001* |
| A2 | 14 | 1.17±0.46 | |||
| B1 | 14 | 1.54±0.66 | |||
| B2 | 16 | 1.39±0.56 | |||
| PD | A1 | 16 | 3.16±0.70 | 0.24 | 0.87 (NS) |
| A2 | 14 | 2.99±0.73 | |||
| B1 | 14 | 2.93±0.74 | |||
| B2 | 16 | 3.01±0.95 | |||
| CAL | A1 | 16 | 3.18±0.72 | 0.13 | 0.94 (NS) |
| A2 | 14 | 3.03±0.79 | |||
| B1 | 14 | 3.03±0.60 | |||
| B2 | 16 | 3.04±0.92 | |||
*Value of P is considered statistically significant P<0.05, P>0.05 (NS). ANOVA – Analysis of variance; NS – Not significant; P – Probability; n – Number of participants; F – Fisher in ANOVA test; PI – Plaque index; BOP – Bleeding on probing; PD – Probing depth; CAL – Clinical attachment level; SD – Standard deviation; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Table 5a.
Intergroup comparison of the change in salivary calprotectin from baseline to 8 weeks posttreatment
| Parameter | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| Salivary calprotectin | A1 | 16 | 1.17±1.08 | 12.74 | <0.001* |
| A2 | 14 | 1.62±1.01 | |||
| B1 | 14 | 6.54±2.90 | |||
| B2 | 16 | 7.99±6.63 | |||
*Value of P is considered statistically significant P<0.05. ANOVA – Analysis of variance; P – Probability; n – Number of participants; F – Fisher in ANOVA test; SD – Standard deviation; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Table 5b.
Intergroup comparison of the change in RBS from baseline to 8 weeks posttreatment
| Parameter | Study groups | n | Mean±SD | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| F | P | ||||
| RBS | Group A1 | 16 | 93.62±4.33 | 282.9 | <0.001* |
| Group A2 | 14 | 93.64±3.69 | |||
| Group B1 | 14 | 164.28±13.68 | |||
| Group B2 | 16 | 162.68±11.17 | |||
*Value of P is considered statistically significant P<0.05. ANOVA – Analysis of variance; P – Probability; n – Number of participants; F – Fisher in ANOVA test; SD – Standard deviation; RBS – Random blood sugar; A1 – Patients with periodontitis receiving scaling and root planing; A2 – Patients with periodontitis receiving scaling and root planing as an adjunct to low-level laser therapy; B1 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing; B2 – Patients with periodontitis associated with type 2 diabetes mellitus receiving scaling and root planing as an adjunct to low-level laser therapy
Correlation between salivary calprotectin levels with other parameters from baseline to 8 weeks showed a significant association with PI and RBS [Table 6].
Table 6.
Correlation between calprotectin levels at baseline and 8 weeks posttreatment with other parameters
| Time interval | Parameter | Clinical parameters | Spearman’s correlation | P |
|---|---|---|---|---|
| Baseline | Calprotectin levels | PI | 0.19 | 0.13 (NS) |
| BOP | −0.04 | 0.74 (NS) | ||
| PD | −0.05 | 0.68 (NS) | ||
| CAL | −0.10 | 0.43 (NS) | ||
| RBS | 0.52 | <0.001* | ||
| 8 weeks | Calprotectin levels | PI | 0.25 | <0.05* |
| BOP | 0.03 | 0.81 (NS) | ||
| PD | −0.97 | 0.46 (NS) | ||
| CAL | −0.11 | 0.38 (NS) | ||
| RBS | −0.33 | <0.05* |
Spearman correlation coefficient. *Value of P is considered statistically significant P<0.05, P>0.05 (NS). P – Probability; NS – Not significant; RBS – Random blood sugar; PI – Plaque index; BOP – Bleeding on probing; PD – Probing depth; CAL – Clinical attachment level
DISCUSSION
The first phase of periodontal treatment involves the elimination of bacterial deposits and colonization by the removal of supra- and subgingival biofilms.[31] To strengthen its effects, LLLT was recommended for its photochemical role as an anti-inflammatory, biostimulatory property.[32,33]
In the present study, clinical parameters and salivary calprotectin levels were evaluated 8 weeks posttreatment. As there was precise orientation of collagen bundle fibers, this is considered a suitable interval for primary evaluation of initial nonsurgical therapy.[34,35]
In the present study, there was no significant difference in the demographic characteristics (age, gender, and BMI) between all the study groups indicating the proper random assignment of the participants into the groups to prevent selection bias.
In this study, there was no significant difference in the clinical parameters (PI, BOP, PD, and CAL) at baseline, thus the obtained results are considered a direct effect of the intervention done.
Inter- and intragroup comparisons of PI and BOP showed a statistically significant difference from baseline to 8 weeks in all the groups. This result can be attributed to the reduction of local factors such as plaque and calculus by SRP and improving clinical condition in groups receiving LLLT supporting its anti-inflammatory role. This significant difference was in accordance with Mauri-Obradors et al.,[36] Nguyen et al.,[37] Saglam et al.,[38] and Euzebio Alves et al.,[39] however, these studies were conducted with a follow-up period ranging from 3 to 6 months.
There is a significant difference in PD and CAL in groups receiving SRP alone (Groups A1 and B1) from baseline to 8 weeks posttreatment. This change can be due to reduced inflammation and the formation of reattachment following SRP. This is in agreement with studies conducted by Jayakumar Sunandhakumari et al.,[40] Cugini et al.,[41] and Haffajee et al.,[24] with the follow-up periods ranging from 1 month to 12 months.
There was a significant reduction in PD and CAL in groups receiving LLLT (Groups A2 and B2) from baseline to 8 weeks posttreatment. This improvement could be attributed to the effective removal of pocket lining, hemostasis and coagulation of periodontally inflamed soft tissues,and bactericidal action(selectively targeting the black pigmented bacteria). This is in accordance with studies conducted by Calderín et al.,[25] Giannopoulou et al.,[42] whereas Nguyen et al.,[37] Euzebio Alves et al., (2013),[39] showed similar results with the studies who's follow-up periods ranged from 3 to 6 months.
However, intergroup comparison showed no significant difference in the mean changes of PD and CAL in all the groups. This may be attributed to mechanical therapy (SRP alone) failing to eliminate pathogenic bacterial colonies in the soft
tissue and areas with deep periodontal pockets, furcations, and root depressions. In groups receiving LLLT, its role is still controversial. The results of the present study were in accordance with Calderín et al.,[25] Giannopoulou et al.,[42], however studies by Nguyen et al.,[37] Euzebio Alves et al.,[39] also showed similar results with follow-up periods ranging from 3 months to 6 months.
In the present study, inter- and intragroup comparisons of random blood sugar showed a statistically significant difference from baseline to 8 weeks posttreatment in all the groups. In diabetic groups (Groups B1 and B2), this significant difference is indicative of the positive effect of nonsurgical periodontal therapy on the glycemic control. This is in accordance with the studies Mauri-Obradors et al.,[36] Abduljabbar et al.,[43] Sun et al.,[44] and Sgolastra et al.,[45] however, the above studies have used HbA1c as a monitoring test for diabetes.
Esposito et al.[46] reported that hyperglycemia acutely increases circulating cytokine concentration by oxidative mechanism. In the present study,a positive correlation was observed between salivary calprotectin and RBS at all time intervals. This could be attributed to the chronic hyperglycemia itself. This is in accordance with Gao et al.,[21] and Pradeep et al.,[20] who reported a correlation between calprotectin concentration in GCF and levels of HbA1c.
Intragroup comparison of salivary calprotectin levels in the present study showed a significant difference from baseline to 8 weeks posttreatment after SRP. This reduction may be related to improvement in the inflammatory condition of the patients. This is in accordance with Kim et al.,[23] who also established similar results, where as the studies by Gao et al.,[21] Andersen et al.,[19] observed similar findings in GCF with a follow-up period ranging from 3 to 6 months.
In the present study, there was a significant difference in salivary calprotectin levels from baseline to 8 weeks posttreatment among groups receiving LLLT. This can be attributed to the beneficial effect of LLLT on reducing the systemic inflammatory condition which occurred as a result of periodontitis and T2DM.
Future studies are recommended in larger sample with histological analysis with correlations between calprotectin levels and periodontal soft tissue status. Here, RBS values have been taken into consideration which when replaced with HBA1C would have been beneficial. Multiple sections of LLLT rather than a single section would have benefited the study.
CONCLUSIONS
LLLT as an adjunct to SRP is effective in controlling salivary calprotectin levels, and calprotectin may be used as a prognostic marker for periodontitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol 2000. 1997;14:112–43. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 2.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 3.Kido J, Kido R, Suryono , Kataoka M, Fagerhol MK, Nagata T. Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysaccharide via the CD-14-Toll-like receptor-nuclear factor kappaB pathway. J Periodontal Res. 2003;38:557–63. doi: 10.1034/j.1600-0765.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 4.Suryono, Kido JI, Hayashi N, Kataoka M, Nagata T. Calprotectin expression in human monocytes: Induction by Porphyromonas gingivalis lipopolysaccharide, tumor necrosis factor-α, and interleukin-1β. J Periodontol. 2005;76:437–42. doi: 10.1902/jop.2005.76.3.437. [DOI] [PubMed] [Google Scholar]
- 5.Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, Zwadlo G, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–2. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 6.Terasaki F, Fujita M, Shimomura H, Tsukada B, Otsuka K, Otsuka K, et al. Enhanced expression of myeloid-related protein complex (MRP8/14) in macrophages and multinucleated giant cells in granulomas of patients with active cardiac sarcoidosis. Circ J. 2007;71:1545–50. doi: 10.1253/circj.71.1545. [DOI] [PubMed] [Google Scholar]
- 7.Brun JG, Ulvestad E, Fagerhol MK, Jonsson R. Effects of human calprotectin (L1) on in vitro immunoglobulin synthesis. Scand J Immunol. 1994;40:675–80. doi: 10.1111/j.1365-3083.1994.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 8.Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem. 1983;134:1–6. doi: 10.1111/j.1432-1033.1983.tb07522.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Hou J, Meng H, Zhang X, Zheng Y, Peng L. Proinflammatory effects and mechanisms of calprotectin on human gingival fibroblasts. J Periodontal Res. 2017;52:975–83. doi: 10.1111/jre.12465. [DOI] [PubMed] [Google Scholar]
- 10.Simard JC, Girard D, Tessier PA. Induction of neutrophil degranulation by S100A9 via a MAPK-dependent mechanism. J Leukoc Biol. 2010;87:905–14. doi: 10.1189/jlb.1009676. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishikawa Y, Kajiura Y, Lew JH, Kido JI, Nagata T, Naruishi K. Calprotectin induces IL-6 and MCP-1 production via toll-like receptor 4 signaling in human gingival fibroblasts. J Cell Physiol. 2017;232:1862–71. doi: 10.1002/jcp.25724. [DOI] [PubMed] [Google Scholar]
- 13.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 14.Narumi K, Miyakawa R, Ueda R, Hashimoto H, Yamamoto Y, Yoshida T, et al. Proinflammatory proteins S100A8/S100A9 activate NK cells via interaction with RAGE. J Immunol. 2015;194:5539–48. doi: 10.4049/jimmunol.1402301. [DOI] [PubMed] [Google Scholar]
- 15.Golden BE, Clohessy PA, Russell G, Fagerhol MK. Calprotectin as a marker of inflammation in cystic fibrosis. Arch Dis Child. 1996;74:136–9. doi: 10.1136/adc.74.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer HB, Odegard S, Fagerhol MK, Landewé R, van der Heijde D, Uhlig T, et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1093–7. doi: 10.1136/ard.2006.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baillet A, Trocmé C, Berthier S, Arlotto M, Grange L, Chenau J, et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology (Oxford) 2010;49:671–82. doi: 10.1093/rheumatology/kep452. [DOI] [PubMed] [Google Scholar]
- 18.Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkötter C, Foell D, et al. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155:62–6. doi: 10.1111/j.1365-2133.2006.07198.x. [DOI] [PubMed] [Google Scholar]
- 19.Andersen E, Dessaix IM, Perneger T, Mombelli A. Myeloid-related protein (MRP8/14) expression in gingival crevice fluid in periodontal health and disease and after treatment. J Periodontal Res. 2010;45:458–63. doi: 10.1111/j.1600-0765.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- 20.Pradeep AR, Martande SS, Singh SP, Suke DK, Raju AP, Naik SB. Correlation of human S100A12 (EN-RAGE) and high-sensitivity C-reactive protein as gingival crevicular fluid and serum markers of inflammation in chronic periodontitis and type 2 diabetes. Inflamm Res. 2014;63:317–23. doi: 10.1007/s00011-013-0703-3. [DOI] [PubMed] [Google Scholar]
- 21.Gao H, Xu J, He L, Meng H, Hou J. Calprotectin levels in gingival crevicular fluid and serum of patients with chronic periodontitis and type 2 diabetes mellitus before and after initial periodontal therapy. J Periodontal Res. 2021;56:121–30. doi: 10.1111/jre.12800. [DOI] [PubMed] [Google Scholar]
- 22.Haririan H, Andrukhov O, Pablik E, Neuhofer M, Moritz A, Rausch-Fan X. Comparative analysis of calcium-binding myeloid-related protein-8/14 in saliva and serum of patients with periodontitis and healthy individuals. J Periodontol. 2016;87:184–92. doi: 10.1902/jop.2015.150254. [DOI] [PubMed] [Google Scholar]
- 23.Kim HD, Kim S, Jeon S, Kim SJ, Cho HJ, Choi YN. Diagnostic and prognostic ability of salivary MMP-9 and S100A8 for periodontitis. J Clin Periodontol. 2020;47:1191–200. doi: 10.1111/jcpe.13349. [DOI] [PubMed] [Google Scholar]
- 24.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–34. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 25.Calderín S, García-Núñez JA, Gómez C. Short-term clinical and osteoimmunological effects of scaling and root planing complemented by simple or repeated laser phototherapy in chronic periodontitis. Lasers Med Sci. 2013;28:157–66. doi: 10.1007/s10103-012-1104-5. [DOI] [PubMed] [Google Scholar]
- 26.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45(Suppl 20):S149–61. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 27.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 28.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 29.Mombelli A, van Oosten MA, Schurch E, Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 30.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi KK, Pavaskar R, Cappetta EG, Drew HJ. Effectiveness of adjunctive use of low-level laser therapy and photodynamic therapy after scaling and root planing in patients with chronic periodontitis. Restor Dent. 2019;39:14. doi: 10.11607/prd.4252. [DOI] [PubMed] [Google Scholar]
- 32.Slot DE, Jorritsma KH, Cobb CM, Van der Weijden FA. The effect of the thermal diode laser (wavelength 808-980 nm) in non-surgical periodontal therapy: A systematic review and meta-analysis. J Clin Periodontol. 2014;41:681–92. doi: 10.1111/jcpe.12233. [DOI] [PubMed] [Google Scholar]
- 33.Kreisler M, Al Haj H, d'Hoedt B. Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med. 2005;37:350–5. doi: 10.1002/lsm.20252. [DOI] [PubMed] [Google Scholar]
- 34.Biagini G, Checchi L, Miccoli MC, Vasi V, Castaldini C. Root curettage and gingival repair in periodontitis. J Periodontol. 1988;59:124–9. doi: 10.1902/jop.1988.59.2.124. [DOI] [PubMed] [Google Scholar]
- 35.Segelnick SL, Weinberg MA. Reevaluation of initial therapy: When is the appropriate time? J Periodontol. 2006;77:1598–601. doi: 10.1902/jop.2006.050358. [DOI] [PubMed] [Google Scholar]
- 36.Mauri-Obradors E, Merlos A, Estrugo-Devesa A, Jané-Salas E, López-López J, Viñas M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized controlled trial. J Clin Periodontol. 2018;45:345–53. doi: 10.1111/jcpe.12858. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen NT, Byarlay MR, Reinhardt RA, Marx DB, Meinberg TA, Kaldahl WB. Adjunctive non-surgical therapy of inflamed periodontal pockets during maintenance therapy using diode laser: A randomized clinical trial. J Periodontol. 2015;86:1133–40. doi: 10.1902/jop.2015.150152. [DOI] [PubMed] [Google Scholar]
- 38.Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: A randomized, controlled clinical trial. Lasers Med Sci. 2014;29:37–46. doi: 10.1007/s10103-012-1230-0. [DOI] [PubMed] [Google Scholar]
- 39.Euzebio Alves VT, de Andrade AK, Toaliar JM, Conde MC, Zezell DM, Cai S, et al. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clin Oral Investig. 2013;17:87–95. doi: 10.1007/s00784-012-0703-7. [DOI] [PubMed] [Google Scholar]
- 40.Jayakumar Sunandhakumari V, Sadasivan A, Koshi E, Krishna A, Alim A, Sebastian A. Effect of nonsurgical periodontal therapy on plasma levels of IL-17 in chronic periodontitis patients with well controlled type-II diabetes mellitus-A clinical study. Dent J (Basel) 2018;6:E19. doi: 10.3390/dj6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 42.Giannopoulou C, Cappuyns I, Cancela J, Cionca N, Mombelli A. Effect of photodynamic therapy, diode laser, and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J Periodontol. 2012;83:1018–27. doi: 10.1902/jop.2011.110281. [DOI] [PubMed] [Google Scholar]
- 43.Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: A systematic review. Lasers Med Sci. 2017;32:449–59. doi: 10.1007/s10103-016-2086-5. [DOI] [PubMed] [Google Scholar]
- 44.Sun QY, Feng M, Zhang MZ, Zhang YQ, Cao MF, Bian LX, et al. Effects of periodontal treatment on glycemic control in type 2 diabetic patients: A meta-analysis of randomized controlled trials. Chin J Physiol. 2014;57:305–14. doi: 10.4077/CJP.2014.BAC262. [DOI] [PubMed] [Google Scholar]
- 45.Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: A meta-analysis of randomized clinical trials. J Periodontol. 2013;84:958–73. doi: 10.1902/jop.2012.120377. [DOI] [PubMed] [Google Scholar]
- 46.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]