Abstract
Context:
Status of bone–implant interface or osseointegration can be assessed by using resonance frequency analysis (RFA), which measures implant stability. A modified implant surface can significantly enhance osseointegration and reduce healing period. Platelet-rich fibrin (PRF) consists of fibrin mesh with entrapped platelets and leukocytes that release a huge number of growth factors which contribute to wound healing and tissue regeneration.
Aims:
The present study aims to evaluate the effect of PRF on osseointegration in terms of implant stability.
Settings and Design:
This was a split-mouth randomized clinical trial.
Materials and Methods:
Sixty surgical sites were divided randomly into two groups. In Group 1 (thirty sites), PRF was placed in osteotomy sites before implant placement whereas no PRF was placed in Group 2 (thirty sites). Stability was measured using RFA in terms of implant stability quotient (ISQ) at baseline, 1 week, 1 month, and 3 months.
Statistical Analysis:
Intergroup comparison was done using Mann–Whitney U-test. Intragroup comparison was done using Friedman's test followed by pairwise comparison using Wilcoxon signed-rank test.
Results:
On intergroup comparison, Group 1 showed higher values for ISQ which were statistically significant (P < 0.05) at 1 week and 1 month. No significant difference (P > 0.05) was found at baseline and 3 months. Intragroup comparison and further pairwise comparison revealed a highly significant difference for values between all pairs of time intervals (P < 0.01) with higher values at 3 months.
Conclusions:
PRF has a significant effect on osseointegration of dental implants during the early healing period prior to loading.
Keywords: Dental implants, implant stability, osseointegration, platelet-rich fibrin, resonance frequency analysis
INTRODUCTION
The modern age of dentistry has experienced a boom with the introduction of dental implants. The use of dental implants has become one of the most reliable procedures for the treatment of either partially or completely edentulous jaws.[1] Rehabilitation of edentulous jaws with dental implant placement not only avoids damage to adjacent teeth but also helps the patient to achieve and maintain function for a long period.[2] While rehabilitating a lost dentition with the help of implants, the ultimate goal for a dentist is to achieve osseointegration. The direct contact between living bone tissue and a dental implant without any interposing connective tissue on a histological and microscopical level is termed as osseointegration.[3] With the principle of osseointegration and high clinical success rates, dental implants nowadays are being extensively used with a broader range of application and in challenging situations.
The quality and quantity of peri-implant bone and the nature of the bone–implant interface greatly influence the bone–implant connection and simultaneous load transmission in an implant-supported prosthesis. Implant stability with no micromotion is of at most requirement for successful osseointegration during an early healing phase. Conventionally, placement of the implant is followed by an unloading period for 3–4 months in the mandible and 6–8 months in the maxilla.[4,5] With an increased understanding of bone physiology, healing together with the advent of new system and techniques, old protocols have been significantly challenged and replaced with new ones. Presently healing period can be significantly reduced to 6–8 weeks with a modified implant surface.[6] A modified implant surface either in design or change in surface chemistry can improve bone–implant contact (BIC) and healing allowing the clinician to immediately load the implant. Another method of enhancing BIC is by addition of molecules or growth factors to the implant surface. A significant amount of literature supports the application of various surgical additives either platelet-rich plasma (PRP),[7] bone morphogenetic protein,[8] or growth factors[9,10,11] in accelerating osseointegration.
Autologous platelet-rich fibrin (PRF) is a second-generation platelet concentrate. PRF consists of slow polymerizing fibrin mesh with entrapped platelets and leukocytes.[12] Activated platelets release a huge number of growth factors, namely vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epidermal growth factor, insulin-like growth factor-1, and hepatocyte growth factor. These factors contribute to wound healing and effective soft and hard-tissue regeneration after tissue injury.[13,14] Activated leukocytes release VEGF, TGF-β, and other anti-inflammatory cytokines which enhance chemotaxis and angiogenesis. A higher platelet and leukocyte concentrate in a fibrin mesh acts like a scaffold which not only is osteogenic but also anti-microbial, inexpensive, and easy to prepare.[15] Application of autologous PRF has been known to enhance bone and soft-tissue healing around dental implants.[16,17]
Status of bone–implant interface can be assessed by using resonance frequency analysis (RFA). For yielding long-term positive outcomes in terms of loading and healing, achieving and maintaining implant stability is crucial. Implant stability and osseointegration have been assessed quantitatively in the past by using primitive techniques either ranging from percussion and mobility testing to invasive methods such as reverse torque test. RFA is a noninvasive, user-friendly, and most reliable method at present to measure implant stability. RFA has high clinical predictability with the relative ease of application and repeatability.[18]
The present study aims to compare and evaluate the effect of autologous PRF on osseointegration of dental implants in terms of implant stability by using RFA method.
MATERIALS AND METHODS
The present study is a 3-month split-mouth randomized clinical trial. The study enrolled a total of 21 patients with partial or complete edentulous state requiring rehabilitation with dental implants. Sample size was determined using the mean and standard deviation values from literature using the formula
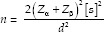
where Zα is the z variate of alpha error, i.e., a constant with value 1.96, Zβ is the z variate of beta error, i.e., a constant with value 0.84, s is standard deviation, and d is true difference between groups. Written informed consent was obtained from each participant. Ethical clearance was obtained from the Institutional Ethical Committee.
Inclusion criteria
Systemically healthy patients who are either partially edentulous with a single tooth missing or free end situations bilaterally or completely edentulous (at least 6 months postextraction) were included in the study. Patients should have adequate bone volume (at least 5 mm width and 10 mm height) and bone density with sufficient mesiodistal and interocclusal space. The surgical site should have a minimum amount of bone loss, no purulent exudates, adequate soft-tissue health, and quantity. All surgical sites were matched for bone density determined using cone-beam computed tomography machine (Carestream CS 3D-900) in gray values. The patient should physically and psychologically be able to tolerate conventional implant surgical procedure and with a sufficient platelet count (>200,000/mm3) for PRF preparation.
Exclusion criteria
Patients with a significant medical history which contradicts placement of dental implants such as diabetes or other diseases affecting bone metabolism; patients currently on antibiotics, steroids, hormonal therapy, or medications known to affect bone metabolism; patients with a history of radiotherapy or chemotherapy, coagulation defects, or currently on anticoagulant treatment; pregnant or lactating women; and patients with poor oral hygiene (full-mouth plaque score >30), habits such as alcohol intake and smoking >10 cigarettes per day, insufficient vertical interarch space, temporomandibular joint disorder, and parafunctional habits were excluded from the study.
A total of 60 bone density-matched surgical sites were enrolled in the study and were divided into two groups. In Group 1 (thirty surgical sites), PRF was placed in the implant osteotomy sites before implant placement. In Group 2 (thirty surgical sites), no PRF was placed in implant osteotomy sites before implant placement.
PRF was prepared according to Choukroun's protocol. By venipuncture method, 10 ml of blood sample without anticoagulant was obtained in a glass test tube. The test tube was centrifuged immediately at 3000 rpm for 10 min using an IntraSpin centrifuge (Intra-Lock International Inc., Boca Raton, FL, USA). The resultant product consists of three layers with PRF clot in the middle. The PRF clot was removed from the tube with forceps. Remnants of red blood cell were scrapped off subsequently with a gauge. The fibrin clot was compressed for 3 min to obtain a PRF membrane.
All patients were operated under local anesthesia (LA) with 2% lignocaine and 1:100,000 adrenaline. Following administration of LA, a crestal incision was made on both sides of arch. Full-thickness mucoperiosteal flaps were elevated to expose the alveolar crest.
The implant system used in the present study was Nobel Replace Conical Connection implant system (Nobel Biocare). Implants of length 11.5 mm and regular platform with a diameter of 4.3 mm were placed at density-matched surgical sites.
Osteotomy sites were prepared precisely following the drilling sequence as per the manufacturer's instructions. Randomization of test and control sites was performed using a computer-generated random allocation to determine on which side PRF is to be placed. On the PRF side (Group 1), the PRF membrane was placed in the osteotomy site before placement of implant, with implants being rinsed with serum obtained during compression of a fibrin clot. On the non-PRF side (Group 2), implants of the same size were placed but without a PRF membrane. All implants were placed 1 mm below the alveolar crest with a tightening torque >25 Ncm. Cover screw was placed and flaps were approximated using 3-0 braided nonresorbable silk suture material. Both sides were operated in a single appointment.
Patients were advised to follow standard postoperative instructions with antibiotics (amoxicillin 500 mg, 3 times a day), analgesic (ibuprofen 400 mg, 3 times a day) for 5 days, and an antiseptic oral rinse (0.12% chlorhexidine gluconate mouthwash, 2 times a day), which were prescribed for 1 week.
A single examiner blinded to randomization and surgical procedure measured implant stability. Stability was measured using RFA in terms of implant stability quotient (ISQ) by using Penguin device (bredent Penguin RFA) after connecting smart pegs or transducers to the implant. ISQ was measured in both mesiodistal and buccolingual directions and mean was calculated. ISQ measurements were performed at baseline (immediately after implant placement), 1 week, 1 month, and 3 months postoperatively.
Data were subjected to statistical analysis using the Statistical Package for social sciences (IBM SPSS Statistics for Windows, version 26.0. IBM Corp., Armonk, New York, USA). Descriptive statistics such as frequencies and percentage for categorical data, mean and standard deviation for numerical data have been depicted. Normality of numerical data was checked using Shapiro–Wilk test and was found that the data did not follow a normal curve; hence, nonparametric tests have been used for comparisons. Intergroup comparison was done using Mann–Whitney U-test. Intragroup comparison was done using Friedman's (for >2 observations) followed by pairwise comparison using Wilcoxon signed-rank test. For all the statistical tests, P < 0.01 was considered to be statistically highly significant and P < 0.05 was considered to be statistically significant, keeping α error at 5% and β error at 20%, thus giving a power to the study as 80%.
RESULTS
All 21 patients completed the study without any dropouts and with no reports of any adverse effects. Healing was uneventful without any postoperative complications. The mean age of participants was 42 ± 14.40, with minimum and maximum age being 23 and 70 years, respectively. Among the 21 participants, 11 were males and 10 were females. The present study had a split-mouth design, hence age and gender cannot be confounders. In terms of the distribution of participants based on the number of implants placed, 57.1%, i.e., 12 participants, had 2 implants placed and 42.9%, i.e., 9 participants, had 4 implants placed. In terms of jaws which received the implants, 33.3%, i.e., 7 participants, had 2 implants in mandible; 23.8%, i.e., 5 participants, had 2 implants in maxilla; and 42.9%, i.e., 9 participants, had 2 implants each placed in both maxilla and mandible.
Intergroup comparison of ISQ [Table 1] revealed a statistically nonsignificant difference between two groups (P > 0.05) at baseline and 3 months. There was a statistically significant difference seen for values between two groups (P < 0.05) with higher ISQ values in Group 1, 50.28 ± 5.41 and 59.56 ± 4.51, than Group 2, 46.84 ± 6.43 and 57.12 ± 3.82 at 1 week and 1 month, respectively.
Table 1.
Intergroup comparison of implant stability quotient values
| Group | n | Mean±SD | Median | Mann-Whitney U value | Z | P of Mann-Whitney U-test | |
|---|---|---|---|---|---|---|---|
| ISQ baseline | 1 | 30 | 25.135333±1.260891 | 25.230000 | 425.500 | −4.085 | 0.717# |
| 2 | 30 | 25.059333±2.611289 | 25.305000 | ||||
| ISQ 1 week | 1 | 30 | 50.287000±5.418054 | 51.140000 | 288.000 | −2.398 | 0.016* |
| 2 | 30 | 46.847000±6.437912 | 47.415000 | ||||
| ISQ 1 month | 1 | 30 | 59.564667±4.510371 | 59.675000 | 296.00 | −2.278 | 0.023* |
| 2 | 30 | 57.127667±3.829209 | 56.500000 | ||||
| ISQ 3 months | 1 | 30 | 67.784000±6.106217 | 69.140000 | 414.000 | −0.533 | 0.594# |
| 2 | 30 | 69.926900±3.566833 | 70.065000 |
#Nonsignificant difference (P>0.05); *Statistically significant difference (P<0.05). Z – Z value for nonparametric test utilizing Mann-Whitney U-test of intergroup comparison; ISQ – Implant stability quotient; n – Number of surgical sites; P – Probability value; SD – Standard deviation
On intragroup comparison by using Friedman's test in Group 1 [Table 2] and Group 2 [Table 3], a statistically highly significant difference was seen for the values between the time intervals (P < 0.01) with higher values at 3 months. Further pairwise comparison using Wilcoxon signed-rank test for Group 1 [Table 4] and Group 2 [Table 5], revealed a statistically highly significant difference for the values between all the pairs of time intervals (P < 0.01).
Table 2.
Intragroup comparison in Group 1 using Friedman’s test
| n | Mean±SD | Minimum | Maximum | Median | Mean rank | χ 2 | P value of Friedman test | |
|---|---|---|---|---|---|---|---|---|
| ISQ baseline | 30 | 25.13533±1.260891 | 23.2200 | 27.1200 | 25.23000 | 1.00 | 87.760 | 0.000** |
| ISQ 1 week | 30 | 50.28700±5.418054 | 40.0000 | 59.2300 | 51.14000 | 2.00 | ||
| ISQ 1 month | 30 | 59.56466±4.510371 | 48.5000 | 67.7300 | 59.67500 | 3.07 | ||
| ISQ 3 months | 30 | 67.78400±6.106217 | 57.9000 | 77.2300 | 69.14000 | 3.93 |
**Statistically highly significant difference (P<0.01). ISQ – Implant stability quotient; n – Number of surgical sites; P – Probability value; SD – Standard deviation; χ2 – Chi Square
Table 3.
Intragroup comparison in Group 2 using Friedman’s test
| n | Mean±SD | Minimum | Maximum | Median | Mean rank | χ 2 | P value of Friedman test | |
|---|---|---|---|---|---|---|---|---|
| ISQ baseline | 30 | 25.05933±2.6112897 | 20.1100 | 29.8700 | 25.3050 | 1.00 | 90.000 | 0.000** |
| ISQ 1 week | 30 | 46.84700±6.4379126 | 34.7000 | 58.7300 | 47.4150 | 2.00 | ||
| ISQ 1 month | 30 | 57.12766±3.8292096 | 52.2300 | 68.2300 | 56.5000 | 3.00 | ||
| ISQ 3 months | 30 | 69.92690±3.5668332 | 63.3500 | 77.1300 | 70.0650 | 4.00 |
**Statistically highly significant difference (P<0.01). ISQ – Implant stability quotient; P – Probability value; SD – Standard deviation; χ2 – Chi Square; n – Number of surgical sites
Table 4.
Pairwise intragroup comparison in Group 1 using Wilcoxon signed-rank test
| Time pairs | Z | P value of Wilcoxon signed-rank test |
|---|---|---|
| ISQ 1 week-ISQ baseline | −4.786 | 0.000** |
| ISQ 1 month-ISQ baseline | −4.786 | 0.000** |
| ISQ 3 month-ISQ baseline | −4.786 | 0.000** |
| ISQ 1 month-ISQ 1 week | −5.150 | 0.000** |
| ISQ 3 months-ISQ 1 week | −5.260 | 0.000** |
| ISQ 3 months-ISQ 1 month | −5.083 | 0.000** |
**Statistically highly significant difference (P<0.01). Z – Z value for nonparametric test utilizing Wilcoxon signed-rank test of pairwise comparison; ISQ – Implant stability quotient; P – Probability value
Table 5.
Pairwise intragroup comparison in Group 2 using Wilcoxon signed-rank test
| Time pairs | Z | P value of Wilcoxon signed-rank test |
|---|---|---|
| ISQ 1 week-ISQ baseline | −4.782 | 0.000** |
| ISQ 1 month-ISQ baseline | −4.782 | 0.000** |
| ISQ 3 month-ISQ baseline | −4.782 | 0.000** |
| ISQ 1 month-ISQ 1 week | −4.810 | 0.000** |
| ISQ 3 months-ISQ 1 week | −4.787 | 0.000** |
| ISQ 3 months-ISQ 1 month | −4.784 | 0.000** |
**Statistically highly significant difference (P<0.01). Z – Z value for nonparametric test utilizing Wilcoxon signed-rank test of pairwise comparison; ISQ – Implant stability quotient; P – Probability value
DISCUSSION
With a concomitant increase in life expectancy and age-related tooth loss, the contemporary world has seen an extreme increase in population with partial and complete edentulism. With drawbacks associated with both removable and tooth-supported prostheses, there has been an increased inclination toward the use of dental implants. Constant researches have been carried out to enhance and fasten the osseointegration process. This allows the clinician to load the implant as soon as possible resolving the functional and esthetic problem of the patient faster. The present study aimed to evaluate the effect of topical application of PRF on osseointegration of dental implants.
Among the 60 implants placed in 21 participants, all of them survived the duration of the study (3 months) which were well osseointegrated. 100% survival rate in the present study could be attributed to various factors that have a direct and indirect effect including careful case selection, which were thoroughly taken care of. Status of bone–implant interface could be assessed precisely using RFA which is relatively noninvasive and more predictable. The results of the present study demonstrated that PRF application increased the stability of implant significantly during 1 week and 1 month of early healing.
Previously growth factors derived from platelets and first-generation platelet concentrate like PRP have been applied and were found to increase osseointegration. In an animal study by Fontana et al., histomorphometric analysis of implants exposed to PRP had a greater amount of peri-implant bone formed than controls.[19] In a study by Quesada-García et al. and Kundu and Rathee, implants were placed in human participants with PRP and concluded that PRP significantly increased the stability of dental implants.[20,21] Anitua et al. found enhanced osseointegration via environmental scanning electron microscopy and histomorphic analysis when the surface of implants was covered with a preparation rich in growth factors.[22] On the one hand, where there were studies that supported, there were studies like the one by Monov et al. and Ergun et al. who found that growth factors rich in platelets have no significant effects on osseointegration.[23,24]
In the present study, there was a highly significant increase in ISQ values in both groups from baseline to 3 months. After preparation of an implant osteotomy site, the purely mechanical engagement of implant into the site provides primary stability. During the healing phase, an eventual remodeling around implant replaces the mechanical stability with secondary or biological stability. The increase in ISQ values can be explained by the process of contact osteogenesis and maturation of peri-implant woven bone to lamellar bone.[25,26] The results of the present study are in agreement with the study by Diana et al. who also found a significant increase in implant stability in both groups test (with PRF) and control over 3 months. However, between groups, no significant difference was found.[27]
On intergroup comparison, a statistically significant difference in ISQ values was found at 1 week and 1 month with high values in Group 1 than Group 2. This difference might be attributed to the fact that PRF accelerates healing and ultimately osseointegration during the early healing period. PRF has a matrix of polymerized fibrin with entrapped platelets, leukocytes in a tetra molecular structure. These platelets are activated by implant surface which locally releases growth factors that enhance wound healing and attract undifferentiated mesenchymal cells to the peri-implant injury site.[28] PRF releases the maximum amount of TGF-β1 at day 14 and PDGF-AB at day 7. Increased alkaline phosphatase activity which enhances proliferation and differentiation of osteoblast has also been seen to peak at day 14. For maturation of bone, deposition of calcium should take place in the extracellular matrix via collagen synthesis by osteoblast. TGF-β1 increases collagen synthesis.[29] PDGF also upregulates collagen synthesis in osteoblast and also expresses an increased chemotactic effect on several connective tissue cells including osteoblast.[30] This explains the significant results in Group 1 at 1 week and 1 month than Group 2 from a nonsignificant baseline ISQ value.
Highly complex interactions between platelets, fibroblast, inflammatory cells, and their products of inflammation form the basis of osseointegration. Hence, the notion of applying PRF to osteotomy sites to promote these processes seems biologically feasible. Mesenchymal stem cell proliferation and differentiation to osteoblast by activating the RunX2 gene have been demonstrated by Li et al.[31] A similar finding of stem cell differentiation to osteoblast by using PRF was obtained by Dohan Ehrenfest et al. and Li et al.[32,33] Stem cells with PRF formed bone with a density similar to that of the spine, which later was confirmed by histological assessments.[34] A study by Fredes et al. concluded that PRF induced bone healing and neossification in patients with a surgical defect in anterior skull base surgery.[35]
Although scientific data are sparse concerning PRF having a direct effect on bone healing around implants in comparison to the established fact that PRF results in less inflammation with improved soft-tissue healing, there is an enormous amount of data that need to be extrapolated. The present study could be one such to highlight the positive effects of PRF on bone healing which could contribute to an educated clinical decision-making.
Oncu E et al. (2015) in their study also found significant mean ISQ values at 1 week and 1 month postoperatively in implants placed with PRF.[36] The results of the present study are in accordance with another study by Oncu E et al. (2019) who also found a significant difference in ISQ values at 1 week and 1 month in implants coated with PRF.[28] This study also had a follow-up of 3 months, but unlike, the present study is a split-mouth design, where much of interindividual variability can be removed, thereby increasing the power of study compared to a whole-mouth design.[37]
Oncu E et al.(2016) conducted a study in an animal model and concluded that the application of PRF increased the rate and amount of new bone formation (BIC) at 2, 3, and 4 weeks.[38] In a split-mouth study, where ISQ was measured in implants with and without PRF exclusively in the posterior maxilla, Tabrizi et al. found that implant stability increased in the test group at 2, 4, and 6 weeks postoperatively. The present study is complementary to the above mentioned studies.[39]
The limitations of the present study include a shorter evaluation period and early follow-up. Unless there are some parameters that can objectively assess bone formation around implants directly attributable to PRF, just measuring the RFA leaves the study a trifle incomplete, considering there is bound to be a surgical clot in both groups. However, the primary aim of the study was to find whether PRF enhanced osseointegration at bone density-matched sites. At present, the most definite and predictable way to assess bone–implant interface is by measuring RFA in terms of ISQ. Another demerit would be a small sample size; a larger sample size would have given a proper representation of population and generalization of results. The present clinical results need to validated by the gold standard method, i.e., using histological evaluation.
CONCLUSIONS
Within the limitations of the study, the results demonstrate that PRF significantly increased implant stability at 1 week and 1 month of osseointegration. This period corresponds to the early healing period. Thus, PRF has a significant effect on the osseointegration of dental implants during the early healing period prior to loading. However, to support these results, further clinical and histological studies are required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zohrabian VM, Sonick M, Hwang D, Abrahams JJ. Dental implants. Semin Ultrasound CT MR. 2015;36:415–26. doi: 10.1053/j.sult.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Buser D, Chen ST, Weber HP, Belser UC. Early implant placement following single-tooth extraction in the esthetic zone: Biologic rationale and surgical procedures. Int J Periodontics Restorative Dent. 2008;28:441–51. [PubMed] [Google Scholar]
- 3.Jayesh RS, Dhinakarsamy V. Osseointegration. J Pharm Bioallied Sci. 2015;7:S226–9. doi: 10.4103/0975-7406.155917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigl P. Implant prosthodontics: What next? Quintessence Int. 2003;34:653–69. [PubMed] [Google Scholar]
- 5.Brånemark R, Ohrnell LO, Nilsson P, Thomsen P. Biomechanical characterization of osseointegration during healing: An experimental in vivo study in the rat. Biomaterials. 1997;18:969–78. doi: 10.1016/s0142-9612(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 6.Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: A systematic review. Clin Oral Implants Res. 2009;20(Suppl 4):172–84. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 8.Sykaras N, Triplett RG, Nunn ME, Iacopino AM, Opperman LA. Effect of recombinant human bone morphogenetic protein-2 on bone regeneration and osseointegration of dental implants. Clin Oral Implants Res. 2001;12:339–49. doi: 10.1034/j.1600-0501.2001.012004339.x. [DOI] [PubMed] [Google Scholar]
- 9.Lynch SE, Buser D, Hernandez RA, Weber HP, Stich H, Fox CH, et al. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants.Results of a pilot study in beagle dogs. J Periodontol. 1991;62:710–6. doi: 10.1902/jop.1991.62.11.710. [DOI] [PubMed] [Google Scholar]
- 10.Lind M. Growth factor stimulation of bone healing.Effects on osteoblasts, osteomies, and implants fixation. Acta Orthop Scand Suppl. 1998;283:2–37. [PubMed] [Google Scholar]
- 11.Stefani CM, Machado MA, Sallum EA, Sallum AW, Toledo S, Nociti H., Jr Platelet-derived growth factor/insulin-like growth factor-1 combination and bone regeneration around implants placed into extraction sockets: A histometric study in dogs. Implant Dent. 2000;9:126–31. doi: 10.1097/00008505-200009020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Stellos K, Kopf S, Paul A, Marquardt JU, Gawaz M, Huard J, et al. Platelets in regeneration. Semin Thromb Hemost. 2010;36:175–84. doi: 10.1055/s-0030-1251502. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Pan S, Dangaria SJ, Gopinathan G, Kolokythas A, Chu S, et al. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed Res Int. 2013;2013:638043. doi: 10.1155/2013/638043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boora P, Rathee M, Bhoria M. Effect of platelet rich fibrin (PRF) on peri-implant soft tissue and crestal bone in one-stage implant placement: A randomized controlled trial. J Clin Diagn Res. 2015;9:C18–21. doi: 10.7860/JCDR/2015/12636.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamad H, Kenway E, Shinanvi U, Salem A, Ahmed F. Efficacy of platelet rich fibrin (PRF) Membrane in immediate dental implant. Manoura J Dent. 2014;1:78–84. [Google Scholar]
- 18.Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998;11:491–501. [PubMed] [Google Scholar]
- 19.Fontana S, Olmedo DG, Linares JA, Guglielmotti MB, Crosa ME. Effect of platelet-rich plasma on the peri-implant bone response: An experimental study. Implant Dent. 2004;13:73–8. doi: 10.1097/01.id.0000116455.68968.29. [DOI] [PubMed] [Google Scholar]
- 20.Quesada-García MP, Prados-Sánchez E, Olmedo-Gaya MV, Muñoz-Soto E, Vallecillo-Capilla M, Bravo M. Dental implant stability is influenced by implant diameter and localization and by the use of plasma rich in growth factors. J Oral Maxillofac Surg. 2012;70:2761–7. doi: 10.1016/j.joms.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Kundu R, Rathee M. Effect of platelet-rich-plasma (PRP) and implant surface topography on implant stability and bone. J Clin Diagn Res. 2014;8:C26–30. doi: 10.7860/JCDR/2014/9177.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anitua E, Orive G, Pla R, Roman P, Serrano V, Andía I. The effects of PRGF on bone regeneration and on titanium implant osseointegration in goats: A histologic and histomorphometric study. J Biomed Mater Res A. 2009;91:158–65. doi: 10.1002/jbm.a.32217. [DOI] [PubMed] [Google Scholar]
- 23.Monov G, Fuerst G, Tepper G, Watzak G, Zechner W, Watzek G. The effect of platelet-rich plasma upon implant stability measured by resonance frequency analysis in the lower anterior mandibles. Clin Oral Implants Res. 2005;16:461–5. doi: 10.1111/j.1600-0501.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 24.Ergun G, Egilmez F, Cekic-Nagas I, Karaca IR, Bozkaya S. Effect of platelet-rich plasma on the outcome of early loaded dental im-plants: A three-year follow-up study. J Oral Implantol. 2013;39:256–63. [Google Scholar]
- 25.Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces.An experimental study in the dog. Clin Oral Implants Res. 2004;15:381–92. doi: 10.1111/j.1600-0501.2004.01082.x. [DOI] [PubMed] [Google Scholar]
- 26.Sadeghi R, Rokn AR, Miremadi A. Comparison of implant stability using resonance frequency analysis: Osteotome versus conventional drilling. J Dent (Tehran) 2015;12:647–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Diana C, Mohanty S, Chaudhary Z, Kumari S, Dabas J, Bodh R. Does platelet-rich fibrin have a role in osseointegration of immediate implants.A randomized, single-blind, controlled clinical trial? Int J Oral Maxillofac Surg. 2018;47:1178–88. doi: 10.1016/j.ijom.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Öncü E, Erbeyoğlu AA. Enhancement of immediate implant stability and recovery using platelet-rich fibrin. Int J Periodontics Restorative Dent. 2019;39:e58–63. doi: 10.11607/prd.2505. [DOI] [PubMed] [Google Scholar]
- 29.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Yosif AM, Al-Hijazi A. Evaluation of the effect of autologous platelet rich fibrin ma-trix on osseointegration of the titanium im-plant: Immunohistochemical evaluation for PDGF-I and IGF-A. J Bagh College Dentistry. 2013;25:70–5. [Google Scholar]
- 31.Li Q, Reed DA, Min L, Gopinathan G, Li S, Dangaria SJ, et al. Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2. Int J Mol Sci. 2014;15:8509–25. doi: 10.3390/ijms15058509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dohan Ehrenfest DM, Doglioli P, de Peppo GM, Del Corso M, Charrier JB. Choukroun's platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch Oral Biol. 2010;55:185–94. doi: 10.1016/j.archoralbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Geng Y, Lu L, Yang T, Zhang M, Zhou Y, et al. Platelet-rich fibrin-induced bone marrow mesenchymal stem cell differentiation into osteoblast-like cells and neural cells. Neural Regen Res. 2011;6:2419–23. [Google Scholar]
- 34.Wang Z, Weng Y, Lu S, Zong C, Qiu J, Liu Y, et al. Osteoblastic mesenchymal stem cell sheet combined with Choukroun platelet-rich fibrin induces bone formation at an ectopic site. J Biomed Mater Res B Appl Biomater. 2015;103:1204–16. doi: 10.1002/jbm.b.33288. [DOI] [PubMed] [Google Scholar]
- 35.Fredes F, Pinto J, Pinto N, Rojas P, Prevedello DM, Carrau RL, et al. Potential effect of leukocyte-platelet-rich fibrin in bone healing of skull base: A pilot study. Int J Otolaryngol. 2017;2017:1231870. doi: 10.1155/2017/1231870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Öncü E, Alaaddinoğlu EE. The effect of platelet-rich fibrin on implant stability. Int J Oral Maxillofac Implants. 2015;30:578–82. doi: 10.11607/jomi.3897. [DOI] [PubMed] [Google Scholar]
- 37.Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split-mouth studies: What statisticians and clinicians should know. Stat Med. 2009;28:3470–82. doi: 10.1002/sim.3634. [DOI] [PubMed] [Google Scholar]
- 38.Öncü E, Bayram B, Kantarci A, Gülsever S, Alaaddinoğlu EE. Positive effect of platelet rich fibrin on osseointegration. Med Oral Patol Oral Cir Bucal. 2016;21:e601–7. doi: 10.4317/medoral.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabrizi R, Arabion H, Karagah T. Does platelet-rich fibrin increase the stability of implants in the posterior of the maxilla.A split-mouth randomized clinical trial? Int J Oral Maxillofac Surg. 2018;47:672–75. doi: 10.1016/j.ijom.2017.07.025. [DOI] [PubMed] [Google Scholar]


