Abstract
Objectives
The aim of this study was to determine the safety and efficacy of same-day discharge (SDD) after transcatheter aortic valve replacement (TAVR) during the COVID-19 pandemic.
Background
The COVID-19 pandemic has placed significant stress on health care systems worldwide. SDD in highly selected TAVR patients can facilitate the provision of essential cardiovascular care while managing competing COVID-19 resource demands.
Methods
Patient selection for SDD was at the discretion of the local multidisciplinary heart team, across 7 international sites. The primary outcome was a composite of cardiovascular death, stroke, myocardial infarction, all-cause readmission, major vascular complications, and new permanent pacemaker (PPM) implantation.
Results
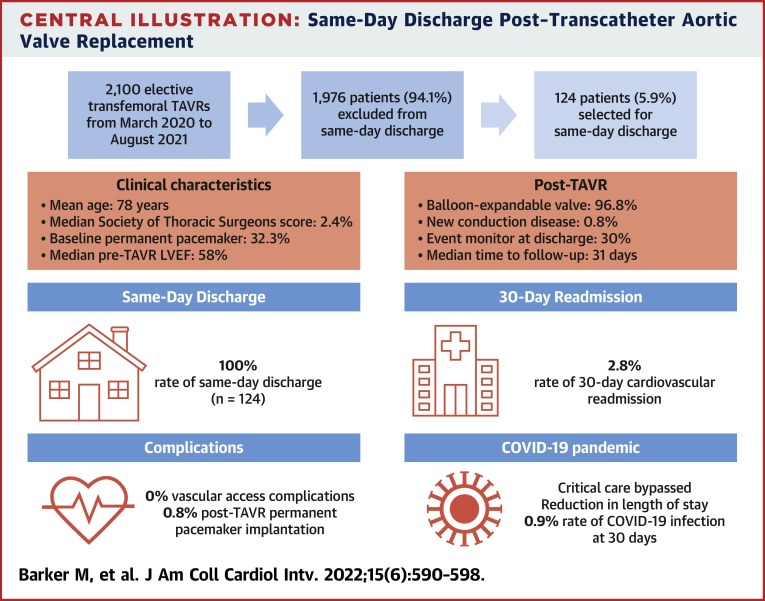
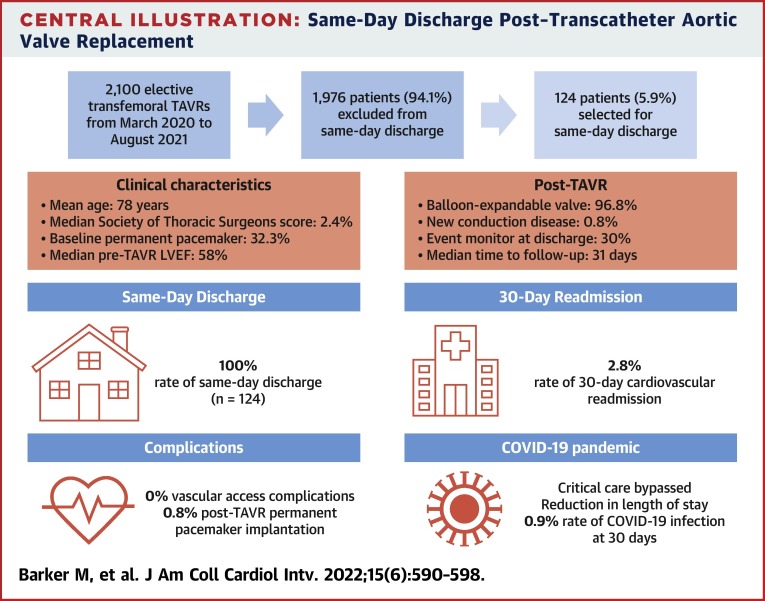
From March 2020 to August 2021, 124 of 2,100 patients who underwent elective transfemoral TAVR were selected for SDD. The average age was 78.9 ± 7.8 years, the median Society of Thoracic Surgeons score was 2.4 (IQR: 1.4-4.2), and 32.3% (n = 40) had preexisting PPMs. There were no major vascular complications, strokes, or deaths during the index admission. One patient (0.8%) required PPM implantation for complete heart block and was discharged the same day. No patient required a PPM between discharge home and 30-day follow-up. The composite of cardiovascular death, stroke, myocardial infarction, all-cause readmission, major vascular complications, and new PPM at 30 days occurred in 5.7% patients (n = 6 of 106).
Conclusions
SDD post-TAVR is safe and feasible in selected patients at low risk for adverse clinical events postdischarge. This strategy may have a potential role in highly selected patients even when the COVID-19 pandemic abates.
Key Words: COVID-19 pandemic, same-day discharge, transcatheter aortic valve replacement
Central Illustration
The COVID-19 pandemic has placed considerable stress on health care systems and has resulted in delays in many cardiovascular procedures, including transcatheter aortic valve replacement (TAVR).1, 2, 3, 4 Cardiovascular societies worldwide have provided guidance to maintain essential structural interventional procedures while minimizing critical care bed use and burden on hospitals.1, 2, 3, 4 Given the high morbidity and mortality associated with delays in treating patients with severe symptomatic aortic stenosis, continued access to structural interventional procedures is essential.5
The safety of next-day discharge (NDD) post-TAVR using a standardized clinical care pathway has been well established through multicenter studies. Knowledge translation programs focused on achieving safe NDD, such as the Edwards Benchmark program, have implemented this approach globally.6 , 7 This strategy consists of team-based quality improvement initiatives aimed at facilitating implementation of best practices for TAVR, from admission to discharge. In particular, clinical care pathways lead to a decreased need for critical care monitoring, rapid reconditioning, and avoidance of in-hospital complications, with excellent safety outcomes.6, 7, 8, 9
Same-day discharge (SDD) post-TAVR in carefully selected patients may allow maintenance of TAVR volumes while preserving necessary hospital resources to address the pandemic as cases wax and wane. Small single-center case series during the COVID-19 pandemic have demonstrated that SDD post-TAVR is feasible in highly selected patients; however, the data remain limited.10, 11, 12, 13
In this international multicenter observational study, we aimed to evaluate the safety and feasibility of SDD in highly selected patients who underwent elective transfemoral TAVR during the COVID-19 pandemic.
Methods
Study design and patient population
This was a multicenter observational study of patients treated at 7 international sites during the COVID-19 pandemic: Vancouver General Hospital (Vancouver, British Columbia, Canada), Emory University Hospital Midtown (Atlanta, Georgia), AMITA Alexian Brothers Medical Center (Elk Grove Village, Illinois), Rochester Regional Health (Rochester, New York), Rutgers Robert Wood Johnson Medical School (New Brunswick, New Jersey), CentraCare Heart and Vascular Center (St. Cloud, Minnesota), Belfast Health and Social Care Trust (Belfast, United Kingdom), and South Tees Hospital NHS Foundation Trust (Middlesbrough, United Kingdom). From March 2020 to August 2021, all patients undergoing elective transfemoral TAVR were evaluated by a multidisciplinary heart team to determine their eligibility for SDD. The decision to pursue SDD was at the discretion of local multidisciplinary heart teams and based on patient selection criteria outlined later. This study was approved by the Institutional Review Board.
SDD clinical care pathway
The validated 3M TAVR clinical care pathway for NDD with a streamlined pre-, peri-, and postprocedural approach has been implemented globally as part of the Edwards Benchmark program (in 15 countries at >100 sites).6 In our study, patient selection for SDD was at the discretion of each site, using selected elements of the previously validated NDD clinical care pathway.6
Patient selection
All patients undergoing elective transfemoral TAVR were potentially eligible for SDD. Preprocedural requirements included adequate social support and either virtual or in-person access to the TAVR program in case of readmission. Both balloon-expandable and self-expanding transcatheter heart valves (THVs) were included at the discretion of individual sites. Patients with preexisting conduction disease, including bundle branch block (BBB) and high-grade atrioventricular (AV) block (second-degree type II and third-degree) were excluded unless they had pre-existing permanent pacemakers (PPMs). Patients with first-degree or second-degree type I AV block were included at the discretion of individual sites. If deemed ineligible for SDD, patients during this time period were considered for NDD or further monitoring per standard institutional practice.
Procedural considerations
Patients were admitted to hospital on the day of their procedures and underwent routine transfemoral TAVR in a cardiac catheterization laboratory or a hybrid operating room. In all eligible SDD patients, procedures were completed before noon. Local anesthesia (2% lidocaine) and minimal procedural sedation (midazolam, fentanyl, propofol, or dexmedetomidine) were administered per local institutional guidelines.
Vascular access
Additional central venous access was avoided unless deemed necessary by the operator. Ultrasound guidance was used for all femoral vascular access. Femoral punctures were routinely preclosed using 2 Perclose ProGlide devices (Abbott Vascular) or per institutional practice at the individual operator’s discretion.
Rapid ventricular pacing
The method of pacing was at the operator’s discretion. Options included using the left ventricular wire or using a 5-F femoral venous pacemaker for temporary pacing. If present, the patient’s own PPM or defibrillator could be used. The temporary pacemaker was removed in the procedure room in most cases if deemed appropriate by the operator. After valve deployment, at Rochester Regional Health, rapid atrial pacing was performed in all patients (except those with atrial fibrillation or flutter or preexisting PPMs) for the development of the Wenckebach phenomenon to assess candidacy for SDD.
Additional procedural considerations
Urinary catheters were avoided if possible. Following the procedure, protamine was administered to achieve a completion activated clotting time of 150 to 200 seconds. Following valve deployment, limited on-table transthoracic echocardiography (TTE) was performed to assess for valve position, left ventricular function, presence of perivalvular leak, gradient across the THV, and presence of a pericardial effusion.
Postprocedure
Post-TAVR, patients bypassed critical care monitoring and were admitted to the cardiac catheterization laboratory recovery unit. Nurse-led mobilization occurred 4 hours after adequate vascular access hemostasis. Electrocardiography was performed immediately postprocedure and at 4 hours to assess for new conduction abnormalities, including intraventricular conduction delay, BBB, or AV block. All patients underwent complete TTE prior to discharge, either on table or in the recovery unit. All patients deemed eligible for SDD on the basis of preprocedural criteria were reviewed by the local multidisciplinary heart team, including the bedside nurse and the valve clinic coordinator. Postprocedural requirements for SDD were left to the discretion of each individual site. The absence of vascular complications and bleeding, defined by the Valve Academic Research Consortium 3 criteria and return to baseline mobilization, was essential.14 The presence of conduction disease as a barrier to SDD and the use of extended event monitors were left up to the individual site’s discretion. Patients who met these criteria were discharged ≥6 hours following successful femoral hemostasis.
Post-TAVR follow-up
Prior to discharge, the follow-up plan and THV clinic information were communicated to both the patient and the family or caregiver. Patients had virtual or in-person follow-up appointments with the THV clinic on postdischarge day 1. Standard follow-up with TTE occurred virtually or in person on postdischarge day 30. If the appointment occurred virtually, TTE was performed by a local referring provider closer to the patient’s residence if the patient resided far from the THV center. Patients were instructed to call the THV clinic if any nonurgent issues arose postdischarge. If there were any urgent issues, they were instructed to return to the emergency department.
Endpoints
The primary endpoint of this study was a composite of cardiovascular death, stroke, myocardial infarction, all-cause readmission, major vascular complications, and new PPM implantation. Secondary outcomes were each component of the primary outcome taken separately, all-cause mortality, and COVID-19 infection and hospitalization within 30 days post-TAVR.
Statistical analysis
The primary analysis of this study was conducted in a descriptive manner. The mean ± SD (or median and IQR as appropriate) was reported for all continuous characteristics. Binary and categorical variables were summarized as frequency (percentage). Data management and statistical analysis were conducted using SAS (SAS Institute).
Results
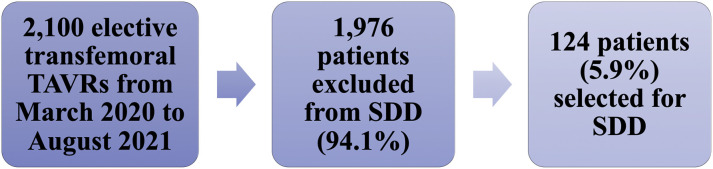
From March 2020 to August 2021, a total of 2,100 patients underwent elective transfemoral TAVR at 7 international sites during the COVID-19 pandemic. Of these, 124 patients (5.9%) underwent successful transfemoral TAVR prior to noon and were selected for SDD (Figure 1 ). Specific reasons for exclusion from SDD for the remaining 1,976 patients were not recorded given the unpredictable and challenging times during which this study was conducted.
Figure 1.
Consolidated Standards of Reporting Trials Diagram
Patient selection for same-day discharge. SDD = same-day discharge; TAVR = transcatheter aortic valve replacement.
Baseline characteristics
Baseline characteristics are summarized in Table 1 . The average age was 78.9 ± 7.8 years, 29% were women (n = 36), and the mean body mass index was 29.1 ± 5.4 kg/m2. The median Society of Thoracic Surgeons score was 2.4 (IQR: 1.4-4.2), and 32.3% of patients had preexisting PPMs (n = 40). All included patients with preexisting high-grade AV block and complete right BBB or left BBB (LBBB) had preexisting PPMs.
Table 1.
Baseline Characteristics (N = 124)
| Age, y | 78.9 ± 7.8 |
| Female | 36 (29.0) |
| CKD (eGFR <60 mL/min/1.73 m2) | 35 (28.2) |
| Diabetes | 42 (33.9) |
| Hypertension | 103 (83.1) |
| BMI, kg/m2 | 29.1 ± 5.4 |
| Smoking | 50 (40.3) |
| Former | 41 (82.0) |
| Current | 9 (18.0) |
| Prior stroke/TIA | 19 (15.3) |
| Peripheral arterial disease | 19 (15.3) |
| Coronary artery disease | 76 (61.3) |
| Atrial fibrillation | 38 (30.7) |
| Prior PCI | 39 (31.5) |
| Prior CABG | 30 (24.2) |
| Prior AVR | 8 (6.5) |
| STS PROM | 2.40 (1.40-4.22) |
| NYHA functional class >II | 75 (60.5) |
| CCS class >II | 32 (28.8) |
| Preexisting PPM or ICD | 40 (32.3) |
| Complete RBBB | 3/34 (8.8) |
| Complete LBBB | 4/34 (11.8) |
| High-grade AVB (second-degree type II and above) | 5/12 (41.7) |
| Albumin, g/L (n = 25) | 43.6 ± 3.7 |
| Pre-TAVR EF, % | 58.0 (55.0-60.0) |
| Bicuspid valve | 10 (8.1) |
| Mean AV gradient | 42.0 (33.0-51.0) |
| AV gradient (n = 77) | 0.40 ± 0.12 |
| AR > moderate | 5/75 (6.7) |
Values are mean ± SD, n (%), median (IQR), or n/N (%).
AR = aortic regurgitation; AV = aortic valve; AVB = atrioventricular block; AVR = aortic valve replacement; BMI = body mass index; CABG = coronary artery bypass grafting; CCS = Canadian Cardiovascular Society; CKD = chronic kidney disease; EF = ejection fraction; eGFR = estimated glomerular filtration rate; ICD = implantable cardioverter-defibrillator; LBBB = left bundle branch block; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; PPM = permanent pacemaker; RBBB = right bundle branch block; STS PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR = transcatheter aortic valve replacement; TIA = transient ischemic attack.
Procedural data
Procedural data are summarized in Table 2 . Overall, 96.8% of patients (n = 120) received balloon-expandable valves. No patients received general anesthesia. The majority of patients received both local anesthesia and minimal procedural sedation (n = 100 [80.7%]), while the remainder received only local anesthesia (n = 24 [19.3%]). All patients underwent complete TTE prior to discharge. Thirty-seven patients (29.8%) were discharged home with prolonged rhythm monitoring (implantable loop recorder or event monitor) (Table 2). The pattern of practice varied across sites included in the study. At Rochester Regional Health and Rutgers Robert Wood Johnson Medical School, all patients were discharged with prolonged rhythm monitoring. The median time from initial puncture to predischarge electrocardiography was 6.35 hours (Table 2).
Table 2.
Procedural Characteristics (N = 124)
| Conscious sedation and local anesthesia | 100 (80.7) |
| Local anesthesia only | 24 (19.3) |
| Pacing through the LV wire | 26 (21.0) |
| Contrast used, mL | 50.0 (25.0-75.0) |
| Total procedural time, min) (n = 94) | 71.0 (44.0-83.0) |
| Total radiation dose, cGy (n = 101) | 361.0 (184.0-664.0) |
| Fluoroscopy time, min (n = 100) | 12.4 (9.2-16.4) |
| Same-day TTE | 124 (100) |
| AV mean gradient on TTE | 5.0 (3.5-7.0) |
| Type of valve | |
| Balloon expandable | 120 (96.8) |
| Self-expandable | 4 (3.2) |
| Event monitor | 37 (29.8) |
| Time from initial puncture to predischarge ECG, h (n = 61) | 6.35 (5.75-7.37) |
Values are n (%) or median (IQR).
AV = aortic valve; ECG = electrocardiography; LV = left ventricular; TTE = transthoracic echocardiography.
Patient outcomes
No major vascular complications, strokes, or deaths occurred during the index admission (Table 3 ). One patient (0.8%) required PPM implantation for postprocedural complete heart block after receiving a self-expanding valve; however, that patient was still discharged the same day. Otherwise, there were no new conduction abnormalities post-TAVR, including LBBB, right BBB, or new AV block (Table 3). No patients required PPMs between discharge home and 30-day follow-up. All patients had either virtual or in-person follow-up visits on postdischarge day 1. The median time to routine follow-up was 31 days (IQR: 24-41 days) (Table 3).
Table 3.
Patient Outcomes
| Periprocedural outcomes (index admission) | (n = 124) |
| Procedural success | 124 (100) |
| Same-day discharge | 124 (100) |
| Death | 0 (0) |
| Stroke/TIA | 0 (0) |
| New PPM implantation | 1 (0.8) |
| Major vascular complications | 0 (0) |
| New complete LBBB | 0 (0) |
| New complete RBBB | 0 (0) |
| New high-grade AVB (second-degree type II and above) | 1 (0.8) |
| Postdischarge follow-up | (n = 106) |
| Time follow-up, d | 31.0 (24.0-41.0) |
| NYHA functional class >II | 3 (2.8) |
| CCS class >II | 1/67 (1.5) |
| Post-TAVR EF, % | 60.0 (55.0-60.0) |
| AV mean gradient | 8.0 (6.0-10.0) |
| 30-d outcomes | (n = 106) |
| Composite outcome | 6 (5.7) |
| Death from all causes | 1 (0.9) |
| Cardiovascular death | 0 (0.0) |
| Stroke/TIA | 1 (0.9) |
| All-cause readmission | 6 (5.7) |
| Cardiovascular readmission | 3 (2.8) |
| Major vascular complications | 0 (0) |
| New PPM implantation | 0 (0) |
| Myocardial infarction | 0 (0) |
| COVID-19 infection | 1 (0.9) |
Values are n (%), median (IQR), or n/N (%).
Abbreviations as in Table 1.
The composite of cardiovascular death, stroke, myocardial infarction, all-cause readmission, major vascular complications, and new PPM at 30 days occurred in 5.7% of patients (n = 6 of 106) (Table 3). There were no cardiovascular deaths at 30 days; however, 1 patient (0.9%) was readmitted with a spontaneous subarachnoid hemorrhage on anticoagulation for atrial fibrillation, resulting in death postcraniotomy (Table 3). The rate of all-cause readmission at 30 days was 5.7% (n = 6 of 106), with a cardiovascular readmission rate of 2.8% (n = 3 of 106) (Table 3). Reasons for cardiovascular readmission included heart failure requiring intravenous diuretic agents, chest pressure and dizziness with no clear cause identified, and transient neurologic symptoms with spontaneous recovery, consistent with a transient ischemic attack. The additional 3 admissions were for reasons unrelated to recent TAVR: spontaneous subarachnoid hemorrhage, epistaxis following a routine COVID-19 nasopharyngeal swab, and anxiety. One patient (0.9%) contracted COVID-19 within 30 days post-TAVR (Table 3).
Discussion
This multicenter observational study demonstrates the safety of SDD post-TAVR in carefully selected patients (Central Illustration ). Only 1 patient required PPM implantation for complete heart block, and this was done during the index hospitalization without delaying discharge. Importantly, no patient required a PPM after index admission up to 30 days of follow-up. Furthermore, there were no major vascular access complications or cardiovascular mortality at 30 days. There was a low rate of 30-day cardiovascular readmission (2.8% [3 of 106]).
Central Illustration.
Same-Day Discharge Post–Transcatheter Aortic Valve Replacement
The main findings of this study are highlighted, including patient selection, clinical characteristics, and patient outcomes. LVEF = left ventricular ejection fraction; TAVR = transcatheter aortic valve replacement.
This is the first multicenter study to investigate the safety of SDD post-TAVR. Prior single-center case series have demonstrated the feasibility of SDD post-TAVR in highly selected patients, with a low rate of complications postdischarge.10, 11, 12, 13 In contrast, this multicenter study spanned 3 countries: Canada, the United States, and the United Kingdom. Patient selection was left at the discretion of each multidisciplinary heart team. We demonstrate a low rate of complications at 30 days with a high follow-up rate (106 of 124 [85%]), which is comparable with those observed in studies investigating the safety of NDD. The 3M TAVR and FAST-TAVI (Feasibility and Safety of Early Discharge After Transfemoral Transcatheter Aortic Valve Implantation) studies had rates of 5.7% and 4.3% for PPM implantation post-TAVR, respectively, and rates of 5.7% and 3.7% for cardiovascular readmission at 30 days, compared with a 0.8% PPM rate and a 2.8% cardiovascular readmission rate in this cohort.6 , 7 Although these studies were larger, with more lenient inclusion criteria, the safety observed with SDD in our study is encouraging.
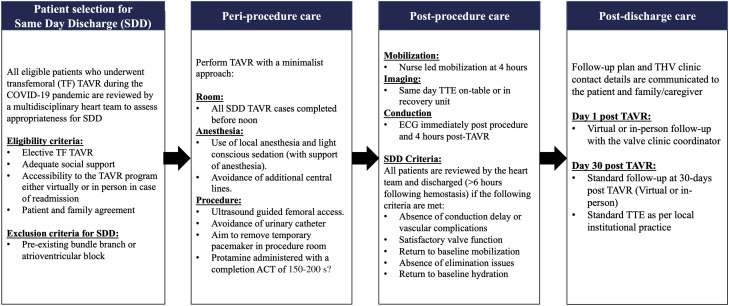
The centers included in this study were experienced, with a minimalist NDD approach to TAVR, and therefore had established clinical pathways that were adaptable to SDD. The use of a streamlined pre-, peri-, and postprocedural clinical pathway for NDD post-TAVR in the 3M TAVR study demonstrated excellent safety and efficacy outcomes at low-, medium-, and high-volume TAVR centers.6 Combining the approaches used by all 7 international sites in this study, we propose a streamlined SDD clinical pathway to facilitate outpatient TAVR (Figure 2 ). Patients with adequate social support and access to the THV clinic as determined by the local multidisciplinary heart team would be eligible for SDD. Preprocedural counseling by both the nursing and medical teams and early involvement of patient family members and caregivers in preparation for expedited discharge was essential for implementation of SDD. As postoperative conduction abnormalities remain a concern for morbidity, patients with preexisting conduction disease should be excluded from SDD.15 This SDD pathway provides the framework to allow minimalist TAVR to continue while preserving essential hospital resources during the COVID-19 pandemic and reducing the risk for nosocomial coronavirus infection by decreasing the length of stay. It allows rapid patient mobilization and bypass of crucial critical care or telemetry ward monitoring post-TAVR. Broader applications of adjunctive telehealth options including virtual visits and continuous cardiac monitoring catalyzed by the pandemic could further enhance this pathway. Given the encouraging safety outcomes of this study, SDD may have an ongoing role in highly selected patients as the COVID-19 pandemic abates.
Figure 2.
SDD Clinical Pathway to Facilitate Outpatient TAVR
Proposed standardized clinical care pathway for SDD, including eligibility criteria and periprocedural, postprocedural, and postdischarge care. ACT = activated clotting time; ECG = electrocardiography; TF = transfemoral; THV = transcatheter heart valve; TTE = transthoracic echocardiography; other abbreviations as in Figure 1.
Postoperative conduction disease, including high-grade AV block and BBB, remains a concern for morbidity. The presence of pre-existing conduction disease increases the risk for postoperative electric complications.15 , 16 Balloon-expandable THVs have been shown to have a lower incidence of delayed conduction abnormalities at 1 to 2 weeks post-TAVR compared with self-expanding THVs.17 , 18 Our selected patient population had an overall low risk for conduction disease, as none had preexisting BBB, 32.3% had preexisting PPMs, and 96.8% received balloon-expandable THVs. In this study, we demonstrate that SDD in patients without pre-existing high-risk conduction disease is safe, as none required delayed PPM implantation after index hospitalization or was admitted with conduction-related issues at 30 days. The role of ambulatory continuous cardiac monitoring has been previously investigated in patients with new-onset persistent LBBB post-TAVR.16 In the MARE (Ambulatory Electrocardiographic Monitoring for the Detection of High-Degree Atrio-Ventricular Block in Patients With New-Onset Persistent Left Bundle Branch Block After Transcatheter Aortic Valve Implantation) study, a high incidence of arrhythmic events was observed at 1 year, including bradyarrhythmias in one-fifth of patients.16 In our study, although there were no patients with new-onset persistent LBBB, 29.8% of patients (n = 37) had implantable loop recorders or event monitors. The data provided by the MARE study could be extrapolated to increase the safety of SDD in low-risk patients with borderline conduction disease, such as new first-degree AV block or intraventricular conduction delay.16 However, given the overall low frequency of conduction abnormalities demonstrated in this selected group of patients, extended rhythm monitoring may be necessary only in the setting of high-risk features postoperatively.
Study limitations
Patients included in this study were deemed at low risk for vascular complications and postoperative conduction abnormalities and were carefully selected for SDD by a multidisciplinary heart team, so the findings should not be generalized to the broader transfemoral TAVR population. The majority of patients received balloon-expandable THVs, so the findings should not be generalized to patients receiving self-expanding valves, as there is a higher risk for delayed conduction abnormalities. As this study was conducted during the COVID-19 pandemic, with significant resource limitations and time constraints associated with data collection, the reasons for exclusion from SDD and a control group (NDD) were not included in the study. A study using a matched control group with the proposed standardized pathway from this study would further elucidate the role of SDD post-TAVR as the pandemic abates.
Conclusions
This multicenter study demonstrates the safety of SDD post-TAVR in highly selected patients at low risk for postoperative conduction disease and vascular complications. This strategy may have a potential role in highly selected patients even when the COVID-19 pandemic abates.
Perspectives.
WHAT IS KNOWN? The safety of NDD post-TAVR has been well established through multicenter studies, and the practice has been implemented globally as part of the Edwards Benchmark program.
WHAT IS NEW? SDD post-TAVR in patients deemed at low risk for vascular complications and postoperative conduction abnormalities is safe and was an effective strategy to allow maintenance of TAVR volumes while preserving necessary hospital resources during the COVID-19 pandemic.
WHAT IS NEXT? Using the proposed SDD clinical pathway, this strategy may have a potential role in highly selected patients even when the COVID-19 pandemic abates.
Funding Support and Author Disclosures
Dr Sathananthan is a consultant to Edwards Lifesciences and Medtronic; and has received speaker fees from Edwards Lifesciences and NVT. Dr Devireddy has received consulting fees from Edwards Lifesciences, Medtronic, ReCor Medical, and Shockwave Medical. Ms Keegan is a consultant for Edwards Lifesciences, Medtronic, and Abbott Vascular. Dr Grubb is a speaker, proctor, and principal investigator for Edwards Lifesciences and Medtronic; and receives grants and educational funding to her employer from Edwards Lifesciences and Medtronic. Dr Spence has received consulting fees and/or institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr Muir is a proctor for Edwards Lifesciences and Abbott Vascular; and has received departmental grants from Edwards Lifesciences and Abbott Vascular. Dr Russo is a speaker, proctor, and principal investigator for Edwards Lifesciences; and receives research funding to his employer from Edwards Lifesciences. Dr Pineda-Salazar receives research funding to her employer from Edwards Lifesciences. Ms Smith has received research funding to her employer from Edwards Lifesciences. Dr Dahle is a speaker, proctor, and principal investigator for Edwards Lifesciences and Medtronic. Dr Meier is supported by the Swiss National Science Foundation (grant P2LAP3_199561). Dr Akodad has received research funding from Medtronic, Biotronik, and Fédération Française de Cardiologie. Dr Nestelberger has received research support from the Swiss National Science Foundation (P400PM_191037/1), the Swiss Heart Foundation (FF20079), the Prof Dr Max Cloëtta Foundation, Margarete und Walter Lichtenstein-Stiftung (3MS1038), the University of Basel, and the University Hospital Basel; and has received speaker honoraria and consulting honoraria from Siemens, Beckman Coulter, Bayer, Ortho Clinical Diagnostics, and Orion. Dr Lauck is a consultant to Edwards Lifesciences. Dr Webb is a consultant to and receives unrestricted grant support from Medtronic, Edwards Lifesciences, and Abbott Vascular. Dr Wood has received consulting fees and/or institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Wood D.A., Sathananthan J., Gin K., et al. Precautions and procedures for coronary and structural cardiac interventions during the COVID-19 pandemic: guidance from Canadian Association of Interventional Cardiology. Can J Cardiol. 2020;36:780–783. doi: 10.1016/j.cjca.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood D.A., Mahmud E., Thourani V.H., et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: from the North American society leadership. Can J Cardiol. 2020;36:971–976. doi: 10.1016/j.cjca.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood D.A., Mahmud E., Thourani V.H., et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: from the North American society leadership. J Am Coll Cardiol. 2020;75:3177–3183. doi: 10.1016/j.jacc.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreini D, Arbelo E, Barbato E, et al. ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Eur Heart J. 2022 In press. [DOI] [PMC free article] [PubMed]
- 5.Malaisrie S.C., McDonald E., Kruse J., et al. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98:1564–1571. doi: 10.1016/j.athoracsur.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Wood D.A., Lauck S.B., Cairns J.A., et al. The Vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. J Am Coll Cardiol Intv. 2019;12:459–469. doi: 10.1016/j.jcin.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Barbanti M., van Mourik M.S., Spence M.S., et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. 2019;15:147–154. doi: 10.4244/EIJ-D-18-01197. [DOI] [PubMed] [Google Scholar]
- 8.Ichibori Y., Li J., Davis A., et al. Feasibility and safety of adopting next-day discharge as first-line option after transfemoral transcatheter aortic valve replacement. J Invasive Cardiol. 2019;31:64–72. doi: 10.25270/jic/18.00300. [DOI] [PubMed] [Google Scholar]
- 9.Kamioka N., Wells J., Keegan P., et al. Predictors and clinical outcomes of next-day discharge after minimalist transfemoral transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2018;11:107–115. doi: 10.1016/j.jcin.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Perdoncin E., Greenbaum A.B., Grubb K.J., et al. Safety of same-day discharge after uncomplicated, minimalist transcatheter aortic valve replacement in the COVID-19 era. Catheter Cardiovasc Interv. 2021;97:940–947. doi: 10.1002/ccd.29453. [DOI] [PubMed] [Google Scholar]
- 11.Rai D., Waqas Tahir M., Chowdhury M., et al. Transcatheter aortic valve replacement same-day discharge for selected patients: a case series. Eur Heart J Case Rep. 2021;5(2):ytaa556. doi: 10.1093/ehjcr/ytaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo M.J., Okoh A.K., Stump K., et al. Safety and feasibility of same day discharge after transcatheter aortic valve replacement post COVID-19. Structural Heart. 2021;5(2):182–185. doi: 10.1080/24748706.2020.1853861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pop A.M., Barker M., Hickman L., et al. Same day discharge during the COVID-19 pandemic in highly selected transcatheter aortic valve replacement patients. Structural Heart. 2021;5(6):596–604. doi: 10.1080/24748706.2021.1988780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genereux P., Piazza N., Alu M.C., et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77:2717–2746. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Rodes-Cabau J., Ellenbogen K.A., Krahn A.D., et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. 2019;74:1086–1106. doi: 10.1016/j.jacc.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Rodes-Cabau J., Urena M., Nombela-Franco L., et al. Arrhythmic burden as determined by ambulatory continuous cardiac monitoring in patients with new-onset persistent left bundle branch block following transcatheter aortic valve replacement: the MARE study. J Am Coll Cardiol Intv. 2018;11:1495–1505. doi: 10.1016/j.jcin.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Sathananthan J., Ding L., Yu M., et al. Implications of transcatheter heart valve selection on early and late pacemaker rate and on length of stay. Can J Cardiol. 2018;34:1165–1173. doi: 10.1016/j.cjca.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Vlastra W., Chandrasekhar J., Muñoz-Garcia A.J., et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation: from the CENTER-collaboration. Eur Heart J. 2019;40:456–465. doi: 10.1093/eurheartj/ehy805. [DOI] [PubMed] [Google Scholar]