Abstract
Introduction
The rapid rollout of coronavirus disease 2019 (COVID-19) vaccines for a large proportion of the population necessitates a strong emphasis on safety. Complementary to the existing spontaneous reporting system, The Netherlands Pharmacovigilance Centre Lareb conducted patient-reported cohort event monitoring (CEM).
Objective
The primary aim was to investigate differences in the frequencies of any and commonly reported, ‘well-known’, systemic adverse events following immunization (AEFIs) with four COVID-19 vaccines (Pfizer’s Comirnaty®, Moderna’s Spikevax®, AstraZeneca’s Vaxzevria® and the Janssen vaccine). As a secondary aim, we analyzed the frequencies of well-known systemic adverse events after the first and, if applicable, second COVID-19 vaccinations, taking into account age, sex and prior COVID-19 infection.
Methods
Patient-reported outcomes (PROs) in the Netherlands starting in February 2021 were analyzed using a prospective cohort design.
Results
Data of 27,554 participants who received one vaccination and 20,682 participants who received complete immunization were analyzed. The percentage of patients reporting any AEFI was high and ranged from approximately 53% for the Pfizer vaccine to approximately 94% for the Moderna vaccine. The frequency of serious AEFIs was low, with the highest frequency found for the AstraZeneca vaccine (0.228%). AEFIs were most often experienced by participants receiving the first dose of the AstraZeneca and Janssen vaccines and the second dose of the Moderna vaccine; the Pfizer vaccine was associated with the lowest rate of AEFIs. Participants with a COVID-19 history before vaccination experienced commonly reported systemic AEFIs more frequently after the first vaccination than after the second vaccination. Women and young people experienced more AEFIs than men and older people, respectively.
Conclusions
The analysis of a large cohort provides important information about the rates of AEFIs across age groups, among brands of vaccines and between those with and without prior COVID-19 infection. Participants reported a high number of AEFIs in general, but the frequency of serious AEFIs was low.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-022-01151-w.
Key Points
| Near real-time safety surveillance of COVID-19 vaccines during vaccine rollout is necessary. |
| A web-based prospective cohort analysis of patient-reported outcomes is useful for vaccine surveillance. |
| Well-known systemic adverse events following immunization (AEFIs) were most often experienced by participants receiving the first dose of the AstraZeneca vaccine and the Janssen vaccine and the second dose of the Moderna vaccine. |
| The frequency of serious AEFIs was low (approximately 0.228% for the first dose of the AstraZeneca vaccine). |
| Participants with a history of COVID-19 infection, women and younger people experienced at least one well-known systemic AEFI more often than their counterparts. |
Introduction
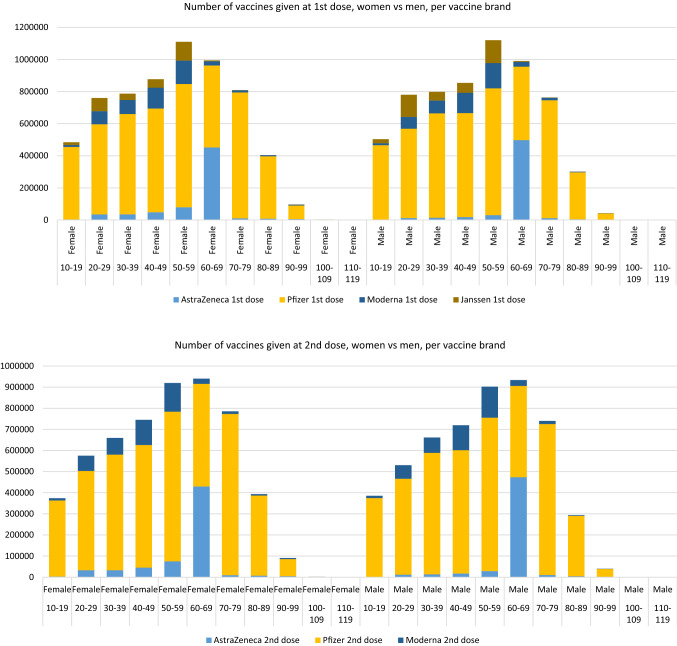
In 2020, the world was shocked by the rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and concerned about the high morbidity and mortality rates after infection [1, 2]. This necessitated the rapid development of vaccines to contain the spread of this virus. At the end of 2020, the first vaccines were granted preliminary approval by the European Medicines Agency (EMA). Starting in January 2021, a large-scale vaccination program was rolled out in the Netherlands. At present, this vaccination program includes four vaccines: two based on viral vectors (AstraZeneca’s Vaxzevria® [3] and the Janssen vaccine [4]) and two with an mRNA basis (Pfizer’s Comirnaty® [5] and Moderna’s SpikeVax® [6]). The number of vaccines given in the Netherlands, stratified per dose, age and sex, is shown in Fig. 1. Detailed information on vaccines given is provided in Electronic Supplementary Material (ESM) 1. The rapid rollout of vaccines for a large proportion of the population necessitated a strong emphasis on safety. Complementary to the existing spontaneous reporting system, The Netherlands Pharmacovigilance Centre Lareb conducted patient-reported cohort event monitoring (CEM). It is considered complementary because it is better suited to capture comprehensive safety data over time and the impacts of more frequent adverse events. Moreover, in contrast to the spontaneously reported data, the denominator of the studied cohort is known, so adverse event frequencies can be calculated. Lareb developed Lareb Intensive Monitoring (LIM), a web-based tool for collecting patient-reported outcomes during the H1N1 pandemic in 2009/2010 [7]. The LIM system is used annually for the monitoring of influenza and, more recently, pneumococcal vaccine data [8].
Fig. 1.
Number of vaccines given at first and second dose, stratified for women and men, per vaccine brand. Vaccination data from the COVID-19 vaccination Information and Monitoring System (CIMS) until 07-10-2011. Data provided by the National Institute for Public Health and the Environment (RIVM)
The aim of this study was to investigate differences in the frequency of systemic adverse events following immunization (AEFIs) for four COVID-19 vaccines (Pfizer’s Comirnaty®, Moderna’s Spikevax®, AstraZeneca’s Vaxzevria® and the Jansen vaccine). As a secondary aim, we focused on the frequency of well-known systemic adverse events after the first and, if applicable, second COVID-19 vaccination, taking into account age, sex and prior COVID-19 infection.
Methods
Setting and Study Population
For data collection, patient-reported outcomes were captured in LIM, a CEM system. This study utilized a web-based prospective cohort design to analyze patient-reported outcomes (PROs). Data collection started in February 2021. All Dutch residents older than 16 years of age who were vaccinated with a COVID-19 vaccine under the Dutch immunization program during the study period from February to 16 August 2021 were eligible to participate in this study. Eligible participants were included if they provided informed consent and had access to the internet.
Data Collection
Participants were invited to participate in this study through flyers at vaccination sites, supported by posters and media attention. Data were collected by means of online questionnaires, which were also usable in a (LIM) web app system [9]. Participants were able to register after they had received an invitation for participation no more than 2 days after being vaccinated with the first dose.
After registration, participants completed the baseline questionnaire, which contained questions about participant characteristics (age, sex, height, weight), date of vaccination, brand of vaccine, comorbidities, concomitant medications, use of any antipyretic drugs several hours before or after vaccination, and history of COVID-19. Throughout the entire study, participants receive a total of six online questionnaires about events attributed to COVID-19 vaccination over a period of 6 months. We included data from five questionnaire timepoints after the baseline questionnaire. This corresponds with a period of 3 months after the first vaccination. The timing of the second vaccination varied for vaccine brands in the Netherlands; For the Pfizer vaccine, a vaccination interval of 6 weeks was used between the first and second vaccination, for Moderna 4 weeks and for AstraZeneca 12 weeks [10]. For those who received a second dose, the date of the second vaccination and brand of vaccine was obtained. In the current analysis, data from the first questionnaire, which was sent 7 days after the vaccination date, and data after the second vaccination, if applicable, were analyzed. We asked about events attributed to vaccination, and referred to reported reactions as AEFIs. An event attributed to vaccination was defined as any atypical medical event that followed immunization and does not necessarily have a causal relationship with the use of the vaccine. Adverse events were defined as any unfavorable or unintended sign, abnormal laboratory finding, symptom or disease [11]. The core data elements from the questionnaire can be found in ESM 2.
Reported Adverse Events Following Immunization (AEFIs)
In the questionnaires used in the study, there were a number of closed questions to ascertain whether well-known AEFIs (local reactions and well-known systemic AEFIs) were experienced. In addition to these closed questions, an open question with a free-text option to specify any other AEFI was available. All reported AEFIs were coded using Medical Dictionary for Regulatory Activities (MedDRA®) terminology 23.0 and 24.0 [12]. The seriousness of the AEFI was coded according to the Council for International Organizations of Medical Sciences (CIOMS) criteria; AEFIs resulting in death, or which are life-threatening; require inpatient hospitalization or prolongation of existing hospitalization; result in persistent or significant disability or incapacity; result in a congenital anomalies; or other [13]. We coded adverse events of special interest (AESIs) according to a listing provided by the Brighton Collaboration [14].
Reported Well-Known Systemic AEFIs
In this study, we focused on differences in the frequencies of the following well-known systemic AEFIs as reported by patients: arthralgia, myalgia, nausea, pyrexia, headache, malaise, chills, fatigue, and fever (body temperature 38 °C or higher).
History of COVID-19 Infection
Participants were asked whether they had previously had COVID-19. The four answer options were ‘Yes, confirmed with a positive polymerase chain reaction (PCR) test,’ ‘Yes, not confirmed with a test’, ‘Most likely, not confirmed with a test’ and ‘No’. For previous COVID-19 infection, we took into account only those with confirmed test results. Those who answered ‘yes, not confirmed with a test’ and ‘most likely, not confirmed with a test’ were excluded from this analysis.
Statistical Analysis
We used descriptive statistics to describe the characteristics of the total study group and the group of participants with complete vaccination data, including numbers of participants, age group distributions, comorbidities, percentages of participants experiencing (serious) AEFIs (totals and after first and second dose) and numbers of participants experiencing serious AEFIs.
To see if there was a difference between participants who registered on the day of vaccination and those who registered later, up to 2 days after being vaccinated with the first dose, we performed a sensitivity analysis.
For the participants with complete vaccination data, a heatmap of the percentage of participants who reported at least one well-known systemic AEFI was generated using the ggplot 2 3.3.3 package in R version 4.0.3; the data were stratified by age group and sex. The same strata were used to calculate the percentages of participants with a reported body temperature of 38.0 °C or higher. Other R packages used were Reshape2 version 1.4, Tidyr version 1.1.2, and Dplyr version 1.0.2, as well as Adobe Illustrator cc 2019.
Results
Figure 2 shows the characteristics of the participants in the study in a flow-chart. In total, 27,594 participants were initially enrolled. For 27,554 participants, data after vaccination were available. For 20,682 participants, data after a complete immunization were available, meaning information about the first and second doses of the AstraZeneca, Pfizer or Moderna vaccines or the single dose of the Janssen vaccine was available.
Fig. 2.
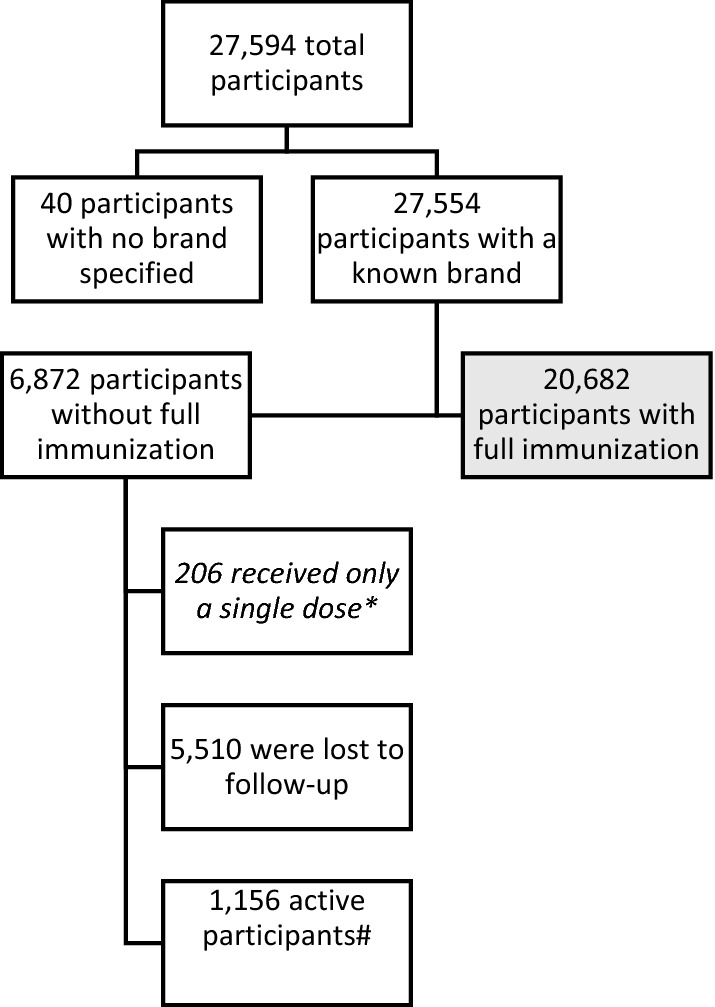
Flowchart of the number of participants in the study. *Only a single dose of the AstraZeneca, Pfizer or Moderna vaccine was administered to patients, possibly due to the protocol in the Netherlands for patients with previous COVID-19 infection [13] or the decision to not receive a second dose. #Active participants had not received a second dose at the time of data extraction but were still eligible to receive a second vaccine and continue in the study
Table 1 shows the descriptive overview of the total study group by vaccine brand. Table 2 shows a descriptive overview of participants who received complete vaccination by vaccine brand; these data are stratified by age, sex and COVID-19 history before vaccination in Figs. 2, 3 and 4, respectively. Table 3 shows the descriptive overview for participants who registered on the day of vaccination.
Table 1.
Descriptive characteristics by vaccine brand
| Vaccine | AstraZeneca | Janssen | Moderna | Pfizer |
|---|---|---|---|---|
| N. participants receiving at least one dose | 8782 | 2458 | 3426 | 12,888 |
| N. participants receiving 2nd dose | 5556 | NA | 2441 | 10,227 |
| Men | 1282 (14.6) | 699 (28.4) | 1154 (33.7) | 7487 (58.1) |
| Women | 7500 (85.4) | 1759 (71.6) | 2272 (66.3) | 5401 (41.9) |
| Age group 12–20 years | 139 (1.6) | 48 (2) | 42 (1.2) | 140 (1.1) |
| Age group 21–65 years | 8538 (97.2) | 2402 (97.7) | 3317 (96.8) | 3270 (25.4) |
| Age group 66–80 years | 96 (1.1) | 8 (0.3) | 67 (2) | 6586 (51.1) |
| Age group > 80 years | 9 (0.1) | 0% | 0% | 2892 (22.4) |
| Reported at least 1 AEFI after dose 1 | 8100 (92.2) | 2015 (82.0) | 2799 (81.7) | 5819 (45.2) |
| Reported at least 1 AEFI after dose 2 | 2691 (48.4) | NA | 2056 (85.2) | 3599 (35.4) |
| Reported at least 1 serious AEFI after dose 1 | 20 (0.228) | 2 (0.081) | 3 (0.088) | 11 (0.085) |
| Reported at least 1 serious AEFI after dose 2 | 0 | NA | 2 (0.083) | 6 (0.059) |
Data reported as n (%)
AEFI adverse events following immunization, NA not applicable
Table 2.
Characteristics of the participants with complete immunization—corresponds to the heatmaps in Figs. 3, 4 and 5
| Vaccine | AstraZeneca | Janssen | Moderna | Pfizer |
|---|---|---|---|---|
| N. participants with complete immunization* | 5556 | 2458 | 2441 | 10227 |
| Age distribution | ||||
| 0–29 years | 570 (10.26) | 390 (15.87) | 197 (8.07) | 176 (1.72) |
| 30–39 years | 773 (13.91) | 383 (15.58) | 413 (16.92) | 404 (3.95) |
| 40–49 years | 1054 (18.97) | 581 (23.64) | 759 (31.09) | 418 (4.09) |
| 50–59 years | 1553 (27.95) | 1059 (43.08) | 967 (39.61) | 452 (4.42) |
| 60–69 years | 1575 (28.35) | 41 (1.67) | 82 (3.36) | 586 (5.73) |
| 70+ years | 31 (0.56) | 4 (0.16) | 23 (0.94) | 8191 (80.09) |
| Men | ||||
| Women | 4708 (84.74) | 1759 (71.56) | 1611 (66) | 3874 (37.88) |
| History of COVID-19 infection confirmed by testing | 374 (6.73) | 270 (10.98) | 154 (6.31) | 273 (2.67) |
| Reported any AEFI | 5143 (92.57) | 2015 (81.98 | 2286 (93.65) | 5394 (52.74) |
| Reported any AEFI after dose 1 | 5044 (90.78) | 2015 (81.98) | 1953 (80.01) | 4094 (40.03) |
| Reported any AEFI after dose 2 | 5044 (48.43) | 2015 (0) | 1953 (85.17) | 4094 (35.71) |
| Reported at least 1 well-known systemic AEFI* | 5044 (88.32) | 2015 (77.14) | 1953 (87.1) | 4094 (39.31) |
| Reported at least 1 well-known systemic AEFI* after dose 1 | 4772 (85.89) | 1896 (77.14) | 1363 (55.84) | 2617 (25.59) |
| Reported at least 1 well-known systemic AEFI*after dose 2 | 2173 (39.11) | NA | 1920 (78.66) | 2696 (26.36) |
| Reported fever after dose 1 | 1746 (31.43) | 544 (22.13) | 105 (4.3) | 89 (0.87) |
| Reported fever after dose 2 | 192 (3.46) | NA | 546 (22.37) | 203 (1.98) |
| Comorbidities (MedDRA System Organ Class) | ||||
| Immune system disorders | 95 (1.71) | 23 (0.94) | 79 (3.24) | 208 (2.03) |
| Respiratory, thoracic and mediastinal disorders | 480 (8.64) | 109 (4.43) | 266 (10.9) | 938 (9.17) |
| Hepatobiliary disorders | 12 (0.22) | 4 (0.16) | 8 (0.33) | 29 (0.28) |
| Nervous system disorders | 66 (1.19) | 21 (0.85) | 32 (1.31) | 193 (1.89) |
| Psychiatric disorders | 222 (4) | 117 (4.76) | 111 (4.55) | 186 (1.82) |
| Cardiac disorders | 221 (3.98) | 33 (1.34) | 81 (3.32) | 1959 (19.16) |
| Vascular disorders | 590 (10.62) | 139 (5.66) | 166 (6.8) | 2942 (28.77) |
| Renal and urinary disorders | 27 (0.49) | 2 (0.08) | 23 (0.94) | 236 (2.31) |
| Metabolism and nutrition disorders | 168 (3.02) | 10 (0.41) | 63 (2.58) | 835 (8.16) |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | 43 (0.77) | 20 (0.81) | 27 (1.11) | 239 (2.34) |
| Pregnancy, puerperium and perinatal conditions | 1 (0.02) | 3 (0.12) | 33 (1.35) | 12 (0.12) |
| Other | 627 (11.29) | 199 (8.1) | 315 (12.9) | 1305 (12.76) |
Data reported as n (%)
AEFI adverse events following immunization, NA not applicable
*Fever (body temperature 38 °C or higher), arthralgia, myalgia, nausea, pyrexia, headache, malaise, chills, fatigue
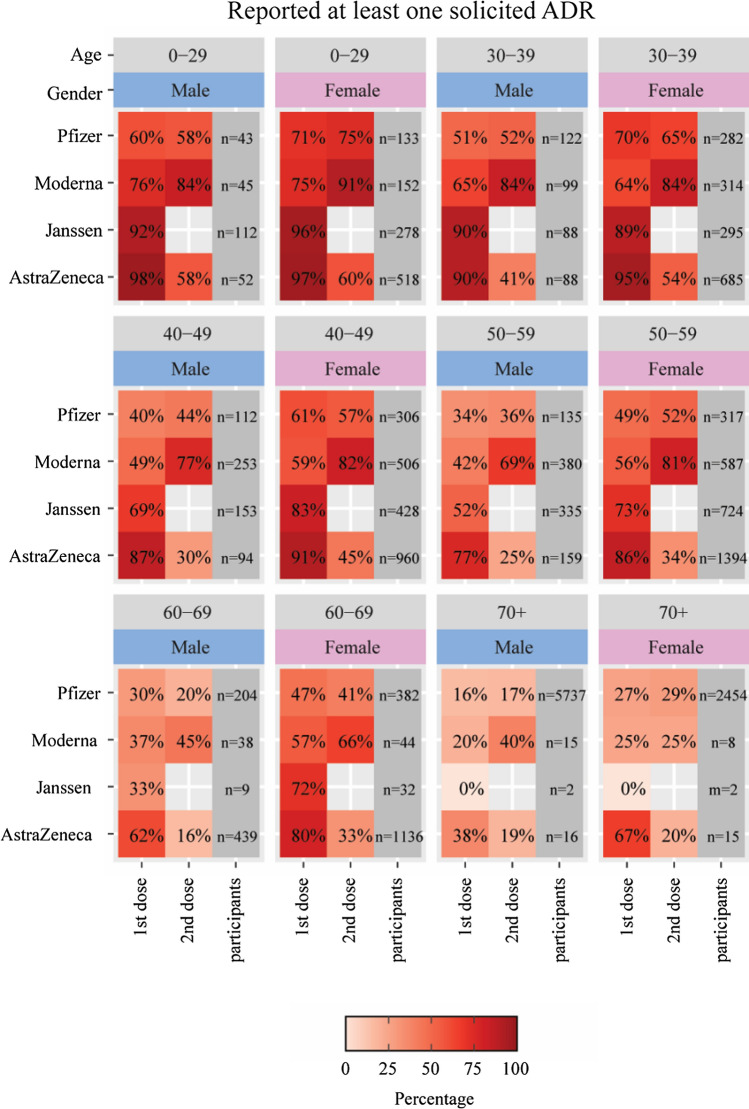
Fig. 3.
Percentages of participants who reported at least one of the following well-known systemic adverse events following immunization (AEFIs) after the first and second doses, stratified by age and sex: arthralgia, myalgia, nausea, pyrexia, headache, malaise, chills, fatigue, and fever (body temperature 38 °C or higher)
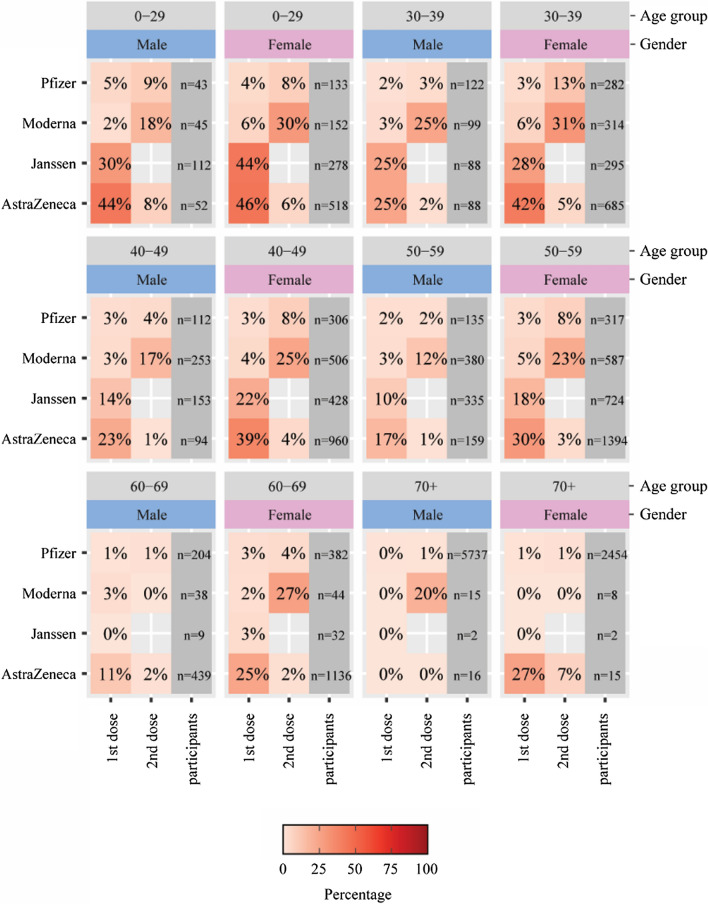
Fig. 4.
Percentages of participants who reported a body temperature of 38.0 °C or higher after the first and second doses of a COVID-19 vaccine, stratified by age and sex
Table 3.
Sensitivity analysis for participants registering on the day of vaccination
| Vaccine | AstraZeneca | Janssen | Moderna | Pfizer |
|---|---|---|---|---|
| N. participants with complete immunization* | 1870 | 1078 | 1164 | 3420 |
| Age distribution | ||||
| 0–29 years | 207 (11.07) | 157 (14.56) | 99 (8.51) | 83 (2.43) |
| 30–39 years | 323 (17.27) | 174 (16.14) | 197 (16.92) | 193 (5.64) |
| 40–49 years | 364 (19.47) | 265 (24.58) | 373 (32.04) | 192 (5.61) |
| 50–59 years | 484 (25.88) | 457 (42.39) | 454 (39) | 181 (5.29) |
| 60–69 years | 482 (25.78) | 23 (2.13) | 32 (2.75) | 188 (5.5) |
| 70+ years | 10 (0.53) | 2 (0.19) | 9 (0.77) | 2583 (75.53) |
| Men | ||||
| Women | 1519 (81.23) | 735 (68.18) | 716 (61.51) | 1167 (34.12) |
| History of COVID-19 infection confirmed by testing | 114 (6.1) | 112 (10.39) | 49 (4.21) | 105 (3.07) |
| Reported any AEFI | 1702 (91.02) | 847 (78.57) | 1071 (92.01) | 1815 (53.07) |
| Reported any AEFI after dose 1 | 1665 (89.04) | 847 (78.57) | 883 (75.86) | 1394 (40.76) |
| Reported any AEFI after dose 2 | 1665 (48.72) | 847 (0) | 883 (84.28) | 1394 (36.17) |
| Reported at least 1 well-known systemic AEFI* | 1665 (85.56) | 847 (73.84) | 883 (85.4) | 1394 (39.5) |
| Reported at least 1 well-known systemic AEFI* after dose 1 | 1555 (83.16) | 796 (73.84) | 588 (50.52) | 872 (25.5) |
| Reported at least 1 well-known systemic AEFI*after dose 2 | 734 (39.25) | NA | 912 (78.35) | 910 (26.61) |
| Reported fever after dose 1 | 487 (26.04) | 196 (18.18) | 31 (2.66) | 26 (0.76) |
| Reported fever after dose 2 | 55 (2.94) | NA | 242 (20.79) | 74 (2.16) |
| Comorbidities (MedDRA System Organ Class) | ||||
| Immune system disorders | 33 (1.76) | 10 (0.93) | 41 (3.52) | 84 (2.46) |
| Respiratory, thoracic and mediastinal disorders | 152 (8.13) | 54 (5.01) | 129 (11.08) | 310 (9.06) |
| Hepatobiliary disorders | 7 (0.37) | 3 (0.28) | 4 (0.34) | 9 (0.26) |
| Nervous system disorders | 20 (1.07) | 11 (1.02) | 11 (0.95) | 71 (2.08) |
| Psychiatric disorders | 87 (4.65) | 54 (5.01) | 48 (4.12) | 71 (2.08) |
| Cardiac disorders | 79 (4.22) | 12 (1.11) | 39 (3.35) | 606 (17.72) |
| Vascular disorders | 194 (10.37) | 63 (5.84) | 62 (5.33) | 898 (26.26) |
| Renal and urinary disorders | 8 (0.43) | 2 (0.19) | 12 (1.03) | 79 (2.31) |
| Metabolism and nutrition disorders | 49 (2.62) | 9 (0.83) | 28 (2.41) | 278 (8.13) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 14 (0.75) | 10 (0.93) | 12 (1.03) | 74 (2.16) |
| Pregnancy, puerperium and perinatal conditions | 1 (0.05) | 2 (0.19) | 16 (1.37) | 6 (0.18) |
| Other | 186 (9.95) | 80 (7.42) | 144 (12.37) | 435 (12.72) |
AEFI adverse events following immunization, NA not applicable
*Fever (body temperature 38 °C or higher), arthralgia, myalgia, nausea, pyrexia, headache, malaise, chills, fatigue
The percentage of patients reporting any AEFI was high and ranged from approximately 53% for the Pfizer vaccine to approximately 94% for the Moderna vaccine. However, these high percentages include local reactions at the injection site. A list of all reported AEFIs per vaccine can be found in ESM 3. The numbers of participants who experienced an AEFI considered serious were, however, low, after both the first (n = 36 in total) and second doses (n = 8 in total). The highest percentage (0.228%) was found after the first dose of the AstraZeneca vaccine. Reported serious AEFIs (n = 81 in total) by vaccine brand are shown in Table 4.
Table 4.
Reported serious AEFIs by vaccine (MedDRA preferred terms)
| Serious AEFI | AstraZeneca | Janssen | Moderna | Pfizer | Total |
|---|---|---|---|---|---|
| Abdominal discomfort | 1 | 1 | |||
| Abortion missed | 1 | 1 | |||
| Abortion spontaneous | 1 | 1 | 1 | 3 | |
| Acute myocardial infarction | 1 | 1 | |||
| Anaphylactic reaction | 1 | 1 | |||
| Angina pectoris | 1 | 1 | |||
| Appendicitis | 1 | 1 | |||
| Arrhythmia | 1 | 1 | |||
| Arthritis | 1 | 1 | |||
| Asthma | 1 | 1 | |||
| Atrial fibrillation | 1 | 1 | |||
| Atrioventricular block complete | 1 | 1 | |||
| Blood loss anaemia | 1 | 1 | |||
| Cerebral infarction | 1 | 1 | 2 | ||
| Cerebrovascular accident | 1 | 1 | |||
| Chills | 1 | 1 | |||
| Diarrhoea | 1 | 1 | 2 | ||
| Dizziness | 1 | 1 | |||
| Dyspnoea | 3 | 1 | 3 | 7 | |
| Epilepsy | 1 | 1 | |||
| Epistaxis | 1 | 1 | |||
| Fall | 1 | 1 | |||
| Gait disturbance | 1 | 1 | |||
| Gastric ulcer | 1 | 1 | |||
| Headache | 1 | 1 | |||
| Hyperpyrexia | 1 | 2 | 3 | ||
| Hypersensitivity | 1 | 1 | |||
| Hypertension | 1 | 1 | |||
| Hypoaesthesia | 1 | 1 | |||
| Hypotension | 1 | 1 | |||
| Internal haemorrhage | 1 | 1 | |||
| Limb discomfort | 1 | 1 | |||
| Loss of consciousness | 1 | 1 | |||
| Malaise | 2 | 1 | 1 | 4 | |
| Muscle spasms | 1 | 1 | |||
| Myalgia | 1 | 1 | |||
| Myocardial infarction | 2 | 2 | |||
| Nausea | 1 | 1 | |||
| Oxygen saturation decreased | 1 | 1 | |||
| Pallor | 1 | 1 | |||
| Palpitations | 1 | 1 | |||
| Paraesthesia oral | 1 | 1 | |||
| Pneumonia | 1 | 1 | |||
| Pruritus | 1 | 1 | |||
| Pulmonary embolism | 2 | 2 | |||
| Pulmonary pain | 1 | 1 | |||
| Pyrexia | 2 | 1 | 3 | ||
| Rash | 1 | 1 | |||
| Rash pruritic | 1 | 1 | |||
| Respiratory arrest | 1 | 1 | |||
| Restlessness | 1 | 1 | |||
| Retinal detachment | 1 | 1 | |||
| Tachycardia | 1 | 1 | |||
| Tinnitus | 1 | 1 | |||
| Transient global amnesia | 1 | 1 | |||
| Transient ischaemic attack | 3 | 3 | |||
| Tremor | 1 | 1 | |||
| Vision blurred | 1 | 1 | |||
| Vitreous floaters | 1 | 1 | |||
| Vomiting | 1 | 1 | |||
| Total | 30 | 12 | 9 | 30 | 81 |
AEFI adverse events following immunization
The reported AESIs per vaccine brand are shown in Table 5. The most frequently reported AESI was COVID-19 after vaccination (17 cases for the AstraZeneca vaccine, 3 for the Pfizer vaccine, and 1 for the Janssen vaccine), followed by hypersensitivity reactions (9 cases for the AstraZeneca vaccine, 8 for the Pfizer vaccine, and one for the Moderna vaccine). Note that not all these reactions adhered to the criteria for a serious reaction. An anaphylactic reaction was reported only once for the Janssen vaccine.
Table 5.
Reported AESI per vaccine (MedDRA preferred terms)
| AESI | AstraZeneca | Pfizer | Moderna | Janssen |
|---|---|---|---|---|
| COVID-19 infection | 17 | 3 | 0 | 1 |
| Hypersensitivity | 9 | 8 | 1 | 0 |
| Arrhythmia | 6 | 7 | 1 | 0 |
| Epilepsy | 2 | 1 | 2 | 0 |
| Facial paralysis | 1 | 0 | 1 | 0 |
| Hypersomnia | 1 | 0 | 1 | 0 |
| Myocardial infarction | 0 | 2 | 0 | 0 |
| Product administration error | 1 | 0 | 0 | 1 |
| Pulmonary embolism | 2 | 0 | 0 | 0 |
| Acute myocardial infarction | 1 | 0 | 0 | 0 |
| Anaphylactic reaction | 0 | 0 | 0 | 1 |
| Atrioventricular block complete | 0 | 1 | 0 | 0 |
| Cerebrovascular accident | 0 | 1 | 0 | 0 |
| Facial paresis | 1 | 0 | 0 | 0 |
| Myocarditis | 0 | 1 | 0 | 0 |
| Pericarditis | 0 | 1 | 0 | 0 |
| Petit mal epilepsy | 0 | 1 | 0 | 0 |
| Platelet count decreased | 0 | 0 | 1 | 0 |
| Respiratory arrest | 1 | 0 | 0 | 0 |
| Seizure | 0 | 1 | 0 | 0 |
| Thrombosis | 1 | 0 | 0 | 0 |
| Vasculitis | 1 | 0 | 0 | 0 |
| Total | 44 | 27 | 7 | 3 |
AESI adverse events of special interest
Figure 3 (corresponding to Table 2) shows a heatmap of the percentages of well-known systemic AEFIs for the first and second doses across vaccine brands and age groups and stratified by sex for participants who received complete vaccination. Figure 4 shows a similar heatmap specifically for a body temperature increase above 38 °C.
Well-known systemic AEFIs were most often experienced by participants receiving the first dose of the AstraZeneca vaccine and the Janssen vaccine and the second dose of the Moderna vaccine; the Pfizer vaccine was associated with the lowest rate of AEFIs. Participants vaccinated with the Moderna vaccine experienced at least one well-known systemic AEFI more often after the second vaccination than after the first vaccination. For the AstraZeneca vaccine, participants experienced more AEFIs after the first dose than after the second dose. For the Pfizer vaccine, there was little difference between vaccine doses between men and women and among age groups. The age distribution of the participants and the number of men and women differed by vaccine. There were more women among the participants who received the AstraZeneca, Moderna and Janssen vaccines. In contrast, more male participants received the Pfizer vaccine, and these participants were older than those who received the other vaccines. Men and the elderly experienced fewer AEFIs than women and younger people, respectively.
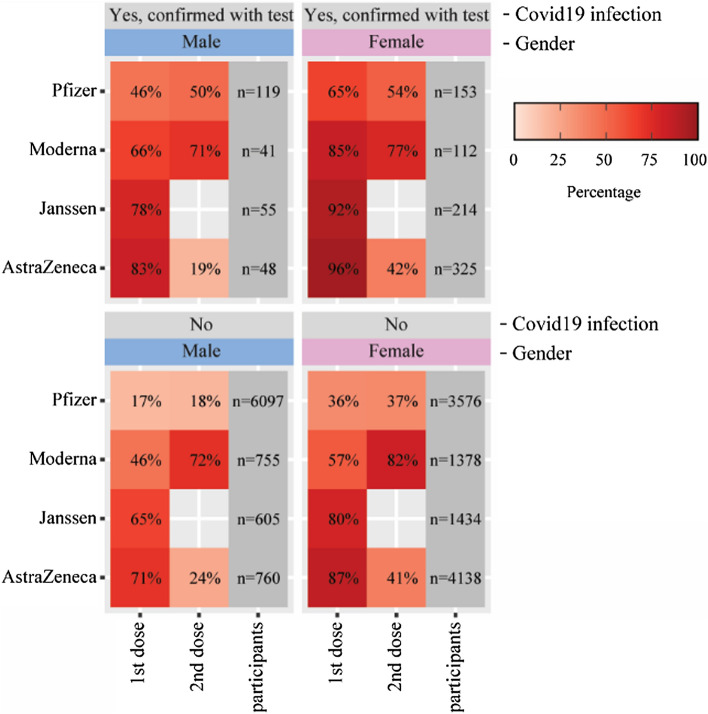
Figure 5 shows the percentage of participants who reported at least one well-known systemic AEFI stratified by COVID-19 history prior to vaccination. Participants who had COVID-19 (confirmed with a positive test) experienced one or more well-known systemic AEFIs more often after the first dose than after the second dose. This pattern was observed in both men and women and for all vaccine brands. For the Pfizer vaccine, this was also the case after the second dose.
Fig. 5.
Percentages of participants who reported at least one of the following well-known systemic adverse events following immunization (AEFIs) stratified by a history of COVID-19 prior to vaccination: arthralgia, myalgia, nausea, pyrexia, headache, malaise, chills, fatigue, and fever (body temperature 38 °C or higher)
Discussion
Clinical trials of vaccines prior to marketing authorization obtain key information on AEFIs and specifically AESIs. During the rollout of the COVID-19 vaccines, a larger and more diverse population was vaccinated, making it possible to study the safety profile in a real-world setting [15].
With the LIM system, a web-based tool for collecting patient-reported outcomes, important insight into the occurrence of AEFIs after the first and second doses of different COVID-19 vaccines was obtained.
A large prospective cohort study from the UK that collected patient-reported data through an app found that systemic AEFIs were reported by 13.5% of participants after the first dose of the Pfizer vaccine and 22% after the second dose. For the AstraZeneca vaccine, the number of systemic AEFIs was higher after the first dose (33% of participants) than after the second dose. Systemic AEFIs were more common among individuals with previous COVID-19 infection than among those without known past infection for both vaccines [16], similar to the results in our study. Chapin-Bardales et al. [17] analyzed mRNA vaccine data obtained from an active surveillance system for COVID-19 vaccine recipients in the US. They also found that systemic reactions frequently occurred after the first dose, but they were more frequently reported after the second dose among both Pfizer and Moderna vaccine recipients. This study also showed a higher frequency of AEFIs after the second dose of the Moderna vaccine, while for the first and second doses of the Pfizer vaccine, such AEFIs were comparable. Similar to Menni et al. [16], we postulate that the increased reactogenicity relates to increased immunogenicity after previous COVID-19 infection. Previous studies have shown that COVID-19 vaccines increase immunogenicity in individuals with past infection, and these individuals rapidly develop higher antibody titers than those without previous infection [18, 19].
Differences in reactogenicity among vaccine brands have been described, with the Pfizer vaccine associated with the lowest rate of reactogenicity [16, 17]. In a previous study, we investigated AEFIs related to local reactogenicity and fever in this cohort. Based on the multivariate analysis, we concluded that the Pfizer mRNA vaccine was associated with the lowest rate of reactogenicity, and the viral vector vaccine from AstraZeneca was associated with the highest rates of general reactogenicity and pyrexia after the first dose. Additionally, those with a history of COVID-19, females and younger individuals had increased odds of reactogenicity after vaccination [20].
It is well known that women and young people experience more AEFIs than men and older people, respectively. Age and sex are associated with differences in the immune response of vaccinated persons [21–23].
Our study found a low frequency of serious AEFIs, which is in accordance with the results of premarketing clinical trials [24–26].
Strengths and Limitations
The strengths of this study include the large study population for which we could obtain information about complete immunization status, vaccine brand and COVID-19 history.
The Netherlands Pharmacovigilance Centre Lareb has long experience with CEM using the LIM web app for the collection of patient-reported outcomes. The well-known AEFIs in this study were easily recognizable by the patient and probably did not warrant contact with a medical healthcare professional, so these effects could be reliably reported by patients themselves. However, there is a potential risk for loss to follow-up among those with AEFIs with serious outcomes. For serious outcomes, additional follow-up was requested if necessary. This could include asking participants for hospital discharge letters or other medical information in order to assess the association between the vaccination given and the reported adverse reaction.
Participants could register for the study within 48 hours after vaccination. This may have introduced selection bias since subjects experiencing an adverse reaction shortly after vaccination may have been more prone to participate. However, a sensitivity analysis for participants who registered on the day of vaccination showed that the percentage of reported AEFIs is in the same range as for the total cohort.
In addition, for the calculation of incidence we included only patients who agreed to participate in the study and this could have an impact on representativeness of the sample.
In most longitudinal studies, there is some attrition over the course of the study [27]. This was also the case in our study, where for a group of patients, there was no information about the second dose. This may have been because patients either received only one vaccine or were lost to follow-up. For patients with a history of confirmed COVID-19, the protocol for COVID-19 vaccination was adjusted from two recommended doses of the AstraZeneca, Pfizer or Moderna vaccines to one dose [28].
There is heterogeneity of the population vaccinated with different vaccine brands in the Netherlands. There were also unequal age and sex distributions among the four vaccine brands in our study. Our cohort is not entirely representative of the vaccinated population. For the Pfizer vaccine, the study population was predominated by men and an older age group compared with the other vaccines. This was largely due to the vaccination strategy in the Netherlands at the time the study started [29]. Age and sex are associated with differences in the immune response of vaccinated persons [21].
Conclusion
This analysis of a large cohort provides important information about the occurrence of AEFIs in different age groups who received different vaccine brands with and without prior COVID-19. Participants reported a high number of AEFIs in general, but the frequency of serious AEFIs was low.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Funding for the cohort event monitoring of COVID-19 vaccine safety was granted by the Dutch Ministry of Health, Welfare and Sport. The Dutch Ministry of Health, Welfare and Sport had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflict of interest
The authors AK, JJ, LB and FH declare no relevant conflicts of interest.
Availability of data and material
The datasets for this manuscript are not publicly available because of the Lareb data protection policy. Requests to access the datasets should be directed to the first author and will be granted upon reasonable request.
Code availability
The SQL statements for the data used in this article are not publicly available because of the Lareb data protection policy. Requests to access the datasets should be directed to the first author and will be granted upon reasonable request.
Author contributions
The original study protocol was designed by all authors. The queries and dataset were established by JJ. Data analysis was performed by JJ, AK and FHL. The design of the manuscript was determined by all authors. All authors contributed to the final data analysis and to manuscript drafting and revision. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Ethical approval and consent to participate
If a study in the Netherlands is subject to the Medical Research Involving Human Subjects Act (WMO), it must undergo a review by an accredited Medical Research Ethics Committee or the central committee on research involving human subjects (CCMO). After submission to an accredited review committee (METC Brabant), this study was deemed not to fall under the WMO act. Participants in the study provided a written statement of consent to participate at the time of registration.
Consent for publication
Participants in the study provided a written statement of consent at the time of registration for their data to be used for the purpose of this research and publication of study results.
References
- 1.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. doi: 10.1016/s1473-3099(20)30195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency. SmPC Vaxzevria 2021. https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf. Accessed 14 July 2021.
- 4.European Medicines Agency. SmPC Covid-19 vaccine Janssen. 2021. https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf. Accessed 14 July 2021.
- 5.European Medicines Agency.SmPC Comirnaty. 2021. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf. Accessed 14 July 2021.
- 6.European Medicines Agency. SmPC Spikevax. 2021. https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf. Accessed 14 July 2021.
- 7.Harmark L, van Hunsel F, Hak E, van Grootheest K. Monitoring the safety of influenza A (H1N1) vaccine using web-based intensive monitoring. Vaccine. 2011;29(10):1941–1947. doi: 10.1016/j.vaccine.2010.12.123. [DOI] [PubMed] [Google Scholar]
- 8.van Balveren-Slingerland L, Kant A, Harmark L. Web-based intensive monitoring of adverse events following influenza vaccination in general practice. Vaccine. 2015;33(19):2283–2288. doi: 10.1016/j.vaccine.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Harmark L. Web-based Intensive Monitoring; a patient based pharmacovigilance tool: Rijksuniversiteit Groningen; 2012.
- 10.Gezondheidsraad. Interval tussen de eerste en tweede vaccinatie. 2021. https://www.gezondheidsraad.nl/documenten/adviezen/2021/04/12/interval-tussen-de-eerste-en-tweede-vaccinatie. Accessed 17 Jan 2021.
- 11.World Health Organization. Causality Assessment Of An Adverse Event Following Immunization (AEFI) - User manual for the revised WHO classification WHO. 2013. https://www.who.int/vaccine_safety/publications/aevi_manual.pdf. Accessed 01 July 2021.
- 12.The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Welcome to MedDRA. 2021. https://www.meddra.org/. [DOI] [PMC free article] [PubMed]
- 13.CIOMS working Group VIII. Practical Aspects of Signal Detection in Pharmacovigilance: Report of CIOMS Working Group VIII. Geneva2010. Report No.: 9290360828.
- 14.Brighton Collaboration. COVID-19 AESI list. 2020. https://brightoncollaboration.us/wp-content/uploads/2021/01/COVID-19-updated-AESI-list.pdf. Accessed 11 Oct 2021.
- 15.Meurs L, Kant A. Cohort event monitoring to assess safety of COVID-19 vaccines using patient reported events, a protocol template from the ACCESS project. 2020. https://vac4eu.org/wp-content/uploads/2021/02/3a.Cohort-event-monitoring-to-assess-safety-of-COVID-19-vaccines-using-patient-reported-events-a-protocol-template-from-the-ACCESS-project.pdf. Accessed 12 Oct 2021.
- 16.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325(21):2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 18.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolfes LH, Kant A, van Balveren L, Van Hunsel F. Cohort event monitoring of COVID-19 vaccine reactogenicity in the Netherlands using patient reported outcomes (P026). In: Drug Safety—20th ISoP annual meeting “integrated pharmacovigilance for safer patients” 8–10 November 2021 Muscat, Oman (Hybrid meeting). 2021.10.1007/s40264-021-01129-0
- 21.Herve C, Laupeze B, Del Giudice G, Didierlaurent AM, Da Silva FT. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinti M, Appay V, Campisi J, Frasca D, Fulop T, Sauce D, et al. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol. 2016;46(10):2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/s0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustavson K, von Soest T, Karevold E, Røysamb E. Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health. 2012;12(1):918. doi: 10.1186/1471-2458-12-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rijksoverheid. Waarom is 1 prik voldoende als ik corona heb gehad? 2021. https://www.rijksoverheid.nl/onderwerpen/coronavirus-vaccinatie/vraag-en-antwoord/waarom-is-1-prik-voldoende-als-ik-corona-heb-gehad. Accessed 29 Sept 2021.
- 29.Rijksoverheid. 2021. Vaccinatieplanning. https://www.rijksoverheid.nl/onderwerpen/coronavirus-vaccinatie/volgorde-van-vaccinatie-tegen-het-coronavirus. Accessed 14 July 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.