Abstract
Background
Healthcare systems internationally need to consider new models of care to cater for the increasing numbers of people with asthma. Telehealthcare interventions are increasingly being seen by policymakers as a potential means of delivering asthma care. We defined telehealthcare as being healthcare delivered from a distance, facilitated electronically and involving the exchange of information through the personalised interaction between a healthcare professional using their skills and judgement and the patient providing information.
Objectives
To assess the effectiveness of telehealthcare interventions in people with asthma.
Search methods
We searched in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO; this was supplemented by handsearching of respiratory journals. We also searched registers of ongoing and unpublished trials.
Selection criteria
We selected completed randomised controlled trials of telehealthcare initiatives aiming to improve asthma care.
Data collection and analysis
Two review authors independently appraised studies for inclusion and extracted data and performed meta‐analyses. We analysed dichotomous variables to produce an odds ratio (OR) and continuous variables to produce a mean difference.
Main results
We included 21 trials in this review. The 21 included studies investigated a range of technologies aiming to support the provision of care from a distance. These included: telephone (n = 9); video‐conferencing (n = 2); Internet (n = 2); other networked communications (n = 6); text Short Messaging Service (n = 1); or a combination of text and Internet (n = 1). Meta‐analysis showed that these interventions did not result in clinically important improvements in asthma quality of life (minimum clinically important difference = 0.5): mean difference in Juniper's Asthma Quality of Life Questionnaire (AQLQ) 0.08 (95% CI 0.01 to 0.16). Telehealthcare for asthma resulted in a non‐significant increase in the odds of emergency department visits over a 12‐month period: OR 1.16 (95% CI 0.52 to 2.58). There was, however, a significant reduction in hospitalisations over a 12‐month period: OR 0.21 (95% CI 0.07 to 0.61), the effect being most marked in people with more severe asthma managed predominantly in secondary care settings.
Authors' conclusions
Telehealthcare interventions are unlikely to result in clinically relevant improvements in health outcomes in those with relatively mild asthma, but they may have a role in those with more severe disease who are at high risk of hospital admission. Further trials evaluating the effectiveness and cost‐effectiveness of a range of telehealthcare interventions are needed.
Keywords: Humans, Asthma, Asthma/therapy, Internet, Randomized Controlled Trials as Topic, Telemedicine, Telemedicine/methods, Telephone
Plain language summary
Telehealthcare interventions for long‐term asthma
Asthma is a common condition, affecting an estimated 300 million people worldwide. Its symptoms include cough, breathlessness, wheeze and associated limitation in activity.
Increases in the prevalence of long‐term conditions such as asthma are presenting considerable challenges to health services internationally and traditional models of healthcare are struggling to cope. Emerging information and communication technologies (ICTs) have the potential to ameliorate some of the challenges being posed through enabling and supporting patient care at a distance. Collectively termed 'telehealthcare' services, these interventions include the use of the telephone, videoconferencing, text‐message (also known as Short Message Service, SMS), instant messaging, email and the Internet to facilitate remote patient monitoring and decisions on care by healthcare professionals.
The potential benefits of telehealthcare include greater accessibility for patients, reduced time and cost expenditure associated with travelling, earlier detection of disease exacerbations and associated reduced risk of hospital admissions for asthma. These interventions are, however, not without risk and it is therefore important to study the effectiveness of such telehealthcare initiatives rigorously.
We undertook a systematic review of the literature, searching for randomised controlled trials that have either been published or are in progress, which studied the impact of telehealthcare on asthma outcomes.
Our searches identified a large body of trial evidence and also a substantial body of work in progress. This revealed that telehealthcare initiatives are unlikely to be of benefit in improving quality of life for the majority of people with relatively mild asthma, but that these interventions may prove useful in preventing exacerbations and hospital admissions in people with more severe asthma. We believe it is important for more research to be done to establish the cost‐effectiveness of these interventions.
Background
Description of the condition
There is no gold standard objective definition of asthma; its diagnosis is clinical, based on the presence of characteristic symptoms (wheeze, breathlessness, chest tightness and nocturnal or exercise‐induced cough) and of variable airflow obstruction (BTS/SIGN 2008). The features of asthma are so heterogeneous that, in both children and adults, it seems that what is currently termed 'asthma' is unlikely in the future to be regarded as a single disease entity (Lancet 2006).
Much research is still needed to answer the following three fundamental questions:
What is asthma?
Who gets asthma and why?
Which factors predict exacerbations and treatment response? (Lancet 2008)
The Global Initiative for Asthma (GINA), run in collaboration with the World Health Organization and the U.S. National Heart Lung and Blood Institute (NHLBI) and National Institute for Health (NIH), estimates that 300 million people have asthma (GINA 2003). Asthma is thus now a very common long‐term condition and there has been an increase in prevalence in recent decades (Anderson 2007; ISAAC 2006; Pearce 2000). The highest prevalence rates, as high as 30%, are amongst certain age groups in economically developed English‐speaking countries (Anandan 2010; Punekar 2009; Simpson 2010). However, there has also been an increase in asthma prevalence in many economically‐developing countries (ISAAC 2001; ISAAC 1998; ISAAC 2004; Marks 2001). These increases affect both children and adults.
Worldwide asthma presents substantial challenges. The high disease burden demands improvements in the development of and access to treatments (Anandan 2009; Gupta 2003; Simpson 2010). Patterns of help‐seeking behaviour are also relevant, as delayed reporting is associated with greater morbidity and the need for costly emergency care. There is also a significant indirect cost burden associated with asthma through school and work absences.
Description of the intervention
Telehealthcare interventions may help to address some of the above challenges by enabling remote delivery of patient‐centred care, facilitating timely access to health advice and medications, prompting self‐monitoring and medication compliance, and educating patients on trigger avoidance (Car 2003; Car 2004a; Car 2004b; McLean 2009a).
Terminology in this area is evolving rapidly and there is significant overlap between expressions such as 'telehealthcare', 'telemedicine', 'telehealth' and 'telenursing' (Busey 2008; HRSA 2008; Lorentz 2008; Mahen 2006). For the purposes of this review, we have chosen to describe the interventions under study as 'telehealthcare'. This emphasises the use of remote information and communication technologies (ICTs) for supporting the active care of people with asthma rather than, for example, inter professional communication, passive information provision (as in traditional online health tools) or unsupported patient self‐monitoring through technology. Another way of looking at this is that telehealthcare concerns what is known as B2C or business‐to‐consumer, i.e. professional to patient communication, rather than B2B or business‐to‐business, i.e. inter professional communication, which is also commonly referred to as 'telemedicine'.
Telehealthcare also avoids the use of professional role‐based terms such as telenursing (implying that remote care is delivered by a nurse) or telemedicine (implying that care is delivered by a doctor). It is thus compatible with the multidisciplinary nature of contemporary chronic disease management. This review therefore focuses on studies which evaluate remote technological interventions that are designed to improve the patient's asthma with the help of any of the following: doctor, nurse or allied healthcare professional, from a distance.
'Telehealthcare' has the following key elements, adapted from Miller 2007:
information obtained from the patient, whether voice, video, other audio, electrocardiography, oxygen saturation or other;
electronic transfer of such information over a distance; and
personalised feedback tailored to the patient from a healthcare professional who exercises their skills and judgement.
Interventions captured within the terms telehealthcare include both synchronous and asynchronous (store and forward) technologies. For example, telephone and video‐conferencing enable synchronous consultations, whereas asynchronous communication would, for example, include storing two weeks worth of spirometry results and then sending them on to a nurse who responds by email or telephone.
How the intervention might work
Telehealthcare is a complex intervention and, as such, it is quite difficult to specify exactly why it works or does not work, i.e. what is/are the 'active ingredient(s)' within the intervention (Medical Research Council 2008). Some potential mechanisms through which the use of telehealthcare may enhance the quality of care and achieve cost savings include (adapted from Finkelstein 2000a):
providing patient education and counselling for primary prevention and early detection of disease;
replacing face‐to‐face nursing/doctor visits;
improving adherence to medications and other treatment regimens;
monitoring patients' health parameters remotely;
enabling early detection of incipient disease exacerbation and timely intervention for early symptom management;
reducing unscheduled/unnecessary visits to the physician and emergency room;
preventing repeat hospitalisations.
These mechanisms are theorised to function both alone and together to bring about the effects of telehealthcare interventions. However, we feel that the main task of this review is to uncover whether or not these telehealthcare interventions work and then they can be subsequently scrutinised ‐ perhaps by more theory‐based studies ‐ to elucidate how and why they work or do not work.
Why it is important to do this review
There are now in many parts of the world an increasing array of electronic tools for remotely helping people with asthma and often many are now beginning to be implemented in the absence of an explicit evidence base (McKinstry 2009; McLean 2009a). A recent Cochrane Review of generic teleconsultations compared with face‐to‐face consultations found little evidence of clinical benefit. There were also a lack of analysable data for assessing the cost‐effectiveness of these interventions. The authors concluded that further research is required (Currell 2008).
Another systematic review (Mair 2000) of studies of patient satisfaction with telehealthcare raised a number of important questions, these included:
What types of consultation are suitable for remote consulting?
What are the effects of this mode of healthcare delivery on the clinician‐patient relationship?
How do communication issues affect the delivery of healthcare via telehealthcare?
What are the possible limitations of telehealthcare in clinical practice?
Answers to such questions are urgently needed or we risk blindly implementing a non‐proven way of working which may have a negative effect on patients and professionals.
One commonly used argument for telehealthcare is that long‐term running costs will be lower than in conventional care because disease will be detected and treated early, preventing ensuing morbidity and hospitalisations and allowing patients to be cared for in their own home. However, the initial start‐up costs of telehealthcare may be substantial (Whitten 2002). The cost‐effectiveness of telehealthcare interventions therefore also need to be established.
In asthma, patients often have a high level of responsibility for their own health. In some people it can also be a life‐limiting and challenging disease to manage. Telehealthcare interventions may make this easier for patients by providing timely professionally guided feedback on their condition. Such interventions may help patients to identify and address triggers and to optimise their medication regimens to address the fluctuations in their illness ‐ and at low cost. This is the ideal situation, however such results cannot be presumed and a robust critique of the evidence base is overdue.
Objectives
To review the effectiveness of telehealthcare for people with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included full reports of randomised controlled trials which compared a telehealthcare intervention with usual care or any other control intervention.
Types of participants
We were interested in studies in children and adults with clinician‐diagnosed asthma. We included studies conducted in both primary and secondary care settings. We focused on studies which looked exclusively at people with asthma; people with chronic obstructive pulmonary disease (COPD) were not included as this population is being studied in a separate review (McLean 2009b). There were no exclusions on the basis of age, gender, ethnicity or language spoken.
Types of interventions
We stipulated that there needed to be a focus on the proactive use of ICT to provide the information the health professional requires to make their decisions and then feedback of their advice to the patient. The study of technology needed to be central and its use sustained. These interventions included the following.
Video or telephone links between patient and healthcare professionals in real time or using store‐and‐forward technologies.
Systems of care using Internet‐based telecommunication; these could be synchronous or asynchronous (e.g. Skype®, messaging, email) with healthcare professionals.
Systems of care using both wired and wireless telemetry for monitoring of Peak Expiratory Flow (PEF), spirometry (Forced Expiratory Volume in 1 second (FEV1); Forced Vital Capacity (FVC) respiratory rate, chest movement and oxygen saturations involving feedback to the patient, which had been processed or authorised by a healthcare professional.
Other systems of remote healthcare incorporating patient self‐reporting of symptoms on a questionnaire and information exchange with a professional.
Complex intervention studies, if it was possible to tease out the individual telehealthcare elements.
Professional involvement in care was considered fundamentally important; we thus excluded the following types of interventions.
Remote interventions that were merely educational and so did not include the input of a professional, e.g. electronic information provision in an emergency waiting room. Although this type of passive information provision was excluded, education could have been part of a more complex interactive intervention that might fit the inclusion criteria, e.g. if it included feedback from a professional.
Decision support which functioned without the active input of a healthcare professional.
Types of outcome measures
Primary outcomes
Clinical endpoints:
Asthma quality of life as measured by the Juniper asthma quality of life questionnaire (AQLQ).
Proportion of patients with one or more emergency department attendances for asthma over three and 12 months.
Proportion of patients with one or more hospitalisations for asthma over three and 12 months.
Other primary outcomes:
Symptom control as judged by use of a variety of instruments.
Facilitation of access to care and overcoming barriers to care and how this is achieved.
Adverse events.
Secondary outcomes
Study withdrawal.
Time off school or work.
PEF monitoring and diary monitoring.
Spirometry (FEV1, FVC).
Patient satisfaction.
Costs from the perspective of healthcare providers.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and this was supplemented by handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details. The search was from the database's inception, i.e. 1990 to January 2010). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
telehealth* or tele‐health* or telemedicine* or tele‐medicine* or internet* or computer* or web* or interactive* or telecommunication* or telephone or phone or SMS or tele‐monitor* or telemonitor* or telemanagement or tele‐management or teleconsultation or tele‐consultation or telecare* or tele‐care* or telematic* or telepharmacy or tele‐pharmacy or telenurs* or tele‐nurs* or video or email or e‐mail or "remote consult*" or wireless or bluetooth or tele‐homecare or telehomecare or "remote care" or tele‐support or telesupport or "mobile healthcare" or "computer mediated therapy" or ehealth or e‐health or mhealth or m‐health
Searching other resources
In an attempt to uncover additional relevant published data, grey literature, unpublished data and research in progress we:
contacted authors of the identified articles and asked them to identify other published and unpublished randomised controlled trials (see Table 1);
searched the references of all included articles for further randomised controlled trials;
searched the UK National Institute for Health Research Register: https://portal.nihr.ac.uk/ Pages/ NRRArchive.aspx; and
searched web sites listing ongoing trials: http://clinicaltrials.gov/; http://www.controlled‐trials.com/ and http://www.actr.org.au/ (see Characteristics of ongoing studies).
1. Author contact table.
| Author | Date replied |
| Barbanel | |
| Bynum | 11 February 2010 |
| Chan | 18 March 2009 |
| Chatkin | |
| Clark | |
| Cruz‐Correia | 17 March 2009 |
| De Jongste | |
| Donald | |
| Gruffydd‐Jones | 26 January 2010 |
| Guendelman | |
| Jan | |
| Khan | |
| Kokubu | |
| Ostojic | 27 January 2010 |
| Pinnock | 08 March 2009 |
| Rasmussen | |
| Van der Meer | 27 January 2010 |
| Vollmer | |
| Willems |
Data collection and analysis
Selection of studies
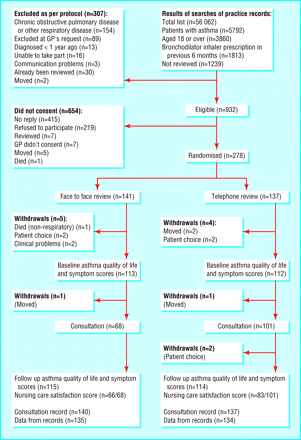
The search strategy above was implemented by SM and DC with support from Liz Arnold (Trials Search Co‐ordinator in the Cochrane Airways Group). We imported identified references into Endnote and deleted duplicates. SM and DC independently checked the titles and abstracts of potentially eligible studies. We obtained full‐text copies of potentially relevant studies and SM and DC assessed their eligibility for inclusion against the criteria outlined above. Disagreements were resolved through discussion between SM and DC or in the case of agreement not being reached, AS arbitrated. We set out reasons for exclusion in Characteristics of excluded studies. For a PRISMA diagram of study selection see Figure 1.
1.
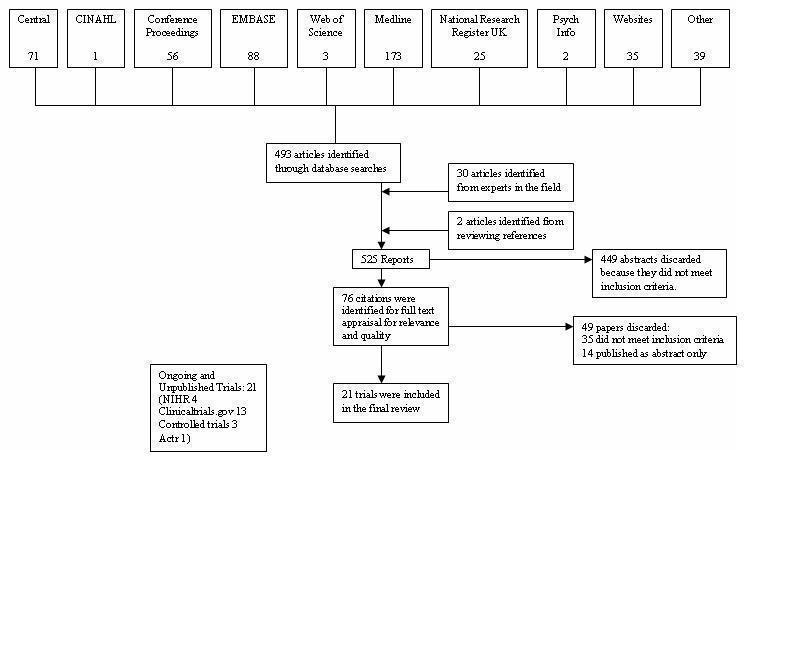
PRISMA flow diagram
Data extraction and management
The following data were, where available, independently extracted from the included studies by two review authors (SM and UN).
Country and setting
Design
Participants (N, mean age, age range)
Description of intervention ‐ system of telehealthcare being investigated and control group management
Outcome measures
Quality of life
Health care utilisation (emergency department visits, hospitalisation)
Symptoms
Access ‐ evidence of facilitated access and improved services or barriers overcome.
Patient satisfaction
PEF monitoring and diary monitoring
Spirometry FEV1 and FVC
Cost data, from the perspective of healthcare providers
Study withdrawal
Adverse events
Assessment of risk of bias in included studies
The quality of each trial was assessed following the Cochrane approach using the methods detailed in section six of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We concentrated on the following parameters to assess quality:
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated intervention adequately prevented during the study (blinding)?
Were incomplete outcome data adequately addressed?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a high risk of bias?
Each parameter was given a judgement as follows:
'Yes' ‐ a low risk of bias, 'No' ‐ a high risk of bias or 'Unclear' ‐ uncertain risk of bias.
Measures of treatment effect
We consider below in detail the clinical and methodological appropriateness of assessing outcomes and synthesising data across studies.
Unit of analysis issues
We calculated summary statistics for our primary outcome measures. For dichotomous variables, we calculated an odds ratio (OR) and for continuous variables we calculated mean differences (MD).
Dealing with missing data
We used the authors' published data. In most cases, data had been analysed on an intention‐to‐treat basis. If a study did not report a particular variable we attempted to contact authors. If data were still unavailable we did not include the study in the meta‐analysis.
Assessment of heterogeneity
We considered the clinical heterogeneity between studies, only deciding to pool data if it was considered clinically meaningful to do so. We assessed the statistical heterogeneity between studies and the likely impact of this heterogeneity on meta‐analysis using the I2 statistic (Higgins 2009). Where this was 40% or less, we used a fixed‐effect model. If the studies were statistically heterogeneous (I2 statistic > 40%) we investigated the potential cause of heterogeneity through subgroup and sensitivity analyses. In such cases, if the heterogeneity could not be explained, we used a random‐effects model.
Assessment of reporting biases
We used funnel plots to assess possible reporting and publication bias.
Data synthesis
We presented pooled data graphically using forest plots. In cases where it was not appropriate or possible to quantitatively pool data, we produced a narrative summary of findings.
Subgroup analysis and investigation of heterogeneity
We investigated the potential causes of heterogeneity using subgroup analysis. Subgroup analysis took account of source of patients, whether a high‐risk secondary care group or potentially lower‐risk primary care population with asthma, and type of intervention (whether telephone, video, web or other networked or text message (SMS)).
Sensitivity analysis
We conducted sensitivity analyses on the basis of risk of bias in studies, excluding studies judged to be at highest risk of bias.
Results
Description of studies
(See Figure 1). Our searches found 525 titles and abstracts and following review we considered 76 to be relevant. After detailed examination of the 76 full texts, 21 trials satisfied our inclusion criteria. In addition, we found 14 ongoing trials that have reported only as abstracts (see Table 2) and a further 21 trials that have yet to report in any format. Two studies had to be translated from Japanese and one from Italian. It was only possible to obtain partial translations of the Japanese reports and so the information is taken from the studies' figures which were published largely in English.
2. Included abstracts.
| Author | Date | Title |
| Anderson | 2007 | Does a www‐based interactive computer program change asthma outcomes, quality of life and asthma knowledge? |
| Bateman | 2000 | A computer‐based home‐monitoring disease management programme, PulmAssist Plus ® (PAP) achieves significant improvement in quality of life and healthcare costs in moderate and severe asthma |
| Cicutto | 2009 | Telephone intervention‐based strategies to increase the completion and use of asthma action plans for adults with asthma |
| Finkelstein | 2005 | Evaluation of home telemanagement in adult asthma patients |
| Finkelstein | 2005 | Impact of home telemanagement in adult asthma |
| Lee | 2005 | Can interactive multimedia increase asthma knowledge? |
| Liu | 2007 | A novel mobile phone‐based self‐care system improves asthma control |
| Neville | 2001 | Assessment for a computer‐assisted assessment and management programme for asthma care |
| Phanareth | 2002 | Using the Internet as a tool for the management of asthma disease |
| Shanovich | 2008 | Nurse case management services provided to supplement a web‐based asthma education program |
| Van den Berg | 2002 | Is the availability of a 24‐hour asthma telephone useful in the implementation of asthma treatment guidelines for children aged 6 to 16 among general practitioners? |
| Van der Meer | 2008 | Internet‐based self‐management improves short‐term asthma control: the SMASHING study |
| Vollmer | 2009 | Use of automated phone calls to support inhaled corticosteroids adherence |
| Wiecha | 2007 | BostonBreathes: a RCT to improve paediatric asthma care with a home‐based interactive website for patient education monitoring and clinical teamwork |
Results of the search
See Figure 1 for details of how we selected the 21 studies that satisfied our inclusion criteria.
Included studies
Two studies used pharmacists as the main deliverer of the telehealthcare intervention (Bynum 2001; Barbanel 2003) and the rest used a combination of doctors (both general practitioners and specialists) and nurses, including specialist nurses.
The most common model for intervention was to have an initial face‐to‐face introductory session and then follow up using telephone, telephone and web, web/other networked system or text message. This approach featured in the following studies: Barbanel 2003; Clark 2007; Donald 2008a; Guendelman 2002; Jan 2007; Ostojic 2005; Willems 2008.
Pinnock 2003 and Donald 2008a published follow‐up papers dealing with the costs and cost‐effectiveness of the interventions (Donald 2008b; Pinnock 2005). Willems 2007a, Willems 2007b and Willems 2008 refer to only one trial of which Willems 2007a is a process evaluation, Willems 2007b publishes cost‐effectiveness data and Willems 2008 is the main report. Kokubu 1999 was expanded on in Kokubu 2000 with the addition of more data and a section on costs; however, this was hard to interpret given the incomplete translations.
In terms of the major telecommunication devices used in the studies, overall nine studies used the telephone (Barbanel 2003; Chatkin 2006; Clark 2007; Donald 2008a; Gruffydd‐Jones 2005; Khan 2004; Pinnock 2003; Pinnock 2007; Vollmer 2006). Two studies used video (Bynum 2001; Chan 2007). In Bynum 2001 videoconferencing was used to deliver education on inhaler technique and in Chan 2007 participants submitted repeated videos for checks of their inhaler technique via modem. One study used text messaging (Ostojic 2005). Two studies used the Internet (Cruz‐Correia 2007; Rasmussen 2005). Other networked systems were used by six trials (de Jongste 2009; Guendelman 2002; Jan 2007; Kokubu 1999; Kokubu 2000; Willems 2008). Van der Meer 2009 used text or internet.
Excluded studies
There were a number of reasons for excluding studies. These are all detailed in Characteristics of excluded studies. Most often studies were excluded because they did not fulfil our definition of telehealthcare, i.e. there was not a two‐way exchange of information between patient and healthcare professional. If the intervention involved only education without feedback or if feedback was only mechanical in nature, e.g. from a peak flow meter and not involving a professional, then the study was excluded. In addition, we excluded studies if they were found not to employ a randomised controlled design or if they were not studying an asthma population.
Risk of bias in included studies
Allocation
Fifteen trials used appropriate randomisation (Barbanel 2003; Bynum 2001; Chan 2007; Clark 2007; Cruz‐Correia 2007; de Jongste 2009; Gruffydd‐Jones 2005; Jan 2007; Khan 2004; Kokubu 2000; Ostojic 2005; Pinnock 2003; Pinnock 2007; Van der Meer 2009; Willems 2008). For the remaining studies the methods of allocation were either unclear (Chatkin 2006; Donald 2008a; Guendelman 2002; Kokubu 1999; Vollmer 2006) or the authors used an inappropriate method such as consecutive randomisation (Rasmussen 2005). The most common method of randomisation was to use a random number table, often computer‐generated; however, other acceptable methods were also used, including tossing a coin. In most studies which included details of concealment, sealed envelopes were used. However, some studies appeared to use a centralised randomisation hub, but this was often unclear.
Blinding
Four studies (Barbanel 2003; Clark 2007; Donald 2008a; Khan 2004) made some attempt to blind researchers as to the group allocation of their participants. The remainder did not and this may have introduced bias. Guendelman 2002 used self‐reporting of outcomes to the nurse co‐ordinator so that the same person was both involved with delivering the intervention and assessing outcomes, thus substantially increasing the risk of bias. In Pinnock 2003, where blinding was not feasible due to the pragmatic nature of the trial, an independent researcher validated a 20% sample of the results.
Incomplete outcome data
Several studies had high drop‐out rates (Bynum 2001; Donald 2008a; Gruffydd‐Jones 2005; Jan 2007, Khan 2004). In Pinnock 2007, the uptake rate and patient population dynamics were being studied as a primary outcome measure because it was a pragmatic phase IV implementation trial (as per Medical Research Council 2008) (see Figure 2).
2.
Selective reporting
Research protocols were not sought for any of the studies. There was nonetheless some evidence of selective reporting of results. In most studies, all outcomes specified in the methods section were reported in the results, however there were some exceptions, for example in Jan 2007 the data on satisfaction were not reported and Cruz‐Correia 2007 did not report quality of life data. These last points do not seem to relate to selective reporting, but rather other problems.
Other potential sources of bias
Variable efforts to recruit from ethnically diverse and marginalised populations may have impacted on the external validity of the findings. There was variable consideration of smoking. Patients with paper diaries filled in more than one day's entry at a single time point thereby opening the data to recall bias. Some studies recruited from academic centres rather than from primary care which may limit the generalisability of findings.
Effects of interventions
Primary outcomes ‐ clinical
Asthma quality of life
The impact of telehealthcare interventions on disease‐specific quality of life was assessed in 14 trials (Chan 2007; Clark 2007; de Jongste 2009; Donald 2008bGruffydd‐Jones 2005; Jan 2007; Khan 2004; Kokubu 2000; Pinnock 2003; Pinnock 2007; Rasmussen 2005; Van der Meer 2009; Vollmer 2006; Willems 2008). The effect of treatment is shown in the forest plot (Figure 3).
3.
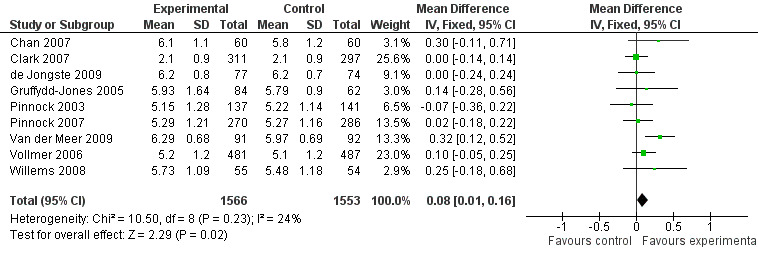
Forest plot of comparison: 1 Asthma quality of life questionnaires, outcome: 1.1 AQLQ Juniper mean scores.
Five of these studies(Clark 2007; Gruffydd‐Jones 2005; Pinnock 2003; Pinnock 2007; Vollmer 2006) used Juniper’s validated Mini‐AQLQ. This instrument contains 15 items which are scored from 7 (no impairment) to 1 (maximum impairment), so high scores indicate better quality of life. Three studies (Rasmussen 2005; Van der Meer 2009; Willems 2008) used Juniper's validated full 32‐item Adult‐AQLQ in which similarly high scores indicate better quality of life. Two studies used Juniper's validated 23‐item Paediatric Asthma Quality of Life Questionnaire (PAQLQ)(Chan 2007; Willems 2008), high scores again indicate better quality of life. Jan 2007 used the Paediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), which is filled in by the patients' parents as did de Jongste 2009 and Khan 2004. There are 13 items in this instrument and in keeping with the other instruments higher scores represent better quality of life and less impairment by asthma (Juniper 1996; Juniper 1999).
As Juniper's quality of life instruments are similarly structured with each question answered on a Likert scale with a minimum value of 1 and a maximum value of 7, we considered it appropriate to perform meta‐analysis of the data derived from these instruments. We performed a meta‐analysis of Chan 2007; Clark 2007; de Jongste 2009; Gruffydd‐Jones 2005; Pinnock 2003; Pinnock 2007; Van der Meer 2009; Vollmer 2006 and Willems 2008. This meta‐analysis of nine studies, yielding a total of 1566 intervention and 1553 control patients, revealed a mean difference of 0.08 point improvement on this scale (95% CI 0.01 to 0.16) in those randomised to intervention compared with controls (see Figure 3). This is lower than the minimal clinically important difference of 0.5 points on the Juniper scale.
It was not possible to include other studies in the meta‐analysis because:
Rasmussen 2005 had used the AQLQ, but could not be included because the data were not normally distributed and so median scores were supplied by the author on request. AQLQ score was 6.42 (IQR 3.62 to 7.00) in the Internet group, 6.31 (IQR 3.98 to 7.00) in the GP group and 6.17 (IQR 1.41 to 7.00) in the specialist group.
Donald 2008b used the Modified Marks Asthma Quality of Life Questionnaire, a validated scale in which a higher score indicated a less detrimental impact on quality of life. A clinically important difference was seen in the intervention group from recruitment to 12 months. There was no clinically important difference seen in the control group in this time.
Kokubu 2000 did not use a validated instrument for the measurement of quality of life. However, they showed a greater improvement in the intervention group than in the control group(P = 0.04). These results, however, need to be interpreted with caution because of the unvalidated nature of the scale and, furthermore, the inability to determine what constituted a minimal clinically important difference.
Jan 2007 used the Juniper's PACQLQ, however insufficient summary statistics were reported for meta‐analysis. Only the caregivers of asthmatic children randomised to the intervention group showed significant improvement after the study compared to before the study.
Khan 2004 used the Juniper's validated PACQLQ. Small increases in median score after the study were not statistically significant for either the control (P = 0.11) or the intervention group (P = 0.6). These data were not normally distributed and so the mean was not used for comparison.
Overall, across all these quality of life studies, these results suggest that telehealthcare does not result in clinically important improvements in quality of life (see Figure 3). The mean difference (fixed‐effect) is 0.08 (95% CI 0.001 to 0.16).
Analysis 1.2 shows a sensitivity analysis (fixed‐effect) of high quality studies at low risk of bias only. This gave a non clinically‐significant mean difference of 0.08 (95% CI 0.00 to 0.16).
1.2. Analysis.

Comparison 1 Asthma quality of life questionnaires, Outcome 2 Sensitivity analysis AQLQ studies judged low risk of bias.
Analysis 1.3 shows a subgroup analysis by mode of communication, i.e. telephone‐based, which suggests that quality of life is not significantly improved by this form of telehealthcare: mean difference 0.04(fixed‐effect) (95% CI ‐0.05 to 0.12).
1.3. Analysis.
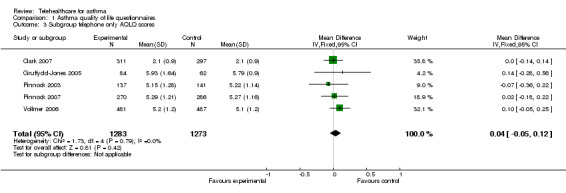
Comparison 1 Asthma quality of life questionnaires, Outcome 3 Subgroup telephone only AQLQ scores.
Analysis 1.4 shows a subgroup analysis by recruitment origin in secondary care. Again, there is no clinically important improvement in quality of life with the use of telehealthcare: non‐significant mean difference (fixed‐effect) 0.11 (95% CI ‐0.08 to 0.30). Analysis 1.5 is a parallel subgroup analysis by recruitment origin in primary care; again there is no significant improvement in quality of life: a fixed‐effect analysis give a mean difference of 0.11 units (95% CI 0.02 to 0.21).
1.4. Analysis.
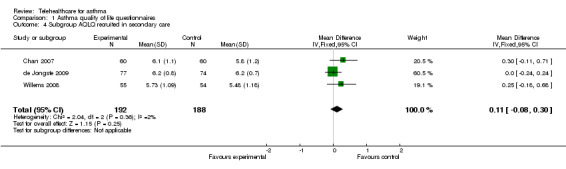
Comparison 1 Asthma quality of life questionnaires, Outcome 4 Subgroup AQLQ recruited in secondary care.
1.5. Analysis.
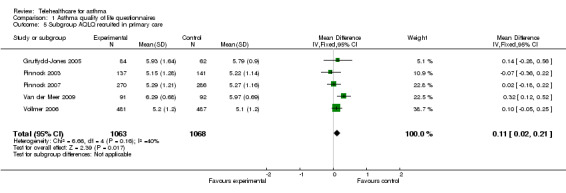
Comparison 1 Asthma quality of life questionnaires, Outcome 5 Subgroup AQLQ recruited in primary care.
We found no evidence of publication bias (see funnel plot in Figure 4).
4.
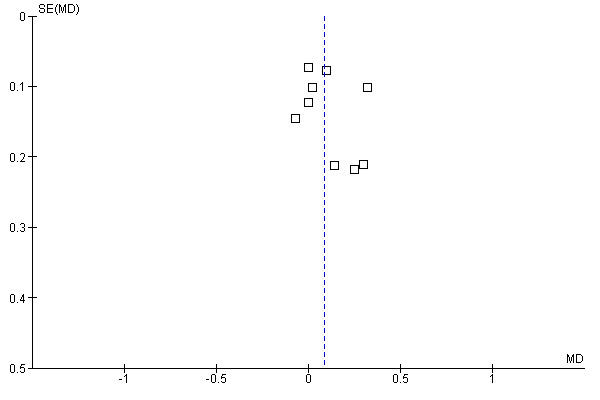
Funnel plot of comparison: 1 Asthma quality of life questionnaires, outcome: 1.1 AQLQ Juniper mean scores.
Emergency department visits
Ten studies reported data on emergency department visits (Chan 2007; Clark 2007; Donald 2008a; Guendelman 2002; Khan 2004; Kokubu 2000; Pinnock 2003; Rasmussen 2005; Vollmer 2006; Willems 2008).
The effect of telehealthcare interventions on emergency department visits over 12 months is shown in Figure 5. This meta‐analysis included five trials (Chan 2007; Donald 2008a; Kokubu 2000; Rasmussen 2005; Willems 2008) representing 619 patients in total. It revealed a non‐significant increase in the odds of emergency department attendance: OR 1.16 (95% CI 0.52 to 2.58).
5.
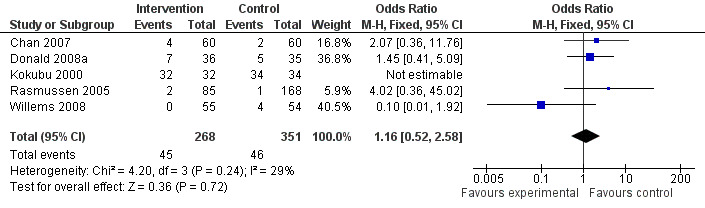
Forest plot of comparison: 2 One or more emergency dept visit in 12 months, outcome: 2.2 Emergency department in 12 months.
In Kokubu 2000, the authors reported a greater reduction of night and daytime emergency room visits per patient in the control group than in the intervention group. It appears from their data that all patients in both arms were admitted to the emergency department at least once at some point during the study; it was therefore not possible to include these data in the meta‐analysis. The absolute numbers of visits (presumably more often than one per patient) are not given in the study's English tables.
Of the other studies not included in the meta‐analysis, Khan 2004 reported emergency department visits over a six‐month interval and so could not be included in the meta‐analysis, but again numbers were very small with only one or two patients attending from each arm over the study period. Clark 2007 reported only within‐group analyses. There were no emergency department consultations in Pinnock 2003. Vollmer 2006 did not distinguish between emergency department care and full hospitalisation, therefore it was not possible to include these results in the meta‐analysis.
de Jongste 2009 reported survival analysis by means of a Kaplan‐Meier curve of time to first prednisolone course, emergency visit or hospitalisation or to whichever came first. There were a total of 31 events in these categories, but the detailed breakdown of data was not reported and so the data could not be included in meta‐analysis. However, the time to the first emergency department visit was considered comparable across the two arms of the study (P = 0.13).
The remaining trials (Barbanel 2003; Bynum 2001; Chatkin 2006; Cruz‐Correia 2007; Donald 2008b; Gruffydd‐Jones 2005; Kokubu 1999; Jan 2007; Ostojic 2005; Pinnock 2007; Van der Meer 2009) did not include data on emergency department visits.
We produced a funnel plot (see Figure 6) for the studies which contained data on emergency department visits over 12 months. It is difficult to determine reliably whether publication bias was an issue as the plot only included data from five studies.
6.
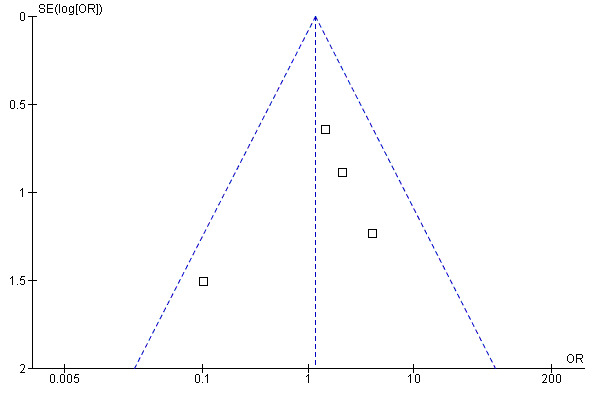
Funnel plot of comparison: 2 One or more emergency dept visit in 12 months, outcome: 2.2 Emergency department in 12 months.
Analysis 2.3 and Analysis 2.4 show emergency department visits over 12 months separated according to the origin of their patients, whether from primary or secondary care. Willems 2008 is not included because patients in this study had a mix of origins. Note the very wide confidence intervals in these data, which is a function of there being very few events in these studies.
2.3. Analysis.
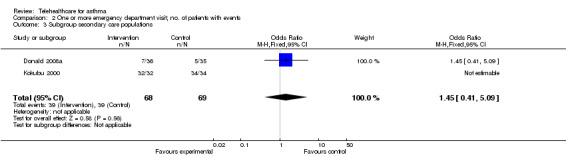
Comparison 2 One or more emergency department visit; no. of patients with events, Outcome 3 Subgroup secondary care populations.
2.4. Analysis.
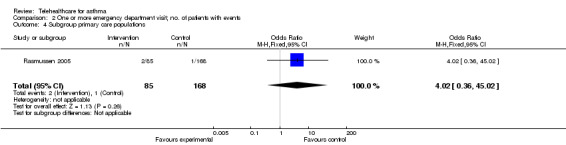
Comparison 2 One or more emergency department visit; no. of patients with events, Outcome 4 Subgroup primary care populations.
Hospitalisations
Six studies (Chan 2007; Donald 2008b; Guendelman 2002; Kokubu 2000; Ostojic 2005; Rasmussen 2005) presented data on hospitalisations. For two studies (Guendelman 2002; Ostojic 2005) these hospitalisations occurred over a three‐month period. For the remaining four studies the hospitalisations were recorded as occurring over a 12‐month period (Chan 2007; Donald 2008b; Kokubu 2000; Rasmussen 2005).
Meta‐analysis of the two studies (Guendelman 2002; Ostojic 2005) that reported data from hospitalisations over three months of study duration is shown in the forest plot in Figure 7. This includes data from 138 patients. Overall, there was no significant difference in the odds of hospitalisation in the intervention groups when compared to the control group (OR 0.47, 95% CI 0.010 to 36.46). The confidence intervals were very wide. The funnel plot of these data (Figure 8) shows no evidence of publication bias.
7.

Forest plot of comparison: 1 Hospitalisation, outcome: 1.2 Proportion hospitalised in 3 months of study.
8.
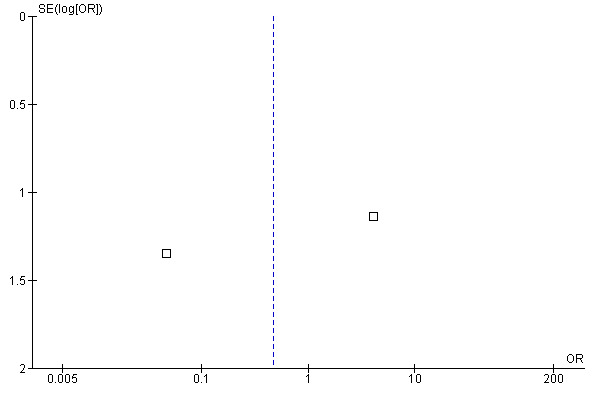
Funnel plot of comparison: 2 Proportion hospitalised in 12 months of study, outcome: 2.1 Proportion hospitalised in 3 months of study.
Meta‐analysis of the four studies (Chan 2007; Donald 2008b; Kokubu 2000; Rasmussen 2005) which reported on hospitalisation within 12 months of randomisation is shown in Figure 9. This includes data from 499 patients. This gave an OR of 0.21 (95% CI 0.07 to 0.61) indicating that telehealthcare reduces the risk of hospitalisation. Chan 2007, Donald 2008b and Rasmussen 2005, however, cross the line of no difference. These studies are large and of reasonable quality. In contrast, Kokubu 2000 which contributes a weight of 55.6%, shows a clearly beneficial effect. This trial focused on patients with severe asthma. Patients who had visited the night emergency department room three times or more within a year in spite of corticosteroid therapy were selected and so these patients were not representative of a typical asthma population. In the Kokubu study, 2/32 intervention patients were hospitalised and 11/34 of the control patients were hospitalised. Overall, it therefore seems as though telehealthcare interventions may prevent hospitalisations in selected high‐risk populations studied over long timescales. Figure 10 suggests no evidence of publication bias, although given the small number of studies it is important to interpret this plot with caution. Analysis 3.5 repeats the meta‐analysis without the Kokubu study this time resulting in a non‐significant reduction in the risk of admission (OR 0.30, 95% CI 0.07 to 1.25). This was done as a sensitivity analysis to show how dependent the results of the 12‐month hospitalisation study had been on one study which may have had some unknown methodological flaws as we only had access to a partial translation of this trial report.
9.

Forest plot of comparison: 3 Proportion hospitalised in 12 months of study, outcome: 3.2 Proportion hospitalised in 12 months of study.
10.
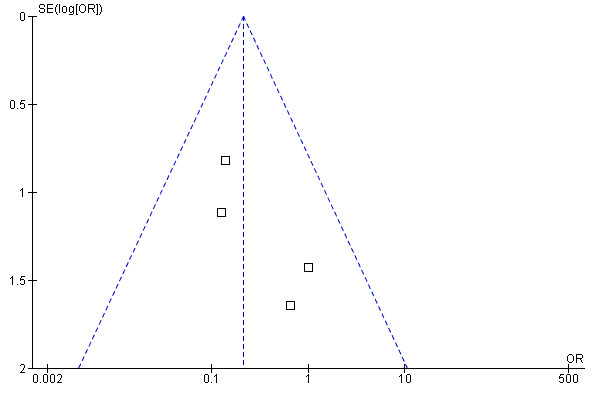
Funnel plot of comparison: 3 Proportion hospitalised in 12 months of study, outcome: 3.2 Proportion hospitalised in 12 months of study.
3.5. Analysis.
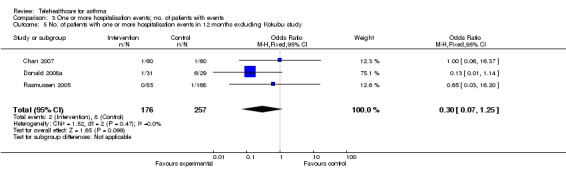
Comparison 3 One or more hospitalisation events; no. of patients with events, Outcome 5 No. of patients with one or more hospitalisation events in 12 months excluding Kokubu study.
There were no hospitalisations during the period of follow up in Pinnock 2003 or in the Khan 2004 study. Numbers of hospitalisations were indistinguishable from emergency department visits in Vollmer 2006. Chatkin 2006 reported hospitalisations over the three‐month period of its trial, but it was unclear as to whether only data for the control group were presented and so the study was not included in the meta‐analysis.
de Jongste 2009 reports survival analysis (Kaplan‐Meier curves) of time to first hospitalisation for both groups with P = 0.13 for intention‐to‐treat analysis. From the curve approximately 5% of the intervention group were hospitalised once or more and 15% of the control group, however as these figures are rough visual estimates they were not used in the meta‐analysis.
Gruffydd‐Jones 2005 did not clearly report on the number of hospitalisations, referring instead to mean length of inpatient stay. Cruz‐Correia 2007 did not report on hospitalisation as an outcome. Jan 2007 and Pinnock 2007 did not report on hospitalisations.
Overall, hospitalisation was an infrequent outcome. In the forest plot for hospitalisations in studies over a three‐month period(Figure 7) it can be seen that telehealthcare is not associated with a reduction in hospital admissions. However, there is a reduction over 12 months(Figure 9). This may in particular point to a role for telehealthcare to reduce hospitalisation in high‐risk individuals. The funnel plot in Figure 10 does not show any clear publication bias.
To test for the causes of heterogeneity according to the a priori defined subgroups in the Methods section, we analysed results from the participants originating in secondary care, then participants originating in primary care(Analysis 3.3; Analysis 3.4).
3.3. Analysis.
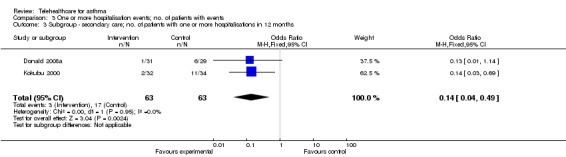
Comparison 3 One or more hospitalisation events; no. of patients with events, Outcome 3 Subgroup ‐ secondary care; no. of patients with one or more hospitalisations in 12 months.
3.4. Analysis.
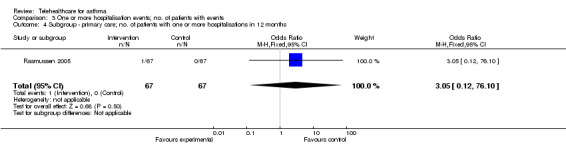
Comparison 3 One or more hospitalisation events; no. of patients with events, Outcome 4 Subgroup ‐ primary care; no. of patients with one or more hospitalisations in 12 months.
Other primary outcomes
Symptoms
The following 17 studies reported on symptoms as an outcome measure: Barbanel 2003; Chan 2007; Chatkin 2006; Clark 2007; de Jongste 2009; Gruffydd‐Jones 2005; Guendelman 2002; Jan 2007; Khan 2004; Kokubu 1999; Ostojic 2005; Pinnock 2003; Pinnock 2007; Rasmussen 2005; Van der Meer 2009; Vollmer 2006; Willems 2008.
In Barbanel 2003, symptom scores improved in the intervention group when compared to the control group over the three months of the study; the difference between groups at three months adjusted for baseline scores was 7.0 (95% CI 4.4 to 9.5, P < 0.001), on a scale of 10 to 40. This difference remained significant when adjustments were made for missing data.
Chan 2007 reported the number of symptom‐free days recorded by each group, but the difference between groups was not significant (P = 0.13, our calculation).
In the Clark 2007 study, there was a small drop in the average number of nights with night‐time symptoms per month experienced by the women following the intervention (from 5.1 to 3.7, i.e. ‐1.4 nights). However, across groups there was no difference in average number of nights with night‐time symptoms per month: control group 3.8 nights, intervention group 3.7 nights.
Wheezing‐related sleep disturbances were studied by Chatkin 2006, but they did not use a validated questionnaire.
de Jongste 2009 calculated the within‐group percentage of symptom‐free days. This was found to improve significantly with P < 0.001 in both groups. The authors speculate that this may be due to frequent monitoring and telephone contacts in both arms, which could not be further improved by adding the nitric oxide telemonitoring. There was no difference across groups, the baseline‐adjusted difference in mean percentage of symptom‐free days was 0.3%, (SD ‐10% to 11%, P = 0.95).
Guendelman 2002 reported asthma control problems between groups as not significantly different at 12 weeks (P = 0.07).
Khan 2004 reported that there was no significant difference between groups in their primary outcome of wheezing in the last three months.
Gruffydd‐Jones 2005 reported the mean of individual changes in the Asthma Control Questionnaire (ACQ), a validated questionnaire, over 12 months. The clinic group changed by ‐0.11 (‐0.32 to 0.11) and the telephone group by ‐0.18 (0.38 to 0.02), this representing a non‐significant improvement in asthma control (P = 0.35 when adjusted for baseline differences) (a negative change in ACQ is an improvement).
Between group differences were significant in Jan 2007 for the Paediatric Asthma Control Test scores change from baseline at 12 weeks: the intervention group had a significant decrease of night‐time (P = 0.028) and daytime (P = 0.009) symptoms compared with the children in the control group. There were no between‐group comparisons in this study.
Kokubu 2000's method for analysing the symptom score remains obscured by the lack of a full translation of the Japanese paper.
Ostojic 2005 used a bespoke symptom score which produced significant results across scores for the study group and control group, demonstrating that the control group had more symptoms during the study. Scores for cough were 1.42 (SD = 0.28) for the study group and 1.85 (SD = 0.43), P = 0.028, and scores for sleep quality were: study group 0.85 (SD = 0.32) and control group 1.22 (SD = 0.23), P = 0.021. However, it is not reported whether this symptom scoring system had been validated.
Pinnock 2003 measured symptom scores using the Short Q asthma morbidity score three months after randomisation and found these to be similar in the two groups. Face‐to‐face consultations had a mean score of 1.96 (SD 1.96) and telephone consultations had a mean score of 2.10 (SD 2.16), difference 0.41 (‐0.41 to 0.68) (P = 0.62), i.e. a non‐significant difference.
Pinnock 2007 used the validated ACQ and found a non‐significant mean difference of 0.12 (‐0.06 to 0.31) (P = 0.19) for the telephone option versus face‐to‐face only.
Rasmussen 2005 reported an improvement in symptoms in the Internet versus specialist groups: OR 2.64 (95% CI 1.43 to 4.88, P = 0.002); and also in the Internet versus GP groups: OR 3.26 (95% CI 1.71 to 6.19, P < 0.001). Here the Internet group showed the greatest likelihood of improvement over six months.
Van der Meer 2009 used the validated ACQ to compare groups. The Internet group showed greater improvement of asthma control than did the usual care group: change from baseline ‐0.54 versus ‐0.06; adjusted difference ‐0.47 (CI ‐0.64 to ‐0.3) which was just slightly smaller than a clinically relevant difference of ‐0.5 (where negative change represent improvements).
Vollmer 2006 assessed several markers of symptom status including the Asthma Therapy Assessment Questionnaire (ATAQ), asthma impact score, self‐reported health status, self‐assessed severity of asthma in the past four weeks and nocturnal waking in the past four weeks. No significant difference between the telephone and usual care groups were found for any of these outcomes (ATAQ P = 0.56, asthma impact score P = 0.46, self‐reported health status P = 0.08, self‐assessed severity of asthma in the past four weeks P = 0.22 and nocturnal waking in past four weeks P = 0.84).
Willems 2008 recorded the changes in asthma symptoms of coughing, production of sputum and shortness of breath/wheezing, however no statistically significant differences in improvement in any of the symptoms were observed.
The above results suggest that symptom scores may be improved with telehealthcare. However, in many cases there was no difference between groups. Future research should use validated scoring systems which can then be pooled in meta‐analysis to give a more accurate picture of the extent to which telehealthcare interventions improve symptom scores.
Improved access
Improved access was clearly demonstrated in the Pinnock 2003 study. Of patients randomised to receive the telephone review, 74% of patients were reviewed, whereas only 48% of patients in the surgery‐only group, were reviewed. There was therefore a significant improvement of 26% (95% CI 14% to 37%; P < 0.001). Asthma‐related morbidity at three months (in terms of acute exacerbations of asthma) P = 0.68; emergency bronchodilation P = 0.97; and steroid courses for asthma P = 0.64) were not significantly different across groups, and neither was quality of life (Juniper scores P = 0.69). And so in this study access was improved with no loss in quality and at no additional cost (see later section on cost).
Access in Pinnock 2007 was also improved: the proportion reviewed was 66.4% of patients in the telephone option group compared with 53.8% in the face‐to‐face only group.
Chan 2007 treated the control group with an ambulatory asthma clinical pathway with six visits scheduled over the period of 12 months after the initial visit. The intervention group had three in‐person visits at 0, 26 and 52 weeks and the rest as virtual visits using video‐conferencing technology.
In Gruffydd‐Jones 2005, 35% more asthma patients received their annual review in the telephone group than in the clinic group and the cost of the telephone group was less.
In Vollmer 2006, there was evidence of a negative reaction to automated computerised calling and the intervention was more successfully delivered in the live caller arm (P < 0.001)
Adverse events
In Gruffydd‐Jones 2005 study, two patients in the telephone triage arm died. Following contact with the author it was confirmed that these were not asthma‐related deaths.
Overall, studies did not report adverse events, with the exception of Rasmussen 2005 which found that an increase in corticosteroid dose was likely to result in oral candidiasis or dysphonia. In this study at follow up an increase in inhaled corticosteroids was found in all groups, but significantly more patients in both the Internet and specialist groups were using inhaled corticosteroids than in the general practice group.
Secondary outcomes
Study withdrawal
See also incomplete outcome data in Characteristics of included studies.
Barbanel 2003: lost one patient when (s)he moved away.
Bynum 2001: 49 students were randomised. Three did not attend any visit, eight had never used a metered dose inhaler (MDI) before and so did not meet the inclusion criteria (the study does not explain why they were not screened out before randomisation) and two students did not attend the follow‐up visit.
Chan 2007: 127 children were assessed for eligibility: one did not meet the inclusion criteria, five refused to participate and one was not able to participate. The study report does not give further details of why not. One hundred and twenty children were randomised, 11 were lost to follow up, seven discontinued because they moved and so 55 were included in the analysis for the control group and 47 remained for analysis in the intervention group.
Chatkin 2006: 293 patients were screened for participation, four were excluded for not fulfilling the inclusion criteria, eight for not responding to the telephone calls and 10 for not returning their inhaler disk to the study office. In the final analysis control group n = 131 and intervention group n = 140.
Clark 2007: 808 women provided baseline data and mailed a consent form back following the mailing of 2336 invitation letters. Four hundred and twenty‐four were randomised to the intervention group and 384 to the control group. There was then attrition of 133 participants from the intervention group and 87 participants from the control group. The reasons for this were not reported.
Cruz‐Correia 2007: 21 patients were included from 28 patients assessed in the outpatient clinic. Patients were only included if they used the Internet. Sixteen patients provided their opinions on the monitoring instruments. Four patients did not use the monitoring tools because of technical problems with the instruments.
de Jongste 2009: 151 children were randomised and four children were excluded, two due to non‐compliance, one because he or she had been inappropriately included and did not have allergy and the final child had moved abroad and was unable to transfer data. All others completed the study.
Donald 2008a: 660 patients were assessed for eligibility, 385 could not be contacted, 154 did not want to take part in the study 31 were excluded and 19 did not attend the first meeting. Following randomisation there were 36 in the intervention group with 31 left at the end of the study for analysis and 35 controls, of which 29 were left in the final analysis. The study did not discuss why participants withdrew following randomisation.
Gruffydd‐Jones 2005: 97 patients were randomised to the control group, only 82 attended their first visit and 62 completed the study, of which 28 completed all visits and 34 completed two visits only. Ninety‐seven patients were similarly randomised to the telephone group, 90 attended their baseline visit, three patients left the area, two patients died and 84 completed all three visits. An explanation for the final patient was missing from the patient flow diagram.
Guendelman 2002: Reasons for withdrawing from the study included moving out of the area (n = 3) or life crisis (n = 4). Five families who dropped out were unavailable for contact by the study authors to find out their reasons.
Jan 2007: 184 families were eligible with access to the Internet. Five families declined to participate as they were "too busy", "not interested" or found it "too complex to perform the diary card". Fifteen participants were excluded because of either a request from the parents or a lack of data due to Internet failure.
Khan 2004: 310 children were enrolled in this study, 266 completed the follow‐up questionnaires. The reasons for non‐response were not explored although in two cases a search of the telephone directory enabled the discovery of a new address.
Kokubu 1999: Two patients withdrew from the intervention group and one from the control group, however the reasons for this are not described.
Kokubu 2000: Five patients withdrew from the intervention group and four from the control group but the reasons for this are not described in the report according to the translation we have available.
Ostojic 2005: Patients unfamiliar with text SMS or without consistent access to a cell telephone were excluded. After enrolment no patient withdrew from the study.
Pinnock 2003: See flow diagram (Figure 11) reproduced under licence.
11.
Pinnock 2007: This is a phase IV implementation study and so there is no selection of participants. It instead takes place within the real‐world situation of general practice where patients move, die or their asthma becomes more active or becomes inactive or is re‐diagnosed as COPD or patients refuse to take part. This reflects the real‐world population. See Figure 2, reproduced under licence.
Rasmussen 2005: 300 subjects were enrolled who fulfilled the criteria for asthma, 253 subjects completed the trial. Reasons for the withdrawals were not given.
Van der Meer 2009 recruitment was undertaken from 37 general practices and continued until there were 200 patients entered in the study. Results were available for 183 patients. Twenty patients did not complete the three‐month questionnaire, eight patients were lost to follow up and nine patients withdrew consent.
Vollmer 2006: It is difficult to assess withdrawal in this study as it was devised to compare usual care with automated telephone outreach and the outcome measures were taken from a representative sample. However, participation statistics were low for the intervention arm, only 38% participated in the first round of calls, 32% in the second round and 18% in the third round. Overall 47% of the intervention group had one call and 12.1% completed all three calls. Compared with those who did not participate in any call, participants were more likely to be female, take more inhaled corticosteroids and report worse asthma‐specific quality of life at baseline. Interestingly 59.9% of the live‐caller arm completed at least one call and 27.6% all three calls, suggesting that patients preferred a live call to the automated calls.
Willems 2008: 274 patients were assessed as potentially eligible from patient records. Eighteen did not have a house phone connection and so were excluded as not meeting inclusion criteria. One hundred and forty‐seven refused to participate because of the following reasons: no time (n = 63), not interested (n = 28), no symptoms (n = 18), too confronting (n = 13) and other reasons (n = 25). Therefore, 109 were enrolled, seven were lost to follow up, six refused participation continuation and one person emigrated.
Time off school or work
Clark 2007 reported the yearly average number of days missed work per month as 3.0 (SD = 7.1) for the control group and 2.3 (SD = 6.2) for the intervention group (P = 0.14).
In the Donald 2008a trial, 24 intervention group participants worked or studied and lost 10 days over 12 months, and 25 of the control group participants lost a total of 11 days; the difference between the groups was not significant (P = 0.58).
In Guendelman 2002, the odds of missing school in the past six weeks in the Health Buddy group were 0.74 (95% CI 0.37 to 1.5).
In summary, it appears that these telehealthcare interventions did not reduce time off work or school.
PEF monitoring and diary monitoring
Chan 2007; Jan 2007; Willems 2008; Ostojic 2005 report PEF flow monitoring.
The initial improvements in inhaler technique seen in both arms of Chan 2007 can be attributed to the monitoring of participants by healthcare professionals. As monitoring by the health professionals dropped in the second 26 weeks of the study to a telephone call once a week in the control group, the participants' inhaler technique scores dropped off. Mean peak flow (+/‐SD) as a percentage of personal best was reported as 91.6% (+/‐ 27.2) in the intervention group and 100% (+/‐17.6) in the control group at the end of the study.
Monitoring, or more accurately "prompting", was also important in Chatkin 2006, a study in Brazil. A twice‐weekly telephone call made by a trained student nurse to the patients in the experimental arm resulted in an inhaler adherence rate of 74.3 % whereas the rate in the control arm was only 51.9% (number needed to treat to benefit 4.5, i.e. telephone calls to 4.5 patients were required to prevent one non‐adherence or missed inhaler dose). The content of the telephone call was individualised to each patient.
In keeping with this advantage of monitoring, Kokubu 1999 also found that those patients who checked their peak flow most regularly tended to have the best peak flows. Patients who did not check or transmit peak flow data regularly remained poorly controlled.
In Jan 2007, at week 12, children in both groups had a significantly increased PEF. Between‐group differences are reported as non‐significant.
Willems 2008 reports rank correlations of lung function values (PEF and FEV1) with morning symptoms and evening symptoms as moderate to high. Absolute values were not reported in the study.
Peak flow in L/min was measured by Ostojic 2005 as mean PEF by time of day(morning, afternoon, evening) none of which were significant. However, PEF variability (%) in the text message intervention group (16.12 +/‐ 6.93) and the control group (27.24 +/‐ 10.01) showed a significant difference (P = 0.049).
Therefore, overall it can be seen that telehealthcare improved PEF in some studies, but that this was not a consistent finding. However monitoring in itself appeared to generate an advantage.
Spirometry FEV1/FVC
Chan 2007, de Jongste 2009, Ostojic 2005, Rasmussen 2005 and Van der Meer 2009 reported data on FVC and FEV1 as follows.
There were no differences between groups in FVC, forced expiratory volume in 1 second or forced expiratory flow in mid‐expiratory phase at the end of the Chan 2007 study.
FEV1 was similar at baseline in both groups in de Jongste 2009 and there was no significant difference in change from baseline over the course of the study between the groups: both groups improved.
Neither FVC nor FEV1 as a percentage of predicted was significantly different across the groups in Ostojic 2005.
Rasmussen 2005 reported odds ratios for improved FEV1 > 300 ml over baseline of 3.26 (1.50 to 7.11) for Internet versus specialist, with a significance of P = 0.002. The odds ratio of Internet versus GP group was also significant at P < 0.001(OR 4.86, 95% CI 1.97 to 11.94). These odds ratios were calculated after six months of intervention.
Van der Meer 2009 reported that mean pre‐bronchodilator FEV1 changed by 0.24 L for the Internet and ‐0.01 L for the usual care group thus indicating an improvement in the telehealthcare arm's FEV1.
Patient satisfaction
In the Cruz‐Correia 2007 study, questionnaires were used to assess patient satisfaction and it was found that overall patients preferred the web‐based system for monitoring their asthma to the paper‐based system.
Gruffydd‐Jones 2005 triaged patients in the intervention group by telephone call then allocated follow‐up appointments accordingly. Of patients in this group 88% expressed a strong preference for care in this form compared to the previous standard form.
Pinnock 2005a is an additional report of patients who had completed the RCT comparing telephone and face‐to‐face consultations for routine asthma reviews; they were sent a questionnaire to better understand their preference for future reviews. Thirty‐three percent preferred telephone consultations for future reviews, 17% preferred surgery and 50% expressed no preference. Thematic analysis of the free‐text responses identified five themes including convenience of telephone consultations, specific problems with telephone consultations (e.g. confidentiality when phoning from a public place), the human dimension of face‐to‐face encounters, that the mode of consultation might change according to the clinical situation and wider implications such as using email for attachment of PEF information. In summary, patient satisfaction appeared to be high, but these newer approaches did not appear to suit all patients.
In Bynum 2001 and Pinnock 2003 there was no significant difference in the satisfaction scores of each of the arms (Bynum 2001 P = 0.132 and Pinnock 2003 P = 0.51)
Kokubu 2000 asked the following satisfaction related questions:
| Yes (%) | No (%) | Don't know (%) | |
| Your self management of asthma has improved with regard to medicines regularly and/or coping? | 74 | 11 | 13 |
| This system is useful for control of your asthma? | 89 | 0 | 11 |
| AirWatch measuring and/or data transfer is a burden to you? | 11 | 70 | 19 |
| Telephone consulting by your nurse is a burden to you? | 4 | 92 | 4 |
| Do you feel your privacy is disturbed? | 0 | 89 | 11 |
What did you think about the frequency of telephone consulting?
Adequate 87%
Too much 7%
Too few 3%
No response 3%
Data are reproduced from Kokubu 2000 with permission from the authors.
It was therefore clear that the telehealthcare patients in the Kokubu 2000 study were overall very satisfied.
Insufficient detail was reported by Chan 2007 to interpret the satisfaction scores published.
Willems 2007a found no clinically important differences between a satisfaction questionnaire administered at four months and again at 12 months. Both arms of the trial used a PEF monitor and both arms answered the satisfaction questionnaire. Only 4% of patients experienced "a lot" of symptoms over the previous months. Forty percent of patients said that they would like to use the monitor again in the future; 36% said maybe. Ninety‐four percent of patients were either much or very much appreciative that lung function deterioration is immediately noticed by the nurse. Eighty‐four percent said that they felt "not at all less safe contacting the nurse instead of the doctor". Eighty‐seven percent found that they had a qualitative improvement by playing an active role in their asthma management. Sixty‐five percent felt safer while using the monitor. Eighty‐four percent said the monitor did not interfere with their daily activities. With regards to questions on the application of the monitor both children and adults were highly satisfied with using the monitor, 87% finding it "not difficult at all" to perform the spirometry test.
Costs from the healthcare perspective
This refers to the costs of providing the intervention compared with differences in outcomes between the intervention and control groups (all currency conversions were undertaken in February 2010 to US dollars).
In Pinnock 2005 the cost to the practices of face‐to‐face asthma reviews was $17.31 each per consultation achieved as there was a higher default rate than for telephone reviews. Telephone reviews reached more patients at $11.20 per consultation achieved. Access was therefore improved at lower cost per consultation, however overall costs were similar because more patients were interviewed in the telephone group.
In Pinnock 2007, the cost per patient reviewed was significantly lower in the telephone option group; $15.63 versus $19.85. This generated a cost saving per patient reviewed of between $4.02 and $4.41 per patient reviewed (i.e. per six‐monthly review achieved).
Data on costs were published in the Donald 2008b paper. The overall cost of delivering the intervention from the healthcare perspective was $1693.91, i.e. the additional six phone calls each over the study time period for all intervention patients. These calls resulted in the intervention group having six readmissions overall, as opposed to the control group's 20 readmissions to hospital. The total cost of the hospital readmissions in the control group was $35,878.52. Therefore there was a significant cost saving solely on the basis of reduced hospital admissions. The intervention group also showed a clinically significant increase in quality of life scores over the 12‐month follow‐up period in comparison to no change in the control group's scores. Formal cost‐effectiveness analysis looking at Quality Adjusted Life Years (QALYs) or Disability Adjusted Life Years (DALYs) was not undertaken.
In the Kokubu 2000 study, it was estimated that a saving of $7074 per year per patient would be achieved if they were to use the telehealthcare system rather than conventional treatment, these savings largely being achieved by a reduction in hospitalisation costs.
In Willems 2007b, costs from the societal perspective were calculated by costing from the healthcare perspective and adding the cost of over‐the‐counter medication, family informal care and productivity losses due to time off work. Cost‐effectiveness data were calculated from the costs and the quality of life data from EQ‐5D utility (adults and children) and the SF‐6D utility (adults only). Cost‐effectiveness depends on what society is prepared to pay per QALY gained. Among adults the healthcare costs were a mean of $695.54 higher in the intervention group. After adjustment for baseline differences by multiple regression an average 0.03 QALY were gained from the intervention. This equates to $42,520.39 (31,035 Euros) per QALY gained from the societal perspective. Among children the healthcare costs were $829.56 higher in the intervention group. On average 0.01 QALY were gained from the intervention, equating to $80,437.16 (59,071 Euros) per QALY from the societal perspective.
Overall it therefore appears that the studies which analysed costs found that where hospitalisation was prevented, costs were favourable to continuing the intervention. However, this did not hold true for all studies.
Discussion
Summary of main results
This review included 21 trials measuring the effects of telehealthcare interventions. Overall, this review indicates that telehealthcare‐based interventions do not have an appreciable impact on disease‐specific quality of life or risk of emergency department attendance for asthma; they may, however, result in a reduction in the risk of hospitalisation for asthma, particularly in high‐risk populations.
This latter finding is consistent with the high hopes that policymakers hold for telehealthcare in terms of its ability to prevent asthma patients requiring hospital admission. However, this meta‐analysis is highly reliant on the results from the Kokubu and Donald studies. The Kokubu study selected a group of patients who had very poorly controlled asthma, requiring oral steroids at least three times in the previous 12 months or having had a previous hospital admission, and so they were much more likely to be admitted to hospital at baseline than patients in other studies which were based in primary care. In addition, our knowledge of this study is incomplete due to difficulties in translating this article. When the data were analysed without the Kokubu study the rate of hospitalisations over 12 months became non‐significant (see Analysis 3.5; P = 0.1). Similarly, the Donald study recruited from a group of adults who had been admitted to hospital with their asthma at some point previously (see Analysis 3.3 ‐ secondary care subgroup). These two studies together suggest that telehealthcare might be most useful for high‐morbidity asthma groups selected from secondary care over longer intervals (i.e. greater than 12 months). However, overall hospitalisations represent infrequent events and so generalisation from this limited number of patient outcomes should only be undertaken cautiously.
Symptom scores data were inconsistent and often reported as within‐group changes rather than across‐group changes. In some instances telehealthcare related to improved symptoms, but mostly the reporting of data was not robust enough to draw any clear conclusions.
There were few adverse events. There is always a risk in reducing the level of care from face‐to‐face to telehealthcare that if patients are inexpertly triaged they will receive insufficient support for their needs and their safety will be compromised. We, however, found no evidence of this having happened. This suggests that authors were aware of this risk and managed it appropriately.
This review did identify a tendency for patients to abandon the technology and cease self‐monitoring when they felt well; for example in Chan 2007 children's adherence to submission of inhaler technique videos decreased over time. This observed pattern calls into question not only what works for asthma in telehealthcare and in what contexts, but for whom too. It seems that many of the primary care population with asthma do not see themselves as having a chronic illness needing constant medication but as having an occasional acute inconvenience ‐ this is a separate issue that is best explored using qualitative designs (Gallagher 2009; Mort 2008).
Overall completeness and applicability of evidence
Nine of the 21 studies included in this review studied the telephone as an intervention. However, Tulu 2007 found that only 6.6% of the telemedicine projects listed in the Telemedicine Information Exchange survey used the "Plain Old Telephone System" (sic). It would seem that more modern technologies such as video‐conferencing, Skype® and web 2.0 technologies are attracting interest but have not yet made it into the asthma literature.
There were several trials which had a very low participation rate for collection of their follow‐up data, e.g. approximately 38% in Vollmer 2006 and 27% in Delaronde 2005.
Studies varied in their recruitment either from hospital outpatients or emergency departments in which case patients with more severe forms of asthma were recruited than in primary care where the patients with milder asthma were recruited. The type of patient in the study had implications for the findings as we saw with the Kokubu and Donald studies.
There is a plethora of ongoing research and research that has as yet only been published in abstract form. This emerging literature includes a number of studies looking at networked telehealthcare tools. It is worth noting that in the situations in which such solutions might prove most useful, e.g. remote and rural settings, there may be anticipated difficulties with broadband linkage required for networks. Similarly, frail, elderly people who could benefit from telehealthcare to help to maintain their independence may lack the cognitive skills to be able to adapt to network interfaces and so have difficulties using the technology at all.
The research in this review has mostly taken place in a developed world setting with the equivalent of primary and secondary care, sometimes transferring the responsibility for care from secondary to primary, or setting up a type of preventative secondary care. As such it is likely to be broadly transferable to other developed world settings. It is quite possible that some types of telehealthcare may work well in the developing world as well. This is because the developing world often has good mobile telephone network coverage. However, the devices for interfacing with patients require reliable electric power and skilled labour which might be more difficult to secure in such countries.
Quality of the evidence
In general, the biases seen included a lack of proper randomisation, problems with allocation concealment and a lack of overt statements of specific methodological processes, such as blinding. Therefore many of the risk of bias tables were often populated with the judgement 'unclear' due to insufficient information.
The decision to structure Rasmussen 2005 with three trial arms is problematic when it comes to interpreting findings and synthesising across studies.
Using "adherence" as an outcome measure infers a link which is not yet necessarily established for telehealthcare; that improved adherence, a process measure, will improve hard clinical endpoints such as numbers consulting at the emergency department or patients being hospitalised. Increased use of process measure outcomes during trials will deepen understanding of why specific interventions might be successful when others are not. Process measures also help clarify how well the trial is being executed.
In other instances, data are presented in such a way that it is not clear whether there was a clinically significant decrease in days and nights with symptoms or time off work. Results were presented as within‐group differences over the time period and frequently statistical significance was quoted but not interpreted. For example, see Clark 2007 where the control group's symptoms decrease by more than the treatment group's.
Potential biases in the review process
The lack of full translations for the Kokubu 1999 and Kokubu 2000 papers may mean that some information is missing.
Leaving out qualitative trials may mean that some studies are excluded from which useful lessons could be learnt (Shepperd 2009). Qualitative data has an important role to play when studies deliver complex interventions. These kinds of studies help us understand the uptake or otherwise of telehealthcare interventions. However, the techniques for systematically reviewing such studies are in their infancy.
Future updates of this review are expected to include more networked and Internet‐based technologies and not repeat the dominance of this literature by the ordinary telephone.
Agreements and disagreements with other studies or reviews
There are several reviews (Botsis 2008; Garcia‐Lizana 2007) which consider the evidence of studies of telemedicine, telecare and telehealth although the inclusion criteria are variable and they are largely concerned with chronic diseases other than asthma, in particular for elderly populations.
A review by Duvvuri 2007 focused on asthma and ICTs, especially the world wide web. Its inclusion criteria were broader than for this Cochrane Review in that the authors searched for decision support and patient education tools as well as the technologies we would have classified under our narrower definition of telehealthcare. Studies were summarised in narrative form and no meta‐analysis was attempted. Overall, Duvvuri was favourable to the increasing use of telehealthcare to help manage the increasing worldwide burden of asthma. They also drew attention to some favourable cost‐effectiveness and patient‐satisfaction analysis. In addition, they identified the remaining hurdle of physician licensing and reimbursement when care is delivered via telehealthcare. This is particularly an issue in countries with insurance‐based healthcare.
Whitten 2002 was a systematic review of cost‐effectiveness studies of telemedicine interventions; they highlighted that the relative cost‐benefit calculations are fundamentally affected by the context in which the intervention is implemented. The example they gave was that they recognised that a service which is highly cost‐effective in the Highlands of Scotland is unlikely to be so in the urban environment of Manchester, England where accessibility and quality of local services is much higher. In total, they found 55 articles with cost data and only 24 of these met the quality criteria for inclusion in their review. Twenty out of 24 were simple cost comparisons and there were no studies of cost utility analysis: they concluded that this made it impossible to say which telemedicine interventions truly represented "value for money." The cost data presented above for this Cochrane Review includes two cost comparisons and a full cost utility analysis in terms of "cost per QALY gained" in children and adults.
Authors' conclusions
Implications for practice.
This review has found 21 randomised controlled trials researching the effects of telehealthcare intervention for asthma. In addition, we found 21 ongoing studies and unpublished trials. Despite some lack of consistency in the way telehealthcare has been researched thus far (see below), this represents a substantial body of reasonably high quality research in different settings internationally and assessing different technologies. Some positive conclusions can be drawn. Telehealthcare improves access and is no worse than normal care in carefully selected and triaged primary and secondary care patients. It does not, however, appear to have the desired impact on quality of life and these interventions have little or no significant impact on emergency department visits. However, given careful candidate selection conditions, telehealthcare may reduce hospital admission rates and associated costs. There is also some evidence for improved symptoms in telehealthcare trial arms where symptoms are dealt with quickly and exacerbations are prevented in a way not open to the control arm.
Implications for research.
One of the problems with telehealthcare research that we have seen in this review is that the control arm is not always usual care, but often receives an enhanced input from the clinicians ‐ for example, a greater number of visits or face‐to face contacts and so this results in greater adherence to medications and surveillance of disease fluctuations in both arms of the trial and improved outcomes for both arms. Telehealthcare fits into the Medical Research Councils's model of a "complex intervention" (Medical Research Council 2008) and as such it seems that each telehealthcare intervention is very diverse. This can make it very hard to pinpoint the "active ingredients" of a telehealthcare intervention. Confusion also comes from the different modes of delivery within the bracket of telehealthcare. For example, both Internet‐ and telephone‐based "helplines" improve access to clinicians for patients. In this example it is the function of improving access which leads to reduced hospitalisations ‐ and so far the evidence shows that this is not to the detriment or advantage of quality of life. The form of the intervention, whether telephone or Internet, seems to have less of an impact than the function of the access which results in timely advice to prevent exacerbation in a way that outpatient clinics cannot deliver in comparison. It is important then in future studies that interventions are described as fully as possible, that relevant process or intermediary measures are studied, and consideration is also given to embed qualitative work within these complex intervention trials to help assess how these interventions exert their effects (Shepperd 2009).
Future telehealthcare interventions studied are likely to be even more complex and include the features of web 2.0. Such research is important to find how best to harness these innovative technologies without inadvertently causing harm. It is, however, important that these interventions are patient‐centred, and that they are developed through close consultation both with patients, but also with healthcare professionals to maximise the chances of success (Catwell 2009a; Catwell 2009b).
What's new
| Date | Event | Description |
|---|---|---|
| 19 June 2012 | Amended | Author (JC) affiliations updated. Minor copy edits made. |
Acknowledgements
Margaret Fletcher translated the Ricci paper from Italian: one of the ongoing research projects.
We are grateful to the members of the External Steering Group for NHS Connecting for Health Evaluation Programme for their advice.
Data and analyses
Comparison 1. Asthma quality of life questionnaires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AQLQ Juniper mean scores | 9 | 3119 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.01, 0.16] |
| 2 Sensitivity analysis AQLQ studies judged low risk of bias | 8 | 2151 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.00, 0.16] |
| 3 Subgroup telephone only AQLQ scores | 5 | 2556 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.05, 0.12] |
| 4 Subgroup AQLQ recruited in secondary care | 3 | 380 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.08, 0.30] |
| 5 Subgroup AQLQ recruited in primary care | 5 | 2131 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.02, 0.21] |
1.1. Analysis.
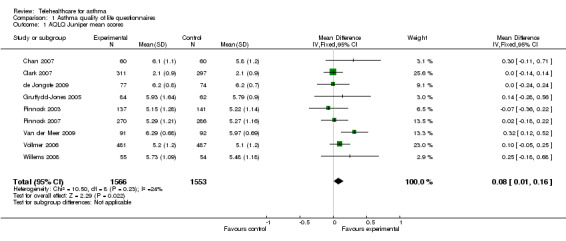
Comparison 1 Asthma quality of life questionnaires, Outcome 1 AQLQ Juniper mean scores.
Comparison 2. One or more emergency department visit; no. of patients with events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emergency department visit in 3 months | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.16, 1.39] |
| 2 Emergency department visit in 12 months | 5 | 619 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.52, 2.58] |
| 3 Subgroup secondary care populations | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.41, 5.09] |
| 4 Subgroup primary care populations | 1 | 253 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.02 [0.36, 45.02] |
2.1. Analysis.
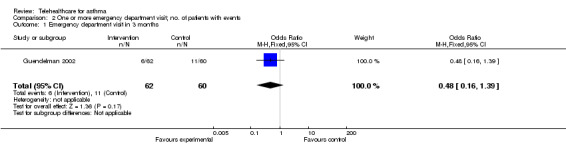
Comparison 2 One or more emergency department visit; no. of patients with events, Outcome 1 Emergency department visit in 3 months.
2.2. Analysis.
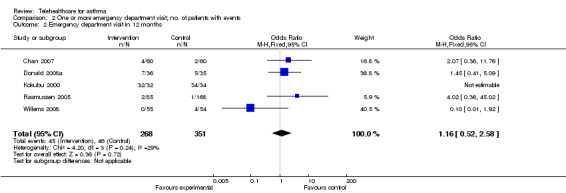
Comparison 2 One or more emergency department visit; no. of patients with events, Outcome 2 Emergency department visit in 12 months.
Comparison 3. One or more hospitalisation events; no. of patients with events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 One or more hospitalisation event in 3 months of study | 2 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.01, 36.46] |
| 2 One or more hospitalisation event in 12 months of study | 4 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.61] |
| 3 Subgroup ‐ secondary care; no. of patients with one or more hospitalisations in 12 months | 2 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.49] |
| 4 Subgroup ‐ primary care; no. of patients with one or more hospitalisations in 12 months | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.12, 76.10] |
| 5 No. of patients with one or more hospitalisation events in 12 months excluding Kokubu study | 3 | 433 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.07, 1.25] |
3.1. Analysis.
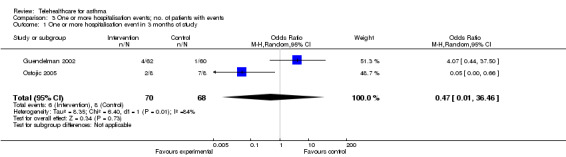
Comparison 3 One or more hospitalisation events; no. of patients with events, Outcome 1 One or more hospitalisation event in 3 months of study.
3.2. Analysis.

Comparison 3 One or more hospitalisation events; no. of patients with events, Outcome 2 One or more hospitalisation event in 12 months of study.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barbanel 2003.
| Methods | Randomised controlled trial | |
| Participants | 24 adults from a socioeconomically deprived, ethnically mixed area in the United Kingdom (Tower Hamlets in London) with GP diagnosed asthma | |
| Interventions | Following attendance at a 3‐day training course on asthma care at the London Chest Hospital, a number of pharmacists were allocated a group of adults to educate. The pharmacists then delivered an educational session on asthma topics and reviewed inhaler technique, and use of a Peak Expiratory Flow Meter, taking a minimum of 45 minutes. They gave the patients supporting literature and a self‐management plan. The pharmacists then phoned the participants on a weekly basis for 3 months in order to give encouragement, answer questions and encourage patients to return to the pharmacy with any problems. The control group received no input from the pharmacist |
|
| Outcomes | Primary outcome measure was asthma symptoms as measured using the North of England Asthma Symptoms Scale completed at baseline and 3 months after intervention | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomised to intervention or control groups” |
| Allocation concealment (selection bias) | Low risk | "randomised using sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unable to blind participants from intervention or control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant moved away and their data were imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Bynum 2001.
| Methods | Randomised controlled trial | |
| Participants | 15 intervention group adolescents and 21 control group adolescents were recruited in rural Arkansas, United States, a socioeconomically deprived area which includes Health Professional Shortage Areas. Adolescents with asthma, ages 12 to 19, predominantly African‐American in origin, were recruited from high schools and randomly assigned to each group. | |
| Interventions | Patients received an assessment and training on their use of a MDI via video conferencing: telepharmacy Control: Participants were given the manufacturer's leaflet from the MDI packet |
|
| Outcomes | 1. MDI technique as assessed against a checklist 2. Patient satisfaction with the technology |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The participants were assigned to either a telepharmacy counselling group or a control group using a random number chart” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of outcome assessors, unable to blind from intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 49 students were randomised, 36 were available for evaluation. Students had different reasons for dropping out, 2 did not attend any visit, 8 had never used the inhaler and so should not have been randomised as they did not meet the inclusion criteria 2 students did not attend the follow up. |
| Selective reporting (reporting bias) | High risk | Questions 3 and 11 in the satisfaction rating questionnaire were analysed separately following reliability analysis. This had not been a feature of pre‐specified analysis |
| Other bias | High risk | Incentive fee of $20 for just the intervention arm of the study. |
Chan 2007.
| Methods | Randomised controlled trial | |
| Participants | 120 children aged 6 to 17 with persistent asthma | |
| Interventions | This group used an Internet‐based asthma website where they could interact with asthma clinicians. Store and forward, i.e. asynchronous monitoring via the website was used to collect and to submit a daily asthma diary, inhaler technique video and Peak Expiratory Flow meter recording twice a week for the first 6 weeks and then weekly thereafter. The group had 3 face‐to‐face visits and 3 virtual visits via video technology. Follow up was conducted by e‐mail by the nurse case manager. This was a 12‐month study. Control: This group had 6 face‐to‐face visits with a nurse case manager. The nurse manager would then contact the patients via telephone 2 times per week for 6 weeks then weekly to review the asthma action and home management plans and assess the symptom diary and provide feedback. Control group also received education regarding their asthma and both groups had 24/7 access to their case manager in case of emergency. |
|
| Outcomes | 1.Therapeutic adherence, i.e. data on use of inhaled corticosteroids 2. Symptom diary adherence 3. Disease control: emergency department visits, hospitalisations, unscheduled asthma‐related clinic visits and use of rescue therapy, i.e. beta agonist inhalers 4. Asthma knowledge scores 5. Paediatric asthma quality of life scores measured for caregivers and for patients. (Paediatric Asthma Quality of Life Questionnaires were Juniper's AQLQ) 6. Inhaler technique scores for both dry powder inhalers and MDIs with valved holding chambers |
|
| Notes | In the selection of patients there was considerable emphasis on the recruitment of “willing” volunteers within the required category of asthma severity and it was checked to make sure that patients were not planning on moving out of the region during the 12 months of the study. These adjustments could bias the results as the usual attrition seen in other studies and potentially clinically relevant would be minimised. However, randomisation appears to have been appropriate. The nurse case manager who was assessing the patients’ inhaler technique was feeding back to the patient and so was not blinded to the allocation of the patient. Similarly, case manager records were used for the majority of other outcomes but it is not stated who extracted the data from the records. There is also an ethical issue in that the computers were removed from the families who were experiencing virtual visits at the end of the study period ‐ might these families have become dependent on this technology? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients underwent block randomisation with a table of random numbers” |
| Allocation concealment (selection bias) | High risk | Table of random numbers ‐ not adequately concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcome assessors not blinded to allocation of patients |
| Incomplete outcome data (attrition bias) All outcomes | High risk | From 60 randomised to each arm, only data from 55 in the control group and 47 in the intervention group were analysed. Also no diary entry was recorded for 60% to 80% of study days. And recording patterns were different in each group ‐ patients completed symptom diary entries only every 2.8 days in the virtual group and 4.8 days in the control group. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting, no deviation from protocol in reporting results |
| Other bias | High risk | Some selection of patients before randomisation, choosing those who would be likely to participate fully in the study and this gives potential bias to results |
Chatkin 2006.
| Methods | Prospective, multicentre, randomised controlled trial | |
| Participants | Aged at least 12 with moderate or severe persistent asthma, according to Global Initiative for Asthma (GINA) in Porto Alegre, Brazil; 271 patients were randomised into 2 groups. The participants were put forward by physicians from all over the country (it does not state whether primary or secondary care physicians) for the trial, in this way there were patients from 15 states of the country. | |
| Interventions | Both groups received three packages of salmeterol/fluticasone for 3 months supplied by a drug company and routine care from their own physician. The participants in the intervention group received a phone call once every 2 weeks to promote treatment adherence. Control: The control group received initial and final telephone calls for collection of demographic information only |
|
| Outcomes | 1. Adherence ‐ percentage of participants taking 85% or more of the prescribed doses of salmeterol/fluticasone. Adherence was measured according to the number of inhalations that were recorded on the disks. This was obtained from the disks that were returned to the office. They excluded patients who did not return the devices. 2. The difference in adherence between the control and telephone intervention groups |
|
| Notes | All participants entered into the study were required to have a residential telephone number not only a mobile, this may have been a source of bias. In addition, exclusion criteria were: mild persistent asthma, pregnancy or breast feeding, intention to move during the course of the study, regular use or recent past abuse of alcohol or illicit drugs and clinically significant active general medical condition; 4 patients were excluded due to these criteria. Little information was given in the study report regarding randomisation and blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 patients were not included because they did not return their drug disks and 8 for not responding to the telephone calls |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Drugs were supplied for free to the intervention group by a drugs company as oppose to the real‐world situation where patients would have to pay the high cost of their inhaler drugs themselves. Also patients adhere more when they know they are being monitored, however, this cannot be controlled for. |
Clark 2007.
| Methods | Randomised controlled trial | |
| Participants | 808 female patients over the age of 18, with physician‐diagnosed asthma in the University of Michigan Health System, United States of America, were randomly assigned to 2 groups. Some of them came from speciality clinics. Patients had to have had active symptoms in the past 12 months and had been enrolled in one of the participating asthma related clinics. They were also required to have no extenuating medical or mental conditions and access to telephone. 424 women were randomised to the intervention group and 384 to the control group. | |
| Interventions | Standard asthma education which does not emphasise sex and gender issues was provided at the time of the clinic visit. The intervention was a behavioural education programme delivered by a nurse health educator through telephone counselling. Based on social cognitive theory, women were introduced to a problem‐solving process to undertake in association with their asthma management plan. At baseline the patient's level of self‐management was determined and then telephone counselling was tailored to that level. Sex and gender‐role related asthma problems were assessed and women were encouraged to keep a diary with a Peak Expiratory Flow Meter to monitor their condition. Six educational telephone calls were made over the 12‐month study period to the intervention women. Control: The usual care group received treatment based on National Asthma Education Prevention Program guidelines as well as telephone follow up for the purposes of monitoring only |
|
| Outcomes | 1. Frequencies of daytime and night‐time asthma symptoms 2. Days and nights that the woman had missed work or study 3. Self‐reported emergency department visits, hospitalisations, unscheduled urgent visits to a clinic and scheduled clinic visits in the 12 months before the study (i.e. at baseline) and at study follow up, were recorded 4. Medical record data for asthma emergency department visits and hospitalisation from a Data Warehouse during the corresponding time periods 5. Sex and gender role‐related queries were made relating to symptoms and the menstrual cycle, pre‐menstrual syndrome, contraceptive pill, hormone replacement therapy and urinary incontinence. Asthma problems relating to housework, washing or cleaning products, fragrances, cosmetics and hair products, exposures through child care and symptoms associated with social and sexual activity. 6. The Juniper's Mini Asthma Quality of Life Questionnaire was used to measure a woman's quality of life 7. A scale of self‐confidence for asthma management 8. The Zimmerman Scale was used to assess the level of a woman's self‐regulation ability |
|
| Notes | The authors acknowledge that more women with persistent asthma were assigned to the treatment group (P = 0.003) The impact of this is difficult to anticipate: as the women in the intervention group were sicker, it may have limited the impact of the intervention. Conversely, the intervention may have been perceived as more effective as the women had greater scope for improvement. The major potentially confounding variable of smoking was not assessed in this study; data on smoking rates were not collected. Medical record data were collected for the corresponding time periods and compared with self reports ‐ data triangulation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomisation processes were based on random length permuted blocks” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Participants' physicians were blind to the assignment of their patients in this study” “Data collectors were blind to the assignment of the women to the study arms” It would not have been possible to blind the women from the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “There were no differences in dropouts due to demographic variables, disease severity and important outcomes between the two groups.” |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified outcomes are reported |
| Other bias | High risk | “It happened that more women with persistent asthma were assigned to the treatment group. As noted this fact could have made intervention results more difficult to achieve given that the women were sicker conversely it could have provided more room for women to improve.” Smoking rates were not assessed |
Cruz‐Correia 2007.
| Methods | Randomised cross‐over study | |
| Participants | 21 adults aged 16 to 65, from Porto, Portugal, with a previous medical diagnosis of asthma were included | |
| Interventions | Patients received web‐based monitoring for 4 weeks then paper monitoring for 4 weeks or vice versa in random sequence ‐ a cross‐over trial. The web‐based application was named "Portal for Assessment and Self‐management of Asthma" and it included information on frequently asked questions, asthma self‐management and enabled patients to fill in the Asthma quality of life test and record PEF/FEV1. The technology provided immediate feedback, automatic messages and alerts to both patients and doctors to enable therapeutic decisions. This information contributed to an interactive asthma plan. Control: The control group recorded PEF/FEV1 in a paper diary and had a paper based action plan |
|
| Outcomes | Outcome measures 1. Patient opinions, in the form of positive and negative comments, reports of problems relating to the internet connection, importance of different features that the different diaries offered and patients' willingness to monitor their asthma 2. The time taken to fill in the internet and paper diaries 3. Adherence to monitoring tools |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was performed using a computer generated algorithm” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding, both patients and data collectors unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two patients dropped out, one lost access to the Internet during the study period the other moved to another city |
| Selective reporting (reporting bias) | High risk | Data collected on quality of life but not reported |
| Other bias | High risk | Some patients reported filling in their paper diaries for several days at once, this was not possible with the Internet system |
de Jongste 2009.
| Methods | Prospective, open‐label, randomised, multicentre, parallel‐group trial | |
| Participants | 151 children aged 6 to 18 years with stable mild/moderate asthma treated with 200 to 1000 microgram of inhaled budesonide or equivalent for 2 months before randomisation, recruited from 5 academic centres and 12 general hospitals. Children had RAST class 2 or higher or a positive skin prick test for at lease one airborne allergen. | |
| Interventions | The intervention group received an nitric oxide airway inflammation monitor to perform measurements daily. Data were transmitted to the co‐ordinating centre. All children (intervention and controls) recorded symptoms on a palmtop electronic diary. All parents were phoned every 3 weeks between visits and medication was adapted according to mean nitric oxide and cumulative symptom scores over the previous 3 weeks. | |
| Outcomes | Children of both groups were seen at randomisation and at 3, 12, 21 and 30 weeks. All parents were phoned every 3 weeks between visits and medication (steroids) was adapted according to an algorithm which included symptoms and mean expired nitric oxide. 1. Expired nitric oxide was performed before and after salbutamol, as a measure of airways inflammation 2. Adverse events were recorded 3. Pediatric Asthma Caregiver Quality of Life Questionnaire with Standardized activities was administered at first and last visits 4. Primary end point was change from baseline of percentage symptom‐free days during the last 12 weeks 5. FEV1 and reversibility 6. Prednisone courses 7. Emergency visits 8. Hospitalisations |
|
| Notes | All nitric oxide analysers were checked for drift. Intention‐to‐treat analysis was performed for all subjects who were enrolled. In addition they performed a per protocol analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomised at their first visit, stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four children, 2 for non‐compliance, 1 for inappropriate inclusion (no allergy) and 1 for moving abroad and being unable to transfer data. Therefore study population for evaluation was 147 children. "Only periods with at least 50% valid daily scores were analysed." Is later explained as 79% valid diary entries over the whole study period. It is not explained what is done for the remaining periods. |
| Selective reporting (reporting bias) | High risk | Full details of number of emergency department visits are not reported |
| Other bias | High risk | Children only recruited from academic centres or general hospitals therefore may not be receiving standard primary care |
Donald 2008a.
| Methods | randomised parallel group trial | |
| Participants | This Australian study recruited 71 adults aged between 18 and 55 years who had been admitted to one or both of 2 teaching hospitals with a primary diagnosis of asthma. Adults were excluded if they had another chronic respiratory condition, an unstable medical condition, a cognitive or intellectual disability, psychiatric illness or were unable to speak English. All patients received a Peak Expiratory Flow (PEF) meter and identical instructions on how to record their results. | |
| Interventions | All participants received a PEF meter and instructions on how to record their results. The nurse then used the first week's record to determine the participant's best PEF rate. All participants attended a face‐to‐face session with an asthma nurse educator and received advice on the pathophysiology of asthma, medications, triggers and self management. They were then provided with an Asthma Action Plan. The intervention group received 6 follow‐up telephone calls from the nurse educator to ask about their current asthma symptoms and give advice on their management Control: The control group was encouraged to continue with self‐management and usual GP care |
|
| Outcomes | 1. Hospital admissions at recruitment 2. Written plan and PEFM ownership 3. Delivery of management sessions 4. Health care utilisation 5. Days lost from work or study 6. Exacerbations requiring use of oral corticosteroids 7. Costs from the healthcare perspective, i.e. costs of providing the intervention compared with differences in outcomes between the intervention and control groups |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “All participants were telephoned weekly by a researcher (blinded to participant allocation)”. However, neither the participant nor the nurse were blinded to allocation ‐ they could not be. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study is under‐powered. A sample of 100 patients had been calculated to provide statistical power, however only 71 participants were randomised and only 44 replies received at 6 months and 49 at 12 months. No report of how data were modified given that fewer than required by power calculation filled in questionnaires at 6 and 12 months |
| Selective reporting (reporting bias) | Unclear risk | ? use of unusual statistical tests to modify data |
| Other bias | High risk | 660 patients were assessed for eligibility, 385 were not contactable, 154 declined to participate, 31 were excluded and 19 failed to attend the baseline meeting. There needs to be consideration of selection bias, however, as the authors recognise, 55% of potential participants could not be contacted therefore their reasons for not taking part cannot be established nor can their characteristics be compared to the study group. |
Donald 2008b.
| Methods | This was the same study as Donald 2008a with additional data and calculations performed with regards to cost, i.e. outcome 7 in the above table. | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
Gruffydd‐Jones 2005.
| Methods | Randomised controlled trial | |
| Participants | 194 patients were recruited from a General Practice in England. If the patient was aged 17 to 70 and on the practice asthma list they were eligible. Exclusion criteria were being housebound, refusing consent and not having a telephone. | |
| Interventions | Intervention: Patients were telephoned at 6‐monthly intervals by an asthma nurse who asked the Royal College of Physicians' 3 risk stratification questions: In the last month/week: 1. Have you had difficulty sleeping because of your asthma symptoms (including cough)? 2. Have you had your usual asthma symptoms during the day (cough, wheeze, chest tightness or breathlessness)? 3. Has your asthma interfered with your usual activities? The patient was also asked 2 extra questions related to a high risk of asthma death. 1. Have you ever needed treatment in intensive care for your asthma? 2. Have you been admitted to hospital with your asthma within the last year? If patients answered “no” to all these questions they were considered low risk and an Asthma Action Plan was formulated with advice regarding what to do if control deteriorated. If patients answered yes to any question they were deemed “high risk” and a clinic visit arranged. When control was stable for 3 months patients returned to telephone asthma review. Control: “Usual care” consisted of 6‐monthly check‐ups (at baseline, 6 months and 12 months) by asthma appointment with a diploma level asthma nurse. Symptom scores, inhaler technique and PEF meter measurements were checked and patients given an Asthma Action Plan. Follow up was according to clinical need and reminders were issued to defaulters. |
|
| Outcomes | Outcome measures: 1. 6‐question Asthma Control Questionnaire 2. Mini‐asthma quality of life questionnaire 3. Evidence of mild exacerbation (increase in the number of times the reliever was used above baseline of > 1, on 2 consecutive days) 4. Evidence of severe exacerbation (oral steroid use or hospitalisation) 5. Economic evaluation from the perspective of the NHS |
|
| Notes | This study only involved one practice which may mean that the results are of limited generalisability. In addition the power of the study was reduced by high uneven drop‐out rates across the groups. Another source of bias is the fact that the assessors were not blinded to which group the patients had been randomised to. However, this study is representative of real‐world conditions when there are high non‐attendance rates for asthma follow‐up interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using random number tables on a one to one basis and stratified according to severity |
| Allocation concealment (selection bias) | High risk | Random number tables unlikely to conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | “It was not possible to blind the patients or nurses to the groups into which the patients were randomised” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 20 withdrawals in the control group after the first visit, mainly due to non‐attendance and 6 in the telephone group, one of which was due to non‐attendance. As this trial is as real‐world as possible the fact that there was a high non‐attendance rate was taken account of in analysing the costs. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Careful account is taken of the non‐attendance and a post‐hoc analysis performed to consider the possibility of attrition bias |
Guendelman 2002.
| Methods | Randomised controlled trial | |
| Participants | Patients: 134 patients were recruited from a primary care clinic in California, United States of America. Children were eligible for inclusion in the study if they were 8 to 16 years of age, had an English‐speaking caregiver, a telephone to the house and persistent asthma. | |
| Interventions | Intervention: Patients first received a teaching session on PEF measurement and how to manage their medication according to the result. Children were then randomised. The intervention group received the Health Buddy device, a computerised interactive asthma self‐management and education program which connected to the Internet and asked every day about asthma status, peak flow and medication. Responses were downloaded to the nurse co‐ordinator overnight. The devices were interactive and gave immediate feedback on questions regarding asthma symptoms, medications, PEF and other items. Control: The control group used a paper asthma diary. All children returned for 2 follow‐up visits at 6 and 12 weeks when they received further standardised teaching from the nurse co‐ordinator. |
|
| Outcomes | Outcome measures 1. Limitation in activity 2. Asthma symptoms including coughing and wheezing 3. Missed school days 4. PEFR in yellow or red zone i.e. sub‐optimal, below the normal (green) zone 5. Healthcare utilisation: emergency department visits or hospitalisations |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information, "randomised" |
| Allocation concealment (selection bias) | Low risk | "Following baseline interview the nurse opened a sealed envelope containing the treatment assignment" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Self‐reported outcomes were assessed by the nurse co‐ordinator, no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Baseline characteristic of children who did and did not complete the trial did not differ", 66 patients were randomised to the intervention group and after 12 weeks 62 patients remained. 68 patients were randomised to the control group and after 12 weeks only 60 remained. Reasons for dropping out of the study included moving out of the area n = 3 or life crisis n = 4. 5 familles who dropped out were uncontactable. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes are reported |
| Other bias | High risk | Incentive fees were paid to study participants; may limit generalisability of study |
Jan 2007.
| Methods | Randomised controlled trial | |
| Participants | This study was conducted in Taiwan where 73% of adults have personal computers and 54% of families have internet access. Children were eligible for inclusion in the study if they were between the ages of 6 and 12 years, had access to the Internet by their caregivers and had a physician’s diagnosis of asthma. Other chronic conditions such as bronchopulmonary dysplasia were excluded. 164 paediatric asthma patients were enrolled. | |
| Interventions | The intervention group participants were given “Blue Angel for Asthma Kids”, an Internet‐based paediatric asthma monitoring program for asthmatic children plus their parents. The system has symptom and peak flow diaries and individual Asthma Action Plan suggestions based on the GINA (Global Initiative for Asthma) guidelines. These data can be shared with the patient’s physician who can give feedback via telephone or email. The peak flow meter provided to the families could measure PEF and FEV1 and attached via a USB (Universal Serial Bus) connection to a computer. Control: Traditional treatment in an outpatient allergy and asthma clinic accompanied by a PEF meter and diary. This group also received asthma education as part of usual care including verbal and printed information. They were also given an Asthma Action Plan to aid decision making. |
|
| Outcomes | Outcome measures: 1. PEF records 2. Symptom diaries 3. Paediatric Quality of Life test was completed at baseline and after 12 weeks 4. Childhood Asthma Control Test, at baseline and 12 weeks 5. Caregiver Survey of Asthma Knowledge, before and after the trial 6. Measurement of patients’ adherence to treatment 7. Adherence to asthma diaries |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “children and their caregivers, who were randomised” |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information is given as to how the outcomes of the groups were collected and whether outcome assessors were blinded to the allocation of the patients. This would, of course, not have been possible for the outcomes that were recorded by the Internet program but other outcomes which were recorded using questionnaires at baseline and 12 weeks. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study power calculation required 100 children in each arm. 99 were randomised to the intervention arm and 97 were randomised to the control arm. Of the original sample of 196 children there were 184 invited to participate. 5 families were "too busy", " not interested" or found it "too complex to perform the diary card" 15 (6 control and 9 intervention participants) were excluded either at their request or because there was a lack of data due to internet failure. 7 families who dropped out were unavailable for comment. "baseline characteristics of children who did not complete the trial did not differ from those who did" The study reports that 82 intervention participants and 71 control participants returned for 12‐week follow up. This leave 4 participants unaccounted for. |
| Selective reporting (reporting bias) | High risk | Satisfaction questionnaires data not shown |
| Other bias | Low risk | No other bias apparent |
Khan 2004.
| Methods | Randomised controlled trial | |
| Participants | 310 children with asthma who were discharged from a hospital emergency department in Sydney, Australia | |
| Interventions | Parents of children in the intervention group received a telephone consultation by an experienced asthma nurse educator within 2 weeks of discharge. This consultation emphasised the advice given to the parents at the time of discharge. These calls lasted an average of 13 min (range 5 to 44 minutes). Parents of both the intervention and control groups received written materials regarding facts about asthma, use of spacers, management of exercise induced asthma and when to contact a doctor. Control: This group did not receive the follow‐up telephone call, however they did receive written material regarding asthma at baseline, before their discharge from the emergency department |
|
| Outcomes | Outcome measures: 1. Number of days of wheezing in the last 3 months 2. Possession and use of a written asthma action plan during a crisis 3. Use of preventer medication 4. Increased asthma knowledge scores (Newcastle Asthma Knowledge Questionnaire) 5. Parental quality of life scores (Juniper Caregiver's Quality of Life Questionnaire) 6. Number of visits to GP/paediatricians 7. Number of attendances at emergency department and admissions to hospital with asthma in the previous 6 months |
|
| Notes | This study may have had insufficient power to show real differences between the groups as the children mainly had mild episodic symptoms. The context of following up an emergency department visit is also important, as it can be considered as a kind of "window of opportunity" to educate patients and children and parents in both arms by giving them written and verbal information regarding control of asthma. The effect of this is that patients and their parents may be more receptive to the messages regarding medication adherence and control in the acute situation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An asthma educator randomly assigned children to either intervention or control using a list of random numbers that had been provided to her” |
| Allocation concealment (selection bias) | Unclear risk | "children's details were faxed to an asthma educator working in New South Wales" sounds as though this might be an attempt at central randomisation but this is not made explicit |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessment by postal questionnaire, therefore single‐blinded. Not possible to blind participants to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 310 children enrolled in study, 266 (86%) completed the follow‐up questionnaire. 22 had changes their address and 22 were non‐responders. 130 control children completed follow up and 155 intervention children completed follow up. "Children lost to follow‐up were similar demographically and in terms of asthma severity when compared to those who completed the study". |
| Selective reporting (reporting bias) | Low risk | "All analyses were specified a priori" |
| Other bias | High risk | 49% of parents were excluded (exclusion criteria not published). Parents whose English was inadequate to complete the questionnaires were also excluded, this might mean that the population in greatest need were excluded as non‐English speakers are often socioeconomically deprived populations. |
Kokubu 1999.
| Methods | Randomised controlled trial | |
| Participants | Patients: This study looked at 53 patients, all adults with asthma, in 2 parallel groups, in Japan. 26 were randomised to the intervention group, 27 to the control group. Patients were included if they had visited the emergency department more than twice in the last year. Exclusion criteria excluded patients with COPD, heart failure and other diseases as potential confounders. | |
| Interventions | Intervention: A telehealthcare system was set up with an electronic Peak Flow Meter which measured both PEF and FEV1 and could store up to 500 readings in its memory. The nurse introduced the patients to the system and then provided telephone follow up available 24 hours if required. The nurse was overseen by a physician who had determined the patient’s best PEF and written an asthma plan. The nurse reported to the physician monthly according to the data they received from the meter. The physician saw the patient regularly to review the action plan. Control: No information given |
|
| Outcomes | Outcome measures:
|
|
| Notes | This was very hard to assess given the limited translation of this study which was available. The majority of figures and graphs were in English and the limited translation for Cochrane stated “no description” for most of the relevant headings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Kokubu 2000.
| Methods | Randomised controlled trial | |
| Participants | Patients: 75 adults with asthma, in Japan, were studied in an intervention group of 37 initially (reducing to 32) and a control group of 38 (reducing to 34). Patients were recruited from 17 medical institutions, a multi‐centre trial over a period of 6 months. Patients were selected who had visited the night emergency department room 3 times or more within a year in spite of corticosteroid therapy. | |
| Interventions | This group was managed with telehealthcare. The nurse under the physicians’s supervision monitored the patient at home via telephone, providing them with advice in managing exacerbations and proper use of a controlled management plan. Control: Standard out‐patient care without telehealthcare |
|
| Outcomes | Outcomes:
|
|
| Notes | As with Kokubu 1999, this was again a limited translation of this study and so risk of bias was very hard to assess. The majority of figures and graphs were in English and so the results can be gleaned from these. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with telephone to the registration centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | “non‐blinding method” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intervention group began as a group of 37, reducing to 32 and the control group of 38, reducing to 34 but no reasons for this are given. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Ostojic 2005.
| Methods | Randomised controlled study | |
| Participants | 16 patients with asthma from a respiratory clinic, in Croatia, 9 males, mean age 24.6 +/‐ 6.5 years, were enrolled. All had asthma and all had experience of Global System for Mobile Communications’ Short Message Service (GSM SMS, i.e. text). Exclusion criteria included a history of smoking, chronic bronchitis or emphysema or inconsistent access to a cell phone with text. | |
| Interventions | Following a 1‐hour education session with a specialist at the clinic during which they discussed symptoms of asthma, and their inhaler technique was addressed, the patients were given a Peak Flow Meter and instructed in its use. Patients were told to note PEF, medication use and symptoms in a paper diary. PEF was to be done 3 times a day, then those patients in the text group would send their results daily to a computer in the asthma centre. Both groups were treated according to GINA guidelines but the text group received weekly instructions by text from an asthma specialist on adjustments of therapy and invitations, when required, to come in for an extra office visit. Control: The controls also kept a daily diary of peak flow and symptoms, however their results were only reviewed by the physician at the end of the study period on attending the physician’s office |
|
| Outcomes | Outcomes: 1. Office pulmonary function test measurements 2. Patient’s daily records of PEF and symptoms 3. Details of asthma medication 4. PEF variability (defined as the difference between the maximal and minimal PEF measurements of a day divided by the maximal PEF for that day) 5. Cost and reliability of text |
|
| Notes | As this was a very small study of only 16 patients all of whom were young and already familiar with text and none of whom smoked it already presents a somewhat biased picture of the asthma population. This is reflected in the 99% compliance rate with the text transmission of PEF rate measurements. In a more representative sample of the general asthmatic population you would anticipate this figure to be much lower. This is the major weakness of this study. There was also no blinding in the study, however efforts were made to randomise appropriately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomised by computer” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | In does not appear that outcome assessors were blinded as to patient allocation. It would have been impossible to blind patients as to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data; there were no study withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Pinnock 2003.
| Methods | Pragmatic, multicentre, randomised controlled trial | |
| Participants | 278 people with asthma (people who had requested a prescription for a bronchodilator inhaler in the last 6 months) were contacted for asthma review at four UK general practices. None of these adults had been reviewed in the previous 11 months. | |
| Interventions | Telephone review was used for 137 patients, with the asthma nurse. The nurse tried up to 4 times to contact the patients. Control: Face‐to‐face reviews for 141 patients in the surgery also with asthma nurse, one invitation was sent in the usual manner. Content of the review was as the nurse deemed appropriate. |
|
| Outcomes | Outcomes: 1. Comparisons were made of the proportion of patients reviewed in each arm within three months of randomisation 2. Time taken to review patients in each, arm i.e. length of consultation 3. Asthma morbidity according to the validated “short Q” score 4. Asthma related quality of life as measured by the Juniper mini asthma quality of life questionnaire 5. Patient satisfaction of nursing care 6. Overall cost of respiratory care from the perspective of the NHS. This included costs of the healthcare professionals, any hospital costs (respiratory outpatients, accident and emergency, hospital admissions), as provided by the Personal Social Services Research Unit. This included cost of all respiratory care, surgery, telephone, home visits and out of hours care for all respiratory conditions as recorded in the patients’ notes. Prescribing costs from the British National Formulary. All costs were in pounds sterling for the year 2000‐2001. 7. Total cost of providing the review service, including costs of the asthma specialist practice nurse (source: Personal Social Services Research Unit) and Telephone costs (British Telecom). 8. Cost per consultation achieved in the 2 groups and also costs per missed appointments and telephone calls that were not answered |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were centrally randomised in blocks of 10 to ensure that approximately equal numbers of patient were allocated to each arm of the study |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding (performance bias and detection bias) All outcomes | High risk | “a researcher, blinded to allocation visited each of the practices and validated a random 20% sample of consultation data and data retrieved from records”. Although the patients and investigators could not be blinded to the interventions and, in most cases the outcome assessors, were not blinded to group allocation either, this was an attempt to ensure that outcome data were not biased. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of patients reviewed by each of the research methods was a primary outcome measure and was clearly reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Pinnock 2005.
| Methods | This was the same study as Pinnock 2003 with additional data and calculations performed with regards to cost, i.e. outcomes 6 to 8 in the above table | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
Pinnock 2007.
| Methods | Phase IV Implementation study (as per Medical Research Council 2008) | |
| Participants | Patients: One large English general practice on 3 sites was involved in this trial. During the 12‐month study patients with asthma were offered a review service according to the allocation of the group with which they were registered. | |
| Interventions | Intervention: The patients who were allocated to the intervention group were given a telephone option for their asthma review service. They were identified from the practice computer database and sent 3 invitations over the study period. They could book either a telephone or face‐to‐face review both at a pre‐arranged time. Patients who did not respond to the 3 invitations were phoned and reviewed opportunistically. Control: The control group were recalled to face‐to‐face only asthma reviews using invitations by post or as memos with repeat prescriptions. There was no option of telephone reviews and no systematic attempt was made to phone non‐ attenders opportunistically. There was a second control arm of usual care who had an established asthma clinic with no systematic recall. Invitations would only be issued in response to clinical need. |
|
| Outcomes | Outcomes: 1. Proportion reviewed: proportion of patients with active asthma who had received a dedicated asthma review within the previous 15 months 2. Asthma morbidity and enablement, as assessed by the following postal questionnaires: mini asthma quality of life questionnaire, Asthma Control Questionnaire, Modified Patient enablement Instrument and Asthma Bother Profile. 3. Adverse events, both clinical, e.g. asthma deaths and near‐fatal asthma attacks and organisational, e.g. complaints 4. Time, cost and mode of review were all documented |
|
| Notes | The population was not fixed in this implementation study as in a trial, therefore new diagnoses, changes in disease status, moves into and away from the practice were all included in the allocated service provision of the group with which they were registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was decided by the toss of a coin |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | The nurses were aware of allocation, however there were quality control checks blinded to allocation which confirmed accuracy of data transfer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Real‐world implementation study, therefore the uptake rate by patients is part of the study, routine asthma review was provided for 66.3% of patients in the telephone only group and 53.8% in the face‐to‐face only group |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Randomisation only took place in 2 of the 3 practices due to various considerations |
Rasmussen 2005.
| Methods | Randomised controlled trial with one intervention arm and 2 control arms | |
| Participants | Patients: This study was set in Copenhagen, Denmark. In 2001, a random sample of subjects was sent a questionnaire to diagnose asthma. A power calculation was performed. Letters were posted until 300 adults aged 18 to 45 had been diagnosed with asthma on the basis of a combination of respiratory symptoms and at least one objective measurement (i.e. hyperresponsiveness to methacholine or peak expiratory flow variability) | |
| Interventions | Intervention: This group were given electronic diary, an asthma action plan and a decision support system for the physician. Patients were given a Peak Flow Meter and taught how to fill in a daily diary and respond to the computer’s advice. Physicians gave instructions via e‐mail or telephone to the participant. Control 1. Specialist care: The specialists taught the patients how to adjust their medication on the basis of a peak flow meter and written action plan Control 2: GP: The GP group were asked to contact their GP and pass on a letter describing the study and giving the test results. GPs in Copenhagen had been sent a circular about asthma and GINA guidelines in the past. |
|
| Outcomes | Outcomes: 1. Quality of Life as assessed by AQLQ 2. Other questionnaire based outcomes: asthma self‐care, smoking, education, salary, sick leave and hospitalisations. Respiratory symptoms current medication, compliance and adverse reactions. 3. Lung function as carried out at baseline and 6 months and airway responsiveness with methacholine |
|
| Notes | The selection of participants was unusual as they came directly from the community and not from previous diagnosis by a physician or GP as in most other studies. This has the benefit of standardised diagnosis and avoids under‐diagnosis which may be a problem in the general population, however it may have implications when synthesising results from other studies. Participants in all 3 groups had to cover the costs of the medication prescribed, this may have been a problem for some patients and caused a bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Patients were randomised consecutively” |
| Allocation concealment (selection bias) | Low risk | “sealed envelope technique” |
| Blinding (performance bias and detection bias) All outcomes | High risk | Impossible to blind participants and no evidence of attempts to blind outcome assessors or data analysers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 300 patients randomised, 253 patiens completed both the screening and follow‐up visits. |
| Selective reporting (reporting bias) | High risk | Not all of the results from the questionnaires above are reported |
| Other bias | High risk | See notes above |
Van der Meer 2009.
| Methods | Randomised controlled trial | |
| Participants | 200 adults with physician‐diagnosed asthma, age 18 to 50, prescription of inhaled corticosteroids for at least 3 months in the previous year | |
| Interventions | There was a 2‐week baseline period where patients familiarised themselves with the technology before randomisation. Then patients who were randomised to receive intervention used an Internet‐based self‐management program. They measured FEV1 daily and reported the highest of 3 measurements before taking their medication. They complete the Asthma Control Questionnaire (ACQ) once a week and reported symptoms via internet or text. Patients monitored their asthma using the special website or via text on a mobile phone then used an Internet‐based asthma treatment plan and online education, including asthma news, frequently asked questions and other information. Patients could also communicate with a specialised asthma nurse either using the web or telephone. The ACQ score fed into an algorithm and patients received one of 4 treatment messages. Control patients had access to the part of the website on which a diary of symptoms and exacerbations was kept. |
|
| Outcomes | 1. Educational outcomes including asthma knowledge assess via the 12‐item Consumer Asthma Knowledge Questionnaire 2. Inhaler technique (standardised checklist of the Dutch Asthma Foundation) 3. Average number of medication changes per patient 4. Healthcare provider contacts including physician visits, telephone contacts and web communications 5. Clinical outcomes including 32‐item Juniper adult AQLQ, Asthma Control Questionnaire, symptom‐free days, pre‐bronchodilator FEV1, daily inhaled corticosteroid dose and exacerbations |
|
| Notes | The study was performed in Dutch. It would have been useful to know the seasonal time of year at which the results were recorded as asthma can worsen in cold weather or with pollen counts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly assigned using a computer‐generated permuted‐block scheme" |
| Allocation concealment (selection bias) | Unclear risk | "Allocation took place by computer after collection of the baseline data ensuring concealment of allocation." It is not clear whether this was central allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of intervention, outcome assessor or data analyser |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 200 adults were randomised, after 12 months there remained 92 in the control group and 91 in the intervention group. 9 patients withdrew consent and 8 were lost to follow up. They analysed complete cases and did not impute missing values |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of further systematic bias |
Vollmer 2006.
| Methods | Randomised clinical trial | |
| Participants | Patients: Central to this study was the aim of identifying individuals at greatest risk for acute exacerbations. Patients from Kaiser Permanente Northwest KPNW, a large group health maintenance organisation in Portland Oregon, were used for this study. Patients were eligible if they were aged 18 or older on January 1st 2003 and were on the KPNW asthma registry or had had at least 180 days of anti‐asthma medication dispensed. 850 individuals who had COPD listed on their problem list were excluded. Resulting sample size was 6948 patients. | |
| Interventions | Intervention: This consisted of 3 rounds of telephone calling about 5 months apart. The calls consisted of a series of questions to assess any recent emergency care for which they had not had follow up; their current stage of asthma control and medication use and whether they could identify a primary care provider whom they usually saw for asthma care. Patients were asked questions to screen for COPD. Next they were given optional tailored feedback regarding their overall level of asthma control and use of medications. Suggestions might include advice on the control of night waking or the need to continue inhaled corticosteroids daily. An offer of further KPNW services and a repeat call in 5 months was given. 3389 patients received calls using automated speech recognition technology. 192 patients received the call from a live caller. The call generated alerts for the provider as to which participants were at high risk of a future exacerbation, thereby hopefully triggering follow up. Controls: Received usual care, i.e. no additional telephone calls |
|
| Outcomes | Primary outcome measures: data were collected by surveys of patients and a selection of providers. Baseline data came from administrative data, KPNW data and a survey mailed to a random sample of 549 health plan members in November 2002. This had an 83% response rate. Follow‐up data came from a survey of 1583 randomly selected participants 1 month after final call, response rate 65% 1. Healthcare utilisation (KPNW data) 2. Medication use (Asthma Therapy Assessment Questionnaire (ATAQ), dispensing of antiasthma medications) 3. Quality of life (ATAQ, 5‐point scale reflecting the number of control problems in the last month, mini‐Juniper Asthma Quality of Life Questionnaire and Asthma Impact survey) 4. Demographic data, measures of health status, self‐management practices, attitudes about asthma and satisfaction with care |
|
| Notes | At first the strength of this study seems to be its large number of participants. However, on closer inspection it transpires that the data sources are often fragmented and chosen as representative data or randomly selected representative group rather than actual data from the full number of participants. Little effort seems to have been made to confirm that the samples used were indeed representative of the groups they were selected to represent, i.e. no confirmation of baseline data etc. In addition, the very low participation rate in the intervention calls is a high risk factor for the introduction of bias Also of note was the ethically dubious decision not to inform participants that they were taking part in research. The authors justified this by stating this was "in order to mimic the results of real‐world implementation.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No detail given as to who amassed results from questionnaires and whether blinding procedures were in place |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data was only collected from a random sample of both the intervention and usual care groups, i.e. incomplete. For healthcare utilisation data, it was only reported for patients who had had at least 6 months of cover by the plan. |
| Selective reporting (reporting bias) | High risk | "although the overall intent‐to‐treat analyses gave non significant results, post hoc analyses that compared the control participants to participants who actually used the intervention found numerous significant, albeit small differences." |
| Other bias | High risk | Very low participation rates in intervention. Follow‐up data were collected from only 38% of participants. |
Willems 2007a.
| Methods | This paper reported process outcomes from the study whose full results were published in Willems 2008 | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
Willems 2007b.
| Methods | This paper reported cost effectiveness of the study whose full results were published in Willems 2008 | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
Willems 2008.
| Methods | Single‐centre, prospective, randomised controlled trial | |
| Participants | Maastricht in the Netherlands, 56 children and 53 adults, 42% of the children and 50% of the adults were female. Mean ages: children,11: adults, 46 | |
| Interventions | Intervention group used an asthma monitor with modem at home with an asthma nurse as the main caregiver, i.e. a telemonitoring kit based on peak‐flow measurements. There was a baseline visit to the asthma nurse when the patient received education about the telemonitoring protocol. Patients were asked to perform daily PEFR and more often in exacerbations. The nurse could increase and decrease asthma medication and involve a doctor if necessary. Control group received regular outpatient care: 3 to 6‐monthly medical check‐ups by their lung specialist or paediatrician |
|
| Outcomes | 1. Diary of clinical asthma symptoms (cough, sputum production, shortness of breath and wheezing) 2. Asthma‐related medical consumption (health care utilisation and self‐reported medication use) 3. Feasibility as assessed by ease of recruitment, and by the occurrence of technical and logistic problems 4. Spirometry data ‐ which could be stored and analysed after several weeks as the monitor had a sufficient memory 5. Quality of life: as assessed by questionnaires ‐ European Quality of Life‐5D, Short Form 36, Asthma Quality of Life Questionnaire, Paediatric Asthma Quality of Life Questionnaire and Health Related Quality of Life Measure for Children. If available, children received paediatric versions. 6. Patient satisfaction in the intervention group as assessed by a questionnaire based on the satisfaction questionnaire developed by Finkelstein et al 7. Cost effectiveness from the society perspective: cost in Euros per QALY gained |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization took place on patient level after stratification by age (ages 7‐18 vs 18 years and older) as regular care differs between these age groups. The asthma nurse used a list of random numbers to allocate the patients to one of the two treatment arms" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants could not be blinded, the nurse practitioner was not blinded to the allocation of the participants as they received monthly transfers of the monitor data, and there was no evidence of outcome assessor blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 109 patients were randomised, 5 were lost to follow up. There were technical problems and where data transfer was missed the nurse practitioner attempted to contact the patients by telephone however this was not possible in 21% of missed data transfers. At baseline there was 100% compliance with filling in the questionnaires, for subsequent measurements response rate was 85% to 92% for questionnaires and 81% to 90% for diaries. 28% of PEF data transfers from adults and 18% from children were missed |
| Selective reporting (reporting bias) | Low risk | No apparent selective reporting |
| Other bias | High risk | Smoking was not taken account of, neither was the sex imbalance among the children. The nurse practitioner could only be reached during working hours. |
ACQ = Asthma Control Questionnaire AQLQ = Asthma Quality of Life Questionnaire ATAQ = Asthma Therapy Assessment Questionnaire COPD = chronic obstructive pulmonary disease FEV1 = forced expiratory volume in 1 second MDI = metered dose inhaler PEF = peak expiratory flow PEFM = peak expiratory flow meter PEFR = peak expiratory flow rate SMS = Short Message Service (text) RAST = radioallergosorbent test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergman 2008 | Prospective cohort study |
| Boone 2002 | Intervention is purely educational with no feedback from a healthcare professional |
| Burkhart 1996 | Trial of educational technique not telehealthcare as defined |
| Burkhart 2007 | No feedback given to the patients who improve their self‐monitoring |
| Chan 2003 | Only 10 patients, non‐randomised pilot study |
| Chandler 1990 | RCT but only 3 asthma patients ‐ therefore sample size is too small, pilot trial |
| Delaronde 2005 | Not randomised ‐ patient choice of intervention |
| Finkelstein 1998 | Not RCT, pilot study only |
| Finkelstein 2000 | Not RCT, discussion piece |
| Finkelstein 2001 | Not RCT |
| Finkelstein 2007 | Not RCT |
| Huss 1992 | Education only, not telehealthcare |
| Huss 2003 | Education only, not telehealthcare |
| Jaana 2009 | Systematic review |
| Joseph 2007 | Programme delivered to African‐American high school students at school with supervision, not telehealthcare |
| Joshi 2009 | Prospective, non‐randomised pre‐post study |
| Karp 2000 | Not limited to asthma; asthma data can not be separated. |
| Le 2007 | Education only; not telehealthcare |
| Lin 2009 | Convenience sampling method |
| Maiolo 2003 | Before and after study design |
| Malone 2004 | Same study as Chan 2007 |
| Marcin 2004 | Convenience sampling of children |
| McCLure 2008 | Focus is on data collection methods not telehealthcare |
| Patel 2009 | No medical advice given over telephone ‐ therefore not telehealthcare |
| Pinnock 2007b | Qualitative study |
| Porter 2006 | Not telehealthcare; takes place in emergency health department |
| Reddel 1998 | Study aims to assess only the quality of the spirometry |
| Smith 2009 | Discussion article |
| Sockrider 2006 | RCT of telehealthcare but protocol was to report after 12 months and only interim results from 9 months have been published |
| van den Berg 2002 | The phone line was for GPs to contact hospital for information regarding asthma guidelines |
| Wise 2007 | No results; describes the system for obtaining them |
| Young 2003 | Not asthma; range of other conditions |
| Zamith 2009 | Not randomised |
RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12606000400561.
| Trial name or title | Improving childhood asthma management through a telemedicine monitoring network |
| Methods | A pilot formal randomised control trial |
| Participants | 120 children with asthma who have had at least one admission to hospital or one emergency department or paediatric or GP visit for asthma requiring steroid rescue within the previous 12 months. SMS patients must have a mobile phone. Participants will be identified by discharge reports from participating hospitals. Age 3 to 16. |
| Interventions | There will be 3 groups of patients: a) regular care ‐ GP/paediatrician/hospital emergency services, b) telemedicine ‐ twice a week automated telephone calls to the family, c) nurse support ‐ telephone call by an asthma nurse every 2 weeks. SMS Monitoring will also be undertaken, 40 adolescents with asthma will be randomised to monitoring via mobile phone using SMS and a control group receives regular care. |
| Outcomes | Health resource utilisation over 6 months, health economic assessment over a 6‐month period, school days missed for children and days off work for parents, medication use and health‐related quality of life |
| Starting date | 11 September 2006 |
| Contact information | Mary Jackson, Department of Respiratory Medicine, Royal Children's Hospital, Herston Rd, Herston, Brisbane, QLD, Australia Mary_Jackson@health.qld.gov.au |
| Notes | — |
Apter NCT00115323.
| Trial name or title | Comparison of two medication adherence strategies to improve asthma treatment adherence |
| Methods | Treatment, randomised, open‐label, parallel‐assignment, efficacy study |
| Participants | Recruitment will be from practices serving low‐income and minority individuals. Age 18 to 90 and receiving treatment for asthma at one of the participating clinics, current use of prescribed inhaled corticosteroids, evidence of reversible airflow obstruction as follows: FEV1 less than 80% at the time of study entry or within 3 years prior to study entry. An increase of greater than 15% and 200ml in FEV1 with asthma treatment over the last 3 years or evidence of reversible airflow obstruction on administration of albuterol. |
| Interventions | Comparison of a problem‐solving intervention with an attention control intervention to improve and sustain asthma self management in a clinical setting. There will be strategies to address contextual factors related to adherence. |
| Outcomes | Adherence to prescribed inhaled steroid regimen measured at week 26, FEV1 and quality of life factors |
| Starting date | May 2005 |
| Contact information | Apter, University of Pennsylvania, Philadelphia, Pennsylvania 19104 |
| Notes | This study does not appear to have a proper control arm and as such would not be eligible to be included in the systematic review and meta‐analysis. |
Bendeer NCT00958932.
| Trial name or title | Telecommunication Enhanced Asthma Management TEAM |
| Methods | Treatment randomised, single‐blind (investigator), placebo control, parallel assignment |
| Participants | 1000 parents of 3 to 12‐year old children in Colorado who require daily corticosteroid inhaler |
| Interventions | Speech recognition calls are tailored to specific situations, e.g. new or re‐issued corticosteroids. Filling an initial or an active prescription. Follow up after an asthma exacerbation. Moderate exercise. |
| Outcomes | medication adherence and urgent care visits |
| Starting date | Sept 2009 |
| Contact information | Bruce Bendeer, National Jewish Health, Denver, Colorado, USA, 80206 |
| Notes | — |
Clover N0702196597.
| Trial name or title | Self‐management of chronic conditions using telemedicine |
| Methods | Immediate transfer of peak flow from a mobile phone to a remote server with monitoring software, i.e. distribution of most recent readings or trend analysis is calculated and fed back to mobile phone |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Starting date | — |
| Contact information | Tim Clover, T+ Medical Ltd, The Magdalen Centre, Oxford Science Park, Oxford, OX4 4GA |
| Notes | Health Technology Devices Programme HTD 244 |
Finkelstein CRISP.
| Trial name or title | Inhaled steroid adherence in moderate and severe asthma |
| Methods | The Internet‐based Home Asthma Telemonitoring (HAT) system, designed to give continuous individualised help to asthma patients following their self‐care plans and to notify their healthcare providers of changes. Patients will take their PEF daily and answer symptom questions on asthma status and an asthma nurse case manager will find out if patients are not following self‐care plans and if there are any clinically significant conditions. She can then contact the patient and give advice about dealing with an exacerbation. |
| Participants | 240 inner‐city adult asthma patients for 12 months |
| Interventions | Home asthma telehealthcare is designed to provide continuous tailored help to asthma patients |
| Outcomes | Clinical outcomes as measured by electronic peak flow, adherence to self‐care |
| Starting date | 2005 |
| Contact information | Finkelstein |
| Notes | — |
Friedman CRISP.
| Trial name or title | Impact of a telecommunication system in childhood asthma |
| Methods | Telephone Linked Communications for Asthma is a computer‐based telecommunications system than monitors educates and counsels asthmatic children and their parents through regular automated conversations and reports finding to primary care |
| Participants | Children aged 5 to 16 with mild to moderate asthma |
| Interventions | A computer‐based telecommunication system is used to help parents and children keep their asthma under control. It converses with parents and children weekly at home. It provides education and counselling appropriate to the self‐monitoring and medication use of the child. This information is stored in a database and passed on to the primary care provider. |
| Outcomes | Changes in PEF, quality of life and lung function and social outcomes including the impact of the child's asthma on the family. Health services outcomes include utilisation of urgent care services and unscheduled office visits. Attitudes of children, parents and providers analysed using qualitative and quantitative questioning. |
| Starting date | 1999 |
| Contact information | Friedman |
| Notes | — |
Garbutt NCT 00860834.
| Trial name or title | Parents, pediatricians and asthma telephone coaches partner to improve control of asthma in children (The PARTNER Study) |
| Methods | Randomised, single‐blind with active control, parallel‐assignment, efficacy study |
| Participants | 1000 children aged 5 to 12 years old their parents and their community paediatricians |
| Interventions | Asthma education will be given to community paediatricians and then an asthma coach will phone parents of children who have asthma to support and give active support to achieving asthma goals with partnership from the community paediatrician |
| Outcomes | Asthma control, asthma‐related quality of life, urgent care events, adherence to guideline recommended asthma maintenance care behaviours, cost‐effectiveness |
| Starting date | August 2008 |
| Contact information | Jane Garbutt and Robert Srunk, Washington University School of Medicine, St Louis, Missouri, USA 63110 |
| Notes | This is a larger more general population than in the other Garbutt study |
Garbutt NCT00660322.
| Trial name or title | Using the telephone to improve care in childhood asthma |
| Methods | 12‐month randomised controlled trial |
| Participants | 362 children aged 5 to 12 under care of community paediatricians |
| Interventions | A series of brief telephone calls with a trained coach to help the parent manage the child's asthma care. The coach teaches self‐management skills, helps the parent with asthma medications, supports the parent and provides reminders for outpatient appointments. |
| Outcomes | Parental asthma related quality of life and urgent care events for asthma over one year |
| Starting date | Jan 2004 |
| Contact information | Jane Garbutt, Washington University School of Medicine |
| Notes | This is a low‐income, urban neighbourhood |
Gustafson NCT00214383.
| Trial name or title | Internet Telehealth for Pediatric Asthma Case Management CHESS |
| Methods | Randomised controlled intervention trial, 12 months |
| Participants | Parents of children age 4 to 12 with asthma |
| Interventions | Internet telehealth ‐ CHESS with nurse case management |
| Outcomes | Improve adherence to controller medications Improve asthma control and reduce health care utilisation |
| Starting date | May 2004 |
| Contact information | Gustafson, University of Wisconsin |
| Notes | — |
Gustafson NCT00993590.
| Trial name or title | Mobile CHESS Research on Emergency Medical Services for Children |
| Methods | Supportive care, randomised, open‐label, placebo control, parallel‐assignment, efficacy study |
| Participants | 400 low‐income teenagers with asthma‐related emergency care or asthma hospitalisation in the last 12 months. They also receive medical care from the managed care organisations participating in the study. |
| Interventions | This group will receive a smart phone with the ability to contact their case managers and primary provider, to communicate with the managed care organisation case managers, their peers, share information regarding health status, receive reminders to take medications and set up follow up. To receive feedback and tailored advice on their asthma plan and the use of asthma resources. And also give access to asthma educational materials and provide monthly study outcome data monthly for 12 months |
| Outcomes | Asthma control test scores over 12 months, asthma‐related healthcare utilisation and school absenteeism |
| Starting date | June 2008 |
| Contact information | Gustafson, Centre for Health Enhancement Systems Studies (CHESS), Milwaukee, Wisconsin, USA 53214 |
| Notes | — |
Mayers NCT00562081.
| Trial name or title | The Virtual Asthma Clinic |
| Methods | Phase IV, prevention, randomised, single‐blind (subject), placebo control, parallel‐assignment efficacy study over 12 months |
| Participants | Physician diagnosis of asthma, FEV1 of at least 50% of predicted at baseline, evidence of 12% increase in FEV1 following inhaled bronchodilator. Ages 18 to 70. |
| Interventions | Standardised education to all participants, baseline pulmonary function tests and instructions on using a peak flow monitor. One arm will receive active therapy using the internet site or a placebo therapy using a web‐based intervention. The active website will monitor the intervention group participants' asthma profile daily, with access to a certified asthma educator. IF patients do not log on for 7 days with poor control or 14 days with good control they will receive a telephone call. |
| Outcomes | The 15 D and AQLQ at baseline, 6 months and 12 months, spirometry at 12 months. Symptom surveys at log in by the intervention groups. |
| Starting date | March 2005 |
| Contact information | Mayers, University of Alberta Hospital, Edmonton, Alberta, Canada, T6G 2B7 |
| Notes | 15 D and AQLQ are questionnaires |
Moldrup NCT00917410.
| Trial name or title | Mobile phone text for optimising asthma treatment |
| Methods | Supportive care, randomised, open‐label, placebo control, single group assignment |
| Participants | Adults with asthma age 18 to 45 |
| Interventions | The SMS (text) tool on mobile phones can be used to monitor asthma. Sequences of SMS messages were sent to the intervention group. They contained monitoring questions and a reminder to take preventive medication. |
| Outcomes | Asthma control test, EQ‐5D, use of health services and used of preventive medicine |
| Starting date | November 2007 |
| Contact information | Moldrup, University of Copenhagen, Copenhagen, Denmark 2100 |
| Notes | This study is noted as having been suspended |
NCT00149474.
| Trial name or title | Peak flow monitoring in older adults with asthma |
| Methods | 5 year demonstration and education project, randomised |
| Participants | 260 adults aged 50 or older with asthma, using asthma medications, with a greater than 12% increase in FEV1 after 2 puffs of inhaled beta agonist |
| Interventions | Assessment of the value of peak flow monitoring over symptoms monitoring in this age group. And compare 3 parallel asthma education programmes for older adults. |
| Outcomes | Frequency and cost of health care utilisation for asthma and asthma‐specific quality of life |
| Starting date | August 1994 |
| Contact information | No contact information provided, performed in Portland |
| Notes | This study has apparently been completed |
Osman N0411013273.
| Trial name or title | A randomised controlled trial of benefits of specialist review or telephone follow up after an Accident and Emergency attendance for asthma |
| Methods | Randomised controlled study of the benefits of follow up |
| Participants | 300 patients recruited from A&E at Aberdeen Royal Infirmary |
| Interventions | Control group receive normal present care, group A out‐patient clinic review, group B telephone follow up with mailed information group D out‐patient clinic review and telephone follow up with mailed information. |
| Outcomes | A&E attendance, GP attendance, medication, symptoms and quality of life |
| Starting date | 1 October 1997 |
| Contact information | Liesl Osman, University of Aberdeen, med078@abdn.ac.uk |
| Notes | — |
Partridge N0016132017.
| Trial name or title | Proposal to study whether we can reduce hospital attendance by those with respiratory conditions without compromising care by the use of telephone consultation |
| Methods | Replacement of traditional face‐to‐face consultations with telephone consultations |
| Participants | 100 patients taken from the lung disease and asthma clinics |
| Interventions | Telephone consultations |
| Outcomes | Patient satisfaction, number of those telephoned needing face‐to‐face follow up. Costs of face‐to‐face consultation, disease profile of 2 study arms |
| Starting date | 13 August 2003 |
| Contact information | Prof Martyn R Partridge, Respiratory Medicine, NHLI, Charing Cross Hospital, Fulham Palace Road, London m.r.partridge@imperial.ac.uk |
| Notes | — |
Perry NCT00964301.
| Trial name or title | Telemedicine education for rural children with asthma |
| Methods | Treatment non‐randomised, parallel assignment efficacy study |
| Participants | Low‐income minority children,age 7 to 17, with asthma in the Delta region of Arkansas |
| Interventions | Interactive school‐based telemedicine program, education delivered via teleconference at school. Monthly sessions for a year |
| Outcomes | Asthma symptoms control, activity level, family/child emotional health, asthma knowledge, self‐efficacy and quality of life in intervention participants and their caregivers |
| Starting date | August 2009 |
| Contact information | Perry T, Arkansas Children's Hospital Research Institute, perrytamarat@uams.edu |
| Notes | — |
Ricci 2001 In progress.
| Trial name or title | A telehealthcare system for home monitoring of respiratory function in children affected by bronchial asthma |
| Methods | Controlled randomised trial |
| Participants | Participants from a family paediatrician and a paediatric allergist recruited over a 24 month period in Pavia, Italy. 20 patients in each arm ages 10 to 16. |
| Interventions | Intervention group performed daily spirometry and a daily symptom diary via the telephone |
| Outcomes | Resuscitation of patients with asthma, medical intervention ‐ re‐hospitalisation or emergency dept visit, cost, quality of life of patients and of their families, therapeutic schemes |
| Starting date | 2001 |
| Contact information | A. Ricci, Dipartimento di Scienze Pediatriche, Universita degli Studi, Pavia |
| Notes | Study has published its methodology |
Ryan NCT 00512837.
| Trial name or title | Mobile phone based structured intervention, the CYMPLA trial |
| Methods | Supportive care, randomised, single‐blind (investigator), active control, parallel assignment |
| Participants | 312 12 years and older patients with poorly controlled asthma who speak English and have a mobile phone |
| Interventions | Patients in the mobile phone group will monitor their asthma daily using their mobile phone to record symptoms medication and lung function. They will receive instantaneous feedback to their phone providing a visual indication of asthma control and prompts about therapy. They will have web‐based access with their clinician to all readings. An asthma nurse will guide them using BTS in order to gain control. |
| Outcomes | Change in asthma control between baseline and 6 months as measured by Asthma Control Questionnaire 24. The ACQ measures 0 good control to 6. Secondary outcome measures ‐ mean difference in ACQ at 3, 6, 24 and 36 months. Mean difference in mini asthma‐related quality of life questionnaire. |
| Starting date | November 2007 |
| Contact information | Dermot Ryan, University of Aberdeen, dermotryan@doctors.org.uk |
| Notes | Data undergoing statistics processing |
Sparrow NCT00232557.
| Trial name or title | Telecommunications system in asthma |
| Methods | Randomised, open‐label, active control, parallel‐assignment, efficacy study |
| Participants | Adults with asthma receiving treatment with one or more daily controller medications. With FEV1 bronchodilator response of at least 12%. |
| Interventions | Telephone linked communications systems will be used to provide education, behavioural counselling and monitoring of clinical status |
| Outcomes | Medication adherence, quality of life, utilisation of urgent care services, oral corticosteroid use |
| Starting date | August 2004 |
| Contact information | Sparrow, Dept of Veterans Affairs, VA Boston HealthCare System, Boston, Massachusetts, United States 02130 |
| Notes | Study scheduled to finish in December 2009 |
Strunk NCT00910585.
| Trial name or title | Coaching in childhood asthma |
| Methods | Prevention, randomised, open‐label, active control, parallel‐assignment, efficacy study |
| Participants | Mothers of 191 Afro‐American children with asthma aged 2 to 8, hospitalised for an acute exacerbation and with Medicaid insurance |
| Interventions | Coaching in person and via telephone to provide behavioural and social support |
| Outcomes | Rehospitalisation over next 2 years |
| Starting date | January 1997 |
| Contact information | Strunk, Washington University School of Medicine, St Louis, Missouri, USA, 63110 |
| Notes | — |
Vollmer NCT 00414817.
| Trial name or title | Telephone‐based program to promote inhaled corticosteroid adherence among individuals with asthma |
| Methods | Supportive care, randomised, open‐label, active control (usual care), parallel‐assignment efficacy study over a 19‐month period |
| Participants | 14,064 participants with asthma over the age of 18 |
| Interventions | A telephone‐based intervention which uses interactive voice recognition technology to remind people with asthma to take their medication and order refills when appropriate. The technology also gives recorded asthma education and can transfer participants to a pharmacist to arrange a prescription. Participants will receive between 1 and 8 phone calls during the study. |
| Outcomes | Approximately 2000 participants will be sent a questionnaire at the study entry and again at the end of the study to assess quality of life, respiratory health, asthma control, depression, inhaler use beliefs and satisfaction with the intervention. Electronic medical record data will be used to help determine adherence rates |
| Starting date | June 2007 |
| Contact information | Vollmer, Kaiser Permanente NW, Centre for Health Research, KPNW, Portland, Oregon United States |
| Notes | — |
Wouters NCT 00411346.
| Trial name or title | Patient Research In Self‐Management of Asthma (PRISMA) |
| Methods | Randomised controlled trial comparing a nurse‐led telemonitoring programme versus regular care in asthmatic outpatients |
| Participants | 7 years or older, asthma severity of stage I to III as described by GINA, competent to use an asthma monitor and possessing a household phone connection |
| Interventions | Lung function tests including PEF is recorded into an electronic monitor and transfers the data to a central database so that a nurse using protocols can supervised the disease status of patients and manage their medication accordingly |
| Outcomes | Asthma‐specific quality of life at 1 year, asthma symptoms at 1 year, generic quality of life at 1 year, direct and indirect costs during 1 year, satisfaction and feasibility at 1 year |
| Starting date | January 2003 |
| Contact information | Emiel Wouters, Dept of Respiratory Medicine, University Hospital Maastricht, Limburg, Netherlands 6202 AZ |
| Notes | The study is finished but no publications are yet listed |
A&E = Accident and Emergency department ACQ = Asthma Control Questionnaire AQLQ = Asthma Quality of Life Questionnaire FEV1 = forced expiratory volume in 1 second
Contributions of authors
AS conceived this review
SM wrote the protocol with oversight from JL, CP and AS.
DC selected studies and assisted with the first part of data extraction
UN completed data extraction.
SM and AS led the writing of this review with contributions from the other co‐authors
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Connecting for Health Evaluation Programme (NHS CFHEP 001), UK.
Edinburgh MRC Trials Methodology Hub (G0800803), UK.
NHS Education for Scotland Clinical Academic Fellowship, UK.
Chief Scientist's Office Scotland Small Grant, UK.
Declarations of interest
All of the authors are working on other projects in telehealth and e‐health funded by the NHS Connecting for Health Evaluation Programme 001 Extension and JL is in addition working on a Medicaid funded project. SM is funded by the Chief Scientist's Office. AS worked with Pinnock et al and is a co‐author on the 2003, 2005 and 2007 papers included in this review.
This report is independent research supported by the National Institute of Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute of Health Research or the Department of Health.
Edited (no change to conclusions)
References
References to studies included in this review
Barbanel 2003 {published data only}
- Barbanel D, Eldridge S, Griffiths C. Can a self‐management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58:851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bynum 2001 {published data only}
- Bynum A, Hopkins D, Thoma A, Copeland N, Irwin C. The effect of telepharmacy counselling on metered‐dose inhaler technique among adolescents with asthma in rural Arkansas. Telemedicine Journal and eHealth 2001;7(3):207‐16. [DOI] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan D, Callahan C, Hatch‐Pigott V, Lawless A, Proffitt L, Manning N, Schweikert M, Malone F. Internet based home monitoring and education of children with asthma is comparable to ideal office‐based care: results of a 1‐year asthma in‐home monitoring trial. Pediatrics 2007;119:569‐78. [DOI] [PubMed] [Google Scholar]
Chatkin 2006 {published data only}
- ChatkinJ, Blanco D, Scaglia N, Wagner M, Fritscher C. Impact of a low cost and simple intervention in enhancing treatment adherence in a Brazilian asthma sample. Journal of Asthma 2006;43:263‐6. [DOI] [PubMed] [Google Scholar]
Clark 2007 {published data only}
- Clark N, Gong M, Wang S, Lin X, Bria W, Johnson T. A randomized trial of a self‐regulation intervention for women with asthma. Chest 2007;132:88‐97. [DOI] [PubMed] [Google Scholar]
Cruz‐Correia 2007 {published data only}
- Cruz‐Correia R, Fonseca J, Lima L, Araujo L, Delgado L, et al. Web‐based or paper‐based self‐management tools for asthma‐patients opinions and quality of data in a randomized crossover study. Studies in Health Technology and Informatics 2007;127:178‐89. [PubMed] [Google Scholar]
de Jongste 2009 {published data only}
- Jongste JC, Carraro S, Hop WC, The CHARISM Study Group and Baraldi E. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. American Journal of Respiratory and Critical Care Medicine 2009;179:93‐7. [DOI] [PubMed] [Google Scholar]
Donald 2008a {published data only}
- Donald K, McBurney H, Teichtahl H, Irving L. A pilot study of telephone based asthma management. Australian Family Physician 2008;37(3):170‐3. [PubMed] [Google Scholar]
Donald 2008b {published data only}
- Donald K, McBurney H, Teichtahl H, Irving L, Browning C, Rubinfeld A, et al. Telephone based asthma management; financial and individual benefits. Australian Family Physician 2008;37(4):212‐75. [PubMed] [Google Scholar]
Gruffydd‐Jones 2005 {published data only}
- Gruffydd‐Jones K, Hollinghurst S, Ward S, Taylor G. Targeted routine asthma care in general practice using telephone triage. British Journal of General Practice 2005;55:918‐23. [PMC free article] [PubMed] [Google Scholar]
Guendelman 2002 {published data only}
- Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self management behaviors of inner city children. Archives of Pediatric Adolescent Medicine ry 2002;156:114‐20. [DOI] [PubMed] [Google Scholar]
Jan 2007 {published data only}
- Jan RL, Wang J, Huang M, Tseng SM, Su HJ, Liu LF. An internet based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine and eHealth 2007;13(3):257‐68. [DOI] [PubMed] [Google Scholar]
Khan 2004 {published data only}
- Khan M, O'Meara M, Stevermuer T, Henry R. Randomised controlled trial of asthma education after discharge from an emergency department. Journal of Paediatrics and Child Health 2004;40:674‐7. [DOI] [PubMed] [Google Scholar]
Kokubu 1999 {published data only}
- Kokubu F, Suzuki H, Sano Y, Kihara N, Adachi M. Tele‐medicine system for high‐risk asthmatic patients. Arerugi 1999;48(7):700‐12. [PubMed] [Google Scholar]
Kokubu 2000 {published data only}
- Kokubu F, Nakajima S, Ito K, Makino S, Kitamura S, Fukuchi Y, et al. Hospitalization reduction by an asthma telemedicine system. Arerugi 2000;49(1):19‐31. [PubMed] [Google Scholar]
Ostojic 2005 {published data only}
- Ostojic V, Cvoriscec B, Ostojc S, Reznikoff D, Stipic‐Markovic A, Tudjman Z. Improving asthma control through telemedicine a study of Short‐Message Service. Telemedicine and eHealth 2005;11(1):28‐35. [DOI] [PubMed] [Google Scholar]
Pinnock 2003 {published data only}
- Pinnock H, Bawden R, Proctor S, Wolfe S, Scullion J, Price D, et al. Accessibility, acceptability, and effectiveness in primary care of routine telephone review of asthma: pragmatic randomised controlled trial. BMJ 2003;326:477‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinnock 2005 {published data only}
- Pinnock H, McKenzie L, Price D, Sheikh A. Cost‐effectiveness of telephone or surgery asthma reviews: economic analysis of a randomised controlled trial. British Journal of General Practice 2005;55:119‐24. [PMC free article] [PubMed] [Google Scholar]
Pinnock 2007 {published data only}
- Pinnock H, Adlem L, Gaskin S, Harris J, Snellgrove C, Sheikh A. Accessibility, clinical effectiveness and practice costs of providing a telephone option for routine asthma reviews: phase IV controlled implementation study. British Journal of General Practice 2007;57:714‐22. [PMC free article] [PubMed] [Google Scholar]
Rasmussen 2005 {published data only}
- Rasmussen L, Phanareth K, Nolte H, Backer V. Internet‐based monitoring of asthma: a long term randomized clinical study of 300 asthmatic subjects. Journal of Allergy and Clinical Immunology 2005;115(6):1137‐42. [DOI] [PubMed] [Google Scholar]
Van der Meer 2009 {published data only}
- Meer V, Bakker MJ, Hour W, Rabe KF, Sterk PJ, Kievit J, Assendelft WJ, Sont JK. Internet‐based self management plus education compared with usual care in asthma. Annals of Internal Medicine 2009;151:110‐20. [DOI] [PubMed] [Google Scholar]
Vollmer 2006 {published data only}
- Vollmer W, Krishner M, Peters D, Drane A, Stibolt T, Hickey T, et al. Use and impact of an automated telephone outreach system for asthma in a managed care setting. American Journal of Managed Care 2006;12(12):725‐33. [PubMed] [Google Scholar]
Willems 2007a {published data only}
- Willems D, Joore M, Hendriks J, Wouters E, Severens J. Cost effectiveness of a nurse‐led telemonitoring intervention based on peak expiratory flow measurements in asthmatics: results of a randomised controlled trial. Cost Effectiveness and Resource Allocation 2007;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willems 2007b {published data only}
- Willems D, Joore M, Hendriks J, Duurling R, Wouters E, Severens J. Process evaluation of a nurse‐led telemonitoring programmer for patients with asthma. Journal of Telemedicine and Telecare 2007;13:310‐17. [DOI] [PubMed] [Google Scholar]
Willems 2008 {published data only}
- Willems DC, Joore MA, Hendriks JJE, Nieman FHM, Severens JL, Wouteres EFM. The effectiveness of nurse‐led telemonitoring of asthma results of a randomized controlled trial. Journal of Evaluation in Clinical Practice 2008;14:600‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bergman 2008 {published data only}
- Bergman D, Sharek PJ, Kekgran K, et al. The use of telemedicine access to schools to facilitate expert assessment of children with asthma. International Journal of Telemedicine Applications 2008;159:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boone 2002 {published data only}
- Boone D, Counsell P, et al. Effect of computer‐assisted education on inhaler technique and knowledge in children with asthma. European Respiratory Society Annual Congress 2002:P2062.
Burkhart 1996 {published data only}
- Burkhart P. Effect of contingency management on adherence to peak flow monitoring in school age children with asthma. University of Pittsburgh 1996 PHD, p 276.
Burkhart 2007 {published data only}
- Burkhart PV. Testing an Intervention to Promote Children's Adherence to Asthma Self‐Management. Journal of Norsing Scholarship 2007;39(2):133‐140. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
- Chan D, Callahan C, Sheets S, Moreno C, Malone F. An Internet‐based store and forward video home telehealth system for improving asthma outcomes in children. American Journal of Health‐System Pharmacy 2003;60:1976‐81. [DOI] [PubMed] [Google Scholar]
Chandler 1990 {published data only}
- Chandler M, Clifton D, Louis B, Coons S, Foster T, Phillips B. Home monitoring of theophylline levels: a novel therapeutic approach. Pharmacotherapy 1990;10(4):294‐300. [PubMed] [Google Scholar]
Delaronde 2005 {published data only}
- Delaronde S, Peruccio D, Bauer B. Improving asthma treatment in a managed care population. American Journal of Managed Care 2005;11(6):361‐8. [PubMed] [Google Scholar]
Finkelstein 1998 {published data only}
- Finkelstein J, Hripcsak G, Cabrera M. Telematic system for monitoring of asthma severity in patients' homes. Studies in Health Technology and Informatics 1998;52 (Pt 1):272‐6. [PubMed] [Google Scholar]
Finkelstein 2000 {published data only}
- Finkelstein J, Cabrera M, Hripcsak G. Internet‐based home asthma telemonitoring: can patients handle the technology?. Chest 2000;179(2):148‐55. [DOI] [PubMed] [Google Scholar]
Finkelstein 2001 {published data only}
- Finkelstein J, O'Connor G, Friedmann RH. Development and implementation of the home asthma telemonitoring HAT system to facilitate asthma self‐care. Medinfo 2001;10(1):810‐14. [PubMed] [Google Scholar]
Finkelstein 2007 {published data only}
- Finkelstein J, Gangopadhyay A. Using machine learning to predict asthma exacerbations. AMIA Annual Symposium Proceedings/AMIA Symposium 2007;11:955. [PubMed] [Google Scholar]
Huss 1992 {published data only}
- Huss K. Computer education for asthmatics: what effects?. Journal of Nursing Care Quality 1992;6(3):57‐66. [DOI] [PubMed] [Google Scholar]
Huss 2003 {published data only}
- Huss K. Computer game for inner city children does not improve asthma outcomes. Journal of Pediatric Health Care 2003;17(2):72‐8. [DOI] [PubMed] [Google Scholar]
Jaana 2009 {published data only}
- Janna M, Pare G, Sicotte C. Home telemonitoring for respiratory conditions: a systematic review. American Journal of Managed Care 2009;14(5):313‐20. [PubMed] [Google Scholar]
Joseph 2007 {published data only}
- Joseph CL, Peterson E, Havstad S, Johnson CC, Hoerauf S, Stringer S, et al. A web‐based tailored asthma management program for urban African‐American high school students. American Journal of Respiratory and Critical Care Medicine 2007;175(9):888‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joshi 2009 {published data only}
- Joshi A, Weng W, Lichenstein R, Arora M, Sears A. Prospective tracking of a pediatric emergency department e‐kiosk to deliver asthma education. Health Informatics Journal 2009;15(4):282‐95. [DOI] [PubMed] [Google Scholar]
Karp 2000 {published data only}
- Karp W, Grigsby R, McSwiggan‐Hardin M, Pursley‐Crotteau S, Adams LN, Bell W, et al. Use of telemedicine for children with special healthcare needs. Paediatrics 2000;105:843‐7. [DOI] [PubMed] [Google Scholar]
Le 2007 {published data only}
- TT Holbrook JT, et al. Effectiveness and acceptability of computer‐based asthma education. American Thoracic Society International Conference May 18‐23:A93.
Lin 2009 {published data only}
- Lin SP, Yang HY. Exploring key factors in the choice of e‐health using an asthma care mobile service model. Telemedicine and eHealth 2009;15(9):884‐9. [DOI] [PubMed] [Google Scholar]
Maiolo 2003 {published data only}
- Maiolo C, Moamed El, Fiorani CM, Lorenzo A. Home telemonitoring for patients with severe respiratory illness: the Italian experience. Journal of Telemedicine and Telecare 2003;22:1372‐8. [DOI] [PubMed] [Google Scholar]
Malone 2004 {published data only}
- Malone F, Calahan C, Chan DS, Sheets, MS Person DA. Caring for children with asthma through teleconsultation: "ECHO‐Pac, the electronic children's hospital of the Pacific". Telemedicine Journal and eHealth 2004;10(2):138‐46. [DOI] [PubMed] [Google Scholar]
Marcin 2004 {published data only}
- Marcin J, Ellis J, Mawis R, Nagrampa E, Nesbitt TS, Dimand RJ. Using telemedicine to provide pediatric subspeciality care to children with special healthcare needs in an underserved rural community. Pediatrics 2004;113:1‐6. [DOI] [PubMed] [Google Scholar]
McCLure 2008 {published data only}
- McClure LA, Harrington K, Graham H, Gerald LB. Internet‐based monitoring of asthma symptoms peak flow meter reading and absence data in a school based clinical trial. Clinical Trials 2008;5:31‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2009 {published data only}
- Patel R, Saltoun C, Grammer L. Improving asthma care for the elderly: as randomised controlled trial using a simple telephone intervention. Journal of Asthma 2009;46:30‐5. [DOI] [PubMed] [Google Scholar]
Pinnock 2007b {published data only}
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Understanding the potential role of mobile phone‐based monitoring on asthma self‐management: qualitative study. Clinical Experimental Allergy 2007;37(5):794‐802. [DOI] [PubMed] [Google Scholar]
Porter 2006 {published data only}
- Porter SC, Forbes P, Feldman HA, Goldmann DA. Impact of patient‐centered decision support on quality of asthma care in the emergency department. Pediatrics 2006;117(1):e33‐42. [DOI] [PubMed] [Google Scholar]
Reddel 1998 {published data only}
- Reddel H, Ware S, Salome C, Henkins C, Woolcock A. Pitfalls in processing home electronic spirometric data in asthma. European Respiratory Journal 1998;12:853‐8. [DOI] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith SM, Elkin SL, Partridge MR. Technology and its role in respiratory care. Primary Care Respiratory Journal 2009;18(3):159‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sockrider 2006 {published data only}
- Sockrider M, Abramson S, Brooks E, Caviness C, Pilney S, Koerner C, et al. Delivering tailored asthma family education in a pediatric emergency department setting: a pilot study. Pediatrics 2006;117:S135‐S144. [DOI] [PubMed] [Google Scholar]
van den Berg 2002 {published data only}
- Berg NJ, Have WH. Is the availability of a 24 hour asthma‐telephone useful in the implementation of asthma treatment guidelines for children aged 6‐16 years among general practitioners?. Conference Abstract European Respiratory Society Annual Congress 2002:P2051.
Wise 2007 {published data only}
- Wise M, Gustafson DH. Internet telehealth for pediatric asthma management: integrating computerised and case manager features for tailoring a web‐based asthma education program. Health Promotion Practice 2007;8(3):282‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2003 {published data only}
- Young TL, Ireson C. Effectiveness of school‐based telehealthcare in urban and rural elementary schools. Pediatrics 2003;112(5):1088‐94. [DOI] [PubMed] [Google Scholar]
Zamith 2009 {published data only}
- Zamith M, Cardoso T, Matias I, Marques Gomes MJ. Home telemonitoring of severe chronic respiratory insufficiency and asthmatic patients. Revista Portuguesa de Pneumologia 2009;15(3):385‐417. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12606000400561 {published data only}
- Improving childhood asthma management through a telemedicine monitoring network. Ongoing study 11 September 2006.
Apter NCT00115323 {published data only}
- Comparison of two medication adherence strategies to improve asthma treatment adherence. Ongoing study May 2005.
Bendeer NCT00958932 {published data only}
- Telecommunication Enhanced Asthma Management TEAM. Ongoing study Sept 2009.
Clover N0702196597 {published data only}
- Self‐management of chronic conditions using telemedicine. Ongoing study —.
Finkelstein CRISP {published data only}
- Inhaled steroid adherence in moderate and severe asthma. Ongoing study 2005.
Friedman CRISP {published data only}
- Impact of a telecommunication system in childhood asthma. Ongoing study 1999.
Garbutt NCT00660322 {published data only}
- Using the telephone to improve care in childhood asthma. Ongoing study Jan 2004.
Garbutt NCT 00860834 {published data only}
- Parents, pediatricians and asthma telephone coaches partner to improve control of asthma in children (The PARTNER Study). Ongoing study August 2008.
Gustafson NCT00214383 {published data only}
- Internet Telehealth for Pediatric Asthma Case Management CHESS. Ongoing study May 2004.
Gustafson NCT00993590 {published data only}
- Mobile CHESS Research on Emergency Medical Services for Children. Ongoing study June 2008.
Mayers NCT00562081 {published data only}
- The Virtual Asthma Clinic. Ongoing study March 2005.
Moldrup NCT00917410 {published data only}
- Mobile phone text for optimising asthma treatment. Ongoing study November 2007.
NCT00149474 {published data only}
- Peak flow monitoring in older adults with asthma. Ongoing study August 1994.
Osman N0411013273 {published data only}
- A randomised controlled trial of benefits of specialist review or telephone follow up after an Accident and Emergency attendance for asthma. Ongoing study 1 October 1997.
Partridge N0016132017 {published data only}
- Proposal to study whether we can reduce hospital attendance by those with respiratory conditions without compromising care by the use of telephone consultation. Ongoing study 13 August 2003.
Perry NCT00964301 {published data only}
- Telemedicine education for rural children with asthma. Ongoing study August 2009.
Ricci 2001 In progress {published data only}
- A telehealthcare system for home monitoring of respiratory function in children affected by bronchial asthma. Ongoing study 2001.
Ryan NCT 00512837 {published data only}
- Mobile phone based structured intervention, the CYMPLA trial. Ongoing study November 2007.
Sparrow NCT00232557 {published data only}
- Telecommunications system in asthma. Ongoing study August 2004.
Strunk NCT00910585 {published data only}
- Coaching in childhood asthma. Ongoing study January 1997.
Vollmer NCT 00414817 {published data only}
- Telephone‐based program to promote inhaled corticosteroid adherence among individuals with asthma. Ongoing study June 2007.
Wouters NCT 00411346 {published data only}
- Patient Research In Self‐Management of Asthma (PRISMA). Ongoing study January 2003.
Additional references
Anandan 2009
- Anandan C, Gupta R, Simpson CR, Fischbacher C, Sheikh A. Epidemiology and disease burden from allergic disease in Scotland: analyses of national databases. Journal of the Royal Society of Medicine 2009;102(10):431‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anandan 2010
- Anandan C, Nurmatov U, Sheikh A. Is the prevalence of asthma declining, systematic review of epidemiological studies. Allergy 2010;65(2):152‐67. [DOI] [PubMed] [Google Scholar]
Anderson 2007
- Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax 2007;62(1):85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Botsis 2008
- Botsis T, Hartvigsen G. current status and future perspectives in telecare for elderly people suffering from chronic diseases. Journal of Telemedicine and Telecare 2008;14:195‐203. [DOI] [PubMed] [Google Scholar]
BTS/SIGN 2008
- British Thoracic Society, Scottish Intercollegiate Guidelines Network. British Guideline on Asthma. National Guideline 2008.
Busey 2008
- Busey C, Michael P. Telehealth ‐ opportunities and pitfalls. Journal of the American Dietetic Association 2008;108(8):1296‐301. [DOI] [PubMed] [Google Scholar]
Car 2003
- Car J, Sheikh A. Telephone consultations. BMJ 2003;326:966‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2004a
- Car J, Sheikh A. Email consultations in health care: 1 ‐ scope and effectiveness. BMJ 2004;329:435‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2004b
- Car J, Sheikh A. Email consultations in health care: 2 ‐ acceptability and safe application. BMJ 2004;329:439‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catwell 2009a
- Catwell L, Sheikh A. Evaluating eHealth interventions: the need for continuous systemic evaluation. Plos Medicine 2009;6(8):e1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catwell 2009b
- Catwell L, Sheikh A. Information technology (IT) system users must be allowed to decide on the future direction of major national IT initiatives. But the task of redistributing power equally amongst stakeholders will not be an easy one. Informatics in Primary Care 2009;17(1):1‐4. [DOI] [PubMed] [Google Scholar]
Currell 2008
- Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002098] [DOI] [PubMed] [Google Scholar]
Duvvuri 2007
- Duvvuri VRSK, Jianhong W. Information and communication technology developments in asthma management: a systematic review. Indian Journal of Medical Science 2007;61(4):221‐41. [PubMed] [Google Scholar]
Finkelstein 2000a
- Finkelstein J, Friedman RH. Potential role of telecommunication technologies in the management of chronic health conditions. Disease Management Health Outcomes 2000;2:57‐63. [Google Scholar]
Gallagher 2009
- Gallagher M, Worth A, Sheikh A. Clinical allergy has much to gain from engagement with qualitative research. Allergy 2009;64(8):1117‐9. [DOI] [PubMed] [Google Scholar]
Garcia‐Lizana 2007
- Garcia Lizana F, Sarria‐Santamera A. New technologies for chronic disease management and control: a systematic review. Journal of Telemedicine and Telecare 2007;13:62‐8. [DOI] [PubMed] [Google Scholar]
GINA 2003
- Masoli M, Fabian D, Holt S, Beasley R. Global Burden of Asthma. The Global Initiative for Asthma 2003.
Gupta 2003
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clinical and Experimental Allergy 2004;34(4):520‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
HRSA 2008
- USA Health Resources and Services Administration. http://www.hrsa.gov/telehealth/ (accessed 26 September 2008).
ISAAC 1998
- International Study of Asthma and Allergies in Childhood Steering Committee. Worldwide variations in the prevalence of asthma symptoms: ISAAC. European Respiratory Journal 1998;12:315‐35. [DOI] [PubMed] [Google Scholar]
ISAAC 2001
- Stewart AW, Mitchell EA, Pearce N, Strachan D, Weiland S, on behalf of the ISAAC Steering Committee. The relationship of per capita gross national product to the prevalence of symptoms of asthma and other atopic diseases in children (ISAAC). International Journal of Epidemiology 2001;30:173‐9. [DOI] [PubMed] [Google Scholar]
ISAAC 2004
- Weiland SK, Björkstén B, Brunekreef B, Cookson WO, Mutius E, Strachan DP. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. European Respiratory Journal 2004;24(3):406‐12. [DOI] [PubMed] [Google Scholar]
ISAAC 2006
- Asher MI, Montefore S, Bjorksten B, Lai C, Strachan DP, Weilland SK, Williams H and the ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis and eczema in childhood: ISAAC Phases One and Three repeat multi country cross‐sectional surveys. Lancet 2006;368:733‐43. [DOI] [PubMed] [Google Scholar]
Juniper 1996
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Quality of Life Research 1996;5:27‐34. [DOI] [PubMed] [Google Scholar]
Juniper 1999
- Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. European Respiratory Journal 1999;14:32‐8. [DOI] [PubMed] [Google Scholar]
Lancet 2006
- Lancet 2006. A plea to abandon asthma as a disease concept. Lancet 2006;368(9537):705. [DOI] [PubMed] [Google Scholar]
Lancet 2008
- Lancet. Asthma: still more questions that answers. Lancet 2008;372(9643):1009. [DOI] [PubMed] [Google Scholar]
Lorentz 2008
- Lorentz M. Telenursing and home healthcare. Home Healthcare Nurse 2008;4:26. [DOI] [PubMed] [Google Scholar]
Mahen 2006
- Mahen MM, Allen A. E‐Health & Telehealth Glossary. http://telehealth.net/glossary.html 2006 (accessed 26 September 2008).
Mair 2000
- Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ 2000;320:1517‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 2001
- Marks G. Commentary: geographical heterogeneity of asthma. International Journal of Epidemiology 2001;30:179‐80. [DOI] [PubMed] [Google Scholar]
McKinstry 2009
- McKinstry B, Pinnock H, Sheikh A. Telemedicine for management of patients with COPD?. Lancet Aug 2009;374(9691):672‐3. [DOI] [PubMed] [Google Scholar]
McLean 2009a
- McLean S, Sheikh A. Does telehealthcare offer a patient‐centred way forward for the community‐based management of long‐term respiratory disease?. Primary Care Respiratory Journal 2009;18(3):159‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 2009b
- McLean S, Nurmatov U, Liu J, Pagliari C, Car J, Sheikh A. Telehealthcare for COPD. Cochrane Database of Systematic Reviews Unpublished. [DOI] [PMC free article] [PubMed]
Medical Research Council 2008
- Craig P, Dieppe P, MacIntyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. Medical Research Council 2008.
Miller 2007
- Miller E. Solving the disjuncture between research and practice; telehealth trends in the 21st Century. Health Policy 2007;82:133‐41. [DOI] [PubMed] [Google Scholar]
Mort 2008
- Mort M, Finch T, May C. Making and unmaking telepatients: identity and governance in new health technologies. Science, Technology and Human Values 2008;34(9):8‐33. [Google Scholar]
Pearce 2000
- Pearce N, Sunyer J, Cheng S, Chinn S, Bjorksten B, et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. European Respiratory Journal 2000;16:420‐6. [DOI] [PubMed] [Google Scholar]
Pinnock 2005a
- Pinnock H, Madden V, Snellgrove C, Sheikh A. Telephone or surgery asthma reviews? Preferences of participants in a primary care randomised controlled trial. Primary Care Respiratory Journal 2005;14:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Punekar 2009
- Punekar Y, Sheikh A. Establishing the incidence and prevalence of clinician‐diagnosed allergic conditions in children and adolescents using routinely collected data from general practices. Clinical and Experimental Allergy 2009;39(8):1209‐16. [DOI] [PubMed] [Google Scholar]
Shepperd 2009
- Shepperd S, Lewin S, Strauss S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions?. Plos Medicine 2009;6(8):e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2010
- Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. Journal of the Royal Society of Medicine 2010;103(3):98‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulu 2007
- Tulu B, Chatterjee S, Maheshewari M. Telemedicine taxonomy: a classification tool. Telemedicine Journal and e‐Health 2007;13(3):349‐58. [DOI] [PubMed] [Google Scholar]
Whitten 2002
- Whitten PS, Mair FS, Haycox A, May CR, WIlliams TL, Hellmich S. Systematic review of cost effectiveness studies of telemedicine interventions. BMJ 2002;324:1434‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]


