Abstract
Background:
Mild traumatic brain injury (mTBI) is common in civilians and highly prevalent among military service members. mTBI can increase health risk behaviors (e.g., sensation seeking, impulsivity) and addiction risk (e.g., for alcohol use disorder (AUD)), but how mTBI and substance use might interact to promote addiction risk remains poorly understood. Likewise, potential differences in single vs. repetitive mTBI in relation to alcohol use/abuse have not been previously examined.
Methods:
Here, we examined how a history of single (1×) or repetitive (3×) blast exposure (blast-mTBI) affects ethanol (EtOH)-induced behavioral and physiological outcomes using an established mouse model of blast-mTBI. To investigate potential translational relevance, we also examined self-report responses to the Alcohol Use Disorders Identification Test-Consumption questions (AUDIT-C), a widely used measure to identify potential hazardous drinking and AUD, and used a novel unsupervised machine learning approach to investigate whether a history of blast-mTBI affected drinking behaviors in Iraq/Afghanistan Veterans.
Results:
Both single and repetitive blast-mTBI in mice increased the sedative properties of EtOH (with no change in tolerance or metabolism), but only repetitive blast potentiated EtOH-induced locomotor stimulation and shifted EtOH intake patterns. Specifically, mice exposed to repetitive blasts showed increased consumption “front-loading” (e.g., a higher rate of consumption during an initial 2-h acute phase of a 24-h alcohol access period and decreased total daily intake) during an intermittent 2-bottle choice condition. Examination of AUDIT-C scores in Iraq/Afghanistan Veterans revealed an optimal 3-cluster solution: “low” (low intake and low frequency), “frequent” (low intake and high frequency), and “risky” (high intake and high frequency), where Veterans with a history of blast-mTBI displayed a shift in cluster assignment from “frequent” to “risky,” as compared to Veterans who were deployed to Iraq/Afghanistan but had no lifetime history of TBI.
Conclusions:
Together, these results offer new insight into how blast-mTBI may give increase AUD risk and highlight the increased potential for adverse health risk behaviors following repetitive blast-mTBI.
Keywords: addiction, alcohol, AUDIT-C, blast, traumatic brain injury, Veteran
INTRODUCTION
Traumatic brain injury (TBI) affects every segment of the population and can lead to significantly decreased quality of life and social and occupational functioning. This is particularly important for Veterans, who experience disproportionate rates of physical and mental illness compared with civilian counterparts (Hoerster et al., 2012; Trivedi et al., 2020). Critically, US Veterans of the conflicts in Iraq and Afghanistan (Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND)) exhibit especially high rates of mild traumatic brain injury (mTBI; labeled the “signature injury” of these conflicts) (DVBIC, 2019; Hoge et al., 2008a; Hoge et al., 2008b; Tanielian & Jaycox, 2008). OEF/OIF/OND mTBI rates are estimated at 10–25% (approximately 400,000 Veterans diagnosed since 2000); most commonly repetitive in nature, blast exposure (via detonation of high explosives) is the primary source of mTBI (blast-mTBI) in this population, and a major source of morbidity among Veterans enrolled in the VA health care (Hendrickson et al., 2018; Tanielian & Jaycox, 2008; Wenger et al., 2018). A history of mTBI is associated with increased health risk behaviors (e.g., sensation/novelty seeking, impulsivity, risk taking, irritability/aggression, Elder & Cristian, 2009, Halbauer et al., 2009, Hendrickson et al., 2018, Miller et al., 2013, Olson-Madden et al., 2012, Peskind et al., 2011, Petrie et al., 2014, Schindler et al., 2017, Tanielian & Jaycox, 2008, Travis Seidl et al., 2015) and substance use/misuse (e.g., alcohol use disorder (AUD)) (Adams et al., 2012; Grossbard et al., 2017; Miller et al., 2013; Petrie et al., 2014), potentially compounding negative outcomes following injury/trauma, but how mTBI and substance use might interact to promote addiction risk remains poorly understood. Likewise, potential differences in single vs. repetitive mTBI in relation to alcohol use/abuse have not been previously examined.
Previous work in animal models supports the notion of brain injury as a risk factor for adverse health risk behaviors, including substance misuse/abuse (Cernak et al., 2001; Lim et al., 2015; Lowing et al., 2014; Muelbl et al., 2018; Nawarawong et al., 2019; Perez-Garcia et al., 2019; Schindler et al., 2017; Schindler et al., 2020; Vonder Haar et al., 2019). Indeed, using our established mouse model of blast exposure (Huber et al., 2013; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler et al., 2020), we previously demonstrated increased novelty-seeking behavior at acute (7 days) and chronic (1 month) time points following injury (Schindler et al., 2017). Likewise, increased risk-like behavior has also been reported following mild impact TBI (Krukowski et al., 2020; Mouzon et al., 2014). Results from animal models are more varied in relation to potential addiction risk following brain injury and depend on the type and severity of TBI exposure. For example, a moderate impact TBI increased ethanol (EtOH)-induced sedation and decreased voluntary EtOH consumption (Lowing et al., 2014), whereas mild impact or blast with body shielding resulted in increased cocaine, oxycodone, and alcohol intake and/or seeking behaviors (Lim et al., 2015; Muelbl et al., 2018; Nawarawong et al., 2019; Vonder Haar et al., 2019). Critically, no study to date has compared single vs. repetitive blast exposure nor examined potential mTBI-induced differences in EtOH-induced stimulation vs. sedation vs. voluntary intake.
Here, we investigated in male mouse potential effects of single (1×) and repetitive (3×) blast exposure on low-dose EtOH locomotor stimulation, high-dose EtOH sedation, tolerance, and metabolism, and finally, voluntary EtOH intake using intermittent 2-bottle choice (herein referred to collectively as measures of “EtOH responsivity”). Using an electronically controlled pneumatic shock tube that models battlefield-relevant open-field blast forces generated by detonation of high explosives (Huber et al., 2013; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler et al., 2020), we found that while both single and repetitive blast exposure increased the sedative properties of high-dose EtOH (with no change in tolerance nor metabolism), only repetitive blast exposure resulted in potentiation of EtOH-induced locomotor stimulation and a shift in EtOH intake patterns (i.e., increased consumption “front-loading” and decreased total daily intake) during intermittent 2-bottle choice (2BC). Next, we examined self-report responses to the Alcohol Use Disorders Identification Test-Consumption Questions (AUDIT-C; a widely used measure to identify risky drinking and potential AUD) (Bush et al., 1998; Crawford et al., 2013; Dawson et al., 2005) in male OEF/OIF/OND Veterans and used a novel unsupervised machine learning approach to investigate whether a history of repetitive blast exposure and mTBI affected drinking behaviors. As predicted, AUDIT-C scores were increased in OEF/OIF/OND Veterans with a history of acute symptomatic blast. Subsequent AUDIT-C cluster analysis identified a 3-cluster solution: “low” (low intake and low frequency), “frequent” (low intake but high frequency), and “risky” (high intake and high frequency) and revealed a significant increase in assignment to the “risky” drinking cluster in Veterans with a history of blast exposure, where the “risky” cluster was characterized by a higher level of combat exposure and numbers of mTBIs with loss of consciousness (LOC). Together, these results suggest disparate trauma effects of single vs. repetitive blast exposure, where only repetitive blast-mTBI is characterized by increased risky drinking self-report in male Veterans and increased EtOH-induced stimulation and consumption front-loading in male mice.
MATERIALS AND METHODS
Animals and mouse model of blast overpressure
Male C57BI/6 mice (Jackson Laboratory) aged 3–4 months (weight 25–33 g; mean 28.5 ± 0.17 g) were used. All animal experiments were carried out in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the VA Puget Sound Institutional Animal Care and Use Committees. The shock tube (Baker Engineering and Risk Consultants) was designed to generate blast overpressures that mimic open-field high explosive detonations encountered by military service members in combat, and the design and modeling characteristics have been described in detail elsewhere (Huber et al., 2013; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler et al., 2020). Briefly, mice were anesthetized with isoflurane (induced at 5% and maintained at 2–3%), secured against a gurney, and placed into the shock tube oriented perpendicular to the oncoming blast wave (ventral body surface toward blast). Sham (control) animals received anesthesia only for a duration matched to blast animals. Repeated blast/sham exposures occurred successively over the course of 3 days (1 per day). The blast overpressure (BOP) peak intensity (psi), initial pulse duration (ms), and impulse (psi■ms) used were in keeping with mild blast TBI (19.9 psi +/− 0.14 psi) [32]. Under these experimental conditions, the overall survival rate exceeded 95%, with blast-exposed mice appearing comparable to sham-exposed mice by inspection 2–4 h postblast exposure as previously reported (Huber et al., 2013; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler et al., 2020). All behavioral tests were conducted starting at 1 month postsham/blast exposure, a time point that allows for the development of blast-induced neuropathology (Goldstein et al., 2014; Rubovitch et al., 2011) and that correlates to a time period where enduring functional and behavioral deficits are detected (Schindler et al., 2017; Schindler et al., 2020). Separate groups of mice were used for EtOH locomotor stimulation, EtOH sedation, and voluntary EtOH consumption (see below for specific details of each procedure), 1× and 3× sham and blast mice were run in parallel, and at least 2 cohorts of mice were used in each behavioral paradigm.
Low-dose EtOH-induced locomotor stimulation
Ethanol-induced locomotor stimulation was investigated over the course of 3 days. On day 1, each animal was preexposed/habituated to the behavioral arena (clean rat cage) for 15 min. On the second day, animals were injected with saline (1.0 ml/kg w/v, i.p.) and were again allowed to explore the behavioral arena for 15 min. Finally, on day 3, animals were injected with EtOH (2.0 g/kg, i.p., 20% w/v) and allowed to explore the behavioral area for 15 min. Activity was recorded from above and analyzed using ANY-maze (Wood Dale, IL). EtOH-induced locomotor stimulation was expressed as a percent increase in distance traveled (EtOH/saline).
High-dose EtOH-induced sedation, tolerance, and metabolism
Ethanol-induced sedation and tolerance were investigated using the loss of righting reflex (LORR) paradigm. Each animal was injected with EtOH (4 g/kg, i.p., 40% w/v) and upon sedation (1–3 minutes later) was placed on its back in a V-shaped trough (any animal that did not lose LORR within 5 min was excluded from the study (n = 2)). Animals were then observed undisturbed for up to 5 hours. When the animal was able to right itself twice within 30 seconds (i.e., flipped from back to stomach in the V-shaped trough), total sedation time was recorded (LORR duration). The identical procedure was repeated 24 h later to assess EtOH tolerance. Blood was collected from the submandibular vein at 10 min and 4 h following EtOH injection to determine blood EtOH concentrations. Blood EtOH concentrations were determined using Bioassay Systems (Hayward, CA) EnzyChrom EtOH Assay Kit as per manufacturer instructions.
Intermittent 2-bottle choice (I2BC)
Starting 4 days prior to the commencement of the I2BC procedure, mice were singly housed, and their usual water bottle removed and replaced with 2 bottles filled each with water. The bottles were 50-mL centrifuge tubes with a #6.5 neoprene rubber stopper and 2.5” ball sipper tube (Antrin). Bottles were weighed immediately prior to placement in the cage and then again at 24-h timepoints (at approximately 5:30 pm) throughout the duration of the study. Each time the bottles were weighed, their position in the cage was reversed to prevent any side bias. One night each week (Wednesday), bottles were also weighed at 8 pm (2 h into the dark cycle) to assess drinking patterns during the acute phase of access. Following 4 days of baseline exposure to the water bottles, animals had access to 1 water bottle and 1 EtOH bottle every Monday, Wednesday, and Friday, and access to only water every Tuesday, Thursday, Saturday, and Sunday. During the first week of EtOH exposure, the EtOH dose was slowly increased (3% on Monday, 6% on Wednesday, and 9% on Friday). For the remaining duration of the study (21 more days), the 20% EtOH dose was used. Body weight was recorded for each animal on Sundays and used to express EtOH intake in g/kg.
Human subjects
These studies were approved by the VA Puget Sound Health Care System Human Subjects Committee and conformed to institutional regulatory guidelines and principles of human subject protection in the Declaration of Helsinki. All participants provided written informed consent prior to any study procedures. The current study used the self-reports of 105 Veterans with a history of blast exposure with acute symptoms and 34 deployed controls with no lifetime history of TBI of any severity (Table 1). As previously reported (Meabon et al., 2016; Peskind et al., 2011; Petrie et al., 2014), blast exposure(s) were reported as mild in nature and ranging from 1 (10%) to 50 or greater (15%) blast exposures. All participants in this report were male. Both males and females were eligible for enrollment and study inclusion. However, in this study we currently have no available data from blast-mTBI women because most mTBI Veteran participants were from combat military occupational specialties. Inclusion criteria for the blast group were as follows: documented hazardous duty in Iraq and/or Afghanistan with the U.S. Armed Forces and at least 1 blast exposure with acute symptoms (e.g., nausea, ringing in ears, blurry vision, hearing loss, unsteady on feet, eyes sensitive to light, headache, alteration of consciousness; occurring within 30 minutes of blast exposure). The VA/Department of Defense/American Congress of Rehabilitation Medicine criteria was used to establish mTBI (for the blast group), with 98% of Veterans within these group meeting criteria for at least 1 mTBI. Exclusion criteria were as follows: moderate-severe TBI, seizure disorder, insulin-dependent diabetes, current diagnosis of alcohol or other substance abuse or dependence (excluding nicotine), schizophrenia or other psychotic disorders, bipolar disorder, dementia, and taking medications likely to affect cognition performance. Inclusion and exclusion criteria for deployed control Veterans were identical with the exception that controls had no lifetime history of TBI of any severity. Exclusionary psychiatric disorders were ruled out by SCID-IV interview (First et al., 1995). Self-report alcohol use was examined using the Alcohol Use Disorders Identification Test-Consumption Questions (AUDIT-C) (Bush et al., 1998; Crawford et al., 2013; Dawson et al., 2005).
TABLE 1.
Study participant demographics and blast exposures
| Deployed control |
Blast exposed |
||
|---|---|---|---|
| Mean ± SEM, (Range) | Mean ± SEM, (Range) | p Value | |
| Demographics | |||
| Number of participants, n | 34 | 105 | |
| Age (years) | 32.7 ± 1.1 (22–46) | 33.6 ±0.8 (21–60) | 0.588 |
| Education (years) | 14.2 ± 0.3 (12–18) | 14.1 ± 0.2 (11–20) | 0.805 |
| Race, nonwhite, n (%) | 9 (26%) | 21 (20%) | 0.476 |
|
| |||
| Blast exposure | |||
| Estimated number of blast exposures with | 19 ± 3 (1 to >100) | ||
| Acute symptoms during military service (lifetime) | Median = 7 | ||
| Estimated number of mTBI with loss of | 0.98 ± 0.2 (0 to 8) | ||
| Consciousness (lifetime) | Median = 1 | ||
| Time since last blast-related | 5.4 ± 0.3 (0.25–13) | ||
| mTBI (years) | Median = 5 | ||
Unsupervised machine learning (cluster analysis)
Individual AUDIT-C question responses were used as features in an unsupervised machine learning model. Using Python and common scientific computing libraries (e.g., scipy, pandas, sk-learn), hierarchical clustering algorithm (Ward method) and k-means (Euclid) were used to assist in optimal cluster number selection. Silhouette, Davies Bouldin, and Calinski Harabasz scores were computed to assess k-means cluster assignments. Cluster stability was assessed using the scores for homogeneity, completeness, and mutual information criterion and a bootstrap approach with repeated random assignment of initial cluster centroids. K = 3 clusters were chosen based on above evaluation metrics. Finally, cluster assignment was compared between deployed controls and Veterans with a history of blast exposure and potential effects of combat and blast exposure on cluster assignment was examined.
Data analysis
As appropriate, data were analyzed using: (i) 2-tailed Student’s t-tests; (ii) 1-way or 2-way (between/within-subjects design) repeated-measures analysis of variance (RM ANOVA), followed by Newman–Keuls multiple comparison tests or Bonferroni post hoc tests, respectively; (iii) chi-square tests were conducted for categorical data (e.g., AUDIT-C). Reported p values denote 2-tailed probabilities of p < 0.05, and nonsignificance (n.s.) indicates p > 0.05. Statistical analyses were conducted using GraphPad Prism 4.0 (GraphPad Software, Inc.) and Python.
RESULTS
EtOH-induced locomotor stimulation is prolonged following repetitive blast exposure
Depending on dose, EtOH can have stimulating and/or sedative effects and can be assessed in mice via EtOH-induced locomotion and loss of righting reflex paradigms, respectively. To investigate whether blast exposure modulates the stimulating effects of EtOH, we first examined the ability of low-dose EtOH (2 g/kg) to increase locomotion (over saline) in C57BL/6 male mice 1 month after they received either 1 (1×) or 3 (3×, 1 per day) blast exposures. EtOH stimulation was expressed as a percent change in distance traveled (EtOH/saline) and was examined in 5-minute bins (15 min total). There were no statistically significant differences between 1× sham-(n = 8) and 3× sham-treated (n = 9) mice, 2-way RM ANOVA: interaction effect F(2, 30) = 0.554, p > 0.05, and thus, 1× and 3× sham animals were pooled together for subsequent analyses related to EtOH stimulation.
Figure 1A shows a significant effect of time postethanol injection, 2-way RM ANOVA: main effect of time F(2, 68) = 19.54, p = 0.0001, Bonferroni multiple comparison test post hoc: sham = 17, blast 1× = 12, blast 3× = 8, and while post hoc analysis revealed no difference in locomotor stimulation at 5 min postethanol injection, a significantly prolonged EtOH effect in 3× but not 1× animals was apparent when examined at 15 min postethanol injection (Figure 1A). We next examined raw distance traveled during the entire 15-min tests across the 3 days of behavioral testing (habituation/baseline, saline, and EtOH) and found a significant effect across groups, 2-way RM ANOVA: interaction effect F(4,68) = 3.359, p = 0.014, Bonferroni multiple comparison test post hoc: sham = 17, blast 1× = 12, blast 3× = 8, Figure 1B). Post hoc analyses demonstrated no differences during the initial habituation/baseline phase (base) but did reveal a significant increase in distance traveled during the final EtOH phase (EtOH) in 3× but not 1× animals. Finally, we examined raw distance traveled in 5-min bins during the each of the behavioral testing days. At baseline, there was a significant effect of time, 2-way RM ANOVA: time effect F(2, 68) = 10.12, p = 0.0005, Bonferroni multiple comparison test post hoc: sham = 17, blast 1× = 12, blast 3× = 8, Figure 1C, but post hoc analyses revealed no differences between groups in any time bin. Likewise, following saline injection, there was a significant interaction effect, 2-way RM ANOVA: interaction effect, F(4, 68) = 4.91, p = 0.0043, Bonferroni multiple comparison test post hoc: sham = 17, blast 1× = 12, blast 3× = 8, Figure 1D, but post hoc analyses revealed no differences between groups in any time bin. Conversely, following EtOH injection, there was a significant group effect, 2-way RM ANOVA: group effect F(2, 68) = 7.762, p = 0.0017, Bonferroni multiple comparison test post hoc: sham = 17, blast 1× = 12, blast 3× = 8, Figure 1E. Post hoc analyses demonstrated increased distance traveled at 5 min in 1× blast mice and at 10 and 15 min in 3× blast mice.
FIGURE 1.
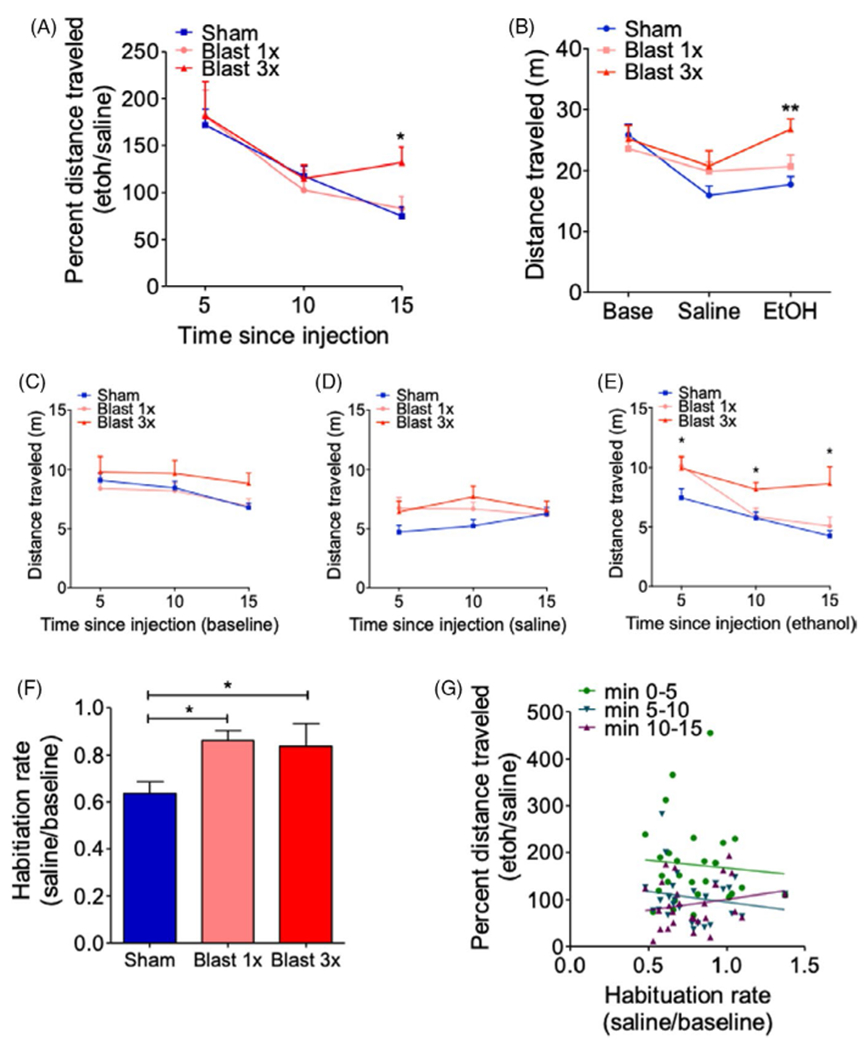
Repetitive blast exposure increases ethanol (EtOH)-induced locomotor stimulation (A) Locomotor stimulating effects of EtOH as expressed by percent distance traveled. Two-way RM ANOVA post hoc Bonferroni multiple comparison test. (B) Distance traveled at baseline and following saline or EtOH administration. Two-way RM ANOVA post hoc Bonferroni multiple comparison test. (C) Distance traveled at baseline in 5-min bins. Two-way RM ANOVA post hoc Bonferroni multiple comparison test. (D) Distance traveled following saline administration in 5-min bins. Two-way RM ANOVA post hoc Bonferroni multiple comparison test. (E) Distance traveled following EtOH administration in 5-min bins. Two-way RM ANOVA post hoc Bonferroni multiple comparison test. (F) Locomotor habituation. One-way ANOVA post hoc Newman–Keuls comparison test. (G) Correlation between EtOH locomotor stimulation and locomotor habituation rate. Spearman correlation. *p ≤ 0.05 **p ≤ 0.001: sham vs. blast. Values represent mean ± SEM
Differences in locomotor habituation can potentially confound EtOH investigations and are especially relevant in paradigms where locomotion is repeatedly measured over a number of days, as is the case here. Indeed, when we computed a habituation rate (saline/base), we found a significant difference in both 1× and 3× blast-exposed mice, 1-way ANOVA: F(2, 36) = 4.946, p = 0.013, Newman–Keuls comparison test post hoc: sham = 17, blast 1× = 12, blast 3× = 8, Figure 1C. Critically, we did not find any significant correlation between habituation rate (saline/base) and EtOH stimulation (EtOH/saline) at any of the 5-min bins (min 0–5: Spearman ρ = 0.153, n.s, n = 27; min 5–10: Spearman ρ = 0.749, n.s, n = 27; min 10–15: Spearman ρ = 0.945, n.s, n = 27), suggesting that our blast effects on EtOH stimulation were not mediated by potentially unrelated differences in locomotor habituation rates. Together, these results demonstrate that repetitive blast exposure increases the duration of the stimulatory effects of EtOH, which is not related to blast-induced deficits in habituation.
Blast exposure increases EtOH-induced sedation without affecting tolerance or metabolism
To investigate whether blast exposure modulates the sedative effects of EtOH, we examined the ability of high-dose EtOH (4 g/kg) to cause loss of righting reflex (LORR) on 2 consecutive days in C57BL/6 male mice 1 month after they received either 1 (1×) or 3 (3×, 1 per day) blast exposures. There were no statistically significant differences between 1× sham- (n = 8) and 3× sham-treated (n = 11) mice, LORR day 1: Student’s unpaired t-test, t(17) = 0.463, p > 0.05; LORR day 2: Student’s unpaired t-test, t(17) = 0.056, p > 0.05, and thus, 1× and 3× sham animals were pooled together for subsequent analyses related to EtOH sedation.
In accordance with previous results [21], a significant increase in LORR duration on the first day of testing was found in both 1× and 3× blast-exposed mice, 1-way ANOVA: F(2, 42) = 9.854, p = 0.0003, Newman–Keuls multiple comparison test post hoc: sham = 19, blast 1× = 13, blast 3× = 13, Figure 2A. A similar increase was found on the second day of repeat testing, 1-way ANOVA: F(2, 42) = 7.413, p = 0.001, Newman–Keuls multiple comparison test post hoc: sham = 19, blast 1× = 13, blast 3× = 13, Figure 2B. We next assessed EtOH tolerance by examining LORR change (day 2 / day 1). Figure 2C shows significant tolerance to repeated EtOH injections for all groups, 1-sample t-test vs. a theoretical mean of 1.0 (no tolerance): sham: t(16) = 3.788, p = 0.001, blast 1×: t(12) = 3.709, p = 0.003, blast 3×: t(12) = 3.66, p = 0.003, and no significant difference in tolerance rate across groups, 1-way ANOVA: F(2, 42) = 1.615, p = 0.211, sham = 17 blast 1× = 13, blast 3× = 13.
FIGURE 2.
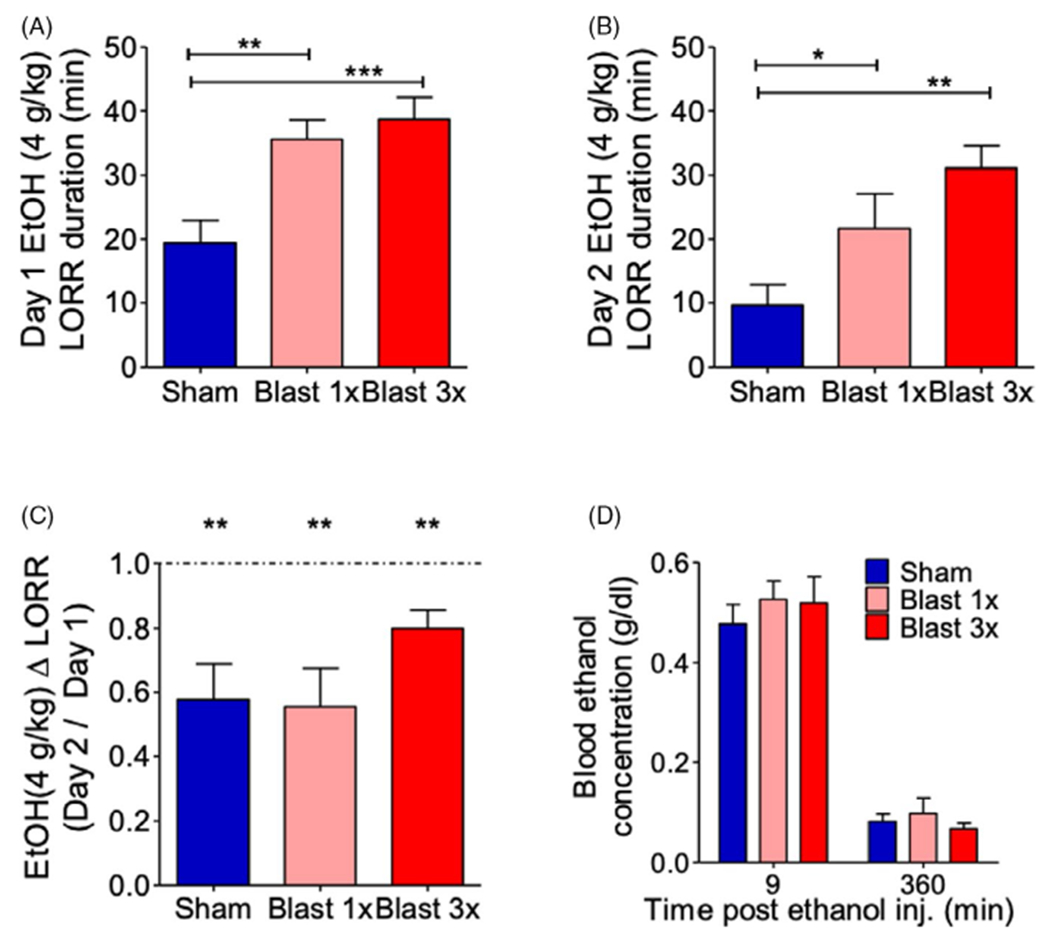
Blast exposure increases ethanol (EtOH)-induced loss of righting reflex. (A, B) EtOH-induced LORR duration on day 1 (A) and day 2 (B) of EtOH administration. One-way ANOVA post hoc Newman–Keuls comparison test. (C) Tolerance to EtOH-induced LORR. One-way ANOVA post hoc Newman–Keuls comparison test. (D) Blood EtOH concentration 9 min and 4 h after EtOH administration. Two-way RM ANOVA post hoc Bonferroni multiple comparison test. *p ≤ 0.05, **p ≤ 0.001, and ***p ≤ 0.0001. Error bars are mean ± SEM
Finally, we examined potential blast-induced changes to EtOH metabolism by measuring blood EtOH concentrations (BEC) at 10 min and 4 hours postinjection. There were no statistically significant differences between 1× sham- (n = 4) and 3× sham-treated (× = 5) mice, 10 min: Student’s unpaired t-test, t(7) = 1.667, p > 0.05; 4 h: Student’s unpaired t-test, t(7) = 1.098, p > 0.05, and thus, 1× and 3× sham animals were pooled together for subsequent analyses related to EtOH metabolism. In accordance with previous results [21], no significant differences were found in EtOH metabolism at time point in either 1× or 3× blast-exposed mice, 2-way RM ANOVA: interaction effect F(2, 15) = 0.427, p > 0.05, sham = 8, blast 1× = 4, blast 3× = 6, Figure 2D.
Repetitive, but not single, blast exposure decreases 24-hour EtOH intake but increases consumption “front-loading”
Previous results have suggested seemingly disparate trauma outcomes in rodents when EtOH self-administration is measured in short-access vs. long-access paradigms. To further investigate these phenomena, we conducted intermittent 2-bottle choice testing with 20% EtOH in C57BL/6 male mice 1 month after they received either 1 (1×) or 3 (3×, 1 per day) blast exposures, where intake measurements were repeatedly taken at time points corresponding to short access (after approximately 2 h of access) and long access (after 24 h of access). Consumption front-loading describes the tendency for some groups to consume significantly more in the acute phase of a self-administration paradigm after a period of forced abstinence and has implications for health risk behaviors such as binge drinking.
Using this paradigm, blast exposure caused a significant decrease in daily EtOH intake across the 3 weeks of testing, 2-way RM ANOVA: main effect of group F(2,192) = 4.417, p = 0.023, sham = 10, blast 1× = 9, blast 3× = 8, Figure 3A, and a significant decrease in average daily intake, 1-way ANOVA: F(2, 24) = 4.632, p = 0.02, Newman–Keuls multiple comparison test post hoc: sham = 10, blast 1× = 9, blast 3× = 8, Figure 3B. Conversely, blast exposure caused a significant increase in daily water intake across the 3 weeks of testing, 2-way RM ANOVA: main effect of group F(2, 192) = 6.711, p = 0.0048, sham = 10, blast 1× = 9, blast 3× = 8, Figure 3C, and a significant increase in average daily intake, 1-way ANOVA: F(2, 24) = 6.711, p = 0.0048, Newman–Keuls multiple comparison test post hoc: sham = 10, blast 1× = 9, blast 3× = 8, Figure 3D. In accordance with these intake results, we found a similar pattern of effects when examining daily EtOH preference (EtOH intake/water intake), 2-way RM ANOVA: main effect of group F(2, 192) = 5.866, p = 0.008, sham = 10, blast 1× = 9, blast 3× = 138, Figure 3E, and average daily preference, 1-way ANOVA: F(2, 24) = 6.038, p = 0.007, Newman–Keuls multiple comparison test post hoc: sham = 10, blast 1× = 9, blast 3× = 138, Figure 3F, across the 3 weeks of testing.
FIGURE 3.
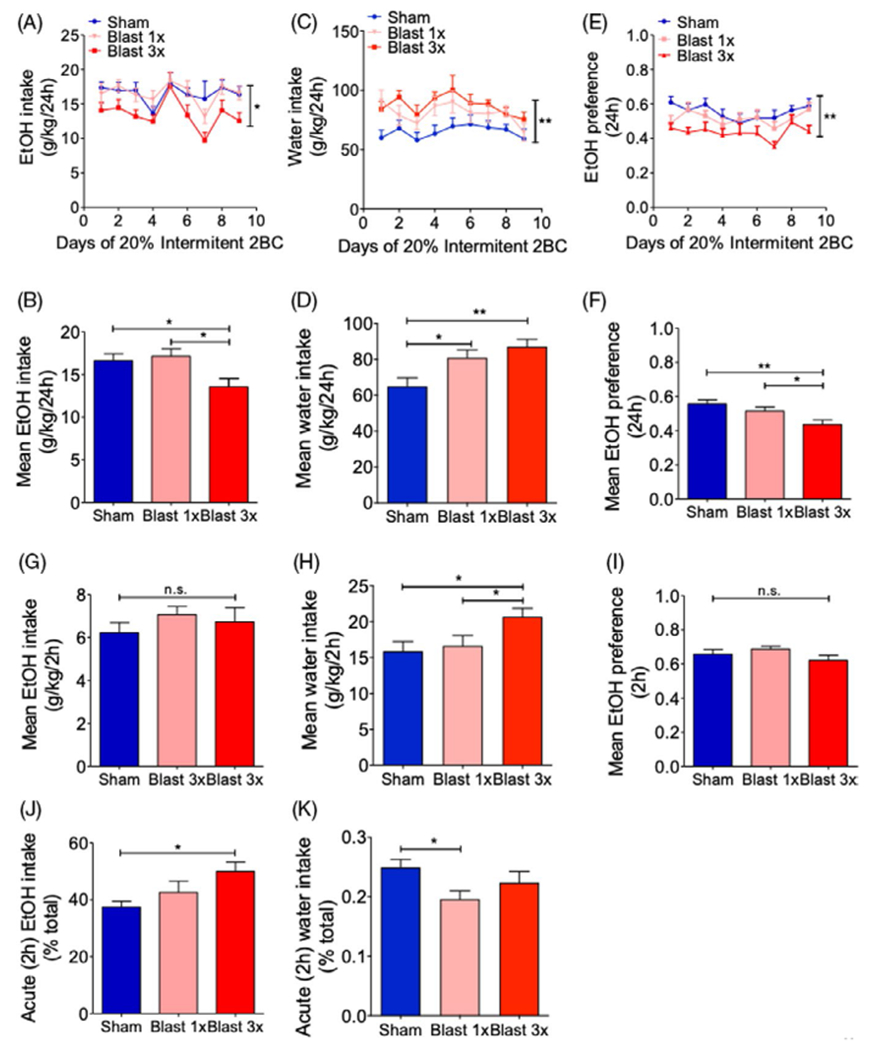
Blast exposure increases ethanol (EtOH) consumption “front-loading.” (A, B) Daily intermittent 2-bottle choice 20% EtOH intake. Two-way RM ANOVA post hoc Bonferroni multiple comparison test (daily) and 1-way ANOVA post hoc Newman–Keuls comparison test (average). (C, D) Daily intermittent water intake. Two-way RM ANOVA post hoc Bonferroni multiple comparison test (daily) and 1-way ANOVA post hoc Newman–Keuls comparison test (average). (E, F) Daily intermittent 2-bottle choice 20% EtOH preference. Two-way RM ANOVA post hoc Bonferroni multiple comparison test (daily) and 1-way ANOVA post hoc Newman–Keuls comparison test (average). (G) EtOH consumption during initial 2 hours of access. One-way ANOVA post hoc Newman–Keuls comparison test. (H) Water consumption during initial 2 hours of access. One-way ANOVA post hoc Newman–Keuls comparison test. (I) EtOH preference during initial 2 hours of access. One-way ANOVA post hoc Newman–Keuls comparison test. (J) EtOH “front-loading” (percent total intake). One-way ANOVA post hoc Newman–Keuls comparison test. (K) Water “front-loading” (percent total intake). One-way ANOVA post hoc Newman–Keuls comparison test. *p ≤ 0.05 **p ≤ 0.001: sham vs. blast. Values represent mean ± SEM
Conversely, when we measured intake levels approximately 2.5 h after reaccess to EtOH (i.e., each Wednesday evening at 6 pm, 2 h into the dark cycle). We found no significant difference between sham- and blast-exposed animals in average EtOH intake, 1-way ANOVA: F(2, 24) = 0.744, p = 0.485; sham = 10, blast 1× = 9, blast 3× = 8, Figure 3G, a significant difference between sham- and blast-exposed animals in average water intake, 1-way ANOVA: F(2, 24) = 3.549, p = 0.045; sham = 10, blast 1× = 9, blast 3× = 138, Figure 3H, and no significant difference in EtOH preference, 1-way ANOVA: F(2, 24) = 1.714, p = 0.201; n = 8–10, Figure 3I, measured after approximately 2.5 h of access. To assess potential changes in consumption “front-loading” (e.g., the tendency to consume higher amounts immediately following a period of forced abstinence), we computed the percent of EtOH consumed within the first 2.5 h of exposure and found that repetitive, but not single, blast exposure caused a significant increase in “front-loading” consumption, 1-way ANOVA: F(2, 24) = 3.975, p = 0.032, Newman–Keuls multiple comparison test post hoc: sham = 10, blast 1× = 9, blast 3× = 138, Figure 3J. Conversely, percent of water consumed within the first 2.5 hours of exposure was significantly decreased in 1× but not 3× blast mice, 1-way ANOVA: F(2, 24) = 3.509, p = 0.046, Newman–Keuls multiple comparison test post hoc: sham = 10, blast 1× = 9, blast 3× = 138, Figure 3K. Together, these results suggest that repetitive blast exposure modifies the pattern of voluntary EtOH intake resulting in increased consumption “front-loading.”
Cluster analysis reveals a change in drinking patterns in Veterans with a history of repetitive blast exposure
A cohort of 105 OEF/OIF/OND Veterans with previous history of blast exposure with acute symptoms (BE) and 34 OEF/OIF/OND Deployed Control (DC) Veterans with no lifetime history of TBI of any severity were studied. Table 1 shows that the BE and DC groups were statistically comparable in terms of age at time of evaluation, education level, and race distribution (nonwhite/white ratio). Veterans in the BE group had an average of 19 ± 3 blast exposures (median = 7) where the average time from their last blast exposure to evaluation was 5.4 ± 0.3 years. Current alcohol use was assessed as previously shown [34] with the Alcohol Use Disorders Identification Test-Consumption Questions (AUDIT-C), a screening measure used to identify individuals who are at risk for problematic drinking [31]. Scores on this measure range from 0 to 12, with 0 indicating no alcohol use and higher scores indicating more risk of unhealthy alcohol use. Previously established study criteria [8, 16, 30] required individuals meeting DSM-IV criteria for alcohol abuse or dependence to be excluded from study. Nonetheless, AUDIT-C scores were significantly higher in BE as compared to DC (χ2 = 20.95, p = 0.021) (Figure 4A).
FIGURE 4.
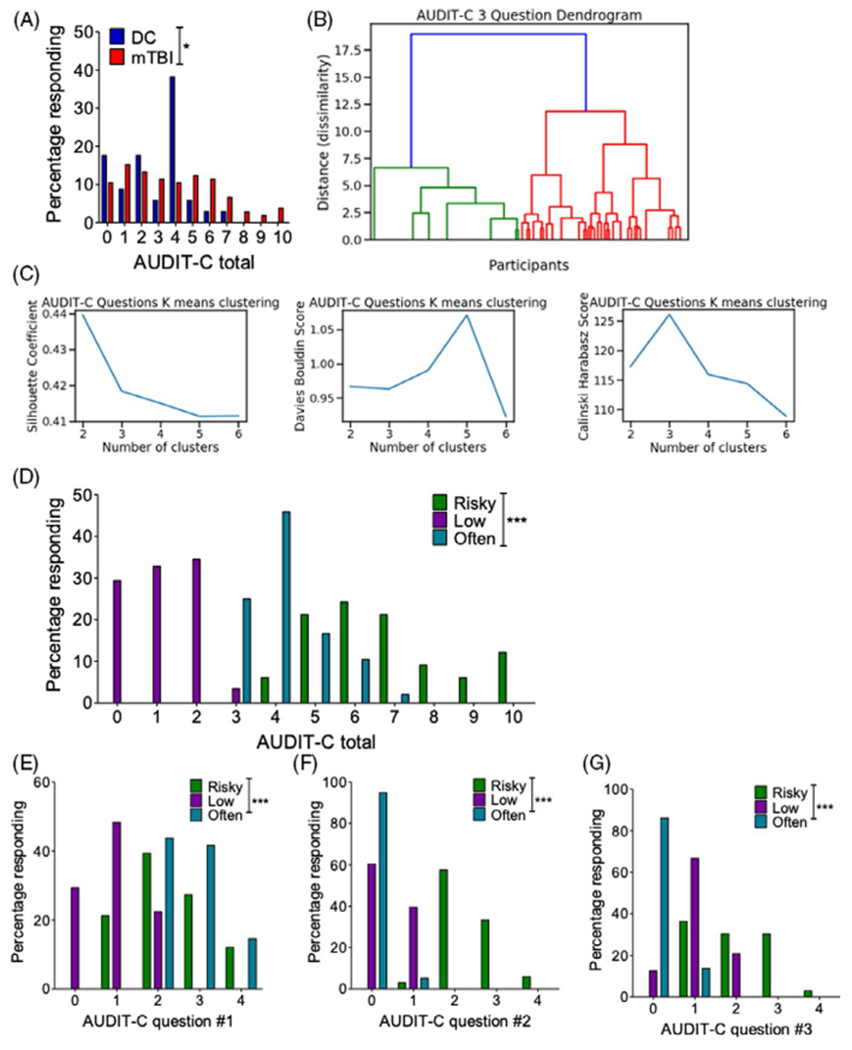
AUDIT-C cluster analysis. (A) Self-report AUDIT-C total scores in Veterans with/without a history of blast exposure with acute symptoms. Chi-square. (B) Hierarchical clustering dendrogram visualization. (C) K-means cluster metrics highlight an optimal 3-cluster optimal. (D) AUDIT-C total scores across the 3 clusters. Chi-square. (D) AUDIT-C total scores across the 3 clusters. Chi-square. (E) AUDIT-C question 1 (i.e., drinking frequency) scores across the 3 clusters. Chi-square. (F) AUDIT-C question 2 (i.e., drinking amount) scores across the 3 clusters. (G) AUDIT-C question 3 (i.e., binge-like drinking) scores across the 3 clusters. Chi-square. *p ≤ 0.05 and ***p ≤ 0.0001. Values represent mean ± SEM
The AUDIT-C consists of 3 Likert-scale questions; while all 3 questions are related to intake patterns, each question probes a different drinking modality—the first question focuses on drinking frequency, the second on drinking quantity, and the third on “binge”-like drinking behavior. Here, we used an unsupervised machine learning approach to examine potential subgroup differences across the AUDIT-C questions. Distance (dissimilarity) from a hierarchical clustering algorithm suggested an optimal cluster number of 3 (Figure 4B), and this was further supported by examining cluster metrics using a K-means clustering algorithm (Figure 4C). Finally, analysis of cluster stability also supported a 3-cluster solution for this data set (Table 2). Using k = 3 with k-means clustering, Veterans were assigned to a single cluster based on their AUDIT-C responses. In support of this cluster assignment, total AUDIT-C scores were significantly different across cluster groups (χ2 = 201.3, p = 0.0001) (Figure 4D). Likewise, individual question AUDIT-C scores were also significantly different across cluster groups (question 1 (drinking frequency): χ2 = 82.99, p = 0.0001; question 2 (drinking quantity): χ2 = 157.7, p = 0.0001; question 3 (binge-like frequency): χ2 = 123.4, p = 0.001). Based on the distribution of scores within each cluster, we labeled the clusters as “low” (n = 58), “often” (n = 48), and “risky” (n = 33). Figure 4D–G shows the outcomes of the cluster assignments: The “low” drinking cluster is characterized by low responses across the 3 AUDIT-C questions, the “often” drinking cluster is characterized by a high response on question 1 (i.e., the question related to drinking frequency) but low responses on questions 2 (i.e., quantity) and 3 (i.e., binge), and the “risky” drinking cluster is characterized by high responses on questions 2 (i.e., the question related to drinking quantity) and 3 (i.e., the question related to binge-like consumption) and intermediate responses on question 1 (i.e., frequency).
TABLE 2.
K-means cluster stability
| k | Homogeneity | Completeness | Adj. mutual info. |
|---|---|---|---|
| 2 | 0.93 | 0.94 | 0.93 |
| 3 | 0.98 | 0.98 | 0.98 |
| 4 | 0.85 | 0.86 | 0.85 |
Comparing cluster assignment between DC and BE Veterans, we found a significant change in cluster assignment (χ2 = 6.326, p = 0.042) toward an increased “risky” group membership in the BE group (Figure 5A). To further understand potential drivers of cluster assignment, we examined whether there were significant differences in combat exposure and/or blast number across the clusters (focusing now only on BE Veterans). We found a significant increase in reported combat exposure, 1-way ANOVA: F(2,103) = 4.725, p = 0.012, Newman–Keuls post hoc: “low” n = 43, “often” n = 32, and “risky” n = 29) (Figure 5B) within the “risky” drinking cluster. Likewise, we found that the risky drinking cluster was also associated with significantly greater number of blast-mTBIs resulting in loss of consciousness, 1-way ANOVA: F(2, 100) = 5.21, p = 0.007, Newman–Keuls comparison test post hoc: “low” n = 42, “often” n = 31, and “risky” n = 29) (Figure 5C), and greater number of blast-mTBIs, 1-way ANOVA: F(2, 101) = 3.895, p = 0.023, Newman–Keuls comparison test post hoc: “low” n = 43, “often” n = 32, and “risky” n = 29, Figure 5D). Together, these results suggest that repetitive blast-mTBI increases alcohol intake and potentially risky drinking behaviors and are in correspondence with current results from our animal model of blast.
FIGURE 5.
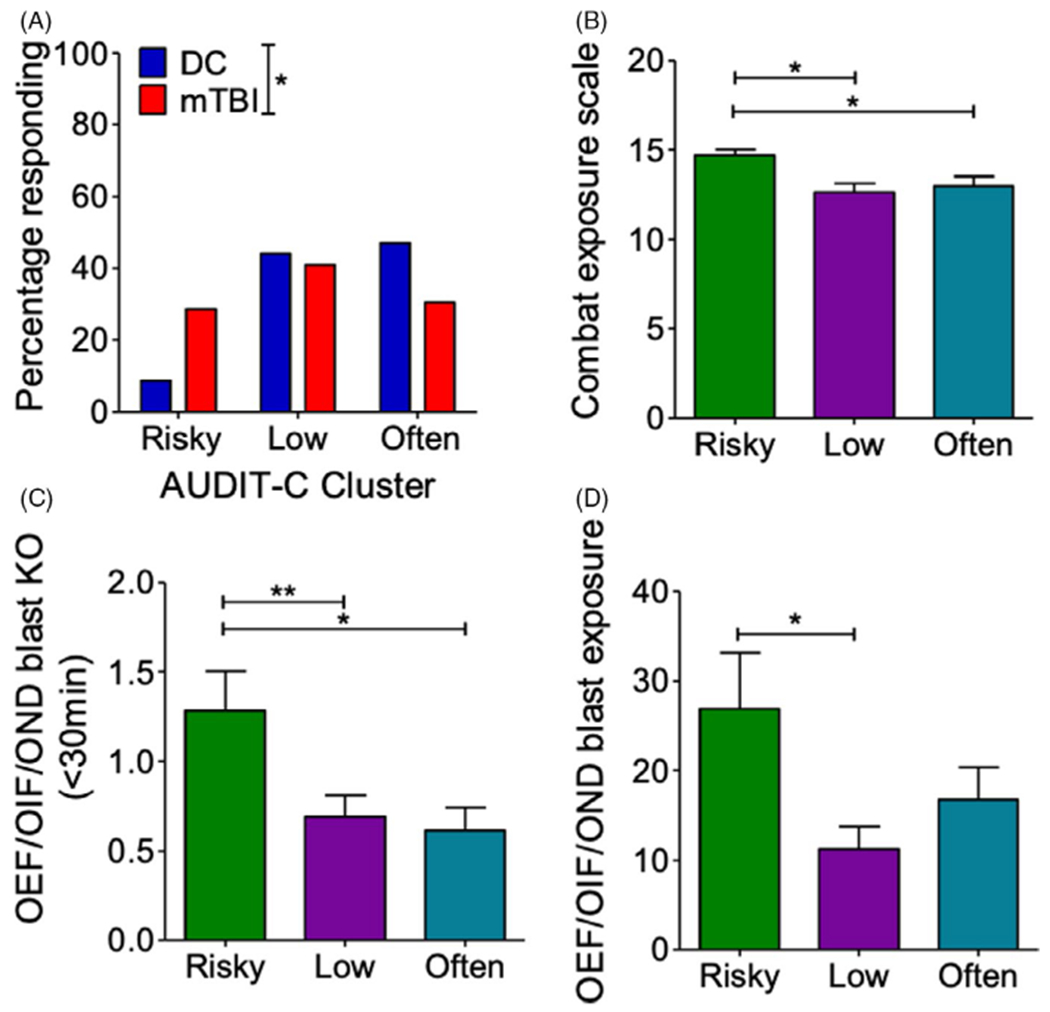
Blast-mTBI results in shift to “risky” drinking cluster. (A) AUDIT-C k-means cluster assignment in Veterans with/without a history of blast exposure with acute symptoms. Chi-square. (B) Combat exposure by cluster assignment in Veterans with a history of blast exposure with acute symptoms. One-way ANOVA post hoc Newman–Keuls comparison test. (C) Blast-mTBI with loss of consciousness (KO) count by cluster assignment in Veterans with a history of blast exposure with acute symptoms. One-way ANOVA post hoc Newman–Keuls comparison test. (D) Blast exposure count by cluster assignment in Veterans with a history of blast exposure with acute symptoms. One-way ANOVA post hoc Newman–Keuls comparison test. *p ≤ 0.05 and **p ≤ 0.01. Values represent mean ± SEM
DISCUSSION
The armed conflicts of OEF/OIF/OND have resulted in an estimated mTBI rate of 10–25% (DVBIC, 2019; Hoge et al., 2008b; Tanielian & Jaycox, 2008). In these conflicts, an estimated 75% of all TBIs reported by service members are a result of blast caused by detonation of high explosives (Tanielian & Jaycox, 2008) and multiple deployments are common (2.77 million service members have served on 5.4 million deployments since 2011) (Wenger et al., 2018), resulting in the increased potential for repetitive blast exposure. As such, blast exposure represents a major potential source of physical and psychological trauma, with implications for subsequent health risk behaviors (e.g., sensation/novelty seeking, impulsivity, risk taking, irritability/aggression). Indeed, mTBI can worsen preexisting psychiatric disorders such as depression and increase and/or exacerbate substance misuse/addiction and other health risk behaviors (Adams et al., 2012; Elder & Cristian, 2009; Grossbard et al., 2017; Halbauer et al., 2009; Hendrickson et al., 2018; Miller et al., 2013; Olson-Madden et al., 2012; Peskind et al., 2011; Petrie et al., 2014; Schindler et al., 2017; Tanielian & Jaycox, 2008; Travis Seidl et al., 2015), potentially compounding negative outcomes following injury and trauma. We previously reported increased PTSD and depression symptoms as well as alcohol use in OEF/OIF/OND Veterans with a history of blast-mTBI as compared to deployed control Veterans with no lifetime history of TBI (Peskind et al., 2011; Petrie et al., 2014), and more recently reported increased Veteran self-report of disinhibition and risk taking behaviors chronically following blast-mTBI (Schindler et al., 2017). Here, we provide evidence demonstrating blast-dose effects in relation to EtOH responsivity in mice and risky drinking behavior in Veterans, highlighting the importance of understanding blast-mTBI history in OEF/OIF/OND Veterans that might be at heightened risk for health risk behaviors related to substance misuse and/or abuse.
Animal models support the notion of brain injury as a risk factor for adverse health risk behaviors, including substance abuse and addiction (Cernak et al., 2001; Lim et al., 2015; Lowing et al., 2014; Muelbl et al., 2018; Nawarawong et al., 2019; Perez-Garcia et al., 2019; Schindler et al., 2017; Schindler et al., 2020; Vonder Haar et al., 2019). Using our established mouse model of repetitive blast exposure (Huber et al., 2013; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler et al., 2020), we previously demonstrated increased novelty seeking in blast-exposed mice (Schindler et al., 2017) and more recently demonstrated acute (30 minutes post) stress responses and chronic (3 months post) PTSD-like outcomes following repetitive blast exposure (Schindler et al., 2020). Likewise, a variety of anxiety and depression-related behavioral outcomes have been demonstrated in rodent models of blast (Cernak et al., 2001; Krukowski et al., 2020; Mouzon et al., 2014; Perez-Garcia et al., 2018; Perez-Garcia et al., 2019). In relation to potential substance misuse and abuse, repetitive blast exposure with body shielding in rats increased voluntary EtOH intake during a short-access challenge session and increased oxycodone seeking following extinction and a period of forced abstinence (Lim et al., 2015; Nawarawong et al., 2019). Likewise, a single mild frontal impact injury resulted in increased cocaine self-administration in rats (Vonder Haar et al., 2019), whereas a single moderate impact injury increased EtOH sedation and decreased voluntary consumption in mice [21]. Finally, we report here that both single blast and repetitive blast increased the sedative properties of EtOH (with no change in tolerance or metabolism), but only repetitive blast potentiated EtOH-induced locomotor stimulation and shifted EtOH intake patterns (i.e., increased consumption “front-loading,” decreased total daily intake) during intermittent 2-bottle choice. As is common for home cage drinking studies, mice were single housed during the intermittent 2-bottle choice procedure, creating a potential confound warranting future investigation to determine the potential for blast x housing interaction effects. Together, these results demonstrate important similarities and differences across TBI models in respect to TBI number, severity, and injury method.
Our result of decreased daily EtOH intake 1 month following repetitive blast-mTBI in mice might seem contrary to our clinical data, suggesting increased EtOH intake self-report in Veterans with a history of blast-mTBI. We interpret these findings to suggest that increased EtOH stimulation and sensitivity following repetitive blast exposure in mice act to limit the overall amount of EtOH consumed (e.g., blast-mTBI mice reach reward and/or intoxication more quickly than shams, thus limiting subsequent opportunities for consumption). In line with this idea, we found that EtOH consumption “front-loading” was significantly increased in our repetitive blast-mTBI mice, highlighting a potentially more “binge”-like intake pattern following repetitive blast exposure. Such “front-loading” behavior is linked to “binge”-like consumption and has been previously reported in other animal models (Sailing et al., 2018; Wilcox et al., 2014) and observed in humans with increased vulnerability to developing problem drinking and alcohol use disorder (Gowin et al., 2017). While the current studies were not originally designed to specifically investigate EtOH binge intake, back translation of the results from our AUDIT-C analysis in Veterans will be enhanced by the specific use of an established animal model of binge drinking (i.e., drinking in the dark) and will be a focus of future investigations.
The findings reported here from our unsupervised cluster analysis of AUDIT-C self-report in Veterans with/without a history of blast-mTBI are in keeping with results from animal models of mTBI. Specifically, frequency of assignment to the “risky” drinking cluster was higher in Veterans reporting a history of repetitive blast-mTBI with loss of consciousness (as compared to blast-mTBI with only altered consciousness). Our cluster analysis findings are a Iso in line with a previous report demonstrating increased odds of frequent binge drinking in Veterans with a history of TBI with loss of consciousness as compared to Veterans with no history of TBI or Veterans with history of TBI without loss of consciousness (Adams et al., 2012). It is important to note that while we tested drinking acquisition in mice using the intermittent 2-bottle choice procedure 1 month following blast-mTBI, it is likely that many of the Veteran study participants had a history of alcohol intake prior to deployment and blast exposure. Likewise, alcohol consumption and binge drinking have been commonly reported by active-duty military personnel and correlate with combat exposure, with increased rates seen in Iraq/Afghanistan (Santiago et al., 2010; Seal et al., 2011; Stahre et al., 2009), so it is possible that the Veteran participants consumed alcohol starting at a more acute time point (e.g., hours or days) postblast than the mice examined at 1 month after in the current study (mice are estimated to mature 45× faster than humans during early adulthood (Fox, 2007)). Thus, it will be important to investigate how prior history of alcohol affects subsequent blast-induced changes to alcohol intake patters and sensitivity in future studies. Indeed, alcohol intake occurring peri-TBI exposure in male rats resulted in worse outcomes as compared to rats with TBI but no previous history of alcohol (Fucich et al., 2019; Mayeux et al., 2015; Teng et al., 2015), supporting the potential for alcohol to impair recovery and/or exacerbate injury.
An AUDIT-C total score of 5 or higher in male Veterans is recommended as a positive screen for potential alcohol misuse and/or dependence (a score of 4 or higher is used for males in the general population), requiring follow-up with a healthcare provider (Bradley et al., 1998; Bush et al., 1998). When examined for its predictive ability, a cutoff of 5 exhibits a high rate of specificity but lower sensitivity in its ability to properly identify patients with substance misuse; indeed, this number was optimized to minimize the burden of false-positive rates on VA providers (Bradley et al., 1998; Bush et al., 1998). Using our cluster-based approach, the “frequent” and “risky” clusters share overlapping AUDIT-C scores of 4–7, raising the possibility that this approach might provide useful additional information to aid in the assessment of whether self-reported drinking behavior should be of concern for the medical provider. These results now require external validation in a larger sample without exclusion criteria related to substance abuse/dependence and/or in a population outside of the VA to determine potential merit of using such a cluster-based approach in a clinical care setting.
How repetitive blast-mTBI might drive subsequent health risk behaviors and addiction risk at the mechanistic level remains unknown. Null results from the current study discount blast-induced changes to EtOH tolerance and metabolism as potential underlying mechanisms. Conversely, we and others have demonstrated mTBI-induced changes to both tonic and phasic dopamine release patterns, as well as neuropathological/inflammatory changes within the mesolimbic system (Sajja et al., 2013; Sajja et al., 2015; Schindler et al., 2017; Vonder Haar et al., 2019). We previously demonstrated a blast-mTBI-induced increase in stimulated phasic dopamine release within the nucleus accumbens, and other reports demonstrate blast-mTBI-induced neuroinflammation and tissue damage within the mesolimbic system [37, 38]. Likewise, mild-to-moderate impact TBI models in rats and mice demonstrate alterations in mesolimbic dopamine receptors and related signal-transduction proteins (Lowing et al., 2014; Vonder Haar et al., 2019). Damage to the mesolimbic system has been associated with deficits in executive function and emotional control, potentially leading to increased health risk behaviors and addiction risk. Blast-induced changes to the structure and/or function of mesolimbic circuits thus pose a potential underlying mechanism related to blast-induced changes in EtOH and drug sensitivity and intake. While the adverse outcomes of trauma are thought to be mediated at least in part though maladaptive changes to the mesolimbic dopamine system, a causal role for mesolimbic dopamine dysfunction in blast-induced behavioral pathology has yet to be established and will be the focus of future investigations.
Together, our results highlight that while a single blast-mTBI can result in changes to the sedating properties of alcohol (without changes in tolerance or metabolism), only repetitive blast-mTBI results in prolonged EtOH-induced locomotor stimulation and “binge”-like consumption “front-loading” in mice and a shift to the “risky” drinking cluster in Veterans. Binge drinking specifically is associated with increased risk for negative consequences related to addiction, criminality, and chronic adverse health outcomes (e.g., obesity, liver damage). Over 400,000 OEF/OIF/OND Veterans have a history of blast exposure and mTBI (most often repetitive), highlighting the potential for significant costs related to blast-mTBI-induced increases in risky drinking patterns and binging. While we are not able to draw causal inferences from our Veteran cohort, these data in combination with data from our animal model strongly support the notion of repetitive blast-mTBI as a driver of health risk behaviors such as risky drinking. Together, these results highlight the importance of understanding blast trauma history in OEF/OIF/OND Veterans who might also be at risk for substance misuse and/or abuse. Additional studies are warranted to further explore potential underlying mechanisms and treatment targets (e.g., the mesolimbic dopamine system) for those with a history of repetitive mTBI and higher potential likelihood of health risk behaviors and addiction risk.
ACKNOWLEDGMENTS
This work was supported by a Department of Veteran Affairs (VA) Basic Laboratory Research and Development (BLR&D) Career Development Award 1IK2BX003258 (AGS), VA Clinical Sciences Research and Development (CSR&D) Career Development Award IK2CX001774 (RCH), a VA BLR&D Merit Review Award 5I01BX002311 (DGC), VA Rehabilitation Research and Development Service Merit Review Award #B77421 (ERP), University of Washington Friends of Alzheimer’s Research (DGC, ERP), UW Royalty Research Fund (DGC), the VA Northwest Mental Illness Research Education and Clinical Center (MAR, ERP, RCH), and the Burroughs Wellcome Fund Postdoctoral Enrichment Program grant #1019873 (BJ). We would like to thank Traci J Webber, Cindy Pekow, DVM, Kari Koszdin, DVM, and Monica Foley for considerable technical assistance and veterinary care.
Funding information
University of Washington; U.S. Department of Veterans Affairs, Grant/Award Number: #B77421 and IK2CX001774; Burroughs Wellcome Fund, Grant/Award Number: 1019873; Biomedical Laboratory Research and Development, VA Office of Research and Development, Grant/Award Number: 1IK2BX003258 and 5I01BX002311
Footnotes
Publisher's Disclaimer: DISCLAIMER
The views expressed in this scientific presentation are those of the author(s) and do not reflect the official policy or position of the U.S. government or the Department of Veterans Affairs.
DATA SHARING
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adams RS, Larson MJ, Corrigan JD, Horgan CM & Williams TV (2012) Frequent binge drinking after combat-acquired traumatic brain injury among active duty military personnel with a past year combat deployment. The Journal of Head Trauma Rehabilitation, 27, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, McDonell MB, Bush K, Kivlahan DR, Diehr P & Fihn SD (1998) The AUDIT alcohol consumption questions: reliability, validity, and responsiveness to change in older male primary care patients. Alcoholism, Clinical and Experimental Research, 22, 1842–1849. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD & Bradley KA (1998) The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test .Archives of Internal Medicine, 158, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X & Savic J (2001) Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Injury, 15, 593–612. [DOI] [PubMed] [Google Scholar]
- Crawford EF, Fulton JJ, Swinkels CM, Beckham JC, VA Mid-Atlantic MIRECC OEF/OIF Registry Workgroup & Calhoun, P.S. (2013) Diagnostic efficiency of the AUDIT-C in U.S. veterans with military service since September 11, 2001. Drug and Alcohol Dependence, 132, 101–106. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS & Zhou Y (2005) Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism, Clinical and Experimental Research, 29, 844–854. [DOI] [PubMed] [Google Scholar]
- DVBIC (2019) DoD Numbers for Traumatic Brain Injury Worldwide [Online], [Accessed]. https://health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Development/Traumatic-Brain-Injury-Center-of-Excellence/DoD-TBI-Worldwide-Numbers
- Elder GA & Cristian A (2009) Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mount Sinai Journal of Medicine, 76, 111–118. [DOI] [PubMed] [Google Scholar]
- First MSR, Gibbon M & Williams J (1995). Structured Clinical Interview for DSM-IV Axis II Disorders-Patient Edition (SCID-IVP, Version 2.0). ed. N.Y.P. Institute. [Google Scholar]
- Fox JG (2007) The mouse in biomedical research. Amsterdam; Boston: Elsevier, AP. [Google Scholar]
- Fucich EA, Mayeux JP, McGinn MA, Gilpin NW, Edwards S & Molina PE (2019) A novel role for the endocannabinoid system in ameliorating motivation for alcohol drinking and negative behavioral affect after traumatic brain injury in rats. Journal of Neurotrauma, 36, 1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, McKee AC & Stanton PK (2014) Considerations for animal models of blast-related traumatic brain injury and chronic traumatic encephalopathy. Alzheimer’s Research &Therapy, 6, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V & Ramchandani VA (2017) Vulnerability for alcohol use disorder and rate of alcohol consumption. American Journal of Psychiatry, 174, 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossbard J, Malte CA, Lapham G, Pagulayan K, Turner AP, Rubinsky AD et al. (2017) Prevalence of alcohol misuse and follow-up care in a national sample of OEF/OIF VA patients with and without TBI. Psychiatric Services, 68, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbauer JD, Ashford JW, Zeitzer JM, Adamson MM, Lew HL & Yesavage JA (2009) Neuropsychiatric diagnosis and management of chronic sequelae of war-related mild to moderate traumatic brain injury. Journal of Rehabilitation Research and Development, 46, 757–796. [DOI] [PubMed] [Google Scholar]
- Hendrickson RC, Schindler AG & Pagulayan KF (2018) Untangling PTSD and TBI: challenges and strategies in clinical care and research. Current Neurology and Neuroscience Reports, 18, 106. [DOI] [PubMed] [Google Scholar]
- Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G & Nelson KM (2012) Health and health behavior differences: U.S. Military, veteran, and civilian men. American Journal of Preventive Medicine, 43,483–489. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI & Koffman RL (2008a) Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. U.S. Army Medical Department Journal, 7–17. [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC & Castro CA (2008b) Mild traumatic brain injury in U.S. Soldiers returning from Iraq. New England Journal of Medicine, 358, 453–463. [DOI] [PubMed] [Google Scholar]
- Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC et al. (2013) Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. Journal of Alzheimer’s Disease, 37, 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Nolan A, Frias ES, Grue K, Becker M, Ureta G et al. (2020) Integrated stress response inhibitor reverses sex-dependent behavioral and cell-specific deficits after mild repetitive head trauma. Journal of Neurotrauma, 37, 1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YW, Meyer NP, Shah AS, Budde MD, Stemper BD & Olsen CM (2015) Voluntary alcohol intake following blast exposure in a rat model of mild traumatic brain injury. PLoS One, 10, e0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon AF, Schindler AG, Meabon JS, Yagi M, Herbert MJ, Banks WA et al. (2020) Nitric oxide synthase mediates cerebellar dysfunction in mice exposed to repetitive blast-induced mild traumatic brain injury. Scientific Reports, 10, 9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowing JL, Susick LL, Caruso JP, Provenzano AM, Raghupathi R & Conti AC (2014) Experimental traumatic brain injury alters ethanol consumption and sensitivity. Journal of Neurotrauma, 31, 1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux JP, Teng SX, Katz PS, Gilpin NW & Molina PE (2015) Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behavioral Brain Research, 279, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF et al. (2016) Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Science Translational Medicine, 8, 321ra6. [DOI] [PubMed] [Google Scholar]
- Miller SC, Baktash SH, Webb TS, Whitehead CR, Maynard C, Wells TS et al. (2013) Risk for addiction-related disorders following mild traumatic brain injury in a large cohort of active-duty U.S. airmen. American Journal of Psychiatry, 170, 383–390. [DOI] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM et al. (2014) Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Annals of Neurology, 75, 241–254. [DOI] [PubMed] [Google Scholar]
- Muelbl MJ, Slaker ML, Shah AS, Nawarawong NN, Gerndt CH, Budde MD et al. (2018) Effects of mild blast traumatic brain injury on cognitive- and addiction-related behaviors. Scientific Reports, 8, 9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawarawong NN, Slaker M, Muelbl M, Shah AS, Chiariello R, Nelson LD et al. (2019) Repeated blast model of mild traumatic brain injury alters oxycodone self-administration and drug seeking. European Journal of Neuroscience, 50, 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson-Madden JH, Forster JE, Huggins J & Schneider A (2012) Psychiatric diagnoses, mental health utilization, high-risk behaviors, and self-directed violence among veterans with comorbid history of traumatic brain injury and substance use disorders. The Journal of Head Trauma Rehabilitation, 27, 370–378. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia G, De Gasperi R, Gama Sosa MA, Perez GM, Otero-Pagan A, Tschiffely A (2018). PTSD-related behavioral traits in a rat model of blast-induced mTBI are reversed by the mGluR2/3 receptor antagonist BCI-838. eNeuro, 5, ENEURO.0357-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia G, Gama Sosa MA, De Gasperi R, Tschiffely AE, McCarron RM, Hof PR et al.(2019) Blast-induced “PTSD”: evidence from an animal model. Neuropharmacology, 145, 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D et al. (2011) Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. NeuroImage, 54(Suppl 1), S76–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K et al. (2014) Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. Journal of Neurotrauma, 31, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E et al. (2011) A mouse model of blast-induced mild traumatic brain injury. Experimental Neurology, 232, 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajja VS, Galloway M, Ghoddoussi F, Kepsel A & Vandevord P (2013) Effects of blast-induced neurotrauma on the nucleus accumbens. Journal of Neuroscience Research, 91, 593–601. [DOI] [PubMed] [Google Scholar]
- Sajja VS, Hubbard WB, Hall CS, Ghoddoussi F, Galloway MP & Vandevord PJ (2015) Enduring deficits in memory and neuronal pathology after blast-induced traumatic brain injury. Scientific Reports, 5, 15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Skelly MJ, Avegno E, Regan S, Zeric T, Nichols E et al. (2018) Alcohol consumption during adolescence in a mouse model of binge drinking alters the intrinsic excitability and function of the prefrontal cortex through a reduction in the hyperpolarization-activated cation current. Journal of Neuroscience, 38, 6207–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago PN, Wilk JE, Milliken CS, Castro CA, Engel CC & Hoge CW (2010) Screening for alcohol misuse and alcohol-related behaviors among combat veterans. Psychiatric Services, 61, 575–581. [DOI] [PubMed] [Google Scholar]
- Schindler AG, Meabon JS, Pagulayan KF, Hendrickson RC, Meeker KD, Cline M et al. (2017) Blast-related disinhibition and risk seeking in mice and combat Veterans: potential role for dysfunctional phasic dopamine release. Neurobiology of Diseases, 106, 23–34. [DOI] [PubMed] [Google Scholar]
- Schindler A, Terry GE, Wolden-Hanson T, Cline MM, Park M, Lee SJ et al. (2020) Repetitive blast promotes chronic aversion to neutral cues encountered in the peri-blast environment. Journal of Neurotrauma, 10.1089/neu.2020.7061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S & Ren L (2011) Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: implications for screening, diagnosis and treatment. Drug and Alcohol Dependence, 116, 93–101. [DOI] [PubMed] [Google Scholar]
- Stahre MA, Brewer RD, Fonseca VP & Naimi TS (2009) Binge drinking among U.S. active-duty military personnel. American Journal of Preventive Medicine, 36, 208–217. [DOI] [PubMed] [Google Scholar]
- Tanielian T & Jaycox LH (2008) Invisible wounds of war: Psychological and cognitive injuries, their consequences, and services to assist recover. Santa Monica, CA: The RAND Center for Military Health Policy Research. [Google Scholar]
- Teng SX, Katz PS, Maxi JK, Mayeux JP, Gilpin NW & Molina PE (2015) Alcohol exposure after mild focal traumatic brain injury impairs neurological recovery and exacerbates localized neuroinflammation. Brain, Behavior, and Immunity, 45, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis Seidl JN, Pastorek NJ, Troyanskaya M & Scheibel RS (2015) Neuropsychological and behavioral correlates of impulsiveness in veterans with and without mild traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 37, 84–91. [DOI] [PubMed] [Google Scholar]
- Trivedi RB, Post EP, Piegari R, Simonetti J, Boyko EJ, Asch SM et al. (2020) Mortality among veterans with major mental illnesses seen in primary care: results of a national study of veteran deaths. Journal of General Internal Medicine, 35, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar C, Ferland JN, Kaur S, Riparip LK, Rosi S.&Winstanley CA. (2019) Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation. European Journal of Neuroscience, 50, 2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger JW, O’Connell C & Cottrell L (2018) Examination of recent deployment experience across the services and components. Santa Monica, CA: RAND Corporation. [Google Scholar]
- Wilcox MV, Carlson VCC, Sherazee N, Sprow GM, Bock R, Thiele TE et al. (2014) Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology, 39, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]


