Abstract
Objective.
Our objective was to model the reciprocal relationships of perceived risk of contracting influenza with and without influenza vaccination, vaccination behavior, and reported influenza illness.
Methods.
We fit a structural equation model to data from a longitudinal survey of adults in the United States collected through the RAND American Life Panel. Data come from Fall and Spring surveys fielded before and after each of 3 influenza seasons, 2016/2017, 2017/2018, and 2018/2019, for a total of 6 waves.
Results.
As expected, reported influenza experience was associated with increased perceived influenza risk in subsequent survey waves. Furthermore, perceived risk was associated with subsequent vaccination behavior, such that vaccination was more common for those with higher perceived unvaccinated influenza risk and lower perceived vaccinated influenza risk. Perhaps surprisingly, both elements of perceived risk were also associated with a greater likelihood of subsequent reported influenza illness. This malleability in illness reports may reflect uncertainty, as more respondents reported being sick but being unsure about whether they had influenza than reported certainty that they had influenza.
Conclusions.
Interventions that influence perceptions about past experience with influenza, including increased testing and informational campaigns about influenza symptoms, could have unanticipated impacts on perceptions of influenza vaccination and vaccination behavior.
Keywords: risk perceptions, health behavior, vaccination, influenza, influenza vaccination, preventive behavior
Introduction
Influenza is responsible for 140,000–810,000 hospitalizations and 12,000–61,000 deaths each year in the United States (US), depending on the transmissibility and virulence of the circulating influenza strains.(Centers for Disease Control and Prevention, 2020) Vaccination is the most effective means available to limit the spread of influenza(Reed et al., 2014) and reduce the severity of outcomes if contracted (Ferdinands et al., 2014; Flannery et al., 2017; Rondy et al., 2017). The influenza vaccine is recommended for everyone ages 6 months or older in the United States, unless an individual has a medical contraindication (Centers for Disease Control and Prevention, 2019). Despite this, typically just over 40% of adults are vaccinated each year in the US(Centers for Disease Control and Prevention, 2017).
An individual’s appraisal of the risk associated with a vaccine-preventable disease is an important and consistent predictor of the likelihood that the individual will choose to be vaccinated (Brewer et al., 2017). The disease appraisal includes the perception of the likelihood that the individual will contract the disease and the perception of the severity of the disease if contracted (Brewer et al., 2007a). Confidence in a vaccine has also been identified as an important predictor of vaccination (Brewer et al., 2017) and includes perceptions of the effectiveness and safety of a vaccine (Chapman and Coups, 1999). One 2015 study found that approximately 40 percent of US adults falsely believe that it is possible to contract influenza from the influenza vaccine (Nyhan and Reifler, 2015); such a belief likely leads to reduced confidence in the vaccine.
Analysis of medical claims data has shown that individuals who have been vaccinated and who contract influenza are less likely to be vaccinated in the future while individuals who are not vaccinated and contract influenza are more likely to be vaccinated in the future (Jin and Koch, 2021). We also found this to be the case using self-reports of illness (Walsh et al., 2020).
One normative framework through which these dynamics can be understood is that individuals update their decision making through a process similar to Bayesian inference (Fischhoff and Beythmarom, 1983). Each influenza season can be thought of as testing hypotheses an individual might have about influenza and the influenza vaccine. In a Bayesian framework, we would expect that individuals who decide to get vaccinated perceive the utility of getting vaccinated to be greater than the utility of not getting vaccinated. The net utility of getting the influenza vaccine can be characterized as:
A Bayesian inference decision-making framework further suggests that when individuals switch vaccination behavior following experiences with influenza, experience of contracting influenza leads individuals to update their perceptions of the likelihood of contracting influenza, which in turn influence vaccination behavior. A Bayesian inference-like process is consistent with the Health Belief Model(Janz and Becker, 1984), and includes perceived susceptibility to disease, perceived severity of disease, and perceived benefit of the health behavior. The Health Belief Model has frequently been applied to understanding influenza vaccination decisions in individuals with high-risk health conditions(Borthwick et al., 2020).
Not included in the Health Belief Model is the uncertainty an individual may have about whether they indeed caught influenza. A Bayesian inference framework suggests the perceptions of the likelihood of a influenza (i.e., their risk perceptions) would influence individuals’ interpretation of uncertain signals that suggest they may have contracted the disease. The symptoms associated with influenza are non-specific, and overlap with those of other infectious diseases including the common cold and now COVID-19(Jutel and Banister, 2013; Rothan and Byrareddy, 2020). In general, patients cannot be certain whether they have had influenza unless they have had a positive influenza test. However, influenza testing varies in sensitivity, and the most commonly used rapid influenza tests (Jester et al., 2018) are not perfectly sensitive, requiring only 80% sensitivity to be approved for use (Centers for Disease Control and Prevention, 2019). As a result, healthcare providers may opt not even to test patients and instead decide to recommend supportive care or antivirals depending on the degree of clinical suspicion. Given the inconsistency of testing approaches for influenza, it is possible that individuals’ perceptions of the likelihood of contracting influenza influences their interpretations of influenza-like-illness.
A recent review of influenza vaccination behavior studies noted that the majority were cross-sectional and retrospective and thus limited by recall bias and a potential desire by participants to reduce dissonance and give responses in support of decisions they had made (Borthwick et al., 2020). Here, we present results from a longitudinal study of adults in the United States conducted over 3 influenza seasons, 2016/2017, 2017/2018, and 2018/2019, in which we asked participants about perceptions of influenza and the influenza vaccine at the start of each season and asked participants about vaccination behavior and experience with influenza at the end of each season. We used these data to test whether individuals interpret influenza-like symptoms, make vaccination decisions, and update perceptions of the likelihood of contracting influenza in a manner consistent with a Bayesian inference-like decision process.
Specifically, our objective was to test the following hypotheses:
H1: (a) Perceived likelihood of contracting influenza if unvaccinated is positively associated with vaccination in future waves and (b) perceived likelihood of contracting influenza if vaccinated is negatively associated with vaccination in future waves.
H2: Contracting influenza is associated with increased perceived likelihood of getting influenza in future waves.
In addition, we sought to examine the following research questions:
RQ1: (a) How certain are individuals in their assessment of whether they had influenza? (b) What influences individuals’ assessment of whether they had influenza?
Methods
Survey
We conducted a longitudinal survey of U.S. adults through the RAND American Life Panel (ALP). The ALP uses a probability-based sampling method to ensure representation of the population of U.S. adults. Participants without regular internet access who agree to participate in the panel are provided internet access. More information about the panel and sampling methods can be found at https://www.rand.org/research/data/alp.html.
Participants were surveyed twice each year, once in the Fall and once in the Spring for three consecutive flu seasons (for six total survey waves), between Fall 2016 and Spring 2019. Each Fall survey included questions about respondent perceptions of the likelihood of getting the flu in the upcoming flu season. Each Spring, respondents were asked about their experiences from the prior flu season, including reports of whether they received the influenza vaccine, whether they experienced influenza-like symptoms, and whether they think they had the flu. Of the 2580 ALP panelists invited, 2,169 completed the baseline survey (84.0% completion rate). Retention rates across the six follow-up surveys were between 91.0% and 83.1%. Respondents were allowed to return if they missed a survey. We conducted the analysis with a sample size of 2,178 observations. This number is slightly higher than the number completing the baseline survey because a small number of panelists began but did not complete the baseline survey, yet still completed the key variables for this analysis and participated in subsequent surveys.
The key variables for this analysis were the respondents’ (i) reports of whether they think they had influenza, (ii) reported vaccination behavior, (iii) reports of whether the respondent experienced fever and cough, and (iv) the perceived probability of getting influenza with and without vaccination.
Note that in all survey questions, we use the term “flu” rather than “influenza”. The terms can be used interchangeably and “flu” is more often used in communication with lay audiences.
Reported Influenza Illness
Specifically, respondents were asked: “Since August [YEAR], have you had an illness that you think was the flu?” Responses were: (1) Yes, (2) No, (3), I got sick, but I don’t know if it was the flu, (4) I thought I had the flu, but later found out it wasn’t the flu, (5) I don’t remember. Those who answered (1), (3), or (4) were then asked: “Did you see or talk to a healthcare provider about having the flu?” (1) Yes, (2) No, (3) I don’t remember. Those who reported seeing a health care provider for influenza were asked “Did a healthcare provider tell you that you had the flu?” (1) Yes, I was told I had the flu, (2) No, I was told I didn’t have the flu, (3) The doctor was unsure whether I had the flu or not, (4) I was not told whether I had the flu or not.
Reported Vaccination Behavior
Respondent reports of whether they received the influenza vaccine were derived from two similar questions. If respondents first indicated that a provider had recommended the influenza vaccine, they were asked, “Did you receive the flu vaccine in response to this recommendation?”. If the respondent either did not indicate such a recommendation or they said that they did not receive the vaccine as a result of a recommendation, they were asked, “Have you been vaccinated for the flu this year (since August [YEAR])?”. Response options were (1) Yes, (2) No, (3) I don’t remember.
Reported Symptoms
To assess influenza-like symptoms that the respondent experienced, we asked: “Since August [YEAR], did you ever have a fever?” Those who did were asked “Did you also have a cough or sore throat at the time you had the fever?” Respondents who did not report fever were asked “Since August [YEAR], did you ever have a cough or sore throat?” Response options for both questions were (1) Yes, (2) No, (3) I don’t remember.
Reported Risk Perceptions
Table 1 shows the questions that we used to construct variables assessing an individual’s perceived probability of getting influenza with and without vaccination, which changed slightly over the course of the study. Respondents to these perceived probability questions were on a clickable numeric probability scale with response options between 0 and 100 percent (Bruine de Bruin and Carman, 2018; Hurd, 2009; Manski, 2004). After the first wave in fall 2016, respondents who had already gotten the influenza vaccine at the time of the survey were not routinely asked their perceived probability of getting the flu if they are not vaccinated. In wave 3, we asked everyone how likely they thought it was that someone who was never vaccinated would get influenza. We used the responses to that question for the perceived probability of getting influenza without vaccination for those who were already vaccinated. In wave 5, those who were already vaccinated were not asked about the perceived probability of getting influenza without vaccination at all (946 observations). We therefore imputed responses for those individuals, as well as responses for waves that individuals did not complete, using a Multivariate Imputation by Chained Equations (MICE) algorithm (Van Buuren and Groothuis-Oudshoorn, 2011), which uses a multiple regression approach for imputing missing values. We used the MICE function and package in R (Van Buuren and Groothuis-Oudshoorn, 2011).
Table 1.
Construction of perceived probability of flu variables
| Perceived Probability of Flu if Vaccinated | Perceived Probability of Flu if Not Vaccinated | |
|---|---|---|
| Wave 1 (Fall 2016) | Asked of everyone: If you do get the flu vaccine this season, what do you think are the chances that you will catch the flu this flu season (starting Fall 2016 through Spring 2017)? | Asked of everyone: If you do not get the flu vaccine this season, what do you think are the chances that you will catch the flu this flu season (starting Fall 2016 through Spring 2017)? |
| Wave 3 (Fall 2017) | Asked of those already vaccinated: What do you think are the chances that you will catch the flu this flu season (between now and April 2018)? | Asked of those already vaccinated: Imagine a person who never gets vaccinated for the flu. What do you think are the chances that they will catch the flu this coming flu season? |
|
Asked of those not already vaccinated:
If you do get the flu vaccine this season, what do you think are the chances that you will catch the flu this flu season (between now and April 2018)? |
Asked of those not already vaccinated: If you do not get the flu vaccine this season, what do you think are the chances that you will catch the flu this flu season (between now and April 2018)? | |
| Wave 5 (Fall 2018) | Asked of those already vaccinated: What do you think are the chances that you will catch the flu this flu season (between now and April 2019)? | NOT ASKED, IMPUTED |
|
Asked of those not already vaccinated: If you do get the flu vaccine this flu season, what do you think are the chances that you will catch the flu this season (between now and April 2019)? |
Asked of those not already vaccinated: If you do not get the flu vaccine this flu season, what do you think are the chances that you will catch the flu this season (between now and April 2019)? |
MICE relies on an assumption that the data are Missing At Random (MAR). In other words, the probability that an observation of a variable Y is missing depends only on observable variables, X, and not on the value of the missing variable Y. In our case, we are missing responses for the perceived probability of influenza without vaccination for respondents who vaccinated early in our Wave 5 survey. This appears to violate the MAR assumption – those who vaccinate early are likely to be the most motivated vaccinators, and may have higher perceived risk of influenza without vaccination than other respondents. If we had only one season’s worth of data, the MAR assumption would almost certainly be violated. However, while getting vaccinated early in the season (reason for missingness) is probably a very good predictor of perceived likelihood of influenza without vaccination, respondents’ prior responses to the same question are likely (we argue) an even better predictor. If the fact that a value is missing provides no additional information about a missing variable’s value over the values of observed variables, the MAR assumption holds. We argue that is the case here. To take advantage of information provided by prior responses to the question, we re-shaped our data into a panel format including lagged variables for previous responses to the perceived probability of influenza questions prior to imputation. In other words, our implementation of MICE assumes that the relationship between Wave 1 and Wave 3 variables is the same as the relationship between Wave 3 and Wave 5 variables. We report comparisons between the distributions of responses to the two likelihood of influenza questions across all seasons after missing variables were imputed in the results section.
Imputation Sensitivity Analyses
We include two imputation sensitivity analyses in the supplementary material. The first included only observations for respondents with complete data or all 6 waves (1,064 complete observations). This excludes any respondents who had already been vaccinated for the current influenza season at the time of the Wave 5 survey, but includes respondents who went unvaccinated that year or who were vaccinated following the survey. In the second sensitivity analysis, we include only four waves of data, and specifically excludes Waves 5 and 6 in which 946 individuals who had already been vaccinated at the time of that fall survey were not asked about their perceived probability of getting influenza.
Statistical Analysis
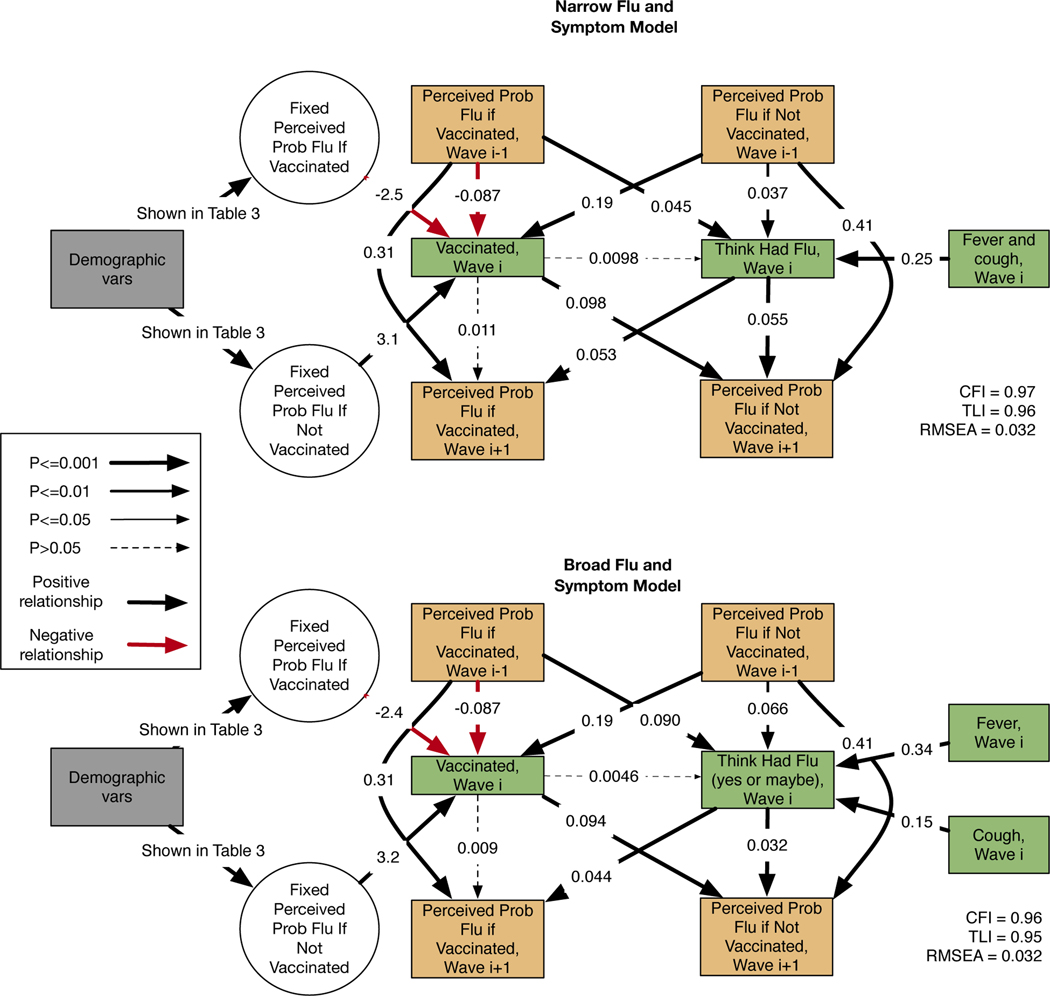
We first conducted a descriptive analysis of the key variables in our data set. Then, we ran a structural equation model (SEM) to examine the relationship between perceived likelihood of getting influenza with and without vaccination on vaccination behavior and whether individuals think they caught influenza, and in turn to estimate the impact of these behaviors and outcomes on future perceptions of the likelihood of getting influenza with and without vaccination. We chose to use SEMs for our analysis instead of simpler regression models because they enable us to model more complex relationships than do simple regression models. Specifically, we wanted to model reciprocal relationships among risk perceptions, vaccination, and reported influenza. This included modeling relationships between variables collected in the same wave, collected in consecutive waves, and collected in consecutive years, which would not have been possible with a simpler regression model. Figure 3 in the results section shows the relationships included in the structural equation models. The latent variables “Fixed Perceived Prob Influenza If Vaccinated” and “Fixed Perceived Prob Influenza If Not Vaccinated” were predicted by the perceived probability of getting influenza if vaccinated in all 3 Fall surveys, and by the perceived probability of getting influenza if not vaccinated in all 3 Fall surveys. This was included to account for stable individual differences in risk perceptions, therefore facilitating isolation of dynamic year-to-year changes. We included demographic variables as predictors for the latent variables for the fixed perceived probabilities. To do this, we used the SEM function in the lavaan package in R (Rosseel, 2012). We use the final iteration of imputed values from MICE to fit the SEM.
Figure 3:
Results for the narrow and broad models. Black arrows indicate positive relationships, while red arrows indicate negative relationships. Orange boxes are variables collected in the fall surveys (at start of flu season, which were odd survey waves) and green boxes are variables collected in the spring surveys (at the end of flu season, which were even survey waves). The reported CFI is the Comparative Fit Index, the TLI is the Tucker Lewis Index, and the RMSEA is the Root Mean Square Error of Approximation.
We present two specifications of the SEM model in the results section, and additional sensitivity analyses in the appendix. To test hypothesis 1, both specifications model the relationship between the perceived likelihood of getting influenza with and without the influenza vaccine on vaccination behavior reported in the following wave. To test hypothesis 2, both specifications modeled relationship between reported influenza and the perceived likelihood of getting influenza with and without vaccination in the subsequent wave. To address research question 1, both specifications model the relationship between the perceived likelihood of getting influenza and the respondent thinking he or she had influenza in the subsequent wave. We included a relationship between influenza-like symptoms (fever and cough or sore throat) and the respondent thinking he/she had influenza as we would expect respondents with such symptoms to be much more likely to report having had influenza compared to respondents who had not experienced such influenza-like symptoms.
We included several additional relationships in the model not directly related to our three core hypotheses. Many prior studies have shown that past vaccination behavior is a very strong predictor of future vaccination behavior (Walsh et al., 2020). We included a relationship between vaccination behavior and perceptions of the likelihood of contracting influenza in the subsequent wave to examine whether the stability of behavior might occur through changes in perceptions that occur over time. Such a dynamic would lend support to the concern that retrospective cross-sectional studies are limited by respondents’ desire to give consistent responses (Borthwick et al., 2020). We included a relationship between vaccination and contracting influenza in the model to account for hypothesized decreased incidence of influenza in vaccinated individuals. As an exploratory analysis, we initially modeled a relationship between reported demographic factors and Wave 1 perceptions of the likelihood of getting influenza. We refined the model to improve the overall fit by including latent variables representing a “fixed perceived probability of flu if vaccinated” and a “fixed perceived probability of flu if not vaccinated”. We ultimately modeled relationships between demographic variables and these latent variables. We discuss the model refinement in more detail in the results section.
The first “Narrow Flu and Symptom Model” used conservative definitions for respondent reports of having gotten influenza, and symptoms associated with influenza. Specifically, we binarized the response to the self-report question about whether the respondent had influenza to be “1” if the respondent answered “yes” and “0” otherwise. We also created a single binary symptom variable indicating whether the respondent reported having both fever and cough or sore throat. In the second “Broad Flu and Symptom Model”, respondents were defined as having had influenza if they answered “Yes” or “I got sick, but I don’t know if it was the flu.” In contrast to the Narrow Flu and Symptom Model, we included fever and cough as separate symptom variables. Across the 6 waves, we imposed constraints in the model such that parameters representing the same relationships among variables in different waves had the same values.
Model Specification Sensitivity Analysis
The purpose of this analysis was to test whether the observed relationship between perceived likelihood of influenza and reported influenza could be explained by respondents’ private information about their likelihood of getting influenza. These analyses are based on the narrow and broad models, and introduce relationships between the perceived likelihood of getting influenza with and without the vaccine and reported fever and cough. We also introduce relationships between reported vaccination and reported fever and cough. If private information does explain the relationship between perceived probability of getting influenza and respondents’ thinking they had influenza, we would expect to see (a) statistically significant relationships between perceived probability of getting influenza and reported symptoms and (b) a decrease in the magnitude of the relationship between perceived probability of getting influenza and the reported influenza.
We assessed model fit using the criteria that a Root Mean Square Error of Approximation (RMSEA) <0.06, Tucker-Lewis Index>0.95 and Comparative Fit Index (CFI) values > 0.95 imply the model fits the data well. When a model had poor fit, we examined the residual variances to include additional relationships in the model to improve the model fit. We note that interpretation of our analysis is limited by the fact that the study was not pre-registered.
Results
Table 2 shows the demographic composition of the survey sample. Of the 2178 respondents in our full sample, 56.5% of respondents were female and 43.5% were male. The average age of respondents was 56 years old. The majority (72.7%) of respondents were non-Hispanic white/Caucasian, 9.2% were Hispanic/White, and 9.0% were Black/African American. Nearly half (47.3%) of respondents had a college or post-graduate degree.
Table 2.
Demographic characteristics of the full sample in Fall 2016 when the Wave 1 survey was collected).
| Variable | Category | Number | Percent |
|---|---|---|---|
| Sex | Male | 948 | 43.5% |
| Female | 1230 | 56.5% | |
| Race/Ethnicity | White/Caucasian, not Hispanic | 1583 | 72.7% |
| Hispanic/White | 201 | 9.2% | |
| Black/African American | 196 | 9.0% | |
| Asian or Pacific Islander | 55 | 2.5% | |
| American Indian or Alaskan Native | 25 | 1.1% | |
| Other | 117 | 5.4% | |
| Education | Less Than High School | 59 | 2.7% |
| High School | 283 | 13.0% | |
| Some College | 806 | 37.0% | |
| College | 555 | 25.5% | |
| Post Graduate | 475 | 21.8% | |
| Variable | Mean | Standard Deviation | |
| Age | 56.0 | 14.0 | |
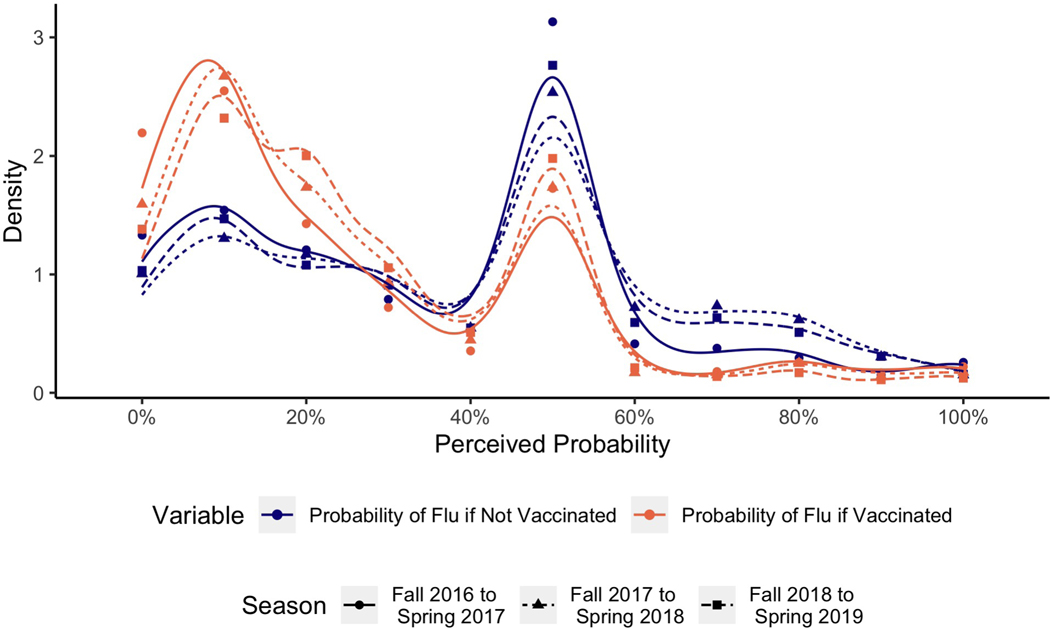
Figure 1 shows the distribution of respondents’ perceived probability of getting influenza with and without vaccination. We see that the overall distribution of both frequencies is fairly stable across the three seasons. Both distributions are bi-modal with one mode at about 10% and another at 50%. Such secondary modes at 50% are common in subjective probability judgments and generally reflect expressions of epistemic uncertainty (Bruine de Bruin and Carman, 2012). In the case of the perceived probability of getting influenza if not vaccinated, the mode at 50% is higher than the mode at 10%. The opposite is true of respondents’ perceived probability of getting influenza if vaccinated, where the mode at 10% is higher than the mode at 50%. The true probability of getting influenza each year is estimated to be 3–14% (Centers for Disease Control and Prevention, 2020), and the estimated effectiveness of the influenza vaccine has ranged between 19–60% over the past decade (Centers for Disease Control and Prevention, 2021).
Figure 1:
Distribution of reported perceptions of probability of getting influenza with and without vaccination by season
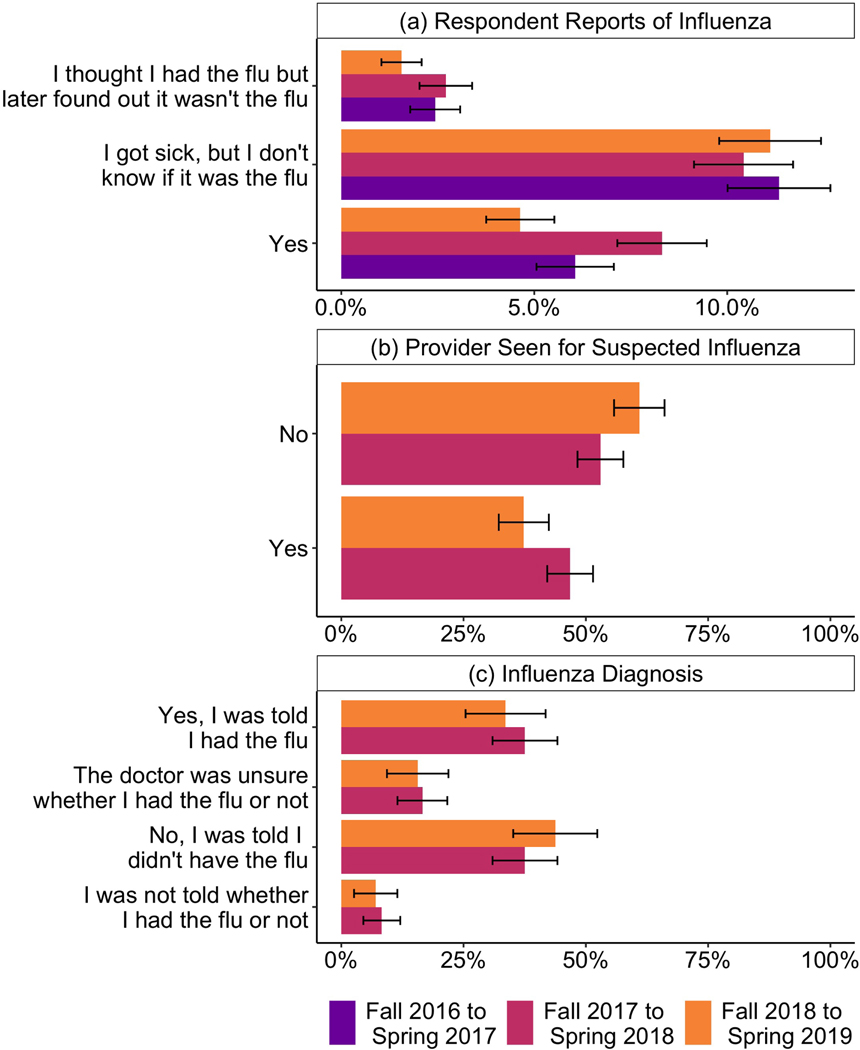
Figure 2 shows responses to three questions about whether respondents had influenza. Figure 2a shows the responses for our Spring survey question: “Since August [year], have you had an illness that you think was the flu.” The response “No” is excluded from the figure, but represents between 78–83% of responses, across the three seasons. Each season, more people report that they were sick with an illness that they are not sure was influenza (11–12% of respondents) than simply answer “Yes” (5–9% of respondents). In addition, about 1–3% of respondents report that they were sick with an illness that they thought was influenza, but later found out was not influenza. Figure 2b shows responses to the question: “Did you see or talk to a healthcare provider about having the flu?” The question was asked in Waves 4 and 6 of all who responded that they thought they had influenza, may have had influenza, or initially thought they had influenza. Less than half these respondents reported seeing a healthcare provider (Figure 2b). Of these, roughly equal numbers reported being told they did have influenza as reported being told they did not have influenza. About a quarter reported that either their provider was unsure whether they had influenza or were not told whether they had influenza (Figure 2c). Hence, there were many opportunities for respondents to interpret an illness as influenza or not.
Figure 2:
(a) Respondents’ reported experience with influenza over three seasons. The “No” response is not shown. (b) Respondent reports of seeing a health care provider for influenza (among those who answered “yes”, “I got sick, but I don’t know if it was the flu.” or “I thought I had the flu but later found out it wasn’t the flu” for whether they had influenza that season.) (c) Respondent reports of what they were told by health care providers about their suspected influenza (among those who saw a health care provider for suspected influenza).
Figure 3 shows the results of the structural equation models. Black lines represent positive relationships, and red lines represent negative relationships. Solid lines represent relationships that are significant at least at the p<0.05 level and the dashed lines indicate relationships that were not statistically significant, with p>0.05. Both the narrow and broad models had the Root Mean Square Error of Approximation (RMSEA) <0.06, Tucker-Lewis Index, and Comparative Fit Index (CFI) values > 0.95, indicating that both models fit the data well(25). We included latent variables for a “fixed perceived probability of getting the flu if vaccinated” and a “fixed perceived probability of getting the flu if not vaccinated”, based on Wave 1, 3, and 5 reports, to improve the model fit and address stable individual differences in risk perception. Without them, the model had high cross-wave residual covariances between reported probabilities of getting influenza with and without vaccination and reported vaccination behavior. The fit for the narrow model without the two fixed latent variables was CFI=0.72, TLI = 0.66 and RMSEA=0.088.
As expected, the fixed (latent) perceived probability of getting the flu if vaccinated is negatively associated with vaccination, and the fixed perceived probability of getting the flu if not vaccinated is positively associated with vaccination. The relationship between perceived risk of disease and vaccination has been well-documented (Brewer et al., 2007b). The good model fit when the latent variables are included (Figure 3) suggests that when respondents report the perceived probability of getting influenza with and without vaccination each Fall, each response varies around an underlying stable perception. Using latent variables constructed using three waves of data produces a better measure of the underlying construct than a single measure. After accounting for this stable construct, the perceived probabilities of getting influenza with and without vaccination from each fall survey also have statistically significant relationships with vaccination behavior as reported in the subsequent spring survey. This suggests that the latent fixed variables do not explain all of the variation in vaccination behavior; perceptions of the probability of getting influenza with and without vaccination vary over time and these changes are also associated with changes in vaccination behavior.
We also find that the greater an individual’s perceived risk of getting influenza with and without the vaccine, the more likely an individual is to report that they had influenza in the subsequent survey wave, under either the narrow or broad definitions of having influenza. In both models, the symptom variables are positively associated with the respondent thinking they had influenza. We find that both fever and cough are independently associated with the belief, and that having a fever is more strongly correlated with a flu self-report than cough or sore throat. Surprisingly, the relationship between being vaccinated and a respondent thinking he or she had influenza is small and not statistically significant in either model. We find that a respondent thinking they had influenza, as reported in a Spring survey, is associated with reporting higher perceived probability of catching influenza with and without vaccination in the subsequent fall survey. Vaccinating is associated with reporting a higher perceived probability of getting influenza if not vaccinated, which means that not vaccinating is associating with reporting a lower perceived probability of getting influenza if not vaccinated in the subsequent fall survey. This could result from unvaccinated individuals who do not get influenza learning from that experience and lowering their perceived probability of getting influenza without vaccination. Note that our model accounts for the relationship between perceived probability of getting influenza reported in consecutive years.
Table 3 shows the relationship between demographic characteristics and the fixed perceived probability of getting influenza with and without vaccination. The fixed perceived probability of getting influenza if not vaccinated is greater with older age while the perceived probability of getting influenza if vaccinated decreases with age. This is consistent with older individuals being more likely to be vaccinated. Women perceive a higher risk of getting influenza with and without vaccination than do men. College graduates perceive the risk of getting influenza with and without vaccination to be lower than do non-college graduates.
Table 3.
Regression Coefficients for the Relationship Between Demographic Variables and Fixed Perceived Probability of Getting Influenza With and Without Vaccination.
| Independent Variable | Dependent Variable | Estimate, narrow model | p-value, narrow model | Estimate, broad model | p-value, broad model |
|---|---|---|---|---|---|
| Age (years) | Fixed Perceived Probability of Influenza if Vaccinated | −0.001 | <0.001 | −0.001 | <0.001 |
| Sex = female (male ref) | 0.030 | <0.001 | 0.030 | <0.001 | |
| College grad | −0.056 | <0.001 | −0.057 | <0.001 | |
| Hispanic (non-Hispanic White ref) | −0.002 | 0.89 | 0.003 | 0.88 | |
| Black/African American (non-Hispanic White ref) | −0.018 | 0.33 | −0.014 | 0.45 | |
| Other race (non-Hispanic White ref) | 0.069 | <0.001 | 0.069 | <0.001 | |
| Age (years) | Fixed Perceived Probability of Influenza if Not Vaccinated | 0.002 | <0.001 | 0.002 | <0.001 |
| Sex = female (male ref) | 0.027 | <0.001 | 0.027 | <0.001 | |
| College grad | −0.026 | <0.001 | −0.028 | <0.001 | |
| Hispanic (non-Hispanic White ref) | 0.02 | 0.24 | 0.024 | 0.17 | |
| Black/African American (non-Hispanic White ref) | −0.03 | 0.089 | −0.028 | 0.10 | |
| Other race (non-Hispanic White ref) | 0.049 | <0.001 | 0.049 | <0.001 |
Imputation Sensitivity Analysis
The full results of the imputation sensitivity analyses are included in the supplementary material. These are the “4-Wave Model” and the “No Imputation Model”. Both are based on the Narrow model. The 4-wave model includes all complete cases from the first 4 waves of data we collected. The relationships between the fixed (i.e. latent) and non-fixed perceived probability of getting influenza with and without vaccination and reported vaccination are qualitatively similar to those in the narrow model. Most relationships remain statistically significant at the p<0.001 level with the exception of the relationship between the perceived probability of influenza if unvaccinated and vaccination in the 4-wave model, which has p=0.0036.
The no imputation model includes 6 waves of data for the complete cases only. In the 5th wave, we did not ask about the perceived likelihood of influenza for individuals who had already been vaccinated at the time of the survey. These individuals, who were vaccinated early in the 2018/2019 influenza season, are not included in the no imputation model. Since they were not only vaccinated, but vaccinated relatively early, they likely represent a disproportionate share of enthusiastic vaccinators in our sample.
Of the four relationships tested in the sensitivity analysis between perceived probability of influenza without vaccination and reported influenza, one drops below statistical significance at the 0.05 level (relationship between the perceived probability of influenza if unvaccinated and influenza in the no imputation model). We suspect this change is related to the fact that this model eliminates many of the most enthusiastic vaccinators from the sample. Two of the other relationships remain significant at the p<0.001 level and one is significant at p=0.0046. The coefficients on these relationships all remain positive and magnitudes are the same or higher in the sensitivity analyses compared to the Narrow model.
Model Specification Sensitivity Analysis
In a set of sensitivity analyses, we added relationships between perceived likelihood of influenza with and without vaccination and symptoms (fever and cough) in both the narrow and broad models. These are the “Narrow Model, Risk Symptoms” and “Broad Model, Risk Symptoms” in the supplementary material. We additionally introduced relationships between reported vaccination and reported symptoms. The purpose of this analysis was to test whether the observed relationship between perceived likelihood of influenza and reported influenza could be explained by respondents’ private information about their likelihood of getting influenza. If private information does explain the perceived probability of getting influenza and respondents’ thinking they had influenza, we would expect to see (a) statistically significant relationships between perceived probability of getting influenza and reported symptoms and (b) a decrease in the magnitude of the relationship between perceived probability of getting influenza and the reported influenza. In the two sensitivity analysis models, the only relationship between perceived probability of getting influenza with or without vaccination and reported symptoms that was statistically significant at the 0.05 level was the relationship between the perceived probability of getting influenza if vaccinated and self reports of cough. Surprisingly, the direction of this relationship was negative – i.e., respondents who reported higher perceived likelihood of influenza if vaccinated were less likely to report cough (p = 0.024). We tested 6 new relationships between perceived likelihood of influenza and reported symptoms in total in these two sensitivity analyses, and therefore interpret this single significant result with caution.
The relationships between perceived probability of getting influenza with and without vaccination and reported influenza were unchanged (to two significant digits) between the main Narrow symptom model and the Narrow Risk Symptoms model and between the main Broad model and the Broad risk symptoms model. These relationships also remained statistically significant at the p<0.001 level, with the exception of the relationship between perceived probability of influenza if unvaccinated and reported influenza in the Narrow model, which had a p-value of 0.0034.
Interestingly, two of the three new relationships between vaccination and reported symptoms were statistically significant in these models. In the Narrow Risk Symptoms model, the relationship between vaccination and reported fever and cough (both symptoms) was negative (p = 0.010). In the Broad Risk Symptoms model, there was a negative relationship between reported vaccination and reported fever (p = 0.032). The relationship between vaccination and cough was not statistically significant (p=0.15). Again, we interpret these sensitivity analysis results with caution, but it is notable that the relationship between vaccination and reported influenza was small and not statistically significant in either our main Narrow or Broad models, but the relationship between vaccination and fever and cough (combined) or fever was statistically significant. This might be expected if reported symptoms are a more valid measure of influenza infection than respondent reports of thinking they had influenza.
This analysis does not exclude the possibility the relationship between perceived likelihood of influenza with and without vaccination and reported influenza is due to private information. However, it is more consistent with an interpretation that perceptions of influenza likelihood influence individuals’ interpretations of ambiguous signals.
Discussion
We tested two hypotheses using unique panel data cutting across six waves and three influenza seasons. Our first hypothesis was H1: (a) Perceived likelihood of contracting influenza if unvaccinated is positively associated with vaccination in future waves and (b) perceived likelihood of contracting influenza if vaccinated is negatively associated with vaccination in future waves. Whereas these hypotheses themselves have been addressed in prior literature, to our knowledge those studies have not had access to such extensive longitudinal data. Our results support these both hypotheses. Perceived likelihood of contracting influenza if not vaccinated was positively associated vaccination behavior reported in the subsequent wave and that perceived likelihood of influenza with vaccination was negatively associated vaccination behavior reported in the subsequent wave. Simultaneously, latent variables constructed from perceived likelihoods reported in all waves were significantly associated with reported vaccination behavior. While the relationship between the likelihoods and subsequent vaccination behavior was expected, we did not include the latent variables in our initial a priori model. The need to include these latent variables to achieve good model fit suggests that yearly perceived likelihoods may be better modeled as variations around a more stable underlying perception. Using latent variables constructed using three waves of data produces a better measure of the underlying construct than a single measure. That the perceived probabilities of getting influenza with and without vaccination from each fall survey also have statistically significant relationships with vaccination behavior reported in the following spring wave suggests that the latent fixed variables do not explain all of the variation in vaccination behavior; perceptions of the probability of getting influenza with and without vaccination vary over time and these changes are also associated with changes in vaccination behavior. To our knowledge, this is a novel documentation and interpretation of influenza risk perception as having both stable and time-varying components. Additional longitudinal studies measuring perceived likelihood of disease and health behavior could test if this observation holds for other health behaviors.
Our second hypothesis was H2: Contracting influenza is associated with increased perceived likelihood of getting influenza in future waves. We found that our results were consistent with this hypothesis, and that individuals’ perceptions of the likelihood of getting influenza appeared to be associated with prior experience contracting influenza, as one would expect from intuitive quasi-Bayesian updating.
Our additional research questions were RQ1: (a) How certain are individuals in their assessment of whether they had influenza? (b) What influences individuals’ assessment of whether they had influenza? Our results suggest that there is substantial uncertainty in respondent reports of influenza. More respondents report being unsure whether or not they had influenza than report having had influenza. Specifically, we found that more people report that they “got sick, but don’t know if it was the flu” than report that they had influenza each year indicating that many respondents are aware of the uncertainty in interpreting influenza-like symptoms. We also found that fewer than half of respondents who reported suspecting that they had influenza saw a health care provider. Of those who did, approximately equal numbers reported being told that they did have influenza as reported being told they did not in fact have influenza.
We found that the perceived probability of getting influenza is positively associated with the likelihood of reporting getting influenza in future survey waves. This suggests that individuals who perceive influenza as more likely more frequently interpret the ambiguous symptoms of an influenza-like-illness as influenza than do people who perceive influenza as rare, which is also consistent with Bayesian inference-like thinking.
To our knowledge, this study is the first demonstration of the potential role of prior expectations (in this case, the perceived likelihood of getting influenza) on interpretation of uncertain influenza-like symptoms. On the other hand, the observed relationship between perceived likelihood of influenza and reported influenza could also be due to private information individuals have about their true influenza risk. We tested for the role of private information by conducting a set of model specification sensitivity analyses to include a relationship between perceived likelihood of influenza and reported symptoms (cough and fever). The results of this analysis suggested that the observed relationship between perceived likelihood of influenza and reported influenza could not be fully explained by private information. However, further studies are warranted to examine how much of the observed variation in perceived risk of influenza is due to individuals’ accurate perceptions based on private information.
We also note that while our study focused on the potential for quasi-Bayesian updating about the probability of influenza based on personal experience, there is also the potential for learning and quasi-Bayesian updating about the costs of influenza and vaccination. For example, some people experience side effects of the influenza vaccine including muscle aches and low-grade fever(Centers for Disease Control and Prevention, 2021). Examining the relationship between such experience and beliefs about the costs of the influenza vaccine, or between experience with influenza and beliefs about costs and severity of contracting the disease is worth future study.
Our results could have implications for other diseases for which clinical confirmation may be uncommon. Other respiratory illnesses with symptoms that overlap with those of influenza, including the common cold and more severe illnesses such as COVID-19 may be particularly worth studying further in this context. In the case of COVID-19, test availability was extremely limited in early stages of the pandemic and continues to be limited in many areas. The earliest reported COVID-19 cases also coincided with the peak of the 2019/2020 influenza season in the US(27). Given the overlap in symptoms between COVID-19 and influenza, there may be considerable uncertainty for those who were ill but untested in the late 2019/2020 influenza season as to whether they had COVID-19, influenza, or something else. This could lead to an incorrect belief in some who had influenza that they had COVID-19, and as a result gained some level of immunity. If influenza and COVID-19 testing are not widely available (or precise), infections with the common cold or with influenza could be interpreted as being COVID-19 within communities, which could lead individuals to over-estimate their local COVID-19 risk.
Informational campaigns about influenza symptoms and influenza testing are often used to encourage appropriate utilization of health care resources during the influenza season and to inform providers’ treatment decisions. Such efforts are also likely to impact individuals’ perceptions of whether they had influenza and could consequently impact vaccination rates. However, the likely net impact of such efforts is unclear and complex. If interventions that impact perceptions of influenza increase the number of unvaccinated people who think they caught influenza or decrease such perceptions among the vaccinated population, those interventions are likely to increase vaccination rates overall. However, if they decrease the number of unvaccinated individuals who think they caught influenza or increase the perception among vaccinated individuals, they could decrease vaccination rates. In that case, it may be important to develop messaging strategies to prevent a decrease in vaccination rates. It could also include more tailored messaging strategies such as informing the public – particularly vaccinated individuals – about the ability of influenza vaccine to reduce the severity of illness even when a vaccinated individual contracts influenza.
Limitations
Interpretation of our analysis should be tempered by the fact is that it was not pre-registered. Based on our results, we infer that perceived risk of disease is associated with greater likelihood of interpreting ambiguous signals about the disease. However, because this relationship has not previously been reported, further (and pre-registered) hypothesis testing studies are needed. In addition, our study was observational but longitudinal. We are therefore able to establish correlation and time order of occurrence, but the lack of ability to rule out other potential causal factors make us unable to draw causal conclusions.
While we present two models that fit the data well, it is possible that other model specifications could fit the data equally well. Our analysis relies on self-reports, and true vaccination behavior, influenza experience, and symptom experience may differ from what respondents report. That said, individuals often choose behaviors based on perceptions, and the results presented here suggest ways that misperceptions could be driving behavior.
Conclusions
Our results from a national United States-based longitudinal panel, using data from six survey waves across three influenza seasons, found that perceived likelihood of contracting influenza if unvaccinated is positively associated with vaccination in future waves, perceived likelihood of contracting influenza if vaccinated is negatively associated with vaccination in future waves, and contracting influenza is associated with increased perceived likelihood of getting influenza in future waves. These results are consistent with prior work (Brewer et al., 2017). In addition, our results suggest that individuals’ preconceptions about the likelihood of getting influenza may impact whether they think that a given illness was in fact influenza. While further work is needed to test this relationship, these results could have implications for other diseases with ambiguous symptoms and where a substantial number of patients do not seek or receive clinical confirmation of their diagnosis.
Supplementary Material
Highlights.
Perceived risk of influenza without vaccination is associated with greater likelihood of vaccinating.
Perceived risk of influenza with vaccination is associated with decreased likelihood of vaccinating.
Experience with influenza is associated with higher future perceived risk of influenza.
Higher perceived risk of influenza may be more associated with interpreting ambiguous symptoms as influenza.
Acknowledgments
This study was funded through support from the National Institute of Allergies and Infectious Diseases (R01AI118705). The views expressed are those of the authors and do not necessarily represent the views of these funders.
Footnotes
Sarah Nowak – Conceptualization, Methodology, Formal Analysis, Writing – Original Draft; Andrew Parker -- Conceptualization, Methodology, Writing – Original Draft, Project administration, Funding acquisition; Courtney Gidengil – Conceptualization, Writing – Original Draft; Andrea Richardson – Methodology, Writing – Review & Editing; Matthew Walsh – Conceptualization, Writing -- Review & Editing;
David Kennedy – Conceptualization, Writing -- Review & Editing; Raffaele Vardavas – Conceptualization, Methodology, Writing – Review & Editing, Project administration, Funding acquisition
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah A. Nowak, Larner College of Medicine at the University of Vermont, Department of Pathology and Laboratory Medicine, Burlington, VT
Andrew M. Parker, RAND Corporation, Pittsburg, PA
Courtney A. Gidengil, RAND Corporation, Boston, MA Boston Children’s Hospital, Boston, MA.
Andrea Richardson, RAND Corporation, Pittsburg, PA.
Matthew Walsh, RAND Corporation, Pittsburg, PA.
David Kennedy, RAND Corporation, Santa Monica, CA.
Raffaele Vardavas, RAND Corporation, Santa Monica, CA.
References
- Borthwick C, O’Connor R, Kennedy L, 2020. Psychological predictors of seasonal influenza vaccination uptake among adults with a high-risk physical health condition: a systematic review. Psychology & Health. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND, 2007a. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychology 26, 136. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A, 2017. Increasing Vaccination: Putting Psychological Science Into Action. Psychol Sci Public Interest 18, 149–207. 10.1177/1529100618760521 [DOI] [PubMed] [Google Scholar]
- Brewer NT, Cuite CL, Herrington JE, Weinstein ND, 2007b. Risk compensation and vaccination: Can getting vaccinated cause people to engage in risky behaviors? Ann. Behav. Med. 34, 95–99. 10.1007/BF02879925 [DOI] [PubMed] [Google Scholar]
- Bruine de Bruin W, Carman KG, 2018. Measuring subjective probabilities: The effect of response mode on the use of focal responses, validity, and respondents’ evaluations. Risk Analysis 38, 2128–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruine de Bruin W, Carman KG, 2012. Measuring risk perceptions: what does the excessive use of 50% mean? Medical Decision Making 32, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2021. CDC Seasonal Flu Vaccine Effectiveness Studies | CDC [WWW Document]. URL https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed 10.14.21).
- Centers for Disease Control and Prevention, 2021. Seasonal Flu Vaccines | CDC [WWW Document]. URL https://www.cdc.gov/flu/prevent/flushot.htm (accessed 10.14.21).
- Centers for Disease Control and Prevention, 2020. Influenza (Flu) [WWW Document]. URL https://www.cdc.gov/flu/about/burden/index.html
- Centers for Disease Control and Prevention, 2019. Vaccine Information Statements (VISs) [WWW Document]. URL https://www.cdc.gov/vaccines/hcp/vis/vis-statements/flu.html
- Centers for Disease Control and Prevention, 2019. Rapid Diagnostic Testing for Influenza: Information for Clinical Laboratory Directors [WWW Document]. URL https://www.cdc.gov/flu/professionals/diagnosis/rapidlab.htm
- Centers for Disease Control and Prevention, 2017. Flu Vaccination Coverage, United States, 2016–17 Influenza Season [WWW Document]. URL https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm
- Chapman GB, Coups EJ, 1999. Predictors of influenza vaccine acceptance among healthy adults. Preventive medicine 29, 249–262. [DOI] [PubMed] [Google Scholar]
- Ferdinands JM, Olsho LE, Agan AA, Bhat N, Sullivan RM, Hall M, Mourani PM, Thompson M, Randolph AG, 2014. Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010–2012. The Journal of infectious diseases 210, 674–683. [DOI] [PubMed] [Google Scholar]
- Fischhoff B, Beythmarom R, 1983. Hypothesis Evaluation from a Bayesian Perspective. Psychol Rev 90, 239–260. 10.1037/0033-295x.90.3.239 [DOI] [Google Scholar]
- Flannery B, Reynolds SB, Blanton L, Santibanez TA, O’Halloran A, Lu P-J, Chen J, Foppa IM, Gargiullo P, Bresee J, 2017. Influenza vaccine effectiveness against pediatric deaths: 2010–2014. Pediatrics 139, e20164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MD, 2009. Subjective probabilities in household surveys. Annu. Rev. Econ. 1, 543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz NK, Becker MH, 1984. The health belief model: A decade later. Health Education & Behavior 11, 1–47. [DOI] [PubMed] [Google Scholar]
- Jester B, Schwerzmann J, Mustaquim D, Aden T, Brammer L, Humes R, Shult P, Shahangian S, Gubareva L, Xu X, Miller J, Jernigan D, 2018. Mapping of the US Domestic Influenza Virologic Surveillance Landscape. Emerg Infect Dis 24. 10.3201/eid2407.180028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin GZ, Koch TG, 2021. Learning by Suffering? Patterns in Flu Vaccination Take-up. American Journal of Health Economics 7, 000–000. [Google Scholar]
- Jutel A, Banister E, 2013. “I was pretty sure I had the’flu”: Qualitative description of confirmed-influenza symptoms. Social Science & Medicine 99, 49–55. [DOI] [PubMed] [Google Scholar]
- Manski CF, 2004. Measuring expectations. Econometrica 72, 1329–1376. [Google Scholar]
- Nyhan B, Reifler J, 2015. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 33, 459–464. [DOI] [PubMed] [Google Scholar]
- Reed C, Kim IK, Singleton JA, Chaves SS, Flannery B, Finelli L, Fry A, Burns E, Gargiullo P, Jernigan D, 2014. Estimated influenza illnesses and hospitalizations averted by vaccination—United States, 2013–14 influenza season. MMWR. Morbidity and mortality weekly report 63, 1151. [PMC free article] [PubMed] [Google Scholar]
- Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG, 2017. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. Journal of Infection 75, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y, 2012. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). Journal of statistical software 48, 1–36. [Google Scholar]
- Rothan HA, Byrareddy SN, 2020. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of autoimmunity 109, 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K, 2011. mice: Multivariate imputation by chained equations in R. Journal of statistical software 45, 1–67. [Google Scholar]
- Walsh MM, Parker AM, Vardavas R, Nowak SA, Kennedy DP, Gidengil CA, 2020. The Stability of Influenza Vaccination Behavior Over Time: A Longitudinal Analysis of Individuals Across 8 Years. Ann Behav Med. 10.1093/abm/kaaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.