Abstract
Diabetes mellitus is a known risk factor for the development of multiple subtypes of dementia and mild cognitive impairment. Recent research identifies a cause-specific diabetes-related dementia with a unique set of characteristics. Currently, there is no standard cognitive assessment battery recommended to specifically assess dementia that is a direct consequence of chronic diabetes, and some evaluations have been used for decades with minimal revisions, regardless of appropriateness. We performed a systematic review of the dementia/cognition evaluation methods most commonly used in the literature for assessing diabetic patients and identified which cognitive domains are typically assessed in this setting, and whether cognitive changes were more reflective of a vascular pathology, Alzheimer’s pathology, or something else entirely. Search results yielded 1089 articles. After screening for appropriateness, a total of 11 full-text articles were assessed. In general, subjects in the reviewed studies were assessed using a variety of testing methods, examining different combinations of cognitive domains. A standard, clear definition of which cognitive domains are the most important to assess in diabetic patients is needed in order to determine what combination of assessment tools are most pertinent. Given the growing subset of the US population, careful reconsideration of cognitive assessment methods is needed to create self-care plans that take into account a specific collection of cognitive challenges for those with diabetes.
Keywords: cognitive assessment screening instrument, cognitive decline, cognitive impairments, diabetes mellitus, diabetes-related complications
Diabetes mellitus (DM) is a risk factor for dementia and mild cognitive impairment (MCI) (1). Negative effects on cognition can occur early in the timeline of diabetes and accelerate over time (2), potentially impacting ability of long-term diabetic patients to provide adequate self-care. This is concerning, given the other complex sequelae of DM, such as renal disease, peripheral vascular disease, and foot ulcers requiring extensive care (3). Maintaining patient ability to self-manage disease can aid in population-level management of diabetes, especially in face of increasing care costs (4); expenditures on diabetes care in the United States increased 26% from 2012 to 2017 (5). Improving patient management by streamlining and harmonizing cognitive evaluations should result in better monitoring and prediction of disease progression and concurrent cognitive decline. However, identifying and properly classifying subtypes of cognitive decline in type 2 DM (T2DM) has proven to be challenging and is difficult to do without conducting extensive neurological and neuroimaging exams. This challenge is particularly evident to clinicians and researchers who struggle to determine the extent of cognitive impairment in these patients, as this also complicates the consent process and introduces uncertainty involving the ability of patients to make their own health care decisions (6).
Cognitive decline as a direct consequence of T2DM is frequently referred to as “diabetes-related dementia,” presenting with a unique pattern of cognitive domain pathologies (7,8). Neuroimaging has demonstrated that diabetes-related metabolic abnormalities also manifest in specific brain areas, consistent with cognitive decline in the areas of memory and executive function greater than experienced in Alzheimer’s (8). In this regard, diabetes-related dementia is more similar to vascular dementia than to Alzheimer’s, although the latter is more common in the broader population (8,9). Evidence suggests that brains of diabetic patients display infarct pathology and lack the classic Alzheimer’s plaque-and-tangle appearance (10). Despite these pathological differences, patients with diabetes-related or Alzheimer’s dementia are often screened to determine cognitive status using the same brief cognitive assessment tests, such as the Mini-Mental State Examination (MMSE) or Mini-Cognitive Assessment Instrument (Mini-Cog) (11).
In diabetic patients without genetic mutations predisposing to other types of dementia, and who develop dementia as a direct consequence of disease, it is not clear if commonly used cognitive assessments capture information needed to identify the unique cognitive characteristics of diabetic dementia. Further, one can ask whether diabetic dementia presents differently from Alzheimer’s disease or vascular dementia. Addressing these questions, and creating disease-specific evaluation methods, could lead to more effective courses of treatment.
This study aims to systematically review the dementia/cognitive evaluation methods commonly used for diabetic patients to identify which cognitive domains are being assessed, whether they are more reflective of a vascular or Alzheimer’s pathology, or whether something else entirely is being examined. This will aid in determining which cognitive evaluations are most pertinent and helpful for assessing the type of dementia that develops in chronic DM patients, especially when trying to assess competency for providing consent or the extent of participation a patient is able to commit.
We did not seek Institutional Review Board approval for this investigation because we did not employ the use of protected health information in this investigation.
Patients/Materials and Methods
We searched Medline and Ovid using the search terms “diabetes mellitus,” “dementia,” “cognition,” and “memory.” Articles were excluded if they lacked at least 2 of these search terms, or did not mention diabetes-related dementia and cognitive assessments. Articles not in English; animal studies; articles focused on biochemical pathways, “renal insufficiency,” or “macrophages”; articles mentioning type 1 DM; articles only addressing the Alzheimer’s form of dementia; articles focused on specific subgroups of people; and articles with subjects not within the United States were excluded. We allowed all article types except biographies, conference notes, datasets, directories, interactive tutorials, legal cases, personal narratives, video-audio media, and webcasts.
The result yielded 1089 articles. Articles were screened for appropriateness by title alone, leaving 526 articles. Abstracts of the remaining articles were designated as “yes” or “no” for further review. Based on these choices, full texts were reviewed. In total, 106 full-text articles were assessed; see Fig. detailing study selection. “Yes” or “no” designations were again assigned, ensuring that articles sufficiently addressed dementia or cognitive changes related to diabetes. When disagreement arose throughout this process, the articles were discussed until agreement was reached. Of these, 11 articles outlined the cognitive tests used for our population of interest and are listed in Table 1. Twenty-two articles from the search, which were not included in the final review, were used as reference material. The various tests used in the studies are detailed in Table 1.
Fig.
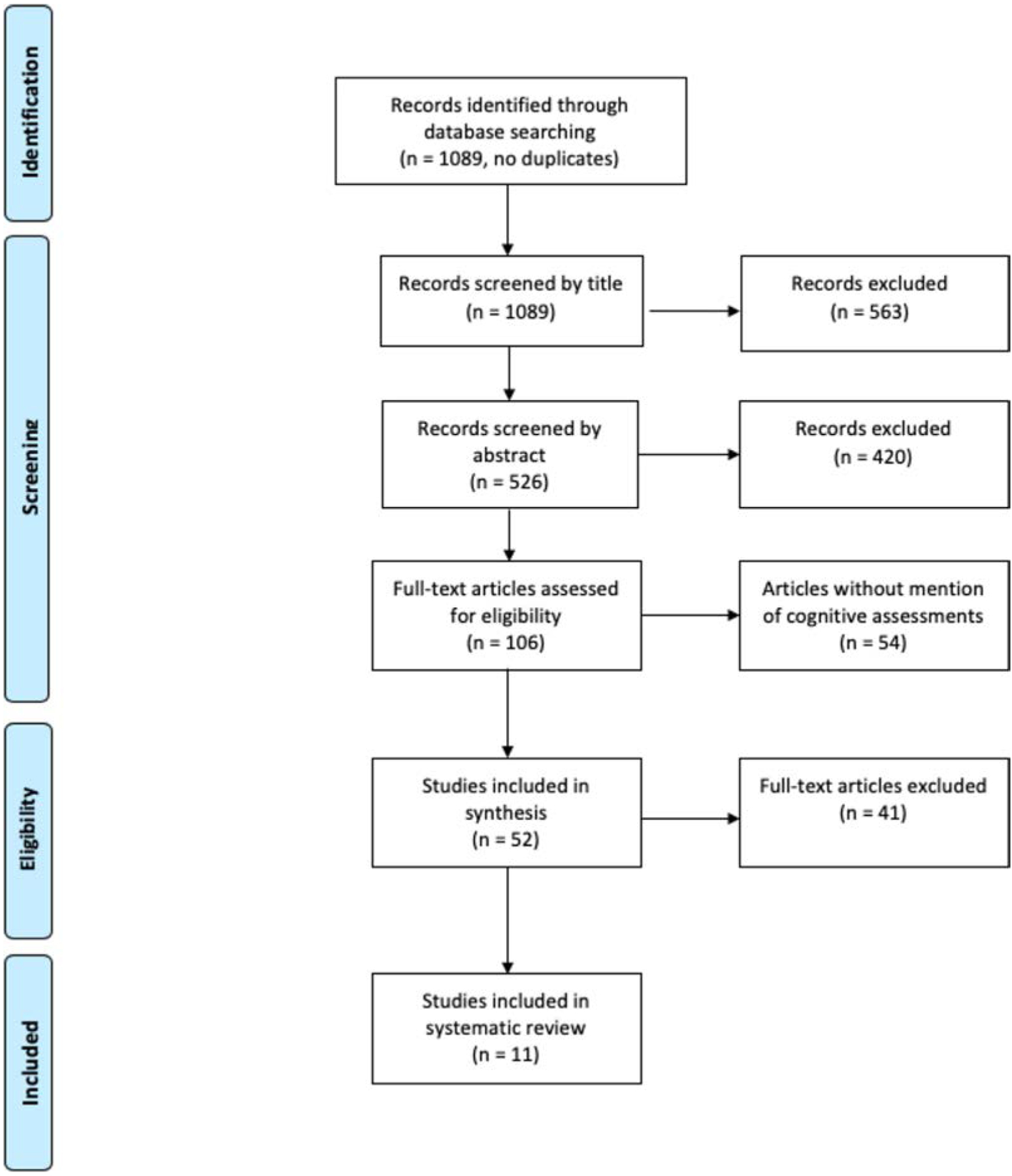
PRISMA flow diagram of article selection process.
Table 1.
Cognitive assessment tools used in selected articles
| Test Name | Description |
|---|---|
| Attentiveness Endurance Test (D2) (45) | This is a European pen-and-paper test of selective attention/processing speed as well as discrimination of visual stimuli. It has been used in the United States since 1998. There are 14 lines, with 47 characters in each line, for a total of 658 items. The participant is given 20 seconds per line and is required to identify the “Ds” that have 2 following dashes, ignoring distractor stimuli that have different dash symbol combinations. The D2 test has been shown to measure visual scanning accuracy and speed with high consistency and validity. |
| Rey’s Auditory Verbal Learning Test (RAVLT or AVLT) (46) | Created in 1964 this is one of the most popular memory word-learning tests in both clinical research and practice. The test involves the repeated presentation of a 15-word list, ‘List A’, followed by rounds of attempted recall. There is also a ‘List B’, that is a 15-word interference list. Presentation of B is followed by recall of list A again, thus testing delayed memory recall and recognition as well as rate of learning. |
| Boston Naming Test (BNT) (46) | This 60-item naming test was constructed in 1983 as a neuropsychological measure for language dysfunction in dementia diagnosis. The test has line drawings of items including both common and uncommon objects. |
| Brixton Test (46) | This is a test of frontal lobe function, specifically of concept or rule realization. The test is in a booklet consisting of 10 numbered circles (1–10) with one of the circles filled in blue. The participant is asked to flip through the pages and try to identify a pattern allowing them to guess where the next blue circle will be located. |
| California Verbal Learning Test (CVLT) (46) | This episodic memory test was constructed in 1987 as the successor to the AVLT, and in an attempt to further assess learning and retrieval strategies of verbal material in clinical and research settings. The concepts have an ‘everyday’ feel (i.e., recall lists comprised of grocery store items), and there is computerized scoring available. |
| CERAD battery episodic memory test (47) (CERAD II) | The battery is composed of 5 subtests originally designed for cognitive domain-specific dementia assessment: an abbreviated 15-item Boston Naming Test, Animal Naming, MMSE, constructional praxis (copying line drawings), and immediate and delayed recall and recognition components based on a word list task. This battery is supposed to be a better measure of overall dementia severity, as compared to separately assessing abilities related to distinct cognitive domains (47). Given that one latent variable of the test accounts for a majority of the test’s score variance, other supplemental tests are needed (48). |
| Clock Drawing Test (CDT) (49) | The CDT can be used as a screening tool to assess executive function and visuospatial abilities in Alzheimer’s and other forms of dementia. The participant is asked to fill in a pre-drawn circle with the face of a clock that indicates a specific time. Abnormalities in number positioning, sequencing, missing elements, incorrect clock hands, and irrelevant components are used to score the drawings. There has been concern over the subjectivity involved in the scoring of this test and automated interpretation methods have been developed to try to address possible scoring biases. |
| Consortium to Establish a Registry for Alzheimer’s Disease Test (50) (CERAD I) | This neuropsychological battery from 1986 consists of the Verbal Fluency test (specifically, animal naming), the Boston Naming Test, the Mini-Mental State Exam (without the serial 7s prompt), Word List, Learning test, Constructional Praxis test, Word List Recall test, Word List Recognition (10 original words with 10 foils) test, and the Constructional Praxis recall test. This battery was chosen based on cognitive functions impaired in Alzheimer’s disease patients. Although the original intent was to use the test for AD staging, it was concluded that this assessment could also distinguish between individuals with normal cognition and individuals with dementia. |
| Digit Span Test (DST) (51,52) | The DST consists of the Digit Span Forward (DSF) and Digit Span Backward (DSB) tests designed to measure immediate attention. The participant is orally given a sequence of digits at 1 second intervals and is asked to recall, in a certain given order, as many of the given digits as possible. The DSF asks participants to recall the digits in the forward order in which they were presented, and the DSB asks participants to recall the digits in a reverse order. There was another added component, Digit Sequencing (DSS), that requires participants to recite the digits in increasing numerical order. There have been concerns about the appropriateness of using a composite DSF, DSB, and DSS score, as research has shown that the memory processes assessed by each component are not similar enough to use as a combined score (52); therefore, it has been suggested that clinicians (and therefore researchers) use scores from each component separately for comparisons between individuals (51). |
| Digit Symbol Substitution Test (DSST) (53) | This test is identical or very similar to the Symbol Digit Modalities Test (SDMT) published in 1973, and is used to assess attention and processing speed. This cognitive test is given on a single sheet of paper, on which the participant is asked to match symbols to numbers and draw the corresponding symbol beneath its number based on a key at the top of the page. The score given is based on the number of correct number/symbol matches in a given amount of time (typically 90 or 120 seconds). This testing method helps to eliminate effects of language, culture, and educational differences. There is still some debate on what is actually being assessed in terms of specific cognitive domains. |
| Guild Memory (Paragraph) Test (54) | Created in 1968, this test battery includes the immediate recall of 2 paragraphs; immediate recall of 10 matching associates; digit span forward and backward; 10 numbered designs exposed for 5 seconds each, after which the participant is shown the designs again in a different order without the associated number, and is asked to recall the number that was originally presented with the shown design; as well as the retention of the 2 paragraphs and as the 10 matching associates. |
| Hopkins Verbal Learning Test (HVLT) (14,55) | The most revised version of this test was created in 2001 and is a verbal learning and memory test with 6 alternative forms, each comprising 12 nouns and 3 semantic categories with 4 words each. There are 3 consecutive learning periods, and after a 20–25 minute break, a delayed recall (free recall of as many words as are remembered) and a recognition (the 12 original words are mixed in with 12 false-positives, 6 semantically similar words, and 6 semantically unrelated words) trial are conducted (55). |
| Instrumental Activities of Daily Living (IADL) scale (25,56,57) | The IADL consists of performance ability assessments that fall into 8 task domains: use the telephone, doing laundry and dressing, shopping and errand running, transportation, meal preparations, medication management, housekeeping, and the ability to manage finances. This scale can be used as an important health indicator in older adults (25). Studies have shown that IADL scores are positively correlated with social participation, and reflect the ability of patients to practice self-care and disease management (56). This scale is meant to detect functional decline or indicate the need for further assessment (57), as cognition is not directly assessed. |
| Immediate and Delayed Word Recall (58) | These word recall tests are episodic memory tasks. For the immediate word recall list, participants are given a list of 10–20 nouns and are asked to recall as many of the words as possible. For the delayed recall test, there is a 5-minute washout period during which participants are asked questions or given cognition items (e.g., object naming, counting backward), before the participant is asked to recall as many of the previously listed nouns as possible (58). |
| Immediate and Delayed Recall of the East Boston Story (East Boston Memory Test) (59) | This is a test of verbal memory and has been used in many epidemiological studies as a screening tool for Alzheimer’s and other dementia-related memory impairments. The test administrator reads a 3-sentence short story, with each sentence containing 2 “idea units.” Scoring is on a scale of 0–6 based on the number of ideas recalled. During the immediate recall test, the participant is read the story and immediately repeats as much of the story as possible. For the delayed portion, participants can be given distraction tasks for 2–5 minutes, before attempting recall of the story. |
| Mini-Mental Status Exam (MMSE) (31) | Developed in 1975, the Mini-Mental Status Exam (MMSE) is one of the most commonly used tests for quickly assessing and tracking cognitive changes. Elements of the MMSE evaluate patient orientation, memory (immediate and short term), attention, language, fluidity of speech, and calculation abilities. This high-sensitivity test can be performed in approximately 10 minutes; however, it has limitations, especially when impairment is either minimal, or very severe as in some cases of dementia. |
| Mosaic Test (60) | Mosaic tiles of different colors and shapes (squares, right-angle triangles, equilateral triangles and diamonds) are given to the participant (60). The participant is given approximately 20 minutes and is allowed to choose the tile sequence to make any desired pattern that looks pleasing to them. Patterns are then classified according to shape and arrangement as well as color arrangement (61). While there could be concern over the classification of participant-generated patterns, there have been validation studies that show this test can be used diagnostically given the objectivity of interpretations under experimental conditions (60). |
| Rey-Osterreith Complex Figure Test (ROCF) (46) | Developed in 1941 by Rey, with standardization in 1944 by Osterrieth, this test is meant for the assessment of visuospatial constructional ability and visual memory, and has also been used for the assessment of prefrontal lobe executive function 46. Participants are required to copy a complex figure by drawing it, then to redraw what they remember, then to redraw again after a 30-minute delay. |
| Spanish English Verbal Learning Test (SEVLT) (62) | The SEVLT was created in 2001 and is a standard verbal learning test with word lists for use in both Hispanic and non-Hispanic U.S. populations (see Rey’s Auditory Verbal Learning Test). |
| Stroop Color-Word (Interference) Test (Original and Modified) (46) | The most commonly used version of this test of attention was developed in 1988. Cards have names of different colors written on them, and the names themselves are written in different colors. The participant is asked to say the actual color of the printed word instead of simply reading the name of the color, which is the dominant, autonomic tendency. The number of cards correctly named within 120 seconds is counted. Processing speed differences attributed to older age can greatly affect test performance. |
| Trail Making Test A (TMT-A) and Trail Making Test B (TMT-B) (63–65) | The trail making tests were developed in 1958 and consist of 2 parts, TMT-A and TMT-B. The TMT-A is more representative of visuoperceptual ability, while TMT-B is more representative of working memory, inhibition control, and task-switching ability (63). Graphomotor speed and visual scanning are also skills used in both test portions (65), and overall the TMT assesses executive function (64). For Part A, participants must draw a connecting line through numbers from 1–25 in consecutive order. For Part B, the participant is asked to connect both numbers and letters in a progressive sequence alternating back and forth between letters and numbers. Central executive functions is supposed to be measured by taking the difference in time between Part B and Part A (or the ration of Part B to A duration), as this is a reflection of cognitive flexibility, responsiveness, and task-set inhibition (64). |
| Verbal (Word) Fluency (VF) (46,66,67) | This executive function test by Benton and Hamsher assesses both phenomic (using words beginning with specific letters) and semantic (a category-based listing of related objects) fluency (66). There are also animal categorical, and phenomic and semantic language fluency portions of the test, but initial letter fluency is particularly used in which participants come up with words with the same beginning letter, typically F, A and S, for approximately 1 minute (46). Education level and age (specifically for the semantic portion) can affect test performance, thus a combination of tests is recommended when evaluating dementia (67). |
| Victoria Stroop Test (68) | The Victoria modification of the Stroop Test was constructed in 1998 as a 5-minute version of the test used to measure executive function. There are 3 tests that each contain 24 items: colored dots, neutral common words, and colored words that are printed in an ink color that does not match the word. For the first test, participants must name the color of the dot printed on the card. In the second test, participants name the color that the common words are printed in. For the third test, participants are asked to name the ink color the word in printed in, not to simply read the name of the color on the card. The time it takes to complete the trial as well as the number of participant errors is used for analysis. Given the shortened administration time, this test has been deemed particularly useful in geriatric and dementia populations, due to the risk in this population of neuropsychological examination fatigue. |
| Weschler Memory Test (Scale) (WAIS, WAIS-R, WAIS III) (46,69) | WAIS-R is the 1981 revised version of the 1955 Wechsler Adult Intelligence Scale (WAIS) used as a measure of intellectual ability. The most recently revised version is the WAIS-III, and it is the instrument used most widely to assess vocabulary (Vocabulary subtest) and visuoconstructional ability (Block Design subset). The WAIS-III adjusts for age, but there is evidence that education and sociocultural factors may affect test performance more than age (46). Verbal subtests include vocabulary, comprehension, information processing, the ability to find similarities, performing arithmetic, and digit span testing. Performance subsets include picture completion and arrangement, block design, digit-symbol coding, and matrix reasoning (69). There are studies that have suggested that the subtests do not easily categorize into verbal and performance intelligence, but instead are better categorized into Verbal Comprehension, Perceptual Organization, Working Memory and Processing Speed (46). |
| Wisconsin Card Sorting Test (WCST) (46) | This test was created in 1993 as a test of prefrontal function (set-shifting ability). The test uses 4 target cards with different faces: one has a single red triangle, one has 2 green stars, one has 3 yellow crosses, and the last card has 4 blue circles. Participants attempt to sort an additional 128 cards based on trial and error attempts—they are told when incorrect categorizations are made, based on whether sorting should be by color, number, or form. After 10 consecutive correct categorizations the sorting rule is changed without the participant’s knowledge. This test may be too stressful for some participants, as live feedback of errors is given. |
| Word List (17) | This is a subtest of the Nuremberg Age Inventory, in which a list of 12 words are read to the participant. Immediately after the list is given, the participant is told to recall as many words as possible. This is repeated again after a short delay (see ‘Immediate and Delayed Word Recall’ above). |
To summarize the assessment tools used in each of the included studies, we relied on the neurocognitive domains recognized by the DSM-5: complex attention, executive function, language, learning and memory, perceptual-motor function, and social cognition (12). For this review, processing speed is considered a sub-category within complex attention. The domains studied by the assessments in each manuscript were also noted (Table 2).
Table 2.
Study descriptions and assessments
| Study | Study Type | Sample | Cognitive Assessments Used | Cognitive Domains Assessed | Dementia Types Addressed |
|---|---|---|---|---|---|
| Catchlove et al (15) | Systematic review | 10 studies with participants ages 30+, having a variety of comorbidities—2 examined subjects with diabetes mellitus | VF, TMT-A & B, WCST, CVLT, ROCF, IADL scale, MMSE | Executive function Learning and memory Perceptual-motor function *Global cognition | Alzheimer’s dementia, diabetes-related, other (moyamoya disease-related, multiple sclerosis-related) |
| Dai et al (14) | Longitudinal cohort study | 72 adults ages 50–85 both with and without type 2 diabetes mellitus | VF, TMT-A & B, CDT, modified HVLT, DSF, DSB | Attention Executive function Learning and memory | Diabetes-related |
| Degen et al (17) | Nested case-control, from the ILSE longitudinal study | 295 patients with mild cognitive impairment or Alzheimer’s dementia born between 1930 and 1932 | Attentiveness endurance test (D2), DSST, Mosaic Test, WAIS (Finding Similarities), VF, Word List† | Attention Executive function Language Learning and memory | Alzheimer’s dementia, diabetes-related |
| Fukasawa et al (7) | Longitudinal cohort study | 29 patients with Alzheimer’s dementia, and 18 patients with type 2 diabetes mellitus | Clinical diagnosis was used to determine diabetes and Alzheimer’s status | - | Alzheimer’s dementia, diabetes-related |
| Lee et al (68) | Cross-sectional, from wave 5 of the Atherosclerosis Risk in Communities (ARIC) study | 2001 diabetic participants with a mean age of 76 years | DSST, DSB, TMT-A & B, VF (word fluency and phenomic fluency), BNT, CERAD I (episodic memory test), CERAD II (animal naming test), WAIS (logical memory tests parts 1 and 2, delayed word recall) | Attention Executive function Language Learning and memory | Diabetes-related |
| Luchsinger et al (69) | Cohort study, from the Diabetes Prevention Program (DPP) study | 2280 participants aged 63.1 +/− 10.7 with type 2 diabetes mellitus years | DSST, VF (animal fluency and letter fluency), SEVLT | Executive function Language Learning and memory | Diabetes-related |
| Murray et al (24) | Observational extension of randomized trial: the ACCORD study | 684 patients with intensive glycemia, and 644 patients with standard glycemia—all with a mean diabetes duration of 10 years, and mean age of 62 | DSST, RAVLT, modified Stroop Color-Word Test, MMSE | Learning and memory Perceptual-motor function *Global cognition | Diabetes-related |
| Palta et al (19) | Nested case-control, from the Ginko Evaluation of Memory Study | 3069 participants aged 72–96 years | WAIS-R(DSF and DSB), TMT-A & B, Stroop Color-Word Test, BNT, WAIS-R (block design), ROCF, CVLT | Attention Executive function Language Learning and memory Perceptual-motor function | Diabetes-related |
| Rajan et al (20) | Cohort study, from the Chicago Health and Aging Project (CHAP) | 7740 adults aged 65+, with mean age 72.3 years | DSST, immediate and delayed recall of the East Boston Story, MMSE | Executive function Learning and memory *Global cognition | Diabetes-related |
| Sadanand et al (18) | Meta-analysis | Ages 50+ | DSF, DSB, DSST, TMT-B, Stroop Color-Word Test, WCST, Brixton test, RAVLT, CVLT, WAIS, CERAD I and II, East Boston Memory test, Guild Paragraph test, VF | Attention Executive function Language Learning and memory | Diabetes-related |
| Wennberg et al (21,70) | Longitudinal cohort study, from the National Health and Aging Trends Study (NHATS) | 7605 Medicare beneficiaries ages 65+ with diabetes mellitus and/or dementia diagnosis | CDT, CERAD I (episodic memory test) | Executive function Learning and memory | Diabetes-related, Alzheimer’s dementia, other‡ |
Abbreviations: AVLT/RAVLT, Rey’s Auditory Verbal Learning Test; BNT, Boston Naming Test; CDT, Clock Drawing Test; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CVLT, California Verbal Learning Test; D2, Attentiveness Endurance Test; DSB, Digit Span Backwards Test; DSF, Digit Span Forward Test; DSST, Digit Symbol Substitution Test; HVLT, Hopkins Verbal Learning Test; IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental Status Exam; ROCF, Rey-Osterreith Complex Figure Test; SEVLT, Spanish English Verbal Learning Test; TMT-A & B, Trail Making Test A and B; WCST, Wisconsin Card Sorting Test; VF, Verbal Fluency Test; WAIS, Weschler Memory Test.
Global cognition assessed specifically by the MMSE.
These tests were taken from the German version of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders.
Diagnosis of dementia was not specified between subtypes, although participants with diabetes were cognitively assessed.
Results
Complex Attention
Complex attention refers to sustained attention over time, divided attention to 2 tasks at a time, selective attention when distractors are present, and processing speed (13). This domain was assessed in several included studies. Dai et al used the Digit Span Test to measure immediate attention as part of their 2-year study examining cerebral blood flow, cognitive decline, and mobility decline in adults with T2DM (14). A systematic review by Catchlove et al investigated the association between level of cognitive function and cerebrovascular changes observed in different diseases leading to cardiovascular reactivity (15). Since cerebrovascular reactivity was assessed in multiple diseases, not all their reviewed studies focused on diabetic patients; however, 2 reviewed papers specifically assessed patients with diabetes, both using part A of the Trail Making Test (TMT-A) to examine processing speed (15). A case-control study looking at diabetes-related dementia, by Chen et al, also used parts of the TMT-A to asses processing speed, as well as using the dot, color, and word portions of the Victoria Stroop Test (16). Degen et al assessed a cohort that underwent the d2 Attentiveness Endurance Test and the Digit Symbol Test for assessment of attention and processing speed (17). Finally, studies included in the meta-analysis by Sadanand et al reported use of the digit span forward and digit symbol substitution tests to evaluate diabetic patients (18).
Executive Function
Executive function encompasses ability to plan and interpret sequences, make decisions, hold information in working memory, error correction, complex inhibition, and mental flexibility (13). Most included studies used tools that assessed aspects of executive function. Dai et al used the Verbal Fluency Test, Trail Making Tests, and the Clock Drawing Test to assess executive function in T2DM adults between the ages of 50 to 85 (14). Catchlove et al referenced 2 studies that assessed this domain: one used the Trail Making Test B (TMT-B) and verbal fluency tests to assess executive function in diabetic patients versus healthy controls, and the other compared diabetic patients with and without hypertension, and used the Wisconsin Card Sorting Test (15). In comparison, 3 of 10 studies reviewed by Catchlove et al assessed participants with the AD form of dementia, and while each used a variety of tests, the only test used in all 3 was the MMSE (15). Degen et al studied a cohort over a 14-year period to investigate effect of diabetes and its duration on neuropsychological functioning (17). They conducted 3 waves of cognitive data collection, using the Mosaic Test and the Finding Similarities portion of the Weschler Intelligence Test Battery to assess reasoning and abstract thinking (17). The Ginkgo Evaluation of Memory study assessing incident dementia in older diabetic adults used the Wechsler Adult Intelligence Scale-R Digit Span Backward, TMT-B, Stroop Color-Word Test, and interference condition tests for executive function (19). Studies examined in a meta-analysis by Sadanand et al incorporated a similar battery of tests used to diagnose AD and other forms of dementia: the TMT-B, Stroop Test, Wisconsin Card Sorting Test, Brixton Test, and Digit Span Backward Test (18). Chen et al used the dot, color, and word portions of the Victoria Stroop Test to look at mental control in T2DM patients, examining results for association with deep gray-matter abnormalities in the brain (16). Rajan et al studied participants with incident and preexisting diabetes enrolled in the Chicago Health and Aging Project, in which executive cognition was assessed with the Symbol Digit Modalities Test (20), more commonly known as the Digit Symbol Substitution Test. The participants in the Wennberg et al longitudinal cohort study were administered the Clock Drawing Test as an assessment of both executive function and visuospatial ability (21). This study looked at patients with diabetes or dementia diagnoses; the diabetes-specific subtype of dementia was not separately assessed.
Language
The language domains refer to fluency that is both semantic (words) and phonemic (sounds), use of grammar and proper word choice, and language comprehension (22). Degen et al’s cohort was assessed using the verbal fluency portion of the 1993 “Leistungsprüfsystem” (17). This test is almost identical to the verbal fluency test developed by Benton and Hammer in 1989, which is seen in papers reviewed by Catchlove et al (15). The verbal fluency test was also chosen by Sadanand et al to assess categorical and phonemic language fluency; all papers in their review used this test, per their study criteria (18). Abner et al used a categorical fluency test with animals to assess infarct neuropathology in diabetic patients with or without AD, at both 2 and 6 years prior to death (23).
Learning and Memory
This domain refers both to immediate memory, such as ability to repeat words or phrases, as well as recent memory, such as ability to encode newly obtained information (13). Chen et al used parts of the Rey-Osterrieth complex figure test (ROCF) to assess short-term memory (16). A revised version of the Hopkins Verbal Learning Test that includes elements of total recall, delayed recall, and retention, was used by Dai et al (14). Degen et al referred to the Word List of the Nuremberg Age Inventory as a test of verbal memory (17). Murray et al referred to the Auditory Verbal Learning Test (AVLT) and the modified Stroop Color-Word Test (24). In Rajan et al, participants were assessed for immediate and delayed recall of the East Boston Story (20). The Sadanand et al’s meta-analysis noted that AVLTs were the most commonly used test of episodic and logical memory (18). More specifically, their meta-analysis included papers using the Rey’s AVLT, California VLT, Weschler Memory Scale, Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test, East Boston Memory Test and the Guild Paragraph Test for this domain (18). Wennberg et al used data from the National Health and Aging Trends database, and their participants underwent the episodic memory test from the CERAD battery (21).
Perceptual-Motor Function
This domain includes visual perception such as facial recognition, hand-eye coordination, purposeful movement based on perception, the ability to imitate learned movements, awareness, and recognition (13). Chung et al, as identified in the review by Catchlove et al, used the ROCF to assess visual-spatial ability, in a prospective study looking at the relationship between inflammation, vasoregulation, and cognitive decline in T2DM patients (25). Chen et al also used the ROCF, as well as the Victoria Stroop Test, to assess spatial ability and its relation to structural magnetic resonance imaging (MRI) brain changes (16). The Digit Symbol Substitution Test, components of which assess psychomotor performance, was used to measure psychomotor function and speed in the ACCORDION MIND study conducted by Murray et al (24), which focused on cognitive testing for vascular cognitive impairment at base-line, 20 months, and 40 months, with repeat brain MRIs taken for visual comparison (24). Wennberg et al used the Clock Drawing Test to assess both executive function and visuospatial ability (21).
Social Cognition
Social cognition refers to the recognition of a range of positive and negative emotions; theory of mind, or the ability to recognize that self and others have intentions, desires, beliefs, and emotions that are driving factors behind actions and interactions (26); and the overall ability to assess another’s mental state (13). Studies that assessed social cognition include Chung et al, who used the Instrumental Activities of Daily Living scale, which is a health indicator for older adults (25). The test was chosen because behavioral changes can be a hallmark of decline in this area of cognition. In the studies reviewed, social cognition was the least frequently assessed domain, corresponding with the least number of domain-specific testing modalities.
Discussion
According to the Centers for Disease Control, approximately 10%, of Americans, have diabetes (27), resulting in a 26% increase in expenditures on diabetes from 2012 to 2017 (5). Roughly 2.3 times as much is spent on healthcare for diabetic patients as for patients without diabetes, and approximately 1 in every 4 dollars spent on healthcare is a direct consequence of diabetes (5).
As individuals age, nonpathologic changes in cognition occur, and separating pathology from cognitive aging is a delicate process. It is widely accepted that cognition exists on a continuum; MCI is often seen as separating normal cognitive aging from early onset of dementia (28). While MCI does not always progress to dementia, it has been suggested that patients with MCI have increased risk of developing dementia later in life. Annually, approximately 8% to 15% of MCI patients progress to some form of dementia (28). Determining which cognitive domains are most important for initial evaluation of etiology is difficult: patients developing issues with cognition, regardless of the cause, often have similar presenting symptoms/difficulties that may involve more than 1 neuropathology (29). Thus, screening requires testing of multiple cognitive domains to reliably detect early MCI (30), which may be present in the early onset of dementia. Therefore, tests such as the MMSE (31), while reflecting global cognition, are suboptimal stand-alone tests for MCI; the MMSE is just 1 example of an assessment tool that focuses on specific domains that can be tested in a variety of ways. More extensive testing is needed to properly identify the pathology (32). Adding to difficulties in assessing MCI in diabetic patients, in particular, is the need to differentiate between diabetes-related dementia, AD, and vascular dementia. In fact, genetic studies reveal multiple layers of complexity when comparing different dementia forms (33). Understanding diabetes-related dementia from a physiological point of view, therefore, is an area of active research (34).
Slow, structural brain changes correlating with diabetes-associated cognitive decline have been identified on MRI (35). Evidence suggests that diabetes-related dementia is more related to executive functioning than memory, although data relating physiological changes to specific affected cognitive areas are less clear (36). Diabetes-related dementia is clinically diagnosed after excluding all other forms of dementia, and postmortem studies have shown unique brain changes in chronic diabetes that are distinct from neuritic plaques and neurofibrillary tangles characteristic of AD (23). Studies have also compared diabetes-related dementia to the vascular dementia subtype as opposed to AD for individuals lacking an ApoE4-related predisposition to AD (9). A clinical diagnosis of Alzheimer’s dementia requires signs of impairment in at least 2 of the following areas: ability to acquire and retain new information, ability to reason and handle complex tasks (having good judgement), visuospatial skills, language skills, and personality/behavior (37). The most common clinical tests used to evaluate possible dementia of any subtype are MMSE, Mini-Cog, Cognitive Abilities Screening Instrument, Montreal Cognitive Assessment, and Clinical Dementia Rating (38). The Montreal Cognitive Assessment is recommended over MMSE for diagnosing AD, as it seems to be more sensitive to cognitive impairment distinctions, specifically impairment of executive and language domains (39). This is particularly useful when differentiating between vascular dementia and AD (37). None of the research articles examined in this review, used the Mini-Cog or the Cognitive Abilities Screening Instrument, demonstrating a possible disconnect between clinical and field data collection.
Although the DSM-5 defines specific cognitive domains used for clinical diagnosis, there were domain definition inconsistencies within and between articles in this review. Cognitive domains appeared to be defined subjectively, based on past research methods, or as conglomerations of 2 or more domains. Selection of the most appropriate cognitive testing instruments is challenging, especially since pathology-specific dementia scores may not always be comparable (40). In general, subjects in the reviewed studies presented with varying composite cognitive scores and levels of impairment, and were assessed using a variety of testing methods. A majority of the examined papers mentioned using at least 2 different cognitive assessment methods, thereby assessing at least 2 different cognitive domains; however, many different combinations of tests were used, and many different combinations of cognitive domains were assessed. We also observed that the same test was used by different studies to assess different cognitive domains, or parts of the same test were used to assess different or multiple cognitive domains.
Assessing cognitive function over time necessitates use of more than 1 cognitive measure to improve accuracy (30), so standardized use of multiple diabetes-specific assessments has the potential to improve the value of cognitive testing results. Taking into account the study population and its disease progression is important to the standardization process when specifically targeting diabetes-specific cognitive changes. The cohort used by Degen et al, for example, was comprised of patients meeting criteria for MCI or Alzheimer’s dementia; however, they excluded participants with mild cognitive disorder and vascular dementia (17). It could be argued that these findings are not applicable to progressive, T2DM-related cognitive decline. Similar incongruencies in the literature in terms of cognitive testing methods, reporting results, and sample selection can unfortunately result in difficulty synthesizing data when diabetes-specific cognitive data is needed. While this phenomenon was observed in some reviews we examined that aimed at the assessment of affected cognitive domains in T2DM (18), there were some limitations. Although this review is meant to be a representative look at the most common testing methods used for identifying a specific subtype of dementia and quantifying cognitive decline in diabetic patients, the initial database search yielded over 1000 articles. It is thus possible that diabetes-specific testing methods may have been missed in this large volume of academic papers. Articles were also limited to 2015 and later, which may exclude studies with more traditional test batteries. Articles not in English were excluded, which could miss other popular cognitive evaluation methods used in other areas of the world. A strength, however, is the variety of study designs assessed.
Cognitive assessments, regardless of method, are subject to biases related to socioeconomic factors such as age, culture, and level of education/health literacy. The Boston Naming Test, for example, has been criticized for having inadequate norms that lead to misclassification and bias against less-educated participants (41). According to the DSM-5, standard neuropsychological testing should take into account age, education, and cultural background when evaluating for neurocognitive diseases (13). In most of studies reviewed, however, there was no stratification by education and/or cultural background in assessing cognitive results. Consideration of cultural and socioeconomic differences not only improves the effectiveness of diabetic interventions, it also improves the ability for patients to engage in more personalized and suitable routines.
Cognitive ability, physical ability, and social participation are equally important for adaptation to self-care methods, especially those that are more effective when client-driven (4). An outpatient study conducted in Slovenia showed that independent diabetes self-management behaviors are influenced by specific cognitive abilities related to planning and problem solving (42). Santos et al found that diabetic patients with cognitive impairment, especially in learning, memory, and executive function domains, were significantly impaired in all self–care tasks (43). However, over 10 cognitive assessment tools were used in the study, and the subtype of dementia (AD vs others) was unmentioned. Those with T2DM and cognitive decline often struggle with proper avoidance of hypoglycemic episodes. The ability of aging diabetic patients to understand and practice effective self-care routines is also important for them to maintain a sense of independence in spite of complex, long-term diabetic sequelae of both central and peripheral vascular diseases (43). Given these concerns, assessment of cognitive abilities of diabetic patients can be seen as a vital component of a personalized treatment regimen, with goals of improved self-management and quality of life (44). To gain a clear understanding of cognitive impairment and decline in diabetic patients and the impact of impairment on self-care and quality of life, it is necessary to properly identify, define, and apply cognitive assessment methods that will target distinctive diabetes decline patterns. This would allow for diabetes-specific recommendations that may differ from those for other forms of dementia, such as AD.
In conclusion, different cognitive assessment methods are used both in clinical and research settings to assess diabetic patients exhibiting MCI or general cognitive decline. It is difficult to distinguish the diabetes-related dementia subtype from other, better-studied subtypes of dementia. While certain cognitive tests were designed to assess specific cognitive domains, we observed that different research groups apply the same tools to assess different cognitive domains, as there is a functional overlap among cognitive tasks. The complexity and multifactorial aspects of cognitive decline make it difficult to decide which cognitive tests sufficiently assess each cognitive domain. As stated by Biessels et al, researchers are faced with the challenge of pinpointing the development of dementia within a spectrum of diabetes-related disease processes that are separate from AD, highlighting the need for agreement between experimental and clinical scientists (42). Multiple tests are required for thorough cognitive assessment.
Currently, specific testing methods that best represent the cognitive domains most affected in diabetes-related dementia cannot be recommended based on this literature review alone because of the nebulous nature of the question posed. The search for a sense of uniformity throughout cognitive assessments depends on what individual researchers are examining on a pathologic level and what they are trying to learn about particular cognitive domains. This literature review highlights the need for further research and a deeper assessment of which cognitive domains should be monitored and targeted in diabetic patients, and which tests most effectively characterize diabetes-specific cognitive changes. Streamlining evaluation of the DM-related dementia type will facilitate earlier application of individualized interventions improving self-care and quality of life for the growing, aging population of diabetic patients.
Acknowledgments
We would like to thank Julie Trumble and Tara Atkins for technical assistance in performing the literature search.
Financial Disclosure:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interest: None reported.
Level of Clinical Evidence: 2
References
- 1.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–491. [DOI] [PubMed] [Google Scholar]
- 2.Ganmore I, Beeri MS. The chicken or the egg? Does glycaemic control predict cognitive function or the other way around? Diabetologia 2018;61:1913–1917. [DOI] [PubMed] [Google Scholar]
- 3.Natovich R, Kushnir T, Harman-Boehm I, Margalit D, Siev-Ner I, Tsalichin D, Volkov I, Giveon S, Rubin-Asher D, Cukierman-Yaffe T. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care 2016;39:1202–1207. [DOI] [PubMed] [Google Scholar]
- 4.Markle-Reid M, Ploeg J, Fraser KD, Fisher KA, Bartholomew A, Griffith LE, Miklavcic J, Gafni A, Thabane L, Upshur R. Community program improves quality of life and self-management in older adults with diabetes mellitus and comorbidity. J Am Geriatr Soc 2018;66:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelbaum PS. Consent in impaired populations. Curr Neurol Neurosci Rep 2010;10:367–373. [DOI] [PubMed] [Google Scholar]
- 7.Fukasawa R, Hanyu H, Shimizu S, Kanetaka H, Sakurai H, Ishii K. Identification of diabetes-related dementia: longitudinal perfusion SPECT and amyloid PET studies. J Neurol Sci 2015;349:45–51. [DOI] [PubMed] [Google Scholar]
- 8.Hanyu H Diabetes-related dementia. Adv Exp Med Biol 2019;1128:147–160. [DOI] [PubMed] [Google Scholar]
- 9.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 2002;51:1256–1262. [DOI] [PubMed] [Google Scholar]
- 10.Pruzin JJ, Schneider JA, Capuano AW, Leurgans SE, Barnes LL, Ahima RS, Arnold SE, Bennett DA, Arvanitakis Z. Diabetes, hemoglobin A1C, and regional Alzheimer disease and infarct pathology. Alzheimer Dis Assoc Disord 2017;31:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;159:601–612. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association, Arlington, VA, 2013. [Google Scholar]
- 13.American Psychiatric Association. Neurocognitive disorders. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 5th ed., American Psychiatric Association, Washington, D.C., 2013. [Google Scholar]
- 14.Dai W, Duan W, Alfaro FJ, Gavrieli A, Kourtelidis F, Novak V. The resting perfusion pattern associates with functional decline in type 2 diabetes. Neurobiol Aging 2017;60:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catchlove SJ, Pipingas A, Hughes ME, Macpherson H. Magnetic resonance imaging for assessment of cerebrovascular reactivity and its relationship to cognition: a systematic review. BMC Neurosci 2018;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Zhang J, Liu X, Wang X, Xu X, Li H, Cao B, Yang Y, Lu J, Chen Z. Abnormal subcortical nuclei shapes in patients with type 2 diabetes mellitus. Eur Radiol 2017;27:4247–4256. [DOI] [PubMed] [Google Scholar]
- 17.Degen C, Toro P, Schönknecht P, Sattler C, Schröder J. Diabetes mellitus type II and cognitive capacity in healthy aging, mild cognitive impairment and Alzheimer’s disease. Psychiatry Res 2016;240:42–46. [DOI] [PubMed] [Google Scholar]
- 18.Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev 2016;32:132–142. [DOI] [PubMed] [Google Scholar]
- 19.Palta P, Carlson MC, Crum RM, Colantuoni E, Sharrett AR, Yasar S, Nahin RL, DeKosky ST, Snitz B, Lopez O, Williamson JD, Furberg CD, Rapp SR, Golden SH. Diabetes and cognitive decline in older adults: the Ginkgo evaluation of memory study. J Gerontol A Biol Sci Med Sci 2017;73:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajan KB, Arvanitakis Z, Lynch EB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Bianco AC, Evans DA. Cognitive decline following incident and preexisting diabetes mellitus in a population sample. Neurology 2016;87:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennberg AMV, Hagen CE, Gottesman RF, Zipunnikov V, Kaufmann CN, Albert MS, Rebok GW, Kasper JD, Spira AP. Longitudinal association between diabetes and cognitive decline: the national health and aging trends study. Arch Gerontol Geriatr 2017;72:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: Classification and criteria changes. World Psychiatry 2013;12:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, Cairns NJ, Yu L, Dodge HH, Xiong C, Masaki K, Tyas SL, Bennett DA, Schneider JA, Arvanitakis Z. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement 2016;12:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray AM, Hsu FC, Williamson JD, Bryan RN, Gerstein HC, Sullivan MD, Miller ME, Leng I, Lovato LL, Launer LJ. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017;60:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung CC, Pimentel D, Jor’dan AJ, Hao Y, Milberg W, Novak V. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology 2015;85:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astington JW, Dack LA. Theory of mind. In: Haith MM, Benson JB, eds. Encyclopedia of Infant and Early Childhood Development, Academic Press, 2008343–356. [Google Scholar]
- 27.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: 2020. [Google Scholar]
- 28.Chehrehnegar N, Nejati V, Shati M, Rashedi V, Lotfi M, Adelirad F, Foroughan M. Early detection of cognitive disturbances in mild cognitive impairment: a systematic review of observational studies. Psychogeriatrics 2020;20:212–228. [DOI] [PubMed] [Google Scholar]
- 29.Arevalo-Rodriguez I, Smailagic N, Roqué IFM, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 2015;2015:Cd010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao K, Di Narzo AF, Ho L, Luo W, Li S, Chen R, Li T, Dubner L, Pasinetti GM. Shared genetic etiology underlying Alzheimer’s disease and type 2 diabetes. Mol Aspects Med 2015;43–44:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr M The validity of the Mosaic test. Amer J Orthopsychiat 2010;9:232–236. [Google Scholar]
- 32.Sutherland GT, Lim J, Srikanth V, Bruce DG. Epidemiological approaches to understanding the link between type 2 diabetes and dementia. J Alzheimers Dis 2017;59:393–403. [DOI] [PubMed] [Google Scholar]
- 33.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol 2015;14:329–340. [DOI] [PubMed] [Google Scholar]
- 34.Sano M, Zhu CW, Grossman H, Schimming C. Longitudinal cognitive profiles in diabetes: results from the National Alzheimer’s Coordinating Center’s Uniform Data. J Am Geriatr Soc 2017;65:2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheen YJ, Sheu WH. Association between hypoglycemia and dementia in patients with type 2 diabetes. Diabetes Res Clin Pract 2016;116:279–287. [DOI] [PubMed] [Google Scholar]
- 36.Shaik S, Anoop V. Differentiating the dementias: a neurological approach. Prog Neurol Psychiatry 2012;16:11–18. [Google Scholar]
- 37.Wolk DA, Dickerson BC. Clinical features and diagnosis of Alzheimer disease. In: DeKosky ST, Wilterdink JL, eds. UpToDate, Waltham, MA: UpToDate, 2020. [Google Scholar]
- 38.Chen YX, Liu ZR, Yu Y, Yao ES, Liu XH, Liu L. Effect of recurrent severe hypoglycemia on cognitive performance in adult patients with diabetes: a meta-analysis. J Huazhong Univ Sci Technol Med Sci 2017;37:642–648. [DOI] [PubMed] [Google Scholar]
- 39.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke 2012;43:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross TP, Lichtenberg PA. Expanded normative data for the Boston Naming Test for use with urban, elderly medical patients. Clin Neuropsychol 1998;12:475–481. [DOI] [PubMed] [Google Scholar]
- 41.Munshi MN. Cognitive dysfunction in older adults with diabetes: what a clinician needs to know. Diabetes Care 2017;40:461–467. [DOI] [PubMed] [Google Scholar]
- 42.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018;14:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos T, Lovell J, Shiell K, Johnson M, Ibrahim JE. The impact of cognitive impairment in dementia on self-care domains in diabetes: a systematic search and narrative review. Diabetes Metab Res Rev 2018;34:e3013. [DOI] [PubMed] [Google Scholar]
- 44.Catalano FL. A preliminary report on a new memory scale. Percept Mot Skills 1968;27:277–278. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Bowers D, Woods AJ. Test of attention. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology, Springer International Publishing, 20181039–1043. [Google Scholar]
- 46.Hall J, O’Carroll RE, Frith CD. Neuropsychology. In: Johnstone EC, Owens DC, Lawrie SM, McIntosh AM, Sharpe M, eds. Companion to Psychiatric Studies, 8th ed., pp 121–140, Churchill Livingstone, 2010. [Google Scholar]
- 47.Mast BT, Gerstenecker A. Screening instruments and brief batteries for dementia. In: Licthtenberg PA, ed. Handbook of Assessment in Clinical Gerontology, Academic Press, 2010503–530. [Google Scholar]
- 48.Strauss M, Fritsch T. Factor structure of the CERAD neuropsychological battery. J Int Neuropsychol Soc 2004;10:559–565. [DOI] [PubMed] [Google Scholar]
- 49.Harbi Z, Hicks Y, Setchi R. Clock Drawing Test interpretation system. Proc Comput Sci 2017;112:1641–1650. [Google Scholar]
- 50.Fillenbaum GG, Beekly D, Edland SD, Hughes JP, Heyman A, van Belle G. Consortium to establish a registry for Alzheimer’s disease: development, database structure, and selected findings. Top Health Inf Manage 1997;18:47–58. [PubMed] [Google Scholar]
- 51.Gignac GE, Reynolds MR, Kovacs K. Digit span subscale scores may be insufficiently reliable for clinical interpretation: distinguishing between stratified coefficient alpha and omega hierarchical. Assessment 2019;26:1554–1563. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds CR. Forward and backward memory span should not be combined for clinical analysis. Arch Clin Neuropsychol 1997;12:29–40. [PubMed] [Google Scholar]
- 53.Jaeger J Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 2018;38:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belkonen S Hopkins verbal learning test. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology, New York: Springer, 20111264–1265. [Google Scholar]
- 55.Tomioka K, Kurumatani N, Saeki K. The differential effects of type and frequency of social participation on IADL declines of older people. PLoS One 2018;13:e0207426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graf C The Lawton instrumental activities of daily living scale. Am J Nurs 2008;108:52–62. quiz 53–62. [DOI] [PubMed] [Google Scholar]
- 57.Ryan JM, Gwenith GF, Hassan H, Jessica DF, Rogers W, David RW. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992–2016. In: Survey Research Center, University of Michigan, ed. Ann Arbor, MI2019. [Google Scholar]
- 58.Gfeller JD, Horn GJ. The East Boston Memory Test: a clinical screening measure for memory impairment in the elderly. J Clin Psychol 1996;52:191–196. [DOI] [PubMed] [Google Scholar]
- 59.Lancu I, Olmer A. The minimental state examination—an up-to-date review. Harefuah 2006;145:687–690. 701. [PubMed] [Google Scholar]
- 60.Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth complex figure test. Nat Protoc 2006;1:892–899. [DOI] [PubMed] [Google Scholar]
- 61.González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc 2001;7:544–555. [DOI] [PubMed] [Google Scholar]
- 62.McMorris T History of research into the acute exercise−cognition interaction: a cognitive psychology approach. In: McMorris T, ed. Cognition Interaction, Academic Press, 20161–28. [Google Scholar]
- 63.Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, Torrents-Rodas D, Garre-Olmo J. The Trail Making Test: association with other neuropsychological measures and normative values for adults aged 55 years and older from a Spanish-speaking population-based sample. Assessment 2015;24. [DOI] [PubMed] [Google Scholar]
- 64.Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Azeredo Passos VM, Giatti L, Bensenor I, Tiemeier H, Ikram MA, de Figueiredo RC, Chor D, Schmidt MI, Barreto SM. Education plays a greater role than age in cognitive test performance among participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). BMC Neurol 2015;15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bayard S, Erkes J, Moroni C. Victoria Stroop Test: normative data in a sample group of older people and the study of their clinical applications in the assessment of inhibition in Alzheimer’s disease. Arch Clin Neuropsychol 2011;26:653–661. [DOI] [PubMed] [Google Scholar]
- 67.Colom R Intelligence assessment. In: Spielberger CD, ed. Encyclopedia of Applied Psychology, Elsevier, 2004307–314. [Google Scholar]
- 68.Lee AK, Rawlings AM, Lee CJ, Gross AL, Huang ES, Sharrett AR, Coresh J, Selvin E. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia 2018;61:1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luchsinger JA, Ma Y, Christophi CA, Florez H, Golden SH, Hazuda H, Crandall J, Venditti E, Watson K, Jeffries S, Manly JJ, Pi-Sunyer FX. Metformin, lifestyle intervention, and cognition in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2017;40:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA 2019;322:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]


