Abstract
Anti-spike monoclonal antibody therapy may be effective for pregnant women with coronavirus disease 2019 (COVID-19).
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is associated with increased risk of hospitalization and death, particularly in certain patients with underlying conditions, including pregnancy.1–6 Anti-spike monoclonal antibodies have received emergency use authorizations from the U.S. Food and Drug Administration for the outpatient treatment of mild-to-moderate COVID-19 in patients at increased risk of severe outcomes.7–10 On May 14, 2021, the U.S. Food and Drug Administration expanded the emergency use authorization to include pregnancy as a qualifying condition for monoclonal antibodies. Guidelines from the National Institutes of Health, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine suggest that pregnant women should be considered candidates for monoclonal antibody therapy.11–13 However, the adoption of these recommendations has been limited by the lack of clinical data on its use in pregnant women.14,15 Here we present a case series of 51 pregnant women who received anti-spike monoclonal antibody therapy for mild-to-moderate COVID-19.
METHODS
This was a retrospective cohort study of pregnant patients who received monoclonal antibody therapy at a single institution from November 6, 2020, through October 30, 2021, who were identified from an institutional COVID-19 treatment registry. Patients diagnosed with mild-to-moderate COVID-19 at our institution were automatically evaluated for eligibility and contacted for education and consent and enrolled in a treatment registry as previously described.16 Patients were considered to have mild-to-moderate COVID-19 if they were symptomatic, had an oxygen saturation higher than 94%, and otherwise did not need admission for COVID-19, for example, due to hemodynamic instability. After approval by the Mayo Clinic Institutional Review Board, the monoclonal antibody treatment registry was queried for pregnant patients,16 and 86 were identified. The medical records of all patients were reviewed, and the data are presented using descriptive methods (Fig. 1).
Fig. 1. Patient inclusion and characteristics.
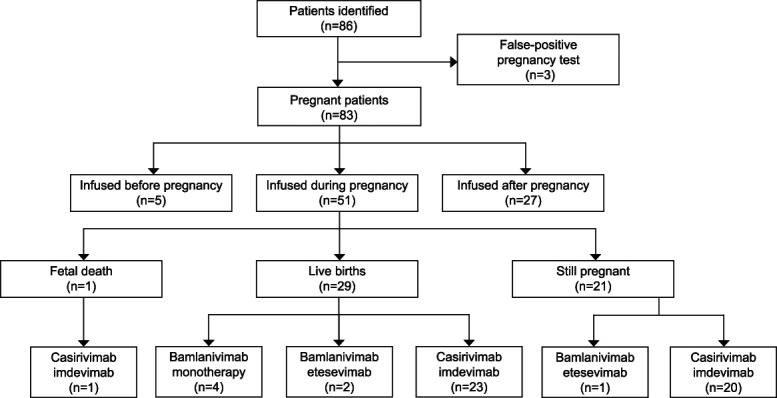
Thilagar. COVID-19 Monoclonal Antibodies in Pregnancy. Obstet Gynecol 2022.
RESULTS
After excluding patients who had false-positive pregnancy test results and those who received monoclonal antibodies after delivery, 51 pregnant women who received monoclonal antibodies for mild-to-moderate COVID-19 during pregnancy were included (Fig. 1). Demographic characteristics of the cohort are displayed in Table 1.
Table 1.
Characteristics of Patients at Time of Monoclonal Antibody Infusion (N=51)

Monoclonal antibody treatment was performed in an outpatient area for individuals who had tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and patients were monitored for 1 hour afterward for immediate adverse effects, with none observed in our cohort. Patients were then evaluated by in the obstetrics department within the next week for any further complications. During the 28-day period after infusion, 10 patients sought care in the emergency department, but only one was related to COVID-19 (dyspnea), with the remainder being related to the pregnancy or underlying conditions. No patients required any additional COVID-19–directed therapy. Four patients were admitted to the hospital while pregnant for reasons unrelated to COVID-19 or monoclonal antibody infusion. At the time of manuscript submission, no patients had pregnancy complications or fetal distress, 21 patients were still pregnant, and 29 had delivered healthy neonates (mean birth weight 3,110 g, mean gestational age 38.6 weeks) now aged 5–310 days (mean age 86 days).
DISCUSSION
This retrospective cohort study of 51 pregnant women who received monoclonal antibody therapy for mild-to-moderate COVID-19 demonstrates no immediate adverse effects, and no patient had COVID-19 progression. Despite these reassuring findings, we suggest continued research with larger populations of diverse patients, longer maternal and infant follow-up, and the examination of efficacy of monoclonal antibodies as SARS-CoV-2 variants of concern emerge.
Footnotes
Financial Disclosure Ryan T. Hurt is a consultant for Nestle Nutrition. Raymund R. Razonable is principal investigator of clinical trials on COVID-19 treatment funded by Gilead (remdesivir), Regeneron (sarilumab, casirivimab-imdevimab), Roche (tocilizumab), and research on Monoclonal Antibody Therapy funded by the Mayo Clinic. They have been on the data safety monitoring board for a Novartis clinical trial. They disclosed that bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab, and sotrovimab are all investigational products authorized for emergency use EUA by the U.S. FDA. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print January 13, 2022.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C590.
REFERENCES
- 1.O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc 2021;10:e019259. doi: 10.1161/JAHA.120.019259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 2021;76:428–55. doi: 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 3.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badr DA, Mattern J, Carlin A, Cordier AG, Maillart E, El Hachem L, et al. Are clinical outcomes worse for pregnant women at >/=20 weeks' gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol 2020;223:764–8. doi: 10.1016/j.ajog.2020.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 2021;225:77.e1–14. doi: 10.1016/j.ajog.2020.12.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with covid-19. N Engl J Med 2021;384:229–37. doi: 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325:632–44. doi: 10.1001/jama.2021.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med 2021;384:238–51. doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021;385:1941–50. doi: 10.1056/NEJMoa2107934 [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health. COVID-19 treatment guidelines: anti-SARS-CoV-2 monoclonal antibodies. 2021. Accessed December 20, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ [Google Scholar]
- 12.American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. Accessed November 27, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics [Google Scholar]
- 13.Society for Maternal-Fetal Medicine. COVID-19 and pregnancy: what maternal-fetal medicine subspecialists need to know. Accessed December 20, 2021. https://s3.amazonaws.com/cdn.smfm.org/media/3238/PDF.pdf [Google Scholar]
- 14.Hirshberg JS, Cooke E, Oakes MC, Odibo AO, Raghuraman N, Kelly JC. Monoclonal antibody treatment of symptomatic COVID-19 in pregnancy: initial report. Am J Obstet Gynecol 2021;225:688–9. doi: 10.1016/j.ajog.2021.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer C, VanHise K, Caskey R, Naqvi M, Burwick RM. Monoclonal antibodies casirivimab and imdevimab in pregnancy for coronavirus disease 2019 (COVID-19). Obstet Gynecol 2021;138:937–9. doi: 10.1097/AOG.0000000000004603 [DOI] [PubMed] [Google Scholar]
- 16.Razonable RR, Aloia NCE, Anderson RJ, Anil G, Arndt LL, Ausman SE, et al. A framework for outpatient infusion of anti-spike monoclonal antibodies to high-risk patients with mild to moderate coronavirus disease-19: the Mayo Clinic model. Mayo Clinic Proc 2021;96:1250–61.doi: 10.1016/j.mayocp.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]


