Abstract
Huang, Z-H, Ma, CZ-H, Wang, L-K, Wang, X-Y, Fu, S-N, and Zheng, Y-P. Real-time visual biofeedback via wearable ultrasound imaging can enhance the muscle contraction training outcome of young adults. J Strength Cond Res 36(4): 941–947, 2022—Real-time ultrasound imaging (RUSI) can serve as visual biofeedback to train deep muscle contraction in clinical rehabilitative settings. However, its effectiveness in resistance training in sports/fitness fields remains unexplored. This article introduced a newly developed wearable RUSI system that provided visual biofeedback of muscle thickening and movement and reported its effectiveness in improving the training outcomes of muscle thickness change (%) during dynamic contraction. Twenty-five healthy young men participated and performed pec fly exercise both with and without RUSI biofeedback. Statistical analysis was conducted to examine the reliability of the measurements and the immediate effects of (a) RUSI biofeedback of muscle contraction and (b) training intensity (50 vs. 80% of 1-repetition maximum [1RM]) on the pectoralis major (PMaj) thickness change measured by ultrasound images. In addition to significantly high inter-contraction reliability (ICC3,1 > 0.97), we observed significantly increased PMaj thickness change for both training intensities upon receiving biofeedback in subjects, compared with without biofeedback (p < 0.001). We also observed significantly larger PMaj thickness change at 80% of 1RM compared with 50% of 1RM (p = 0.023). The provision of visual biofeedback using RUSI significantly enlarged the magnitude of PMaj thickness change during pec fly exercises, potentially indicating that RUSI biofeedback could improve the ability of targeted muscle contraction of PMaj in healthy young adults. To our knowledge, this study has pioneered in applying RUSI as a form of biofeedback during weight training and observed positive effectiveness. Future iterations of the technique will benefit more subject groups, such as athletes and patients with neuromuscular disorders.
Key Words: ultrasound-imaging biofeedback, neuromuscular training, pectoralis major, resistance training, ultrasound biofeedback, fitness training
Introduction
Muscle training has become very common in daily life. However, without appropriate external monitoring during training, instructors (e.g., therapists, coaches, and trainers) and exercisers (e.g., patients, athletes, and trainees) tend to be unaware of which muscle is contracting or to what degree the targeted muscle contraction (or the change of muscle thickness) has reached (10,27). This lack of awareness leads to difficulties in improving proprioception (23) and has been an issue in the practice of rehabilitation therapy, sport training, and physical fitness (25), which needs to be addressed.
Various approaches have been applied to improve the muscle training efficiency. Verbal instruction is commonly used because it is easily accessible in practice (1–3,17,24,25). However, such approach is experience-based and rather subjective. Subtle changes in individualized instruction, such as in context (choice of words) and/or vocal characteristics (e.g., tone of voice, pitch, speaking rate, etc), might mislead exercisers, leading to unintended effects on the activities of the involved muscles (18,25). Previous studies have reported unsatisfactory neuromuscular training outcomes in different populations, ranging from no effectiveness in recreationally trained exercisers (3,25) to less than 50% of effectiveness in patients (2). Compared with verbal instructions, the utilization of external monitoring devices to assist muscle training appeared to have more promising application potential. The measurement modalities of electromyography (EMG), accelerometers, goniometers, linear position transducers, and infrared motion-tracking cameras have been used to explore muscle contraction externally; however, they cannot provide concrete visualization of the actual muscular condition inside the human body.
Meanwhile, real-time ultrasound imaging (RUSI) has the advantages of noninvasiveness, objective assessment, direct and real-time visualization of soft tissue morphologies, elimination of cross-talk from adjacent muscles, and being free of motion artifacts during contraction compared with the EMG technique. The capability of therapeutic application of RUSI during muscle contraction has also offered it the potential to serve as an alternative biofeedback technique. Approximately 81% of physiotherapists reported that they benefit from RUSI biofeedback in a recent survey-based study (22). However, the previous scientific literature evaluating its effectiveness is still scarce. Up to now, RUSI biofeedback has only been used for neuromuscular reeducation of muscles that are difficult to contract voluntarily in therapeutic exercises (4,8,11,14,20,28,29,30); and only a few muscles have been studied, including pelvic floor muscles (20,29), deep abdominal muscles (transversus abdominis and internal oblique) (8,11,14,28), lumbar spine muscles (multifidus muscles) (11,30), and respiratory muscles (diaphragm) (4). In these studies, the biofeedback trainings were all conducted by the conventional cumbersome RUSI machines with redundant cables, resulting in reduced practicality of the RUSI biofeedback intervention. Therefore, a portable RUSI system dedicated to visual biofeedback for muscle training is desirable in the research and clinical communities.
Real-time ultrasound imaging has been validated as a type of metric to characterize the level of muscle activation. For example, in tests where muscle activation was measured using both RUSI and EMG, ultrasonic measurement of thickness change was highly correlated with EMG activity (r = 0.79) in the lumbar multifidus (12). McMeeken et al. (19) also demonstrated that RUSI could measure activation of the transverse abdominis in a wide range (5–80% of maximal voluntary contraction (MVC)) with a significantly correlated relationship between increases in transversus abdominis thickness and EMG activity. These results helped provide evidence that muscle thickness change quantified by RUSI can be considered as a reliable indicator of muscle activation (12,19). Such verified relationship further supports the theory that RUSI could act as an advanced form of biofeedback to regulate the activation level of involved muscles.
To the best of the authors' knowledge, although several studies have applied RUSI biofeedback for rehabilitation purposes to date (4,8,11,14,20,28,29,30), none of the previous studies have explored the feasibility of applying visualized RUSI biofeedback to improve muscle resistance training for sports and fitness purposes. Furthermore, although RUSI biofeedback has already been investigated during bodyweight exercises, its effectiveness in weight exercise remains unknown. Most existing ultrasound-related research has focused on isometric contraction patterns (4,8–11,13,14,20,28,29,30), whereas very few studies have paid attention to isotonic contraction patterns, which is a primary form of muscle training.
Therefore, this study aimed to introduce and validate a newly developed wearable ultrasound imaging system providing visualized RUSI biofeedback of targeted muscle contractions. The objectives of this study were (a) to establish the reliability of the proposed system and the repeated measurement method of calculating the thickness change of the targeted muscle (i.e., pectoralis major [PMaj]) and (b) to evaluate the immediate effect of RUSI biofeedback on isotonic PMaj muscle contraction during a single-joint resistance training exercise in healthy male adults. It was hypothesized that the proposed system and measurement method are reliable, and the thickness change of the PMaj increases with RUSI biofeedback during the pec fly exercise that performed at low-moderate and high loads in healthy young male subjects.
Methods
Experimental Approach to the Problem
A repeated-measure design was used to determine the immediate effects of a newly designed training approach of incorporating a wearable RUSI biofeedback system into PMaj resistance training. The experiment was conducted on an exercise-specific weight machine.
The novel RUSI biofeedback system and training setup can be found in Figure 1. It was mainly composed of (a) a customized ultrasound image acquisition unit and (b) a mobile terminal (Figure 1). Specifically, the image acquisition unit consisted of a customized ultrasound probe (4.5 × 0.7 cm), a signal cable, and a control box (15.6 × 6 × 2 cm). The ultrasound frequency of the probe was 7.5 MHz (35% bandwidth), which is suitable for superficial muscle imaging according to earlier studies (15). The control box contained the components for signal processing and data transmission. The image acquisition unit enabled wireless connection and transmission of real-time ultrasound data to a smartphone via Wi-Fi communication. The application program was installed on a smartphone to display B-mode ultrasound images. The frame rate of the system was 18 frame·s−1 (fps). When delivering RUSI biofeedback, the smartphone's screen displaying the real-time images was properly placed within the scope of subjects' vision by a phone holder. This allowed subjects to comfortably observe the thickening and movement of the examined muscle while performing the required exercise (Figure 2).
Figure 1.
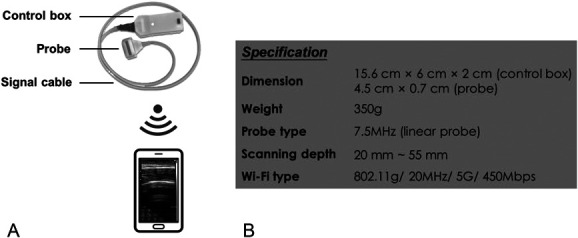
The wearable RUSI biofeedback system: (A) Ultrasound imaging acquisition unit wirelessly connected to the matched smartphone-based application. (B) Specification of the system. RUSI = real-time ultrasound imaging.
Figure 2.
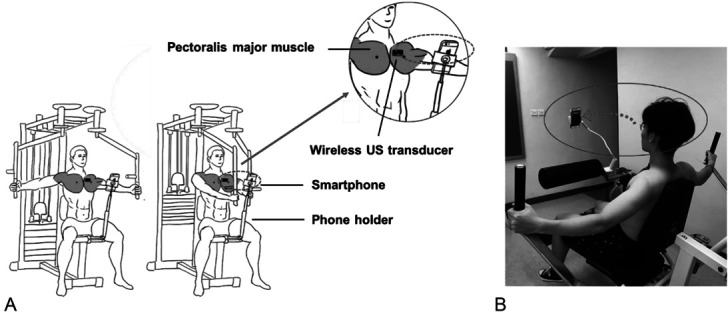
Setup of using the wearable RUSI biofeedback system to guide the subjects' pec fly exercise in a pec-deck machine: (A) Illustrating diagram. (B) Real experiment. RUSI = real-time ultrasound imaging.
For the immediate effect assessment, subjects performed pectoral muscle contraction under 2 conditions in the following order: (a) resistance training without biofeedback and (b) resistance training with biofeedback. For each of the aforementioned biofeedback conditions, 2 randomized training intensities (50 and 80% of 1-repetition maximum [RM]) were allocated, generating 4 experimental conditions per training session for each subject: (a) training with RUSI biofeedback at 50% of 1RM, (b) training with RUSI biofeedback at 80% of 1RM, (c) training without RUSI biofeedback at 50% of 1RM, and (d) training without RUSI biofeedback at 80% of 1RM. One set of 3 repetitions of pec fly exercises was performed under each experimental condition. The captured ultrasound images were provided to subjects during contraction in the training session, whereas offline measurement was performed for muscle thickness of PMaj.
Subjects
Twenty-five healthy young male volunteers were recruited for this study. The sample size of 25 produced a statistical power of 0.924, assuming a medium effect size of 0.5 and 2-sided significance level of 0.05 under a repeated-measures experimental design. Subjects were excluded if they had (a) a history of chest, spine, or upper limb surgery; (b) recent orthopedic or neurological issues; (c) serious chronic disease; or (d) prior experience of RUSI feedback training. Subjects should not have performed any upper-body resistance training for a minimum of 48 hours before the experiment. The age ranges for all subjects are 18–35 years. All subjects were informed of the study purpose and protocol, and signed an approved written informed consent form. Ethical approval was granted by the authority of the Hong Kong Polytechnic University (HSEARS20180418002).
Procedures
Experimental Preparation
In the introductory session, subjects first received verbal instruction on how to perform a standardized pec fly exercise using a pec deck machine (Apollo350, TuffStuff Fitness International Inc., Chino, CA), as specified in Haff et al. (6). To control the possible confounding factors, each repetition was completed in around 5 seconds, maintaining a pace of approximately 2 seconds for each of the concentric and eccentric contractions, as well as a 1-second buffer time at the end of a concentric contraction. Subjects were then introduced to how the biofeedback system functioned, including a 10-minute demonstration of RUSI-guided training and identifying the PMaj in ultrasound images. It was explained to subjects that if isotonic PMaj contraction occurred, they would see the bulking of muscle directly on the screen. In ultrasound images, this muscle bulking is represented by the thickening and movements of the muscle belly, accompanied by the sliding of the fasciae.
Before training, each subject performed warm-up exercises, as described in Hass et al. (7). Afterward, the 1RM for the pec fly exercise for each subject was estimated using a recommended protocol (6). Experimental preparation was finalized by attaching the probe to the left pectoral muscle surface transversely using Scotch tape. To standardize the location of the probe, the placement point was determined as the intersection of the third intercostal space and the midclavicular line, which displays a good view of the PMaj in both resting and contracted states and avoids interference by artifacts from the ribs. The orientation of the probe was fine tuned to ensure that both muscle fibers and fasciae of the PMaj could be imaged sharply in the field of view in ultrasound images.
Experimental Protocol
The overall training protocol is outlined in Figure 3. To minimize the inter-rater variability, the same investigator conducted all the experimental procedures. At the beginning of the training session, each subject was asked to remain still and not move for approximately 30 seconds. The muscle thickness at resting state (i.e., the PMaj was relaxed without any resistance) was obtained as the baseline. After the baseline assessment, all 25 subjects performed 2 sets of training with both a low-moderate load and high load in a randomized order. To accommodate real-life application, the low-moderate load and high load were set as 50 and 80% of 1RM (6), respectively. For each training intensity, subjects performed the pec fly exercise without RUSI biofeedback first, followed by using biofeedback. Each subject performed 3 repetitions consecutively for each of the 2 biofeedback conditions (with and without biofeedback) at the assigned intensity (i.e., 3 contractions per experimental condition and totally 12 contractions per training session for each subject). Such protocol can be easily operated, reduce the likelihood of muscle overuse, and also provide ample data to test the hypothesis (3,25,26). The real-time images of PMaj contraction were kept available for the investigator for all conditions during the experiment, to avoid incorrect motion or technique failure. Besides the ultrasound images, subjects were not given any additional cues on which specific muscle should be emphasized during training. Each of the 4 experimental conditions lasted for about 20 seconds. To minimize the effect of muscle fatigue, a 5-minute rest period between 2 conditions was allowed for each subject (25).
Figure 3.
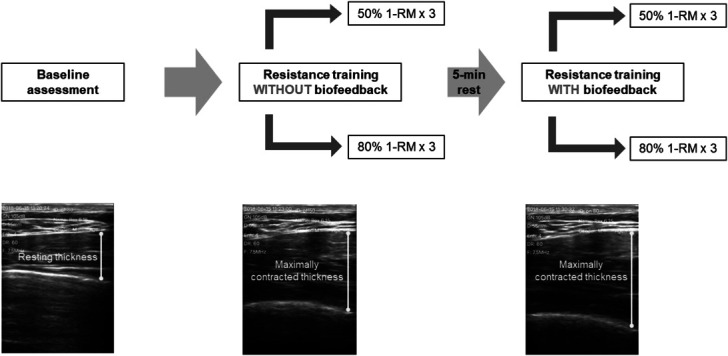
Outline of the methodology: each subject underwent a baseline assessment first, followed by 4 experimental conditions of PMaj resistance training. During the training session, the nonbiofeedback condition was always performed before the biofeedback condition with 2 randomized training intensities. One set of 3 repetitions was performed in each experimental condition. PMaj = pectoralis major.
Outcome Measures
The PMaj thickness during each contraction was measured from the ultrasound images that were captured during the training session. Using ImageJ software (National Institutes of Health, Bethesda) to view the images, the investigator calculated the PMaj thickness by locating the longest length between the tip of the inferior border and the bottom of the superior border using visual approximation (Figure 4). The muscle thickness change (%) represented muscle activation level (4,12,30) and was calculated as the percentage change in PMaj thickness from resting to maximal contraction using the following equation:
Figure 4.
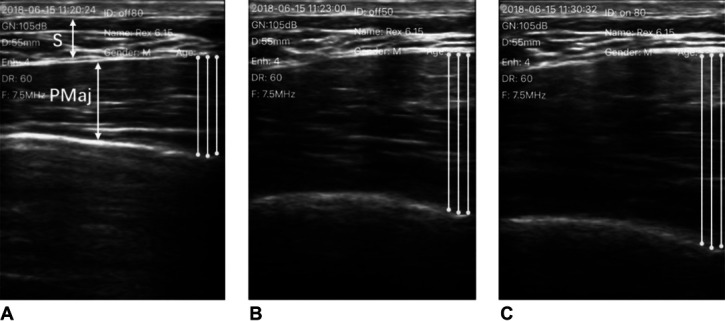
An example of ultrasound images illustrating the PMaj in transverse view in 1 subject (S: subcutaneous soft tissue; PMaj: pectoralis major): (A) The PMaj at rest. (B) Maximally contracted PMaj without RUSI biofeedback. (C) Maximally contracted PMaj with RUSI biofeedback. RUSI = real-time ultrasound imaging.
Data Analyses
To establish the inter-contraction reliability, repeated measurements were performed offline. These measurement data were collected by a third-party rater, who was blinded to the actual experimental condition of the images being measured. During muscle contraction, the thickness increased to a maximal point, before relaxing and returning to the baseline level of the resting state. For the captured ultrasound images of each muscle contraction, the rater looked through all the captured images first and then determined the frame with the maximal muscle thickness as measured in millimeters. The rater performed measurements for 3 frames (i.e., the frame with the maximal thickness and the 2 frames before and after it) for each contraction. The rater also performed 3 measurements (i.e., the location with the maximal thickness, and 0.5 mm to the left and right of that location) for each frame (3 linear measurements) (Figure 4). Thus, each muscle contraction is represented by the average of 9 measurements (3 linear measurements × 3 frames). The results of the 3 contractions were then averaged to represent each of the 4 experimental conditions. For the resting thickness, the investigator viewed all the images captured during the baseline assessment first and randomly selected 3 frames within the period of the baseline for measurement, following the same measurement procedure as outlined above (Figure 4).
Statistical Analyses
Statistical analysis was conducted using the SPSS software (version 21.0, SPSS Inc., Chicago, IL). The level of significance was set at 0.05. To evaluate the reliability of the repeated measurements, the intraclass correlation coefficient (ICC), standard error of measurement (SEM), and coefficient of variation method error (CVME) of the inter-contraction were calculated for each experimental condition. For the immediate effect, 2-way repeated-measures analysis of variance was conducted to analyze the main and interaction effects of (a) “biofeedback” factor (without vs. with RUSI biofeedback) and (b) “intensity” factor (50% of 1RM vs. 80% of 1RM) on PMaj thickness change among the 25 subjects.
Results
A total of 25 healthy young male subjects (age 23.8 years ± 4.1 years; height 172.5 ± 5.1 cm; weight 63.5 ± 6.6 kg; 1RM for pec fly 30.8 ± 7.4 kg) participated in the study. Table 1 summarizes the results of reliability of the repeated measurements in the 25 subjects. Excellent reliability of the measurements was observed, with the ICC(3,1) of all measurements larger than 0.95 (21) in this study. The SEM and CVME of all measurements also ranged from 0.33 to 1.01 and from 2.05 to 3.06%, respectively.
Table 1.
Inter-contraction reliability of measurements in subjects (n = 25).*
| PMaj thickness (mm) | Inter-contraction reliability (N = 25) | ||
| ICC3,1 (95% Cl) | SEM (mm) | CVME (%) | |
| Baseline (resting state) | 0.988 (0.977–0.994) | 0.33 | 2.05 |
| Resistance training without biofeedback | |||
| 50% of 1RM | 0.981 (0.964–0.991) | 0.75 | 2.63 |
| 80% of 1RM | 0.973 (0.949–0.987) | 0.93 | 2.92 |
| Resistance training with biofeedback | |||
| 50% of 1RM | 0.974 (0.926–0.989) | 1.01 | 3.06 |
| 80% of 1RM | 0.972 (0.945–0.987) | 1.00 | 2.91 |
PMaj = pectoralis major; ICC = intraclass correlation coefficient; CI = confidence interval; CVME = coefficient of variation method error; 1RM = 1-repetition maximum.
The immediate effects of with and without RUSI biofeedback on PMaj thickness change for 2 training intensities are illustrated in Figure 5. The values are presented as mean ± SD. Although no significant interaction effect was found between the 2 factors, significant main effects of “biofeedback” (p < 0.001) and “intensity” factors (p = 0.023) in PMaj thickness change were identified. Upon receiving the RUSI biofeedback, the PMaj thickness change significantly increased at both 50% of 1RM (increased from 72 ± 35% to 89 ± 43%; p < 0.001) and 80% of 1RM (increased from 77 ± 37% to 92 ± 41%; p < 0.001). We also observed greater percentage difference in PMaj thickness change for the low-moderate training intensity condition (24%) than for the high training intensity condition (20%). Regarding the main effect of “intensity,” as expected, the mean PMaj thickness change during 80% of 1RM (77 ± 37% without biofeedback and 92 ± 41% with biofeedback) was significantly larger than that for 50% of 1RM (72 ± 35% without biofeedback and 89 ± 43% with biofeedback) regardless of whether biofeedback was provided or not (p = 0.023).
Figure 5.
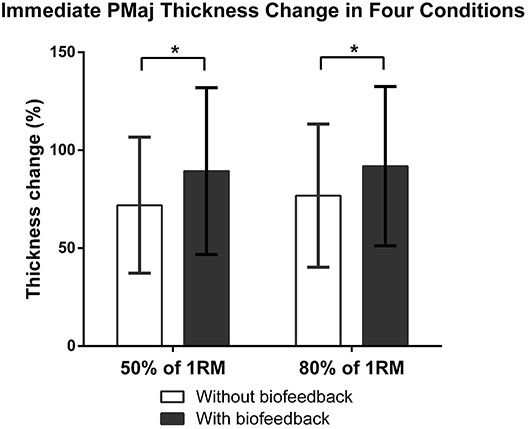
PMaj thickness change under 4 experimental conditions during the training session (immediate effect) (N = 25).
Discussion
The findings of this study supported the theory and reliability of using a novel wearable biofeedback system with RUSI to improve the training outcomes of PMaj thickness change during dynamic exercise. This improved performance in the ability to contract the PMaj muscle with a larger thickness change can be explained by the augmented visual cues that RUSI provided to the subjects. Such cues may have enhanced their proprioceptive awareness during training in this study. The findings can inspire future investigations and applications of wearable RUSI biofeedback systems to augment various types of muscle training.
Excellent reliability of PMaj thickness measurements in resting and maximally contracted states was found and is considered to attain the level of clinical application that required in the clinical guideline (21). This was demonstrated by the achievement of consistent results of muscle thickness change with the manually conducted repeated measurements in this study. In particular, the range of the CVME values (2.05–3.06%) suggested that the influence of variability has been low and acceptable (13). The SEM values in this study (0.33–1.01) have also been close to the previous measurements using digital technology (13). Although the SEM values found in this study were quite low, it is expected that using an automated tracking algorithm (13) could further minimize the SEM values. From a technical standpoint, the workflow can be further improved by integrating the automated image processing techniques into the existing system, to reduce the complexity of image interpretation and make the measurement more accurate and time efficient.
This study observed that the PMaj thickness change significantly increased when RUSI biofeedback was presented to the subjects. This indicated the improved ability to specifically contract the targeted muscle with a larger thickness change. This is in agreement with the findings of other studies on enhancing muscle reeducation and rehabilitative outcomes but which used large conventional ultrasound machines to deliver visual biofeedback in clinical settings (4,8,11,14,20,28,29,30). Henry and Westervelt (8) reported that subjects receiving visual ultrasound cues required fewer trials to perform correct abdominal hollowing exercises than without ultrasound cues. Van et al. (30) concluded that this technique could effectively improve the voluntary contraction of the multifidus in healthy subjects, based on the significant improvement in the muscle thickness change of the multifidus. Although all previous studies applied RUSI biofeedback for rehabilitative isometric exercises of deep muscles, this study further supported its positive effect on the dynamic exercise in a strength and conditioning setting. This study was also the first attempt to investigate its feasibility in the training of a superficial muscle. The mainstream theory that used to explain such enhanced muscle performance has been motor learning, which refers to the human ability to adapt motor movements in response to external stimuli changes (17,24). Research showed that feedback, movement sequence, and instruction can facilitate repetitive exercises during the early stage of motor learning (i.e., cognitive phase) (5). Real-time ultrasound imaging is considered as a type of extrinsic visual feedback, which has been proved to be effective when learners had difficulties in performing or improving the skill through intrinsic feedback (17). In this study, the observed improvement in motor performance may be explained by the fact that in the cognitive phase, the subjects paid attention to the extrinsic feedback by observing how the muscles thicken and move in the real-time RUSI video during contraction. This augmented visual cue gave the subjects a better overall understanding of the physical state of a muscle's contraction and provided information about the changes in muscle morphology. With this information, the subject can adjust the contraction accordingly, which in turn establishes a sensory-motor loop for learning the motor skill required for activating the PMaj and further develop volitional control over the PMaj. It is expected that providing RUSI biofeedback during training may benefit exercisers with poor or decreased proprioception, and future studies are needed to verify this.
A larger magnitude of mean PMaj thickness change was observed in the training at a high intensity level (80% of 1-RM), compared with the low-moderate intensity level (50% of 1RM). This is in accordance with previous findings on verbal instruction (3,25) probably because higher force production and motor unit recruitment are required to cope with heavier loads. Meanwhile, the increase in PMaj thickness change was also significantly larger at 50% of 1RM than at 80% of 1RM when using RUSI biofeedback. This may potentially indicate that the subjects' ability to voluntarily activate PMaj was more sensitive to lower intensity in our study.
To the authors' knowledge, this study is the first to report the usage of the RUSI biofeedback technique in the weight training using a weight machine, instead of using subjects' bodyweight as resistance. Building on previous knowledge and setups, it is encouraging to observe the improved PMaj contraction at both intensities of 50 and 80% of 1RM; however, the previous attempts of using verbal instruction seemed to be less effective at higher intensities. Previous studies have verified the benefits of verbal instruction for bench press at both 50 and 80% of 1RM (25) or a series of intensities (20, 40, 50, 60, and 80% of 1RM) (3) and concluded that resistance-trained individuals have difficulty in consistently increasing PMaj activation measured by EMG at 80% of 1RM when verbally instructed to focus on using only chest muscles during the lifting of weights. Indeed, this might be why the majority of these studies that reported greater muscle activities used a relatively low load intensity, including loads ranging from 30 to 50% of MVC (1,26), a 1.5-kg dumbbell (16), or bodyweight (18) as resistance. Such protocol may limit the practical application value because a load of less than 50% of MVC is not a very common real-life training practice. The positive findings for high loads in this study may provide a solution to this issue. Although 2 predetermined intensities (i.e., 50 and 80% of 1RM) have been verified, it should be noted that this result may still be inconclusive for it to be generalized to other intensities. More efforts and studies are needed. For example, future studies should verify the effect of a number of tested loads and determine the minimum effective training dosage. Having this knowledge would prove valuable for strength and conditioning practitioners when tailoring a weight training protocol for individuals with different fitness levels or training-outcome expectations.
This study has several limitations. It was not a randomized controlled trial, and there was only 1 rater who performed the experiments and executed the measurements. More than 1 rater with double blindness could enable more comprehensive reliability analyses, such as inter-session and inter-rater reliability. All the subjects in this study were young male adults with no experience of chest muscle-related resistance training, which may limit the generalization of the findings. Future studies should recruit weightlifters, recreationally trained individuals, or patients with proprioceptive impairment to further validate whether the ability to improve PMaj contraction still exists in other subgroups. The study design also lacked other confirmatory outcome measures capable of providing more direct assessments of improved muscle activation and performance with RUSI. Multimodal assessments, including EMG measuring myoelectricity, and isokinetic dynamometer controlling movement speed and recording force output throughout a range of motion (ROM), may make the results more convincing. This study only measured the agonist contraction and did not measure the antagonist activity because of the technical limitation of having only 1 probe in the RUSI biofeedback system. Regarding agonist activity, it is expected that as pectoral muscle contraction is increased, presumably the force of the muscle increases and therefore the speed of muscle contraction increases during isotonic exercise; unless the increase in agonist contraction is countered by an increase in antagonist muscle activation. Additional measurements of antagonistic contraction or task speed would help and should be conducted in future studies. Although our study has shed light on the feasibility and importance of RUSI biofeedback in improving resistance training exercises, the long-term effect of the proposal remains unclear. Other parameters can be derived from RUSI, such as static measurements of muscle morphology (e.g., muscle cross-sectional area, pennation angle, and fascicle length) and dynamic measurements of muscle behavior (e.g., changes in pennation angle) (31), to further verify the positive findings of this study. Additionally, measurement errors may have arisen during the data collection. The probe was attached to skin surface by adhesive tape, which may restrict limb movement and deform the examined muscle to a varying degree. Koo et al. (13) also reported that changing the applied pressure of the probe would greatly influence the measured PMaj thickness. Future attempts shall be made to optimize probe attachment and positioning. Our study was designed to observe the natural response when subjects received RUSI feedback. This is why the nonfeedback condition was performed before the feedback condition. However, this may lead to the concern that the order of experimental conditions may affect the training outcomes. Future studies should consider randomizing the experimental conditions to eliminate the learning effect in subjects. Future studies should also investigate the application of RUSI in muscle training with more training protocols and intensities, beyond the 50 and 80% of 1RM of this feasibility study.
Practical Applications
It is expected that strength and conditioning practitioners and therapists can take RUSI biofeedback technique into consideration when designing training plans or protocols. The provision of RUSI biofeedback could increase muscle thickness change during weighted isotonic contraction. This approach can target specific muscle groups, which may enhance localized muscle size and improve blood circulation. As ultrasound technology is more available than ever, it may even be possible to be applied as a biofeedback tool daily. The success of the newly introduced system may pave the way toward providing visual biofeedback of muscle contraction during dynamic exercise in more flexible fitness or gym settings. Its wearable design and portable nature can not only facilitate the exercise and training of patients, athletes, and gym goers but also allow therapists, coaches, and fitness instructors to be freed from using conventional handheld probes. This will further improve the user's experience and enhance the clinical translation of this laboratory work. More optimizations of the system are still needed before implementing real-time visual biofeedback via wearable ultrasound imaging to enhance the muscle contraction training outcome of people in real-life practice and beyond laboratory settings.
Acknowledgments
The authors thank all the subjects who voluntarily participated in this study. Z.-H. Huang and C. Z.-H. Ma contributed equally to this work. Z.-H. Huang contributed to the research concept and study design, literature review, data collection, data analysis and interpretation, statistical analyses, and writing of the manuscript; C. Z.-H. Ma contributed to the research concept and study design, literature review, data collection, data analysis and interpretation, statistical analyses, and editing of the manuscript; L.-K. Wang contributed to the research concept and study design, and data collection; X.-Y. Wang contributed to the research concept and study design, and data collection; S.-N. Fu contributed to the research concept and study design, and editing of the manuscript; Y.-P. Zheng contributed to the research concept and study design, data analysis and interpretation, statistical analyses, and editing of the manuscript.
References
- 1.Bressel E, Willardson JM, Thompson B, Fontana FE. Effect of instruction, surface stability, and load intensity on trunk muscle activity. J Electromyogr Kinesiol 19: e500–e504, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Bump RC, Hurt WG, Fantl JA, Wyman JF. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol 165: 322–329, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Calatayud J, Vinstrup J, Jakobsen MD, et al. Importance of mind-muscle connection during progressive resistance training. Eur J Appl Physiol 116: 527–533, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Cho J-E, Hwang D-Y, Hahn J, Lee W-H. Use of real-time ultrasound imaging for biofeedback of diaphragm motion during normal breathing in healthy subjects. Phys Ther Rehabil Sci 7: 95–101, 2018. [Google Scholar]
- 5.Fitts PM, Posner MI. Learning and skilled performance. In: Human Performance. Belmont, CA: Brooks/Cole, 1967. pp. 8–25. [Google Scholar]
- 6.Haff GG, Triplett NT. Exercise technique for free weight and machine training. In: Essentials of Strength Training and Conditioning. Champaign, IL: Human Kinetics, 2016. pp. 317–351. [Google Scholar]
- 7.Hass CJ, Garzarella L, de Hoyos D, Pollock ML. Single versus multiple sets in long-term recreational weightlifters. Med Sci Sports Exerc 32: 235–242, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Henry SM, Westervelt KC. The use of real-time ultrasound feedback in teaching abdominal hollowing exercises to healthy subjects. J Orthop Sports Phys Ther 35: 338–345, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Hodges PW, Pengel LH, Herbert RD, Gandevia SC. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve 27: 682–692, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Harvey R, Peper E. Case 8 – I thought I was relaxed: The use of SEMG biofeedback for training awareness and control. In: Case Studies in Applied Psychophysiology. West Sussex, United Kingdom: Wiley-Blackwell, 2012. pp. 144–159. [Google Scholar]
- 11.Kermode F. Benefits of utilising real-time ultrasound imaging in the rehabilitation of the lumbar spine stabilising muscles following low back injury in the elite athlete—A single case study. Phys Ther Sport 5: 13–16, 2004. [Google Scholar]
- 12.Kiesel KB, Uhl TL, Underwood FB, Rodd DW, Nitz AJ. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther 12: 161–166, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Koo TK, Wong C, Zheng Y. Reliability of sonomyography for pectoralis major thickness measurement. J Manipulative Physiol Ther 33: 386–394, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Hong SK, Lee Y-S, et al. Is abdominal hollowing exercise using real-time ultrasound imaging feedback helpful for selective strengthening of the transversus abdominis muscle? A prospective, randomized, parallel-group, comparative study. Medicine (Baltimore) 97: e11369–e11369, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YK, Skalski MR, White EA, et al. US and MR imaging of pectoralis major injuries. Radiographics 37: 176–189, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Lim OB, Kim JA, Song SJ, Cynn HS, Yi CH. Effect of selective muscle training using visual EMG biofeedback on infraspinatus and posterior deltoid. J Hum Kinet 44: 83–90, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magill RA, Anderson DI. Instruction and augmented feedback. In: Motor Learning and Control: Concepts and Applications. New York, NY: McGraw-Hill, 2007. pp. 343–372. [Google Scholar]
- 18.Marchant DC, Greig M, Scott C. Attentional focusing instructions influence force production and muscular activity during isokinetic elbow flexions. J Strength Cond Res 23: 2358–2366, 2009. [DOI] [PubMed] [Google Scholar]
- 19.McMeeken JM, Beith ID, Newham DJ, Milligan P, Critchley DJ. The relationship between EMG and change in thickness of transversus abdominis. Clin Biomech (Bristol, Avon) 19: 337–342, 2004. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan PB, Beales DJ. Changes in pelvic floor and diaphragm kinematics and respiratory patterns in subjects with sacroiliac joint pain following a motor learning intervention: A case series. Man Ther 12: 209–218, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Portney LG. Reliability of measurements. In: Foundations of Clinical Research: Applications to Practice. Upper Saddle River, NJ: Pearson/Prentice Hall, 2009. pp. 77–94. [Google Scholar]
- 22.Potter CL, Cairns MC, Stokes M. Use of ultrasound imaging by physiotherapists: A pilot study to survey use, skills and training. Man Ther 17: 39–46, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Rasch PJ. Neural control and motor learning. In: Kinesiology and Applied Anatomy. Philadelphia, PA: Lea & Febiger, 1989. pp. 87–107. [Google Scholar]
- 24.Schmidt RA. Augmented feedback. In: Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics, 2011. pp. 341–372. [Google Scholar]
- 25.Snyder BJ, Fry WR. Effect of verbal instruction on muscle activity during the bench press exercise. J Strength Cond Res 26: 2394–2400, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Snyder BJ, Leech JR. Voluntary increase in latissimus dorsi muscle activity during the lat pull-down following expert instruction. J Strength Cond Res 23: 2204–2209, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Stein C, Schäfer M, Machelska H. Attacking pain at its source: New perspectives on opioids. Nat Med 9: 1003–1008, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Teyhen DS, Miltenberger CE, Deiters HM, et al. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J Orthop Sports Phys Ther 35: 346–355, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JA, O'Sullivan PB, Briffa NK, Neumann P. Assessment of voluntary pelvic floor muscle contraction in continent and incontinent women using transperineal ultrasound, manual muscle testing and vaginal squeeze pressure measurements. Int Urogynecol J Pelvic Floor Dysfunct 17: 624–630, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Van K, Hides JA, Richardson CA. The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J Orthop Sports Phys Ther 36: 920–925, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Zhou GQ, Chan P, Zheng YP. Automatic measurement of pennation angle and fascicle length of gastrocnemius muscles using real-time ultrasound imaging. Ultrasonics 57: 72–83, 2015. [DOI] [PubMed] [Google Scholar]


