Abstract
The rapid increase in the incidence of obesity contributes to a parallel increase in nonalcoholic steatohepatitis (NASH). Monocyte-derived macrophages, recruited from the bone marrow to the liver, promote NASH-related inflammation and fibrosis. In addition, adipose tissue macrophages (ATMs) release pro-inflammatory cytokines (PICs) which stimulate adipose tissue lipolysis liberating free fatty acids (FFAs) that can accumulate in the liver as triglycerides (TGs), thereby inducing steatosis. As such, bone marrow-derived macrophages (BMDMs) function as an essential tool to study the pathogenesis of NASH. BMDMs are primary bone marrow-derived cells which are differentiated into macrophages in vitro in the presence of growth factors. Macrophage colony-stimulating factor (M-CSF) is required for the proliferation and differentiation of committed myeloid progenitors into cells of the macrophage/monocyte lineage. Here, we describe a protocol for the isolation of mouse bone marrow cells and subsequent macrophage differentiation in which bone marrow cells are cultured in the presence of M-CSF, supplemented either by conditioned medium from L929 cells or in purified form. The efficiency of the differentiation is confirmed by immunofluorescent staining of macrophage surface antigen F4/80. The BMDMs serve as an excellent ex vivo model for a variety of studies, including hepatocyte-macrophage and adipocyte-macrophage cross-talk regulating NASH.
Keywords: Bone marrow cell, Macrophage, Nonalcoholic steatohepatitis, Macrophage colony-stimulating factor (M-CSF), L929 cells, F4/80
1. Introduction
NASH is characterized by steatosis, inflammation, and fibrosis [1]. One of the unique features of NASH is extensive hepatic infiltration of macrophages derived from monocytes originating in the bone marrow in addition to the liver-resident macrophages (Kupffer cells) [2]. The macrophages contribute to NASH-associated inflammation as well as fibrosis by releasing factors that activate hepatic stellate cells (HSCs) [3]. In obesity, hypertrophied adipocytes secrete chemotactic factors which recruit pro-inflammatory macrophages [2]. Pro-inflammatory cytokines (PICs), released from macrophages, promote inflammation, and stimulate adipocyte lipolysis resulting in FFA release which accumulate in hepatocytes as TG causing steatosis [2, 4]. Insulin inhibits lipolysis and adipocytes are highly insulin-sensitive. PICs inhibit insulin action by activating pro-inflammatory kinases, such as IκB kinase (IKK) and Janus N-terminal kinase (JNK) which phosphorylate and deactivate insulin receptor supstrate-1 (IRS1) [4]. In this way, macrophages contribute to all aspects of NASH. Therefore, studying macrophages in the context of NASH is of utmost importance, specifically, to analyze cross-talks of macrophages with hepatocytes, HSCs, and adipocytes. Mouse BMDMs represent an ideal mammalian experimental model to study NASH ex vivo.
Pluripotent hematopoietic stem cells (HSCs) in the bone marrow generate blood cells. At first, the HSCs give rise to two progenitor cell lineages, the lymphoid and myeloid stem cells. The myeloid stem cells can differentiate into megakaryoblasts, proerythroblasts, myeloblasts, and monoblasts [5]. The monoblasts can differentiate into monocytes and then macrophages depending upon the presence of growth factors (e.g., M-CSF). In this chapter, we describe a protocol for the isolation of bone marrow cells from a mouse and their subsequent differentiation into BMDMs. The differentiation of bone marrow cells into macrophages can be confirmed by detecting the F4/80 glycoprotein, which has been established as one of the most specific cell-surface markers for murine macrophages [6]. Compared to many other primary cells, BMDMs are homogeneous and can be grown in culture dishes. Furthermore, the BMDMs are transfectable and can proliferate up to 3 weeks without considerable cell death or altered morphology. Macrophages are specialized cells that perform numerous tasks in the immune system, such as phagocytosis, antigen presentation, and cytokine production. BMDMs, generated by our protocol, are useful not only for studying NASH but also interrogating the role of the immune system in NASH [7-9].
2. Materials
2.1. Reagents
70% ethanol.
Sterile, ice-cold Dulbecco’s phosphate buffered saline (DPBS).
10× red blood cell (RBC) lysis buffer (eBioscience).
Dulbecco’s Modified Eagle’s Medium (DMEM), high glucose with fetal bovine serum, non-essential amino acids (NEAA), l-Glutamine, and Penicillin-Streptomycin.
L929 conditioned media (CM) or M-CSF (Gibco).
Cellstripper solution (Corning).
2.2. Equipment and Consumables
L929 mouse fibroblast cells (optional, if not using commercial M-CSF).
Sterile dissecting instruments (forceps, scissors).
10 ml syringes.
25G needles.
Swinging bucket centrifuge with 50 ml tube adapters.
50 ml conical tubes.
10, 25 ml pipettes.
70 μm cell strainers.
CO2 incubator, 37 °C.
Hemocytometer or automated cell counter.
100 mm non-tissue culture treated petri dishes.
Tissue culture dishes/plates.
3. Methods
If not using purified M-CSF, prepare L929 CM: Plate 4.75 × 105 L929 cells/T75 flask containing 50 ml L929 media. After 7 days of incubation at 37 °C in a CO2 incubator, collect media, centrifuge (5 min, 1000 rpm, room temperature), filter through a 0.45 μm filter, and store at −20 °C. Another 50 ml media can be added to each flask and collected 7 days later. Combine with the first batch of media (stored at 4 °C), filter, and store at −20 °C (see Note 1).
Sacrifice a mouse by cervical dislocation and thoroughly soak with 70% ethanol. Prepare a petri dish with ice-cold DPBS.
Using sterile scissors and forceps, make an incision in the skin where the leg meets the hip and cut all the way around the leg. Peel skin down to the foot and remove. Carefully remove the leg by cutting at the hip joint, making sure to keep the femur intact. Surrounding muscle can be removed as needed to better visualize the joint. Remove foot and place leg in the petri dish containing DPBS. Repeat with the second leg.
Prepare a fresh petri dish containing ice-cold DPBS. Remove muscle tissue (and fibula from tibia) from bones and carefully separate femur from tibia, keeping both bones intact (see Note 2). Dip cleaned bones in 70% ethanol and place in a petri dish (see Note 3).
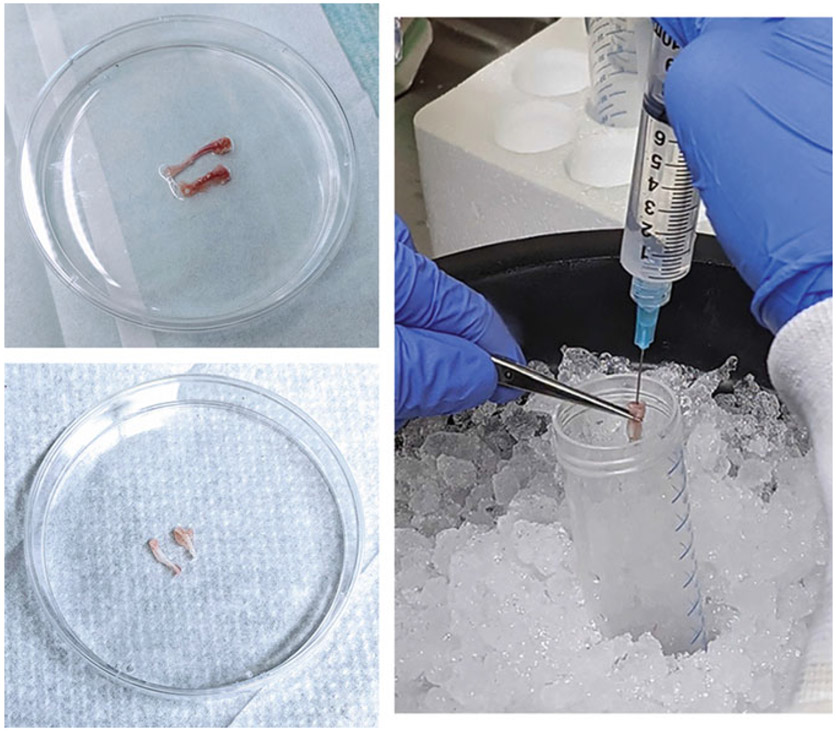
Fill two 10 ml syringes with ice-cold DPBS and attach 25G needles. With a fresh pair of sterile forceps, remove one bone from the petri dish and use a fresh pair of sterile scissors to remove the epiphyses. Holding the bone vertically over an open 50 ml tube on ice, insert the needle into the bone cavity and flush the marrow from the bone with the DPBS into the 50 ml tube. Invert the bone and repeat, running the needle up and down through the cavity to dislodge any remaining marrow. Repeat this process with the remaining bones (see Note 4) (Fig. 1).
Pipet the marrow suspension up and down with a 10 ml pipette to break up the marrow and filter through a 70 μm cell strainer to separate out any remaining clumps.
Centrifuge the cells for 10 min at 500 × g, 4 °C.
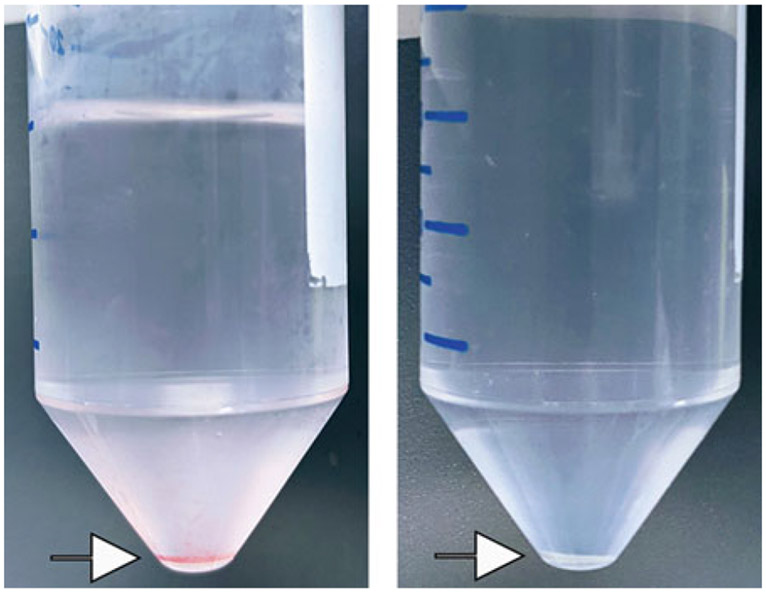
Remove supernatant and resuspend cells in 10 ml 1× RBC lysis buffer. Incubate for 2 min at room temperature. Add 20 ml ice-cold DPBS and centrifuge for 10 min at 500 × g, 4 °C. The resulting pellet should be white (Fig. 2).
Resuspend the cells in 20 ml warm complete media and determine cell concentration with hemocytometer or automated cell counter (see Note 5). Typical yield can range from 3.5 × 107 to 5 × 107 progenitor cells/mouse.
Plate 6 × 106 cells/petri dish in 10 ml complete media containing either 25 ng/ml M-CSF or 10–30% L929 CM (see Notes 6 and 7).
After 2 days, add 10 ml additional complete media as formulated in step 10. After 5 days, remove media and add 10 ml fresh complete media as formulated in step 10.
After 7 days, the cells will be fully differentiated. Aspirate media and wash plates with DPBS. Add 2 ml warm CellStripper solution and incubate at 37 °C for 5 min.
Add 4 ml media to plates and pipette cells into suspension. Centrifuge 10 min at 400 × g, 4 °C. Discard supernatant, resuspend pellet in fresh complete media, and count cells.
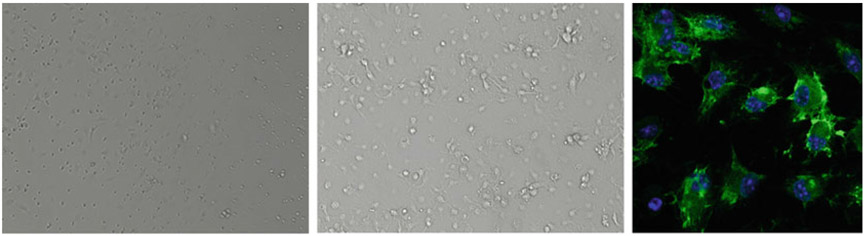
Plate cells in tissue culture treated dishes according to the numbers shown in Table 1 and maintain in a humidified 37 °C CO2 incubator. A representative image of bone marrow-derived macrophages is shown in Fig. 3.
Fig. 1.
Harvesting bone marrow from mouse femur and tibia. Clockwise from top left: Cleaned bones containing marrow, removal of marrow using 25G needle, bones after marrow removal
Fig. 2.
Cell pellets (arrows) before (left) and after (right) RBC lysis
Table 1.
Number of macrophages to be plated according to the vessel size
| Culture vessel | Number of plated cells |
|---|---|
| 24-well plate, 4-chamber slide | 5 × 103 to 1 × 104 cells/well |
| 6-well plate | 1 × 106 cells/well |
| 60 mm dish | 2 × 106 cells/dish |
Fig. 3.
Representative images of bone marrow-derived macrophages (BMDM). From left: bright-field image after three days of differentiation, bright-field image of fully differentiated BMDM, and fully differentiated macrophages stained with F4/80 antibody (green) and DAPI (blue)
4. Notes
L929 media: DMEM (high glucose) + 10% FBS + 1% HEPES +1% penicillin-streptomycin.
To prevent contamination of cultures, all subsequent steps should be conducted in a laminar flow hood.
If the bones have been fractured or cut by mistake on the ends, do not submerge in 70% ethanol.
Use approximately 10 ml/set of leg bones. The bones will appear white once the marrow has been flushed from the bone.
Complete media: DMEM +10% FBS + 1% NEAA +1% penicillin-streptomycin.
If the cells are plated on tissue culture treated dishes, they will be extremely difficult to detach after differentiation without scraping, which can lead to lower viability in the cells.
Optimal concentrations of L929 conditioned medium should be determined by the user, as M-CSF levels in each batch of LCM can vary. FBS concentration should be increased to 15% when using LCM.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under Grant 1R01DK107451-01A1, the National Cancer Institute (NCI) under Grants 1R01CA230561-01A1, 1R01CA240004-01, and 1R01CA244993-01, and the Department of Defense (DOD) under Grant CA170048.
References
- 1.Friedman SL, Neuschwander-Tetri BA et al. (2018) Mechanisms of NAFLD development and therapeutic strategies. Nat Med 24: 908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefere S, Tacke F (2019) Macrophages in obesity and non-alcoholic fatty liver disease: Cross-talk with metabolism. JHEP Rep 1:30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellicoro A, Ramachandran P, Iredale JP, Fallow-field JA (2014) Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 14:181–194 [DOI] [PubMed] [Google Scholar]
- 4.Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA et al. (2014) Regulation of adipocyte lipolysis. Nutr Res Rev 27:63–93 [DOI] [PubMed] [Google Scholar]
- 5.Grove JE, Bruscia E, Krause DS (2004) Plasticity of bone marrow-derived stem cells. Stem Cells 22:487–500 [DOI] [PubMed] [Google Scholar]
- 6.Lin HH, Faunce DE, Stacey M et al. (2005) The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med 201: 1615–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanters E, Pasparakis M, Gijbels MJ et al. (2003) Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest 112:1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle SE, O’Connell RM, Miranda GA et al. (2004) Toll-like receptors induce a phagocytic gene program through p38. J Exp Med 199: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YJ, Cunnick JM, Yi SJ, Kaartinen V et al. (2007) Abr and Bcr, two homologous Rac GTPase-activating proteins, control multiple cellular functions of murine macrophages. Mol Cell Biol 27:899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]