Abstract
The in vitro activity of the everninomicin antibiotic SCH 27899 against 17 isolates of Borrelia spp. was investigated. MICs ranged from 0.06 to 0.5 μg/ml. Time-kill studies with the B31 strain of B. burgdorferi demonstrated ≥3-log10-unit killing after 72 h with concentrations representing four times the MIC. The in vitro activity of four other newer antimicrobial agents, meropenem, cefepime, quinupristin-dalfopristin, and linezolid, was also tested against the B31 strain. Meropenem was the most potent of the latter agents, with an MIC of 0.125 μg/ml.
Compared to those of other common bacterial pathogens, relatively little is known about the pharmacodynamic interactions of antimicrobials with Borrelia species. This lack of information contributes to the controversy regarding optimal treatment regimens for patients with Lyme disease, particularly for late-stage or persistent disease (2, 14). Spirochetes are phylogenetically distinct from both gram-positive and gram-negative bacteria (11), and their susceptibilities to antimicrobials have not been predictable. In particular, the susceptibility profile of Borrelia burgdorferi has not been well characterized because of the difficulty of working with the organism in vitro and the lack of standardized methods for susceptibility testing (5).
This study examined the in vitro activity of several newer antimicrobial agents against B. burgdorferi. The drugs tested were as follows: an everninomicin, SCH 27899 (Schering-Plough Research Institute, Kenilworth, N.J.); an oxazolidinone, linezolid (Pharmacia & Upjohn Company Laboratories, Kalamazoo, Mich.); a streptogramin, quinupristin-dalfopristin (Rhône-Poulenc Rorer Pharmaceuticals, Collegeville, Pa.); a carbapenem, meropenem (Zeneca Pharmaceuticals, Wilmington, Del.); and an extended-spectrum cephalosporin, cefepime (Bristol-Myers Squibb Company, Princeton, N.J.). The high-passage strain B31 of B. burgdorferi was used for these experiments. Further testing was done with SCH 27899 to determine its activity against other Borrelia spp. Included in this group were low (<10 passages in vitro)- and high-passage isolates of the same strain and two mutants lacking the major outer surface proteins OspA and OspB (Table 1). The bactericidal activities of SCH 27899 and meropenem were also compared to those of ceftriaxone and doxycycline, two drugs commonly used to treat Lyme disease.
TABLE 1.
Isolates of Borrelia spp. studied
| Isolate | Geographic origin | Source |
|---|---|---|
| B. burgdorferi | ||
| B31-82,a B31-97 (ATCC 35210), B31 (1p49−)b | New York | Tick (Ixodes scapularis) |
| N40 | New York | Tick (Ixodes scapularis) |
| SH2-82a, SH2-97c | New York | Tick (Ixodes scapularis) |
| HB19-83,a HB19-97, HB19 (1p49−)b | Connecticut | Human blood |
| G-25 | Sweden | Tick (Ixodes ricinus) |
| B. afzelii | ||
| ACA1 | Sweden | Human skin |
| Ip21 | Russia | Tick (Ixodes ricinus) |
| B. garinii | ||
| Ip90-88,a Ip90-97 | Russia | Tick (Ixodes persulcatus) |
| G1-86a | Germany | Human cerebrospinal fluid |
| B. hermsii (HS1) | Washington | Tick (Ornithodoros hermsi) |
| B. turicatae (OZ1) | Texas | Tick (Ornithodoros turicata) |
Low-passage isolate.
Lacks OspA and OspB proteins.
Passaged in mice.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997.)
MICs were determined by the broth microdilution method previously described (5). The ranges of antibiotic concentrations tested were 0.0075 to 1 μg/ml for SCH 27899, ceftriaxone, and meropenem and 0.03 to 2 μg/ml for all other antibiotics. The antibiotics were prepared according to the manufacturers’ directions and diluted twofold in BSK II medium (3). For the determination of microdilution MICs, 100 μl of each concentration to be tested was dispensed into microtiter tray wells in duplicate rows. In addition, 100 μl of BSK II was dispensed into wells of each microtiter tray as growth controls for each strain tested and as negative controls. All wells except negative control wells were inoculated with 10 μl of actively growing cultures of borrelias propagated in BSK II medium and adjusted to yield a final inoculum of ∼106 cells/ml as determined by enumeration with a Petroff-Hausser chamber and dark-field microscopy. The microdilution trays were sealed with sterile plastic adhesive and incubated for 72 h at 34°C. For the determination of macrodilution MICs, 6 ml of BSK containing the appropriate concentrations of antibiotics to be tested was dispensed into sterile polystyrene tubes. The tubes and growth controls were inoculated with B31 to yield a final inoculum concentration of ∼106 cells/ml. The tubes were sealed tightly with screw caps and incubated under the same conditions as the microtiter trays. The lowest concentration of antibiotic that showed inhibition of visual turbidity and lack of color change from pink to yellow of the BSK II medium indicator compared to the growth control was interpreted as the MIC. Color discrimination, particularly with low-passage strains, was improved after the MIC plates and tubes had been held at 4°C for 2 to 3 h (13). In some instances, slowly growing low-passage strains had to be incubated an additional 24 to 48 h before there was sufficient growth of the controls. All MIC determinations were performed in duplicate. The higher MIC value was reported in cases where there were differences between the two values.
B31 was used to determine killing rates for SCH 27899, meropenem, ceftriaxone, and doxycycline in concentrations representing two times the respective microdilution MIC. SCH 27899 and doxycycline were also tested at four times the MIC. Polystyrene tubes containing 6 ml of BSK II medium with the antibiotic concentrations to be tested and one tube without antibiotics were inoculated with 100 μl of an actively growing culture adjusted to yield a final inoculum of ∼106 cells per ml. The tubes were incubated at 34°C. At 0, 8, 24, 48, and 72 h, the cultures were gently mixed and spirochete numbers for each tube were estimated with a Petroff-Hausser counting chamber. The estimated numbers were used to determine the appropriate dilutions needed to provide countable plates following subsurface plating, a technique described elsewhere (5). The medium for subsurface plating consisted of a 2× concentrate of BSK II medium without gelatin, a 3% bottom agarose, and a 2% top agarose. Spirochete suspensions were prepared in 2× BSK II medium, and 100 μl was added to aliquots of molten agarose and 2× BSK II. After being mixed gently, the suspensions were poured immediately onto the surface of 2× BSK II agar. The plates were rotated gently to spread the suspension evenly, and the agarose was allowed to solidify and was incubated in a candle jar at 34°C. The plates were examined and the colonies were counted after 10 to 12 days of incubation. A bactericidal effect was defined by ≥3-log10-unit killing (99.9%) of the final inoculum (10).
MICs of SCH 27899 ranged from 0.06 to 0.5 μg/ml for the isolates of Borrelia spp. tested. The MICs at which 50 and 90% of the isolates were inhibited were 0.25 and 0.5 μg/ml, respectively. These values are similar to what has been reported for staphylococci, streptococci, and enterococci (7, 15). MICs for low- and high-passage isolates of the same strain of B. burgdorferi and those lacking OspA and OspB were all within 3-log2-unit dilutions. The MICs for B31 were >2 μg/ml for linezolid, 1 μg/ml for quinupristin-dalfopristin, 0.125 μg/ml for meropenem, and 1 μg/ml for cefepime. The MIC of ceftriaxone was 0.03 μg/ml, and the MIC of doxycycline was 1 μg/ml. In all instances, macrobroth dilution MIC results were within 2-log2-unit dilutions of those obtained by the microbroth dilution methods.
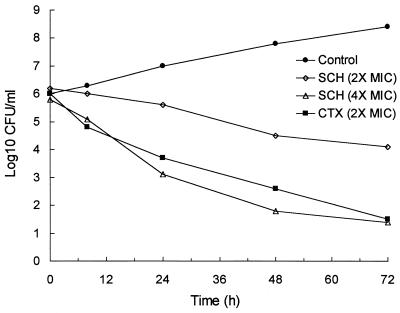
The results from time-kill experiments are shown in Table 2. Ceftriaxone and meropenem were bactericidal at concentrations representing twice their respective MICs, while SCH 27899 and doxycycline were bacteriostatic (<3-log10-unit killing). However, SCH 27899 demonstrated a bactericidal effect at a concentration of 2.0 μg/ml (four times the MIC) that was comparable to that of ceftriaxone at a concentration of twice the MIC (Fig. 1).
TABLE 2.
Time-kill studies with B. burgdorferi B31
| Time (h) | LOG10 CFU/ml
|
||||||
|---|---|---|---|---|---|---|---|
| Control | SCH 27899, 1.0 μg/ml (2× MIC) | SCH 27899, 2.0 μg/ml (4× MIC) | Meropenem, 0.25 μg/ml (2× MIC) | Ceftriaxone, 0.06 μg/ml (2× MIC) | Doxycycline, 2 μg/ml (2× MIC) | Doxycycline, 4 μg/ml (4× MIC) | |
| 0 | 6.0 | 6.2 | 5.8 | 6.3 | 6.0 | 5.9 | 6.2 |
| 8 | 6.3 | 6.0 | 5.1 | 5.7 | 4.8 | 5.8 | 5.8 |
| 24 | 7.0 | 5.6 | 3.1 | 5.1 | 3.7 | 5.7 | 4.8 |
| 48 | 7.8 | 4.5 | 1.8 | 3.2 | 2.6 | 5.3 | 4.4 |
| 72 | 8.4 | 4.1 | 1.4 | 2.3 | 1.5 | 4.8 | 4.0 |
| Log change at 72 h | +2.4 | −2.1 | −4.4 | −4.0 | −4.5 | −1.1 | −2.2 |
FIG. 1.
Time-kill curves with the B31 strain of B. burgdorferi. Ceftriaxone was tested at a concentration of 0.06 μg/ml (two times the MIC). SCH 27899 was also tested at concentrations of 1.0 (two times the MIC) and 2.0 (four times the MIC) μg/ml. CTX (2× MIC), ceftriaxone at two times the MIC; SCH (2× MIC), SCH 27899 at two times the MIC; SCH (4× MIC), SCH 27899 at four times the MIC.
Everninomicins are oligosaccharide antibiotics that are active primarily against gram-positive bacteria (7, 15). The exact mechanism of action has not been fully elucidated; however, recent data suggest that SCH 27899 binds to a ribosomal protein, thereby inhibiting protein synthesis (1). At each time interval of the time-kill studies, B31 cells exposed to SCH 27899 were examined by dark-field microscopy. After 72 h of exposure to 2 μg of SCH 27899/ml, the majority of spirochetes were nonmotile; however, morphologically they appeared intact, with fewer than 20% of cells demonstrating surface blebs. In contrast, approximately 75% of the cells exposed to ceftriaxone and meropenem demonstrated morphological abnormalities, with large and small surface blebs (6). This would support the hypothesis that the antimicrobial activity of SCH 27899 against borrelia does not result from interruption of cell wall synthesis.
Our data demonstrate that SCH 27899 is active in vitro against a wide variety of strains of Borrelia. These include strains causing human Lyme disease (B. burgdorferi, B. afzelii, and B. garinii) and those causing relapsing fever (B. turicatae and B. hermsii). Time-kill studies suggest that killing of the B31 strain of B. burgdorferi by SCH 27899 may be concentration dependent. In vitro time-kill and pharmacodynamic studies have demonstrated dose-related killing of staphylococci and pneumococci (4, 7). Pavia and colleagues (12) recently reported MICs for SCH 27899 ranging from 0.1 to 0.5 μg/ml for six strains of B. burgdorferi, values comparable to what we report. However, they found ceftriaxone to be less potent, with MICs ranging from 0.1 to 0.25 μg/ml for the same strains of B. burgdorferi.
The results of this study confirm that the susceptibility profile of B. burgdorferi is unlike that of either gram-positive or gram-negative bacteria. For example, while the β-lactam antibiotics ceftriaxone and meropenem are highly active against B. burgdorferi, cefepime is less active. Additionally, linezolid (8) and quinupristin-dalfopristin (9), both of which act by inhibiting protein synthesis and have a spectrum of activity similar to SCH 27899, are less active in vitro against B. burgdorferi than SCH 27899.
In summary, this study identified two agents with excellent in vitro activity against B. burgdorferi. Further studies are needed to determine the in vivo activities of these agents in experimental models of borrelia infection.
Acknowledgments
We thank Yaqab Ibrahim for technical assistance and James H. Jorgensen for expert advice.
This research was supported by a grant from Schering-Plough Research Institute.
REFERENCES
- 1.Adrian P V, Klugman K P. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Ribosomal protein L16: the putative target site for everninomicin ( SCH27899) action in Streptococcus pneumoniae, abstr. C-110. [Google Scholar]
- 2.Barbour A G. Lyme disease: the cause, the cure, the controversy. Baltimore, Md: The Johns Hopkins University Press; 1996. Treating Lyme disease: what works and what doesn’t. [Google Scholar]
- 3.Barbour A G, Burgdorfer W, Hayes S F, Peter O, Aeschlimann A. Isolation of a cultivable spirochete from Ixodes ricinus ticks of Switzerland. Curr Microbiol. 1986;8:123–126. [Google Scholar]
- 4.Bauernfeind A, Eberlein E, Jungwirth R. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Comparative bactericidal kinetics of SCH27899 (Ziracin) at various dosages in a pharmacodynamic model, abstr. A-33. [Google Scholar]
- 5.Dever L L, Jorgensen J H, Barbour A G. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J Clin Microbiol. 1992;30:2692–2697. doi: 10.1128/jcm.30.10.2692-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dever, L. L., J. H. Jorgensen, and A. G. Barbour. In vitro activity of vancomycin against the spirochete Borrelia burgdorferi. Antimicrob. Agents Chemother. 37:1115–1121. [DOI] [PMC free article] [PubMed]
- 7.Jones R N, Barrett M S. Antimicrobial activity of SCH 27899, oligosaccharide member of the everninomycin class with a wide gram-positive spectrum. J Clin Microbiol Infect. 1995;1:35–43. doi: 10.1111/j.1469-0691.1995.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones R N, Johnson D M, Erwin M E. In vitro antimicrobial activities and spectra of U-100592 and U-10076, two novel fluorinated oxazolidinones. Antimicrob Agents Chemother. 1996;40:720–726. doi: 10.1128/aac.40.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low D E. Quinupristin/dalfopristin: spectrum of activity, pharmacokinetics, and initial clinical experience. Microb Drug Resist. 1995;1:223–234. doi: 10.1089/mdr.1995.1.223. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. NCCLS document M26-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 11.Paster B J, Dewhirst F E, Weisburg W G, Tordoff L A, Fraser G J, Hespell R B, Stanton T B, Zablen L, Mandelco L, Woese C R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991;173:6101–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavia C, Wormser G, Nowakowski J, Cacciapuoti A. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Comparative in vitro sensitivity of Borrelia burgdorferi to Ziracin ( SCH27899), penicillin, and ceftriaxone, abstr. E-117. [Google Scholar]
- 13.Sadziene A, Thompson P A, Barbour A G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 14.Sigal L H. The Lyme disease controversy: social and financial costs of misdiagnosis and mismanagement. Arch Intern Med. 1997;156:1493–1500. doi: 10.1001/archinte.156.14.1493. [DOI] [PubMed] [Google Scholar]
- 15.Urban C, Mariano N, Mosinka-Snipas K, Wadee C, Charhour T, Rahal J J. Comparative in-vitro activity of SCH 27899, a novel everninomicin, and vancomycin. J Antimicrob Chemother. 1996;37:361–364. doi: 10.1093/jac/37.2.361. [DOI] [PubMed] [Google Scholar]