Abstract
The inhibition of intracellular Leishmania amazonensis growth by 2′,6′-dihydroxy-4′-methoxychalcone (DMC) isolated from Piper aduncum was further enhanced after encapsulation of DMC in polymeric nanoparticles. Encapsulated DMC also showed increased antileishmanial activity in infected BALB/c mice, as evidenced by significantly smaller lesions and fewer parasites in the lesions.
Leishmania spp. are obligate protozoan parasites of the macrophage, where they survive and multiply in the phagolysosome. Depending on the parasite species, different forms of leishmaniasis may develop in the mammalian host, ranging from chronic skin ulcers to fatal visceral disease. The number of infected people is steadily rising in several parts of the world, in part due to the lack of effective drugs which pose no serious toxic side effects (7). The search for new antileishmanial agents is generally hampered by the intracellular location of the parasites. Despite the difficulty of access for soluble substances, the phagolysosomes easily accommodate particulate material by vesicle fusion (2, 22). This feature is being used as a strategy to increase the bioavailability of drugs and reduce their toxicity (8, 14). Amphotericin B has been successfully encapsulated in liposomes, with increased effectiveness and reduced toxicity for visceral (3, 5, 18, 19) and cutaneous (17, 23) leishmaniasis patients. Drug incorporation in nanoparticles of biocompatible polymers has an advantage over other systems due to ease of preparation, a longer shelf life, and greater stability in biological fluids (10). Nanoparticles prepared with biodegradable poly(lactide) have been proposed as a passive system of drug delivery to macrophages that would increase the therapeutic index of leishmanicidal drugs (14). The advantage of this polymer is related to its degradation to lactic acid, which is eliminated in the urine and exhaled as CO2 (1). The antileishmanial activity of chalcones has been previously reported for Leishmania donovani (4). In the search for novel chemotherapeutic agents for the treatment of cutaneous leishmaniasis, we have recently described the effectiveness and selectivity of the 2′,6′-dihydroxy-4′-methoxychalcone (DMC) extracted from the herb Piper aduncum against the promastigote and amastigote forms of Leishmania amazonensis in vitro (20). In the present work, we report on the improvement of the antileishmanial effect of DMC by encapsulation in poly(d,l-lactide) (PLA) nanoparticles in vitro and in vivo.
DMC was purified from P. aduncum (Piperaceae) inflorescences following fractionation of the dichloromethane extract as described by Moreira and colleagues (12) and entrapped in PLA nanoparticles as described previously (15). Briefly, PLA (100 mg) and DMC (10 mg) were dissolved in 10 ml of acetone and mixed with 20 ml of Pluronic F68 aqueous solution (1%) under agitation for 10 min. The organic solvent was eliminated by evaporation. Unloaded nanoparticles were made in the same way by omitting DMC. The average diameters were 130 ± 35 and 168 ± 65 nm for unloaded and DMC-loaded nanoparticles, respectively. The encapsulation rate was 92%. The formulation is stable when stored at 4°C, maintaining its physical and chemical properties for at least 1 month.
To assess the effect of PLA-encapsulated DMC (DMC-PLA) on intracellular amastigotes, mouse peritoneal macrophages were infected with promastigotes of L. amazonensis in eight-chamber Lab-Tek slides (Nunc) at a 1:4 cell ratio for 4 h (20), washed, and cultivated for a further 48 h in the presence of DMC (1 μg/ml), DMC-PLA (5 μg of PLA plus 1 μg of DMC/ml), or empty PLA (5 μg/ml) in Dulbecco’s modified Eagle’s medium plus 5% fetal calf serum. The monolayers were washed and Giemsa stained for parasite counting under light microscopy. Alternatively, infected cultures were cultivated for 6 h in the presence of empty PLA (5 μg/ml) and processed for electron microscopy as described previously (20).
To assess the effect of DMC targeting with PLA nanoparticles in vivo, female BALB/c mice weighing 20 g were infected with 4 × 106 L. amazonensis promastigotes in the rear footpad. Each mouse was treated intraperitoneally with either empty PLA (1 mg), DMC-PLA (1 mg of PLA plus 200 μg of DMC), Glucantime (meglumine antimoniate, 200 μg of Sb; Rhodia), or phosphate-buffered saline (PBS) alone on days 42 and 48 of infection as well as with a 10-fold-smaller subcutaneous dose of the respective material on days 27 and 54. Free DMC was omitted here because treatment with doses as high as 1 mg of DMC did not alter the course of leishmanial lesions in comparison to that for PBS controls (21). Lesion sizes were measured with a dial caliper every 3 to 4 days. At the end of the experiment (day 74), the animals were killed under ether inhalation, and their infected feet were excised, skinned, weighed, and minced in Schneider’s insect medium (Sigma) plus 5% fetal calf serum. The cell suspensions were serially diluted in 100-μl samples and maintained at 26°C for 48 h. The relative parasite loads in the infected feet were estimated by counting the number of promastigotes derived from 10 μg of tissue. Statistical analysis of the data was performed by using the Student t test.
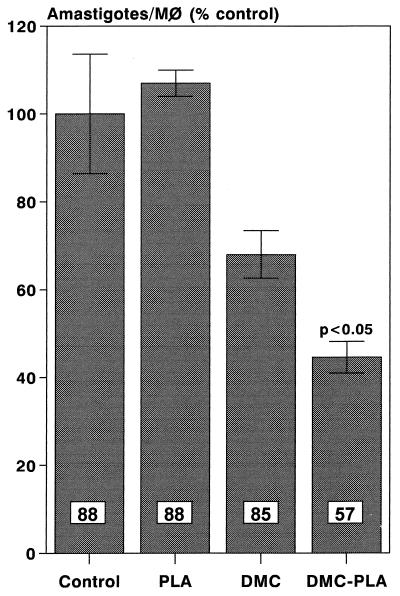
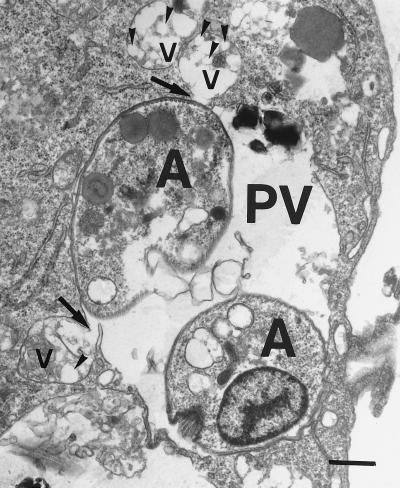
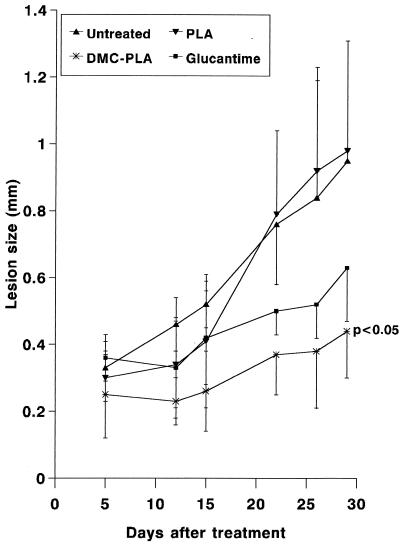
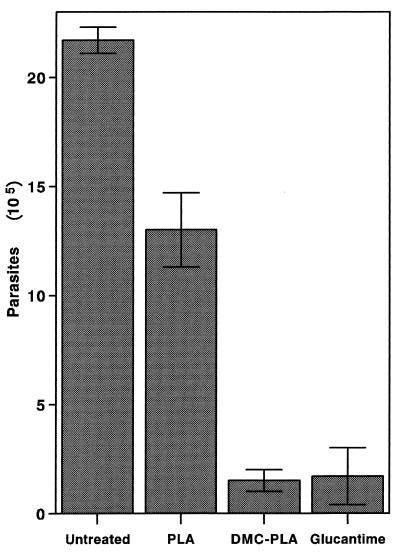
The results in Fig. 1 show that whereas a suboptimal concentration of free DMC (1 μg/ml) induced a 23% reduction in the number of intracellular leishmanias, DMC-PLA produced a 53% reduction. PLA alone did not significantly affect parasite growth inside the macrophages. We consistently observed the fusion of PLA nanosphere-containing vacuoles with the parasitophorous vacuoles of infected macrophages, as exemplified in the electron micrograph shown in Fig. 2. This indicates that the nanoparticles actually reach the parasite site before their degradation and suggests that DMC may be discharged close to the parasites, improving its bioavailability. Previous studies in vivo had demonstrated that local subcutaneous treatment with doses of free DMC as high as 1 mg did not alter the course of leishmanial lesions in comparison to treatment with PBS alone (21). However, when DMC-PLA nanoparticles were used at a dose 5 times smaller, the animals showed significantly smaller lesions (P < 0.05), about 60% the size of lesions in control animals treated with PBS or empty PLA nanoparticles alone (Fig. 3). The DMC-PLA effect was comparable to that of equivalent doses of Glucantine, which is considered the first-choice drug for the treatment of leishmaniasis (13). Thirty days after the initiation of treatment, the parasite load in the lesions was quantitated, demonstrating that the number of parasites in the DMC-PLA group was 90% lower than that in PBS controls, similar to the effect observed with Glucantime (Fig. 4). A partial reduction in the number of parasites was observed in vivo with empty PLA. The parasite load is considered a more accurate indication of the degree of infection than the lesion size, although the latter may be more appropriate for follow-up if the animals remain alive. We have detected live parasites in clinically cured lesions before (6) and have suggested that this discrepancy may be due to a possible anti-inflammatory effect of the drug. Whether empty PLA nanoparticles have intrinsic anti-inflammatory activity is not yet known, but it is possible that cell types other than macrophages may be activated by the PLA in vivo and may indirectly induce parasite death in the lesions. Some nanoparticle carriers may have direct activity against the parasites (isoalkyl cyanoacrylate nanoparticles, for instance, have shown intrinsic activity against trypanosomes [11] and L. donovani [9]), but this may not be the case with PLA, which showed no direct effect on L. amazonensis (Fig. 1) or L. donovani (14).
FIG. 1.
Inhibition of intracellular parasite growth in vitro by DMC after encapsulation in PLA. L. amazonensis-infected macrophages were cultured for 48 h in the presence of 1 μg of free DMC/ml, 5 μg of empty PLA nanoparticles/ml, or the same concentrations of DMC-PLA. Control, medium alone. The percentages of infected macrophages are given inside the bars. The P value of DMC-PLA in relation to DMC is given. Results are means ± standard deviations (SD) (n = 3).
FIG. 2.
Electron micrograph showing the fusion of PLA nanoparticles with the parasitophorous vacuole. Infected macrophages were cultivated for 6 h in the presence of PLA nanoparticles and then processed for electron microscopy. PLA nanosphere-containing vacuoles (v) and one parasitophorous vacuole (PV) containing two amastigotes (A) are shown. Arrows indicate the fusion of PLA-containing vacuoles with the parasitophorous vacuole membrane. Arrowheads point to individual nanospheres. Bar, 0.5 μm.
FIG. 3.
In vivo effectiveness of DMC-PLA treatment. BALB/c mice were infected with L. amazonensis and after 42 days of infection received a total dose of 440 μg of DMC-PLA or Glucantime. Controls received PBS or equivalent doses of empty PLA. Results are means ± SD (n = 5).
FIG. 4.
Parasite load in DMC-PLA-treated mice. BALB/c mice (five per group) were infected and treated as described in the legend to Fig. 3. Thirty days after the initiation of treatment (day 74 of infection), the numbers of parasites in the footpads were estimated. Means ± SD of triplicate samples are shown.
The diameter of particles, between 130 and 170 nm, is compatible with intravenous administration. In fact, DMC-PLA was well tolerated by mice that received intravenous injections. We observed that uninfected BALB/c mice (n = 10) treated with three consecutive daily doses of 5 mg of DMC-PLA showed no change in weight gain or mortality rate after 30 days of follow-up, compared with an untreated group (16).
This study therefore points to the in vivo effectiveness of a novel antileishmanial chalcone and the feasibility and efficacy of its targeting to the specific site by means of PLA nanoparticles. This preparation may well serve as the basis for the development of a substitute for the toxic antileishmanial drugs currently in use.
Acknowledgments
We thank Márcia Attias for helpful advice on the electron microscopy study.
REFERENCES
- 1.Bazile D V, Ropert C, Huve P, Verecchia T, Marland M, Feydman A, Veillard M, Spenlehauer G. Body distribution of fully biodegradable 14C-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials. 1992;13:1093–1102. doi: 10.1016/0142-9612(92)90142-b. [DOI] [PubMed] [Google Scholar]
- 2.Berman J D, Fioretti T B, Dweyer D M. In vivo and in vitro localization of Leishmania within macrophage phagolysosomes: use of colloidal gold as a lysosomal label. J Protozool. 1981;28:239–242. doi: 10.1111/j.1550-7408.1981.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 3.Berman J D. Human leishmaniasis: clinical, diagnostic and chemotherapeutic developments in the last ten years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Christensen S B, Theander T G, Kharazmi A. Antileishmanial activity of licochalcone A in mice infected with Leishmania major and in hamsters infected with Leishmania donovani. Antimicrob Agents Chemother. 1994;38:1339–1344. doi: 10.1128/aac.38.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft S L, Davidson R N, Thornton E A. Liposomal amphotericin B in the treatment of visceral leishmaniasis. J Antimicrob Chemother. 1991;28:111–118. doi: 10.1093/jac/28.suppl_b.111. [DOI] [PubMed] [Google Scholar]
- 6.Da-Silva S A G, Costa S S, Mendonça S C F, Silva E M, Moraes V L G, Rossi-Bergmann B. Therapeutic effect of oral Kalanchoe pinnata leaf extract in murine leishmaniasis. Acta Trop. 1995;60:201–210. doi: 10.1016/0001-706x(95)00128-2. [DOI] [PubMed] [Google Scholar]
- 7.Desjeux P. Leishmaniasis: public health aspects and control. Clin Dermatol. 1996;14:417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 8.Fusai T, Deniau M, Durand R, Bories C, Paul M, Rivollet D, Astier A, Houin R. Action of pentamidine-bound nanoparticles against Leishmania on an in vivo model. Parasite. 1994;1:319–324. doi: 10.1051/parasite/1994014319. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar R, Opperdoes F R, Preat V, Roland M. Drug targeting with polyalkylcyanoacrylate nanoparticles: in vitro activity of primaquine-loaded nanoparticles against intracellular Leishmania donovani. Ann Trop Med Parasitol. 1992;86:41–49. doi: 10.1080/00034983.1992.11812629. [DOI] [PubMed] [Google Scholar]
- 10.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 11.Lherm C, Couvreur P, Loiseau P, Bories C, Gayral P. Unloaded polyisobutylcyanoacrylate nanoparticles: efficiency against bloodstream trypanosomes. J Pharm Pharmacol. 1987;39:650–652. doi: 10.1111/j.2042-7158.1987.tb03446.x. [DOI] [PubMed] [Google Scholar]
- 12.Moreira D L, Guimarães E F, Kaplan M A C. A chromene from Piper aduncum L. Phytochemistry. 1998;48:1075–1077. [Google Scholar]
- 13.Olliaro P I, Bryceson D M. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today. 1993;9:323–328. doi: 10.1016/0169-4758(93)90231-4. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues J M, Jr, Croft S L, Fessi H, Bories C, Devissaguet J P. The activity and ultrastructural localization of primaquine-loaded poly(d,l-lactide) nanoparticles in Leishmania donovani-infected mice. Trop Med Parasitol. 1994;45:223–228. [PubMed] [Google Scholar]
- 15.Rodrigues J M, Jr, Fessi H, Bories C, Puisieux F, Devissaguet J P. Primaquine-loaded poly(lactide) nanoparticles: physicochemical study and acute tolerance in mice. Int J Pharm. 1995;126:253–260. [Google Scholar]
- 16.Rodrigues, J. M., Jr. 1997. Unpublished data.
- 17.Sampaio R N, Marsden P D. Treatment of the mucosal form of leishmaniasis without response to Glucantime, with liposomal amphotericin B. Rev Soc Bras Med Trop. 1997;30:125–128. doi: 10.1590/s0037-86821997000200007. [DOI] [PubMed] [Google Scholar]
- 18.Seaman J, Boer C, Wilkinson R, Jong J, Wilde E, Sondorp E, Davidson R. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin Infect Dis. 1995;21:188–193. doi: 10.1093/clinids/21.1.188. [DOI] [PubMed] [Google Scholar]
- 19.Smith O P, Hann I M, Cox H, Novelli V. Visceral leishmaniasis: rapid response to AmBisome treatment. Arch Dis Child. 1995;73:157–159. doi: 10.1136/adc.73.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Santos E C, Moreira D L, Kaplan M A C, Meirelles M N, Rossi-Bergmann B. The selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob Agents Chemother. 1999;43:1234–1241. doi: 10.1128/aac.43.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres-Santos, E. C., and B. Rossi-Bergmann. 1997. Unpublished data.
- 22.Veras P S T, De Chastellier C, Rabinovitch M. Transfer of zymosan (yeast cell walls) to the parasitophorous vacuoles of macrophages infected with Leishmania amazonensis. J Exp Med. 1992;176:639–646. doi: 10.1084/jem.176.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yardley V, Croft S L. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 1997;41:752–756. doi: 10.1128/aac.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]