Abstract
We report a study that seeks to find a correlation between the overall sensitization potential quantified by the expression of IL-8 by stimulated monocytes and the chemical structure of a model contact allergen, 2,4-dinitrochlorobenzene (DNCB). We show that structure and reactivity of the chemical compounds play an important role in activation of the monocytes and subsequent inflammation in tissue. However, we observed a non-linear correlation between the rate of reaction and biological activity indicating a required balance of stability and reactivity.
Keywords: 1-chloro-2,4-dinitrochlorobenzene (DNCB); Structure Activity Relationship (SAR); Interleukin (IL) – 8; Contact Hypersensitivity; Skin sensitization
1. Introduction
Elucidation of contact hypersensitivity is a longstanding goal in allergy and immune response. The concept of protein reactivity is commonly used to explain the initial step towards sensitization by small molecular weight compounds commonly called contact allergens.1,2 The physicochemical properties of the contact allergens that dictate this initial phase of the pathology includes reactivity, spatial geometry, and steric restraints.3,4 There have been multiple studies highlighting the relationship between chemical reactivity and an external sensitization index such as LLNA.5-8 However, limited studies encompass both the structural and electronic requirements of reactivity, with a direct comparison to in-vivo and in-vitro activity. DNCB (1) is a widely used reference molecule in sensitization models probing pathways and cell types involved in the sensitization process.9-11 Here, we investigate the specific structural requirements for the high sensitization potential of DNCB (1) using a two-pronged strategy encompassing a cell-based assay and examination of parameters defining chemical reactivity.
We report that the rate of reactivity as well as steric elements contribute to a contact allergen’s ability to stimulate IL-8 production in target human THP-1 cells and ultimately sensitivity in vivo. However, the relationship is not linear. Using these experiments, we also show that non-allergenic compounds can be made to activate skin inflammation by controlling their rate of aromatic substitution reaction – the reaction postulated to occur within the skin. In examining contact allergen responses, we sought a reliable in vitro technique for quantifying activity of individual compounds. Historically, contact allergen identification was done by accessing the elicitation phase after painting the skin or injecting the allergen.12-15 However, in the past decades, the field has moved to murine local lymph node assay (LLNA)16-18 and cell-based assays.19-21 These cell-based assays, notably the activation of cultured THP-1 monocyte, minimize animal usage and are translatable to humans.19 DNCB is hypothesized to form covalent bonds through a nucleophilic substitution mechanism with nucleophilic residues of proteins. What intrigued us was that despite the widespread use of DNCB both in culture and in vivo as a model of allergy, very little work examines the mechanism of DNCB or its analogs.
2. Results and Discussion
In the 1930’s, Landsteiner and Jacobs reported a series of papers detailing their sensitization experiments on guinea pigs with DNCB and structurally-similar compounds.15,22-24 Since then, there have been many reports studying the effect of electrophilicity of contact allergens on sensitization potential.25-28,29,30 From these earlier experiments, we ascertained that although reactivity was a critical component to sensitization, sterics play a role in immunological activity and sought to clarify this further. For all initial experiments, we maintained consistency with the field of contact allergens by testing with the human monocyte cell line THP-1 for our cell model and measuring interleukin (IL)-8 expression31-33 as the proxy for the sensitization potential of DNCB and its derivatives. Working from the previous report by Landsteiner in 1935, we first tested the effect of the quality of leaving group of the aromatic compounds.22 The substitution reaction mechanism occurs through a Meisenheimer complex that is stabilized by electronegative leaving groups.34 We chose halogen derivatives of DNCB as an initial examination of this phenomenon (Figure 2a, 1-4). We expected that we would see a correlation between electronegativity and IL-8 expression where the most electronegative leaving group would lead to the highest expression. THP-1 cells were incubated with the compounds at 50 μM concentration, the optimal condition for IL-8 release (Supplementary Figure 1), and the percent of IL-8 expressing cells was determined through either cytokine staining or quantified using an ELISA assay.
Figure 2.
The leaving group halides (a) and non-halide leaving groups (b) were explored. THP-1 cells were incubated with 50 μM of labelled compounds for 10 h and the percentage of IL-8 expressing cells were measured via flow cytometry. * = significance from resting, ¥ = significance from DNCB. *P ≤ 0.05 ***P ≤ 0.001 ****P ≤ 0.001 ¥¥ P ≤ 0.01
Broadly, we observed reduction of IL-8 expression which correlated to the decrease of electronegativity of the leaving group halides. We detected a loss of activity with dinitrophenol (DNP, 5), which confirmed the requirement of a leaving group. Interestingly, we observed that the difference in IL-8 expression between dinitrofluorobenzene (DNFB, 2) and DNCB (1) was not significant (Figure 2a). This could be due to the high reactivity of DNFB, which could result in loss of activity through hydrolysis. Based on our initial observations, we hypothesized that any leaving group which enabled a Meisenheimer complex would likely allow the activation of IL-8 in cells. To test this, we modified DNP with both tosyl (6) and mesyl (7) groups to create DNP based derivative capable of reacting (Figure 2b). Both derivatives 6 and 7 activated cells resulting in IL-8 levels comparable to or higher than that of DNCB (Figure 2). Interestingly, the mesyl 7 resulted in higher activity than the tosyl 6 and the parent compound 1, implying an additive positive effect of having a small and unhindered leaving group. As the leaving group experiments indicated aromatic nucleophilic substitution (SnAr) mechanism, we tested the quality of electron withdrawing substituents. One important element of an SnAR is the ability to correlate reactivity with the electron density at the point of reaction in the molecule.35,36 To test this hypothesis, we chose substituents with varying electron withdrawing capabilities36 including cyano (8 and 12, p 0.66), carboxylic acid (9 and 12, p 0.45), and a methyl ester (10 and 13, p 0.45) at the para and ortho position relative to the leaving group (Figure 3a).
Figure 3.
The quality of electron-withdrawing groups was explored. A series of electron withdrawing groups were substituted in place of nitro group (a) or additional electron-withdrawing groups were substituted in ortho position (b). THP-1 cells were incubated with 50 μM of labelled compounds for 10 h and the percentage of IL-8 expressing cells were measured via flow cytometry. * = significance from resting, ¥ = significance from DNCB. *P ≤ 0.05 **P ≤ 0.01 ****P ≤ 0.0001
Using the same experimental procedure, IL-8 expression was measured. As expected, we observed a decrease in IL-8 expression as the compounds assayed were more electron-rich than DNCB (1, Figure 3a). From this, we concluded that the electron withdrawing capability of the substituents are a determining factor for activity. This scaffold required substituents with electron withdrawing capabilities comparable to the nitro group at the para and ortho position as is the case for DNCB (1). With this in mind, we sought to elucidate the effect of increasing the number of electron withdrawing groups – balancing it with the activity of steric interactions with potential target proteins. To test overall electron withdrawing capacity, we examined a set of compounds which included derivatives with the two nitro groups of the parent compound 1 as well as carboxylic acid and ester groups (Figure 3b, 14-17).
We observed comparable activity of 3,5-dinitro-4-chlorobenzoic acid (14), to DNCB (1). This similarity indicated a cumulative effect of the electron-withdrawing groups in this compound. However, similar derivatives (15-17) were not comparable in activity, implying that sterics and potentially charge density were also critical factors. Investigating this hypothesis further, we synthesized and assayed ester derivatives (Figure 4, 18-21). Although these ester derivatives failed to elicit a response as strong as the one observed from the carboxylic acid (14), we observed an above resting activity with the ortho substituted esters. This suggests the importance of the carboxylic group or nitro group at the para position (Figure 4, 18 and 19). One potential explanation for this difference is that the potential target protein/s has a binding pocket with an H-bonding acceptor.
Figure 4.
IL-8 secretion by THP-1 cells stimulated with the parent compound (1) and the ester derivatives (18-21). THP-1 cells were incubated with 50 μM of labelled compounds for 20 h and the IL-8 secreted to supernatant was quantified using sandwich ELISA * = significance from resting, ¥ = significance from DNCB. **P ≤ 0.01 ***P ≤ 0.001 ****P ≤ 0.0001
Observing such clear relationships between structure and activity, we examined the mechanism/process by comparing the chemical reactivity of these compounds to the biological activity, both experimentally and with models. To simulate the reaction with a protein, we reacted the compounds with cysteine at physiological pH. This reaction is a common proxy for a contact allergen’s ability to generate a response.37 Plotting the log kobs vs normalized IL-8 activity, we observed that there was no simple, linear correlation between electrophilicity of a compound and its cellular activity. High reactivity did not immediately translate to high cellular response. Our model compound (1) was comparable in biological reactivity to the tosyl (6) and 3,5-dinitro-4-chlorobenzoic acid (14). The ester derivatives (15, 17, and 19) showed the highest relative rate of reaction. These compounds, however, resulted in lower IL-8 expression when incubated with THP-1 cells compared to DNCB (1, Figure 5). We also observed that there was a rate of reactivity window where a balance between compound stability and reactivity led to IL-8 expression (Figure 5). We observed that the substituted esters (19-21) were not as potent as DNCB (1) in eliciting IL-8 activity (Figure 3, 4). By plotting the log kobs vs IL-8, we see that the chemical reactivity of these esters is comparable to DNCB (1) but that they elicit low IL-8 expression suggesting steric hindrance at the site of reactivity (Figure 5).
Figure 5.
Correlation IL-8 activity to the rate of reactivity to cysteine. IL-8 activity of compounds was normalized to DNCB (1).
Using a molecular modelling software (Spartan), we calculated the molecular orbitals of the electrophiles using B3LYP/6-31+G (d,p) basis set and generated an electrophilicity map. This was done by mapping the absolute value of the lowest-unoccupied molecular orbital (LUMO) on the electron density. We compared the nucleophilic reactivity of the derivatives at the site of nucleophilic substitution reaction. Plotting the ∣LUMO∣ value vs log k0bs, we saw that there was a loose but direct correlation between the experimental and computational reactivity. Plotting IL-8 activity v ∣LUMO∣ (Supplementary Figure 7) indicated a similar trend as with the experimental plot (Figure 5) where there appeared a required balance of reactivity and stability for optimal IL-8 expression.
Hapten formation is considered the initial step in the pathology of allergic contact dermatitis.3 There are ongoing studies that are targeted to the identification of proteins that react with contact allergens and become immunogenic. Therefore, we wanted to compare proteins that were modified by DNCB to those of some of the derivatives. DNCB usually modifies nucleophilic side chains such as cysteines (Figure 6A and 6B). Proteins modified by DNCB and its derivatives were analyzed by western blotting with anti-DNP antibody that could be used to detect DNP-moieties on the proteins (Supplementary Figure 3). We expected that this experiment would tell us which proteins are labeled and the relative amount of modification. Because compounds 8 and 11 would result in conjugation of a non-DNP moiety, we confirmed the specificity of the anti-DNP antibody with BSA modified with both 8 and 11 (SI). From the western blot, we saw that treatment with compounds 4-7 resulted in a series of gel staining pattern similar to that of DNCB (1, Figure 6C), suggesting that similar proteins are modified. Compounds 8 and 11 however showed no modified proteins in the lysate. This result indicates that although the immune response seen after treating THP-1 cells with the derivatives 4-7 was varied, these compounds seened to modify similar proteins. Treatment with compounds 8 and 11 led to low or resting IL-8 levels on THP-1 cells. These derivatives also showed slow reaction kinetics with cysteine. In this experiment, we also confirmed that these two derivatives did not modify any proteins when added to THP-1 cells, confirming that protein modification is a clear correlation for an immune response on the THP-1 cells.
Figure 6.
Diagram showing DNCB modification to cysteine side chains of cellular protein (a) and the reaction mechanism (b). Proteins modified by DNCB (1) and its derivatives were analyzed by western blotting (c, left). Untreated lane shows no band, confirming that anti-DNP antibody is specific to DNP-like-moieties. Compounds 4, 6, and 7 show band intensity similar to that of DNCB. Proteins were separated using the same material and methods and Coomassie-stained to confirm that the same amount of proteins were used in all lanes (c, right).
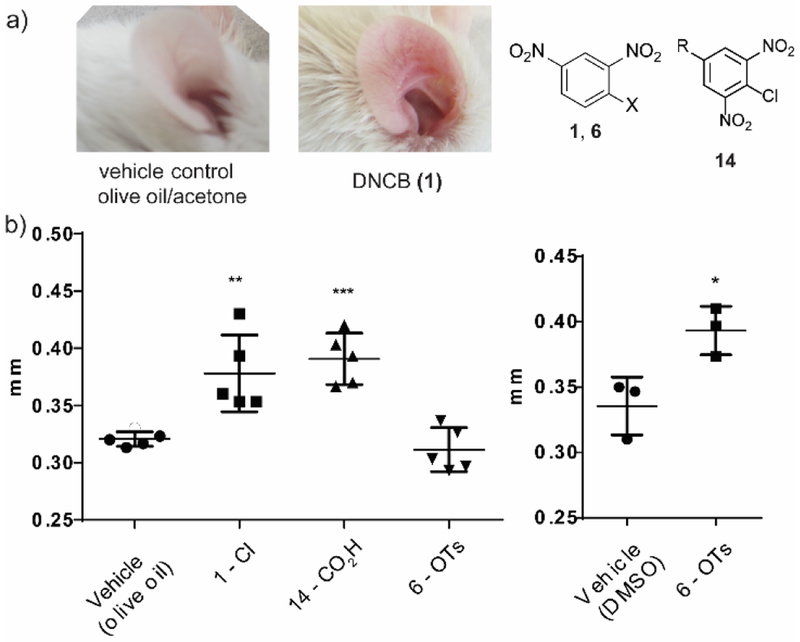
Lastly, we sought to confirm that our cell-based assay results would translate into a mouse model. We obtained male Balb/c mice between 6-7 weeks old. Based on a previously published protocol,38 we sensitized their shaven abdomen for two consecutive days. After a rest period, the mice were challenged on the ear dorsum for five consecutive days. To determine the degree of inflammation, we measured the ear thickness the day after the last compound exposure.
We observed a general inflammation of the mice dorsum with DNCB (1) that was comparable to that of benzoic acid (14). The tosyl (6) induced low inflammation which was unexpected based on the cell-based assay, where we saw comparable IL-8 expression to DNCB (1, Figure 7). We speculated that the compound was unable to cause inflammation at a comparable level with DNCB due to its inability to cross the skin barrier. To investigate this hypothesis, we repeated the experiment using DMSO as a vehicle to ensure skin penetration. In this case, we did observe inflammation that was statistically significant when compared to the control mice– confirming our hypothesis (Figure 7).
Figure 7.
Skin inflammation study on mouse dorsum. (a) Examples of unswollen (left) and swollen (right) mouse dorsum. Mouse was treated with 0.5 % compound solution on day 0 and 1. The mouse was challenged with the same 0.3 % compound solution on day 5, 6, 7 and 8. (b) Ear thickness measured on day 9. * = significance from resting, **P ≤ 0.01 ***P ≤ 0.001 ****P ≤ 0.0001
In this study, we show that there are various aspects to the degree of sensitization potential of DNCB and its derivatives. We show that understanding the reactivity of the compounds can be used to manipulate and predict the in vitro and in vivo response more broadly than screening each compound individually. However, the rate of covalent bond formation of a contact allergen is not linearly correlated with its sensitization potential. This result strongly implies that there are specific protein targets which rely on spatial information along with reactivity to determine activation of an allergic response. We hope to use these findings in the development of DNCB based contact allergen probes for the identification of protein targets in the overall elucidation of the contact allergen pathway. This work may also guide the use of DNCB analogues which elicit stronger reactions than DNCB improving the outcomes of immunological mechanisms probed via DNCB.
3. Methods
3.1. Intracellular cytokine flow cytometry staining
Cytofix/Cytoperm™ kit with GolgiPlug™ was purchased from BD Biosciences (San Jose, CA). Cytokine staining was performed according to BD Biosciences protocol with minor modifications. The test compounds were dissolved in DMSO to make 0.5 M or 1 M concentration and sterile-filtered using 0.22 μm syringe filters. THP-1 cells were harvested and plated in a 24-well plate at the concentration of 106 cells/mL. The cells were subsequently incubated with 50 μM of compounds and 1 μL of BD GolgiPlug™ (protein transport inhibitor containing Brefeldin A). Lipopolysaccharide (LPS, 200 ng/mL) was used as positive control for immune activation. After 10 h incubation at 37 °C with 5 % C02, the cells were washed with FACS buffer (300 μL, 2X). The cell pellet was resuspended in 300 μL of Cytofix/Cytoperm™ solution and incubated on ice for 20 m. The cells were washed with 1X BD Perm/Wash™ Buffer (300 μL, 2X) and then resuspended in 50 μL of 10X BD Perm/Wash™ Buffer. For antibody staining, 5 μL of FITC anti-human IL-8 antibody (BioLegend) was added to each sample and vortexed. After 30 m incubation on ice with rigorous vortexing at the halfway mark, the cells were washed with 1X BD Perm/Wash™ Buffer (300 μL, 3X). IL-8 expression was analyzed using BD Accuri C6 cytometer and BD Accuri C6 software.
3.2. ELISA
Human IL-8 ELISA MAX™ Deluxe was purchased from BioLegend (San Diego, CA). The experiment was carried out according to the manufacturer’s protocol. About 5 × 105 cells were plated in 500 μL of culture media containing 50 μM compounds. LPS at concentration of 200 ng/mL was used for positive control. After 20 h of incubation, the cells were harvested and the supernatant was collected for analysis.
3.3. Western Blot analysis
Conjugation of target protein was performed according to previously published protocol.39-41 Bovine serum albumin (BSA, Millipore Sigma) and the selected compounds were dissolved in a mixture composed of 17 parts 1X PBS and 3 parts 100 % acetonitrile (HPLC grade) and incubated in 37 C for 7 days. To ensure conjugation, 10 μl of the sample was desalted and analyzed using MALDI. To analyze proteins modified by DNCB and its derivatives, THP-1 cells were incubated in RPMI supplemented with 10 % FBS and 50 μM of compounds. After 30 m incubation, cells were lysed with 1x cell lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 % Triton X-100) supplemented with 1 tablet of cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail (Sigma). Cell lysates were quantified using Pierce™ BCA Protein Assay Kit. For western blot analysis, proteins were first separated by 4-15 % Mini-Protean® TGX™ Precast Protein Gels. Then, separated proteins were tank-transferred to PVDF membrane, and primarily stained with mouse anti-DNP antibody (Millipore) that could bind to protein-conjugated DNP moieties.40 After staining with goat anti-mouse IgG (H+L) Secondary Antibody, DyLight 650, the membrane was imaged using Azure c600 Imaging System (Azure Biosystems). To ensure that equal amount of protein was used, cell lysates were separated again in the same condition, fixed in gel-fixing solution (50 % (v/v) ethanol in water with 10 % (v/v) acetic acid) for 1 h, washed in gel-washing solution (50 % (v/v) methanol in water with 10 % (v/v) acetic acid) overnight, stained in Coomassie solution (0.1 % (w/v) Coomassie blue R350, 20 % (v/v) methanol in water with 10 % (v/v) acetic acid) for 3 h, and then destained with gel-washing solution until desired color and band intensity appeared. Band intensity was calculated using ImageJ and normalized to the band intensity of DNCB-treated sample.
3.4. Quantifying sensitization through mice ear thickness experiments
This experiment was run according to literature protocol38 with minor modifications. DNCB and its derivatives were dissolved into 0.5 % (w/v) solutions using a 4:1 by volume acetone/olive mixture or DMSO. Male Balb/c mice was sensitized by exposing the shaven abdomen to 25 μL of the compounds utilizing a different animal for each compound. The negative control was run in tandem, utilizing the vehicle without any of the assayed compounds in solution. Sensitization was performed on day 0 and 1. On day 5,6,7, and 8, the mice were challenged on the dorsum of both ears with 10 μL of 0.3 % (w/v) solutions of the compounds in the same vehicle as the sensitization phase. Ear thickness was measured 24 h after the last compound exposure using a digital micrometer.
3.5. General protocol for rate analysis
The compounds of interest were dissolved in 10 % CH3CN in PBS (1 mM, 1 mL). The reaction was initiated by adding 100 μL of cysteine (10 equiv., 10 mM) in PBS to the fully dissolved compounds. At each time point, 10 μL aliquots of solution were quenched with 0.1 % TFA and the content was analyzed by HPLC. The area under the compound curve and the compound-cysteine curve were calculated and used to generate pseudo first-order rate.
3.6. Computational Details
All computations were performed using Spartan software (Wavefun. Inc.). Ground state geometries and energies were optimized in gas phase using the B3LYP functional and 6-31 G+ (d.p) basis set for all atoms. The electron density, and the composite surface of ∣LUMO∣ mapped onto the electron density were then calculated. Hexyl-2-chloro-3,5-dinitrobenzoate (18), hexyl-4-chloro-2,5-dinitrobenzoate (20), tert-butyl-4-chloro-3,5-dinitrobenzoate (19), and tert-butyl-2-chloro-3,5-dinitrobenzoate (21) were truncated to propyl-2-chloro-3,5-dinitrobenzoate, propyl-4-chloro-2,5-dinitrobenzoate isopropyl-4-chloro-3,5-dinitrobenzoate, isopropyl-2-chloro-3,5-dinitrobenzoate respectively for feasible calculation (Supplementary Table 1).
Supplementary Material
Figure 1.
The overview of this paper. The sensitization potential of DNCB and derivatives is measured employing a variety of techniques
ACKNOWLEDGMENT
The authors would like to thank Yoseline Escalante-Buendia, Brittany Moser and Jainu Ajit for help with mouse experiments.
Funding Sources
The authors acknowledge the financial support provided by National Institute of Health grant DMR-1609946
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information.
Experimental and spectroscopic data of synthesized compounds, supporting methods, 3 supplementary figures and 1 supplementary table.
No competing financial interests have been declared.
REFERENCES
- 1.Ashby J, Basketter DA, Paton D & Kimber I Structure activity relationships in skin sensitization using the murine local lymph node assay. Toxicology 103, 177–194 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Kimber I et al. Alternative approaches to the identification and characterization of chemical allergens. Toxicology in vitro : an international journal published in association with BIBRA 15, 307–312 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Divkovic M, Pease CK, Gerberick GF & Basketter DA Hapten-protein binding: From theory to practical application in the in vitro prediction of skin sensitization. Contact Dermatitis 53, 189–200 (2005). [DOI] [PubMed] [Google Scholar]
- 4.McKinney JD The Practice of Structure Activity Relationships (SAR) in Toxicology. Toxicological Sciences 56, 8–17 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Chipinda I et al. Rapid and simple kinetics screening assay for electrophilic dermal sensitizers using nitrobenzenethiol. Chemical Research in Toxicology (2010). doi: 10.102l/txl00003w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbiya W, Chipinda I, Siegel PD, Mhike M & Simoyi RH Substituent effects on the reactivity of benzoquinone derivatives with thiols. Chemical Research in Toxicology (2013). doi: 10.1021/tx300417z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chipinda I et al. Pyridoxylamine reactivity kinetics as an amine based nucleophile for screening electrophilic dermal sensitizers. Toxicology (2014). doi: 10.1016/j.tox.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbiya W, Chipinda I, Simoyi RH & Siegel PD Reactivity measurement in estimation of benzoquinone and benzoquinone derivatives’ allergenicity. Toxicology (2016). doi: 10.1016/j.tox.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwa Y, Niwa H, Kohmura J, Ou DW & Yokoyama MM Studies on the dinitrochlorobenzene (DNCB) sensitization test. Ann Allergy 48, 108–112 (1982). [PubMed] [Google Scholar]
- 10.Popov A et al. Contact allergic response to dinitrochlorobenzene (DNCB) in rats: Insight from sensitization phase. Immunobiology 216, 763–770 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Zhang EY, Chen AY & Zhu BT Mechanism of dinitrochlorobenzene-induced dermatitis in mice: Role of specific antibodies in pathogenesis. PLoS ONE 4, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnusson B & Kligman AM The identification of contact allergens by animal assay. The guinea pig maximization test. The Journal of investigative dermatology 52, 268–276 (1969). [DOI] [PubMed] [Google Scholar]
- 13.Buehler EV Occlusive patch method for skin sensitization in guinea pigs: The Buehler method. Food and Chemical Toxicology 32, 97–101 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Frankild S, Volund A, Wahlberg JE & Andersen KE Comparison of the sensitivities of the Buehler test and the guinea pig maximization test for predictive testing of contact allergy. Acta Dermato-Venereologica 80, 256–262 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Landsteiner K & Jacobs J Studies on the Sensitization of Animals With Simple Chemical Compounds. Ii. The Journal of experimental medicine 64, 643–657 (1936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basketter DA et al. Use of the local lymph node assay for the estimation of relative contact allergenic potency. Contact Dermatitis 42, 344–348 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Rovida C et al. The local lymph node assay (LLNA). Current protocols in toxicology / editorial board, Maines Mahin D. (editor-in-chief) … [et al. Chapter 20, Unit 20.7 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Basketter DA, Gerberick GF, Kimber I & Loveless SE The local lymph node assay: A viable alternative to currently accepted skin sensitization tests. Food and Chemical Toxicology 34, 985–997 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Sharma NS et al. Perspectives on non-animal alternatives for assessing sensitization potential in allergic contact dermatitis. Cellular and Molecular Bioengineering 5, 52–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi H et al. Development of an in vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT). II. An inter-laboratory study of the h-CLAT. Toxicology in vitro : an international journal published in association with BIBRA 20, 774–784 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Python F, Goebel C & Aeby P Assessment of the U937 cell line for the detection of contact allergens. Toxicology and Applied Pharmacology 220, 113–124 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Landsteiner K & John J Studies on the sensitization of animals with simple compounds. The Journal of experimental medicine 643–657 (1935). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landsteiner K & Di Somma AA Studies on the sensitization of animals with simple compounds : V. Sensitization to diazomethane and mustard oil. The Journal of experimental medicine 68, 505–512 (1938). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landsteiner K & Chase MW Studies on the sensitization of animals with simple compounds : IX. Skin sensitization by injection of conjugates. The Journal of experimental medicine 73, 431–438 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delaine T, Hagvall L, Rudbäck J, Luthman K & Karlberg AT Skin sensitization of epoxyaldehydes: Importance of conjugation. Chemical Research in Toxicology 26, 674–684 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Manabe Y et al. 1-Fluoro-2,4-dinitrobenzene and its derivatives act as secretagogues on rodent mast cells. European Journal of Immunology 47, 60–67 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Roberts DW & Aptula AO Electrophilic reactivity and skin sensitization potency of SnAr electrophiles. Chemical Research in Toxicology (2014). doi: 10.1021/tx400355n [DOI] [PubMed] [Google Scholar]
- 28.Rosenkranz HS, Klopman G, Zhang YP, Graham C & Karol MH Relationship between allergic contact dermatitis and electrophilicity. Environmental Health Perspectives 107, 129–132 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisen HN & Belman S Studies of Hypersensitivity to Low Molecular Weight Substances: Ii. Reactions of Some Allergenic Substituted Dinitrobenzenes with Cysteine or Cystine of Skin Proteins. Journal of Experimental Medicine 98, 533–549 (1953). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisen HN, Kern M, Newton WT & Helmreich E A Study of the Distribution of 2,4-Dinitrobenzene Sensitizers Between Isolated Lymph Node Cells and Extracellular Medium in Relation to Induction of Contact Skin Sensitivity. Journal of Experimental Medicine 110, 187–206 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott H et al. High-resolution transcriptional profiling of chemical-stimulated dendritic cells identifies immunogenic contact allergens, but not prohaptens. Skin Pharmacology and Physiology 23, 213–224 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Mitjans M et al. Use of IL-8 release and p38 MAPK activation in THP-1 cells to identify allergens and to assess their potency in vitro. Toxicology in Vitro 24, 1803–1809 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Nukada Y, Ito Y, Miyazawa M, Sakaguchi H & Nishiyama N The relationship between CD86 and CD54 protein expression and cytotoxicity following stimulation with contact allergen in THP-1 cells. J Toxicol Sci 36, 313–324 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Meisenheimer J Ueber Reactionen aromatischer Nitrokorper. Justus Liebigs Annalen der Chemie 323, 205–246 (1902). [Google Scholar]
- 35.Hammett LP Effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 59, 96–103 (1937). [Google Scholar]
- 36.McDaniel DH & Brown HC An Extended Table of Hammett Substituent Constants Based on the Ionization of Substituted Benzoic Acids. Journal of Organic Chemistry 23, 420–427 (1958). [Google Scholar]
- 37.Gerberick GF et al. Development of a peptide reactivity assay for screening contact allergens. Toxicological Sciences 81, 332–343 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Röse L, Schneider C, Stock C, Zollner TM & Döcke WD Extended DNFB-induced contact hypersensitivity models display characteristics of chronic inflammatory dermatoses. Experimental Dermatology 21, 25–31 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Aleksic M et al. Investigating protein haptenation mechanisms of skin sensitisers using human serum albumin as a model protein. Toxicology in Vitro (2007). doi: 10.1016/j.tiv.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 40.Hirota M et al. Modification of cell-surface thiols elicits activation of human monocytic cell line THP-1: Possible involvement in effect of haptens 2,4-dinitrochlorobenzene and nickel sulfate. The Journal of Toxicological Sciences (2009). doi: 10.2131/jts.34.139 [DOI] [PubMed] [Google Scholar]
- 41.Aleksic M et al. Mass spectrometric identification of covalent adducts of the skin allergen 2,4-dinitro-l-chlorobenzene and model skin proteins. Toxicology in Vitro (2008). doi: 10.1016/j.tiv.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 42.Crossland RK & Servis KL A Facile Synthesis of Methanesulfonate Esters. Journal of Organic Chemistry 35,3195–3196 (1970). [Google Scholar]
- 43.Contreras Carballada P et al. Variation of the viologen electron relay in cyclodextrin-based self-assembled systems for photoinduced hydrogen evolution from water. European Journal of Organic Chemistry 6729–6736 (2012). doi: 10.1002/ejoc.201200886 [DOI] [Google Scholar]
- 44.Denehy E, White JM & Williams SJ Electronic structure of the sulfonyl and phosphonyl groups: A computational and crystallographic study. Inorganic Chemistry 46, 8871–8886 (2007). [DOI] [PubMed] [Google Scholar]
- 45.van der Aar EM et al. Enzyme kinetics and substrate selectivities of rat glutathione S-transferase isoenzymes towards a series of new 2-substituted 1-chloro-4-nitrobenzenes. Xenobiotica; the fate of foreign compounds in biological systems 26, 143–155 (1996). [DOI] [PubMed] [Google Scholar]
- 46.Shang J et al. Aromatic triazole foldamers induced by C-H…X (X = F, Cl) intramolecular hydrogen bonding. Journal of Organic Chemistry 79, 5134–5144 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Haydon DJ et al. Creating an antibacterial with in vivo efficacy: Synthesis and characterization of potent inhibitors of the bacterial cell division protein FTSZ with improved pharmaceutical properties. Journal of Medicinal Chemistry 53, 3927–3936 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Hiari YM, Qaisi AM, El-Abadelah MM & Voelter W Synthesis and Antibacterial Activity of Some Substituted 3-(Aryl)- and 3-(Heteroaryl)indoles. Monatshefte fur Chemie - Chemical Monthly 137, 243–248 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.