Abstract
Pantomime has a long tradition in clinical neuropsychology of apraxia. It has been much more used by researchers and clinicians to assess tool-use disorders than real tool use. Nevertheless, it remains incompletely understood and has given rise to controversies, such as the involvement of the left inferior parietal lobe or the nature of the underlying cognitive processes. The present article offers a comprehensive framework, with the aim of specifying the neural and cognitive bases of pantomime. To do so, we conducted a series of meta-analyses of brain-lesion, neuroimaging and behavioural studies about pantomime and other related tasks (i.e. real tool use, imitation of meaningless postures and semantic knowledge). The first key finding is that the area PF (Area PF complex) within the left inferior parietal lobe is crucially involved in both pantomime and real tool use as well as in the kinematics component of pantomime. The second key finding is the absence of a well-defined neural substrate for the posture component of pantomime (both grip errors and body-part-as-tool responses). The third key finding is the role played by the intraparietal sulcus in both pantomime and imitation of meaningless postures. The fourth key finding is that the left angular gyrus seems to be critical in the production of motor actions directed towards the body. The fifth key finding is that performance on pantomime is strongly correlated with the severity of semantic deficits. Taken together, these findings invite us to offer a neurocognitive model of pantomime, which provides an integrated alternative to the two hypotheses that dominate the field: The gesture-engram hypothesis and the communicative hypothesis. More specifically, this model assumes that technical reasoning (notably the left area PF), the motor-control system (notably the intraparietal sulcus), body structural description (notably the left angular gyrus), semantic knowledge (notably the polar temporal lobes) and potentially theory of mind (notably the middle prefrontal cortex) work in concert to produce pantomime. The original features of this model open new avenues for understanding the neurocognitive bases of pantomime, emphasizing that pantomime is a communicative task that nevertheless originates in specific tool-use (not motor-related) cognitive processes.
<Please insert Graphical abstract here>
Keywords: pantomime, apraxia, tool use, technical reasoning, left inferior parietal lobe
Osiurak et al. describe neurocognitive bases of pantomime of tool use through a series of meta-analyses of brain-lesion, neuroimaging and behavioural studies.
Graphical Abstract
Graphical Abstract.
Introduction
Limb apraxia—hereafter shortened as apraxia—refers to a high-level motor disorder that cannot be attributed to basic sensorimotor or comprehension deficits, and which disturbs the ability to imitate meaningless postures, produce symbolic gestures and/or use tools.1–4 Tool-use disorders can be investigated with real tool use (familiar or novel), single tool use and pantomime of tool use—hereafter shortened as pantomime (for a description of these tasks, see Table 1). Pantomime is a gold standard with a long tradition in clinical neuropsychology,1,5–7 which has been much more used by researchers and clinicians to assess tool-use disorders than real tool use.8,9 The main reason is that it can be viewed as a proxy for studying real tool-use disorders because the deficits are more salient than during real tool use.10–19 A secondary reason is its practical convenience. Surprisingly, even though pantomime has attracted considerable interest, it remains incompletely understood. Additionally, it has given rise to several controversies, such as the involvement of the left inferior parietal lobe (IPL) or the nature of the underlying cognitive processes. In this context, a comprehensive framework may be welcomed to shed some light on the rich and fascinating literature on pantomime.
Table 1.
Description of tasks and hypotheses
| Task | Description |
|---|---|
| Real tool use | The individual actually uses a toola (e.g. hammer) with an object a (e.g. nail). In some cases, several tools or objects are presented, and the individual must select the correct one to actually perform the tool-use action expected (i.e. tool selection). |
| Familiar tool use | Only familiar tools are presented. Familiar tool use differs from ‘activity of daily living’70 in which the individual has to perform a sequence of familiar tool-use actions. Note also that in familiar tool-use tasks, the individual has to use tool-object pairs. By contrast, activity-of-daily-living tasks involve multiple tools and objects, implying the selection of the tools and objects that are appropriate to perform the appropriate sequence of actions. |
| Novel tool use | The task can consist in using familiar tools in a non-conventional way (e.g. driving a screw with a knife) or in selecting, making and/or using novel tools to solve mechanical problems. |
| Single tool use | The individual grasps a tool presented in isolation and shows how to use it. |
| Pantomime of tool use | The individual demonstrates the use of a tool presented in isolation without holding it in hand. |
| Verbal modality | The name of the tool or a verbal description of the corresponding tool-use action is provided. |
| Visual modality | A picture of a tool or the real tool itself is shown. |
| Imitation modality | The examiner performs the tool-use action without holding the tool in hand. |
| Hypothesis | |
| Gesture engram | This hypothesis is grounded on the idea that all tool-use situations—including pantomime—critically require specific motor programs specifying the features of the movements to be performed to use a specific tool. These motor programmes have been labelled with different terms, such as visuo-kinesthetic motor engram,20 action lexicon,214 gesture engram112 or manipulation knowledge.135 We will hereafter use the generic term motor engram and, as a result, will call this perspective the gesture-engram hypothesis. |
| Communicative | This hypothesis highlights the important—if not exclusive—role of communicative/language skills in pantomime. |
| Mixed | This non-exclusive hypothesis suggests that both gesture engrams and communicative skills contribute to the production of pantomime. In other words, gesture engrams and communicative skills are complementary. |
| Technical reasoning | This hypothesis assumes that all tool-use situations—including pantomime—require reasoning about the physical properties of tools and objects to generate a mental simulation of the mechanical action appropriate to perform the task. Then, this mental simulation orients the selection, planning and online control of the appropriate motor actions within the motor-control system. This hypothesis does not exclude that additional cognitive processes can be at play in pantomime because of the absence of specific information. |
The term tool will be hereafter used for the implement that performs an action (e.g. hammer) and the term object for the recipient of the action (e.g. nail).
The present article aims at offering such a renewed framework, with the aim of specifying the neural and cognitive bases of pantomime. To do so, we conducted a series of meta-analyses of brain-lesion, neuroimaging and behavioural studies (see Supplementary Methods), which allowed us to test several hypotheses, and specifically the two dominant hypotheses in the field: The gesture-engram hypothesis and the communicative hypothesis (Table 1). The influential gesture-engram hypothesis assumes that gesture engrams are critical to any tool-use situations. In this frame, pantomime is the ideal task to investigate the integrity of motor engrams.7,20–26 By contrast, the communicative hypothesis questions the autonomy of apraxia from aphasia in highlighting the important—if not exclusive—role of communicative/language skills in pantomime.8,12,27–29 Our analyses provide conclusive support for a third alternative, namely that pantomime is a communicative task that nevertheless originates in specific tool-use (not motor-related) cognitive processes. This conclusion may appear trivial for some contributors, who have already stressed that pantomime is at the crossroad between tool use and communicative skills30 and that it should be more fruitful to consider the two hypotheses as complementary (i.e. the mixed hypothesis31). However, our conclusion offers a subtle but perhaps crucial nuance, in ruling out the gesture-engram component (hence the ‘not motor-related’ above) and in arguing for a rival hypothesis, namely, the technical-reasoning hypothesis32,33 (Table 1). In broad terms, the alternative hypothesis proposed here goes beyond the mixed hypothesis. To capture its essence, one may be instructed to temporarily discard the idea that pantomime is a clinical task aiming to assess the integrity of so-called motor engrams. We acknowledge that this statement is provocative, notably because of the strong dominance of the motor-engram hypothesis in the literature. In addition, challenging the concept of gesture engrams amounts to question the very concept of apraxia. After all, what may a high-level motor disorder become if we do not hypothesize the existence of a high-level motor component such as motor engrams? We will come back to this possibility later. For the sake of simplicity, we will temporarily keep this assumption aside to explore alternative ways of understanding the neurocognitive origins of pantomime. Keeping this in mind, let us begin by defining pantomime.
Definition
Pantomime refers here to a mime consisting in pretending to use a tool as if it was held in hand (e.g. pretending to brush teeth with a toothbrush or to pound a nail with a hammer). Although pantomime can be considered as gesture, it must not be confounded with other expressive and communicative movements.34 Thus, pantomime does not concern conventionalized movements that are embedded in a situation, such as symbolic gestures (e.g. waving goodbye), nor does it concern a gesture accompanying speech.35,36 It must also not be confounded with signs that are used like words in connected discourse (e.g. sign language37).
The function of pantomime
The question of the function of pantomime is important. Yet, it has received little attention to date, reflecting that it is mainly viewed as a clinical task. The motor-engram hypothesis has been developed based on the idea that pantomime is a proxy for studying real tool-use disorders. Thus, the error scoring system describes the errors committed on parameters that are transposable to real tool use13,38–42 (e.g. hand posture, arm trajectory, amplitude, timing). The motor-engram hypothesis does not elaborate on the function of pantomime, otherwise than as an ideal task to assess the integrity of motor engrams. Yet, we rarely pantomime in everyday life. Just take a few minutes to think about how many times you have produced a pantomime over the last 3 days. You may realize that this number is very low and that it systematically concerned a means of communication (e.g. a kind of circumlocution to explain to someone which tool you are looking for). The rarity of pantomime in daily life suggests that it is not a routine, but rather an improvized and creative act,34,43–46 and this is not inconsequential. The gesture-engram hypothesis does not ignore that pantomime induces additional cognitive or motor-control demands compared to real tool use15,38 (e.g. the absence of external cues). However, the creative nature of pantomime questions the idea that there is a strict correspondence between the errors committed during pantomime and those committed during real tool use (e.g. posture errors could have distinct cognitive origins in pantomime versus real tool use).
The communicative hypothesis highlights that the function of pantomime is to inform observers about the manner of using a tool. In this vein, Goodglass and Kaplan34 proposed a scoring system describing communicative responses during pantomime [e.g. gestural enhancement, vocal overflow, body-part-as-object, hereafter called body-part-as-tool (BPT), based on our definitions of the terms tool and object, see Table 1]. Except the BPT responses that have since become popular, this proposal has not been adopted even by the proponents of the communicative hypothesis themselves. Yet, this description is very close to the clinical reality and reflects relatively well the individuals’ engagement in this communicative act, which they may perceive as a mime game. The communicative function of pantomime becomes even more obvious when we move from neuroscience to anthropology. Many attempts have been made to elucidate the adaptive value of pantomime in the human lineage and its potential co-evolution with tool-use skills.47–50 Pantomime has been suggested to be an early form of teaching (i.e. proto-language48) that could have been useful to transmit technical content and thus favour the emergence of cumulative technological culture in our lineage.32,51,52 Teaching can be defined as behaviour that facilitates learning in others.53 Teaching—or more precisely direct active teaching53—can take the form of verbal explanation or pointing movements. The teacher can also repeat or slow down the sequence of actions, or provide a demonstration punctuated by exaggerated movements.53 This latter aspect is reminiscent of the exaggerated amplitude of movements commonly reported during pantomime.9,54,55 Some have explored whether this exaggeration could result from the lack of external cues and feedback,56–61 without providing conclusive evidence for this. Others have already stressed that this exaggeration could be viewed as an attempt to facilitate the recognition of the tool-use action by the observers.9 In broad terms, this confirms that pantomime is a communicative act, which is improvized and creative. The emphasis put here on the communicative and creative aspects may appear exaggerated, but we argue that it is not: It might be the only way of envisioning an appropriate interpretation of pantomime. Pantomime must not be merely conceived as a real tool-use task minus some components (e.g. the possibility of holding the tool in hand or of performing a real mechanical action). The absence of these components imposes specific demands on individuals, who must decide, more or less consciously, which demonstration is expected (e.g. cutting or peeling with a knife) and which movement parameters increase the recognition of their demonstration by observers (e.g. arm amplitude). This is not to say that pantomime does not share any cognitive component with real tool use. Instead, this emphasis allows us to keep in mind the specificity of pantomime compared to real tool use, which might be essential to apprehend the neurocognitive bases of pantomime. Before discussing in more details the dissimilarities between pantomime and real tool use, let us begin by their commonalities.
Pantomime and real tool use: the left area PF
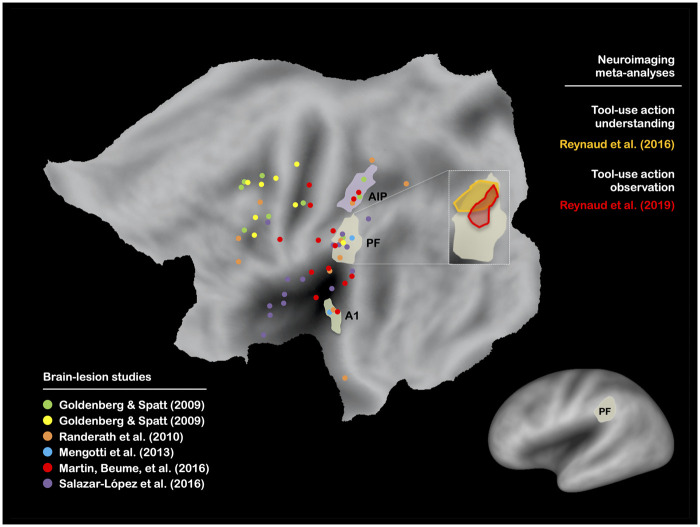
A significant body of evidence has indicated a strong association in left brain-damaged (LBD) patients between performance in pantomime and real tool use, when real tool use is assessed by asking patients to use either familiar (i.e. familiar tool use,15,58,62–71 for an association between single tool use and familiar tool use, see De Renzi and Lucchelli6, Neiman et al.58 and Buchmann and Randerath72) or novel tools.66,67,72–75 This association has also been corroborated by kinematic analyses.54,55,61 The question is whether this behavioural association reflects shared neural substrates between pantomime and real tool use. To address this question, we present the results from the five brain-lesion studies76–80 (six conditions) that have explored real tool use in LBD patients (familiar tool use, n = 5; novel tool use, n = 1; Supplementary Table 1). As shown in Fig. 1, the only brain area that is systematically associated with impaired performance in real tool use is the left area PF81 (Area PF complex) within the left IPL. We also report the results from the 16 brain-lesion studies9,16–19,25,26,30,31,78,82–87 (19 conditions) that have investigated pantomime in LBD patients (verbal modality, n = 4; visual modality, n = 9; imitation modality, n = 6; Supplementary Table 2). The results are less straightforward than for real tool use because no brain area is systematically associated with impaired performance (Figs 2–4). This indicates that pantomime is more multidetermined cognitively than real tool use. We will come back to this crucial point below. Importantly, this analysis also reveals that only the left area PF is involved whatever the modality (Fig. 2A) as well as in at least 50% of the studies when each modality is taken separately (Figs 2B and 3A and B). The same conclusion is drawn when the studies are divided into those controlling for lesion volume or not (Fig. 4A and B). This finding is consistent with the long tradition in neuropsychology, which has repeatedly stressed the involvement of the left IPL, containing the area PF, in pantomime (but see Refs.8,23,82,88–90).
Figure 1.
Brain-lesion studies of real tool use. Maximum lesion overlap locations are represented on the PALS-B12 left hemisphere (flat map, main figure; very inflated map, mini figure) atlas surface configuration.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 Only the brain areas that have been reported in at least 50% of the conditions included here are represented (see Supplementary Methods). The main findings obtained by two neuroimaging meta-analyses on tool-use action understanding111 and tool-use action observation114 are also shown.
Figure 2.
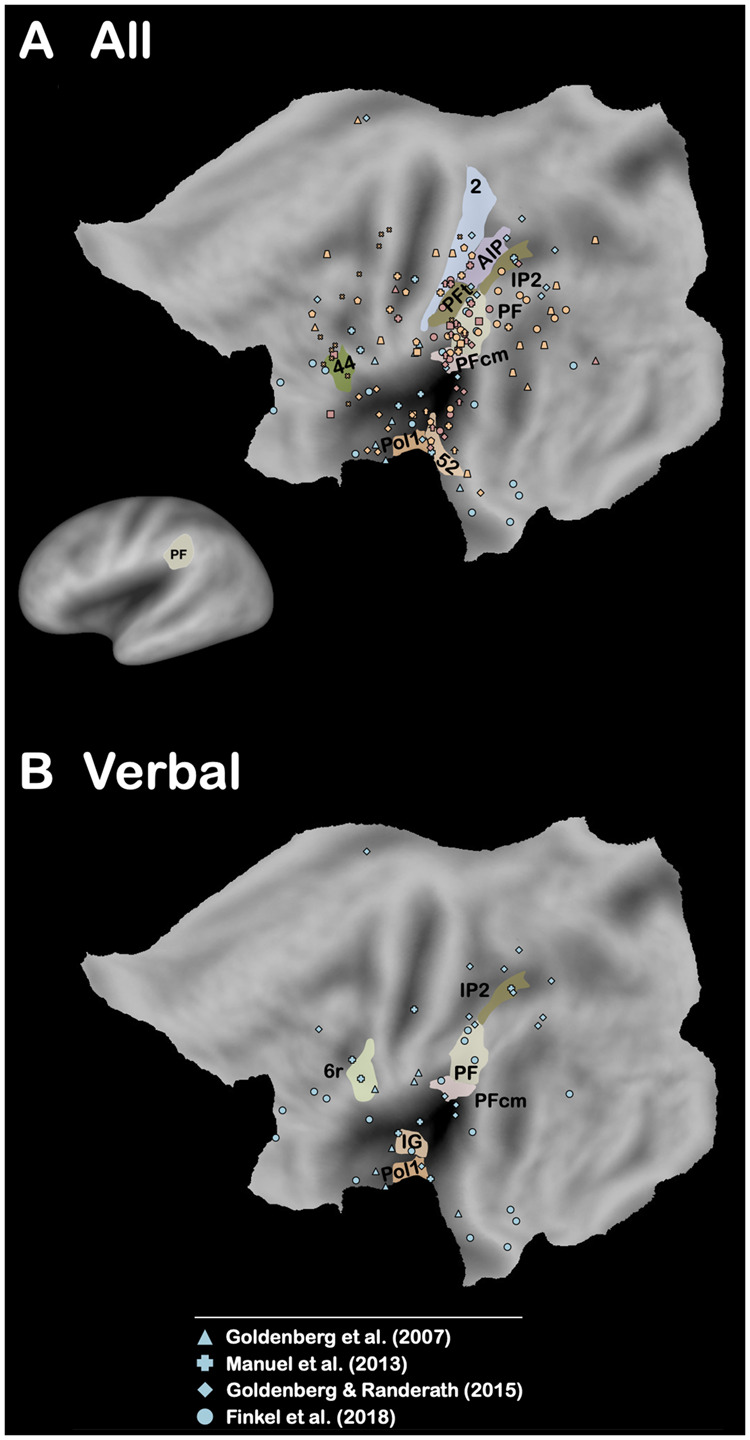
Brain-lesion studies of pantomime of tool use (All and Verbal). Maximum lesion overlap locations are represented on the PALS-B12 left hemisphere (flat maps, main figures; very inflated map, mini figure) atlas surface configuration.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 (A)Only the brain areas that have been associated with the three modalities (verbal, visual and imitation) are represented. (B) Only the brain areas that have been reported in at least 50% of the conditions included for the verbal modality are represented (see Supplementary Methods).
Figure 3.
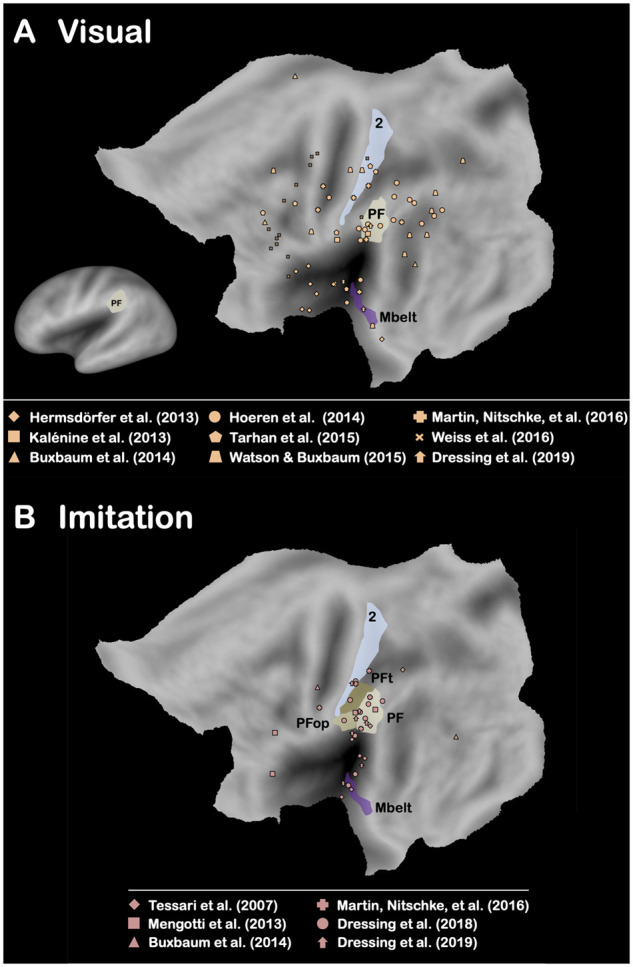
Brain-lesion studies of pantomime of tool use (Visual and Imitation). Maximum lesion overlap locations are represented on the PALS-B12 left hemisphere (flat maps, main figures; very inflated map, mini figure) atlas surface configuration.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 (A) Only the brain areas that have been reported in at least 50% of the conditions included for the visual modality are represented. (B) Only the brain areas that have been reported in at least 50% of the conditions included for the imitation modality are represented (see Supplementary Methods).
Figure 4.
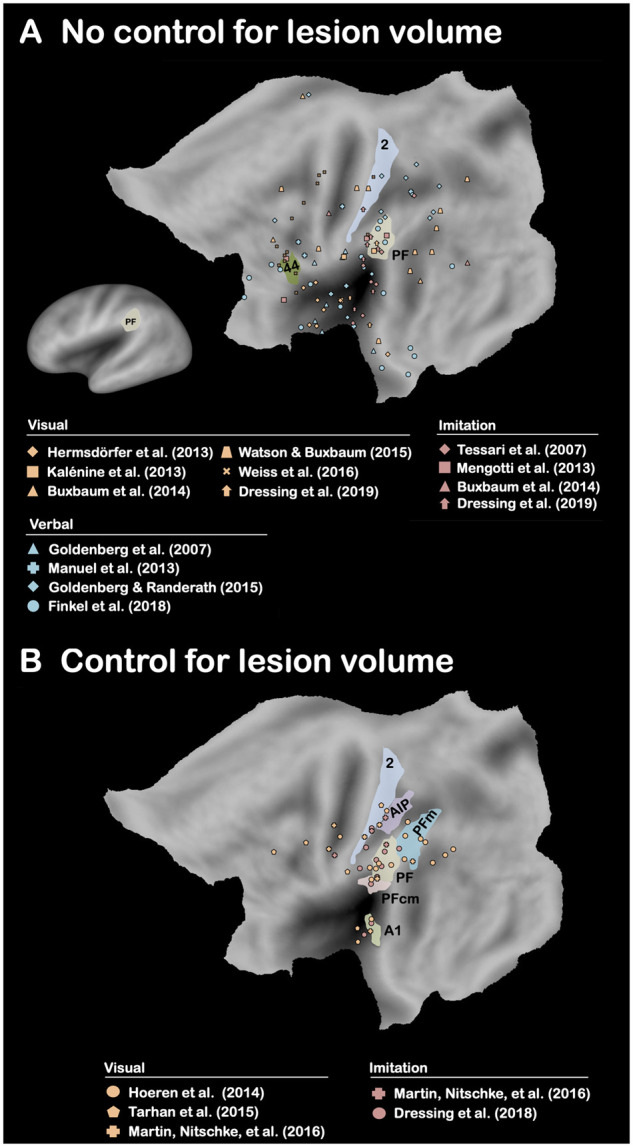
Brain-lesion studies of pantomime of tool use (No control and Control for lesion volume). Maximum lesion overlap locations are represented on the PALS-B12 left hemisphere (flat maps, main figures; very inflated map, mini figure) atlas surface configuration.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 (A) Only the brain areas that have been reported in at least 50% of the conditions in which there was no control for lesion volume are represented. (B) Only the brain areas that have been reported in at least 50% of the conditions in which there was a control for lesion volume are represented (see Supplementary Methods).
We conducted an additional meta-analysis of neuroimaging studies on pantomime23,37,91–107 (verbal modality, n = 10; visual modality, n = 11; Supplementary Table 3) to explore whether this finding can be extended to healthy participants. As shown in Fig. 5, two brain areas were activated in both modalities: The left intraparietal sulcus (IPS; IP0, IP2, LIPd, MIP) and the left inferior frontal gyrus (IFG; IFJp). The left area PF is also involved in the verbal modality but not in the visual modality. We do not have any satisfactory interpretation for this discrepancy (e.g. difference in terms of ‘processing depth’ between the two modalities). Therefore, we will not elaborate further on this point, which nevertheless temper our conclusions. Regardless, these results partly confirm that the left area PF is activated when healthy participants produce pantomime.
Figure 5.
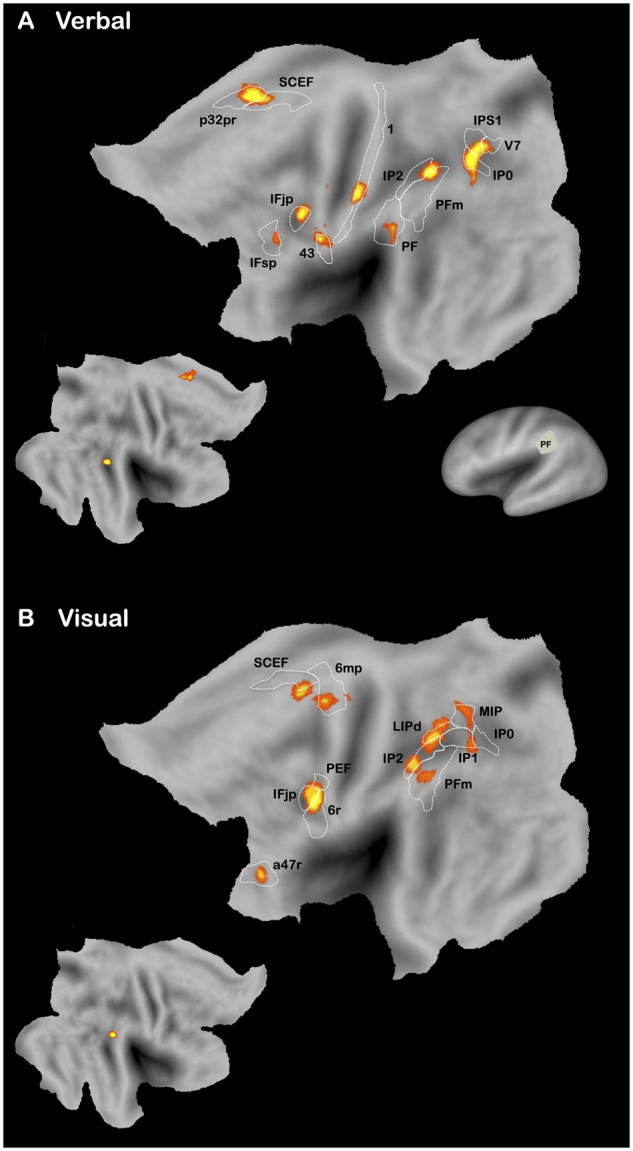
Neuroimaging studies of pantomime of tool use (Verbal and Visual). ALE maps are represented on the PALS-B12 left (main figures) and right (mini figures) hemisphere atlas surface configurations.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 (see Supplementary Methods).
The communicative hypothesis is not a good candidate to account for the central role of the left area PF in both pantomime and real tool use as well as the behavioural association between both tasks. This hypothesis holds that communicative/language skills are critical to pantomime but not to real tool use. Therefore, the neural bases of pantomime should mainly concern the cortical structures involved in communication/language skills [i.e. ventral structures such as temporal lobes (TL)], and no strong behavioural association should be found between pantomime and real tool use. Concerning the former point, it is noteworthy that most of LBD patients have middle cerebral artery lesions, which impact several brain areas involved in language skills (e.g. superior temporal sulcus or the left IFG) but also spare the polar portions of the temporal cortex that are known to play a key role in communicative skills. As a result, in LBD studies, the role played by communicative skills could be potentially underestimated. We will come back later to this point.
The gesture-engram hypothesis may appear more likely to explain these findings, in suggesting that the central role of the left area PF in both pantomime and real tool use and their behavioural association reflect the involvement of gesture engrams. Remind that gesture engrams refer to motor programs containing information about hand–tool relationships (i.e. motor actions) for the use of familiar tools. However, the evidence reported above does not provide support for this interpretation.
First, the behavioural association between pantomime and real tool use in LBD patients has been found not only for familiar tool use but also for novel tool use. Brain-lesion studies also indicate that the left area PF is associated with both familiar and novel tool use (evidence also indicates a strong behavioural link between familiar tool use and novel tool use66,67,73,108–110). This is also consistent with evidence showing that a similar cerebral network is recruited when healthy participants pantomime both familiar and novel tools.102 However, gesture engrams are thought to be involved only in the real use or pantomime of familiar tools so that no strong link should be reported between the real use or pantomime of both familiar and novel tools. Therefore, the gesture-engram hypothesis is inappropriate to account for these findings, which corroborate that pantomime is an act that is improvized based on the current situation (i.e. familiar or novel).
Second, the gesture-engram hypothesis suggests that patients with tool-use disorders meet difficulties in executing the appropriate motor actions to use familiar tools. However, another interpretation is that these patients execute the motor actions that are appropriate to realize the inappropriate mechanical action (i.e. tool–object relationship) they intend to perform. The problem is that the pantomime task is not suited to disentangle between these two interpretations because only the motor action can be observed. Thus, it is impossible to determine whether the difficulty concerns the representation of the motor action or of the mechanical action. Interestingly, real tool-use tasks can also assess the ability to select appropriate tools and objects (i.e. tool selection). Impaired tool selection cannot be interpreted in terms of motor-engram deficits, since the individual does not have to perform the motor action associated with the use during this selection. Instead, impaired tool selection necessarily reveals difficulties in understanding the appropriate mechanical action to realize. This is all the more true for novel tool-use tasks in which individuals are asked to select and even sometimes make novel tools to solve mechanical problems. Therefore, the behavioural association found between pantomime and real tool use (both familiar and novel tool use) seems to indicate difficulties in forming an accurate representation of the mechanical action to be performed, even when no tool is held, or no mechanical action is concretely performed, as in pantomime.
This interpretation is also consistent with two recent meta-analyses of neuroimaging studies on tool use. The first meta-analysis111 included studies in which healthy participants had to focus on the appropriateness of either the motor action (e.g. hand posture-tool; called GESTURE in this meta-analysis; no pantomime studies included) or the mechanical action (e.g. orientation of the tool in function of the object; called ACTION in this meta-analysis; no pantomime studies included). The findings indicate that the IPS was preferentially activated for motor actions, and the left area PF for mechanical actions (Fig. 1). This finding clearly challenges the gesture-engram hypothesis, which suggests that the left IPL and not the IPS is central to gesture engrams.20,93,112,113 The second meta-analysis114 included studies on non-tool-use action observation (i.e. observing someone grasping an object) and tool-use action observation (i.e. observing someone using a tool with an object). The results confirmed the now classical fronto-parieto-temporal action observation network.115–117 More relevant to our concerns, the contrast tool-use action observation minus non-tool-use action observation revealed a specific activation of the left area PF (Fig. 1). Again, this highlights that this brain area is involved in the processing of mechanical actions between tools and objects, since the presence of mechanical actions was the only difference between the two types of action observation.
Taken together, these findings suggest that the left area PF is central for the processing of mechanical actions irrespective of whether the tool-use action is familiar or novel. This invalidates former interpretations in terms of gesture engrams, since these engrams are thought to contain information about motor actions for familiar tool use. The idea that an impaired processing of mechanical actions can disturb pantomime may sound odd, since the only aspect of the action that we can observe is the motor action. Nevertheless, this is consistent with the ideomotor principle,118–122 according to which motor actions are necessarily guided by the realization of an expected effect. In the case of pantomime, this perceptual effect is the mechanical action even if this mechanical action is performed with tools and objects that are nevertheless absent. We will discuss this aspect in more details below.
Technical reasoning
Technical reasoning is a causal and analogical reasoning, which is directed towards the physical world.33,123–127 It is based on mechanical knowledge, which contains information about physical principles/mechanical actions. Technical reasoning is much more than spatial reasoning, which can consist, for example, in determining whether a car can pass between two trees or whether two puzzle pieces can be arranged together. It also involves the material dimension of physical objects (e.g. sharpness, hardness), which is the prerequisite for the understanding of physical principles/mechanical actions (e.g. lever, cutting). In this respect, people reason technically as soon as they must perform ‘mental making’, either by using tools but also by making them or building constructions.128
The function of technical reasoning is to generate technical solutions (i.e. potential mechanical actions) in selecting the appropriate tools and objects to solve physical problems, which can be familiar (e.g. to cut an apple) or novel (e.g. to get a ball that rolled under a couch). The outcome of technical reasoning is a mental simulation of the mechanical action to be performed (e.g. the motion of a knife on an apple). However, it does not deal with the selection and on-line control of the most appropriate motor actions to realize the mentally generated mechanical action. This is the function of the motor-control system, which is blind to the goal of the action (i.e. tool use or object transport). If someone has the idea of performing back-and-forth movements with a knife on an apple, this is the expected effect, which constrains the motor actions chosen by the motor-control system. Likewise, if someone intends to move an object from one location to another, the expected effect is the motion of the object, which constrains the motor actions chosen. In broad terms, the technical-reasoning hypothesis is akin to the ideomotor principle, and assumes that no specific tool-use motor programmes (i.e. gesture engrams) are required to specify how to manipulate familiar tools.3
The left area PF is the key neural substrate of technical reasoning, which is consistent with its involvement in any tool-use situations (e.g. familiar tool use, novel tool use), including pantomime, as well as in situations in which people must reason about physical events. In a way, technical reasoning can be viewed as a kind of ‘mechanical imagery’ (and not of spatial imagery, which is restricted to spatial reasoning; see above). By contrast, the motor-control system is supported by more superior structures within the dorso-dorsal stream (e.g. IPS) and is recruited for the processing of motor actions (i.e. motor imagery), as evidenced in the aforementioned meta-analysis of neuroimaging.111 Thus, damage to the dorso-dorsal stream can lead to impaired processing of motor actions, as described in specific forms of apraxia (e.g. motor apraxia). However, the technical-reasoning hypothesis posits that the nature of the difficulties met by LBD patients in pantomime and real tool use after damage to the left area PF is more technical because it concerns the understanding of mechanical actions. In this respect, the term atechnia129–131 may better reflect these difficulties (see below).
The technical-reasoning hypothesis shares some resemblance with other accounts that have suggested the existence of a potential non-lexical route between object structure and their potential use66,67,132 (e.g. affordance, inference of function from structure). However, it differs crucially from them, in assuming that technical reasoning is involved in any situation that needs mental making, including the use of physical tools. Thus, technical reasoning is not limited to novel tool use or a kind of compensatory strategy when gesture engrams are impaired.67,72,79,112,133–136 It is also not a synonym with ‘structural affordance’, which concerns the ability to extract a potential motor action (e.g. power or precision grip) from the mere observation of the structure of an object.24,137–139 For the technical-reasoning hypothesis, this is the specific role of the motor-control system (i.e. egocentric, hand–tool relationships or hand–object relationships in the case of a non-tool-use action such as object transport). Indeed, structural object properties can also be processed to generate mechanical actions between external objects (i.e. allocentric, tool–object relationships). In this case, it involves technical reasoning, even when the external object is the body of the user itself (e.g. brushing teeth with a toothbrush).
Kinematics: motion of the tool not of the body
The kinematic component of pantomime refers to the global shape of the movement trajectory. This component is commonly considered as critical140 because this is what makes a demonstration recognizable or not by observers. Thus, when a quantitative approach is used (e.g. 2, 1 or 0 point for each pantomime), the quality of kinematics is roughly associated with the number of points given (i.e. from recognizable to unrecognizable34,67,141). The gesture-engram hypothesis posits that the kinematics component is contained within gesture engrams, so impaired gesture engrams are responsible for the production of motor actions characterized by incorrect kinematics. Although this proposal is viable at a theoretical level, the evidence described above against it leads us to envisage another interpretation.
The technical-reasoning hypothesis predicts that the mental simulation of the mechanical action guides motor actions during pantomime (i.e. ideomotor principle). In other words, it is the kinematics of the mechanical action that constrains the kinematics of motor actions needed to realize the mechanical action given. Therefore, given that the generation of mechanical actions could be mainly supported by the left area PF, this brain area should be preferentially involved in the kinematic component of pantomime. We tested this prediction by exploring the results of five brain-lesion studies,16,17,25,31,85 which have investigated the lesion sites associated with kinematic errors (verbal, n = 2; visual, n = 1; imitation, n = 2; Fig. 6A; Supplementary Table 4). As shown, only the left area retroinsular cortex and the left area PF were reported in four of the five studies. In broad terms, as suggested by the technical-reasoning hypothesis and the ideomotor principle, the left area PF could be responsible for the processing of mechanical actions (i.e. motion of the tool) rather than of motor actions (i.e. motion of the body).
Figure 6.
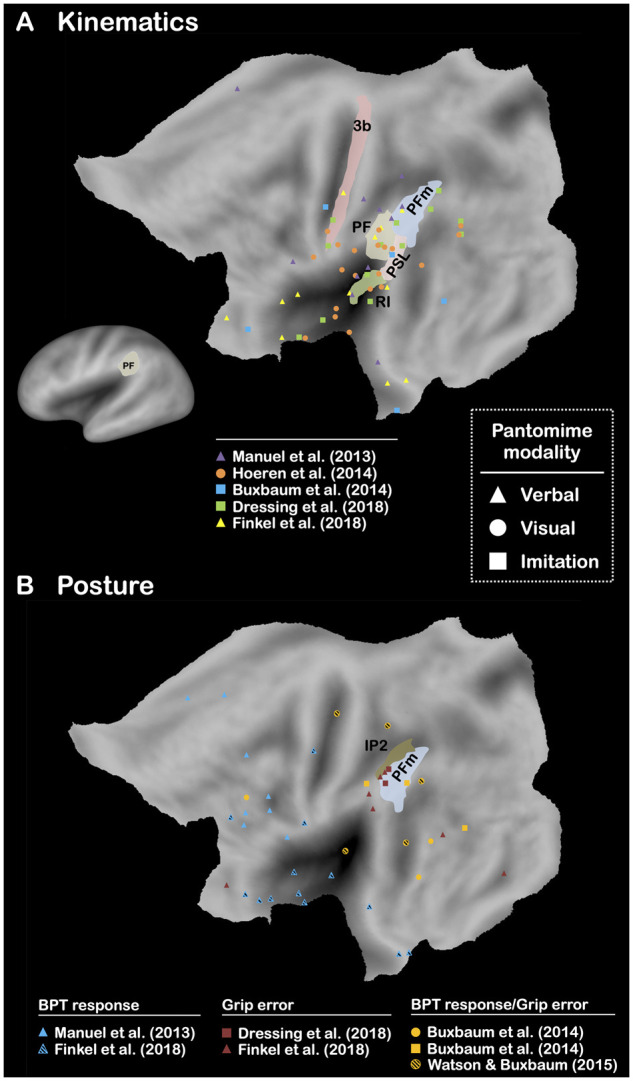
Brain-lesion studies of pantomime of tool use (kinematics and posture components). Maximum lesion overlap locations are represented on the PALS-B12 left hemisphere (flat maps, main figures; very inflated map, mini figure) atlas surface configuration.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 (A) Only the brain areas that have been reported in 50% of the conditions included are represented. (B) Here, the 50% refers to either BPT response and BPT response/grip error (n = 5 studies), or grip error and BPT response/grip error (n = 5 studies; see Supplementary Methods).
Posture: an experimental artefact?
The posture component (i.e. grip) is considered as the second key component of pantomime. The neural bases associated with posture errors have been subject to debate, even by the same research groups, who have found discrepancies over time (e.g. left IPL112,142; Left IPL and IPS13; left posterior temporal gyrus17; left IPL19). In Fig. 6B, we present the results of four brain-lesion studies17,19,31,85 in LBD patients (five conditions) that have investigated the lesion sites associated with posture errors, and more particularly with either grip errors or grip errors/BPT responses (verbal, n = 1; visual, n = 2; imitation, n = 2; Supplementary Table 4). The pattern of results corroborates the aforementioned discrepancies, with no clear lesion sites except perhaps the IPS, which is found in both studies on grip errors only. Although this pattern of results must be considered with caution given the low number of studies (n = 4), it contrasts with the more straightforward results obtained for the kinematic component. This also questions the gesture-engram hypothesis or at least some of its version, which predicts an association between posture errors and the left IPL or temporal structures. At best, it can be hypothesized that the posture component is supported by the IPS, which would be consistent with its role in motor control and grip planning. However, the discrepancy of these results also leads us to consider another interpretation.
An intriguing aspect of the posture component is that posture errors found during pantomime are rarely reported in LBD patients in real tool use. Indeed, only few cases of patients have been reported with severe posture errors in real tool use (e.g. grasping a tool upside-down),71,143,144 which contrasts with the frequent occurrence of posture errors found in the pantomime tasks. Posture errors (e.g. grasping a tool upside-down) can increase when LBD patients are asked to actually grasp a tool (single tool use), while the tool is presented with the handle pointing away from the patient’s body145 (note that such errors can also be observed in healthy young participants under dual-task conditions146). However, these errors disappear dramatically in real tool-use tasks.71 In broad terms, the posture errors reported in pantomime cannot be viewed as an indicator of posture errors in real tool use, which suggests that pantomime is not a strict proxy for studying real tool-use disorders. Additional evidence supports this conclusion. Indeed, it has been shown that the most significant characteristics of a natural prehension movement are diminished or absent during pantomime of grasping movements.147–149 At best, hand aperture matches or indicates the diameter of the object grasped but does not represent the real motor action.
These findings stress that individuals meet difficulties in performing the posture component during pantomime. One potential interpretation is that it is cognitively demanding. Another non-exclusive interpretation that is in line with the communicative hypothesis is that the posture component is not crucial for pantomime, because its recognition is mainly based on the kinematic component. Interestingly, both individuals and examiners seem to share the same belief: To be recognizable—or to obtain a higher score—it is better to favour the kinematic component. This is consistent with the goal-directed imitation model,150 which has been initially developed for action imitation. According to this model, the individual does not imitate a movement as a whole but rather decomposes it into its separate aspects. Then, these aspects are hierarchically ordered, and the highest aspect becomes the individual’s main goal. The corollary is that the lowest aspects are not necessarily well produced, particularly if there is no sufficient cognitive resource. This model has already been discussed in the literature on imitation of meaningless postures.151,152 Here we propose to extend it to the case of pantomime. Thus, if we consider that (i) pantomime is a communicative act, (ii) the kinematic component is the highest aspect for recognition and (iii) pantomime is a creative, cognitively demanding act, then this can explain why the posture component is not favoured during pantomime. As a result, it is easier to understand why it is difficult to identify its neural bases.
Body part as tools
As mentioned above, BPT responses were initially described by Goodglass and Kaplan34 as a kind of communicative responses. Since, there has been an intense debate as to whether BPT responses are pathognomonic of apraxia.153–156 The main reason is that BPT responses can be found in many pathologies that are not characterized by real tool-use disorders [e.g. autism157,158; Schizophrenia159–161; but also after right brain damage42,153,155 (RBD)]. More intriguingly, healthy participants can also produce BPT responses31,34,153,162 and even sometimes in high proportion163 (e.g. 30%). Heilman and Rothi10 stressed that, when a BPT response occurs, it is fundamental to reinstruct the individuals that they have to pretend to hold the tool in hand, and not to shape the hand as if it was the actual tool.13,154,164 It is only when the individual does not modify the response that a BPT response becomes pathologic. It is true that the instructions given are crucial because they can modulate the occurrence of BPT responses163 and that reinstructing the individual can lead to a decrease of BPT responses in healthy partcipants.154,164 However, reinstructing LBD patients with tool-use disorders does not guarantee that they understand the nuance given the high co-occurrence of aphasic deficits in these patients. Regardless, the fact that healthy participants—when not reinstructed—perform BPT responses confirms the communicative nature of pantomime, the individuals spontaneously attempting to produce a demonstration that can be easier to be recognized by an observer. In this respect, BPT responses should not be viewed as an artefact or errors to be controlled to make pantomime a better proxy for studying real tool-use disorders. Instead, they should be considered as an intrinsic aspect of pantomime, which reflects the interpretation made by the individual of the goal of the task: To communicate.
In this line, a key prediction is that it should be difficult to identify a specific neural basis for BPT responses from brain-lesions studies, because they do not characterize a particular deficit. To test this prediction, we report the results of the four brain-lesion studies16,17,19,31 (five conditions) that have explored the neural bases of either BPT responses or grip errors/BPT responses (verbal, n = 2; visual, n = 2; imitation, n = 1; Fig. 6B; Supplementary Table 4). As shown, no specific lesion site is associated with BPT responses, corroborating that BPT responses are not pathognomonic of impaired pantomime.153 A potential interpretation of these findings is that BPT responses heavily depends on the nature of the tools presented, some of them clearly favouring the occurrence of BPT responses31,153,165 (e.g. scissors). Thus, the presence of such tools could be insufficient or not controlled, which can prevent from creating a good measure of these responses and, therefore, to obtain a significant link between them and lesion sites. One of the studies discussed here controlled for this aspect and found an increased frequency of BPT responses after damage to left ventral structures31 (i.e. inferior frontal cortex and anterior temporal cortex). For the authors, this confirms the link between BPT responses and communicative skills. Although we agree that BPT responses can be a manifestation of the communicative nature of pantomime, the conclusion drawn by the authors is unclear, because it suggests that impaired communicative skills increase the frequency of communicative responses. Predicting the inverse relationship sounds more viable at a theoretical level, namely, less BPT responses after damage to the cerebral structures underlying communicative skills. This illustrates the difficulty in interpreting the neurocognitive origins of BPT responses.
Motor control and body schema
Planning and on-line control of motor actions are supported by the motor-control system, which involves dorso-dorsal structures and notably the IPS.166 Planning and controlling motor actions need to dynamically keep track of the spatial relations between different body parts,167–169 which is referred to as body schema.170,171 Motor actions are obviously not directed only towards tool-use actions and can also concern non-tool-use actions (e.g. grasping or meaningless gestures). Therefore, deficits of the motor-control system/body schema should disturb both tool-use and non-tool-use tasks (e.g. imitation of meaningless postures). The general role of the motor-control system/body schema for action has been repeatedly stressed in the literature on apraxia25,106,112,125,172–175 on the basis of evidence indicating a behavioural association between pantomime and imitation of meaningless postures in LBD patients.12,13,65,72,176 This association is nevertheless not systematic.6,177,178 As shown in Figs 2–4, lesions to some IPS areas seem to be associated with pantomime performance. Results of neuroimaging studies are more straightforward, in stressing a clear activation of IPS during pantomime (Fig. 5). In order to explore this aspect in more details, we report the results of the 10 brain-lesions studies17,25,26,30,78,84,179–182 (14 conditions) that have investigated the lesion sites associated with imitation of meaningless postures (Hand/finger postures, n = 5; Hand postures, n = 5; Finger postures, n = 4; Fig. 7A; Supplementary Table 5). As shown, we found only two studies in which the left area PF was found to be associated with impaired imitation,180,182 confirming its specific role for tool use. This also invalidates the idea that real tool use and imitation of meaningless postures strictly requires the same neurocognitive skills (i.e. categorical apprehension of spatial relationships).3,183,184 More particularly, the results highlight the involvement of the left angular gyrus (AG; PFm, PGi), which we will discuss in more details in the next section, and of the left IPS (AIP, IP2). In broad terms, these results confirm that the control-motor system is involved in both tool-use and non-tool-use actions (i.e. object transport, imitation of postures).
Figure 7.
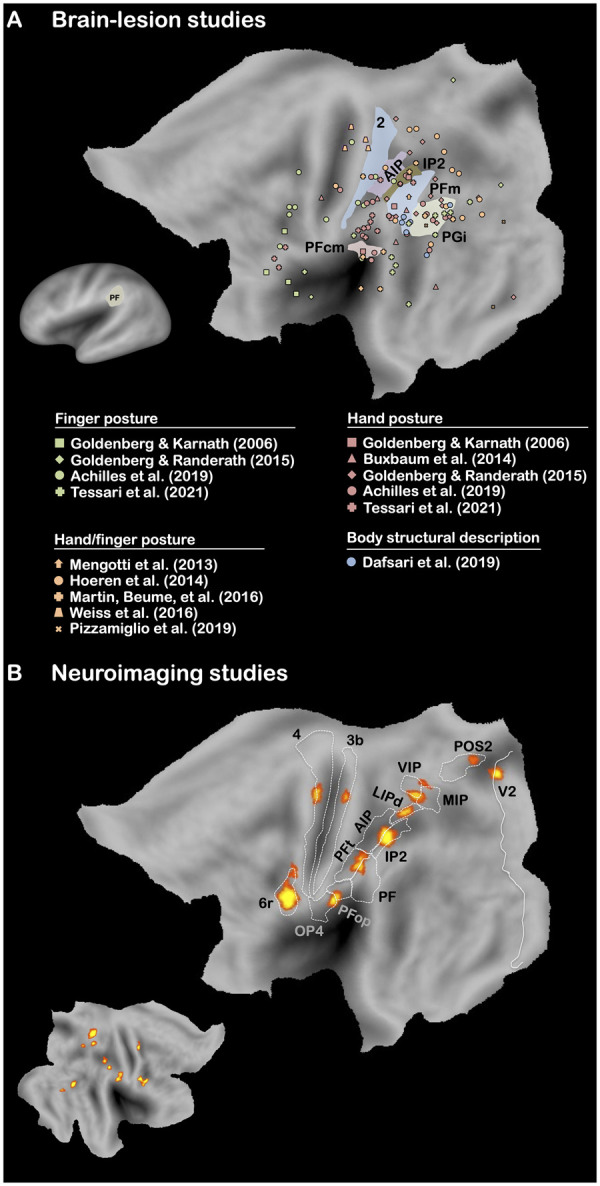
Brain-lesion and neuroimaging studies of imitation of meaningless postures. Maximum lesion overlap locations and ALE maps are represented on the PALS-B12 left (main figures; very inflated map, mini figure) and right (mini-figure) hemisphere atlas surface configurations.249 Brain areas are identified using anatomical labels from the parcellation of Glasser et al.81 For brain-lesions studies, only the brain areas that have been associated with the three types of posture (hand/finger, hand, finger) are represented (see Supplementary Methods).
Body structural description
Body schema is dedicated to the processing of motor actions that an individual can perform to interact with the physical world. However, the body can also be represented as an external object. These body representations can correspond to either lexical-semantic representations of the body (e.g. names, functions or relations with familiar tools; i.e. body semantics) or to a topological map of locations that defines body part boundaries and proximity relationships170,171 (i.e. body structural description). Here, we will focus on this latter form of body representations. Patients with impaired body structural description meet difficulties in pointing accurately to body parts either on their own body or on the examiner’s body/manikin. This deficit is commonly called autotopoagnosia. In imitation of meaningless postures, the individual is sometimes asked to perform postures towards the body. Thus, some of the errors committed can reflect difficulties in pointing accurately to body parts. In other words, imitation of meaningless postures could need body structural description. This possibility has repeatedly been suggested in the literature.179,184–189 Evidence also stresses that the LBD patients who are impaired to imitate meaningless postures also encounter difficulties in reproducing these postures on a manikin.185 In other words, imitation of meaningless postures could be based on both body schema and body structural description.
Interestingly, several studies have reported that impaired imitation of meaningless postures could be observed after damage to the left AG.87,174,177 This is corroborated by the results reported above, which also point out that the left AG is associated with difficulties in imitation of meaningless postures in LBD patients (Fig. 7A). Thus, the left AG could be strongly involved in body structural description. This prediction is confirmed by a recent study,190 which demonstrated the co-occurrence of autotopoagnosia and apraxia as well as the involvement of the left AG in the task assessing body structural description (Fig. 7A).
To investigate further this point, we conducted a meta-analysis of neuroimaging studies on the 13 studies152,191–202 (19 conditions) that have explored imitation of meaningless postures (Hand/finger postures, n = 1; Hand postures, n = 5; Finger postures, n = 13; Fig. 7B; Supplementary Table 6) for a similar analysis on both meaningless and meaningful gestures.203 The results indicate a relatively symmetric and bilateral network, which contrasts sharply with the left lateralization observed for tool-use actions111,114 including pantomime. Importantly, there was a clear involvement of the IPS (AIP, IP2, LIPd, VIP, MIP), which corroborates the role of the motor-control system/body schema in imitation of meaningless postures. However, no preferential activation of the left AG was reported, contrary to what we found for brain-lesion studies. This finding stresses the frequent discrepancy that can be reported between brain-lesion and neuroimaging studies7,203,204 (see also above for pantomime). Nevertheless, the discrepancy noted here could make sense and even help us better understand the neurocognitive bases of imitation of meaningless postures. Indeed, several authors have already stressed that neuroimaging studies can provide divergent results because of the experimental constraints imposed by the scanner.16,82,99 More specifically, the postures produced in the scanner usually correspond to hand or finger postures that are not directed towards the body or, at best, towards the supine trunk of the body,191,195 whereas in clinical testing postures are directed towards other body parts82 (e.g. mouth, face). This constraint could reduce the requirement of body structural description and explain the absence of activation of the left AG in neuroimaging studies.
Studies interested in pantomime generally do not distinguish between tool-use actions directed towards (e.g. toothbrush, comb) and away from the body (e.g. hammer, knife). However, tool-use actions directed towards the body could require the additional involvement of body structural description.205–207 This is consistent with a recent study, which showed that pantomiming a tool-use action directed towards versus away from the body involves distinct cognitive processes.208 Future research is needed to test this prediction, which could explain why the left AG has been sometimes found to be associated with tool-use disorders.13,30,77,174
Semantic knowledge
Performance on pantomime tasks is difficult to measure,209 notably for a clinician who cannot systematically videotape it and ask help from a colleague for the scoring. This difficulty lies in the fact that an incredible number of productions can be performed with the same tool (e.g. a knife can be used to cut or peel an apple, or to spread butter), which do not necessarily correspond to the so-called prototypical production expected by the examiner.131,210,211 This is all the more true when only the name of the tool is given, without allowing the individual to see the real tool or have more information about the tool-use action expected. Again, this questions the idea that pantomime is a good proxy for studying real tool-use disorders. Interestingly, this variability in the production can also be viewed as very instructive, reminding us that pantomime puts additional demands on individuals, who must decide what is expected by the examiner, while it does not necessarily correspond to the way they usually use the tool in their daily life (e.g. pretending to read newspapers while the individual is not used to read them but to light fire with them). This is also true for single tool use, because even if, in this case, the tool can be grasped during the demonstration, the absence of the corresponding object prevents individuals from knowing which specific mechanical action is expected. In sum, to solve this problem, individuals must use their knowledge about the social usages associated with tools and objects, which refer to the function and the context of use of the tool that are globally shared by the members of the social group to which they—including the examiner—belong.
Knowledge about social usages associated with tools and objects refers to what is commonly called functional or contextual knowledge.133,212 We will use the more generic term semantic knowledge. This knowledge was once considered as crucial for the real use of familiar tools.66,213,214 However, a large body of evidence has demonstrated that this knowledge is neither necessary nor sufficient for real tool use.215–219 For instance, patients with semantic dementia, who are characterized by selective semantic deficits, perform in the same range as healthy matched controls on novel tool use,133,220,221 which corroborates that their technical-reasoning skills are preserved. The fact that, in semantic dementia, the atrophy mainly affects the polar portions of the TLs—and not the left IPL and, therefore, not the left area PF—is also consistent with this pattern. Patients with semantic dementia also perform relatively well in familiar tool-use tasks, when no choice is allowed (i.e. only the tool and its corresponding object).220 Nevertheless, because of semantic deficits, these patients can fail to recognize the tools and objects presented, putting them in a situation very close to a novel tool-use task. In fact, for them, familiar tool use may even be more difficult than novel tool use. Indeed, the instructions commonly given in familiar tool use is to ask the individual to show how to use a tool and an object together. Thus, no goal is provided. Instead, it must be inferred from the structure of the tool and the object provided. By contrast, in novel tool-use tasks, the goal is explicitly given by the examiner or can be understood more easily from practice trials, since it does not change during the task133,221 (e.g. to lift a cylinder; to extract a target out from a box). Thus, unexpected responses can be reported in patients with semantic dementia in no-choice conditions of familiar tool-use tasks. This is particularly likely when the mechanical action between a tool and its target is opaque. For instance, patients with semantic dementia can insert a key into a padlock without turning the key.220 It is not obvious to identify that a padlock possesses a hidden mechanical system by looking at it. In this case, semantic knowledge can be relevant in providing the information that it is something commonly used to lock. Such errors can also be found in choice conditions of familiar tool-use tasks, the patient being unable to identify what is the mechanical action expected.220 In a way, even if semantic knowledge is not a generator of mechanical actions—this is the function of technical reasoning—, it can nevertheless be helpful to decide which mechanical action is expected in a social context such as the clinical setting. In line with this, it is noteworthy that, in these patients and contrary to LBD patients with tool-use disorders, the difficulties observed in clinical tasks are not found in their daily lives, in which they can use their own tools and objects in order to achieve the goals they set themselves.222
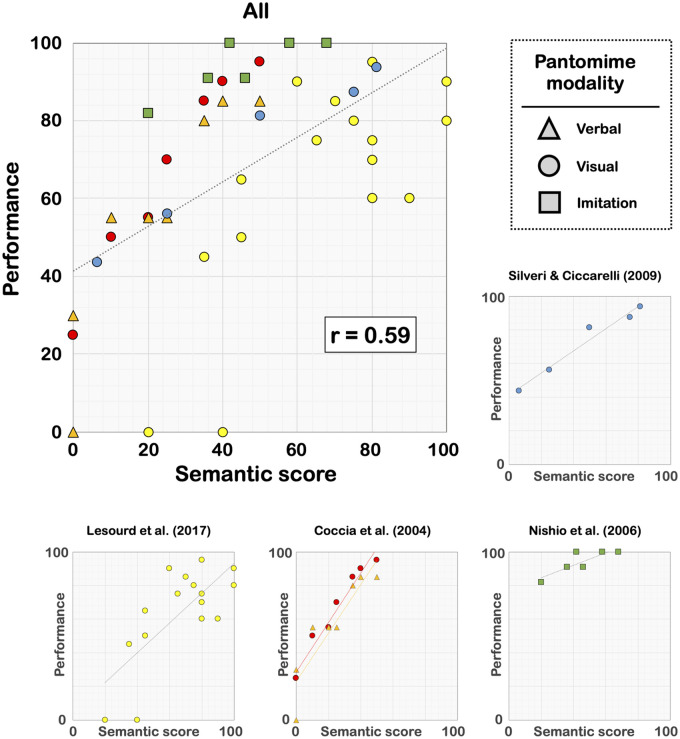
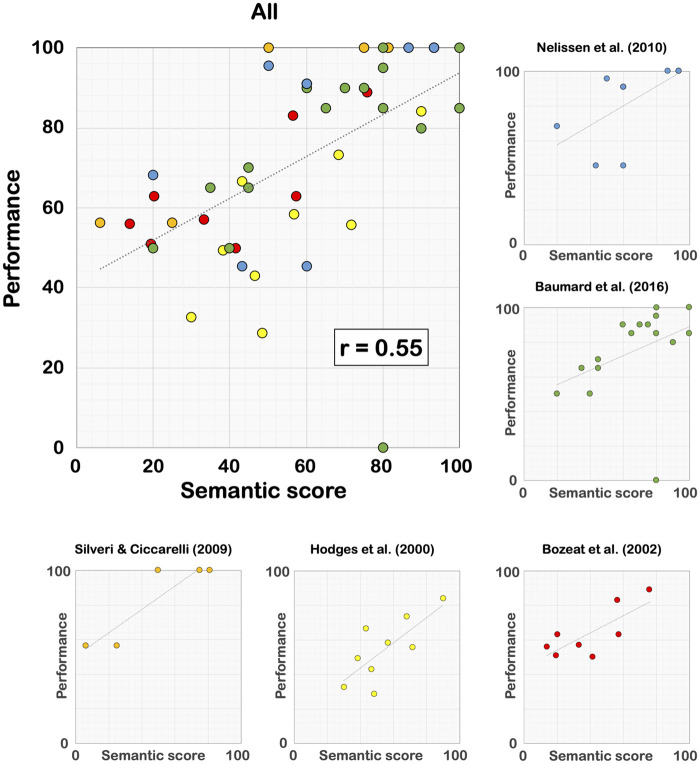
The difficulties in recognizing familiar tools can have an even greater deleterious effect when tools are presented in isolation, such as in pantomime or single tool use. As explained above, for a patient with selective semantic deficits, a familiar tool-use task can become a novel tool-use task, which can be more difficult to solve because of the absence of explicit goal. However, in pantomime or single tool use, the individual cannot infer at all the potential mechanical action expected given that no additional object is provided. Interestingly, in this case, the patient can use a specific strategy consisting in attempting to show how to use the tool presented in isolation with the objects available in the clinical context.218,223 Therefore, a key prediction is that patients with selective semantic deficits should be impaired in pantomime and single tool use, and that the performance in these tasks should be associated with the severity of semantic deficits. We explored these predictions by collecting the data from eight behavioural studies133,211,219,220,224–227 (Supplementary Table 7), in which patients with selective semantic deficits (i.e. semantic dementia or herpetic encephalitis) have been assessed on both semantic tasks and pantomime (n = 4) or single tool use (n = 5). In all these studies, patients performed worse than healthy matched controls, validating our first prediction. Our second prediction was also correct since a robust link was observed between the performance on pantomime/single tool use and the severity of semantic deficits (Figs 8 and 9). Interestingly, this link has also been reported in LBD patients.66,73 Taken together, these findings emphasize that semantic knowledge can play a crucial role in pantomime, in helping the individual identify the mechanical action that is ‘socially’ expected (for somewhat similar interpretations, see Randerath et al.,15 Hoeren et al.25 and Goldenberg and Randerath30).
Figure 8.
Link between semantic knowledge and pantomime in patients with selective semantic deficits. Performance was systematically converted into percentages. In the study of Coccia et al.,225 the eight points reported for each modality refer to two patients who were assessed over a 4-year longitudinal period. The coefficients r are Pearson correlation coefficients (see Supplementary Methods).
Figure 9.
Link between semantic knowledge and single tool use in patients with selective semantic deficits. Performance was systematically converted into percentages. The coefficients r are Pearson correlation coefficients (see Supplementary Methods).
From language to communication
The communicative hypothesis has been sometimes confounded with the language hypothesis, which assumes that tool use and language might be based on common cognitive processes as suggested by the concept of asymbolia.12,27,28 It is known for a long time that the prevalence of apraxia is higher in patients with aphasia.1,38,78,228 In this vein, auditory comprehension tasks strongly correlate with pantomime.30,68,109 There is also evidence that tool use and language are co-lateralized and that this co-lateralization is independent of the manual dominance.104,144,178,229 However, the language network differs from the tool-use network, with no involvement of the left area PF.230 A recent study also showed that language and tool-use disorders involve different neural substrates.79 As a result, the most likely hypothesis is that the lesions responsible for tool-use disorders very frequently encroach on the brain areas specialized for language,228 creating an illusory correlation in many studies. As discussed above, semantic knowledge could be critically involved in pantomime. Thus, semantic knowledge could be the only linguistic component that is shared with tool use, and particularly, pantomime.
In the psychology and neuroscience literature, communication skills are generally considered as supported by theory-of-mind skills,231 which allow the individual to think about others’ mental states (i.e. perspective taking). Interestingly, in children, there is a co-evolution between pantomime and theory-of-mind skills.232 Pantomime is also impaired in some pathologies that are characterized by theory-of-mind deficits, such as autism spectrum disorders157,158,233,234 or schizophrenia.160,161,235 Indeed, as any communicative act, producing a pantomime requires taking the perspective of the observer. As explained above, if the pantomime concerns a familiar tool, semantic knowledge is needed to identify which is the associated social usage and, as a result, the demonstration that is the most likely to be recognized. If the tool is unfamiliar, it can also be needed to identify what is the most relevant to mime from the observer’s perspective. Regardless, in both cases, perspective taking is required. Interestingly, the theory-of-mind network includes the anterior portions of the TLs,236 thereby suggesting that semantic knowledge could be involved in theory of mind236 as suggested above. In this respect, this possibility could stimulate a renewed interest for some aspects of pantomime that have been so far mainly concerned as methodological problems. For instance, if the presence of exaggerated amplitude in pantomime is a characteristic shared by a great number of individuals (to make the demonstration easier to recognize), the question could be to investigate the cognitive specificity of the individuals who do not show such exaggeration. Thus, a possibility could be that exaggerated amplitude in pantomime—and more generally performance—is associated with theory-of-mind skills and particularly perspective-taking skills. This possibility is partly supported by research on autism spectrum disorders, which has reported an association between pantomime and communication scales.234 We say partly because, on the other hand, the difficulties met by patients with schizophrenia do not seem to be explained by their communication disorders and could rather reflect the presence of subtle tool-use disorders strictly speaking.161 Regardless, investigating the communicative aspect of pantomime could open new avenues on the interaction between tool use, semantic knowledge and theory of mind.46,237
Severity versus disconnection
Significant evidence has shown that performance is worse in pantomime than in real tool use. This pattern has been found not only for LBD patients,15,54,60,71,73,109 but also in Alzheimer’s disease238 or in other pathologies such as autism spectrum disorders or schizophrenia (see above), where difficulties are generally found in pantomime but not in real tool use. Two hypotheses have been proposed to account for this pattern15,65: The severity hypothesis versus the disconnection hypothesis. The severity hypothesis is based on the assumption that pantomime is a good proxy for studying real tool-use disorders. Simply, pantomime possesses a better sensitivity to tool-use disorders than real tool use. As discussed so far, this assumption is invalid, because it minimizes the communicative nature of pantomime, which can lead people to produce a demonstration that does not fully correspond to what they can do during real tool use. The disconnection hypothesis posits that pantomime and real tool use are based on distinct cognitive processes. In a way, this hypothesis is close to the communicative hypothesis, according to which pantomime is supported by communicative/language skills and not tool-use skills strictly speaking, and vice versa for real tool use. An important prediction derived from the disconnection hypothesis is that we should also find the opposite pattern, namely, difficulties in real tool use but not in pantomime. This pattern has been reported in two case-studies,239,240 which are subject to methodological flaws.45 In other words, evidence rules out the disconnection hypothesis. Instead, it seems that pantomime shares some specific tool-use processes with real tool use, but it also requires additional cognitive processes. This explains why pantomime is more sensitive to diverse pathologies because it is more multidetermined at a cognitive level.
A neurocognitive model of pantomime
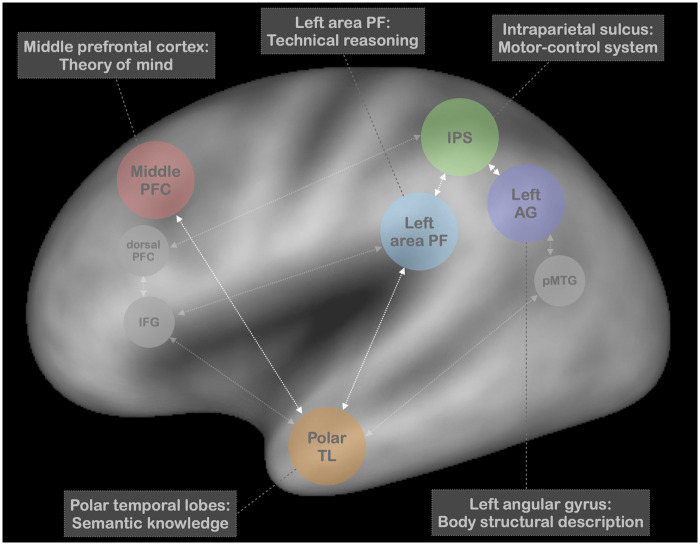
This invites us to put forward a new neurocognitive model of pantomime, which is based on the key findings discussed above (Fig. 10; see also Osiurak et al.131 and Baumard et al.210). Pantomime requires the generation of a mechanical action through technical reasoning (notably the left area PF). The mental simulation of this mechanical action guides the selection of the appropriate motor actions within the motor-control system (notably the IPS). If tools are used towards the body, body structural description is needed (notably the left AG). When the tool is familiar, semantic knowledge (notably the polar TLs) must be recruited to specify the social usages associated with the tool and, thus, to select the appropriate mechanical action to be performed. Finally, theory-of-mind skills might also be involved for perspective taking (notably the middle prefrontal cortex115). In this context, pantomime and real tool use might be both impaired after damage to the left area PF because they both crucially rely on technical reasoning. Difficulties in both tasks can also be found after damage to the IPS, because of the obvious requirement of the motor-control system. However, the difficulties met during real tool use do not concern the selection and generation of appropriate mechanical actions but rather the ability to perform appropriate motor actions in order to realize the mechanical action generated (e.g. incorrect grip144). Both tasks can also be impaired after damage to the left AG if the real use or demonstration is directed towards the body. Impaired semantic knowledge might have a more deleterious effect in pantomime than in real tool use even if, as discussed above, some subtle difficulties can also be reported in this latter case. Finally, theory-of-mind skills might play a critical role in pantomime because of its communicative nature. These skills can also be recruited during real tool use if the individual is asked to ‘teach’ someone else how to make the tool-use action. In this case, they could be essential to help the individual take the perspective of someone else to facilitate the learning (see above). However, theory-of-mind skills do not intrinsically impact on the individual’s ability to actually use tools with objects.
Figure 10.
A neurocognitive model of pantomime of tool use. This model assumes that technical reasoning (notably the left area PF), the motor-control system (notably the intraparietal sulcus), body structural description (notably the left angular gyrus), semantic knowledge (notably the polar TLs) and potentially theory of mind (notably middle prefrontal cortex) work in concert to produce pantomime. The white arrows indicate the privileged connections between the main brain areas described in the model. Three other brain regions are also represented given their potential involvement in pantomime: Inferior frontal gyrus (IFG), dorsal prefrontal cortex (dorsal PFC) and posterior middle temporal gyrus (pMTG).
We acknowledge that the idea that pantomime is cognitively multidetermined is not new.15,131,211 In the same vein, previous accounts have already proposed that pantomime relies on working memory.15,45,46,131 Thus, a working-memory component involving the dorsal prefrontal cortex can be added to our model (Fig. 10). Nevertheless, this implies that difficulties in pantomime should also be reported in patients with dysexecutive syndrome/prefrontal lobe lesions. However, empirical evidence seems to indicate that it is not necessarily the case.110 In addition, this also suggests that pantomime could also be impaired in RBD patients. Even if the difficulties are generally greater in LBD patients than in RBD patients, RBD patients performed frequently worse than healthy matched controls.42 However, these difficulties can also be explained by other deficits, such as neglect.241 In sum, future work is needed to specify whether a specific working-memory component involving the dorsal prefrontal cortex must be added to the model because of the multidetermined nature of pantomime. Note that other interpretations can also be made about the involvement of the dorsal prefrontal cortex in pantomime. For instance, even in relatively simple motor imagery tasks, the dorsal prefrontal cortex along with the IPS has been found to be recruited to form a hierarchical system for flexible, context-adaptive and goal-directed behaviour.242 This hierarchical system might be particularly useful for pantomime to help the individual be aware of what they are doing as well as adjust their production to the demands of the context (i.e. self-monitoring).
Two additional brain areas deserve to be mentioned in our neurocognitive model, even if the present findings do not allow us to generate specific hypotheses about their role in pantomime at a cognitive level (Fig. 10). The first is the IFG, which has been repeatedly found to be involved in pantomime.31,82 This brain area has been proposed to play a role in the selection of competing alternatives from semantic memory.82 Thus, the left IFG along with the polar TLs might form a ventral network supporting communicative skills,31 which is consistent with the presence of poor gestural expression (i.e. gesture accompanying speech) in patients with lesions in anterior temporal and inferior frontal regions.36 This interpretation is also supported by other studies combining fMRI and DTI approaches, which have suggested that the left IFG could play a role of explicit cognitive control in testing possible combinations to extract meaning.106,242 The second is the posterior middle temporal gyrus, which has also been found to be involved in pantomime tasks.17,19 This brain area has been proposed to be specialized in the ‘postural component’ of gesture engrams.17 This proposal is not supported by the present findings, which have not stressed the specific involvement of the posterior middle temporal gyrus in the posture component of pantomime. Another interpretation is that this brain area (along with AG) contributes to semantic control, which refers to the strategic retrieval of semantic information.243,244 For example, it has been shown that the posterior middle temporal gyrus is preferentially recruited in high semantic control tasks (e.g. associative matching, neighbour judgement) compared to low semantic control tasks (e.g. word-picture matching).244 The absence of specific involvement of the left posterior middle temporal gyrus in our different meta-analyses might suggest that the pantomime task does not require high semantic control. Future work is needed to test this possibility.
The neurocognitive model proposed here differs from previous ones in emphasizing the key role of technical reasoning in pantomime. It also aims to identify the brain areas that could be central (hence the ‘notably’) for each cognitive function described (e.g. technical reasoning, body structural description). Importantly, it acknowledges that all the cognitive functions discussed here are necessarily supported by wider brain networks composed of other brain areas, although these latter areas might be less central for the cognitive function concerned. For instance, even if we assume that the left area PF plays a central role in technical reasoning, this kind of reasoning certainly requires other brain areas that might be, nevertheless, not specific to technical reasoning (for discussion about the potential role of the left IFG in technical reasoning, see Reynaud et al.111,114). In this way, our neurocognitive model is in line with previous accounts that have suggested that pantomime might emerge from the interactions between the different brain areas of the ventral, ventro-dorsal and dorso-dorsal streams.85,106,245
Apraxia or atechnia?
The literature on apraxia has been dominated for a long time by the gesture-engram hypothesis.7,20,25,93,99,112,113,135,141,214 The findings presented here challenge this hypothesis and bring support for the rival technical-reasoning hypothesis (for discussion see Refs.33,122–124,127,246,247). More specifically, the neurocognitive model developed above excludes the existence of gesture engrams and assumes that technical reasoning, the motor-control system, body structural description, semantic knowledge and potentially theory of mind work in concert to produce pantomime. Therefore, this model raises the issue of which clinical manifestations should fall within the scope of apraxia, which owes its existence to the assumption that humans possess a high-level motor component such as gesture engrams. However, if such a component turned out not to exist, then there would be no reason to keep on using the term apraxia for describing pantomime deficits or, more broadly, tool-use disorders. An alternative could be to call these disorders atechnia in reference to technical reasoning, as initially suggested by Gagnepain129 (see also Le Gall130). However, this proposal is not fully satisfactory because pantomime can be impaired not only after technical-reasoning deficits, which could create confusion. Regardless, in line with the enlightened work of Goldenberg,3 the field of apraxia could be more deeply renewed if we adopt the idea that most of the difficulties that we refer to as ‘apraxia’ do not reflect a motor disorder but rather the secondary repercussions of non-motor cognitive disorders on skilled motor performance.248
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This work was performed within the framework of the LABEX CORTEX (ANR-11-LABX-0042) of Université de Lyon, within the programme ‘Investissements d’Avenir’ (ANR-11- IDEX-0007) operated by the French National Research Agency (ANR).
Data availability
Data sharing is not applicable to this article as no new data were created or analysed.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- 2 =
Area 2
- 3b =
Area 3b
- 4 =
Area 4
- 6mp =
Area 6mp
- 6r =
rostral area 6
- 43 =
Area 43
- 44 =
Area 44
- 52 =
Area 52
- A1 =
primary auditory cortex
- A47r =
area anterior 47r
- AG =
angular gyrus
- AIP =
anterior intraparietal area
- BPT =
body-part-as-tool
- GOADI =
goal-directed imitation
- IFG =
inferior frontal gyrus
- IFJp =
area IFJp
- IFSp =
area IFSp
- IG =
insular granular complex
- IPL =
inferior parietal lobe
- IP0 =
intraparietal area 0
- IP1 =
intraparietal area 1
- IP2 =
intraparietal area 2
- IPS =
intraparietal sulcus
- LBD =
left brain damage
- LIPd =
lateral intraparietal area dorsal
- Mbelt =
medial belt complex
- MIP =
medial intraparietal area
- p32pr =
area p32 prime
- Pol1 =
posterior insular area 1
- PF =
area PF complex
- PFC =
prefrontal cortex
- PFcm =
area PFcm
- PFm =
area PFm complex
- PFop =
area PF opercular
- PFt =
area PFt
- PGi =
area PGi
- PALS-B12 =
population average landmark- and surface-based atlas
- PEF =
premotor eye field
- POS2 =
parieto-occipital sulcus area 2
- pMTG =
posterior middle temporal gyrus
- OP4 =
area OP4/PV
- RBD =
right brain damage
- RI =
retroinsular cortex
- TL =
temporal lobe
- V2 =
second visual area
- V7 =
seventh visual area
- VIP =
ventral intraparietal complex
References
- 1.Liepmann H. Drei Aufsätze Aus Dem Apraxiegebiet. Berlin: Karger; 1908. [Google Scholar]
- 2.De Renzi E, ed. Apraxia. In: Boller F, Grafman J, eds. Handbook of neuropsychology. Amsterdam: Elsevier; 1989:245–263. [Google Scholar]
- 3.Goldenberg G. Apraxia: The cognitive side of motor control. New York: Oxford University Press; 2013. [DOI] [PubMed] [Google Scholar]
- 4.Osiurak F, Rossetti Y.. Definition: Limb apraxia. Cortex. 2017;93(3):228. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind N. Disconnexion syndromes in animals and man: Part II. Brain. 1965;88(3):585–644. [DOI] [PubMed] [Google Scholar]
- 6.De Renzi E, Lucchelli F.. Ideational apraxia. Brain. 1988;111(5):1173–1185. [DOI] [PubMed] [Google Scholar]
- 7.Niessen E, Fink GR, Weiss PH.. Apraxia, pantomime and the parietal cortex. NeuroImage: Clinical. 2014;5:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberg G. Pantomime of object use: A challenge to cerebral localization of cognitive function. Neuroimage. 2003;20:S101–S106. [DOI] [PubMed] [Google Scholar]
- 9.Hermsdörfer J, Li Y, Randerath J, Roby-Brami A, Goldenberg G.. Tool use kinematics across different modes of execution. Implications for action representation and apraxia. Cortex. 2013;49(1):184–199. [DOI] [PubMed] [Google Scholar]
- 10.Heilman KM, Rothi L.. Apraxia. In: Heilman KM, Rothi LJG. eds. Clinical neuropsychology. New York: Oxford University Press; 1993:141–163. [Google Scholar]
- 11.Rothi LJG, Heilman KM.. Apraxia: The neuropsychology of action. East Sussex: Psychology Press; 1997. [Google Scholar]
- 12.Goldenberg G, Hartmann K, Schlott I.. Defective pantomime of object use in left brain damage: Apraxia or asymbolia? Neuropsychologia. 2003;41(12):1565–1573. [DOI] [PubMed] [Google Scholar]
- 13.Buxbaum LJ, Kyle KM, Menon R.. On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain Res Cogn Brain Res. 2005;25(1):226–239. [DOI] [PubMed] [Google Scholar]
- 14.Króliczak G, Cavina-Pratesi C, Goodman DA, Culham JC.. What does the brain do when you fake it? An fMRI study of pantomimed and real grasping. J Neurophysiol. 2007;97(3):2410–2422. [DOI] [PubMed] [Google Scholar]
- 15.Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J.. From pantomime to actual use: How affordances can facilitate actual tool-use. Neuropsychologia. 2011;49(9):2410–2416. [DOI] [PubMed] [Google Scholar]
- 16.Manuel AL, Radman N, Mesot D, et al. Inter- and intrahemispheric dissociations in ideomotor apraxia: A large-scale lesion-symptom mapping study in subacute brain-damaged patients. Cereb Cortex. 2013;23(12):2781–2789. [DOI] [PubMed] [Google Scholar]
- 17.Buxbaum LJ, Shapiro AD, Coslett HB.. Critical brain regions for tool-related and imitative actions: A componential analysis. Brain. 2014;137(Pt 7):1971–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarhan LY, Watson CE, Buxbaum LJ.. Shared and distinct neuroanatomic regions critical for tool-related action production and recognition: Evidence from 131 left-hemisphere stroke patients. J Cogn Neurosci. 2015;27(12):2491–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson CE, Buxbaum LJ.. A distributed network critical for selecting among tool-directed actions. Cortex. 2015;65:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heilman KM, Rothi LJ, Valenstein E.. Two forms of ideomotor apraxia. Neurology. 1982;32(4):342–346. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12(3):211–231. [DOI] [PubMed] [Google Scholar]
- 22.Buxbaum LJ, Kyle K, Grossman M, Coslett HB.. Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–423. [DOI] [PubMed] [Google Scholar]
- 23.Króliczak G, Frey SH.. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex. 2009;19(10):2396–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buxbaum LJ, Kalénine S.. Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann N Y Acad Sci. 2010;1191:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeren M, Kümmerer D, Bormann T, et al. Neural bases of imitation and pantomime in acute stroke patients: Distinct streams for praxis. Brain. 2014;137(Pt 10):2796–2810. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Nitschke K, Beume L, et al. Brain activity underlying tool-related and imitative skills after major left hemisphere stroke. Brain. 2016;139(Pt 5):1497–1516. [DOI] [PubMed] [Google Scholar]
- 27.Finkelnburg F. Sitzung der Niederrheinischen Gesellschaft in Bonn. Medizinische Section. Berliner Klinische Wochenschrift. 1870;7:449–450; 460–462. [Google Scholar]
- 28.Duffy RJ, Duffy JR.. Three studies of deficits in pantomimic expression and pantomimic recognition in aphasia. J Speech Hear Res. 1981;24(1):70–84. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg G. Facets of pantomime. J Int Neuropsychol Soc. 2017;23(2):121–127. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg G, Randerath J.. Shared neural substrates of apraxia and aphasia. Neuropsychologia. 2015;75:40–49. [DOI] [PubMed] [Google Scholar]
- 31.Finkel L, Hogrefe K, Frey SH, Goldenberg G, Randerath J.. It takes two to pantomime: Communication meets motor cognition. NeuroImage: Clinical. 2018;19:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osiurak F, Reynaud E.. The elephant in the room: What matters cognitively in cumulative technological culture. Behav Brain Sci. 2019;43:e156. [DOI] [PubMed] [Google Scholar]
- 33.Osiurak F, Lesourd M, Navarro J, Reynaud E.. Technition: When tools come out of the closet. Perspect Psychol Sci. 2020;15(4):880–897. [DOI] [PubMed] [Google Scholar]
- 34.Goodglass H, Kaplan E.. Disturbance of gesture and pantomime in aphasia. Brain. 1963;86(4):703–720. [DOI] [PubMed] [Google Scholar]
- 35.Hogrefe K, Ziegler W, Weidinger N, Goldenberg G.. Non-verbal communication in severe aphasia: Influence of aphasia, apraxia, or semantic processing? Cortex. 2012;48(8):952–962. [DOI] [PubMed] [Google Scholar]
- 36.Hogrefe K, Ziegler W, Weidinger N, Goldenberg G.. Comprehensibility and neural substrate of communicative gestures in severe aphasia. Brain Lang. 2017;171:62–71. [DOI] [PubMed] [Google Scholar]
- 37.Emmorey K, McCullough S, Mehta S, Ponto LLB, Grabowski TJ.. Sign language and pantomime production differentially engage frontal and parietal cortices. Lang Cogn Process. 2011;26(7):878–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haaland KY, Flaherty D.. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984;3(4):370–384. [DOI] [PubMed] [Google Scholar]
- 39.Mozaz MJ. Ideational and ideomotor apraxia: A qualitative analysis. Behav Neurol. 1992;5(1):11–17. [DOI] [PubMed] [Google Scholar]
- 40.McDonald S, Tate RL, Rigby J.. Error types in ideomotor apraxia: A qualitative analysis. Brain Cogn. 1994;25(2):250–270. [DOI] [PubMed] [Google Scholar]
- 41.Tate RL, McDonald S.. What is apraxia? The clinician’s dilemma. Neuropsychol Rehabil. 1995;5(4):273–297. [Google Scholar]
- 42.Hanna-Pladdy B, Daniels SK, Fieselman MA, et al. Praxis lateralization: Errors in right and left hemisphere stroke. Cortex. 2001;37(2):219–230. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein K. Language and language disturbances. New York: Grune & Stratton; 1948. [Google Scholar]
- 44.Roy EA, Hall C.. Limb apraxia: A process approach. In: Proteau L, Elliott D, eds. Vision and motor control. Amsterdam: Elsevier; 1992;261–282. [Google Scholar]
- 45.Bartolo A, Cubelli R, Della SS, Drei S.. Pantomimes are special gestures which rely on working memory. Brain Cogn. 2003;53(3):483–494. [DOI] [PubMed] [Google Scholar]
- 46.Bartolo A, Stieglitz Ham H.. A cognitive overview of limb apraxia. Curr Neurol Neurosci Rep. 2016;16(8):75. [DOI] [PubMed] [Google Scholar]
- 47.Arbib MA. Mirror system activity for action and language is embedded in the integration of dorsal and ventral pathways. Brain Lang. 2010;112(1):12–24. [DOI] [PubMed] [Google Scholar]
- 48.Morgan TJH, Uomini NT, Rendell LE, et al. Experimental evidence for the co-evolution of hominin tool-making teaching and language. Nat Commun. 2015;6(1):6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arbib MA. Toward the language-ready brain: Biological evolution and primate comparisons. Psychon Bull Rev. 2017;24(1):142–150. [DOI] [PubMed] [Google Scholar]
- 50.Bohn M, Kordt C, Braun M, Call J, Tomasello M.. Learning novel skills from iconic gestures: A developmental and evolutionary perspective. Psychol Sci. 2020;31(7):873–880. [DOI] [PubMed] [Google Scholar]
- 51.Boyd R, Richerson PJ.. Why culture is common, but cultural evolution is rare. Proc Br Acad. 1995;88:77–93. [Google Scholar]
- 52.Tomasello M, Kruger AC, Ratner HH.. Cultural learning. Behav Brain Sci. 1993;16(3):495–511. [Google Scholar]
- 53.Kline MA. How to learn about teaching: An evolutionary framework for the study of teaching behavior in humans and other animals. Behav Brain Sci. 2015;38:1–71. [DOI] [PubMed] [Google Scholar]
- 54.Clark MA, Merians AS, Kothari A, et al. Spatial planning deficits in limb apraxia. Brain. 1994;117(5):1093–1106. [DOI] [PubMed] [Google Scholar]
- 55.Poizner H, Clark M, Merians AS, Macauley B, Rothi LJG, Heilman KM.. Joint coordination deficits in limb apraxia. Brain. 1995;118(1):227–242. [DOI] [PubMed] [Google Scholar]
- 56.Graham NL, Zeman A, Young AW, Patterson K, Hodges JR.. Dyspraxia in a patient with corticobasal degeneration: The role of visual and tactile inputs to action. J Neurol Neurosurg Psychiatry. 1999;67(3):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wada Y, Nakagawa Y, Nishikawa T, et al. Role of somatosensory feedback from tools in realizing movements by patients with ideomotor apraxia. Eur Neurol. 1999;41(2):73–78. [DOI] [PubMed] [Google Scholar]
- 58.Neiman MR, Duffy RJ, Belanger SA, Coelho CA.. The assessment of limb apraxia: Relationship between performances on single- and multiple-object tasks by left hemisphere damaged aphasic subjects. Neuropsychol Rehabil. 2000;10(4):429–448. [Google Scholar]
- 59.Heath M, Almeida QJ, Roy EA, Black SE, Westwood D.. Selective dysfunction of tool-use: A failure to integrate somatosensation and action. Neurocase. 2003;9(2):156–163. [DOI] [PubMed] [Google Scholar]
- 60.Goldenberg G, Hentze S, Hermsdorfer J.. The effect of tactile feedback on pantomime of tool use in apraxia. Neurology. 2004;63(10):1863–1867. [DOI] [PubMed] [Google Scholar]
- 61.Hermsdörfer J, Hentze S, Goldenberg G.. Spatial and kinematic features of apraxic movement depend on the mode of execution. Neuropsychologia. 2006;44(10):1642–1652. [DOI] [PubMed] [Google Scholar]
- 62.Hécaen H. Les apraxies idéomotrices: Essai de dissociation. In: Hécaen H, Jeannerod M, eds. Du Contrôle Moteur à l’organisation Du Geste. Paris: Masson; 1978. 343–358. [Google Scholar]
- 63.Belanger SA, Duffy RJ, Coelho CA.. An investigation of limb apraxia regarding the validity of current assessment procedures. Clin Aphasiol. 1994;22:191–201. [Google Scholar]
- 64.Foundas AL, Macauley BL, Raymer AM, et al. Ecological implications of limb apraxia: Evidence from mealtime behavior. J Int Neuropsychol Soc. 1995;1(1):62–66. [DOI] [PubMed] [Google Scholar]
- 65.Belanger SA, Duffy RJ, Coelho CA.. The assessment of limb apraxia: An investigation of task effects and their cause. Brain Cogn. 1996;32(3):384–404. [DOI] [PubMed] [Google Scholar]
- 66.Heilman KM, Maher LM, Greenwald ML, Rothi LJG.. Conceptual apraxia from lateralized lesions. Neurology. 1997;49(2):457–464. [DOI] [PubMed] [Google Scholar]
- 67.Goldenberg G, Hagmann S.. Tool use and mechanical problem solving in apraxia. Neuropsychologia. 1998;36(7):581–589. [DOI] [PubMed] [Google Scholar]
- 68.Goldenberg G, Hagmann S.. Therapy of activities of daily living in patients with apraxia. Neuropsychol Rehabil. 1998;8(2):123–141. [Google Scholar]
- 69.Goldenberg G, Daumüller M, Hagmann S.. Assessment and therapy of complex activities of daily living in apraxia. Neuropsychol Rehabil. 2001;11(2):147–169. [Google Scholar]
- 70.Hartmann K, Goldenberg G, Daumüller M, Hermsdörfer J.. It takes the whole brain to make a cup of coffee: The neuropsychology of naturalistic actions involving technical devices. Neuropsychologia. 2005;43(4):625–637. [DOI] [PubMed] [Google Scholar]
- 71.Osiurak F, Aubin G, Allain P, et al. Different constraints on grip selection in brain-damaged patients: Object use versus object transport. Neuropsychologia. 2008;46(9):2431–2434. [DOI] [PubMed] [Google Scholar]
- 72.Buchmann I, Randerath J.. Selection and application of familiar and novel tools in patients with left and right hemispheric stroke: Psychometrics and normative data. Cortex. 2017;94(June):49–62. [DOI] [PubMed] [Google Scholar]
- 73.Jarry C, Osiurak F, Delafuys D, Chauviré V, Etcharry-Bouyx F, Le Gall D.. Apraxia of tool use: More evidence for the technical reasoning hypothesis. Cortex. 2013;49(9):2322–2333. [DOI] [PubMed] [Google Scholar]
- 74.Osiurak F, Jarry C, Lesourd M, Baumard J, Le Gall D.. Mechanical problem-solving strategies in left-brain damaged patients and apraxia of tool use. Neuropsychologia. 2013;51(10):1964–1972. [DOI] [PubMed] [Google Scholar]
- 75.Lesourd M, Budriesi C, Osiurak F, Nichelli PF, Bartolo A.. Mechanical knowledge does matter to tool use even when assessed with a non-production task: Evidence from left brain-damaged patients. J Neuropsychol. 2019;13(2):198–213. [DOI] [PubMed] [Google Scholar]
- 76.Goldenberg G, Spatt J.. The neural basis of tool use. Brain. 2009;132(Pt 6):1645–1655. [DOI] [PubMed] [Google Scholar]
- 77.Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J.. Different left brain regions are essential for grasping a tool compared with its subsequent use. Neuroimage. 2010;53(1):171–180. [DOI] [PubMed] [Google Scholar]
- 78.Mengotti P, Corradi-Dell’Acqua C, Negri GAL, Ukmar M, Pesavento V, Rumiati RI.. Selective imitation impairments differentially interact with language processing. Brain. 2013;136(Pt 8):2602–2618. [DOI] [PubMed] [Google Scholar]
- 79.Martin M, Beume L, Kümmerer D, et al. Differential roles of ventral and dorsal streams for conceptual and production-related components of tool use in acute stroke patients. Cereb Cortex. 2016;26(9):3754–3771. [DOI] [PubMed] [Google Scholar]
- 80.Salazar-López E, Schwaiger BJ, Hermsdörfer J.. Lesion correlates of impairments in actual tool use following unilateral brain damage. Neuropsychologia. 2016;84:167–180. [DOI] [PubMed] [Google Scholar]
- 81.Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldenberg G, Hermsdörfer J, Glindemann R, Rorden C, Karnath HO.. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb Cortex. 2007;17(12):2769–2776. [DOI] [PubMed] [Google Scholar]
- 83.Kalénine S, Shapiro AD, Buxbaum LJ.. Dissociations of action means and outcome processing in left-hemisphere stroke. Neuropsychologia. 2013;51(7):1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiss PH, Ubben SD, Kaesberg S, et al. Where language meets meaningful action: A combined behavior and lesion analysis of aphasia and apraxia. Brain Struct Funct. 2016;221(1):563–576. [DOI] [PubMed] [Google Scholar]
- 85.Dressing A, Nitschke K, Kümmerer D, et al. Distinct contributions of dorsal and ventral streams to imitation of tool-use and communicative gestures. Cereb Cortex. 2018;28(2):474–492. [DOI] [PubMed] [Google Scholar]
- 86.Dressing A, Kaller CP, Nitschke K, et al. Neural correlates of acute apraxia: Evidence from lesion data and functional MRI in stroke patients. Cortex. 2019;120:1–21. [DOI] [PubMed] [Google Scholar]
- 87.Tessari A, Canessa N, Ukmar M, Rumiati RI.. Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain. 2007;130(Pt 4):1111–1126. [DOI] [PubMed] [Google Scholar]
- 88.Kertesz A, Ferro JM.. Lesion size and location in ideomotor apraxia. Brain. 1984;107(3):921–933. [DOI] [PubMed] [Google Scholar]
- 89.Peigneux P. L’apraxie gestuelle: Une approche cognitive, neuropsychologique et par imagerie cérébrale. PhD thesis. Université de Liège, Faculty of Psychology and Sciences. 2000.
- 90.Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philos Trans R Soc Lond B Biol Sci. 2008;363(1499):1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi S, Na DL, Kang E, Lee K, Lee S, Na D.. Functional magnetic resonance imaging during pantomiming tool-use gestures. Exp Brain Res. 2001;139(3):311–317. [DOI] [PubMed] [Google Scholar]
- 92.Ohgami Y, Matsuo K, Uchida N, Nakai T.. An fMRI study of tool-use gestures: Body part as object and pantomime. Neuroreport. 2004;15(12):1903–1906. [DOI] [PubMed] [Google Scholar]
- 93.Johnson-Frey SH, Newman-Norlund R, Grafton ST.. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15(6):681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fridman EA, Immisch I, Hanakawa T, et al. The role of the dorsal stream for gesture production. Neuroimage. 2006;29(2):417–428. [DOI] [PubMed] [Google Scholar]
- 95.Hermsdörfer J, Terlinden G, Mühlau M, Goldenberg G, Wohlschläger AM.. Neural representations of pantomimed and actual tool use: Evidence from an event-related fMRI study. Neuroimage. 2007;36:T109–T118. [DOI] [PubMed] [Google Scholar]
- 96.Higuchi S, Imamizu H, Kawato M.. Cerebellar activity evoked by common tool-use execution and imagery task: An fMRI study. Cortex. 2007;43(3):350–358. [DOI] [PubMed] [Google Scholar]
- 97.Imazu S, Sugio T, Tanaka S, Inui T.. Differences between actual and imagined usage of chopsticks: An fMRI study. Cortex. 2007;43(3):301–307. [DOI] [PubMed] [Google Scholar]
- 98.Montgomery KJ, Isenberg N, Haxby JV.. Communicative hand gestures and object-directed hand movements activated the mirror neuron system. Soc Cogn Affect Neurosci. 2007;2(2):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bohlhalter S, Hattori N, Wheaton L, et al. Gesture subtype-dependent left lateralization of praxis planning: An event-related fMRI study. Cereb Cortex. 2009;19(6):1256–1262.doi:10.1093/cercor/bhn168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vingerhoets G, Acke F, Vandemaele P, Achten E.. Tool responsive regions in the posterior parietal cortex: Effect of differences in motor goal and target object during imagined transitive movements. Neuroimage. 2009;47(4):1832–1843. [DOI] [PubMed] [Google Scholar]
- 101.Péran P, Démonet JF, Cherubini A, Carbebat D, Caltagirone C, Sabatini U.. Mental representations of action: The neural correlates of the verbal and motor components. Brain Res. 2010;1328:89–103. [DOI] [PubMed] [Google Scholar]
- 102.Vingerhoets G, Vandekerckhove E, Honoré P, Vandemaele P, Achten E.. Neural correlates of pantomiming familiar and unfamiliar tools: Action semantics versus mechanical problem solving? Hum Brain Mapp. 2011;32(6):905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wadsworth HM, Kana RK.. Brain mechanisms of perceiving tools and imagining tool use acts: A functional MRI study. Neuropsychologia. 2011;49(7):1863–1869. [DOI] [PubMed] [Google Scholar]
- 104.Vingerhoets G, Alderweireldt AS, Vandemaele P, et al. Praxis and language are linked: Evidence from co-lateralization in individuals with atypical language dominance. Cortex. 2013;49(1):172–183. [DOI] [PubMed] [Google Scholar]
- 105.Mäki-Marttunen V, Villarreal M, Leiguarda RC.. Lateralization of brain activity during motor planning of proximal and distal gestures. Behav Brain Res. 2014;272:226–237. [DOI] [PubMed] [Google Scholar]
- 106.Vry MS, Tritschler LC, Hamzei F, et al. The ventral fiber pathway for pantomime of object use. Neuroimage. 2015;106:252–263. [DOI] [PubMed] [Google Scholar]
- 107.Lausberg H, Kazzer P, Heekeren HR, Wartenburger I.. Pantomiming tool use with an imaginary tool in hand as compared to demonstration with tool in hand specifically modulates the left middle and superior temporal gyri. Cortex. 2015;71:1–14. [DOI] [PubMed] [Google Scholar]
- 108.Bartolo A, Daumüller M, Della SS, Goldenberg G.. Relationship between object-related gestures and the fractionated object knowledge system. Behav Neurol. 2007;18(3):143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Osiurak F, Jarry C, Allain P, et al. Unusual use of objects after unilateral brain damage. The technical reasoning model. Cortex. 2009;45(6):769–783. [DOI] [PubMed] [Google Scholar]
- 110.Goldenberg G, Hartmann-Schmid K, Sürer F, Daumüller M, Hermsdörfer J.. The impact of dysexecutive syndrome on use of tools and tehnical devices. Cortex. 2007;43(3):424–435. [DOI] [PubMed] [Google Scholar]
- 111.Reynaud E, Lesourd M, Navarro J, Osiurak F.. On the neurocognitive origins of human tool use: A critical review of neuroimaging data. Neurosci Biobehav Rev. 2016;64:421–437. [DOI] [PubMed] [Google Scholar]
- 112.Buxbaum LJ. Ideomotor apraxia: A call to action. Neurocase. 2001;7(6):445–458. [DOI] [PubMed] [Google Scholar]
- 113.Van Elk M, van Schie H, Bekkering H.. Action semantics: A unifying conceptual framework for the selective use of multimodal and modality-specific object knowledge. Phys Life Rev. 2014;11(2):220–250. [DOI] [PubMed] [Google Scholar]
- 114.Reynaud E, Navarro J, Lesourd M, Osiurak F.. To watch is to work: A review of neuroimaging data on tool use observation network. Neuropsychol Rev. 2019;29(4):484–497. [DOI] [PubMed] [Google Scholar]
- 115.Van Overwalle F, Baetens K.. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage. 2009;48(3):564–584. [DOI] [PubMed] [Google Scholar]
- 116.Spunt RP, Satpute AB, Lieberman MD.. Identifying the what, why, and how of an observed action: An fMRI study of mentalizing and mechanizing during action observation. J Cogn Neurosci. 2011;23(1):63–74. [DOI] [PubMed] [Google Scholar]
- 117.Spunt RP, Lieberman MD.. Dissociating modality-specific and supramodal neural systems for action understanding. J Neurosci. 2012;32(10):3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Massen C, Prinz W.. Programming tool-use actions. J Exp Psychol Hum Percept Perform. 2007;33(3):692–704. [DOI] [PubMed] [Google Scholar]
- 119.Massen C, Prinz W.. Movements, actions and tool-use actions: An ideomotor approach to imitation. Philos Trans R Soc Lond B Biol Sci. 2009;364(1528):2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Osiurak F, Badets A.. Pliers, not fingers: Tool-action effect in a motor intention paradigm. Cognition. 2014;130(1):66–73. [DOI] [PubMed] [Google Scholar]
- 121.Badets A, Osiurak F.. A goal-based mechanism for delayed motor intention: Considerations from motor skills, tool use and action memory. Psychol Res. 2015;79(3):345–360. [DOI] [PubMed] [Google Scholar]
- 122.Osiurak F, Badets A.. Tool use and affordance: Manipulation-based versus reasoning-based approaches. Psychol Rev. 2016;123(5):534–568. [DOI] [PubMed] [Google Scholar]
- 123.Osiurak F, Jarry C, Le Gall D.. Grasping the affordances, understanding the reasoning: Toward a dialectical theory of human tool use. Psychol Rev. 2010;117(2):517–540. [DOI] [PubMed] [Google Scholar]
- 124.Osiurak F. What neuropsychology tells us about human tool use? The four constraints theory (4CT): Mechanics, space, time, and effort. Neuropsychol Rev. 2014;24(2):88–115. [DOI] [PubMed] [Google Scholar]
- 125.Osiurak F, Rossetti Y, Badets A.. What is an affordance? 40 years later. Neurosci Biobehav Rev. 2017;77(April):403–417. [DOI] [PubMed] [Google Scholar]
- 126.Osiurak F, Heinke D.. Looking for intoolligence: A unified framework for the cognitive study of human tool use and technology. Am Psychol. 2018;73(2):169–185. [DOI] [PubMed] [Google Scholar]
- 127.Osiurak F. The tool instinct. London: Wiley Press; 2020. [Google Scholar]
- 128.Osiurak F, Lasserre S, Arbanti J, et al. Technical reasoning is crucial to avoid reinventing the wheel. Nat Hum Behav. 2021. 10.1038/s41562-021-01159-9 [DOI] [PubMed] [Google Scholar]
- 129.Gagnepain J. Du Vouloir Dire: Du Signe, de l’outil. Bruxelles: De Boeck; 1990.
- 130.Le Gall D. Des Apraxies Aux Atechnies: Propositions Pour Une Ergologie Clinique. Bruxelles: De Boeck; 1998.
- 131.Osiurak F, Jarry C, Le Gall D.. Re-examining the gesture engram hypothesis. New perspectives on apraxia of tool use. Neuropsychologia. 2011;49(3):299–312. [DOI] [PubMed] [Google Scholar]
- 132.Humphreys GW. Objects, affordances …action! Psychologist. 2001;14(8):408–412. [Google Scholar]
- 133.Hodges JR, Bozeat S, Lambon RM, Patterson K, Spatt J.. The role of conceptual knowledge in object use: Evidence from semantic dementia. Brain. 2000;123(9):1913–1925. [DOI] [PubMed] [Google Scholar]
- 134.Norman J. Two visual systems and two theories of perception: An attempt to reconcile the constructivist and ecological approaches. Behav Brain Sci. 2002;25(1):73–144. [DOI] [PubMed] [Google Scholar]
- 135.Buxbaum LJ. Learning, remembering, and predicting how to use tools: Distributed neurocognitive mechanisms: Comment on Osiurak and Badets (2016). Psychol Rev. 2017;124(3):346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Caruana F, Cuccio V.. Types of abduction in tool behavior. Phenomenol Cogn Sci. 2017;16(2):255–273. [Google Scholar]
- 137.Tucker M, Ellis R.. On the relations between seen objects and components of potential actions. J Exp Psychol Hum Percept Perform. 1998;24(3):830–846. [DOI] [PubMed] [Google Scholar]
- 138.Borghi AM, Riggio L.. Sentence comprehension and simulation of object temporary, canonical and stable affordances. Brain Res. 2009;1253:117–128. [DOI] [PubMed] [Google Scholar]
- 139.Thill S, Caligiore D, Borghi AM, Ziemke T, Baldassarre G.. Theories and computational models of affordance and mirror systems: An integrative review. Neurosci Biobehav Rev. 2013;37(3):491–521. [DOI] [PubMed] [Google Scholar]
- 140.Roy EA, Black SE, Blair N, Dimeck PT.. Analyses of deficits in gestural pantomime. J Clin Exp Neuropsychol. 1998;20(5):628–643. [DOI] [PubMed] [Google Scholar]
- 141.Cubelli R, Marchetti C, Boscolo G, Della Sala S.. Cognition in action: Testing a model of limb apraxia. Brain Cogn. 2000;44(2):144–165. [DOI] [PubMed] [Google Scholar]
- 142.Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R.. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41(8):1091–1113. [DOI] [PubMed] [Google Scholar]
- 143.Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y.. A selective impairment of hand posture for object utilization in apraxia. Cortex. 1995;31(1):41–55. [DOI] [PubMed] [Google Scholar]
- 144.Lesourd M, Naëgelé B, Jaillard A, Detante O, Osiurak F.. Using tools effectively despite defective hand posture: A single-case study. Cortex. 2020;129:406–422. [DOI] [PubMed] [Google Scholar]
- 145.Randerath J, Li Y, Goldenberg G, Hermsdörfer J.. Grasping tools: Effects of task and apraxia. Neuropsychologia. 2009;47(2):497–505. [DOI] [PubMed] [Google Scholar]
- 146.Creem SH, Proffitt DR.. Grasping objects by their handles: A necessary interaction between cognition and action. J Exp Psychol Hum Percept Perform. 2001;27(1):218–228. [DOI] [PubMed] [Google Scholar]
- 147.Goodale MA, Meenan JP, Bülthoff HH, Nicolle DA, Murphy KJ, Racicot CI.. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr Biol. 1994;4(7):604–610. [DOI] [PubMed] [Google Scholar]
- 148.Westwood DA, Chapman CD, Roy EA.. Pantomimed actions may be controlled by the ventral visual stream. Exp Brain Res. 2000;130(4):545–548. [DOI] [PubMed] [Google Scholar]
- 149.Laimgruber K, Goldenberg G, Hermsdörfer J.. Manual and hemispheric asymmetries in the execution of actual and pantomimed prehension. Neuropsychologia. 2005;43(5):682–692. [DOI] [PubMed] [Google Scholar]
- 150.Wohlschläger A, Gattis M, Bekkering H.. Action generation and action perception in imitation: An instance of the ideomotor principle. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tessari A, Rumiati RI.. The strategic control of multiple routes in imitation of actions. J Exp Psychol Hum Percept Perform. 2004;30(6):1107–1116. [DOI] [PubMed] [Google Scholar]
- 152.Rumiati RI, Weiss PH, Tessari A, et al. Common and differential neural mechanisms supporting imitation of meaningful and meaningless actions. J Cogn Neurosci. 2005;17(9):1420–1431. [DOI] [PubMed] [Google Scholar]
- 153.Duffy RJ, Duffy JR.. An investigation of body part as object (BPO) responses in normal and brain-damaged adults. Brain Cogn. 1989;10(2):220–236. [DOI] [PubMed] [Google Scholar]
- 154.Raymer AM, Maher LM, Foundas AL, Heilman KM G, Rothi LJ.. Significance of body part as tool errors in limb apraxia. Brain Cogn. 1997;34(2):287–292. [DOI] [PubMed] [Google Scholar]
- 155.Mozaz MJ, Peña J, Barraquer LL, Marti J, Goldstein LH.. Use of body part as object in brain-damaged subjects. Clin Neuropsychol. 1993;7(1):39–47. [Google Scholar]
- 156.Gonzalez Rothi LJ, Mack L, Verfaellie M, Brown P, Heilman KM.. Ideomotor apraxia: Error pattern analysis. Aphasiology. 1988;2(3-4):381–387. [Google Scholar]
- 157.Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB.. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J Int Neuropsychol Soc. 2006;12(3):314–326. [DOI] [PubMed] [Google Scholar]
- 158.Stieglitz Ham H, Bartolo A, Corley M, Rajendran G, Szabo A, Swanson S.. Exploring the relationship between gestural recognition and imitation: Evidence of dyspraxia in autism spectrum disorders. J Autism Dev Disord. 2011;41(1):1–12. [DOI] [PubMed] [Google Scholar]
- 159.Martin P, Tewesmeier M, Albers M, Schmid G, Scharfetter C.. Investigation of gestural and pantomime performance in chronic schizophrenic inpatients. Eur Arch Psychiatry Clin Neurosci. 1994;244(2):59–64. [DOI] [PubMed] [Google Scholar]
- 160.Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S.. Impaired pantomime in schizophrenia: Association with frontal lobe function. Cortex. 2013;49(2):520–527. [DOI] [PubMed] [Google Scholar]
- 161.Walther S, Mittal VA, Stegmayer K, Bohlhalter S.. Gesture deficits and apraxia in schizophrenia. Cortex. 2020;133:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dumont C, Ska B, Joanette Y.. Conceptual apraxia and semantic memory deficit in Alzheimer’s disease: Two sides of the same coin? J Int Neuropsychol Soc. 2000;6(6):693–703. [DOI] [PubMed] [Google Scholar]
- 163.Bartolo A, Della Sala S, Cubelli R.. Effect of test instructions: The example of the pantomime production task. Brain Cogn. 2020;139:105516. [DOI] [PubMed] [Google Scholar]
- 164.Peigneux P, van der Linden M.. Influence of ageing and educational level on the prevalence of body-part-as-objects in normal subjects. J Clin Exp Neuropsychol. 1999;21(4):547–552. [DOI] [PubMed] [Google Scholar]
- 165.Ohnemus EM. Use of Body-Part as Object (BPO) on a Test of Pantomime Expression in Normal Adults. Senior thesis. University of Massachusetts. 1983. [Google Scholar]
- 166.Vingerhoets G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Frontiers Psychol. 2014;5:151–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Desmurget M, Grafton S.. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4(11):423–431. [DOI] [PubMed] [Google Scholar]
- 168.Wolpert D, Ghahramani Z, Jordan M.. An internal model for sensorimotor integration. Science. 1995;269(5232):1880–1882. [DOI] [PubMed] [Google Scholar]
- 169.Wolpert DM, Ghahramani Z.. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(S11):1212–1217. [DOI] [PubMed] [Google Scholar]
- 170.Schwoebel J, Coslett HB.. Evidence for multiple, distinct representations of the human body. J Cogn Neurosci. 2005;17(4):543–553. [DOI] [PubMed] [Google Scholar]
- 171.de Vignemont F. Body schema and body image: Pros and cons. Neuropsychologia. 2010;48(3):669–680. [DOI] [PubMed] [Google Scholar]
- 172.Heilman KM, Rothi LG, Mack L, Feinberg T, Watson RT.. Apraxia after a superior parietal lesion. Cortex. 1986;22(1):141–150. [DOI] [PubMed] [Google Scholar]
- 173.Buxbaum LJ, Giovannetti T, Libon D.. The role of the dynamic body schema in praxis: Evidence from primary progressive apraxia. Brain Cogn. 2000;44(2):166–191. [DOI] [PubMed] [Google Scholar]
- 174.Haaland KY, Harrington DL, Knight RT.. Neural representations of skilled movement. Brain. 2000;123(11):2306–2313. [DOI] [PubMed] [Google Scholar]
- 175.Rumiati RI, Weiss PH, Shallice T, et al. Neural basis of pantomiming the use of visually presented objects. Neuroimage. 2004;21(4):1224–1231. [DOI] [PubMed] [Google Scholar]
- 176.Wang L, Goodglass H.. Pantomime, praxis, and aphasia. Brain Lang. 1992;42(4):402–418. [DOI] [PubMed] [Google Scholar]
- 177.Goldenberg G, Hagmann S.. The meaning of meaningless gestures: A study of visuo-imitative apraxia. Neuropsychologia. 1997;35(3):333–341. [DOI] [PubMed] [Google Scholar]
- 178.Goldenberg G. Apraxia in left-handers. Brain. 2013;136(Pt 8):2592–2601. [DOI] [PubMed] [Google Scholar]
- 179.Goldenberg G, Karnath HO.. The neural basis of imitation is body part specific. J Neurosci. 2006;26(23):6282–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Achilles EIS, Ballweg CS, Niessen E, et al. Neural correlates of differential finger gesture imitation deficits in left hemisphere stroke. Neuroimage Clin. 2019;23:101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pizzamiglio G, Zhang Z, Kolasinski J, et al. A role for the action observation network in apraxia after stroke. Front Hum Neurosci. 2019;13:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tessari A, Mengotti P, Faccioli L, et al. Effect of body-part specificity and meaning in gesture imitation in left hemisphere stroke patients. Neuropsychologia. 2021;151:107720. [DOI] [PubMed] [Google Scholar]
- 183.Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47(6):1449–1459. [DOI] [PubMed] [Google Scholar]
- 184.Sunderland A. Impaired imitation of meaningless gestures in ideomotor apraxia: A conceptual problem not a disorder of action control? A single case investigation. Neuropsychologia. 2007;45(8):1621–1631. [DOI] [PubMed] [Google Scholar]
- 185.Goldenberg G. Imitating gestures and manipulating a mannikin—The representation of the human body in ideomotor apraxia. Neuropsychologia. 1995;33(1):63–72. [DOI] [PubMed] [Google Scholar]
- 186.Goldenberg G, Hermsdörfer J, Spatt J.. Ideomotor apraxia and cerebral dominance for motor control. Brain Res Cogn Brain Res. 1996;3(2):95–100. [DOI] [PubMed] [Google Scholar]
- 187.Goldenberg G. Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia. 1999;37(5):559–566. [DOI] [PubMed] [Google Scholar]
- 188.Sunderland A, Wilkins L, Dineen R, Dawson SE.. Tool-use and the left hemisphere: What is lost in ideomotor apraxia? Brain Cogn. 2013;81(2):183–192. [DOI] [PubMed] [Google Scholar]
- 189.Lane D, Tessari A, Ottoboni G, et al. Body representation in people with apraxia post stroke: An observational study. Brain Inj. 2021;35(4):468–475. [DOI] [PubMed] [Google Scholar]
- 190.Dafsari HS, Dovern A, Fink GR, Weiss PH.. Deficient body structural description contributes to apraxic end-position errors in imitation. Neuropsychologia. 2019;133:107150. [DOI] [PubMed] [Google Scholar]
- 191.Tanaka S, Inui T.. Cortical involvement for action imitation of hand/arm postures versus finger configurations: An fMRI study. NeuroReport. 2002;13(13):1599–1602. [DOI] [PubMed] [Google Scholar]
- 192.Koski L, Iacoboni M, Dubeau MC, Woods RP, Mazziotta JC.. Modulation of cortical activity during different imitative behaviors. J Neurophysiol. 2003;89(1):460–471. [DOI] [PubMed] [Google Scholar]
- 193.Buccino G, Vogt S, Ritzl A, et al. Neural circuits underlying imitation learning of hand actions: An event-related fMRI study. Neuron. 2004;42(2):323–334. [DOI] [PubMed] [Google Scholar]
- 194.Chaminade T, Meltzoff AN, Decety J.. An fMRI study of imitation: Action representation and body schema. Neuropsychologia. 2005;43(1):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mühlau M, Hermsdörfer J, Goldenberg G, et al. Left inferior parietal dominance in gesture imitation: An fMRI study. Neuropsychologia. 2005;43(7):1086–1098. [DOI] [PubMed] [Google Scholar]
- 196.Jackson PL, Meltzoff AN, Decety J.. Neural circuits involved in imitation and perspective-taking. Neuroimage. 2006;31(1):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Suchan B, Melde C, Herzog H, Hömberg V, Seitz RJ.. Activation differences in observation of hand movements for imitation or velocity judgement. Behav Brain Res. 2008;188(1):78–83. [DOI] [PubMed] [Google Scholar]
- 198.Adamovich SV, August K, Merians A, Tunik E.. A virtual reality-based system integrated with fmri to study neural mechanisms of action observation-execution: A proof of concept study. Restor Neurol Neurosci. 2009;27(3):209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Watanabe R, Watanabe S, Kuruma H, Murakami Y, Seno A, Matsuda T.. Neural activation during imitation of movements presented from four different perspectives: A functional magnetic resonance imaging study. Neurosci Lett. 2011;503(2):100–104. [DOI] [PubMed] [Google Scholar]
- 200.Krüger B, Bischoff M, Blecker C, et al. Parietal and premotor cortices: Activation reflects imitation accuracy during observation, delayed imitation and concurrent imitation. Neuroimage. 2014;100:39–50. [DOI] [PubMed] [Google Scholar]
- 201.Vingerhoets G, Clauwaert A.. Functional connectivity associated with hand shape generation: Imitating novel hand postures and pantomiming tool grips challenge different nodes of a shared neural network. Hum Brain Mapp. 2015;36(9):3426–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Watanabe R, Higuchi T, Kikuchi Y, Taira M.. Visuomotor effects of body part movements presented in the first-person perspective on imitative behavior. Hum Brain Mapp. 2017;38(12):6218–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lesourd M, Osiurak F, Baumard J, Bartolo A, Vanbellingen T, Reynaud E.. Cerebral correlates of imitation of intransitive gestures: An integrative review of neuroimaging data and brain lesion studies. Neurosci Biobehav Rev. 2018;95(August):44–60. [DOI] [PubMed] [Google Scholar]
- 204.Faye A, Jacquin-Courtois S, Reynaud E, Lesourd M, Besnard J, Osiurak F.. Numerical cognition: A meta-analysis of neuroimaging, transcranial magnetic stimulation and brain-damaged patients studies. Neuroimage Clin. 2019;24:102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Scotto di Tella G, Ruotolo F, Ruggiero G, Iachini T, Bartolo A.. Towards and away from the body: The relevance of the direction of use in the coding of object-related actions. Q J Exp Psychol (Hove). 2021;74(7):1225–1233. [DOI] [PubMed] [Google Scholar]
- 206.Bartolo A, Claisse C, Gallo F, Ott L, Sampaio A, Nandrino J-L.. Gestures convey different physiological responses when performed toward and away from the body. Sci Rep. 2019;9(1):12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Halsband U, Schmitt J, Weyers M, Binkofski F, Grützner G, Freund HJ.. Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: A perspective on apraxia. Neuropsychologia. 2001;39(2):200–216. [DOI] [PubMed] [Google Scholar]
- 208.Ruotolo F, Iachini T, Ruggiero G, Scotto di Tella G, Ott L, Bartolo A.. The role of mental imagery in pantomimes of actions towards and away from the body. Psychol Res. 2021;85(4):1408–1417. [DOI] [PubMed] [Google Scholar]
- 209.Osiurak F, Jarry C, Baltenneck N, Boudin B, Le Gall D.. Make a gesture and I will tell you what you are miming. Pantomime recognition in healthy subjects. Cortex. 2012;48(5):584–592. [DOI] [PubMed] [Google Scholar]
- 210.Baumard J, Osiurak F, Lesourd M, Le Gall D.. Tool use disorders after left brain damage. Front Psychol. 2014;5:473–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lesourd M, Baumard J, Jarry C, et al. Rethinking the cognitive mechanisms underlying pantomime of tool use: Evidence from Alzheimer’s disease and semantic dementia. J Int Neuropsychol Soc. 2017;23(2):128–138. [DOI] [PubMed] [Google Scholar]
- 212.Buxbaum LJ, Saffran EM.. Knowledge of object manipulation and object function: Dissociations in apraxic and nonapraxic subjects. Brain Lang. 2002;82(2):179–199. [DOI] [PubMed] [Google Scholar]
- 213.Ochipa C, Rothi LJG, Heilman KM.. Ideational apraxia: A deficit in tool selection and use. Ann Neurol. 1989;25(2):190–193. [DOI] [PubMed] [Google Scholar]
- 214.Rothi LJG, Ochipa C, Heilman KM.. A cognitive neuropsychological model of limb praxis. Cogn Neuropsychol. 1991;8(6):443–458. [Google Scholar]
- 215.Buxbaum LJ, Schwartz MF, Carew TG.. The role of semantic memory in object use. Cogn Neuropsychol. 1997;14(2):219–254. [Google Scholar]
- 216.Lauro-Grotto R, Piccini C, Shallice T.. Modality-specific operations in semantic dementia. Cortex. 1997;33(4):593–622. [DOI] [PubMed] [Google Scholar]
- 217.Negri GAL, Rumiati R, Zadini A, Ukmar M, Mahon B, Caramazza A.. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cogn Neuropsychol. 2007;24(8):795–816. [DOI] [PubMed] [Google Scholar]
- 218.Osiurak F, Aubin G, Allain P, Jarry C, Richard I, Le Gall D.. Object utilization and object usage: A single-case study. Neurocase. 2008;14(2):169–183. [DOI] [PubMed] [Google Scholar]
- 219.Silveri MC, Ciccarelli N.. Semantic memory in object use. Neuropsychologia. 2009;47(12):2634–2641. [DOI] [PubMed] [Google Scholar]
- 220.Baumard J, Lesourd M, Jarry C, et al. Tool use disorders in neurodegenerative diseases: Roles of semantic memory and technical reasoning. Cortex. 2016;82:119–132. [DOI] [PubMed] [Google Scholar]
- 221.Lesourd M, Baumard J, Jarry C, et al. Mechanical problem-solving strategies in Alzheimer’s disease and semantic dementia. Neuropsychology. 2016;30(5):612–623. [DOI] [PubMed] [Google Scholar]
- 222.Bozeat S. L., Ralph MA, Patterson K, Hodges JR.. The influence of personal familiarity and context on object use in semantic dementia. Neurocase. 2002;8(1-2):127–134. [DOI] [PubMed] [Google Scholar]
- 223.Sirigu A, Duhamel JR, Poncet M.. The role of sensorimotor experience in object recognition. Brain. 1991;114(6):2555–2573. [DOI] [PubMed] [Google Scholar]
- 224.Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR.. When objects lose their meaning: What happens to their use? Cogn Affect Behav Neurosci. 2002;2(3):236–251. [DOI] [PubMed] [Google Scholar]
- 225.Coccia M, Bartolini M, Luzzi S, Provinciali L. L., Ralph MA.. Semantic memory is an amodal, dynamic system: Evidence from the interaction of naming and object use in semantic dementia. Cogn Neuropsychol. 2004;21(5):513–527. [DOI] [PubMed] [Google Scholar]
- 226.Nishio Y, Kazui H, Hashimoto M, et al. Actions anchored by concepts: Defective action comprehension in semantic dementia. J Neurol Neurosurg Psychiatry. 2006;77(12):1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nelissen N, Pazzaglia M, Vandenbulcke M, et al. Gesture discrimination in primary progressive aphasia: The intersection between gesture and language processing pathways. J Neurosci. 2010;30(18):6334–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.De Renzi E, Motti F, Nichelli P.. Imitating gestures. Arch Neurol. 1980;37(1):6–10. [DOI] [PubMed] [Google Scholar]
- 229.Króliczak G, Piper BJ, Potok W, et al. Praxis and language organization in left-handers. Acta Neuropsychologica. 2020;18(1):15–28. [Google Scholar]
- 230.Alyahya RSW, Halai AD, Conroy P, Lambon MA.. A unified model of post-stroke language deficits including discourse production and their neural correlates. Brain. 2020;143(5):1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Quesque F, Rossetti Y.. What do theory-of-mind tasks actually measure? Theory and practice. Perspect Psychol Sci. 2020;15(2):384–396. [DOI] [PubMed] [Google Scholar]
- 232.Suddendorf T, Fletcher-Flinn C, Johnston L.. Pantomime and theory of mind. J Genetic Psychology. 1999;160(1):31–45. [Google Scholar]
- 233.Rogers SJ, Bennetto L, McEvoy R, Pennington BF.. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Dev. 1996;67(5):2060–2073. [PubMed] [Google Scholar]
- 234.Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, Mostofsky SH.. Dyspraxia in autism: Association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49(10):734–739. [DOI] [PubMed] [Google Scholar]
- 235.Walther S, Eisenhardt S, Bohlhalter S, et al. Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull. 2016;42(6):1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gallagher HL, Frith CD.. Functional imaging of ‘theory of mind.’ Trends Cogn Sci. 2003;7(2):77–83. [DOI] [PubMed] [Google Scholar]
- 237.Baumard J, Lesourd M, Remigereau C, et al. The – weak – role of memory in tool use: Evidence from neurodegenerative diseases. Neuropsychologia. 2019;129(March):117–132. [DOI] [PubMed] [Google Scholar]
- 238.Lesourd M, Le Gall D, Baumard J, Croisile B, Jarry C, Osiurak F.. Apraxia and Alzheimer’s disease: Review and perspectives. Neuropsychol Rev. 2013;23(3):234–256. [DOI] [PubMed] [Google Scholar]
- 239.Motomura N, Yamadori A.. A case of ideational apraxia with impairment of object use and preservation of object pantomime. Cortex. 1994;30(1):167–170. [DOI] [PubMed] [Google Scholar]
- 240.Fukutake T. Apraxia of tool use: An autopsy case of biparietal infarction. Eur Neurol. 2003;49(1):45–52. [DOI] [PubMed] [Google Scholar]
- 241.Dressing A, Martin M, Beume LA, et al. The correlation between apraxia and neglect in the right hemisphere: A voxel-based lesion-symptom mapping study in 138 acute stroke patients. Cortex. 2020;132:166–179. [DOI] [PubMed] [Google Scholar]
- 242.Vry M-S, Saur D, Rijntjes M, et al. Ventral and dorsal fiber systems for imagined and executed movement. Exp Brain Res. 2012;219(2):203–216. [DOI] [PubMed] [Google Scholar]
- 243.Ralph MAL, Jefferies E, Patterson K, Rogers TT.. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42–55. [DOI] [PubMed] [Google Scholar]
- 244.Noonan KA, Jefferies E, Visser M, Lambon Ralph MA.. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013;25(11):1824–1850. [DOI] [PubMed] [Google Scholar]
- 245.Binkofski F, Buxbaum LJ.. Two action systems in the human brain. Brain Lang. 2013;127(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Osiurak F, Badets A.. Use of tools and misuse of embodied cognition: Reply to Buxbaum (2017). Psychol Rev. 2017;124(3):361–368. [DOI] [PubMed] [Google Scholar]
- 247.Osiurak F, Federico G.. Four ways of (mis-)conceiving embodiment in tool use [Published online 2020]. Synthese. doi:10.1007/s11229-020-02960-1. [Google Scholar]
- 248.Osiurak F, Le Gall D.. Apraxia: A gestural or a cognitive disorder? Brain. 2015;138(Pt 3):e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed.