Abstract
Background:
Recovery from coronary artery bypass graft (CABG) surgery is often complicated by depression and insomnia, resulting in poorer health-related quality of life and clinical outcomes. We explored the relationships among depression, insomnia, quality of life, and the impact of a collaborative care (CC) strategy on reducing insomnia in patients post-CABG.
Methods:
Patients with a Patient Health Questionnaire (PHQ-9) ≥10 were randomized to nurse-delivered CC for depression (n=150) or their physician’s usual care (UC; n=152). A convenience sample of non-depressed patient (n=151) served as control group. Using the Hamilton Rating Scale for Depression (HRS-D) sleep questions, we created an ‘insomnia index’.
Results:
63% of depressed vs.12% of non-depressed participants reported insomnia at baseline. Compared to UC, fewer CC participants reported insomnia at 8-months and tended to have a lower insomnia score (Insomnia Index change score-0.95 and −1.47 respectively, p=0.05) with no time-by-randomization interaction, Cohen’s d= 0.22 (95%CI −0.001 – 0.43). Participants with baseline insomnia reported greater improvements in mental health-related quality of life (SF-36 Mental Component Score; −3.32, p=0.02), but insomnia was not a significant moderator of the CC effect.
Conclusions:
This study is the first to examine long-term the impact on insomnia in post-CABG patients treated for depression. Future CC studies may consider including a therapeutic focus for insomnia.
Keywords: cardiac, collaborative care, depression, health-related quality of life, insomnia
Introduction
Coronary artery bypass graft (CABG) surgery is a common treatment for coronary artery disease (1). Although CABG improves patients’ quality of life and reduces both all-cause mortality and myocardial infarctions, like any surgery or major procedure, it is associated with significant risks of re-hospitalization, morbidity, and mortality (2). Furthermore, pre- and post-operative depression affects post-surgical outcomes, is associated with worse health-related quality of life, functional status, major adverse cardiovascular and cerebrovascular events after surgery (3, 4), and is an independent predictor of mortality after CABG (5).
Insomnia is a common symptom reported by patients with depression (6, 7) and contributes to depression treatment response variability (8). Both subjective and objective sleep disturbances predict greater all-cause mortality in older adults (9) and worsened general medical comorbidity including coronary heart disease (CHD) (10). A frequent complaint in the immediate and even late post-operative period following CABG surgery is insomnia (11). While for many of those patients these sleep disturbances are temporary and begin to improve within the first few months after surgery (12, 13), a systematic review found over 50% of patients still experienced persistent sleep disturbances 6-months after surgery (14). Given that many depressed patients following CABG may experience insomnia, and treating insomnia may reduce depression severity (15), treating insomnia in the post-CABG period may also result in better depression outcomes and possibly decreased cardiac morbidity.
Collaborative care is an effective and scalable intervention to improve mood symptoms and quality of life after CABG (16). However, the long-term effect of insomnia on these outcomes in a depressed post-CABG sample, and specifically, the effect of depression-specific collaborative care on insomnia, have not been described.
Using a sample of depressed and non-depressed patients with CHD after CABG surgery who participated in a clinical trial of collaborative care for depression (17), we tested the following hypotheses: (1) The prevalence of insomnia is greater in depressed post-CABG patients with compared with non-depressed post-CABG controls; (2) Collaborative care for depression in post-CABG patients with CHD results in greater improvement in insomnia after 8 months, compared to usual care (UC); and (3) Baseline insomnia moderates the effect of collaborative care (versus UC) on the Mental Component Score (MCS) of the Medical Outcomes Study 36-item Short Form (SF-36), a measure of mental health-related quality of life (mHRQoL).
Methods
We analyzed data from the NIH-funded, randomized controlled effectiveness “Bypassing the Blues” trial, which demonstrated that a post-CABG nurse-led, telephone-delivered collaborative care intervention for depression improves mHRQoL, physical functioning, and mood symptoms at 8-month follow-up, and is cost-effective (16–18). Our goal was to determine the role of insomnia in depression treatment response. Details of the parent trial are briefly discussed below (17). The University of Pittsburgh Institutional Review Board approved the study protocol, and all participants provided written informed consent.
Participants:
Between 2004 and 2007, study nurses screened patients for depression at seven Pittsburgh-area hospitals prior to discharge following CABG surgery with the two-item Patient Health Questionnaire (PHQ-2) (19). Patients who endorsed at least one item were screened again via telephone two weeks after discharge with the PHQ-9 (20). If patients had a PHQ-9 ≥ 10, indicating at least moderate depression (20), and met other eligibility criteria, they were randomized to either the collaborative care intervention (n=150) or the “usual care” group (n=152). Other eligibility criteria included Mini Mental State Exam (MMSE) > 24 (21), ability to be safely treated for depression as an outpatient, absence of non-cardiovascular conditions likely to be fatal within one year, and not currently in treatment with a mental health specialist.
A cohort of patients who screened negative on the PHQ-2 before hospital discharge, were not using antidepressants, met all other protocol eligibility criteria, and scored less than 5 on the 2-week PHQ-9 served as the non-depressed control group (n=151).
Intervention:
Nurse care managers delivered the collaborative care intervention via telephone for eight months. During regular calls, the care managers reviewed skills that participants learned from a self-help depression workbook (22), encouraged adherence with antidepressant pharmacotherapy, if applicable, and behavioral suggestions, and instructed participants how to minimize antidepressant related treatment-emergent side effects. At weekly case review meetings, the care managers presented their patients to the study clinicians, who made treatment recommendations if the participant was not improving. These recommendations, including pharmacotherapy, were then conveyed to the participants and his/her physician who was ultimately responsible for all medication prescriptions and adjustments.
Usual Care:
For ethical reasons, the study team informed both depressed participants randomized to the usual care group and their PCP of the participants’ depression status. However, no treatment advice was provided unless suicidality was detected during a follow-up assessment.
Materials:
Research assessors blinded to the participant’s group assignments completed all assessments over the telephone at baseline and at 8 months.
Insomnia:
We created an insomnia index (scored from 0–6) by summing the early, middle, and late insomnia questions (each question scored from 0–2) of the Hamilton Rating Scale for Depression (HRS-D) (23). In addition to using this as a continuous variable, this index was used as a binary measure with a score ≥ 3 indicating clinically significant insomnia (24).
Psychiatric Symptoms:
We used the PRIME-MD (25) to diagnose depression and anxiety syndromes. Depression severity was assessed at all time points using the HRS-D (26).
Health Related Quality of Life (HRQoL):
We measured HRQoL with the Medical Outcomes Study 36-item Short Form Health Survey (27) and assessed changes in the mental component subscale (MCS) and physical component subscale (PCS), with the MCS as the primary outcome of interest.
Additional Baseline Descriptors:
At study entry, we assessed participants’ sociodemographic characteristics, medical, and psychiatric history.
Statistical Analysis
Demographic and baseline characteristics were described and compared between insomnia status at baseline (No Insomnia vs. Insomnia) using means and standard deviations as well as sample proportions. Two-sample t-tests and chi-square tests of independence were used to assess between-group statistical significance. All models were adjusted for gender, education (high school and beyond), presence of diabetes, percent ejection fraction (EF), and baseline major depression and anxiety, as those measures were significantly different between groups at baseline.
We assessed the effect of the collaborative care intervention versus UC on insomnia over time in two ways: insomnia was treated as a continuous scale at each time point and as a binary variable. Generalized linear mixed models were used to describe the trajectory of insomnia as a function of study arm (collaborative care vs. UC), time point, their interaction, and baseline covariates mentioned above. Contrasts were used to estimate 8-month between-arm differences in insomnia (for continuous outcome) as well as 8-month odds ratio (for binary outcome).
We assessed whether baseline insomnia status moderated the effect of collaborative care (vs. UC) on SF-36 MCS using linear mixed models. Covariates included study arm, baseline insomnia status, their interaction, and baseline covariates mentioned previously. Between-arm differences in 8-mo improvement were estimated along with 95% confidence intervals.
Results
Hypothesis 1: The prevalence of insomnia post CABG is greater in depressed patients compared with non-depressed controls.
Based on an insomnia index score of ≥3, we classified 190 (63%) of the 302 randomized depressed participants as having insomnia at baseline, while 18 (12%) of the 151 non-depressed control participants were classified as having insomnia (p<0.0001).
Furthermore, depressed participants of the insomnia group were more likely to have obtained at least a high school education as compared to the no-insomnia group, but there were no other significant sociodemographic differences between the two groups at baseline (Table 1). Clinically, the depressed insomnia group were less likely to have a diagnosis of diabetes and had a higher percent ejection fraction (EF). Depressed participants with insomnia reported more severe mood symptoms and were more likely to have major depression or an anxiety disorder than those without insomnia and reported significantly lower mHRQoL (SF-36 MCS) compared to the no-insomnia group (Table 1).
Table 1:
Baseline Characteristics for Depressed Group
| All Depressed (N=302) | No Insomnia (N=112) | Insomnia (N=190) | p-value‡ | |
|---|---|---|---|---|
| Age, Mean (SD) | 63.9 (11) | 65.3 (10.4) | 63.1 (11.2) | 0.09 t |
| Male, % (N) | 59 (177) | 57 (64) | 59 (113) | 0.69 c |
| White Race, % (N) | 91 (274) | 95 (106) | 88 (168) | 0.07 c |
| High School Education or beyond, % (N) | 56 (168) | 46 (52) | 61 (116) | 0.01 c |
| Married, % (N) | 68 (205) | 70 (78) | 67 (127) | 0.62 c |
| Perceived Social Support Scale, Mean (SD) | 69.4 (10.9) | 70.5 (10.5) | 68.7 (11.1) | 0.17 t |
| Working, Part-time or full time, % (N) | 38 (115) | 33 (37) | 41 (78) | 0.17 c |
| SF-36 MCS, Mean (SD) | 43 (11.5) | 45.9 (10.4) | 41.3 (11.8) | 0.001 t |
| SF-36 PCS, Mean (SD) | 30.7 (7) | 31.2 (7.1) | 30.4 (7) | 0.31 t |
| Body Mass Index, Mean (SD) | 31 (6.5) | 30.6 (6) | 31.3 (6.8) | 0.36 t |
| Systolic Blood Pressure, Mean (SD) | 133 (22) | 136 (22) | 131 (22) | 0.09 t |
| Diastolic Blood Pressure, Mean (SD) | 70 (14) | 70 (13) | 71 (14) | 0.80 t |
| Hypertension, % (N) | 84 (253) | 86 (96) | 83 (157) | 0.48 c |
| Diabetes, % (N) | 42 (128) | 50 (56) | 38 (72) | 0.04 c |
| Hyperlipidemia, %(N) | 81 (245) | 78 (87) | 83 (158) | 0.24 c |
| Myocardial Infraction, % (N) | 46 (140) | 50 (56) | 44 (84) | 0.33 c |
| Congestive Heart Failure, % (N) | 20 (59) | 21 (24) | 18 (35) | 0.52 c |
| Percent Ejection Fraction, Mean (SD) | 51 (12) | 49 (13) | 52 (12) | 0.04 t |
| Tobacco Use in Last Year, % (N) | 27 (82) | 29 (32) | 26 (50) | 0.67 c |
| PHQ-9 Scores, Mean (SD) | 13.5 (3.4) | 12.6 (2.9) | 14.1 (3.5) | <.0001 t |
| PHQ-9 Scores (insomnia adjusted), Mean (SD) | 11.2 (3.2) | 10.6 (2.8) | 11.6 (3.4) | 0.01 t |
| HRSD, Mean (SD) | 16.2 (7) | 11.6 (5.2) | 18.9 (6.5) | <.0001 t |
| HRSD (insomnia items removed), Mean (SD) | 12.9 (6.1) | 10.5 (5.1) | 14.4 (6.1) | <.0001 t |
|
PRIME MD dx
Major Depression, % (N) |
38 (116) | 24 (27) | 47 (89) | <.0001 c |
| Anxiety Disorder, % (N) | 29 (89) | 20 (22) | 35 (67) | 0.004 c |
| Visit with mental health professional | ||||
| Within last 2 year, % (N) | 3 (9) | 3 (3) | 3 (6) | 0.75 c |
| Treatment for depression from primary care physician | ||||
| Within last 2 year, % (N) | 17 (50) | 16 (18) | 17 (32) | 0.86 c |
| Pharmacotherapy | ||||
| Antidepressants Within last 2 year, % (N) |
24 (73) | 23 (26) | 25 (47) | 0.77 c |
| At Baseline, % (N) | 26 (79) | 23 (26) | 28 (53) | 0.37 c |
| Sleep Medication, %(N) | 6 (19) | 5 (6) | 7 (13) | 0.61 c |
| Benzodiazepines, % (N) | 14 (42) | 14 (16) | 14 (26) | 0.88 c |
c, Pearson’s chi-square test; t, T-test
Abbreviations: HRS-D, Hamilton Rating Scale for Depression; PHQ-9, 9-item Patient Health Questionaire; PRIME MD, Primary Care Evaluation of Mental Disorders; SD, Standard Deviation; SF-36 MCS, Short Form-36, Mental Component Summary; SF-36 PCS, Short Form-36, Physical Component Summary
Hypothesis 2: Collaborative care for depression results in lower levels of insomnia after 8 months, compared to UC.
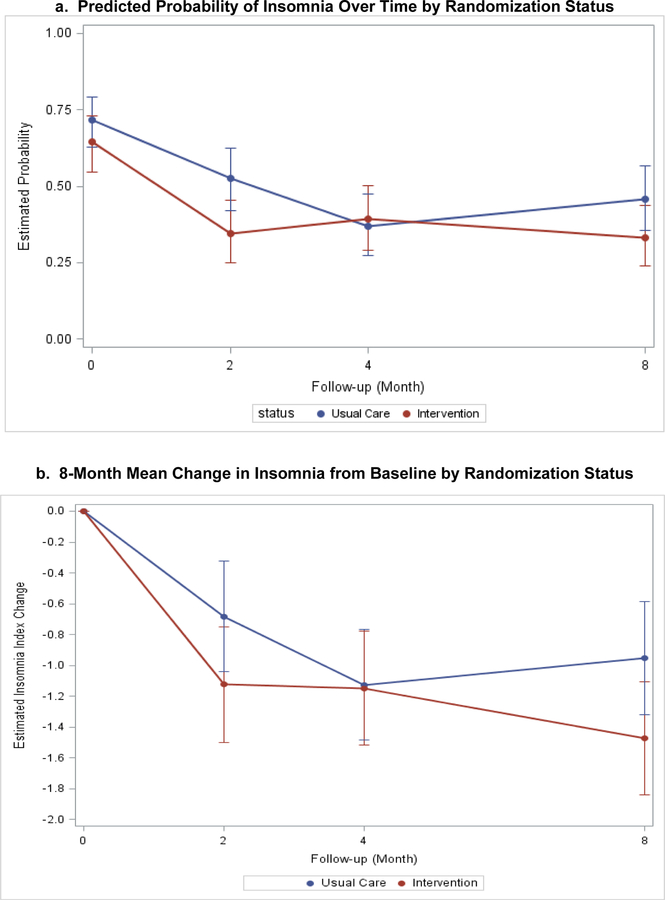
When the insomnia index was tested as a binary variable, the rates of insomnia were similar in both groups at baseline, and this similarity persisted after 8-months. The intervention group did not report a lower rate of insomnia between baseline and 8 month follow-up (OR=0.58, p=0.09). The interaction between time and study arm also did not differ between the groups (Table 2 and Figure 1).
Table 2:
Baseline to 8-Month Mixed Model Estimates of Mean Change Scores by Randomization Status
| Intervention (n=150) | Usual Care (n=152) | Odds Ratio Between Group Difference (95% CI) | P Value | P Value for Interaction b | |
|---|---|---|---|---|---|
| Insomnia Index (Binary) | |||||
| Baseline N (%) | 91 (61%) | 99 (65%) | 0.58 (0.32,1.08)a | 0.09 | 0.20 |
| 8-mo follow-up, % (CI) | 0.33 (0.24, 0.44) | 0.46 (0.35, 0.56) | |||
| Insomnia Index (Continuous) | |||||
| Baseline Mean (SE) | 3.37 (0.15) | 3.50 (0.15) | −0.52 (−1.04, 0.004)c | 0.05 | 0.10 |
| 8-mo follow-up Mean (SE) | 1.90 (0.16) | 2.54 (0.16) | |||
| ∆ Baseline to 8-mo Mean (SE) | −1.47 (0.19) | −0.95 (0.19) | |||
The odds of having insomnia for Intervention group at month 8 for are 0.58 times of the odds for Usual Care group.
P-value comes from the interaction between time and study arm in the linear mixed model.
Cohen’s D and 95%CI: 0.22(−0.001,0.43); NNT and 95% CI: 8.2 (4.1,3059)
Abbreviations: CI, Confidence Interval; NNT, Number Needed to Treat; SE, Standard Error
Figure 1.
a. Predicted Probability of Insomnia Over Time by Randomization Status
b. 8-Month Mean Change in Insomnia from Baseline by Randomization Status
Model-based estimates of change in insomnia index (a: binary; b: continuous variable) by intervention status over 8 months. Point estimates and 95% confidence intervals derived from generalized linear mixed models with study arm, time point, their interaction, gender, education, presence of diabetes, percent ejection fraction, baseline depression, and presence of anxiety as fixed effects.
When insomnia was analyzed as a continuous variable (insomnia score), there was a difference in 8-month improvement for participants randomized to collaborative care compared to the UC group (−1.47 and −0.95, respectively) but the change in score and the time-by-study arm interaction were not significant. (Table 2 and Figure 1). Therefore, there was no significant difference in trajectory over time despite marginally greater reductions in continuous insomnia index scores. A sensitivity analysis exploring the effect of collaborative care (vs UC) on changes in insomnia among participants who met threshold criteria for insomnia at baseline found similar results.
Hypothesis 3: Baseline insomnia will moderate the effect of collaborative care (versus UC) on the SF-36 MCS.
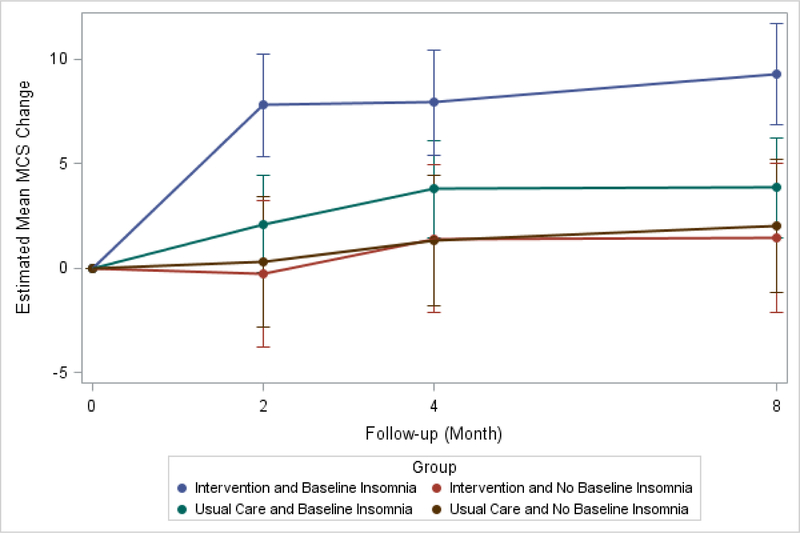
Baseline insomnia was not a statistically significant moderator of the intervention effect (Figure 1). However, insomnia was a non-specific predictor of improvement in MCS (Table 3). Participants with baseline insomnia had greater 8-month improvements in the MCS compared to those without insomnia (−3.32, p=0.02). In addition, there was a significant difference between groups with respect to MCS trajectory over time (p=0.001) (Table 3, Figure 2).
Table 3.
Baseline to 8-Month Mixed Model Estimates of Mean MCS Change by Baseline Insomnia Group
| Insomnia (n=190) | No Insomnia (n=112) | Between Group Difference (95% CI) | P Value | P Value for Interactiona | |
|---|---|---|---|---|---|
| SF36-MCS | |||||
| Adjusted Baseline Mean (SE) | 39.564 (0.82) | 41.71 (1.06) | −3.32 (−6.10, −0.53)b | 0.02 | 0.001 |
| 8-mo follow-up Mean (SE) | 46.18 (0.87) | 44.93 (1.14) | |||
| ∆ Baseline to 8-mo Mean (SE) | 6.54 (0.87) | 3.22 (1.12) | |||
P-value comes from the interaction between time and study arm in the linear mixed model.
Cohen’s D and 95%CI: 0.27 (0.04, 0.50); NNT and 95% CI: 6.56 (3.62, 40.66)
Abbreviations: CI, Confidence Interval; NNT, Number Needed to Treat; SE, Standard Error; SF36-MCS, Short Form 36, Mental Component Score
Figure 2.
8-Month Change from Baseline in Mean SF-36 MCS By Baseline Insomnia and Randomization Status
Model-based estimates of change in SF-36 MCS by intervention status and baseline insomnia over 8 months. Point estimates and 95% confidence intervals derived from mixed models with study arm, time point, baseline insomnia status, all two- and three-way interactions, gender, education, presence of diabetes, percent ejection fraction, baseline depression, and presence of anxiety as fixed effects.
Abbreviation: SF-36 MCS, Short Form-36 Mental Component Summary
Discussion
Following CABG surgery, 63% of depressed patients reported significantly higher rates of insomnia compared to 12% of patients without depression. Given that insomnia is a common symptom of depression, and primary insomnia disorder is frequently comorbid with depression, it may partially explain this observation. However, among the depressed participants, those with insomnia were more likely to have more severe depression at baseline (measured with both the PHQ-9 and the HRS-D), even when controlling for the sleep items. This has been confirmed in the literature, where insomnia severity is associated to depression severity (28). While depression and insomnia can be considered as separate disorders, they do have a bidirectional relationship (29). Depression may be more difficult to treat in the presence of insomnia, and the presence of insomnia is a risk factor for depression (30). Additionally, the risk of persistent insomnia increases the risk of recurrence of depression (23). Therefore, it may be necessary to treat both conditions in order to optimize overall health outcomes. This is especially important in patients with heart disease as insomnia is associated with higher risk of cardiovascular disease (31–34).
Among the depressed participants, those with insomnia at baseline had more education, higher EF rates, lower mHRQoL (as measured with the MCS), and were less likely to be diabetic compared to those without insomnia. Some of these observations were not expected, as education and socioeconomic status are typically associated with better sleep quality (35, 36). Other studies have reported that insomnia is associated with diabetes (37), possibly due to lack of sleep impairing glucose tolerance or sleep disturbances and subsequent daytime sleepiness and napping interfering with healthy behaviors, blood glucose monitoring, and adherence with medications (38, 39). While hypothetical, it is possible that in our study participants with insomnia were not yet diagnosed with diabetes, but still had insulin resistance (40). Interestingly, those with insomnia had higher EF compared to those without insomnia and were not more likely to have a diagnosis of congestive heart failure at baseline, despite the known association of insomnia with heart failure (41, 42). However, the exact nature of the relationship of insomnia and EF is unclear (42), and there may not be a causal relationship between insomnia and the development of heart failure (43).
Although the results were not statistically significant, participants who received collaborative care for depression may have experienced improvement in insomnia, when measured as a continuous variable (even though the collaborative care intervention focused on depression, not specifically addressing insomnia). In patients with mild to moderate depression, manualized behavioral treatments for depression have been shown to improve insomnia (44). Given the relatively high rates of insomnia in our depressed cohort, further study of whether a collaborative care intervention that includes a therapeutic focus specific for insomnia produces more significant changes in insomnia levels may be indicated. Incorporating elements or modules from Cognitive Behavioral Treatment of Insomnia (CBTI) into a CC approach may improve outcomes, and are recommended as first line treatments for insomnia (45, 46). This approach is effective, safe, and has minimal side effects and no medication interactions as compared to pharmacologic treatments (47). Including behavioral elements that focus on sleep restriction and stimulus control may be easily blended into a more personalized CC intervention for depression.
To further refine collaborative care approaches for physical and mental conditions, we explored moderators of treatment effect to guide the development of personalized interventions. Although insomnia did not moderate the effect of collaborative care, it was observed to be a non-specific predictor of improvement in the SF-36 MCS for those receiving collaborative care. The absence of treatment moderation associated with insomnia suggests that collaborative care is efficacious regardless of pre-treatment insomnia. Nevertheless, the observed evidence suggests that depressed post-CABG patients without insomnia may experience a more challenging treatment course, and require more intensive treatment than collaborative care for their depression, such as referral to a mental health specialist.
One limitation of our study is that we defined insomnia using a subjective index of sleep quality, while a discrepancy between self-reported insomnia and objective measures of sleep quality (e.g., polysomnography, actigraphy) has been established (48). This may be especially true in those with underlying sleep pathology like obstructive sleep apnea (49), which was not assessed in the parent study, or those with depression which may negatively bias the reporting of sleep quality (50). The insomnia index also did not capture information about distress related to insomnia or interference in daily activities due to insomnia, limiting a multidimensional assessment of the effect of this symptom. As stated previously, another limitation is that the participants received a depression-specific collaborative care intervention, not one that included an insomnia-specific approach or module. Given that the majority of depressed patients experienced insomnia, future collaborative care interventions for depressed post-CABG patients may consider implementing an insomnia-specific module to address this common problem that frequently interferes with treatment response.
Conclusion
Following CABG surgery, the majority of depressed patients (63%) reported insomnia, while only 12% of non-depressed patients reported insomnia. To the best of our knowledge, this study is the first to describe the prevalence and course of insomnia in this population and investigate the effects of collaborative care for depression on the trajectory of insomnia. Although insomnia did not moderate the effect of collaborative care, it was observed to be a non-specific predictor of improvement in the SF-36 MCS for those receiving collaborative care. While collaborative care for depression showed limited impact on insomnia, given the high prevalence of this symptom among depressed patients after CABG surgery, future clinical trials may consider expanding the focus of depression interventions to include the treatment of insomnia.
Financial Disclosure and acknowledgment:
This work was supported by NIH grants R01 HL70000 (Dr. Rollman) and R34 MH101371 (Dr. Karp) and T32 MH019986 (Dr. Gebara). The authors have no conflict of interest, financial, or otherwise, to report.
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT00091962
References
- 1.Herbison P,Wong CK: Has the difference in mortality between percutaneous coronary intervention and coronary artery bypass grafting in people with heart disease and diabetes changed over the years? A systematic review and meta-regression. BMJ Open 2015; 5:e010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipahi I, Akay MH, Dagdelen S, et al. : Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med 2014; 174:223–230 [DOI] [PubMed] [Google Scholar]
- 3.Stenman M, Holzmann MJ,Sartipy U: Relation of major depression to survival after coronary artery bypass grafting. Am J Cardiol 2014; 114:698–703 [DOI] [PubMed] [Google Scholar]
- 4.Perrotti A, Mariet AS, Durst C, et al. : Relationship between depression and health-related quality of life in patients undergoing coronary artery bypass grafting: a MOTIV-CABG substudy. Qual Life Res 2016; 25:1433–1440 [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal JA, Lett HS, Babyak MA, et al. : Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609 [DOI] [PubMed] [Google Scholar]
- 6.McCall WV, Reboussin BA,Cohen W: Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res 2000; 9:43–48 [DOI] [PubMed] [Google Scholar]
- 7.Sung SC, Wisniewski SR, Luther JF, et al. : Pre-treatment insomnia as a predictor of single and combination antidepressant outcomes: a CO-MED report. J Affect Disord 2015; 174:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troxel WM, Kupfer DJ, Reynolds CF 3rd, et al. : Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J Clin Psychiatry 2012; 73:478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dew MA, Hoch CC, Buysse DJ, et al. : Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med 2003; 65:63–73 [DOI] [PubMed] [Google Scholar]
- 10.Phillips B,Mannino DM: Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med 2007; 3:489–494 [PMC free article] [PubMed] [Google Scholar]
- 11.Ranjbaran S, Dehdari T, Sadeghniiat-Haghighi K, et al. : Poor Sleep Quality in Patients after Coronary Artery Bypass Graft Surgery: An Intervention Study Using the PRECEDE-PROCEED Model. J Tehran Heart Cent 2015; 10:1–8 [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz H,Iskesen I: Objective and subjective characteristics of sleep after coronary artery bypass graft surgery in the early period: a prospective study with healthy subjects. Heart Surg Forum 2007; 10:E16–20 [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz H,Iskesen I: Follow-up with objective and subjective tests of the sleep characteristics of patients after cardiac surgery. Circ J 2007; 71:1506–1510 [DOI] [PubMed] [Google Scholar]
- 14.Liao WC, Huang CY, Huang TY, et al. : A systematic review of sleep patterns and factors that disturb sleep after heart surgery. J Nurs Res 2011; 19:275–288 [DOI] [PubMed] [Google Scholar]
- 15.Gebara MA, Siripong N, DiNapoli EA, et al. : Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depress Anxiety 2018; 35:717–731 [DOI] [PubMed] [Google Scholar]
- 16.Rollman BL, Belnap BH, LeMenager MS, et al. : Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA 2009; 302:2095–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollman BL, Belnap BH, LeMenager MS, et al. : The Bypassing the Blues treatment protocol: stepped collaborative care for treating post-CABG depression. Psychosom Med 2009; 71:217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donohue JM, Belnap BH, Men A, et al. : Twelve-month cost-effectiveness of telephone-delivered collaborative care for treating depression following CABG surgery: a randomized controlled trial. Gen Hosp Psychiatry 2014; 36:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL,Williams JB: The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292 [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL,Williams JB: The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE,McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [DOI] [PubMed] [Google Scholar]
- 22.Katon W: The depression helpbook, Boulder, Colorado Bull Pub Co, 2002 [Google Scholar]
- 23.Dombrovski AY, Cyranowski JM, Mulsant BH, et al. : Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress Anxiety 2008; 25:1060–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SC, Kim JM, Jun TY, et al. : Prevalence and Clinical Correlates of Insomnia in Depressive Disorders: The CRESCEND Study. Psychiatry Investig 2013; 10:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer RL, Williams JB, Kroenke K, et al. : Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994; 272:1749–1756 [PubMed] [Google Scholar]
- 26.Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHorney CA, Ware JE Jr.,Raczek AE: The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31:247–263 [DOI] [PubMed] [Google Scholar]
- 28.Sunderajan P, Gaynes BN, Wisniewski SR, et al. : Insomnia in patients with depression: a STAR*D report. CNS Spectr 2010; 15:394–404 [DOI] [PubMed] [Google Scholar]
- 29.Franzen PL,Buysse DJ: Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci 2008; 10:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dew MA, Reynolds CF 3rd, Houck PR, et al. : Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry 1997; 54:1016–1024 [DOI] [PubMed] [Google Scholar]
- 31.Bertisch SM, Pollock BD, Mittleman MA, et al. : Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018; 41: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javaheri S,Redline S: Insomnia and Risk of Cardiovascular Disease. Chest 2017; 152:435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal B, Makarem N, Shah R, et al. : Effects of Inadequate Sleep on Blood Pressure and Endothelial Inflammation in Women: Findings From the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc 2018; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sofi F, Cesari F, Casini A, et al. : Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 2014; 21:57–64 [DOI] [PubMed] [Google Scholar]
- 35.Grandner MA, Patel NP, Gehrman PR, et al. : Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med 2010; 11:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham TJ, Ford ES, Chapman DP, et al. : Independent and joint associations of race/ethnicity and educational attainment with sleep-related symptoms in a population-based US sample. Prev Med 2015; 77:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Liao D, Pejovic S, et al. : Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care 2009; 32:1980–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottlieb DJ, Punjabi NM, Newman AB, et al. : Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005; 165:863–867 [DOI] [PubMed] [Google Scholar]
- 39.Tsunoda T, Yamada M, Akiyama T, et al. : The Effects of Ramelteon on Glucose Metabolism and Sleep Quality in Type 2 Diabetic Patients With Insomnia: A Pilot Prospective Randomized Controlled Trial. J Clin Med Res 2016; 8:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyykkonen AJ, Isomaa B, Pesonen AK, et al. : Subjective sleep complaints are associated with insulin resistance in individuals without diabetes: the PPP-Botnia Study. Diabetes Care 2012; 35:2271–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanno Y, Yoshihisa A, Watanabe S, et al. : Prognostic Significance of Insomnia in Heart Failure. Circ J 2016; 80:1571–1577 [DOI] [PubMed] [Google Scholar]
- 42.Redeker NS, Knies AK, Hollenbeak C, et al. : Cognitive behavioral therapy for insomnia in stable heart failure: Protocol for a randomized controlled trial. Contemp Clin Trials 2017; 55:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strand LB, Laugsand LE, Dalen H, et al. : Insomnia and left ventricular function - an echocardiography study. Scand Cardiovasc J 2016; 50:187–192 [DOI] [PubMed] [Google Scholar]
- 44.Yon A, Scogin F, DiNapoli EA, et al. : Do manualized treatments for depression reduce insomnia symptoms? J Clin Psychol 2014; 70:616–630 [DOI] [PubMed] [Google Scholar]
- 45.Wilson SJ, Nutt DJ, Alford C, et al. : British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol 2010; 24:1577–1601 [DOI] [PubMed] [Google Scholar]
- 46.Chesson AL Jr., Anderson WM, Littner M, et al. : Practice parameters for the nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999; 22:1128–1133 [DOI] [PubMed] [Google Scholar]
- 47.Morgenthaler T, Kramer M, Alessi C, et al. : Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep 2006; 29:1415–1419 [PubMed] [Google Scholar]
- 48.Baker FC, Maloney S,Driver HS: A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res 1999; 47:335–341 [DOI] [PubMed] [Google Scholar]
- 49.McCall WV, Turpin E, Reboussin D, et al. : Subjective estimates of sleep differ from polysomnographic measurements in obstructive sleep apnea patients. Sleep 1995; 18:646–650 [DOI] [PubMed] [Google Scholar]
- 50.DiNapoli EA, Gebara MA, Kho T, et al. : Subjective-Objective Sleep Discrepancy in Older Adults With MCI and Subsyndromal Depression. J Geriatr Psychiatry Neurol 2017; 30:316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]