Abstract
Aim
The COVID-19 pandemic has prompted governments around the globe to implement various restriction policies, including lockdown, social distancing, and school closures. Subsequently, there has been a surge in sedentary behaviour particularly screen time (ST) together with a significant decline in physical activity that was more marked amongst children and adolescents. Excessive screen exposure in adolescents has been correlated with cardio-metabolic risk factors including obesity, hypertension, high cholesterol, and glucose intolerance that may have adverse morbidity and mortality implications in adulthood. Thus, the current study aimed to synthesize the literature on the relationship between ST of various types and the risk of metabolic syndrome (MetS) in adolescents in the context of the COVID-19 pandemic.
Methods
In August 2021, a systematic search of the literature was undertaken using electronic databases: PubMed, PsycINFO, and the Cochran library. Studies were considered if they met the following key eligibility criteria: (i) Measure of ST as an exposure (TV, computer, videogames, internet, smartphone, tablet), using quantified duration/frequency either self-reported or observed; (ii) Measure of MetS as an outcome with standard definition and/or criteria required to establish MetS diagnosis. The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to assess the risk of bias.
Results
A total of ten studies met the inclusion criteria, and the majority were cross sectional studies. Most studies met fair bias scoring. Overall, the review revealed considerable evidence that suggests a significant negative association between ST and components of MetS among adolescents with dose-response association.
Conclusion
During the pandemic, screen usage may become more prevalent through periods of school closures, lockdowns, social isolation, and online learning classes. Public health policies and health promotion strategies targeting parents are needed to raise awareness of the adverse health effects associated with screen-based sedentary behaviour as a precursor of NCDs. Parent or home focused interventions might be effective in limiting adolescents’ screen exposure, alternatively substituted with an appropriate level of physical activity.
PROSPERO registration number
PROSPERO 2021 CRD42021272436.
Introduction
The ongoing COVID-19 pandemic has impacted billions of children’s and adolescents’ lives in an unprecedented manner [1–3]. To limit the spread of the virus, stringent preventative measures were imposed worldwide. Countries around the globe announced complete lockdown which included school closures for the most part of the year 2020 and extended partially into the following years. Around 1.5 billion children (aged 5–12 year) and youths (aged 13–17 year) were transitioned into virtual learning [4, 5]. Disruption of daily routines, limited mobility and social constraints have considerably increased engagement in sedentary activities especially screen time (ST) [6, 7]. Available evidence indicates that screen-based sedentary behaviours have been associated with unhealthy dietary habits [6], interrupted sleep patterns [8] and limited opportunities for children and adolescents to engage in physical activity [9], all of which comprise a combination of risk factors for metabolic syndrome.
Given the revolutionary advances in digital technologies, the question of how to adequately classify ST remains a challenge [10]. The World Health Organization (WHO) defines ST as “Time spent passively watching screen-based entertainment (TV, computers, mobile devices),” excluding other innovative and modern forms of virtual realities, interactive video-gaming where physical activity or movement is required [11].
The COVID-19 pandemic has caused a marked increase in ST across the globe. A large observational study (n = 8395) in 10 European countries revealed that 69.5% [95%CI: 68.5–70.5] of young adolescents aged 6–18 years have exceeded the recommended limit of ST (>2 h/day) during weekdays and 63.8% during weekend [95%CI: 62.7–64.8]. Children residing in mildly affected countries and those in countries with lower level of restrictions were less likely to exceed that limit (OR = 3.25 [95%CI: 2.38–4.45) and OR = 1.42 [95% CI: 1.07–1.90], respectively) [12]. Similarly, findings from (ABCD) study during the early stages of the pandemic reported a mean (SD) of 7.70 (5.74) h/day of screen use, a more than twofold increase as compared to the pre-pandemic figure [13].
MetS is defined as a set of cardio-metabolic risk factors that includes glucose intolerance, central obesity, hypertension, and dyslipidaemia [14]. Lifestyle factors such as insufficient moderate-to-vigorous physical activity (MVPA), low cardiorespiratory fitness, smoking, and sedentary behaviour are amongst the various possible predictors of MetS in adolescents [15]. According to the American Heart Association (AHA), National Heart, Lung and Blood Institute (NHLBI) and International Diabetes Federation (IDF), the diagnosis of MetS is based on the presence of three of the followings: waist circumference (WC) indicative of central obesity (at least 102 cm in men and 89 cm in women), raised triglyceride (<40 mg/dl) in males, <50 mg/dl in females), raised blood pressure (systolic BP≥ 130 or diastolic BP ≥ 85 mmHg or receiving treatment for hypertension), and raised fasting glucose level (≥100 mg/dL, or diagnosed with type 2 diabetes) [16]. The diagnosis of MetS is usually established after the age of 10 years. In older children and adolescents aged 10–16 years, MetS is diagnosed in the presence of central adiposity (≥90th) and two of the following: triglycerides (TG)≥ 150 mg/dl, HDL-C <40 mg/dl, systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg, fasting plasma glucose (FG) ≥ 100 mg/dl or previously diagnosed type 2 diabetes [17].
MetS in children and adolescents has become a major public health concern, with prevalence reaching as high as 38.9% in the general population and relatively higher in overweight/obese children [18]. Numerous studies [19–21] have suggested that metabolic risk factors in childhood are associated with an increased risk of type 2 diabetes, subclinical atherosclerosis, and cardiovascular disease in adulthood. The pathological process underlying MetS begins in childhood with complex interrelated genetic and environmental factors [22]. Screen-based behaviours and physical inactivity are linked to higher levels of inflammatory biomarkers like interleukin-6 (IL-6) and tumour necrosis factor- (TNF-), which stimulate C-reactive protein (CRP), an important causative pathway leading to dyslipidaemia, insulin resistance, and cardiovascular disease [23]. A study by Strizich et al. showed that lower levels of MVPA were associated with higher glucose/lipid profiles and increased inflammatory biomarkers [24].
According to the current physical activity (PA) guidelines, children and adolescents should be engaged in at least 60 minutes of MVPA and no more than two hours of sedentary recreational ST daily [25]. Nevertheless, restrictions imposed due to the COVID-19 pandemic as well as prolonged missed opportunities in physical education due to school closures are foreseeable to profoundly limit the ability to meet these recommendations.
The association between ST and MetS among adolescents has been investigated in several studies prior to the declaration of the COVID-19 pandemic [26, 27]. However, results were found inconclusive for the most part owing to limited data and generalizability of findings to different types of ST considering the duration, content, and context of exposure [28, 29]. In a recent systematic review, authors pointed out limitations in approving the direct cause and effect relationship between excessive ST and MetS in adolescents [30]. For instance, de Oliveira RG et al. [31] revealed that ST of more than 2h/day during the weekend was significantly associated with a twofold increased risk of MetS, while insignificant association was observed concerning other days of the week. Khan et al. [32], however, observed a positive linear correlation between ST and MetS in children and adolescents, indicating a dose-response relationship for every 2 hours/day increase in ST (OR: 1.29, 95 percent CI 1.12–1.46) [32]. Stiglic and Viner [33] in their review found inadequate and unreliable evidence of association between ST and MetS, though, higher levels of ST were related significantly to an increased caloric intake, lower nutritional food, being obese, and having an overall reduced quality of life.
In light of the evolving pandemic, the prolonged screen-based sedentary behaviour exacerbated by remote learning remains a particular cause of concern. The emergence of MetS in earlier life indicates a serious risk of persistence into adulthood. Identification of contributing risk factors is of a great importance to planning for cost-effective prevention strategies. Therefore, an updated evaluation of available evidence is needed to examine the association between ST and MetS (with dose-response gradient) among adolescents, taking into consideration adjustment of potential confounders such as PA and dietary behaviour.
Thus, the aim of the current systematic review was to summarise the findings of studies that have looked at the quantifiable association between various forms of ST and MetS in adolescents aged 12 to 18 years.
Methods
Protocol and registration
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting this systematic review [34] as shown in S1 Table. The protocol was registered with PROSPERO (CRD42021272436).
Search strategy
A systematic search strategy was conducted using the following electronic bibliographic databases to identify relevant studies: PubMed Central/MEDLINE, Cochrane Library, PsycINFO and google scholar without the use of a filter to limit the date of publication or language. The search was conducted between August 2021 and September 2021. Only currently open access published articles were retrieved. The following keywords were used for the search: “screen time” OR “sedentary behaviour” OR “television” OR “computer” OR “internet” OR “videogames” AND “MetS” OR “cardiometabolic” OR “obesity” AND “adolescents” OR “children” OR “youth” OR “school-aged”. Titles and abstracts of potentially relevant articles were screened by one reviewer to assess relevance and suitability for inclusion. Full-text articles with reference lists were retrieved and examined for appropriateness. Another reviewer backtracked all reviewed articles for double-checking. Any discrepancies or disagreements between reviewers were resolved by either discussion or a third reviewer. RefWorks software was used to remove all the duplicate articles, and any that weren’t removed automatically were manually removed.
Eligibility criteria
We only included studies that fulfilled the following eligibility criteria:
Study design: observational studies (cross-sectional, longitudinal, case-control, cohort).
Population of interest: apparently healthy children and adolescents (12–18) year.
Measure of ST as an exposure: Included studies that reported type of ST (TV, computer, videogames, internet, smartphone, tablet) quantified duration/frequency either self-reported or observed measure.
Measure of MetS as an outcome: Included studies that reported standard definition and/or criteria used to establish MetS diagnosis.
Measure of relationship: examined association between ST and MetS as odds ratio (ORs) or equivalent with their 95% confidence interval (CI).
Exclusion criteria
We excluded reviews in which ST was not defined adequately or where time spent on various forms of screens was not differentiated from other forms of sedentary lifestyle. Studies examining sedentary behaviour but reporting findings for ST separately from other forms of sedentary behaviours were included. Studies were excluded if MetS diagnosis was not defined adequately, not an observational study design, no reporting of ORs or equivalent, studies including adolescents with pathological conditions, population younger than 12 year or older than 18 years, and studies assessing relationship of ST with outcomes other than MetS such as obesity, physical inactivity, or cardiovascular risk.
Study selection
Through systematic search, titles and abstracts were screened independently by two investigators (S.M and R.E), and potentially eligible articles were identified after removal of duplicates. Full text articles were retrieved for studies found to be relevant and compatible with eligibility criteria. Any discrepancies during the selection process were resolved either through consensus or consultation with the third investigator (I.D).
Data extraction
Data extraction and full text review of eligible studies were cross-checked by two independent authors (S.M and R.E) for accuracy. A standardized data extraction table was created, including key characteristics of the identified studies as the following: descriptive study characteristics (author, publication year, country, study design, sample size, age, gender), screen type, exposure, and outcome indicator measures. Results were extracted as risk estimates: Odds Ratio or prevalence ratio with corresponding confidence intervals or z-score of MetS. A P-value of <0.05 was considered as a cut-off for statistical significance.
Quality assessment
The National Institute of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to evaluate the risk of bias [35]. The checklist comprised 14 items for longitudinal research, of which only 11 could be applied to cross-sectional studies. Each item of methodological quality was classified as yes, no, or not reported and based on number of yes as total score, studies were classified according to quality rating: Poor<50%, Fair 50–75% and good >75%. Possible disagreements on the final score were resolved by consensus among the authors. Studies met from 73% to 91% of the quality criteria, with 9 studies (9/10, 90%) meeting good scoring indicating low risk of bias. All studies clearly stated the main aim, population and definition of exposure/outcome. However, two studies (2/10, 20%) did not use key potential confounders in the analysis. Eleven items were applicable to nine studies due to cross-sectional nature of these studies and one perspective cohort study where all 14 items were applicable (Table 1). Details of the NHL Quality assessment questions (Q1-14) are shown in S2 Table.
Table 1. Study quality assessed by the quality assessment tool for observational cohort and cross-sectional studies.
| Author | Items of Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total score | |
| Schaan et al. [36] | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y | NA | NA | Y | 10/11 (91%) |
| Khan M et al. [32] | Y | Y | Y | Y | Y | N | NA | Y | Y | N | Y | NA | NA | Y | 9/11 (82%) |
| Mark E and Janssen [37] | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y | NA | NA | Y | 10/11 (91%) |
| Kang HT et al. [38] | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y | NA | NA | Y | 10/11 (91%) |
| de Oliveira RG et al. [31] | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y | NA | NA | Y | 10/11 (91%) |
| Siwarom S et al. [39] | Y | Y | Y | Y | Y | N | NA | Y | Y | N | Y | NA | NA | Y | 9/11 (82%) |
| Hardy L et al. [40] | Y | Y | Y | Y | N | N | NA | Y | Y | N | Y | NA | NA | Y | 8/11 (73%) |
| Fadzlina A et al. [41] | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y | NA | NA | N | 9/11 (82%) |
| Grøntved A et al. [42] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | 12/14 (86%) |
| de Castro Silveira et al. [43] | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y | NA | NA | Y | 10/11 (91%) |
Total score, number of yes; NA not applicable, N, not present, Y, present.
Quality rating: poor <50%, Fair 50–75%, Good >75%
Data analysis
Synthesis began by summarizing review results and conclusions in note form. Reviews were then grouped by the exposure, which is screen time, and the outcome of interest was measured, which is the MetS and related risk factors. Moreover, we examined the conclusions of the included studies to decide which article came out as plausible. However, we did not enumerate the findings across studies as quantitative summaries should be undertaken at an individual study level rather than at a review level. A descriptive analysis of each included publication was conducted. ST exposure in hrs/day or week and the observed prevalence of MetS in percentages were specified. Adjusted estimates of OR or MetS z-score for the association between ST and MetS with a corresponding 95% CI were obtained. The OR of the dose-response gradient effect was also extracted.
Result
Flow of the studies
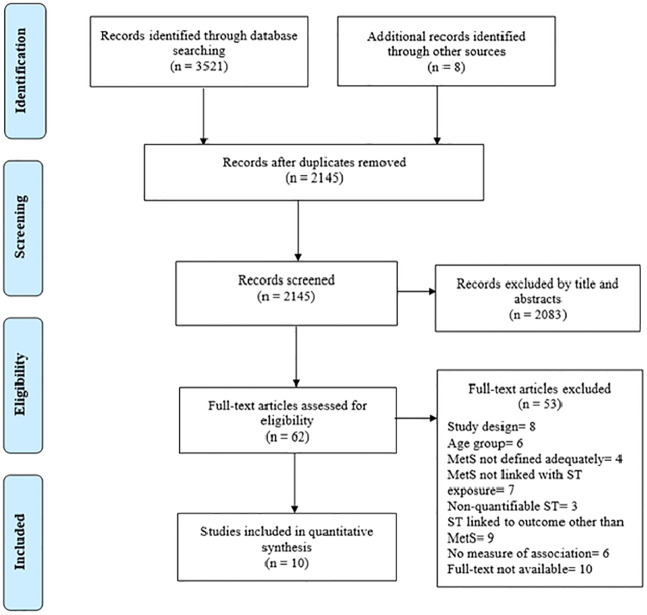
Fig 1 displays a flow chart of study identification and selection. Systematic search on database identified 3521 abstracts; of these, 2137 were excluded during initial screening for unrelated topics, meeting the exclusion criteria and duplicate studies from different databases. Totally, 62 full text articles were assessed to examine their eligibility for inclusion in the current review, and finally, after review of the full texts, ten studies were included in the data extraction.
Fig 1. PRISMA flowchart for review.
Overview of studies
Table 2 presents the general characteristics of the eligible studies. Most studies (9/10, 90%) employed a cross-sectional study design, except for one that implemented a prospective cohort design. In total, data from 41,687 participants was included in this review. The sample size ranged from 474 to 33,900 and the mean age of participants ranged from 12–18 years. Six of the studies have utilized school-based setting [31, 32, 36, 40–42], while the remaining 4 were part of national surveys. The primary objective of included studies was to establish significant association between ST (of any type) and MetS among adolescents, with all studies considering single or multiple exposures like PA, dietary habit or sleep duration associated with ST. Although the scope of the review focused on adolescents at the age of (12–18) year, studies that had a range of below 12 or above 18 were not excluded if the mean age was between 12–18 years. All the studies used subjective measures of daily/weekly hours ST including parental report, self-reported interview questionnaires. The main modalities of ST were TV viewing, computers, videogames, internet, tablets, and smartphones. Nine of the studies (9/10, 90%) utilized multivariable analysis to adjust for covariates [31, 32, 36–40, 42, 43], 8 of which (8/10, 80%) [31, 32, 36–38, 40, 42, 43], found a positive association between ST and MetS. Seven studies (7/10, 70%) proved a dose-response gradient for that association [29, 30, 34–36, 40, 41]. Two studies by Fadzlina et al. [41] and Siwarom et al. [39] found no significant association between ST and MetS. Exposure to ST prior to the age of 2 years was significantly associated with MetS in one of the included studies [39].
Table 2. Summary of characteristics of included studies showing relation between screen time (ST) and Metabolic syndrome (MetS).
| Author; publication year | Country | Study design; Sample size (N) | Mean age at baseline (SD); gender | Screen type | Exposure ST measure |
Outcome measures (MetS) | Association with MetS OR (95%CI) |
Comments |
|---|---|---|---|---|---|---|---|---|
| Schaan et al. (2019) [36] | Brazil | 33,900; cross-sectional | 14.6 year (SD not reported); 59.4% Female | TV view, computers, videogames | Self-reported hours per day | IDF guidelines (WC, SBP, DBP, Fasting blood glucose, Triglycerides; HDL) | ST ≥6 h/day; 1.68 (1.03–2.74). | Prevalence of MetS 2.6% (95%CI: 2.3–3.0), ST remained significantly associated with MetS after adjusting of covariates; age, sex, socioeconomic, PA. Association remained significant MetS remained significant only for adolescents who reported consumption of snacks in front of screens. |
| Khan M et al. (2019) [32] | UAE | 474; cross-sectional | 14.9 ±1.9 years; 47% Female | Computer, television, and video game | Self-reported hours per day | IDF guidelines (WC, SBP, DBP, Fasting blood glucose, Triglycerides; HDL) | ST ≥2 h/day: 2.20 (1.04–4.67) Each hour of increased ST (1.21; 1.08–1.35) |
Prevalence of MetS 8.5% in <2hr/d, 13.4% ≥2 hr/d) Association was adjusted for age, sex, physical education classes, smoking, parental education, daily intake of carbonated drink, fruits, vegetables, milk, fast food |
| Mark E and Janssen (2008) [37] | US | 1803; cross- sectional | 15.9 ± 2.2 years; 50.3% Female | TV, video, computer game | Self-reported hours per day home interview/ mobile exam centre | NCEP ATP II: ≥3 of the following: high triglycerides, high fasting glucose, high WC, high BP, low HDL. | ST ≥5 h/day: 2.90 (1.39–6.02) | Prevalence of MetS 3.7% in≤1 hr/d, 8.4% in ≥5 hr/day. Association was adjusted for age, smoking and PA. |
| Kang HT et al. (2010) [38] | Korea | 845, cross-sectional | 13.4 ± 2.5 years; 46.9% Female | TV time, computer game, internet | Self-reported hours per week | NCEP ATP II: ≥3 of the following: high triglycerides, high fasting glucose, high WC, high BP, low HDL. | ST (≥35 h/week: 2.23 (1.02–4.86) | Prevalence of MetS 7.3%. Association was adjusted for age, sex, household income, residence area. |
| de Oliveira RG et al. (2014) [31] | Brazil | 1,035, cross-sectional | Mean not reported; 56.6% of (12-15y), 43.4% of (16-20y), 54.6% Female | TV, computer, video game, tablet, smartphone | Self-reported hours per day | IDF guidelines (WC, SBP, DBP, Fasting blood glucose, Triglycerides; HDL) | ST> 2 h/day: 1.32 (1.07–1.94) | Prevalence of MetS 4.5% (95% CI: 3.8–5.4). Association was adjusted for demographic, anthropometric nutritional indicators and, lifestyle determinants. |
| Siwarom S et al. (2021) [39] | Thailand | 1934, cross-sectional | 13.40 ± 1.94; 49.7% Female | television watching, computer, smart phone, tablet use | Self-reported hours per week/screen media exposure during the first 2 years of life | IDF, Cook’s, and de Ferranti’s. | MetS by 1 out of 3 definitions: Exposure to screen media during the first 2 years of life: 1.30 (1.01–1.68). No association between total ST & MetS: 1.00 (0.99–1.00) |
Prevalence of MetS 17%, Association of ST and MetS was adjusted for age, sex, foot intake, fruits and vegetables, PA. |
| Hardy L et al. (2010) [40] | Australia | 496, cross-sectional | 15.4 ± 0.4 year; 42% Female | watching television/DVDs/videos and using a computer for recreation | Self-reported hours per day. Adolescent Sedentary Activity Questionnaire |
Metabolic risk factors: Insulin level Glucose level HOMA-IR, HDL-C, LDL-C, Triglyceride, hs-CRP, ALT, GGT l, SBP, DBP |
ST ≥2 h/day Boys: HOMA-IR (adjusted OR, 2.42 (1.11–5.28), insulin levels (adjusted OR, 2.73 (1.43–5.23) Girls: no association |
Prevalence of abnormal biomarker e.g., Insulin in ≥2h/d is 22.7% boys vs, 22.9% girls; HOMA-IR 41.5% boys vs. 46.5% girls. Association was adjusted for BMI, SES (IRDS score), EDNP food score, Tanner score, and CRE (number of laps) |
| Fadzlina A et al. (2014) [41] | Malaysia | 1014, cross-sectional | 12.88 ± 0.33 years; 61.8% Female | Not reported | Self-reported hours per day | IDF guidelines (WC, SBP, DBP, Fasting blood glucose, Triglycerides; HDL) | No association between ST and MetS | Prevalence of MetS 2.6%in total, 10% among overweight. Obese No adjusted model was utilized |
| Grøntved A et al. (2020) [42] | EYHS, Danish cohort | 435, cohort | 15.6 ± 0.4 year; 54.5% Female | TV, computer use | Self-reported hours per day | MetS z-score based on AHA/NHLBI; WC, SBP, DBP, triglycerides, HDL (inverted), fasting glucose, fasting insulin | Total ST > 2 h/day a/w MetS z-score. 0.35 (0.08–0.62) Each 1-hour increment in TV viewing time; syndrome z-score 0.45 (0.14–0.76) |
MetS Z-score for ≤1h (−0.2 ± 2.6), 1-3h (−0.1 ± 2.5) >3 h were (1.2 ± 3.5) Adjusted for age, gender, cohort, parental education level, current smoking status, (MVPA), intake of soft drinks, fruit- and vegetable intake, and family history of cardiovascular disease. |
| de Castro Silveira et al. (2020) [43] | Brazil | 1200, cross-sectional | Up to 17 years, no mean age reported; 56% Female | Not reported | Self-reported hours per day | Continuous metabolic score (CMetS) > 1 as metabolic risk factor Z-score of WC, SBP, glucose, triglyceride, total cholesterol, LDL, HDL |
ST ≥2 h/day; Prevalence Ratio (PR) = 0.99 (0.95–1.03), insignificant association | Prevalence of metabolic risk 14.7%. ST was adjusted for cardiorespiratory fitness measured at time of recruitment yielded significant association. |
Abbreviations: IDF, International Diabetes Federation, NCEP ATP II, National Cholesterol Education Program Adult Treatment Panel; SD, Standard deviation; WC, Waist circumference; SBP, systolic blood pressure, DBP, diastolic blood pressure, PA, physical activity, BMI, Body mass index; SES, socioeconomic status; IRDS, Australian Bureau of Statistics Index of Relative; EDNP, energy-dense nutrient-poor; CRE, cardiorespiratory endurance. EYHS, European Youth Heart Study; AHA, American Heart Association (AHA); NHLBI, and the National Heart, Lung, and Blood Institute. HDL-C, high density lipoprotein cholesterol, LDL-C, low density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; h-s CRP, high sensitivity C-reactive protein; ALT, Alanine Aminotransferase; GGT, Gamma-Glutamyl Transferase.
Screen time
All the studies have examined the measure of time in relation to different screen types, including TV viewing, computers, videogames, internet, tablets and smartphones. Daily or weekly reported hours of ST exposure was the main exposure measure in all studies. In most of the studies, the exposure variable was grouped into categories; two (< 2; > 2 hr/day), three (≤2, 3–5 and ≥ 6h/day) or four subgroups (≤1, 2,3,4, ≥5hr/day), (<2, 2, 4, ≤6), (0–16, 17–24, 25–34, ≥35 hr/week). Odds of MetS were compared according to each response category with least subgroup being as the reference. Seven of the studies have indicated a graded dose-response relationship between ST and MetS. Khan et al. [32] found that for every increased hour of ST, the risk of MetS is increased by 1.21. (1.08–1.35). Similarly, the cohort study of 12 years follow-up [42] showed that for each 1-hour increment in TV viewing time during adolescence, the MetS z-score is increased by 0.45 (95% CI 0.14 to 0.76). Screen media exposure during the first two years of life was independently linked to a 30% increase in MetS in adolescence (OR 1.3, 95% CI: 1.01–1.68) [42].
Prevalence of MetS and ST dose-response effect
The prevalence of MetS among adolescents ranged from 2.6 percent to 17 percent in eight of the included studies (8/10, 80 percent), with a gradually rising trend toward longer ST duration. Three studies reported outcomes of interest as metabolic Z-score [42], multiple metabolic risk factors [40] or continuous metabolic risk score [43]. For instance, Hardy et al. [40] reported more than twofold increase in the risk of abnormal insulin levels (adjusted OR, 2.42; 95% CI, 1.11–5.28) or elevated HOMA-IR (adjusted OR, 2.42; 95% CI, 1.11–5.28) among boys exceeding 2 hours of ST per day. Grøntved et al. [42], on the other hand, utilized the MetS z-score, which was found to be 0.35 (95 percent CI: 0.08–0.62) and that each hour of TV viewing was associated with 0.45 increase in MetS z-score. de Castro Silveira et al. [43] found that exposure to ST ≥ 2h/day when adjusted for cardiorespiratory fitness yielded a significant association with metabolic risk score (High ST/unfit (1.07:1.01–1.13, p = 0.020), Low ST/unfit 1.08: 1.02–1.14, p = 0.011) as compared to unadjusted ST (0.99: 0.95–1.03, P = 0.645). Metabolic risk was also higher in those with low ST/unfit (8%) and high ST/unfit (10%). (7 percent). Khan et al. [32] in their study found that higher prevalence of MetS was noted amongst adolescents who spent two hours or more of ST as compared to those with less than two hours (13.4 percent vs. 8.5 percent), respectively. The study also reported that for each hour increase in ST, the risk of MetS was increased by 21 percent (OR, 1.21; 95% CI: 1.08–1.35). Adolescents who spend more than two hours per day of ST were two times more likely to develop MetS (aOR = 2.20; 95% CI: 1.04–4.67) as compared to those who spend less than 2 hours/day. Concerning the dose-response gradient, six of the included studies have confirmed this hypothesis with 5–6 hours of ST yielding the highest odds of MetS. While most studies utilized a 2-hour cut-off point, for greater accuracy, further subcategorization was performed during the multivariate analysis. In two comparable studies, a twofold increase in the odds of MetS was observed among adolescents who spent more than two hours [34] and five hours per day [40], respectively.
Metabolic syndrome
Seven studies (7/10, 70%) [31, 32, 36–39, 41] used one of the following MetS outcome measure definitions: IDF, NCEP ATP II, Cook’s, or de Ferranti’s. Hardy et al. [40], on the other hand, classified metabolic risk factors including insulin level, glucose level, HOMA-IR, HDL-C, LDL-C, triglyceride, hs-CRP, ALT, GGT, SBP and DBP as isolated outcomes rather than MetS diagnosis. In contrast, in the cohort study of Grøntved et al. [42], the outcome was calculated as a continuous MetS z-score to preserve statistical power and because the number of incident cases of MetS according to the American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) definitions with the additional inclusion of fasting insulin was calculated as a continuous MetS z-score. Furthermore, de Castro Silveira et al. [43] used metabolic risk assessment, which was calculated by adding the Z score of the following parameters: WC, SBP, glucose, triglycerides, total cholesterol, LDL and HDL, where MetS values greater than 1 were considered metabolic risk.
Adjustment of covariates
Age, sex, socioeconomic level, region of residence, physical education classes, cardiorespiratory fitness, nutritional status, smoking, parental education, and BMI were all corrected for in nine of the studies (9/10, 90 percent) [31, 32, 36–40, 42, 43]. Even though seven of the studies [31, 32, 36, 37, 40, 42, 43] computed ORs adjusted for PA level and/or nutritional status, ST remained an independent determinant of MetS, apart from one study where unadjusted ST was found to be an insignificant predictor of metabolic risk [43]. In multivariate analysis, the relationship between screen-based sedentary behaviour and MetS remained significant only for teenagers who conveyed snacking in front of the screens [36].
Discussion
Public health measures related to the COVID-19 pandemic have led to a critical increase in the use of digital screen devices and reliance on remote learning. Screen-based sedentary behaviour is linked to physical inactivity and increased caloric consumption, which are important contributors to obesity and cardio-metabolic risk. Taken together, a better understanding of the association between ST (of different types) and MetS among vulnerable populations i.e., adolescents, is necessary to target preventable causes of premature mortality in later adulthood.
The present systematic review provides a narrative synthesis of data concerning the relationship between ST and MetS among adolescents. The majority of studies indicate a positive and dose-response association between exposure and the outcome of interest. Based on Quality Assessment Tool for Observational and Cross-Sectional Studies, 90 percent of included studies indicated low risk of bias, demonstrating good quality score.
Findings from this review confirm that adolescents engaged in screen-based sedentary behaviour have an increased likelihood of developing MetS. Multivariate analysis demonstrated that ST was a significant independent predictor of MetS. These findings are consistent with Tremblay et al. [44], who reported in their systematic review and meta-analysis of 232 publications a positive correlation between increased levels of sedentary behaviour (especially TV viewing> 2h/day) and cardiometabolic illnesses among children and youth. A notable link was observed between ST and adverse body composition, poor fitness, and low self-esteem [44]. Contrarily, some scholars have argued that ST in adolescents is irrelevant to future health risks. A systematic review [31] showed weak evidence of an association between ST and poor cardiorespiratory fitness, poorer cognitive development, lower educational attainments, poor sleep outcomes, or risk of MetS. However, it is important to note that weak association does not imply absence of correlation. For instance, the authors have reported a lack of literature as the probable cause of such an observation.
Time spent in front of screens (of different types), whether at home or school-based, was obtained as a self-reported measure based on daily/weekly hours. Longitudinal studies [42, 45] established that longer duration or more frequent TV viewing was associated with a higher clustering cardio-metabolic risk score, particularly elevated systolic blood pressure. In contrast, computer-based ST was associated with higher diastolic blood pressure, while a lower level of HDL was objectively associated with longer-accelerometer-derived sedentary time [46]. Sedentary behaviour was not found to be associated with other cardiometabolic risk factors such as triglycerides, HOMA-IR, or glucose level [42].
This systematic review indicates a linear association between ST and MetS, meaning that MetS risk increased in tandem. For instance, spending more than two hours of daily ST triggered an increased risk of MetS in a dose-response manner, with the most harmful effect noted at 5–6 hours per day. Consistent with our findings, longitudinal studies have shown that higher cholesterol levels [47] and higher blood pressure [48] were associated with watching more than two hours of television per day, as compared to those who watch less. Similarly, high levels of self-reported sedentary behaviour including ST were associated with an increased risk of elevated systolic and diastolic blood pressure [48–51], higher glycated haemoglobin (HbA1C) [52], fasting insulin [50, 53], insulin resistance [54], and MetS [55]. With reference to the dose-response gradient, longer duration of TV viewing was significantly correlated with increased risk of MetS/cardiovascular disease risk factors, whether a 2-h cut-point [56, 57], a 3-h [58] or a 4-h [59] cut-point [60] was utilized. Accordingly, adolescents should be urged to limit their daily recreational ST to less than 2 hours per day, as recommended by the American Academy of Paediatrics and the World Health Organization (WHO). This is especially crucial given the likelihood of such a risk persisting into adulthood.
Several studies have conveyed a rising trend of ST and digital technology use during the pandemic [33, 61]. Children and adolescents are considered amongst the most susceptible groups due to their limited self-regulation and liability to peer pressure. For instance, Xiang et al. [62], illustrated a considerable increase in ST during the pandemic among 6–17 years old in Shanghai (+1730 minutes or nearly 30 hours in total). More than a quarter of students revealed an increase in leisure-based ST in addition. Comparably, the median time spent in PA was shown to be reduced from, 540 minutes per week (prior to the pandemic) to 105 minutes per week, with an average reduction of 435 minutes. The prevalence of physical inactivity was almost tripled as compared to the pre-pandemic period (21.3 vs. 65.6%). Another study [63] conducted in Canada exhibited a significant decline of adherence into PA and ST guidelines among 5–17-year-old during the pandemic (PA; from 18% to 35% [64] and ST: from 64% to 11.2% [65], subsequently).
Despite the well-established harmful effects of ST, several academic studies have indicated possible benefits (especially educational outcomes) of ST among younger generations. According to several studies, children and teenagers frequently lack the discipline and insight to limit ST on their own [65]. Thus, taking into account the current challenging crisis [66], it becomes critically vital to address mitigation strategies and encourage a family-centred media use plan that allows us to balance the risks and benefits. Despite the fact that ST has been traditionally linked to sedentary behaviour and increases the risk of negative health outcomes [67], there are always alternative positive ways around the corner. Appropriately used, ST can promote PA during a shelter-in-place [68], such as online PA classes, workout apps for mobile devices or active video games [68]. In this way, a recent systematic review among adolescents found that digital interventions incorporating educational activities, goal setting, self-monitoring, and parental involvement have led to a significant increase in PA [69].
Evidence is validating the role of opportunistic behaviour change counselling by clinical practitioners in the management of sedentary behaviour. A key mitigative strategy is to identify correlates of sedentary time, such as sociodemographic attributes, accessibility, parental behaviour, psychological, PA, and dietary behaviour [70]. Engaging adolescents, families, schools, and social workers in healthy lifestyle choices provides an enabling environment that supports behavioural change. School could be an ideal setting for encouraging physical activity and reducing sedentary behaviour. For instance, Verloigne et al. [71] acclaimed the placing of standing desks in classrooms and promoting stand-up bouts as an interventional approach to increase pupils’ self-efficacy and reduce sedentary behaviour in a pleasurable manner.
It may be possible to use a holistic method that looks at all three levels of the socio-ecological model (intra-individual, inter-individual, physical environment, and policy) to help people move more and make healthy food choices [72]. Effective communication with adolescents and their families enhances digital literacy related to screen types, content, and setting screen limits. A systematic review and meta-analysis on the effect of behavioural interventions in reducing ST revealed that smaller sample sizes and shorter intervention durations were associated with greater impact. Involvement of healthcare professionals in setting goals, feedback, and planning clusters yielded better outcomes in ST reduction [73].
Healthcare workers are advised to initiate early prevention strategies tackling the associated risk factors of NCDs among adolescents. Given the challenging period of COVID-19 [74–78], it has become increasingly important to integrate lifestyle education, health promotion, and community awareness within the management. Screening for early identification of behavioural and metabolic risk factors will help reduce the burden of NCDs later in adulthood. Tools such as screening of baseline PA, ST, Body Mass Index (BMI) and psychological assessment are essential to identify modifiable risk factors and ‘at risk’ children. Accordingly, replacement strategies, weight loss programs, and exercise prescriptions are recommended, taking into account the importance of continuous monitoring and evaluation. Evidence from this study can guide national and public health efforts in planning accessible multisectoral prevention and intervention strategies to tackle the determinants of MetS and NCDs.
Limitations
The evidence in this review was dependent on peer-reviewed journals via scientific databases, not accessing data from unpublished reports from educational institutions, non-profit data, or community services, and maybe subject to publication bias. Due to the apparent high risk of bias (ROB) attributable to clinical and methodological heterogeneity, meta-analysis of data was not performed. Moreover, undefined information in any of the included studies was not confirmed by the related authors, jeopardizing their quality, if any important details were missed. Furthermore, our research was limited to studies published only in English, and the cross-sectional design of the majority of included studies prevented inference of causality, thereby limiting the conclusion drawn regarding the temporal relationship between ST and MetS. Data were gathered by one researcher, and even though the data were carefully checked back to the publication by the second researcher, we did not use two separate extractions. In our narrative synthesis of findings, we aimed to avoid vote-counting of numbers of positive or negative studies to judge the strength of evidence. However, it is possible that our findings reflect methodological or conceptual biases in our included reviews. Finally, the search did not extend to all existing databases. Nonetheless, we performed searches in two primary databases and one secondary database.
Conclusion
The COVID-19 pandemic has resulted in children and adolescents spending more time on digital screen devices, eliciting a profound effect on their cardio-metabolic health and NCDs burden. In brief, our review demonstrated that independent of PA, significant association between ST and MetS was noted among adolescents. This observation has significant public health and clinical implications that demand urgent prevention initiatives targeting young people and their parents. Such interventions aim to enhance early screening of behavioural and metabolic risk factors and increase awareness of potential adverse health impacts related to NCDs. Healthcare providers should consider a promotive, holistic approach, taking into consideration the international recommendation of ST and PA across different age groups. Further community-based research, including longitudinal and RCTs are needed to confirm this primarily observational evidence.
Supporting information
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The publication of this article was funded by Qatar National Library. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dergaa I, Varma A, Tabben M, Malik RA, Sheik S, Vedasalam S, et al. Organising football matches with spectators during the COVID-19 pandemic: What can we learn from the Amir Cup Football Final of Qatar 2020? A call for action. Biology of Sport. 2021;38(4):677–81. doi: 10.5114/biolsport.2021.103568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dergaa I, Abdelrahman H, Varma A, Yousfi N, Souissi A, Ghram A, et al. COVID-19 Vaccination, Herd Immunity and The Transition Toward Normalcy: Challenges with The Upcoming Sports Event. Annals of Applied Sport Science.:0-. [Google Scholar]
- 3.Varma A, Dergaa I, Mohammed AR, Abubaker M, Al Naama A, Mohammed S, et al. COVID-19 and diabetes in primary care–How do hematological parameters present in this cohort?. Expert review of endocrinology & metabolism. 2021. May 4;16(3):147–53. doi: 10.1080/17446651.2021.1909472 [DOI] [PubMed] [Google Scholar]
- 4.Couzin-Frankel J, Vogel G, Weiland M. Not open and shut. Science. 2020. Jul 17;369(6501):241–245. doi: 10.1126/science.369.6501.241 [DOI] [PubMed] [Google Scholar]
- 5.Musa S, Dergaa I, Mansy O. The puzzle of Autism in the time of COVID 19 pandemic:“Light it up Blue”. Psychology and Education Journal. 2021. Jun 2;58(5):1861–73. [Google Scholar]
- 6.Margaritis I, Houdart S, El Ouadrhiri Y, Bigard X, Vuillemin A, Duché P. How to deal with COVID-19 epidemic-related lockdown physical inactivity and sedentary increase in youth? Adaptation of Anses’ benchmarks. Archives of Public Health. 2020. Dec;78:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanderloo LM, Carsley S, Aglipay M, Cost KT, Maguire J, Birken CS. Applying harm reduction principles to address screen time in young children amidst the COVID-19 pandemic. Journal of Developmental & Behavioral Pediatrics. 2020. Jun 1;41(5):335–6. doi: 10.1097/DBP.0000000000000825 [DOI] [PubMed] [Google Scholar]
- 8.Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, Bouaziz B, et al. Sleep quality and physical activity as predictors of mental wellbeing variance in older adults during COVID-19 lockdown: ECLB COVID-19 international online survey. International journal of environmental research and public health. 2021. Jan;18(8):4329. doi: 10.3390/ijerph18084329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, Bouaziz B, et al. Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. doi: 10.5114/biolsport.2021.101605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandya A, Lodha P. Social connectedness, excessive screen time during COVID-19 and mental health: a review of current evidence. Frontiers in Human Dynamics. 2021:45. [Google Scholar]
- 11.World Health Organization. Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. World Health Organization; 2019. [PubMed] [Google Scholar]
- 12.Kovacs VA, Starc G, Brandes M, Kaj M, Blagus R, Leskošek B, et al. Physical activity, screen time and the COVID-19 school closures in Europe–An observational study in 10 countries. European journal of sport science. 2021. Mar 29:1–0. [DOI] [PubMed] [Google Scholar]
- 13.Nagata JM, Cortez CA, Cattle CJ, Ganson KT, Iyer P, Bibbins-Domingo K, et al. Screen time use among US adolescents during the COVID-19 pandemic: findings from the Adolescent Brain Cognitive Development (ABCD) study. JAMA pediatrics. 2022. Jan 1;176(1):94–6. doi: 10.1001/jamapediatrics.2021.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He F, Rodriguez-Colon S, Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Berg A, et al. Abdominal obesity and metabolic syndrome burden in adolescents—Penn State Children Cohort study. Journal of Clinical Densitometry. 2015. Jan 1;18(1):30–6. doi: 10.1016/j.jocd.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele RM, Brage S, Corder K, Wareham NJ, Ekelund U. Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. Journal of applied physiology. 2008. Jul;105(1):342–51. doi: 10.1152/japplphysiol.00072.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA. Diagnosis and management of the metabolic syndrome. Circulation [Internet]. 2005; 112 (17): 2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 17.Rizzo AC, Goldberg TB, Silva CC, Kurokawa CS, Nunes HR, Corrente JE. Metabolic syndrome risk factors in overweight, obese, and extremely obese Brazilian adolescents. Nutrition journal. 2013. Dec;12(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hamad D, Raman V. Metabolic syndrome in children and adolescents. Translational pediatrics. 2017. Oct;6(4):397. doi: 10.21037/tp.2017.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnussen CG, Koskinen J, Chen W, Thomson R, Schmidt MD, Srinivasan SR, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010. Oct 19;122(16):1604–11. doi: 10.1161/CIRCULATIONAHA.110.940809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. New England journal of medicine. 2007. Dec 6;357(23):2329–37. doi: 10.1056/NEJMoa072515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison JA, Glueck CJ, Horn PS, Yeramaneni S, Wang P. Pediatric triglycerides predict cardiovascular disease events in the fourth to fifth decade of life. Metabolism. 2009. Sep 1;58(9):1277–84. doi: 10.1016/j.metabol.2009.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberger J, Daniels SR, Hagberg N, Isasi CR, Kelly AS, Lloyd-Jones D, et al. Cardiovascular health promotion in children: challenges and opportunities for 2020 and beyond: a scientific statement from the American Heart Association. Circulation. 2016. Sep 20;134(12):e236–55. doi: 10.1161/CIR.0000000000000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miranda VP, dos Santos Amorim PR, Bastos RR, Canabrava KL, Júnior MV, Faria FR, et al. Association of lifestyle and body composition on risk factors of cardiometabolic diseases and biomarkers in female adolescents. Mediators of inflammation. 2020. Jul 9;2020. doi: 10.1155/2020/9170640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strizich G, Kaplan RC, Sotres-Alvarez D, Diaz KM, Daigre AL, Carnethon MR, et al. Objectively measured sedentary behavior, physical activity, and cardiometabolic risk in Hispanic youth: Hispanic community health study/study of Latino youth. The Journal of Clinical Endocrinology & Metabolism. 2018. Sep;103(9):3289–98. doi: 10.1210/jc.2018-00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Guidelines on physical activity and sedentary behaviour. https://apps.who.int/iris/bitstream/handle/10665/336656/9789240015128-eng.pdf?sequence=1&isAllowed=y. accessed on August 19, 2021
- 26.Van Ekris E, Altenburg TM, Singh AS, et al. An evidence-update on the prospective relationship between childhood sedentary behaviour and biomedical health indicators: a systematic review and meta-analysis. Obes Rev 2016;17:833 49. doi: 10.1111/obr.12426 [DOI] [PubMed] [Google Scholar]
- 27.Costigan SA, Barnett L, Plotnikoff RC, et al. The health indicators associated with screen-based sedentary behavior among adolescent girls: a systematic review. J Adolesc Health 2013;52:382 92. doi: 10.1016/j.jadohealth.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 28.Straker L, Zabatiero J, Danby S, et al. Conflicting guidelines on young children’s screen time and use of digital technology create policy and practice dilemmas. J Pediatr 2018;202:300 3. doi: 10.1016/j.jpeds.2018.07.019 [DOI] [PubMed] [Google Scholar]
- 29.Sidney S, Sternfeld B, Haskell WL, Jacobs DR, Chesney MA, Hulley SB: Television viewing and cardiovascular risk factors in young adults: the CARDIA study. Ann Epidemiol. 1996, 6 (2): 154–159. doi: 10.1016/1047-2797(95)00135-2 [DOI] [PubMed] [Google Scholar]
- 30.Ekelund U, Brage S, Griffin SJ, Wareham NJ: Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predicts insulin resistance in high-risk individuals. Diabetes Care. 2009, 32 (6): 1081–1086. doi: 10.2337/dc08-1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Oliveira RG, Guedes DP. Physical activity, sedentary behavior, cardiorespiratory fitness and metabolic syndrome in adolescents: systematic review and meta-analysis of observational evidence. PloS one. 2016. Dec 20;11(12):e0168503. doi: 10.1371/journal.pone.0168503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan MA, Shah SM, Shehab A, Ghosal S, Muhairi SJ, Al-Rifai RH, et al. Screen time and metabolic syndrome among expatriate adolescents in the United Arab Emirates. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019. Jul 1;13(4):2565–9. doi: 10.1016/j.dsx.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 33.Stiglic N, Viner RM. Effects of screentime on the health and well-being of children and adolescents: a systematic review of reviews. BMJ open. 2019. Jan 1;9(1):e023191. doi: 10.1136/bmjopen-2018-023191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021. Mar 29;372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Heart, Lung, and Blood institute: Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed on 19 August 2021.
- 36.Schaan CW, Cureau FV, Salvo D, Kohl HW, Schaan BD. Unhealthy snack intake modifies the association between screen-based sedentary time and metabolic syndrome in Brazilian adolescents. International Journal of Behavioral Nutrition and Physical Activity. 2019. Dec;16(1):1–9. doi: 10.1186/s12966-019-0880-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mark AE, Janssen I. Relationship between screen time and metabolic syndrome in adolescents. Journal of public health. 2008. Jun 1;30(2):153–60. doi: 10.1093/pubmed/fdn022 [DOI] [PubMed] [Google Scholar]
- 38.Kang HT, Lee HR, Shim JY, Shin YH, Park BJ, Lee YJ. Association between screen time and metabolic syndrome in children and adolescents in Korea: the 2005 Korean National Health and Nutrition Examination Survey. Diabetes research and clinical practice. 2010. Jul 1;89(1):72–8. doi: 10.1016/j.diabres.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 39.Siwarom S, Aekplakorn W, Pirojsakul K, Paksi W, Kessomboon P, Neelapaichit N, et al. Metabolic syndrome in Thai adolescents and associated factors: the Thai National Health Examination Survey V (NHES V). BMC public health. 2021. Dec;21(1):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardy LL, Denney-Wilson E, Thrift AP, Okely AD, Baur LA. Screen time and metabolic risk factors among adolescents. Archives of pediatrics & adolescent medicine. 2010. Jul 5;164(7):643–9. doi: 10.1001/archpediatrics.2010.88 [DOI] [PubMed] [Google Scholar]
- 41.Fadzlina AA, Harun F, Haniza MN, Al Sadat N, Murray L, Cantwell MM, et al. Metabolic syndrome among 13 year old adolescents: prevalence and risk factors. InBMC public health 2014. Dec (Vol. 14, No. 3, pp. 1–8). BioMed Central. doi: 10.1186/1471-2458-14-S3-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grøntved A, Ried-Larsen M, Møller NC, Kristensen PL, Wedderkopp N, Froberg K, et al. Youth screen-time behaviour is associated with cardiovascular risk in young adulthood: the European Youth Heart Study. European journal of preventive cardiology. 2014. Jan 1;21(1):49–56. doi: 10.1177/2047487312454760 [DOI] [PubMed] [Google Scholar]
- 43.de Castro Silveira JF, Barbian CD, Burgos LT, Renner JD, Paiva DN, Reuter CP. Association between the screen time and the cardiorespiratory fitness with the presence of metabolic risk in schoolchildren. Revista Paulista de Pediatria. 2020. May 22;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tremblay MS, LeBlanc AG, Kho ME, Saunders TJ, Larouche R, Colley RC, et al. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. International journal of behavioral nutrition and physical activity. 2011. Dec;8(1):1–22. doi: 10.1186/1479-5868-8-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wennberg P, Gustafsson PE, Dunstan DW, Wennberg M, Hammarström A. Television viewing and low leisure-time physical activity in adolescence independently predict the metabolic syndrome in mid-adulthood. Diabetes care. 2013. Jul 1;36(7):2090–7. doi: 10.2337/dc12-1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hjorth MF, Chaput JP, Damsgaard CT, Dalskov SM, Andersen R, Astrup A, et al. Low physical activity level and short sleep duration are associated with an increased cardio-metabolic risk profile: a longitudinal study in 8–11 year old Danish children. PloS one. 2014. Aug 7;9(8):e104677. doi: 10.1371/journal.pone.0104677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hancox RJ, Milne BJ, Poulton R. Association between child and adolescent television viewing and adult health: a longitudinal birth cohort study. The Lancet. 2004. Jul 17;364(9430):257–62. doi: 10.1016/S0140-6736(04)16675-0 [DOI] [PubMed] [Google Scholar]
- 48.Dasgupta K, O’Loughlin J, Chen S, Karp I, Paradis G, Tremblay J, et al. Emergence of sex differences in prevalence of high systolic blood pressure: analysis of a longitudinal adolescent cohort. Circulation. 2006. Dec 12;114(24):2663–70. doi: 10.1161/CIRCULATIONAHA.106.624536 [DOI] [PubMed] [Google Scholar]
- 49.Wells JC, Hallal PC, Reichert FF, Menezes AM, Araújo CL, Victora CG. Sleep patterns and television viewing in relation to obesity and blood pressure: evidence from an adolescent Brazilian birth cohort. International Journal of Obesity. 2008. Jul;32(7):1042–9. doi: 10.1038/ijo.2008.37 [DOI] [PubMed] [Google Scholar]
- 50.Dasgupta K. Sex differences in the development of higher systolic blood pressure during adolescence. Cardiology Review. 2008;25(5):54–7. [Google Scholar]
- 51.Lazarou C, Panagiotakos DB, Matalas AL. Lifestyle factors are determinants of children’s blood pressure levels: the CYKIDS study. Journal of human hypertension. 2009. Jul;23(7):456–63. doi: 10.1038/jhh.2008.151 [DOI] [PubMed] [Google Scholar]
- 52.Imayama I, Plotnikoff RC, Courneya KS, Johnson JA. Determinants of quality of life in adults with type 1 and type 2 diabetes. Health and quality of life outcomes. 2011. Dec;9(1):1–9. doi: 10.1186/1477-7525-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ekelund U, Brage S, Froberg K, Harro M, Anderssen SA, Sardinha LB, et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European Youth Heart Study. PLoS medicine. 2006. Dec;3(12):e488. doi: 10.1371/journal.pmed.0030488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sardinha LB, Andersen LB, Anderssen SA, Quitério AL, Ornelas R, Froberg K, et al. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9-to 10-year-old Portuguese children. Diabetes care. 2008. Mar 1;31(3):569–75. doi: 10.2337/dc07-1286 [DOI] [PubMed] [Google Scholar]
- 55.Øverby NC, Margeirsdottir HD, Brunborg C, Anderssen SA, Andersen LF, Dahl-Jørgensen K, Norwegian Study Group for Childhood Diabetes. Physical activity and overweight in children and adolescents using intensified insulin treatment. Pediatric diabetes. 2009. Mar;10(2):135–41. doi: 10.1111/j.1399-5448.2008.00454.x [DOI] [PubMed] [Google Scholar]
- 56.Giussani M, Antolini L, Brambilla P, Pagani M, Zuccotti G, Valsecchi MG, et al. Cardiovascular risk assessment in children: role of physical activity, family history and parental smoking on BMI and blood pressure. Journal of hypertension. 2013. May 1;31(5):983–92. doi: 10.1097/HJH.0b013e32835f17c7 [DOI] [PubMed] [Google Scholar]
- 57.You MA, Son YJ. Prevalence of metabolic syndrome and associated risk factors among Korean adolescents: analysis from the Korean national survey. Asia Pacific Journal of Public Health. 2012. May;24(3):464–71. doi: 10.1177/1010539511406105 [DOI] [PubMed] [Google Scholar]
- 58.Magee CA, Caputi P, Iverson DC. Patterns of health behaviours predict obesity in A ustralian children. Journal of paediatrics and child health. 2013. Apr;49(4):291–6. doi: 10.1111/jpc.12163 [DOI] [PubMed] [Google Scholar]
- 59.Calamaro CJ, Park S, Mason TB, Marcus CL, Weaver TE, Pack A, et al. Shortened sleep duration does not predict obesity in adolescents. Journal of sleep research. 2010. Dec;19(4):559–66. doi: 10.1111/j.1365-2869.2010.00840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carson V, Hunter S, Kuzik N, Gray CE, Poitras VJ, Chaput JP, et al. Systematic review of sedentary behaviour and health indicators in school-aged children and youth: an update. Applied physiology, nutrition, and metabolism. 2016;41(6):S240–65. doi: 10.1139/apnm-2015-0630 [DOI] [PubMed] [Google Scholar]
- 61.Janssen X, Martin A, Hughes AR, Hill CM, Kotronoulas G, Hesketh KR. Associations of screen time, sedentary time and physical activity with sleep in under 5s: A systematic review and meta-analysis. Sleep medicine reviews. 2020. Feb 1;49:101226. doi: 10.1016/j.smrv.2019.101226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang M, Zhang Z, Kuwahara K. Impact of COVID-19 pandemic on children and adolescents’ lifestyle behavior larger than expected. Progress in cardiovascular diseases. 2020. Jul;63(4):531. doi: 10.1016/j.pcad.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerrero MD, Vanderloo LM, Rhodes RE, Faulkner G, Moore SA, Tremblay MS. Canadian children’s and youth’s adherence to the 24-h movement guidelines during the COVID-19 pandemic: A decision tree analysis. Journal of sport and health science. 2020. Jul 1;9(4):313–21. doi: 10.1016/j.jshs.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes JD, Cameron C, Carson V, Chaput JP, Colley RC, Faulkner GE, et al. Results from Canada’s 2018 report card on physical activity for children and youth. Journal of Physical Activity and Health. 2018. Jan 2;15(s2):S328–30. doi: 10.1123/jpah.2018-0454 [DOI] [PubMed] [Google Scholar]
- 65.Sultana A, Tasnim S, Hossain MM, Bhattacharya S, Purohit N. Digital screen time during the COVID-19 pandemic: A public health concern. F1000Research. 2021. Feb 8;10(81):81. [Google Scholar]
- 66.Musa S, Dergaa I, Abdulmalik MA, Ammar A, Chamari K, Saad HB. BNT162b2 COVID-19 Vaccine Hesitancy among Parents of 4023 Young Adolescents (12–15 Years) in Qatar. Vaccines. 2021. Sep;9(9):981. doi: 10.3390/vaccines9090981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lissak G. Adverse physiological and psychological effects of screen time on children and adolescents: Literature review and case study. Environmental research. 2018. Jul 1;164:149–57. doi: 10.1016/j.envres.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization. (2020). Stay physically active during self-quarantine. [WWW document]. URL http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-COVID-19/novel-coronavirus-2019-ncov-technical-guidance/stay-physically-active-during-self-quarantine
- 69.Rose T, Barker M, Jacob CM, Morrison L, Lawrence W, Strömmer S, et al. A systematic review of digital interventions for improving the diet and physical activity behaviors of adolescents. Journal of Adolescent Health. 2017. Dec 1;61(6):669–77. doi: 10.1016/j.jadohealth.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minges KE, Owen N, Salmon J, Chao A, Dunstan DW, Whittemore R. Reducing youth screen time: qualitative metasynthesis of findings on barriers and facilitators. Health Psychology. 2015. Apr;34(4):381. doi: 10.1037/hea0000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verloigne M, Ridgers ND, De Bourdeaudhuij I, Cardon G. Effect and process evaluation of implementing standing desks in primary and secondary schools in Belgium: A cluster-randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity. 2018. Dec;15(1):1–7. doi: 10.1186/s12966-018-0726-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stokols D. Establishing and maintaining healthy environments: Toward a social ecology of health promotion. American psychologist. 1992. Jan;47(1):6. [DOI] [PubMed] [Google Scholar]
- 73.Jones A, Armstrong B, Weaver RG, Parker H, von Klinggraeff L, Beets MW. Identifying effective intervention strategies to reduce children’s screen time: a systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity. 2021. Dec;18(1):1–20. doi: 10.1186/s12966-021-01189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varma A, Dergaa I, Mohammed AR, Abubaker M, Al Naama A, Mohammed S, et al. Covid-19 and diabetes in primary care—How do hematological parameters present in this cohort? Expert Rev Endocrinol Metab. 2021. May;16(3):147–153. doi: 10.1080/17446651.2021.1909472 [DOI] [PubMed] [Google Scholar]
- 75.Dergaa I, Abubaker M, Souissi A, Mohammed AR, Varma A, Musa S, et al. Age and clinical signs as predictors of COVID-19 symptoms and cycle threshold value. Libyan J Med. 2022. Dec;17(1):2010337. doi: 10.1080/19932820.2021.2010337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musa S, Dergaa I, Abdulmalik MA, Ammar A, Chamari K, Saad HB. BNT162b2 COVID-19 Vaccine Hesitancy among Parents of 4023 Young Adolescents (12–15 Years) in Qatar. Vaccines (Basel). 2021. 9(9):981. doi: 10.3390/vaccines9090981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dergaa I, Musa S, Romdhani M, Souissi A, Abdulmalik M, Chamari K, et al. FIFA World Cup 2022: What can we learn from the inspiring Tokyo 2020 Olympic Games held in COVID-19 times?. Biol Sport, 39(4), 1073–1080. doi: 10.5114/biolsport.2022.113293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dergaa I, Ben Saad H, Souissi A, et al. 2022. Br J Sports Med. 2022. [In Press] 1–3. doi: 10.1136/bjsports-2020-103454 [DOI] [PubMed] [Google Scholar]