Summary:
A growing number of studies have demonstrated the risk associated with mild elevations in pulmonary artery (PA) pressure as estimated by echocardiography. On right heart catherization in healthy individuals, the average PA systolic pressure is 21 ± 4 mm Hg; on echocardiography, estimated PA systolic pressure of >30 mm Hg is outside the normal range in most healthy individuals. Elevated PA systolic pressure is present in 40% of all echocardiograms performed clinically and is associated with a five-year mortality of 25-40%. However, current guidelines do not sufficiently highlight risk and risk-reduction approaches for the sizable patient population with elevated pulmonary artery pressures but do not have underlying severe pulmonary vascular disease such as pulmonary arterial hypertension. Increased awareness of this frequently reported high-risk echocardiographic finding, and multi-disciplinary risk-reduction approaches for patients with metabolic and cardiopulmonary comorbidities and elevated pulmonary artery pressures, are urgently needed.
Pulmonary hypertension is a common and highly morbid condition associated with decreased survival. Defined as mean pulmonary artery pressure > 20 mm Hg measured by right heart catherization1, based on the upper limit of normal pulmonary artery pressure in healthy individuals at rest and increased risk associated with mPAP >20 mmHg, it is present in more than three-quarters of all right heart catheterizations performed2,3. However, the pulmonary artery systolic pressure can also be estimated (ePASP) noninvasively with adequate reliability by measuring the velocity of the tricuspid regurgitation (TR) jet using echocardiography. ePASP is estimated by calculating Doppler estimated transtricuspid gradient (TR gradient= 4 x (TR velocity)2) and adding estimated right atrial pressure to it based on the size of the inferior vena cava and collapsibility with sniff 4. Indeed, ePASP is a standard assessment in contemporary echocardiography, and can be measured in about two-thirds of all echocardiograms5,6. Tables 1 and 2 delineate values of ePASP, measured by echocardiography or right heart catheterization, in healthy volunteers and community-based cohorts, respectively. On right heart catherization performed in healthy individuals, the average PASP is 21 ± 4 mm Hg, with an upper limit around 30 mm Hg 7. From echocardiography performed in healthy individuals, the average ePASP is similar to measurements from right heart catheterization, generally in the teens to low-20s mm Hg range on average; ePASP above 30 mm Hg is uncommon, with the exception of young well-trained athletes, high-altitude residents, and the elderly. Notably, ePASP of >30 mm Hg is below the traditional threshold of 40 mm Hg typically cited as concerning for possible PH and actually relates more closely to a mean PA pressure ≥20 mm Hg.8,9 Mean PA pressure in this range (mPAP 20-24 mm Hg) associates independently with increased mortality2, suggesting that ePASP levels <40 mmHg are informative for identifying at-risk patients as well. While an elevated ePASP is a risk marker, absence of a TR jet notably does not rule out elevated pulmonary artery pressure 10. Therefore, in the minority of patients without a measureable ePASP, clinical suspicion of elevated ePASP based on short pulmonary acceleration time on echocardiography, notching in the Doppler flow recording in the right ventricular outflow track, and presence of right ventricular hypertrophy, dilation or dysfunction may be helpful in identifying at-risk patients 10,11.
Table 1:
Values and upper limits of pulmonary artery systolic pressure in selected large cohort studies of echocardiography and right heart catheterization in healthy subjects
| Authors | Population | n | Age (Mean ± SD or median (IQR)) Sex (%Female) |
Modality | Mean or median PASP or RVSP in mmHg (SD or IQR): |
Upper limits, Pulmonary Artery Systolic Pressure |
|---|---|---|---|---|---|---|
| D’Andrea A et al (2011)15 | Healthy control subjects and Highly-trained athletes including endurance- and strength-trained athletes | Healthy Controls n=230 Athletes n=615: Endurance-trained athletes n =370 Strength-trained n=245 |
Healthy controls: 27·5 ± 11.3 years (yrs), 39.1% female Athletes:28·4 ± 10.1 yrs, 36·7% female |
Echocardiogram | Healthy controls: 17·6 ± 4.6* Athletes: Endurance-trained: 26·1 ± 6.6** Strength-trained: 19·4 (8.1)** |
26·8 mm Hg (healthy controls) |
| D’Andrea et al (2020)23 | Healthy controls and Endurance athletes | Healthy Controls n=150 Endurance athletes n=350 |
Healthy controls: 32·4 ± 15·1 yrs, 42·6% female 31·6 ± 4·2 yrs, 41·5% female |
Echocardiogram | Healthy controls: 21·6 ± 3·1* Endurance athletes: 25·5 ± 5·8* |
28mmHg (healthy controls) |
| Ferrara F et al (2016)17 | Healthy volunteers and those undergoing work assessment without evidence of cardiovascular disease, diabetes, obesity, significant valvular disease | n=1168 | 45·1 ± 16 yrs 52.5% female |
Echocardiogram | 20·9 ± 5·9 * | Age <40 yrs: 31 mm Hg Age 40-49 yrs: 32 mm Hg Age 50-59 yrs: 33 mm Hg Age ≥ 60 yrs: 35.6 mm Hg |
| Soria R et al (2016)24 | Systematic review and meta-analysis of echocardiography data on healthy low-altitude and high-altitude study participants from general population | Low-altitude dwellers: n=620 High-altitude dwellers: n=786 |
Low-altitude: Mean age range: 8·8 – 45yrs, 43·3% female (2·9% sex not recorded) High-altitude: Mean age range: 9·5 – 48 yrs, 38·5% female |
Echocardiogram | Low-altitude: 18·4 (95% CI 17·1-19·7) High-altitude: 25·3 (95% CI 24·0 – 26·7) |
Low-altitude: 26mmHg High altitude: 36 mm Hg |
| Yang Y et al (2020)25 | Healthy male volunteers | n=121 | 20 (19, 21) 0% female |
Echocardiogram | At low altitude: 24·2** (20·4, 27·3) | NA |
| Kovacs G et al (2009)7 | Systematic review of right heart catheterization data on healthy individuals | n=1187 | N/a 18·9% female, 20·6% sex notrecorded |
Right heart catheterization | 20·8 ± 4.4 | 29–6 mm Hg |
| Wolsk E et al (2019)19 | Healthy subjects aged 20-80 years from the community (n=60) | n=60 | 50 ± 17 yrs 53·3% female |
Right heart catheterization | 21 ± 5 | 31 mm Hg |
IQR: Interquartile range; PASP: Pulmonary artery systolic pressure; RVSP: Right ventricular systolic pressure; SD: standard deviation; Yrs: Years
RA pressure based on inferior vena cava (IVC) measurements.
Assuming a right atrial (RA) pressure of 5mmHg
Table 2:
Values of pulmonary artery systolic pressure and prevalence and prognosis of elevated pulmonary artery systolic pressure in selected large cohort studies of echocardiography and right heart catheterization in community-based populations
| Authors | Population | n | Age (Mean ± SD or median (IQR)) Sex (%Female) |
Modality | Mean or median PASP or RVSP in mmHg (SD or IQR): |
Prevalence of elevated PASP and prognosis |
|---|---|---|---|---|---|---|
| Brittain E et al (2017)26 | Middle-aged, community-based biracial study cohort with measurable TR jet | n=1311 | 51 (47, 53) yrs 49% female | Echocardiogram | 31 mm Hg (27, 34)* (median 26mmHg assuming RAP 5mm Hg) | 50·0% PASP ≥ 31 mm Hg 6·6% PASP > 40 mm Hg |
| Brittain E et al (2018)27 | HIV-infected and HIV-uninfected Veterans (matched 1:2) enrolled on or after April 1st, 2003 in prospective longitudinal study with PASP measured on echocardiogram | HIV-infected n=2,831 HIV-uninfected n=5,465 |
HIV-infected, no PH: 56 yrs, 2.7% female HIV-infected, PH: 57 years, 1.9% female HIV-uninfected, no PH: 57 yrs, 3.8% female HIV-uninfected, no PH: 58 years, 1.1% female |
Echocardiogram | 36 ± 15 mm Hg (HIV-infected) 36 ± 14 mm Hg (HIV-uninfected) |
28% (HIV-infected) and 27% (HIV-uninfected) PASP > 40 mm Hg Adjusted hazardof mortality 40% higher for PASP 30 mm Hg compared to PASP 15 mm Hg (HIV-infected) Adjusted hazard of mortality 21% higher for PASP 30 mm Hg compared to PASP 15 mm Hg (HIV-uninfected) |
| Choudhary G et al (2013)6 | Community-based, African-American | n=3,282 | 56·1 ± 12.6 yrs 67·5% female | Echocardiogram | 27 (18, 36) ** | 35% PASP > 30 mm Hg 6.8% PASP ≥ 40 mm Hg |
| Lam CS et al (2009)5 | Community-based, white | n=1413 778 without cardiopulmonary disease | 63 ± 11 yrs 57% female | Echocardiogram | 26 (24, 30) ** | 25% >30mmHg Adjusted hazard ratio for mortality 1·46 (overall study population) and 2·74 (subpopulation without cardiopulmonary disease) per 10 mm Hg increase in PASP |
| Leibowitz D et al (2014)28 | Randomly selected subjects 85 year-old subjects | n=300 with measurable TR jet | 85 yrs. 42% female | Echocardiogram | 30·5 ± 9·4 (Median 28·3: IQR 24, 34·9) | 25% PASP ≥ 35 mm Hg Five-year survival significantly worse for PASP > 30 mm Hg |
| Moreira EM et al (2015)29 | Sub-study embedded in a population-based prospective cohort study of middle-aged and older adults | n=2,823 | 76·4 ± 6.2 yrs 59% female | Echocardiogram | 26·3 ± 7·0† (Median 25·3) | ≈25% PASP > 30 mm Hg |
| Teramoto K et al (2020)30 | Adults > 65 years Older free of Prevalent heart failure and with measurable tricuspid regurgitation participating in community-based prospective longitudinal study | n=2,810 | 76·2 ± 5·2 yrs 66% female | Echocardiogram | 28 ± 5 mm Hg** | 18·3% PASP > 32 mm Hg Hazard ratio for heart failure or death 1·55 (95% CI 1·24 – 1·93) with PASP > 32 mm Hg |
HIV: Human immunodeficiency virus; IQR: Interquartile range; LV: Left ventricular; PASP: pulmonary artery systolic pressure; RAP: right atrial pressure; RVSP: right ventricular systolic pressure; SD: standard deviation; TR: tricuspid regurgitant; Yrs: years
Assuming a right atrial pressure of 10 mm Hg
Assuming a right atrial pressure of 5 mm Hg
Derived from TR velocity measurements
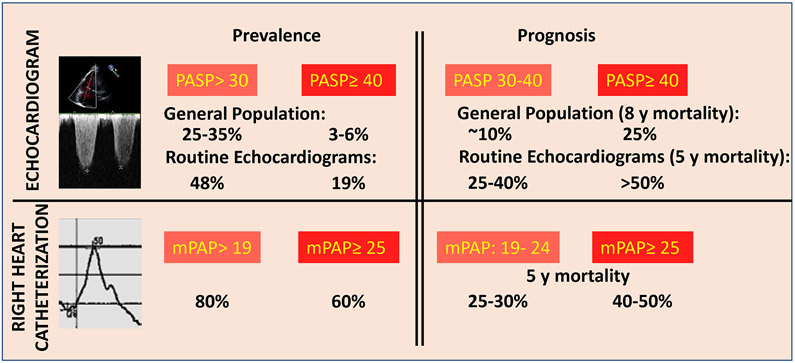
Elevated ePASP > 30 mm Hg is present in over 40% of clinical echocardiograms performed12,13 and in 25% of the adult population on echocardiograms performed in population-based samples5,6. Strikingly, mortality risk rises substantially with an ePASP > 30 mm Hg, confirming the narrow upper bound of the healthy range of pulmonary artery pressures. Five-year mortality in patients with an ePASP of 30-32 mm Hg on clinical echocardiograms is 28·9%, 66% higher than those with a PASP 28-30 mm Hg and 2·5-fold higher than those with an ePASP < 22 mm Hg13. One-year mortality in patients with an ePASP of 30-32 mm Hg is 10·1% 13. In studies conducted in the general population, the risk of mortality rises by about 40% with every 10 mm Hg increase in the PA systolic pressure14 and is notably higher with ePASP ≥28 mm Hg compared to those with lower ePASP5. This results in a 9-10% percent eight-year mortality in the general population with an ePASP of 30-32 mm Hg5, and a 25% ten-year mortality with ePASP ≥40 mm Hg14 . The poor prognosis associated with elevated ePASP on echo is similar to that associated with mean PA pressure on right heart catheterization, where the five-year mortality is about 25% in patients with mean PA pressure between 19-24 mm Hg, and about 40% in patients with mean PA pressure >25 mm Hg2 (Figure 1). Similar to systemic blood pressure, PASP increases with age, related to increased stiffness of the pulmonary vasculature5. While PASP has a modest and significant corelation with age (r value: 0.19-0.48) 5,15-19, elevated PASP cannot be considered benign since, even after adjustment for age, an elevated PASP remains associated with poor outcomes 5,6. Therefore, based on available data, a resting mean PA pressure < 20mmHg or PA systolic pressure < 30mmHg appear to be a healthy (or optimal) pressure in the pulmonary circulation and elevation in ePASP is a common and high-risk finding on echocardiogram.
Figure 1:
Prevalence and prognosis of elevated pulmonary artery pressures by echocardiograms performed in either community based5,6 or referral13 population and right heart catheterizations performed in referral populations2,3. The echocardiograms and right heart catheterizations in the referral populations were clinically indicated. The mortality rates are unadjusted for age or co-morbidities. PASP: Pulmonary Artery Systolic Pressure; mPAP: Mean Pulmonary Artery Pressure; y: year
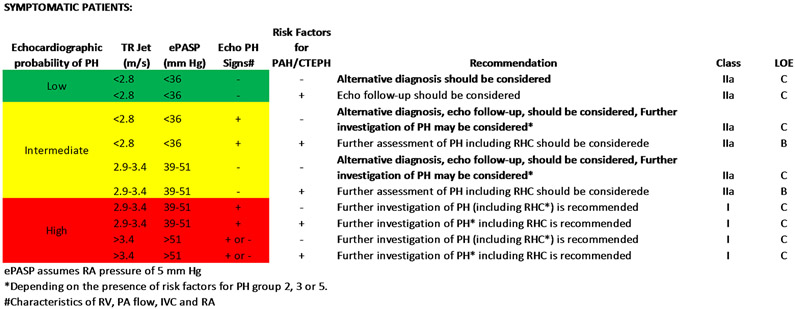
In the contemporary PH guidelines, in symptomatic patients, tricuspid regurgitant velocity measured on echocardiogram, along with other clinical characteristics (Figure 2), plays an important role in the algorithm targeted towards identification of (Group 1 PH) pulmonary arterial hypertension (PAH) and (Group 4) chronic thromboembolic pulmonary hypertension (CTEPH), the two groups of PH patients for which there are well-proven therapeutic interventions.4 While this diagnostic strategy is focused on identifying patients with PAH and CTEPH, a significant gap in recommendations exists for the management of patients with elevated ePASP who do not fall into these distinct diagnostic groups. This is particularly true of symptomatic patients with mildly to moderately elevated ePASP on echocardiogram (PASP 30-50 mm Hg) without clear risk factors or conditions associated with PAH or CTEPH and with obesity, metabolic, and cardiopulmonary co-morbidities. Therefore, patients with mildly elevated ePASP are often continued on non-PH focused management without a thorough consideration of the implication of this high-risk feature. The reasons for the missed opportunity to mitigate risk more aggressively in these vulnerable patients are likely lack of recognition of the poor prognosis associated with even mildly elevated PASP and an absence of well-defined strategies to manage these patients and improve outcomes.
Figure 2:
Summation of the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines on suggested assessment and management of symptomatic patients with suspected pulmonary hypertension defined as mean PA pressure ≥ 25mmHg. Figure adapted from Table 8A and 9 of the 2015 ESC/ERS guidelines.4
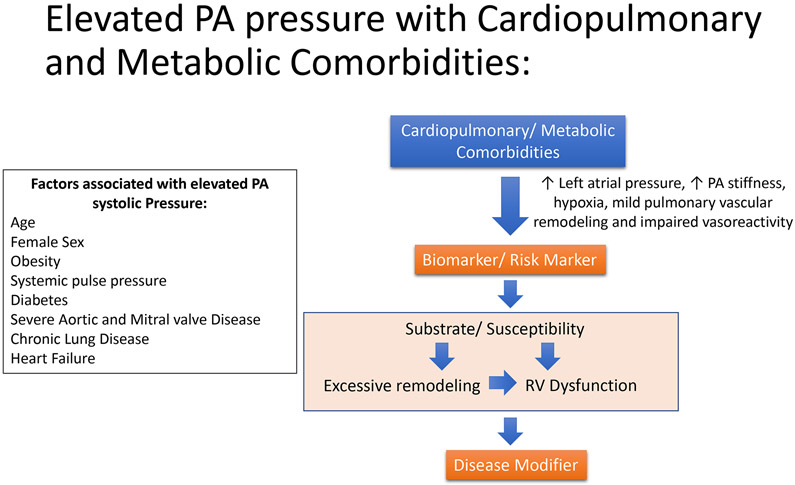
A consideration of the pathophysiological basis for the poor outcomes associated with elevated PA pressures can inform the development of appropriate strategies for risk reduction. In select groups of PH patients, such as those with PAH and Group 4 PH, elevation of PA pressure is primarily caused by underlying pulmonary vascular disease with a clear pathophysiological link between the primary disease and consequent morbidity and mortality. Not surprisingly, these patients benefit from pulmonary vasoactive medications and other interventions specifically aimed at the underlying pulmonary vascular disease. However, since these conditions are rare, in the vast majority of individuals, elevated PA pressures are likely a consequence of increased left atrial pressure, increase in PA stiffness, and pulmonary vascular remodeling and impaired vasoreactivity in the setting of cardiopulmonary and metabolic disorders, perhaps acting in combination in any given patient. As demonstrated by Wolsk et. al. fluid infusion resulting in an acute increase in pulmonary capillary wedge pressure, a surrogate for left atrial pressure, can increase PASP even in healthy individuals 19. About 22% and 78% of patients have elevated pulmonary capillary wedge pressures (>15 mm Hg) in settings of mean PAP 19-24 mm Hg and mean PAP >25 mm Hg, respectively2,3. Therefore, increased left atrial pressure, frequently resulting from LV diastolic dysfunction that is known to be associated with cardiopulmonary and metabolic diseases, is an important reason for elevated PA pressure. Increasing age also corelates with elevations in PA systolic pressure due to pathophysiological mechanisms such as an increased incidence of diastolic dysfunction and increase in PA stiffness. Age, gender, cardiopulmonary and metabolic comorbidities have been independently associated with increased prevalence of elevated ePASP6, and it is common to have multiple cardiopulmonary comorbidities present in the same patient. Yet, at the same time, elevated PA pressure is associated with poor outcomes independent of age and co-morbidities14. These contrasting observations can be reconciled if we consider elevation of PA pressure as both a risk marker on its own and as a risk modifier in the presence of co-morbidities, wherein an elevation of pulmonary vascular resistance with consequent right heart dysfunction adds to the burden of the primary disease (Figure 3). Indeed, even mildly elevated PA pressures are associated with evidence of right ventricular dysfunction that is associated with poor outcomes in the presence or absence of comorbid conditions12.
Figure 3:
Relationship of comorbidities and risk factors with elevated pulmonary artery systolic pressure. In the context of a patient with cardiopulmonary and metabolic comorbidities, elevated pulmonary artery systolic pressure may serve as both a biomarker and disease modifying factor.
PH guidelines already provide algorithms for appropriate and timely referral to specialized PH centers for patients with severe pulmonary vascular disease. However, due to differences in the underlying pathophysiology outlined above, the majority of patients with elevated PASP will not benefit from pulmonary vasoactive medications but will require more multidimensional management strategies. While echocardiographic ePASP measurement has moderate precision compared to right heart catheterization 20, an elevated ePASP serves as a marker to identify a population of patients in need of careful clinical assessment and more intensive risk factor modification. We believe that it is time to develop risk-reduction programs for patients with elevated PASP that will be widely applicable to these at-risk patients and that need not be confined to PH centers. Disease management and risk-reduction approaches are commonly used in other high-risk conditions, such as preventive cardiology clinics for coronary artery disease management and metabolic clinics for diabetes. Accumulating evidence has already identified risk factors for elevated ePASP (Figure 3); these include comorbidities with well-defined management approaches but which cross multiple specialties (cardiology, pulmonary, endocrine), resulting in fragmentation of care and lost opportunities for an integrative approach to management.
There is a strong rationale to evaluate and aggressively manage patients with elevated PA pressures using the key principles outlined in Figure 4. One model for care in these patients is a multidisciplinary PH clinic to evaluate patients with elevated ePASP and, in those without identifiable underlying severe pulmonary vascular disease, manage their cardiopulmonary and metabolic risk factors in a coordinated manner. Experience with such a clinic at a non-tertiary care hospital has shown that an appropriate PH work-up can be completed in the majority of patients, and the most common interventions, such as optimizing diuretic doses for volume overload and optimizing oxygen requirements and treatment for sleep disordered breathing,21 may be associated with reduced hospitalizations in this population. Such a strategy may also reduce the inappropriate use of pulmonary vasodilator medications in those patients with elevated PA pressures lacking a clear indication for their use, such as those with group 2 pulmonary hypertension. These clinics could also serve as platforms for registries and clinical trials that could be developed to accumulate evidence in support of specific risk reduction approaches for elevated PASP. This model would allow for better capture of the sizeable population of at-risk patients that are overlooked at present22. It may also afford the opportunity for both earlier diagnosis of patients with pulmonary vascular disease and, through early intervention and risk factor management, the prevention of progression of mildly elevated pulmonary artery pressures to severe PH.
Figure 4:

Principles for management of patients with elevated pulmonary artery (PA) systolic pressure based on potentially modifiable pathophysiologic factors
In summary, current data shows that patients with a PASP > 30 mm Hg are at risk for increased morbidity and mortality, which may potentially relate to underlying multimorbidity, the elevated pressure within the pulmonary circulation itself, and its consequent effects on the heart. While we do not advocate for screening general population for elevated PASP, this is a common high-risk finding on clinical echocardiograms that often receives inadequate attention. Clinicians should be aware of the adverse prognosis associated with elevated PASP, identify those with suspected group 1 and group 4 PH for specialty referral, and address treatable and/or modifiable factors such as manifest volume overload, systemic hypertension, hypoxia, diabetes, obesity, and obstructive sleep apnea in those who are not appropriate for referral. Given the complexity of this evaluation and management strategy, a multidisciplinary approach to evaluate and manage these patients would, however, be ideal. Finally, there is a crucial need for more data to improve our understanding of the problem of elevated PASP at a population level and to determine the most appropriate screening and risk-reduction strategies.
Key Message:
Estimated pulmonary artery systolic pressure (ePASP) > 30 mm Hg is outside the normal range and is reported in about 40% of clinically indicated echocardiograms
Elevated ePASP is associated with 25-40% 5-year mortality and is frequently associated with cardiopulmonary and metabolic co-morbidities
Current pulmonary hypertension (PH) guidelines outline assessment and referral approaches to identify Group 1 and Group 4 PH, which are relatively rare diseases, but management of the large number of patients with elevated ePASP associated with cardiopulmonary and metabolic co-morbidities is not well addressed.
We propose an approach of thorough multidisciplinary assessment and aggressive risk factor management to mitigate risk in this high-risk population.
Acknowledgements:
R01HL139613-01, R01HL1535-02, U54HL119145, R21HL145420; Cardiovascular Medical Research Education Foundation (CMREF), and McKenzie Family Charitable Trust, Boston Biomedical Innovations Center to BAM. VA CSR&D grant I01CX001892, and NHLBI R01HL148727 to GC. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Search Strategy:
For Healthy Subjects studies:
Search: (pulmonary artery systolic pressure) AND (echocardiography) AND (healthy): 124 results
Search: (pulmonary artery catheter) OR (right heart catheter) AND (healthy) from 2008 – 2020 (i.e. since Kovacs review in 2009): 155 results
Healthy subjects were as defined in the individual studies but generally excluded persons with known or suspected cardiopulmonary diseases
For Community studies:
(echocardiography) AND (pulmonary artery pressure) AND (community)
(echocardiography) AND (pulmonary artery pressure) AND (population-based)
(echocardiography) AND (pulmonary artery pressure) AND (cross-sectional)
(echocardiography) AND (pulmonary artery pressure) AND (general population)
(echocardiography) AND (pulmonary artery pressure) AND (prospective longitudinal)
Community studies included persons recruited from particular community settings as noted, who may or may not have cardiopulmonary or other diseases prevalent in the given community.
References were reviewed and other studies known to the authors were also included.
Studies included in systematic reviews were not reduplicated.
Conflict of interest statement: Dr. Maron reports grants and personal fees from Actellion, outside the submitted work; In addition, Dr. Maron has a patent US Patent 9,605,047 issued, a patent US Patent PCT/US2019/059890 pending, a patent Provisional 62475955 pending, and a patent Provisional 029672 pending.
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. The European respiratory journal 2019; 53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maron BA, Hess E, Maddox TM, et al. Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016; 133(13): 1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assad TR, Maron BA, Robbins IM, et al. Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA cardiology 2017; 2(12): 1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). The European respiratory journal 2015; 46(4): 903–75. [DOI] [PubMed] [Google Scholar]
- 5.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009; 119(20): 2663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhary G, Jankowich M, Wu WC. Prevalence and clinical characteristics associated with pulmonary hypertension in African-Americans. PloS one 2013; 8(12): e84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. The European respiratory journal 2009; 34(4): 888–94. [DOI] [PubMed] [Google Scholar]
- 8.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 2004; 126(4): 1313–7. [DOI] [PubMed] [Google Scholar]
- 9.Syyed R, Reeves JT, Welsh D, Raeside D, Johnson MK, Peacock AJ. The relationship between the components of pulmonary artery pressure remains constant under all conditions in both health and disease. Chest 2008; 133(3): 633–9. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary JM, Assad TR, Xu M, et al. Lack of a Tricuspid Regurgitation Doppler Signal and Pulmonary Hypertension by Invasive Measurement. Journal of the American Heart Association 2018; 7(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2010; 23(7): 685–713; quiz 86-8. [DOI] [PubMed] [Google Scholar]
- 12.Huston JH, Maron BA, French J, et al. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA cardiology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strange G, Stewart S, Celermajer DS, et al. Threshold of Pulmonary Hypertension Associated With Increased Mortality. Journal of the American College of Cardiology 2019; 73(21): 2660–72. [DOI] [PubMed] [Google Scholar]
- 14.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in African Americans: Jackson Heart Study. Circulation Heart failure 2014; 7(4): 558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Andrea A, Naeije R, D'Alto M, et al. Range in pulmonary artery systolic pressure among highly trained athletes. Chest 2011; 139(4): 788–94. [DOI] [PubMed] [Google Scholar]
- 16.D'Andrea A, Naeije R, Grunig E, et al. Echocardiography of the pulmonary circulation and right ventricular function: exploring the physiologic spectrum in 1,480 normal subjects. Chest 2014; 145(5): 1071–8. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara F, Rudski LG, Vriz O, et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. International journal of cardiology 2016; 223: 736–43. [DOI] [PubMed] [Google Scholar]
- 18.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104(23): 2797–802. [DOI] [PubMed] [Google Scholar]
- 19.Wolsk E, Bakkestrom R, Kristensen CB, et al. Right Ventricular and Pulmonary Vascular Function are Influenced by Age and Volume Expansion in Healthy Humans. Journal of cardiac failure 2019; 25(1): 51–9. [DOI] [PubMed] [Google Scholar]
- 20.D'Alto M, Romeo E, Argiento P, et al. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. International journal of cardiology 2013; 168(4): 4058–62. [DOI] [PubMed] [Google Scholar]
- 21.Jankowich M, Hebel R, Jantz J, Abbasi S, Choudhary G. Multispecialty pulmonary hypertension clinic in the VA. Pulmonary circulation 2017; 7(4): 758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maron BA, Choudhary G, Khan UA, et al. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circulation Heart failure 2013; 6(5): 906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Andrea A, Radmilovic J, Carbone A, et al. Speckle tracking evaluation in endurance athletes: the "optimal" myocardial work. Int J Cardiovasc Imaging 2020; 36(9): 1679–88. [DOI] [PubMed] [Google Scholar]
- 24.Soria R, Egger M, Scherrer U, Bender N, Rimoldi SF. Pulmonary artery pressure and arterial oxygen saturation in people living at high or low altitude: systematic review and metaanalysis. Journal of applied physiology (Bethesda, Md : 1985) 2016; 121(5): 1151–9. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Liu C, Yu S, et al. Pulmonary function tests at low altitude predict pulmonary pressure response to short-term high altitude exposure. Respir Physiol Neurobiol 2020; 282: 103534. [DOI] [PubMed] [Google Scholar]
- 26.Brittain EL, Nwabuo C, Xu M, et al. Echocardiographic Pulmonary Artery Systolic Pressure in the Coronary Artery Risk Development in Young Adults (CARDIA) Study: Associations With Race and Metabolic Dysregulation. Journal of the American Heart Association 2017; 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brittain EL, Duncan MS, Chang J, et al. Increased Echocardiographic Pulmonary Pressure in HIV-infected and -uninfected Individuals in the Veterans Aging Cohort Study. Am J Respir Crit Care Med 2018; 197(7): 923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibowitz D, Gilon D, Jacobs JM, Stessman-Lande I, Stessman J. Pulmonary artery systolic pressure and mortality in the oldest old. Cardiology 2014; 129(2): 111–6. [DOI] [PubMed] [Google Scholar]
- 29.Moreira EM, Gall H, Leening MJ, et al. Prevalence of Pulmonary Hypertension in the General Population: The Rotterdam Study. PloS one 2015; 10(6): e0130072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teramoto K, Santos M, Claggett B, et al. Pulmonary vascular dysfunction among people aged over 65 years in the community in the Atherosclerosis Risk In Communities (ARIC) Study: A cross-sectional analysis. PLoS Med 2020; 17(10): e1003361. [DOI] [PMC free article] [PubMed] [Google Scholar]