Abstract
The catechin epigallocatechin gallate showed the strongest activity of the six tea catechins tested against Helicobacter pylori (MIC for 50% of the strains tested, 8 μg/ml). It had bactericidal activity at pH 7 but not at pH ≤5.0. In infected Mongolian gerbils, H. pylori was eradicated in 10 to 36% of the catechin-treated animals, with significant decreases in mucosal hemorrhage and erosion. Tea catechins, therefore, may have therapeutic effects on H. pylori infection.
The association between Helicobacter pylori infection and upper gastrointestinal diseases such as chronic gastritis, peptic ulceration, and gastric cancer has been widely investigated (13, 22, 25). H. pylori is sensitive to various antibiotics in vitro (5, 11). However, clinical trials with such an antibacterial agent alone have mostly failed to eradicate H. pylori (3, 17). Although the new triple therapy consisting of the combined use of two antibiotics and a proton pump inhibitor suppressing acid secretion shows a high eradication rate and a low incidence of harmful side effects (2, 19), some problems remain. In recent years, for instance, an increased occurrence of metronidazole- and/or clarithromycin-resistant strains of H. pylori has become a problem (12, 15, 18). This problem might be amplified in Asia and Africa, where many people are infected with H. pylori (1, 6, 16). Even if the widespread use of antibiotics were feasible economically and logistically in these countries, it would most certainly lead to increased resistance of not only H. pylori but also other pathogenic bacteria. Therefore, a nonantibiotic agent which is both highly effective and safe might be of utmost importance for the eradication of both antibiotic-susceptible and -resistant strains of H. pylori.
Recent studies have presented data that show a variety of biological activities of tea catechins, compounds which constitute about 15% (dry weight) of green tea (7). It has been reported that tea catechins have antibacterial activity against various foodborne pathogenic bacteria (8). Thus, it seems reasonable to explore the possibility of using tea catechins, harmless compounds extracted from green tea, for eradication of H. pylori. In this study, we investigated the antibacterial activity of catechins against H. pylori in vitro and in vivo and the in vivo effect of these compounds on the gastric mucosal injury induced by this organism in Mongolian gerbils.
Bacterial strains.
Two standard strains (ATCC 43504 and ATCC 43629) and 108 clinical isolates (YMA1 to YMA108) of H. pylori were used. The clinical isolates were obtained from gastric biopsy specimens from patients with gastritis and peptic ulcer, and their identification was based on standard biochemical tests (14). Stock cultures were stored at −80°C in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with 10% heat-inactivated horse serum (Nacalai Tesque, Kyoto, Japan).
Catechins.
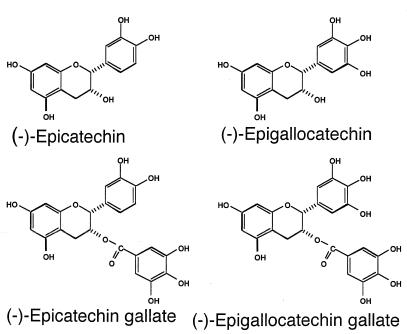
Epigallocatechin gallate (EGCg), epicatechin gallate, epigallocatechin, epicatechin, crude catechin (Polyphenon 70SR), and crude theaflavin were from Mitsui Norin (Shizuoka, Japan). Each was more than 98% pure, excluding the crude compounds. The structural formulas of tea catechins are shown in Fig. 1. Polyphenon 70SR contains a total of 73.4% catechins, including EGCg (33.5%), epicatechin gallate (10.1%), epigallocatechin (17.4%), and epicatechin (8.6%). Theaflavins are made up of the dimers of catechins which are produced during the manufacture of black tea.
FIG. 1.
Chemical structures of tea catechins.
In vitro studies.
MICs were determined by a broth microdilution method with Mueller-Hinton broth supplemented with 10% heat-inactivated horse serum and an inoculum of 5 × 104 CFU per well. Broth microdilution plates were prepared in house and stored at −80°C until use. After bacterial inoculation, plates were incubated for 72 h and then MICs were determined.
Bactericidal actions were determined by using an in vitro killing assay. A bacterial suspension of an H. pylori clinical isolate, YMA78 (100 μl, 107 CFU) was inoculated into 10 ml of culture medium containing EGCg concentrations equal to 0, 0.25, 1, 2, and 4 times the MIC (32 μg/ml) and incubated for 48 h at 37°C in an atmosphere of 5% O2–10% CO2–85% N2 with reciprocation. Samples (100 μl) were taken 0, 3, 6, 12, 24, and 48 h after the start of incubation for viable-cell counting. Viability was measured by the plate colony count technique. Colonies were counted after 5 days of incubation. Effects of pH on the antibacterial activity of EGCg were assessed with time-kill curves at pH values of 7.0 (67 mM Sorensen phosphate buffer), 5.0, and 4.0 (30 mM citrate buffer) by using the method reported previously (23). The final concentrations of EGCg were 0, 250, and 500 μg/ml. The cultures were incubated at 37°C for 80 min with reciprocation. Samples (100 μl) were taken 0, 20, 40, and 80 min after the start of incubation for viable-cell counting.
Animals and inoculation with H. pylori.
Seven-week-old, specific-pathogen-free, male Mongolian gerbils (MGS/sea; body weight, 40 to 50 g) purchased from Seac Yoshitomi (Fukuoka, Japan) were used in this study. The gerbils were housed in animal facilities, fed a sterilized commercial rodent diet (CE-2; Japan CLEA, Tokyo, Japan), and allowed free access to sterilized distilled water. Each animal was fasted for 24 h and inoculated orally with a suspension of H. pylori ATCC 43504 (500 μl, 108 CFU) by using a feeding needle (9). After inoculation, each animal was kept without food and water for 4 h.
Evaluation of catechins in vivo. (i) Experiment A.
Gerbils were randomly divided into four groups of 9 or 10 after bacterial inoculation. Four weeks after bacterial inoculation, each group of gerbils was fed a different diet (i.e., containing 0, 0.5, 1, or 2% catechins) for 2 weeks. These diets and water were provided ad libitum throughout the test period. After the animals were fasted for 24 h, their stomachs were excised and cut along the greater curvature for macroscopic observation. The stomachs were then homogenized in 10 ml of saline. A 100-μl aliquot each of serial dilutions of the homogenate was spread on an M-BHM PYLORI AGAR plate (Nikken Bio Medical Laboratory, Kyoto, Japan). The plates were incubated for 5 days, and the colonies were counted.
(ii) Experiment B.
Gerbils were randomly divided into three groups of 10 or 11 after bacterial inoculation. Six weeks after inoculation, the gerbils were treated for 2 weeks as follows: (i) control without catechins, (ii) 1% catechin-containing diet, and (iii) 1% catechin-containing diet plus 0.5% catechin-containing water. After the gerbils were fasted for 24 h, their stomachs were excised and homogenized. Colonies were counted as described above.
Statistical analysis.
A one-way analysis of variance (ANOVA), Scheffe’s test, and the Kruskal-Wallis test were used. The level of significance selected was P < 0.05.
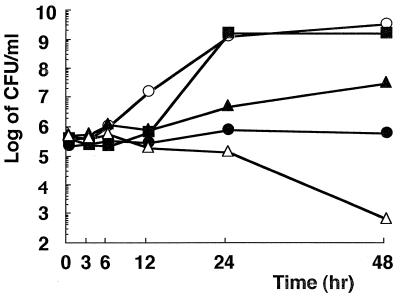
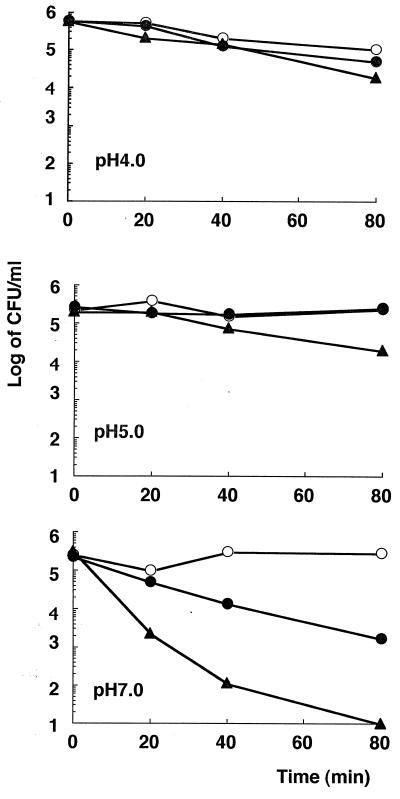
The MICs of six catechins against H. pylori isolates are presented in Table 1. All of the catechins showed activity against H. pylori, and EGCg showed the strongest activity of all of the catechins tested. The bactericidal action of EGCg was also examined. Figure 2 shows representative data obtained with strain YMA78 exposed to 8, 32 (MIC), 64, and 128 μg of EGCg per ml. Bacteriostatic and bactericidal effects were observed at concentrations equal to 2 and 4 times the MIC, respectively. Although the mechanism of this action is still obscure, structure-activity relationship studies indicated that the antibacterial activities of catechins were predominantly related to the gallic acid moiety and the number of hydroxyl groups (10; Fig. 1). It has also been reported that catechins damage the membrane lipid bilayer (10). Catechins probably damage the membrane of H. pylori, but further aspects of their effect, such as morphological changes, should be tested. Moreover, EGCg inhibits the urease activity and motility of H. pylori (data not shown), which may contribute to its antibacterial activity in vivo. As shown in Fig. 3, H. pylori YMA78 survived for 80 min in all of the buffers used at pHs 4.0 to 7.0. EGCg showed a dose-dependent bactericidal action at pH 7.0 but only a weak effect at pHs 4.0 and 5.0, indicating that the bactericidal action of EGCg against H. pylori was pH dependent. The activity of antibiotics such as clarithromycin decreases under acidic conditions (4). These data suggest that the bactericidal action of EGCg is weakened by acidic conditions.
TABLE 1.
Susceptibility of H. pylori isolates to tea catechins
| Druga | No. of strains | MIC(s) (μg/ml)b
|
||
|---|---|---|---|---|
| Range | For 50% of isolates | For 90% of isolates | ||
| Polyphenon 70SR | 110 | 2–128 | 32 | 64 |
| EGCg | 110 | 1–64 | 8 | 32 |
| ECg | 55 | 1–256 | 16 | 32 |
| EGC | 55 | 8–256 | 64 | 128 |
| EC | 55 | 16–1,024 | 256 | 512 |
| Crude theaflavin | 55 | 2–128 | 32 | 64 |
ECg (−)-epicatechin gallate; EGC, (−)-epigallocatechin; EC, (−)-epicatechin.
MICs were determined by the broth microdilution method in Mueller-Hinton broth supplemented with 10% heat-inactivated horse serum. Test strains were inoculated at 5 × 104 CFU per well of the microtiter plate.
FIG. 2.
Effect of EGCg on H. pylori viability in liquid medium. H. pylori YMA78 was cultured microaerobically in Mueller-Hinton broth supplemented with 10% heat-inactivated horse serum at 37°C with reciprocation in the presence of EGCg at concentrations of 128 (▵), 64 (●), 32 (▴), 8 (■), and 0 (○) μg/ml. Culture samples (100 μl) were taken at the times indicated, and viability was measured by the plate colony count technique.
FIG. 3.
Effect of EGCg on H. pylori viability in buffers at various pHs. H. pylori YMA78 was incubated microaerobically in each buffer at 37°C with reciprocation in the presence of EGCg at concentrations of 500 (▴), 250 (●), and 0 (○) μg/ml. Viability was determined at each time point indicated.
The effect of catechin on H. pylori colonization in vivo is shown in Table 2. In experiment A, H. pylori was eradicated in about 10% of the gerbils in each of the catechin-fed groups whereas H. pylori was detected in all of the control gerbils. When a one-way ANOVA was performed to compare the number of H. pylori cells in the stomachs of the gerbils (excluding those in which the bacteria had been eradicated), significant differences among the four groups were found. However, on comparison of each group with the control by using Scheffe’s test, only the 0.5% catechin-fed group showed a significant decrease. In experiment B, H. pylori was eradicated in 10% of the gerbils in the 1% catechin-containing diet group (catechin at 70 mg/head/day) and 36.4% of the gerbils given the 1% catechin-containing diet plus 0.5% catechin-containing water (catechin at 100 mg/head/day) while H. pylori was detected in all of the control animals. Excluding the gerbils in which the bacteria had been eradicated, a one-way ANOVA was used to compare the numbers of H. pylori cells in the stomachs and no significant differences among the three groups were found. In our infection model, tea catechins showed an antibacterial effect but only at a low eradication rate of 10 to 36.4%. Similarly, eradication therapies using single antibiotics also result in failure in humans despite their in vitro efficacy (3, 17). The pH dependency of antibacterial activity may be one of the factors producing such low eradication rates. Combinations of catechins with a proton pump inhibitor which neutralizes the acidity in the stomach might be effective, as in the new triple therapy.
TABLE 2.
In vivo effect of catechin on H. pylori colonization
| Expt and group | Bacterial count (log CFU/stomach)a | Clearance rateb (%) |
|---|---|---|
| A | ||
| Control | 5.54 ± 0.59 | 0/9 (0) |
| 0.5% Catechin diet | 4.38 ± 0.51c | 1/10 (10) |
| 1.0% Catechin diet | 4.89 ± 0.59 | 1/9 (11) |
| 2.0% Catechin diet | 5.05 ± 0.73 | 1/9 (11) |
| B | ||
| Control | 5.12 ± 0.29 | 0/11 (0) |
| 1.0% Catechin diet | 4.77 ± 0.47 | 1/10 (10) |
| 1.0% Catechin diet + 0.5% catechin water | 5.04 ± 0.26 | 4/11 (36) |
The bacterial counts of each group (excluding the gerbils in which the bacteria had been eradicated) were added, and the average was calculated. The results are expressed as the mean ± the standard deviation. A one-way ANOVA and Scheffe’s test were used for statistical analysis.
The clearance rate shows the number of gerbils in which H. pylori was not detected in the gastric mucosa.
Statistically significant reduction (P < 0.01) compared with the control.
Another reason why catechins are not significantly effective in the eradication of H. pylori might be the short gastric-transit time of these agents. The effect on eradication was enhanced when the catechins were administrated in both the diet and drinking water, while no additional effect was obtained with a higher-dose catechin diet. Thus, further studies on efficacy may be warranted in which catechins are combined with a proton pump inhibitor and a drug delivery system which will prolong the gastric-transit time is used. Macroscopic findings on the gastric mucosa of the H. pylori-infected gerbils in the first in vivo experiment are shown in Table 3. At 6 weeks after H. pylori infection, notable changes in the antral and fundic mucosa, such as hemorrhages and erosions, were observed in all control animals. The hemorrhage scores and the scores of injury to the gastric mucosa were significantly decreased (P < 0.01 versus the control) in all of the catechin-fed groups, although the bacteria were not eradicated in most of the animals tested. This decrease may have derived primarily from the antibacterial and antiurease effects of tea catechins, although it is possible that other actions for which catechins are known, such as antioxidative (21) or anti-inflammatory (24) effects or inhibition of gastric acid secretion (20), also contribute, in part, to this efficacy.
TABLE 3.
Effect of catechin on gastric mucosal injury
| Treatment | Mean scorea ± SD
|
|
|---|---|---|
| Hemorrhageb | Gastric mucosal injuryc | |
| Control | 3.1 ± 0.28 | 3.2 ± 0.29 |
| 0.5% Catechin diet | 1.5 ± 0.34c | 1.0 ± 0.33c |
| 1.0% Catechin diet | 1.3 ± 0.41c | 1.1 ± 0.46c |
| 2.0% Catechin diet | 1.3 ± 0.33c | 1.0 ± 0.37c |
The stomachs of the gerbils in experiment A in Table 2 were used, and the Kruskal-Wallis test was performed.
Hemorrhage of the gastric mucosa was scored as follows: 0, no bleeding; 1, a small bleeding spot; 2, multiple small bleeding spots; 3, a bleeding area; 4, multiple bleeding areas.
Injury to the gastric mucosa was scored as follows: 0, normal; 1, edematous; 2, erosion; 3, multiple erosions; 4, ulcers. Statistically significant reduction (P < 0.01) versus the control.
In conclusion, tea catechins have an antibacterial effect against H. pylori and may have a therapeutic effect against gastric mucosal injury induced by this organism. A new, safe, and effective therapeutic regimen against H. pylori infection may be contrived by the use of catechins combined with a proton pump inhibitor, possibly in a delivery system which prolongs the gastric-transit time of catechins.
Acknowledgments
We thank Y. Hara (Food Research Institute, Mitsui Norin Co., Ltd.) for kindly providing catechins and T. Ishigami (Food Research Institute, Mitsui Norin Co., Ltd.) for his skillful technical collaboration.
This study was partly supported by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences in Japan.
REFERENCES
- 1.Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham D Y. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760–766. doi: 10.1016/0016-5085(92)90156-s. [DOI] [PubMed] [Google Scholar]
- 2.Axon A T R, Moayyedi P. Eradication of Helicobacter pylori: omeprazole in combination with antibiotics. Scand J Gastroenterol. 1996;31(Suppl. 215):82–89. [PubMed] [Google Scholar]
- 3.Chiba N, Rao B V, Rademaker J W, Hunt R H. Meta-analysis of the efficacy of antibiotic therapy in eradicating Helicobacter pylori. Am J Gastroenterol. 1992;87:1716–1727. [PubMed] [Google Scholar]
- 4.Debets-Ossenkopp Y J, Namavar F, MacLaren D M. Effects of an acidic environment on the susceptibility of Helicobacter pylori to trospectomycin and other antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1995;14:353–355. doi: 10.1007/BF02116532. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin C S, Blake P, Blincow E. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pyloridis. J Antimicrob Chemother. 1986;17:309–314. doi: 10.1093/jac/17.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Graham D Y, Adam E, Reddy G T, Agarwal J P, Agarwal R, Evans D J, Jr, Malaty H M, Evans D G. Seroepidemiology of Helicobacter pylori infection in India: comparison of developing and developed countries. Dig Dis Sci. 1991;36:1084–1088. doi: 10.1007/BF01297451. [DOI] [PubMed] [Google Scholar]
- 7.Hara Y. Antioxidants in tea and their physiological functions. In: Hiramatsu M, et al., editors. Food and free radicals. New York, N.Y: Plenum Press; 1997. pp. 49–65. [Google Scholar]
- 8.Hara Y, Ishigami T. Antibacterial activities of tea polyphenols against foodborne pathogenic bacteria. J Jpn Soc Food Sci Technol. 1989;36:996–999. [Google Scholar]
- 9.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31(Suppl. 9):24–28. [PubMed] [Google Scholar]
- 10.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 11.Lambert T, Megraud F, Gerbaud G, Courvalin P. Susceptibility of Campylobacter pyloridis to 20 antimicrobial agents. Antimicrob Agents Chemother. 1986;30:510–511. doi: 10.1128/aac.30.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling T K W, Cheng A F B, Sung J J Y, Yiu P Y L, Chung S S C. An increase in Helicobacter pylori strains resistance to metronidazole: a five-year study. Helicobacter. 1996;1:57–61. doi: 10.1111/j.1523-5378.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 14.Marshall B J, Royce H, Annear D I, Goodwin C S, Pearman J W, Warren J R. Original isolation of Campylobacter pyloridis from human gastric mucosa. Microbios Lett. 1984;25:83–88. [Google Scholar]
- 15.Matsumoto S, Washizuka Y, Matsumoto Y, Tawara S, Ikeda F, Yokota Y, Karita M. Appearance of a metronidazole-resistant Helicobacter pylori strain in an infected-ICR-mouse model and difference in eradication of metronidazole-resistant and -sensitive strains. Antimicrob Agents Chemother. 1997;41:2602–2605. doi: 10.1128/aac.41.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mégraud F, Rabbé M B, Denis F, Belbouri A, Hoa D Q. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27:1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens J C, Dekker W, Lightvoet E E, Blok P. Treatment failure of norfloxacin against Campylobacter pylori and chronic gastritis in patients with nonulcerative dyspepsia. Antimicrob Agents Chemother. 1989;33:256–257. doi: 10.1128/aac.33.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Midolo P D, Lambert J R, Turnidge J. Metronidazole resistance: a predictor of failure of Helicobacter pylori eradication by triple therapy. J Gastroenterol Hepatol. 1996;11:290–292. doi: 10.1111/j.1440-1746.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 19.Misiewicz J J, Harris A W, Bardhan K D, Levi S, O’Morain C, Cooper B T, Kerr G D, Dixon M F, Langworthy H, Piper D. One week triple therapy for Helicobacter pylori: a multicentre comparative study. Gut. 1997;41:735–739. doi: 10.1136/gut.41.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami S, Muramatsu M, Otomo S. Gastric H+,K+-ATPase inhibition by catechins. J Pharm Pharmacol. 1992;44:926–928. doi: 10.1111/j.2042-7158.1992.tb03238.x. [DOI] [PubMed] [Google Scholar]
- 21.Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 22.Parsonnet J, Gary D F, Daniel P V, Yuan C, Joseph H V, Norman O, Richard K S. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 23.Shibata K, Ito Y, Hongo A, Yasoshima A, Endo T, Ohashi M. Bactericidal activity of a new antiulcer agent, ecabet sodium, against Helicobacter pylori under acidic conditions. Antimicrob Agents Chemother. 1995;39:1295–1299. doi: 10.1128/aac.39.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suganuma M, Okabe S, Sueoka E, Iida N, Komori A, Kim S, Fujiki H. A new process of cancer prevention mediated through inhibition of tumor necrosis factor α expression. Cancer Res. 1996;56:3711–3715. [PubMed] [Google Scholar]
- 25.Warren J R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]