Abstract
The growing use of neoadjuvant chemotherapy to treat advanced-stage high-grade serous ovarian cancer (HGSOC) creates an opportunity to better understand chemotherapy-induced mutational and gene expression changes. Here we performed a cohort study including 34 patients with advanced stage IIIC or IV HGSOC to assess changes in the tumor genome and transcriptome in women receiving neoadjuvant chemotherapy. RNA-sequencing and panel DNA-sequencing of 596 cancer-related genes was performed on paired FFPE specimens collected before and after chemotherapy, and differentially expressed genes (DEGs) and CNVs in pre- and post-chemotherapy samples were identified. Following tissue and sequencing quality control, the final patient cohort consisted of 32 paired DNA and 20 paired RNA samples. Genomic analysis of paired samples did not reveal any recurrent chemotherapy-induced mutations. Gene expression analyses found that most DEGs were upregulated by chemotherapy, primarily in the chemotherapy resistant specimens. AP-1 transcription factor family genes (FOS, FOSB, FRA-1) were particularly upregulated in chemotherapy resistant samples. CNV analysis identified recurrent 11q23.1 amplification, which encompasses SIK2. In vitro, combined treatment with AP-1 or SIK2 inhibitors with carboplatin or paclitaxel demonstrated synergistic effects. These data suggest that AP-1 activity and SIK2 copy number amplification are induced by chemotherapy and may represent mechanisms by which chemotherapy resistance evolves in HGSOC. AP-1 and SIK2 are druggable targets with available small molecule inhibitors and represent potential targets to circumvent chemotherapy resistance.
Statement of Significance
Genomic and transcriptomic analyses identify increased AP-1 activity and SIK2 copy number amplifications in resistant ovarian cancer following neoadjuvant chemotherapy, uncovering synergistic effects of AP-1 and SIK2 inhibitors with chemotherapy.
Keywords: ovarian cancer, chemotherapy, copy number variations, AP-1, c-Jun, transcriptomics, neoadjuvant chemotherapy, carboplatin, paclitaxel, SIK2
Introduction
High grade serous ovarian cancer (HGSOC) is the most common subtype of ovarian cancer with a high mortality rate. In recent years, the paradigm for the treatment of advanced stage HGSOC has shifted from a focus on primary debulking surgery followed by adjuvant chemotherapy to the more frequent use of neoadjuvant chemotherapy (NACT) followed by an interval debulking surgery. This approach has demonstrated non-inferior outcomes with reduced patient morbidity1. Most large genomic studies of HGSOC, such as The Cancer Genome Atlas (TCGA), have focused on chemotherapy naïve tumors and therefore cannot assess chemotherapy’s impact on the tumor2. Neoadjuvant chemotherapy provides an opportunity to better understand the genomic and transcriptomic effects of chemotherapy by enabling direct comparison of paired untreated and treated tumor samples collected from the same patient.
Although HGSOC is one of the most chemotherapy sensitive solid tumors, with 80% of women responding to their initial treatment, the majority have a recurrence of metastatic disease3. There is currently no clinically useful way to predict a patient’s response to NACT before treatment; however, genomic and transcriptomic studies have begun to reveal the changes that occur following NACT. In a study that compared 10 patients who received NACT with excellent response and 10 patients who received NACT with poor response, nonsense mutations in CSMD3 and PIK3CA were only seen in patients with poor response4. Transcriptomic analysis of tumors post-NACT has found increased immune cell transcripts compared to pre-NACT samples, suggesting that chemotherapy response is associated with immune recruitment and remodeling5. A study of patients with matched pre- and post-NACT tumor samples found that pre-NACT transcriptomes were enriched for cell growth pathways and post-NACT transcriptomes for drug transport and peroxisome pathways6. Another study that employed targeted panel genomic and transcriptomic analyses found that NACT is associated with changes in cell cycle progression and DNA damage response7. A study focused on patients with minimal residual disease after NACT demonstrated upregulation of fatty acid oxidation and epithelial to mesenchymal transition signature genes in residual tumor cells8.
We investigated a cohort of matched pre- and post-NACT tumors using RNA-sequencing and targeted panel DNA-sequencing to better understand the effect of neoadjuvant chemotherapy on the genome and transcriptome of HGSOC tumors. Our analyses found evidence of transcriptomic remodeling associated with NACT, as well as for genomic instability that gives rise to recurrent amplifications following NACT. This includes the identification of potentially clinically relevant roles for increased AP-1 signaling and SIK2 amplification in driving the initial responses of tumors to carboplatin and paclitaxel.
Materials and Methods
Clinical cohort
One hundred and fifty-three patients with Federation Internationale de Gynecolgie et d’Obstetrique (FIGO) 2013 advanced stage IIIC or IV high grade serous ovarian cancer who received neoadjuvant chemotherapy between January 2007 and June 2019 were identified in the University of Chicago ovarian cancer database. In accordance with the Common Rule, all patients in this database provided written informed consent under a University of Chicago IRB approved protocol for use of their archived tissue in future research studies. Fifty patients with high-quality tissue samples collected before and after NACT were selected for analysis. All patients received both carboplatin and paclitaxel for neoadjuvant chemotherapy. Patients underwent an interval debulking surgery consisting of hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and other intra-abdominal procedures to remove the maximum amount of tumor. Caucasian women made up the majority of the cohort at 58%, followed by African American women at 28%, Hispanic women at 10%, and Asian women at 4%. Platinum response was defined as resistant if recurrence occurred within 6 months of primary therapy or sensitive if more than 6 months elapsed before disease progression. All patients signed an IRB approved (University of Chicago) consent for the use of their tissue at the time of their surgery. Pre- and post-chemotherapy samples were submitted to Tempus for DNA and RNA sequencing.
Pathological evaluation and sample preparation
Formalin-fixed paraffin embedded (FFPE) tissue blocks were cut into 10 μm sections from the patient’s pre-neoadjuvant chemotherapy biopsy and interval debulking surgery. Samples were assessed by two gynecologic pathologists (RL, DC) to confirm high grade histology, stage, and chemotherapy response score (CRS). Samples were also evaluated by a pathologist to ensure at least 20% tumor cellularity and some specimens were macrodissected to increase tumor cellularity. Germline DNA was collected at the time of surgery from peripheral blood mononuclear cells or FFPE sections free of tumor.
DNA and RNA extraction and sequencing
Targeted panel sequencing was performed on FFPE samples using the Tempus xT panel, a hybrid capture next generation sequencing panel that encompasses clinically-relevant and known cancer driver genes (Table S1). Total tumor nucleic acid was extracted by digesting sections with proteinase K. Each tumor and germline sample had 100ng of DNA mechanically sheared by a Covaris ultrasonicator to an average size of 200 base pair (bp). Libraries were then prepared using KAPA Hyper Prep Kit, hybridized to the xT probe set, and were then amplified with KAPA HiFi HotStart ReadyMix. Remaining nucleic acid was purified to RNA with DNase-I digestion. One-hundred ng of RNA was input to the Kapa RNA Hyperprep kit hybridized to IDT xGen Exome Research Panel version 1 probes. Amplification of cDNA was performed with KAPA HiFi HotStart ReadyMix. Sequencing to an average target depth of 500x was performed on Illumina HiSeq 4000 (version 2) or NovaSeq6000 (version 3). For quality control, each sample was required to have 95% of target bases sequenced to at least 300x.
DNA analysis
Germline data was filtered using the Broad institute hard filter recommendations. Briefly, sequence quality was evaluated by FastQC (v0.11.8, RRID:SCR_014583) and sequences trimmed with Trimmomatic (v0.36, RRID:SCR_011848). Trimmed sample sequence files were mapped to GRCh38 with BWA MEM (v0.7.17, RRID:SCR_010910) and merged. Duplicates were marked with sambamba (v0.6.8) and base quality score recalibration performed using the GATK 4.1.6 tools BaseRecalibrator and ApplyBQSR (RRID:SCR_001876). Germline variants of known clinical interest in ovarian cancer were selected and cBioportal’s (SCR_014555) MutationMapper used to generate lollipop plots. Somatic data was annotated with ANNOVAR and reads passing filters were selected for analysis. This included mutations with a variant allele frequency (VAF) of >20% and population variant allele frequency of <5% in women. To estimate mutational burden, exonic SNVs with a VAF>5% that were not in the COSMIC database (RRID:SCR_002260) were included. CNVs were detected from panel sequencing data using SynthEx (v1.0.5) and significantly enriched CNVs detected using GISTIC2.0 (v2.0.23, RRID:SCR_000151).
RNA analysis
RNA-seq data was aligned and quantified using Kallisto (v0.46.1, RRID:SCR_016582) against human genome build hg38. Raw counts were normalized using RPKM. Given high interpatient heterogeneity, GFOLD was used to determine which genes were differentially expressed in pre and post chemotherapy samples. GFOLD (0.01) was used, meaning in 99% of cases the fold change of a gene is above the calculated GFOLD value. A cut off of >±2 was used to consider expression significantly altered by chemotherapy. STRING database (RRID:SCR_005223) was used for extracting and visualizing GO terms. R version 4.0.2 (RRID:SCR_000432) with consensusOV package was used to determine molecular subtypes; Complex Heatmap (RRID:SCR_017270) and ggplot2 (RRID:SCR_014601) packages were used for visualizations. Genes in the AP-1 pathway were extracted from the Pathway Interaction Database (PID_AP1_PATHWAY; M167). Kaplan-Meier survival curves were generated with the Kaplan Meier Plotter using HGSOC datasets GSE14767, GSE15622, GSE18520, GSE19829, GSE23554, GSE26193, GSE26712, GSE26751, GSE30161, GSE3149, GSE51373, GSE63885, GSE65986, GSE9891, and the TCGA.
Cell lines and culture conditions
OVCAR-8 cells were obtained from Marcus Peter (Northwestern University, Chicago, USA, RRID: CVCL_IM65), TYK-nu cells from Gottfried Konecny (Ronald Reagan UCLA Medical Center, RRID:CVCL_1776), OVCAR-4 cells from Charles River Fredrick National Laboratory for Cancer Research (RRID:CVCL_1627), and PEO-1 cells from Scott Kaufmann (Mayo Clinic, Rochester, MN, RRID:CVCL_2686). OVCAR-8 cells were grown in DMEM (10-013-CV) (Corning) with 10% Fetal Bovine Serum (35-010-CV, FBS) (Fisher Scientific), 1% MEM nonessential amino acids (25-025-CI) (Corning) and 1% MEM Vitamins (25-020-CI) (Corning). TYK-nu cells were grown in MEM (10-022-CV) (Corning) with 10% FBS, 1% MEM nonessential amino acids (25-025-CI) (Corning) and 1% MEM Vitamins (25-020-CI) (Corning). OVCAR-4 cells were grown in RPMI with 10% Fetal Bovine Serum (35-010-CV, FBS) (Fisher Scientific). PEO-1 cells were grown in DMEM (10-013-CV) (Corning) with 10% Fetal Bovine Serum (35-010-CV, FBS) (Fisher Scientific), 1% MEM nonessential amino acids (25-025-CI) (Corning) and 1% MEM Vitamins (25-020-CI) (Corning). Cells were grown in 5% CO2 incubator at 37°C. All cells were mycoplasma negative and their identity verified regularly by short tandem repeat profiling (IDEXX) (OVCAR-8 and TYK-nu Feb 2020, OVCAR-4 June 2019, PEO-1 July 2019). Cells were passaged 2-10 times after unfreezing prior to performing experiments.
Antibodies
C-Jun antibody (1:1000, ab31419, Abcam, RRID:AB_731605) or c-Jun antibody (1:1000, 2315, Cell Signaling, RRID:AB_490780), c-Fos antibody (1:500, sc-166940, Santa Cruz, RRID:AB_10609634), anti-thrombospondin 1 (THBS1) antibody (1:100, sc-59887, Santa Cruz, RRID:AB_793045), GAPDH antibody (1:1000, 2118, Cell Signaling, RRID:AB_561053), SIK-2 antibody (1:1000, 6919S, Cell Signaling, RRID:AB_10830063), phospho-Akt (Ser473) antibody (1:1000, 9271, Cell Signaling, RRID:AB_329825), and AKT antibody (1:1000, 9272, Cell Signaling, RRID:AB_329827).
Apoptosis assay with flow cytometry
OVCAR-8 cells 1) treated with 5μM of SIK-2 inhibitor (HG-9-91-01, MedChem Express, HY-15776) for the indicated time, 2) with stable knockout of SIK-2, or 3) OVCAR-8 cells transfected with SIK-2 siRNA or NT siRNA (30nM; 24hours, Dharmacon), were treated with carboplatin (100μM; 72 hours) or paclitaxel (100nM; 24 hours). Cells were then trypsinized and washed with staining buffer (BD, 554657). 500,000 cells were re-suspended in binding buffer (Apoptosis Detection Kit, BioLegend, 640932) and stained with Annexin V (APC) and PI (PE) for 15 min at room temperature. Flow cytometry analysis was then carried out using CytoFLEX Flow Cytometer (Beckmann Coulter). Data analysis was carried out using FlowJo 10.7.1 (RRID:SCR_008520).
Proliferation assay
OVCAR8 cells were seeded at 10,000 cells per well in a black-wall 96-well plate and allowed to adhere for 24 hours before addition of treatment (Paclitaxel [100 nM], Carboplatin [100 μM], SIK-2i [5 μM]). After 24 hours or 48 hours, nuclei were visualized by addition of Hoechst 33258 (1:5000) and wells fluorescently imaged with a Nikon Eclipse Ti2 to extract the Hoechst+ area fraction.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections (5 μm) were deparaffinized with xylene and rehydrated with a graded series of ethanol. For THBS1, the slides were stained using Leica Bond RX automated stainer. Epitope retrieval solution I (Leica Biosystems, AR9640) was used for 20-minute treatment. Anti-THBS1 (1:100) was applied on tissue sections for one-hour incubation and the antigen-antibody binding was detected with Bond polymer refine detection (Leica Biosystems, DS9800). For trichrome stain, slides were placed in Bouin’s solution at 55-66°C for 1 hour and then washed in running water until yellow color disappeared. Next, slides were stained with Weigert’s Iron Hematoxylin for 3 minutes and washed in running water for 3 minutes. Then, slides were stained with Biebrich Scarlet-Acid Fuschin solution for 3 minutes and rinsed with deionized water. Finally, slides were differentiated in Phosphotungstic-Phosphomolybdic acid for 3 minutes and immersed slides into aniline blue solution for 5 minutes.
Immunoblotting
Cells were lysed using SDS lysis buffer, and protein estimation was carried out using a BCA Protein Assay Kit (Thermo Scientific, 23224). Equal protein amounts were resolved using 4-20% SDS gel and transferred onto nitrocellulose membrane. Signals were detected using 1:3000 dilutions of either horseradish peroxide linked anti-mouse (Cell Signaling Technology, 7076, RRID:AB_330924) or anti-rabbit (Cell Signaling Technology, 7074, RRID:AB_2099233) secondary antibodies and subsequently developed with ECL substrate (Bio-Rad, 1705061) or Pico (Thermo Fisher, 34579). Images were acquired using G: Box (Syngene).
Dual luciferase assay
200,000 OVCAR8 cells in 6 well plates were co-transfected with AP-1 reporter (1.5 μg pAP-1-Luc plasmid containing seven AP-1 enhancer elements; Agilent) and pRL-CMV-Renilla plasmids (0.1 ng; Promega) using 6 μl of Lipofectamine 2000. Cells were treated with paclitaxel or carboplatin at indicated treatment for 24 hours two days after transfection. Cells were lysed, and luminescence measured using a Berthold Technologies Lumat LB 9507 per manufacturer’s instructions (Promega Dual Luciferase Reporter Assay).
MTT assay
For inhibitor assays, 10,000 cells were seeded into 96 well plates on day 1 and treated with chemotherapy and inhibitor on day 2. MTT assay (Thiazolyl blue tetrazolium bromide, M2128, Sigma) was performed 72 hours after treatment. TYK-nu and OVCAR-8 cells were treated with constant ratios of drug combinations (carboplatin and SR11302; paclitaxel and SR11302) in serial dilutions and combination indices calculated with Compusyn software.
The data generated in this study are available within the article and its supplementary data files. Expression profile data analyzed in this study were obtained from Gene Expression Omnibus (GEO) at GSE14767, GSE15622, GSE18520, GSE19829, GSE23554, GSE26193, GSE26712, GSE26751, GSE30161, GSE3149, GSE51373, GSE63885, GSE65986, GSE9891.
Results
We identified a cohort of 50 consecutive patients from our ovarian cancer database with advanced stage IIIC and IV HGSOC treated with neoadjuvant carboplatin and paclitaxel. These women were not eligible for primary debulking surgery because of their significant tumor burden on imaging and clinical exam. Tumor tissue was biopsied before NACT and at the interval debulking surgery. After removal of cases that were exceptional responders9 with minimal tumor tissue at the second surgery and tumors that failed library preparation or sequencing (Methods), the final patient cohort consisted of 32 paired DNA and 20 paired RNA samples from pre- and post-NACT, with 18 patients contributing both paired DNA and RNA, 14 patients only contributing DNA, and 2 patients only contributing RNA (Fig. 1A, Fig. S1A, Table 1, Table S2-3).
Fig. 1. Transcriptomic and genomic characterization of HGSOC response to neoadjuvant chemotherapy.
(A) Overview of cohort and sample collection time points. Representative H&E of matched pairs of pre- and post-NACT samples from tumors with poor pathologic response to therapy (CRS 1) or excellent response to therapy (CRS 3; 40× magnification). (B) RNA-sequencing of whole transcriptome showed that samples cluster based on treatment status (pre- or post-NACT) rather than chemo-sensitivity, as visualized with t-SNE. CRS = chemotherapy response score; NACT = neoadjuvant chemotherapy; t-SNE = t-distributed stochastic neighbor embedding. (C) Molecular subtype2 of indicated patients before and after NACT. Patient molecular subtypes are plastic and frequently change after NACT.
Table 1:
Clinical characteristics of the neoadjuvant chemotherapy high grade serous ovarian cancer patient cohort
| Characteristics, n (%) | DNA-seq patients (32) | RNA-seq patients (20) |
|---|---|---|
| Median age at diagnosis, years (range) | 60 (43-86) | 61 (46-80) |
| Median cycles of neoadjuvant chemotherapy (range) | 3 (3-6) | 3 (3-4) |
| FIGO stage | ||
| IIIC | 21 (66%) | 16 (80%) |
| IVA | 2 (6%) | 0 |
| IVB | 9 (28%) | 4 (20%) |
| Primary disease site | ||
| Ovary | 17 (53%) | 12 (60%) |
| Fallopian tube | 15 (47%) | 8 (40%) |
| Peritoneum | 0 | 0 |
| Tumor debulking status | ||
| optimal | 26 (81%) | 16 (80%) |
| suboptimal | 6 (19%) | 4 (20%) |
| Chemotherapy response score * | ||
| 1 | 15 (48%) | 8 (42%) |
| 2 | 8 (26%) | 7 (37%) |
| 3 | 8 (26%) | 4 (21%) |
| Primary platinum response | ||
| Sensitive | 18 (56%) | 12 (60%) |
| Resistant | 14 (44%) | 8 (40%) |
| Median Progression free survival, days (range) | 460 (246-1693) | 521 (199-1257) |
| Median overall survival, days (range) | 734 (292-2248) | 720 (292-2248) |
1 patient with unknown score
Three patients (10%) carried a BRCA1 and one patient (4%) a BRCA2 germline mutation (Fig. S1B); both BRCA1 patients were chemotherapy sensitive and the BRCA2 patient was chemotherapy resistant. Patients were nearly evenly split between platinum sensitive (>6 months to recurrence after the end of chemotherapy) and resistant (<6 months to recurrence) disease. Whole transcriptome t-SNE analysis established that clustering of samples was driven by pre- or post-chemotherapy status, but not chemotherapy sensitivity (Fig. 1B). Increased epithelial marker expression was not correlated with a lower pathologic tumor content (Fig. S1C). We determined each patient’s TCGA molecular subtype10 pre- and post-NACT and found that 50% of patients underwent a subtype change (Fig. 1C) consistent with previous reports6. There was no correlation between chemotherapy resistance and pre-NACT TCGA subtype (p=0.9), post-NACT subtype (p=0.4), or change in subtype (p=0.6). As HGSOC molecular subtypes were initially identified from chemotherapy naïve patient specimens and are imperfect prognostic indicators2, these data suggest that tumors are highly plastic and surviving tumor cells adapt to chemotherapy by changing gene expression11.
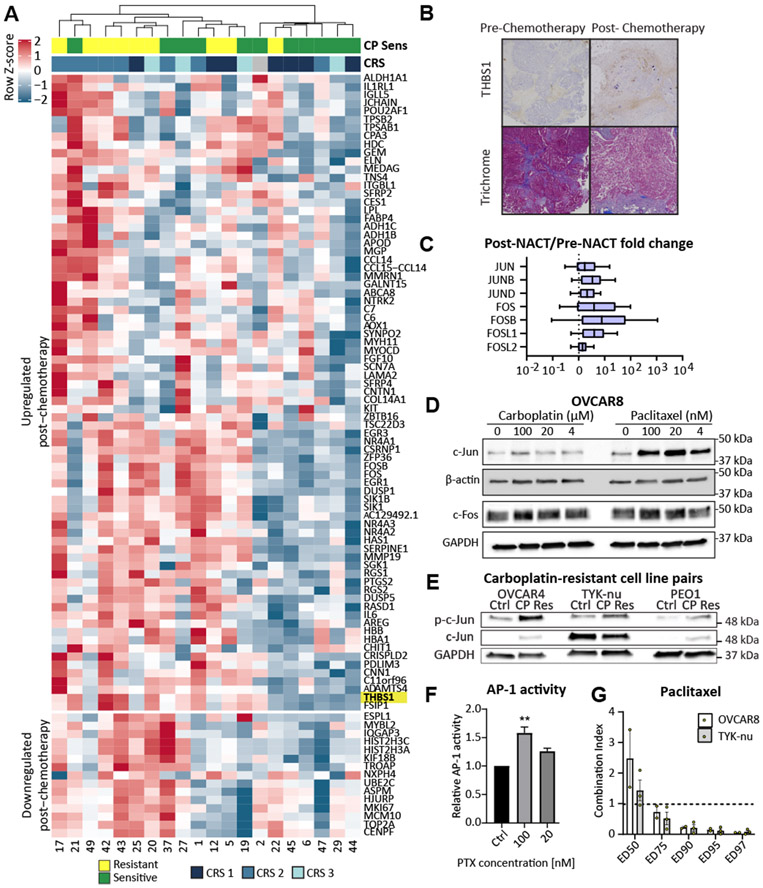
Analyzing all patients, 77 genes were upregulated after chemotherapy and 15 genes were downregulated (Fig. 2A). To identify differentially expressed genes (DEGs) associated with chemotherapy response, we performed a paired analysis of each patient’s pre- and post-NACT sample using GFOLD (Tables S4-8). When samples were clustered based on read counts, clustering occured by pre or post chemotherapy status and not chemotherapy response. However, when clustering was based on DEGs, the samples did cluster by response as well as chemotherapy status (Fig. 2A, Fig. S2A). We validated previous observations that FOS, NR4A1, NR4A3, NTRK2, SFRP2, and SFRP4 are upregulated following NACT7. When categorizing patients by platinum sensitivity, we identified 141 genes exclusively upregulated in chemotherapy resistant patients compared to only 7 genes in chemotherapy sensitive patients (Table S9), consistent with findings in triple-negative breast cancer that shares many genomic features with HGSOC12. Genes downregulated following NACT included several genes associated with DNA replication and the cell cycle (e.g., MKI67, MCM10, TOP2A, and CENP). Upregulated genes included extracellular matrix genes (e.g., COL14A1, THBS1; Fig. 2B), metabolic genes (e.g., ALDH1A1, FABP4, AOX1), cytokines (e.g., CCL14 and IL6), and transcriptional regulators. In particular, genes involved in the AP-1 transcription factor network, including FOS, FOSL1, and FOSB, showed consistently high expression following NACT (Fig. 2C, Fig. S2B-C). Supporting a functional role of AP-1 family members in chemotherapy resistance, we found that the core AP-1 genes were more highly expressed in chemoresistant patients (Fig. S2D, Table S10). Consistent with our findings, Jimenez-Sanchez et al5 identified similar increases in the expression of AP-1 pathway genes in platinum resistant patients following chemotherapy using a microarray (Fig. S2E).
Fig. 2. Neoadjuvant chemotherapy is associated with increased AP-1 activity.
(A) Hierarchical clustering of differentially expressed genes following NACT (>±2 GFOLD) finds that patients cluster by platinum sensitivity. (B) Pre and post-chemotherapy IHC (40x) demonstrating increased THBS1 (thrombospondin) expression after chemotherapy. THBS is an extracellular matrix protein whose gene expression is upregulated post chemotherapy, as highlighted in Fig.1A. Trichrome staining demonstrates stable quantity of blue staining collagen regardless of chemotherapy status. (C) Expression of core AP-1 transcription factors is consistently elevated after NACT, including c-Jun and c-Fos. (D) Treatment of OVCAR8 cells with paclitaxel, but not carboplatin, for 24 hours leads to a dose-dependent increase in c-Jun protein levels. C-Fos levels are unaffected. (E) On immunoblot Carboplatin (CP) resistant OVCAR4, TYK-nu, and PEO1 cells have elevated levels of phosphorylated c-Jun (Ser63) compared to control cells. (F) AP-1 transcriptional activity as assessed with a luciferase reporter plasmid incorporating multiple AP-1 binding sites is increased in OVCAR8 cells following treatment with paclitaxel at the indicated concentrations for 24 hours. (G) Combination of PTX with the AP-1 inhibitor SR11302 leads to synergistic cytotoxicity of OVCAR8 and TYK-nu cells at large effect sizes (ED = Effective dose; ED75-ED97). Combination index of less than 1 indicates synergistic activity of AP-1 inhibitor with paclitaxel. CP sens = platinum sensitive. **ANOVA p < 0.01.
Based on the consistently elevated expression of AP-1 family members in the chemoresistant post-treatment samples, we further investigated their functional role in mediating chemoresistance. The activator protein 1 (AP-1) transcription factor is a dimeric leucine-zipper protein composed of elements from the JUN, FOS, ATF and MAF protein families, which play important roles in cell proliferation and invasion13. While AP-1 expression is predominantly tumor-promoting, some family members can act as tumor suppressors depending on heterodimer composition, cell type, and tumor microenvironment14. Treatment of HGSOC cell lines with carboplatin and paclitaxel led to an increase in total and phosphorylated c-Jun (Fig. 2D, S3A-B). Platinum-resistant cell lines generated by long-term, in vitro treatment of OVCAR4, TYK-nu, and PEO1 cells with carboplatin had significantly elevated levels of c-Jun phosphorylation (Fig. 2E). Paclitaxel, but not carboplatin, increased AP-1 transcriptional activity, as assessed with a luciferase reporter system (Fig. 2F, Fig. S3C). Treatment of ovarian cancer cells with an AP-1 inhibitor (SR1130215) combined with paclitaxel or carboplatin led to a synergistic increase in cell death (Fig. 2G, S3D), which was present at lower kill fractions for paclitaxel than carboplatin. Moreover, elevated expression of both c-Jun and c-Fos are consistently associated with worse overall and progression-free survival in several large-scale transcriptomic analyses of patients with epithelial ovarian cancer (Fig. S3E-H). Combined, these data suggest that both carboplatin and taxane-based chemotherapies select cells with elevated AP-1 transcriptional activity.
Panel sequencing of cancer-associated genes identified between one and fourteen nonsynonymous mutations per patient; twelve genes were mutated in two or more patients (Fig. 3A, Tables S11-12). No gene demonstrated a recurrent gain in mutation following NACT, suggesting that NACT does not recurrently alter specific genes. There were no significant differences in average mutational burden pre- and post-NACT and mutational burden was not associated with chemotherapy resistance or CRS (Fig. S4A). Patients showed both an increase and decrease in mutational burden following NACT, which is in contrast to breast cancer which demonstrates either no change or decrease in mutations16. Consistent with other studies, we found that the most common mutational class was driven by the aging-related C>T transition signature caused by spontaneous deamination of 5-methylcytosine (Fig. 3B)9. As expected, TP53 mutations were the primary recurrent mutation found, identified in all but one patient. Consistent with the TCGA and other analyses, we identified mutations in NF1 (9%), BRCA1 (3%) and BRCA2 (3%)2 in several patients; other mutations present in multiple patients included APOB, MTOR, CREBBP, NRG1, PAX3, and SALL2. Reduced NF1 expression is associated with decreased 5-year survival and chemotherapy resistance in multiple cancer types, while APOB (9%) is recurrently mutated in hepatocellular carcinoma, and MTOR (9%) mutations are common in renal cell cancers. CREBBP mutations in acute lymphoblastic leukemia, NRG1 fusions in non-small cell lung cancer, PAX3 overexpression in gliomas, and epigenetic silencing of SALL2 in breast cancer are all genomic events that have been associated with chemotherapy resistance17.
Fig. 3. Neoadjuvant chemotherapy is not associated with the acquisition of somatic mutations.
(A) Oncoprint of all somatic mutations detected in at least two patient samples. Left upper triangle in each square corresponds to pre-NACT sample and right lower triangle corresponds to post-NACT tissue. Mutational types are indicated by color. The frequency of mutations within our cohort is compared to the frequency of mutations in the TCGA analysis of HGSOC. VAF = variant allele frequency. (B) Proportion of each mutational class in all coding genes detected in the entire patient cohort, divided based on pre or post –NACT.
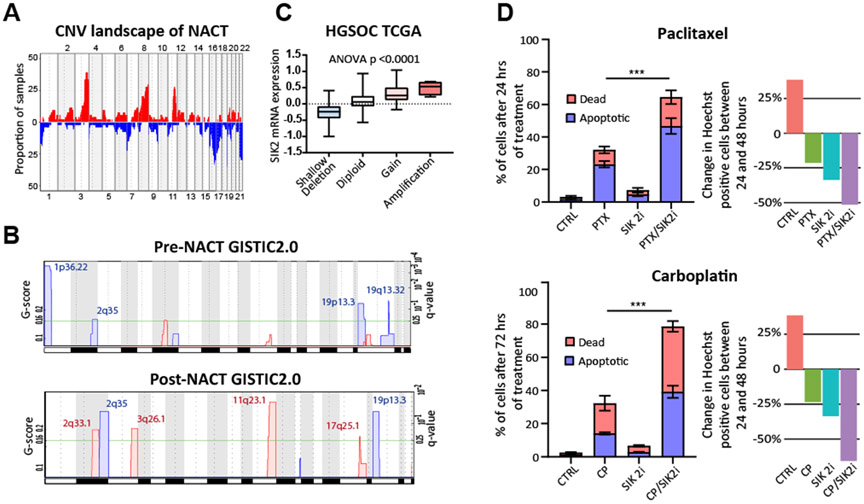
Copy number profiles of patient samples generally recapitulated those identified in TCGA and other analyses2, with frequent amplification of chromosome 3q and 8q and deletion of chromosomes 16 and 17 (Fig. 4A and Table S13). In general, both pre- and post-NACT specimens were characterized by widespread genomic instability. To identify recurrently amplified and deleted chromosomal regions, we performed CNV analysis with GISTIC2.018 software tool. We identified several regions that were recurrently amplified or deleted following NACT, emphasizing that NACT leads to the induction or selection of conserved copy number alterations in HGSOC. The most prominent were amplification of 2q33.1 and 11q23.1 and deletion of 19p13.3 and 2q35 (Fig. 4B). Relatively few to no copy number gains or losses were associated with response to NACT or associated with recurrence one year after NACT, independent of whether pre- or post-NACT samples were interrogated (Fig. S4B). The most-significant amplification was noted in 11q23.1, which encompasses salt-inducible kinase 2 (SIK2), a serine/threonine kinase that is associated with paclitaxel and platinum resistance in ovarian cancer19,20 (Table S14). Genomic amplification of SIK2 has not been described as a mechanism driving SIK2 upregulation, but our analysis shows that the expression of SIK2 is strongly associated with copy number status (p<0.0001, Fig. 4C, Fig. S4C). The treatment of OVCAR8 HGSOC cells with carboplatin or paclitaxel and a SIK2 inhibitor led to a significant synergistic increase in cell death (Fig. 4D). Addition of SIK2 inhibitor HG-9-91-0119 or blocking SIK2 with siRNA against SIK2 inhibited AKT activity (Fig. S4D). Genomic amplification of SIK2 in response to NACT may be a novel mechanism by which HGSOC evolves resistance to cytotoxic therapies.
Fig. 4. Recurrent copy number variations associated with neoadjuvant chemotherapy.
(A) The frequency of CNVs in the patient cohort captures recurrent CNVs observed in the TCGA analysis of HGSOC such as amplification of 3q and 8q and deletion of chromosomes 16 and 22. (B) Comparing TCGA HGSOC cohort normalized SIK2 mRNA expression to copy number alteration data reveals increased SIK2 expression with copy number gain or amplification. (C) GISTIC2.0 analysis of differentially amplified (red) and deleted (blue) regions identifies recurrent CNVs present both pre- and post-NACT, including frequent amplification of SIK2 in 11q23.1 which contains 75 total genes (Table S14). Chromosome locations are indicated on the x-axis. (D) Treatment of OVCAR8 cells with a combination of paclitaxel (PTX, 100 nM) or carboplatin (CP, 100 μM) and a SIK2 inhibitor (HG-9-91-01; 5 μM) leads to synergistic cytotoxicity based on 2-way ANOVA and Tukey’s test. Proliferation data demonstrating percent change in the Hoechst positive area between the 24 and 48 hour post treatment time points is shown in the panels on the right ***ANOVA p < 0.001. Supplementary Figures
Discussion
Using a combination of transcriptomic and genomic analyses, we found that NACT is associated with significant transcriptomic remodeling, including upregulation of AP-1 transcriptional networks. Although tumors continue to evolve mutations during NACT, no recurrently mutated genes associated with NACT were identified in our patient cohort. In contrast, we found evidence that NACT is associated with recurrent copy number alterations, including amplification of 11q23.1 which is associated with increased expression of SIK2. The selection criteria for NACT is inconsistent, but patients triaged to NACT typically have a higher disease burden, increased ascites, and are more medical complex than primary debulking surgery candidates1. Although our study provides insight into the molecular mechanisms underlying chemotherapy response in HGSOC, fully understanding the adaptive and evolutionary processes underlying the response of tumor cells to NACT will require an analysis of clonal dynamics and epigenetic processes. In particular, single-cell techniques will be useful to understand NACT-associated transcriptional remodeling by unraveling the selection pressure of NACT on pre-existing tumor cell populations from the induction of specific gene expression programs in tumor cells (or stromal) sub-populations.
Our data suggest that AP-1 activity is induced by chemotherapy and may contribute to the survival of cancer cells in response to cytotoxic agents. AP-1 is a druggable target for which multiple classes of small molecule inhibitors have been developed. Several AP-1 inhibitors have undergone clinical testing for inflammatory conditions, including rheumatoid arthritis. Therefore, it is possible to consider therapeutically targeting AP-1 in the context of NACT for the purpose of overcoming chemoresistance. In addition, work from our group and others have demonstrated that SIK2 is a therapeutically tractable mediator of platinum and paclitaxel sensitivity in ovarian cancer. Indeed, a small molecule inhibitor of SIK2 (GRN-300) is currently under investigation for the treatment of recurrent ovarian, primary peritoneal, and fallopian tube cancers (NCT04711161).
Supplementary Material
Acknowledgements
We thank Gail Isenberg for help with editing the manuscript. We appreciate Martin Miller Ph.D. Cancer Systems Biology Lab, CRUK, Cambridge University for sharing RNA sequencing results. This project was supported by a grant from the National Cancer Institute (R01CA169604 to E Lengyel) and funding from Tempus for panel sequencing (E Lengyel and M Eckert).
Abbreviations:
- AP-1
Activator protein 1
- CNV
Copy number variation
- CP
Carboplatin
- CP sens
Carboplatinum sensitive
- CRS
Chemotherapy response score
- ED
Effective dose
- DEG
Differentially expressed genes
- FFPE
Formalin-fixed paraffin embedded
- HGSOC
High grade serous ovarian cancer
- NACT
Neoadjuvant chemotherapy
- PTX
Paclitaxel
- SNV
Single nucleotide variant
- TCGA
The Cancer Genome Atlas
- t-SNE
t-distributed stochastic neighbor embedding
- VAF
Variant allele frequency
Footnotes
Conflict of Interest Declaration: Research funding for preclinical studies from Abbvie and Arsenal Bioscience outside of the scope of this work (EL).
References
- 1.Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MKB, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol 2018;19:1680–7 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC Jr., Beral V, et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 2015;15:668–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, Zhao L, Rojas C, Bateman NW, Yao H, Lara OD, et al. Molecular Analysis of Clinically Defined Subsets of High-Grade Serous Ovarian Cancer. Cell reports 2020;31:107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiménez-Sánchez A, Cybulska P, Mager KL, Koplev S, Cast O, Couturier D-L, et al. Unraveling tumor–immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat Genet 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasgow MA, Argenta P, Abrahante JE, Shetty M, Talukdar S, Croonquist PA, et al. Biological Insights into Chemotherapy Resistance in Ovarian Cancer. Int J Mol Sci 2019;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arend RC, Londono AI, Montgomery AM, Smith HJ, Dobbin ZC, Katre AA, et al. Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma. Mol Cancer Res 2018;16:813–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artibani M, Masuda K, Hu Z, Rauher PC, Mallett G, Wietek N, et al. Adipocyte-like signature in ovarian cancer minimal residual disease identifies metabolic vulnerabilities of tumor-initiating cells. JCI Insight 2021;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015;521:489–94 [DOI] [PubMed] [Google Scholar]
- 10.Chen GM, Kannan L, Geistlinger L, Kofia V, Safikhani Z, Gendoo DMA, et al. Consensus on Molecular Subtypes of High-Grade Serous Ovarian Carcinoma. Clin Cancer Res 2018;24:5037–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drapkin BJ, Minna JD. Studying Lineage Plasticity One Cell at a Time. Cancer Cell 2020;38:150–2 [DOI] [PubMed] [Google Scholar]
- 12.Berger AC, Korkut A, Kanchi RS, Hegde AM, Lenoir W, Liu W, et al. A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell 2018;33:690–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengyel E, Wang H, Stepp E, Juarez J, Wang Y, Doe W, et al. Requirement of an upstream AP-1 motif for the constitutive and phorbol ester-inducible expression of the urokinase-type plasminogen activator receptor gene. J Biol Chem 1996;271:23176–84 [DOI] [PubMed] [Google Scholar]
- 14.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 2003;3:859–68 [DOI] [PubMed] [Google Scholar]
- 15.Mishra DK, Kim MP. SR 11302, an AP-1 Inhibitor, Reduces Metastatic Lesion Formation in Ex Vivo 4D Lung Cancer Model. Cancer Microenviron 2017;10:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C, Gao R, Sei E, Brandt R, Hartman J, Hatschek T, et al. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018;173:879–93 e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research N, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 2013;45:1113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 2011;12:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miranda F, Mannion D, Liu S, Zheng Y, Mangala LS, Redondo C, et al. Salt-Inducible Kinase 2 Couples Ovarian Cancer Cell Metabolism with Survival at the Adipocyte-Rich Metastatic Niche. Cancer Cell 2016;30:273–89 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AA, Lu Z, Jennings NB, Etemadmoghadam D, Capalbo L, Jacamo RO, et al. SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell 2010;18:109–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.