Abstract
Objective:
To evaluate the risk for developing a substance use disorder (SUD, alcohol or drug abuse or dependence) in individuals with high-functioning autism spectrum disorder (ASD).
Methods:
Subjects with high-functioning ASD were derived from consecutive referrals to a specialized ambulatory program for ASD at a major academic center from 2007 to 2016. Age-matched controls and attention deficit hyperactivity disorder (ADHD) comparison subjects were derived from three independent studies of children and adults with and without ADHD using identical assessment methodology. Cox proportional hazard models were used to analyze the prevalence of SUD (alcohol or drug use disorder). Age of onset of SUD was analyzed with linear regression models.
Results:
Our sample included 230 controls, 219 subjects with ADHD, and 230 subjects with ASD. The mean age for the ASD subjects was 20.0 ± 10.3 years. Among ASD subjects, 69% had a lifetime prevalence of ADHD, and the ASD subjects had significantly higher rates of other psychiatric psychopathology compared to ADHD and control subjects (p<0.001) ASD subjects were at significantly decreased risk for developing a SUD compared to ADHD (Hazard ratio (HR)=0.22, p<0.001) and control subjects (HR=0.62, p=0.04). The age of onset of a SUD was significantly older in ASD subjects, mean age 21.7 years, when compared to ADHD and control subjects (both p<0.005).
Conclusions:
Individuals with ASD are at decreased risk to develop a SUD, and when they do, the onset is significantly later than ADHD and controls.
Keywords: Autism spectrum disorder, Psychiatric comorbidity, Substance use disorder, Risk
Autism spectrum disorders (ASD) are chronic, neurodevelopmental disorders characterized by impairment in social communication, social interactions, and restricted interests [1]. ASD begins in childhood and functional impairment often persists into adulthood [2,3]. A recent systematic review estimated there were 52 million cases of ASD globally, and ASD was associated with substantial disability [4]. A sizeable proportion of individuals with ASD have intact cognitive and language abilities and are referred to as high functioning ASD.
While recent research on high functioning ASD has documented high levels of psychiatric comorbidity with disruptive mood and anxiety disorders [5,6], much less is known about the comorbidity with substance use disorders (SUD). To our knowledge only two studies to date, one with adolescents and another with adults, have specifically examined co-occurring SUD in individuals with ASD seeking psychiatric treatment [7,8]. In both studies individuals with ASD when compared to individuals without ASD were significantly less likely to use substances [7,8].
Results from studies examining the risk for SUD in non-referred populations with ASD, however, have been mixed. A case-control study of adults with ASD found they were less likely than controls to have a lifetime history of SUD [9]. Likewise, a population-based registry study of adults with ASD within a large health care system found adults with ASD were significantly less likely than controls to have an alcohol use disorder or self-report alcohol use or cigarette use [10]. However, no difference in risk for a drug use disorder was found between adults with ASD and controls in this study. In contrast, two population-based registry studies found non-referred individuals with ASD were two to four times more likely to have a SUD when compared to controls [11,12].
The limited research on the prevalence of SUD in clinically referred and non-referred individuals with ASD, and mixed findings on risk for SUD in this population call for additional research on this issue. SUD is associated with substantial morbidity and disability [13,14] and further insights into the risk for SUD in high functioning ASD could help design programs to mitigate this risk. Furthermore, adults with ASD and SUD are particularly affected by this comorbidity since they have been found to have a poorer quality of life and a higher number of care needs when compared to adults with SUD only [15].
The main aim of this study was to evaluate the risk for developing SUD, including cigarette smoking, in individuals with high-functioning ASD. To this end we assessed the risk for SUD in individuals seeking psychiatric treatment for high-functioning ASD and compared them with age-matched ADHD and unaffected controls. Based on the literature, we hypothesize that individuals with high functioning ASD will be at lesser risk to develop a SUD compared to ADHD and unaffected control subjects.
Methods
We conducted a retrospective medical chart review of consecutive referrals of intellectually capable subjects with ASD (high functioning ASD) to a specialized ambulatory program for ASD at a major academic center from 2007-2016. Individuals referred to this program had presumptive or established diagnoses of ASD. We included subjects who had a SUD assessment documented as part of a structured interview conducted during their evaluation. We excluded subjects with established or suspected diagnosis of intellectual disability as per clinical evaluation. We received institutional review board approval to review, analyze, and report anonymously on these subjects.
The ASD subjects were then age-matched with attention-deficit hyperactivity (ADHD) and control (no ADHD or ASD) comparison subjects to evaluate risk for SUD and age of onset of SUD (Fig. 1). The ADHD and control comparison subjects were derived from three independent case control studies. Specifically, 1) and 2) were prospective controlled studies of boys and girls 6-17 years of age with and without Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R) ADHD [16,17], and 3) was a prospective controlled family study of men and women 18-55 years of age with and without Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) ADHD [18]. For each study, available biological parents and siblings of probands (and children of probands in the adult ADHD study) were also ascertained and assessed. All three studies excluded probands with inadequate command of the English language, ASD, or full-scale IQ <80.
Fig. 1.
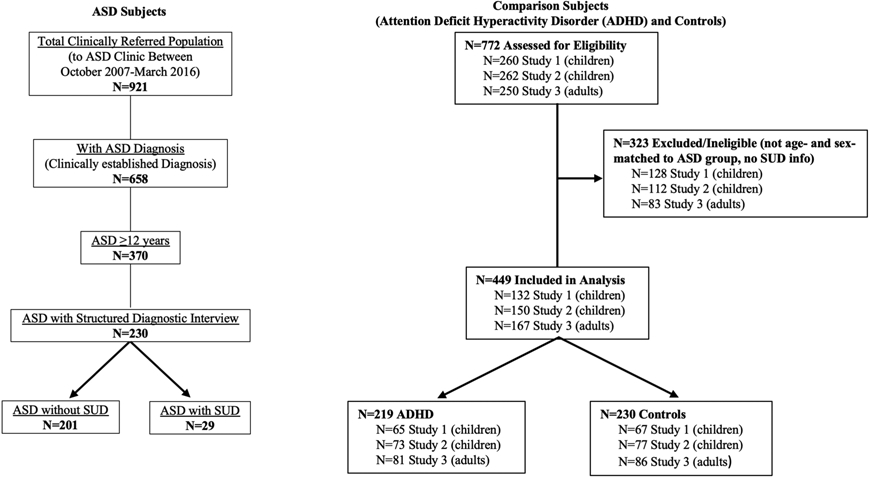
Study Subject Selection Process (Comparison subjects age-matched to Autism Spectrum Disorder (ASD) subjects)
Assessment Procedures
Psychiatric and SUD Diagnoses
All subjects, including the ASD subjects who were derived from a clinical program, had structured interviews. The structured interviews were conducted by trained and supervised psychometricians with bachelor’s or master’s degrees in psychology or a related field. For the ADHD studies, interviewers were blind to the ascertainment status of the families. For the ASD subjects, interviewers were blind to any a priori information as to the subject’s specific complaints or clinical diagnoses apart from their knowledge that they had been referred to the clinic.
Subjects <18 years of age were evaluated using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children – Epidemiologic Version (K-SADS-E) [19]. The K-SADS-E is a semi-structured interview that generates current and lifetime Axis-I diagnoses according to DSM-III-R/IV criteria in children and adolescents. Subjects ≥18 years of age were evaluated using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) [20] supplemented with modules from the K-SADS-E to assess childhood diagnoses. The diagnoses for psychiatric disorders and SUD were DSM-III-R based for subjects assessed before 1994 and DSM-IV based thereafter. Age of onset for SUD and cigarette smoking were derived from structured interviews and defined as the age at which subjects first met criteria for a SUD and first used cigarettes. Since the K-SADS-E and other structured interviews lack a module to evaluate ASD, this was assessed differently as described in detail below. For subjects <18 years of age, independent interviews occurred with parents, and direct interviews with children >12 years of age. For ASD subjects ≥18 years of age, a primary caretaker (if available) also completed the structured diagnostic interview.
ASD Diagnosis
ASD Sample
Patients seen within the ASD program were assessed for ASD through a detailed psychiatric diagnostic interview with a board-certified psychiatrist experienced in evaluating ASD and comorbid psychiatric disorders. Diagnosis of ASD was established by applying the prevailing DSM based diagnostic criteria for autism (DSM-IV Pervasive Developmental Disorders: autistic disorder, Asperger’s disorder, or pervasive developmental disorder-not otherwise specified [PDD-NOS] or DSM 5: ASD). The psychiatric diagnostic interview was conducted with the subject and parent/guardian(s)/significant other, and information was incorporated from multiple sources when available (e.g., psychiatric records, schools, social services).
ADHD and Control Sample
Subjects in the ADHD studies were assessed for ASD through a structured interview that was adopted based on DSM-III-R diagnostic criteria for PDD. ASD was defined as subjects meeting criteria for autistic disorder or PDD-NOS.
Structured Interview Rater Reliability
Rater reliability was established by having experienced, board-certified child and adult psychiatrists and licensed clinical psychologists who were blinded to any prior information diagnose subjects from audiotaped interviews. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was 0.98 [17]. The kappa coefficient for SUD was 1.00 [17]. Rater reliability for the ASD module for the ADHD studies was established by having an independent rater with expertise in the diagnosis of ASD listen to audiotapes of 20 randomly selected modules with or without a diagnosis of ASD. Based on these audiotapes, the reliability with the rater was kappa=0.90. Rater reliability for the diagnosis of ASD for the subjects with ASD was established by an independent rater and the final diagnostic rating made by a clinician-reviewer was kappa=0.88 [6].
IQ Assessment
Full scale IQ was measured using the Wechsler Intelligence Scale for Children – Third Edition (WISC-III) [21] for ADHD and control subjects <18 years of age, Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) [22] for ADHD and control subjects ≥18, and Wechsler Abbreviated Scale of Intelligence (WASI) [23] for ASD subjects. For ASD subjects without a WASI a board certified psychiatrist evaluated IQ based on current and childhood history. As noted above, individuals with ASD with intellectual impairment were excluded, and intellectual impairment was determined based on conceptual (academic) adaptive functioning that required additional support.
Socioeconomic Status Assessment
We measured socioeconomic status (SES) using the 5-point Hollingshead scale [24]. A higher score indicates being of lower SES.
Statistical Method
There were 230 ASD subjects from the ASD program with information regarding SUD (alcohol or drug abuse or dependence or cigarette smoking). Given the wide age range of subjects from the clinic (12-59 years of age), we matched ADHD and control subjects to the ASD group by age using frequency matching. The ASD group had 144 subjects (63%) 12-17 years of age and 86 subjects (37%) 18-59 years of age. We derived ADHD and control subjects 12-17 years of age from the child ADHD studies, and subjects 18-59 from the adult ADHD study. For our ADHD group, the child ADHD studies did not have enough children with ADHD to sample 144 subjects 12-17 years of age, so we included all 138 available subjects and randomly selected 81 subjects 18-59 years of age from the adult ADHD study to keep the group at 63% adolescents and 37% adults. For the control group, we randomly selected children and adults without ADHD which included 144 subjects 12-17 years of age, and 86 subjects 18-59 years of age. Our final sample consisted of 230 ASD, 219 ADHD, and 230 control subjects.
We examined differences in demographic characteristics, rates of psychopathology, and age at onset for any SUD (alcohol or drug use disorder), alcohol use disorder (abuse or dependence), drug use disorder (abuse or dependence), and cigarette smoking among the groups using linear, ordered logistic, and logistic regression models for continuous, ordinal, and binary outcomes, respectively. Lifetime prevalence of any SUD, alcohol use disorder, drug use disorder, and cigarette smoking were analyzed using Kaplan-Meier failure functions and Cox proportional hazards models to account for the age of onset of SUD. We used the earliest age at onset in computing the survival time for cases and the age at assessment as the time of censoring for noncases.
Analyses were performed using regression models with robust standard errors to account for the non-independence of family members. Tests were two-tailed and performed at the 0.05 alpha level using Stata (Version 15.1) [25]. Data are presented as percentages, absolute numbers, or mean ± standard deviation (SD) unless otherwise described.
Results
Sociodemographic Characteristics
There were significant differences between ASD, ADHD, and control subjects in sex, SES, and IQ, but not in age or race (Table 1). The mean age for each group was 20.0 ± 10.3 years for ASD subjects, 21.6 ± 12.1 years for ADHD subjects, and 19.6 ± 8.5 years for control subjects. ASD subjects had a significantly higher proportion of males and lower IQ compared to ADHD and control subjects, and those in the control group had a lower SES compared to the other two groups. The differences in sex, but not SES or IQ, were considered clinically significant in addition to statistically significant, and thus all subsequent analyses controlled for sex.
Table 1.
Demographic characteristics of control, ADHD, and ASD subjects. Control and ADHD subjects were frequency-matched by age to ASD subjects
| Controls N=230 |
ADHD N=219 |
ASD N=230 |
Test Statistic | P-Value | |
|---|---|---|---|---|---|
| Age (years) | 19.6 ± 8.5 | 21.6 ± 12.1 | 20.0 ± 10.3 | F(2, 644)=1.90 | 0.15 |
| Socioeconomic Status† | 1.7 ± 0.8 | 1.9 ± 1.0a* | 2.0 ± 1.0a* | χ22=6.49 | 0.04 |
| Full Scale IQ† | 113.7 ± 11.7 | 110.6 ± 13.4a* | 102.4 ± 17.7a***b*** | F(2, 538)=20.22 | <0.001 |
| N (%) | N (%) | N (%) | |||
| Male | 97 (42) | 108 (49) | 182 (79)a***b*** | χ22=66.83 | <0.001 |
| Caucasian† | 209 (94) | 206 (96) | 187 (93) | χ22=2.10 | 0.35 |
Compared to controls.
Compared to ADHD.
P<0.05
P<0.005
P<0.001
Smaller sample size. Socioeconomic status: Controls: N=227, ADHD: N=214, ASD: N=191; Full Scale IQ: Controls: N=230, ADHD: N=218, ASD: N=125; Caucasian: Controls: N=222, ADHD: N=214, ASD: N=201
Psychopathology
Among ASD subjects, 69% had a lifetime prevalence of ADHD (N=155 out of 225 subjects, 5 patients did not have this information). Lifetime prevalence of other psychopathology (excluding ASD, ADHD, SUD, and cigarette smoking) within each group was 96% for ASD subjects, 89% for ADHD subjects, and 43% for control subjects (χ22=144.99; p<0.001). ASD subjects had significantly higher rates of other psychopathology compared to ADHD and control subjects, and ADHD subjects had significantly higher rates compared to control subjects. When looking at specific psychopathology, ASD subjects had significantly higher rates of bipolar disorder and multiple (≥2) anxiety disorders compared to ADHD and control subjects (Table 2). Additionally, ASD subjects had significantly higher rates of conduct disorder compared to control subjects, but significantly lower rates of conduct disorder compared to ADHD subjects. ADHD subjects had significantly higher rates of all examined psychopathology compared to control subjects.
Table 2.
Lifetime rates of psychiatric comorbidity
| Controls N=230 |
ADHD N=219 |
ASD N=230 |
Test Statistic |
P-Value | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Bipolar disorder | 1 (<1) | 30 (14)a*** | 51 (22)a***b* | 18.54 | <0.001 |
| Major depressive disorder | 51 (22) | 138 (63) a*** | 138 (61) a*** | 88.55 | <0.001 |
| Multiple (≥2) anxiety disorders† | 19 (12) | 66 (42)a*** | 145 (70)a***b*** | 88.97 | <0.001 |
| Oppositional defiant disorder/Antisocial personality disorder † | 20 (9) | 118 (54) a*** | 116 (52)a*** | 83.47 | <0.001 |
| Conduct disorder † | 7 (3) | 48 (22) a*** | 27 (12) a*b*** | 36.23 | <0.001 |
Compared to Controls.
Compared to ADHD.
P<0.05
P<0.005
P<0.001
Smaller sample size. Multiple anxiety disorders: Controls=156, ADHD=156, ASD=206; ODD/ASPD & Conduct: ASD=224-225
Any SUD
Lifetime prevalence of any SUD in ASD (N=29, 13%) was similar to controls (N=436, 19%, p≥0.05) but lower than observed with ADHD (N=78, 36%, p<0.001). This pattern remained the same when subjects were stratified by age (12 to 17 years: ASD: N=1, <1%, Controls: N=7, 5%, ADHD: N=16, 12%, p=0.003; 18 years and older: ASD: N=28, 33%, Controls: N=36, 42%, ADHD: N=62, 77%, p<0.001).
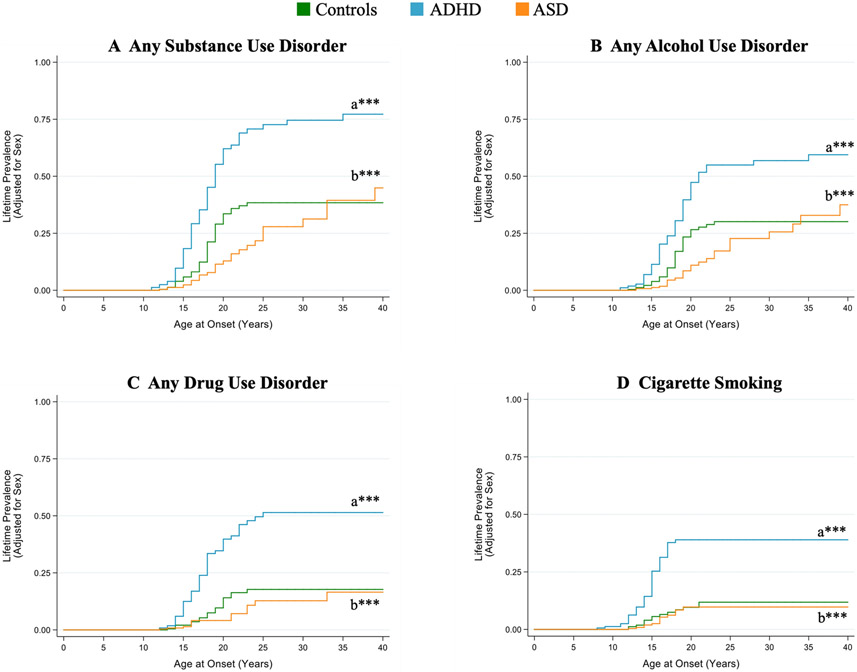
Significant group differences were noted in the Cox proportional hazards model (χ22=58.83; p<0.001) (Figure 2A). The risk of developing any SUD was significantly lower in ASD subjects compared to ADHD subjects (Hazard ratio [HR]=0.22, 95% confidence interval [CI]: 0.15, 0.34; p<0.001) and control (HR=0.62; 95% CI: 0.39, 0.98; p=0.04) subjects.
Fig. 2.
Kaplan-Meier failure functions for A) any substance use disorder (alcohol or drug abuse or dependence), B) any alcohol use disorder (abuse or dependence), C) any drug use disorder (abuse or dependence), and D) cigarette smoking
Sample sizes vary. Any SUD: Controls N=229, ADHD N=219, ASD N=230; Any AUD: Controls N=230, ADHD N=218, ASD N=230; Any DUD: Controls N=230, ADHD N=219, ASD N=230; Cigarette Smoking: Controls N=163, ADHD N=154, ASD N=230
aCompared to Controls. bCompared to ADHD
*P<0.0125, **P<0.005, ***P<0.001
Significant group differences were noted in age at any SUD onset (Table 3). ASD subjects were significantly older when they developed any SUD compared to ADHD and control subjects (p<0.005).
Table 3.
Age at onset of substance use disorders in control, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD) subjects
| Controls N=42 |
ADHD N=78 |
ASD N=29 |
Test Statistic | P-Value | |
|---|---|---|---|---|---|
| Any Substance Use Disorder Age at Onset (Years) | 17.4 ± 2.6 | 16.9 ± 3.8 | 21.7 ± 7.9a**b** | F(2, 148)=5.68 | 0.004 |
| Alcohol Use Disorder Age at Onset (Years)†,* | 17.4 ± 2.5 | 16.8 ± 3.1 | 22.5 ± 7.9a**b*** | F(2, 123)=6.69 | 0.002 |
| Drug Use Disorder Age at Onset (Years)† | 17.9 ± 2.8 | 17.0 ± 3.0 | 19.2 ± 5.8 | F(2, 83)=2.31 | 0.11 |
| Cigarette Smoking Age at Onset (Years)† | 15.8 ± 3.0 | 14.3 ± 2.1 | 15.7 ± 2.1 | F(2, 73)=2.50 | 0.09 |
Compared to controls.
Compared to ADHD.
P<0.0125
P<0.005
P<0.001
Smaller sample size. Alcohol Use Disorder: Controls: N=35. ADHD: N=64, ASD: N=26; Drug Use Disorder: Control: N=19, ADHD: N=52, ASD: N=13; Cigarette Smoking: Controls: N=14, ADHD: N=47, ASD: N=13
Two ADHD subjects were removed from this analysis because they had outlying onsets (both ≥35 years; mean age of onset was 17.5 years when outliers were included)
Alcohol Use Disorder
Lifetime prevalence of an alcohol use disorder in ASD (N=26, 11%) was similar to controls (N=36, 16%, p≥0.05) but significantly lower than observed with ADHD (N=65, 29%, p<0.001). Significant group differences were noted in the Cox proportional hazards model (χ22=39.03; p<0.001) (Figure 2B). The risk for developing an alcohol use disorder in ASD subjects was significantly lower compared to ADHD subjects (HR=0.26, 95% CI: 0.17, 0.41; p<0.001). There was a trend toward decreased risk for an alcohol use disorder when ASD were compared to controls (HR=0.62, 95% CI: 0.38, 1.03; p=0.06).
Significant differences among the groups were found for age at alcohol use disorder onset (Table 3). ASD subjects were significantly older when they developed an alcohol use disorder compared to ADHD and control subjects.
Drug Use Disorder
Lifetime prevalence of drug use disorder in ASD (N=13, 6%) was similar to controls (N=19, 8%, p≥0.05) but significantly lower than observed with ADHD (N=53, 24%, p<0.05). Significant group differences were noted in the Cox proportional hazards model (χ22=45.38; p<0.001) (Figure 2C). ASD subjects were at significantly decreased risk for developing a drug use disorder compared to ADHD subjects (HR=0.18, 95% CI: 0.10, 0.34, p<0.001) and there was no significant difference in the risk of a drug use disorder between ASD and control subjects (HR=0.69, 95% CI: 0.34, 1.42; p=0.32).
We did not find significant differences among the groups when looking at age at drug use disorder onset (Table 3). There were 13 ASD subjects with drug use disorder and the most common type was cannabis (n=9, 69%), followed by cocaine (n=2, 15%), stimulants (n=1, 8%), and sedatives (n=1, 8%).
Cigarette Smoking
Data on cigarette smoking were available for 163 control, 154 ADHD, and 230 ASD subjects. Lifetime prevalence of cigarette smoking in ASD (N=13, 6%) was similar to controls (N=14, 9%, p≥0.05) but significantly lower than observed with ADHD (N=47, 31%, p<0.001). Significant group differences were noted in the Cox proportional hazards model (χ22=45.68; p<0.001) (Figure 2D). ASD subjects were at significantly decreased risk for cigarette smoking compared to ADHD subjects (HR=0.17, 95% CI: 0.08, 0.32; p<0.001). There was no significant difference in risk of cigarette smoking between ASD and control subjects (HR=0.74, 95% CI: 0.33, 1.63; p=0.45).
We did not find significant differences among the groups when looking at age at onset of cigarette smoking (Table 3).
Discussion
The results of our analysis showed that individuals seeking psychiatric treatment with high-functioning ASD were at significantly decreased risk to develop a SUD (alcohol or drug use disorder) when compared to age-matched controls and those with ADHD. Additionally, when individuals with ASD developed a SUD they did so at a significantly older age than ADHD and control subjects. This study brings increased attention to an understudied but important co-occurring disorder with ASD—SUD. Although individuals with high-functioning ASD are at decreased risk to develop a SUD it is important to continue to screen for substance use in this population through young adulthood.
Our finding showing that relative to individuals with ADHD and controls, individuals with high functioning ASD were at significantly decreased risk to develop a SUD (80% and 40% reductions respectively) relative to individuals with ADHD, are consistent with those found in referred adults with ASD [8,9]. Our findings are inconsistent, however, with larger registry studies that have found individuals with ASD to be at elevated risk for SUD when compared to age and sex-matched controls [11,12]. Additional research is needed to help clarify SUD risk among treatment and non-treatment seeking individuals with high functioning ASD.
Although the risks for alcohol use disorder, drug use disorder, and cigarette smoking were statistically different between the ASD and ADHD subjects (all p<0.05), there were no statistical differences in risks between the ASD and control groups. SUD subgroup differences have been examined in non-treatment seeking individuals with ASD with varying results [12,10]. In our study the smaller sample size in subgroups and subsequent variability in the data may have limited our ability to detect statistically significant differences between ASD and control groups. Since it is unclear if individuals with ASD have a substance specific vulnerability, and differences exist in acute and chronic health risks associated with substances, continued research is needed on ASD risk for different types of substance use/disorder.
A noteworthy finding from our study was that individuals with ASD develop SUD at a later age relative to those with ADHD and controls. There was no difference, however, in the age of onset of cigarette smoking between individuals with ASD and those with ADHD or controls. Our findings are discrepant with those of Sizoo et al. (2010) that found no difference in age of onset for SUD between adults with ASD and ADHD, but did report a later age of onset of cigarette smoking in adults with ASD when compared to those with ADHD [8]. In both our study and Sizoo et al., (2010)’s, cigarette smoking began before individuals with ASD developed a SUD [8]. This developmental pattern of substance use with early cigarette use is consistent with the broader literature on patterns of substance use in young people [26].
The consistent pattern of decreased risk for SUD and later onset of SUD among individuals with ASD relative to individuals with ADHD is notable since 69% of individuals with ASD in our sample had a lifetime history of ADHD. This finding suggests that despite a large portion of individuals with ASD having increased risk for SUD due to co-occurring ADHD their ASD and/or other psychiatric co-morbidity may have mitigated this risk. Unfortunately, our sample size limited our ability to formally explore the impact of psychiatric co-morbidity on the relationship between ASD and SUD. Research on the impact of psychiatric co-morbidity on the association between ASD and SUD has been limited to date, although a web-based survey of students entering college who self-identified as having ASD was able to examine associations between a history of alcohol use and ASD co-morbidity [27]. This study found individuals with ASD and a learning disorder when compared to those with ASD only were 2.5 times more likely to have drank alcohol in high school. Additional research to inform clinical care is needed with large samples of individuals with ASD to examine the risk for a SUD in the context of co-occurring psychopathology.
Although the reasons for the decreased risk for SUD and later age of onset of these disorders in individuals with high functioning ASD in our sample remain unclear several explanations are plausible. It is possible that the delay in social development and unique traits related to ASD, such as rule-boundness and cognitive rigidity, may moderate the risk for SUD. For example, adolescents with ASD may not be included in social events where they would have access to substances for misuse. Likewise, adolescents with ASD may lack the social skills and social network needed to obtain substances for misuse from peers or strangers. Alternatively, they may not think it is acceptable to use cigarettes or alcohol until they are old enough to legally do so. Since social skills can impact access to substances, additional research that formally assesses the degree of social impairment when evaluating SUD risk among individuals with ASD is needed.
Study strengths include systematic assessment of all subjects which allowed us to examine for clinical differences between subjects with high-functioning ASD, ADHD, and controls and allowed for the examination of substance specific differences. However, the findings from our study need to be considered in the context of some methodologic limitations. Although subjects were systematically assessed, mediators and moderators of SUD risk, such as perception of risk and family history of alcohol and drug use, were not assessed which would have provided a more comprehensive understanding of the findings. Furthermore, although all subjects were systematically evaluated with similar procedures, the ASD subjects presented for clinical care and the ADHD and control subjects were ascertained through an observational research study. Samples of convenience drawn from clinical samples may have more complicated co-morbidity and/or psychosocial stressors that differ from samples purposefully recruited for research which can impact outcome. For our study, the control and ADHD subjects were largely recruited from clinical settings which decreases unmeasured differences between groups. Additionally, although the ASD subjects were systemically evaluated with structured interviews, the ASD diagnosis was established clinically and diagnostic aids commonly used in research, such as the Autism Diagnosis Observation Schedule, were not applied. The subjects were also ascertained over a broad time frame and the diagnostic criteria used to assess for ASD changed over this period. Despite this, the diagnostic criteria for ASD include autistic traits that are consistent across versions of DSM. Although the DSM versions may differ in categorization of subtypes of ASD, they are substantially consistent in eliciting significant social impairment associated with ASD. Furthermore, our sample only included intellectually capable individuals with ASD which many not generalize to ASD populations with intellectual disability. Another limitation for our study is that we had missing data on cigarette smoking in the control and ADHD groups. However, our sample size was still relatively large, and it did not affect our power to detect differences. Nonetheless, future studies would benefit from groups balanced in sample size when examining cigarette smoking. Lastly, since the sample was mostly Caucasian our findings may not generalize to other ethnic groups.
Despite limitations, our study demonstrated that individuals with high-functioning ASD, when compared to those with ADHD and controls, were at decreased risk to develop a SUD (alcohol or drug use disorder), and when they did develop SUD they did so at an older age.
Funding:
This work was supported by the National Institutes of Health and the National Institute of Drug Abuse (Grant Number: K12DA00357-17) and the Massachusetts General Hospital Pediatric Psychopharmacology Council.
Footnotes
Conflicts of Interest/Competing Interests:
Amy Yule, MD is currently receiving grant support from the NIH (NIDA). She was a consultant to the Phoenix House from 2015 to 2017 and is currently a consultant to the Gavin House and BayCove Human Services (clinical services).
Joseph Biederman, MD, in the past 36 months, receives/received research support from the following sources: Genentech, Headspace Inc., Lundbeck AS, Neurocentria Inc., PamLab, Pfizer Pharmaceuticals, Roche TCRC Inc., Shire Pharmaceuticals Inc., and Sunovion Pharmaceuticals Inc. He is/was a consultant for Aevi Genomics, Alcobra, Akili, Guidepoint, Ironshore, Jazz Pharma, Medgenics, Piper Jaffray, and Shire. He received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. Dr. Biederman had a financial interest in Avekshan LLC, a company that develops treatments for attention deficit hyperactivity disorder (ADHD); his interests were reviewed and managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. Through MGH corporate licensing, he has a US Patent (#14/027,676) for a non-stimulant treatment for ADHD, a US Patent (#10245271) on a treatment of impaired cognitive flexibility, and a patent pending (#61/233,686) on a method to prevent stimulant abuse. Dr. Biederman’s program received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Bracket Global, Ingenix, Prophase, Shire, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH.
Timothy Wilens, MD receives or has received grant support from the following sources: NIH(NIDA). Dr. Timothy Wilens is or has been a consultant for Neurovance/Otsuka, Ironshore, KemPharm and Vallon. Dr. Timothy Wilens has published books: Straight Talk About Psychiatric Medications for Kids (Guilford Press); and co/edited books ADHD in Adults and Children (Cambridge University Press), Massachusetts General Hospital Comprehensive Clinical Psychiatry (Elsevier) and Massachusetts General Hospital Psychopharmacology and Neurotherapeutics (Elsevier). Dr. Wilens is co/owner of a copyrighted diagnostic questionnaire (Before School Functioning Questionnaire). Dr. Wilens has a licensing agreement with Ironshore (BSFQ Questionnaire). Dr. Wilens is Chief, Division of Child and Adolescent Psychiatry and (Co) Director of the Center for Addiction Medicine at Massachusetts General Hospital. He serves as a clinical consultant to the US National Football League (ERM Associates), U.S. Minor/Major League Baseball; Phoenix House/Gavin Foundation and Bay Cove Human Services.
Gagan Joshi, MD is currently receiving research support from F. Hoffmann-La Roche Ltd. as a site Principal Investigator (PI) for two multi-site clinical trials and the Demarest Lloyd, Jr. Foundation. In 2019, he received a speaker’s honorarium from the American Academy of Child and Adolescent Psychiatry, In 2018, he was supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under Award Number K23MH100450, Pfizer Pharmaceuticals, and the Simons Center for the Social Brain as a PI for investigator-initiated studies. He also served on the Governor's Council for Medical Research and Treatment of Autism in New Jersey and as a reviewer and member of an editorial board for the NIMH. He received speaker’s honoraria from the Israeli Society of ADHD and the Canadian Academy of Child and Adolescent Psychiatry.
Maura DiSalvo, MPH, Nina T. Dallenbach, BS, and Daria Taubin, BA declare that they have no conflict of interest.
Availability of data and material:
The datasets generated during and/or analyzed during the current study are not publicly available.
Ethics approval:
The human research committee at Massachusetts General Hospital approved this study, and this study has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate:
For subjects with ADHD and controls, adults provided written informed consent to participate and for children under the age of 18 years, parents provided written informed consent to participate, and children and adolescents provided written assent to participate. For subjects with ASD, we received institutional review board approval to review, analyze, and report anonymously on these subjects and a waiver of informed consent was granted.
Consent for publication:
Not applicable. Information is anonymized and the submission does not include images that may identify any persons.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect postacceptance improvements, or any corrections. Use of this AM is subject to the publisher's embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature's terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- 1.Association AP (2013) Diagnostic and Statistical Manual of Mental Health Disorders, Fifth Edition (DSM-5). American Psychiatric Publishing, Arlington, VA [Google Scholar]
- 2.Murphy CM, Wilson CE, Robertson DM, Ecker C, Daly EM, Hammond N, Galanopoulos A, Dud I, Murphy DG, McAlonan GM (2016) Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 12:1669–1686. doi: 10.2147/NDT.S65455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courchesne E, Campbell K, Solso S (2011) Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 1380:138–145. doi: 10.1016/j.brainres.2010.09.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG (2015) The epidemiology and global burden of autism spectrum disorders. Psychol Med 45 (3):601–613. doi: 10.1017/S003329171400172X [DOI] [PubMed] [Google Scholar]
- 5.Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Kotarski M, Walls S, Biederman J (2010) The Heavy Burden of Psychiatric Comorbidity in Youth with Autism Spectrum Disorders: A Large Comparative Study of a Psychiatrically Referred Population. J Autism Dev Disord 40 (11):1361–1370. doi: 10.1007/s10803-010-0996-9 [DOI] [PubMed] [Google Scholar]
- 6.Joshi G, Wozniak J, Petty C, Martelon MK, Fried R, Bolfek A, Kotte A, Stevens J, Furtak SL, Bourgeois M, Caruso J, Caron A, Biederman J (2013) Psychiatric Comorbidity and Functioning in a Clinically Referred Population of Adults with Autism Spectrum Disorders: A Comparative Study. J Autism Dev Disord 43 (6):1314–1325. doi: 10.1007/s10803-012-1679-5 [DOI] [PubMed] [Google Scholar]
- 7.Santosh PJ, Mijovic A (2006) Does pervasive developmental disorder protect children and adolescents against drug and alcohol use? Eur Child Adolesc Psychiatry 15 (4):183–188. doi: 10.1007/s00787-005-0517-0 [DOI] [PubMed] [Google Scholar]
- 8.Sizoo B, van den Brink W, Koeter M, Gorissen van Eenige M, van Wijngaarden-Cremers P, van der Gaag RJ (2010) Treatment seeking adults with autism or ADHD and co-morbid substance use disorder: prevalence, risk factors and functional disability. Drug and alcohol dependence 107 (1):44–50. doi: 10.1016/j.drugalcdep.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 9.Lever AG, Geurts HM (2016) Psychiatric Co-occurring Symptoms and Disorders in Young, Middle-Aged, and Older Adults with Autism Spectrum Disorder. J Autism Dev Disord 46 (6):1916–1930. doi: 10.1007/s10803-016-2722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, Kripke C (2015) The health status of adults on the autism spectrum. Autism 19 (7):814–823. doi: 10.1177/1362361315577517 [DOI] [PubMed] [Google Scholar]
- 11.Huang JS, Yang FC, Chien WC, Yeh TC, Chung CH, Tsai CK, Tsai SJ, Yang SS, Tzeng NS, Chen MH, Liang CS (2021) Risk of Substance Use Disorder and Its Associations With Comorbidities and Psychotropic Agents in Patients With Autism. JAMA Pediatr 175 (2):e205371. doi: 10.1001/jamapediatrics.2020.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butwicka A, Langstrom N, Larsson H, Lundstrom S, Serlachius E, Almqvist C, Frisen L, Lichtenstein P (2017) Increased Risk for Substance Use-Related Problems in Autism Spectrum Disorders: A Population-Based Cohort Study. J Autism Dev Disord 47 (1):80–89. doi: 10.1007/s10803-016-2914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesney E, Goodwin GM, Fazel S (2014) Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 13 (2):153–160. doi: 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T (2015) The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One 10 (2):e0116820. doi: 10.1371/journal.pone.0116820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronenberg LM, Goossens PJ, van Etten DM, van Achterberg T, van den Brink W (2015) Need for care and life satisfaction in adult substance use disorder patients with and without attention deficit hyperactivity disorder (ADHD) or autism spectrum disorder (ASD). Perspect Psychiatr Care 51 (1):4–15. doi: 10.1111/ppc.12056 [DOI] [PubMed] [Google Scholar]
- 16.Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, Mennin D, Marrs A, Ouellette C, Moore P, Spencer T, Norman D, Wilens T, Kraus I, Perrin J (1996) A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Archives of General Psychiatry 53 (5):437–446 [DOI] [PubMed] [Google Scholar]
- 17.Biederman J, Monuteaux M, Mick E, Spencer T, Wilens T, Klein K, Price JE, Faraone SV (2006) Psychopathology in females with attention-deficit/hyperactivity disorder: A controlled, five-year prospective study. Biological Psychiatry 60 (10):1098–1105 [DOI] [PubMed] [Google Scholar]
- 18.Faraone SV, Biederman J, Spencer TJ, Mick E, Murray K, Petty C, Adamson JJ, Monuteaux MC (2006) Diagnosing adult attention deficit hyperactivity disorder: Are late onset and subthreshold diagnoses valid? Am J Psychiatry 163 (10):1720–1729 [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini PJ (2000) Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS). J Am Acad Child Adolesc Psychiatry 39 (1):49–58 [DOI] [PubMed] [Google Scholar]
- 20.First M, Spitzer R, Gibbon M, Williams JB (1997) Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press, Washington, D.C. [Google Scholar]
- 21.Wechsler D (1991) Manual for the Wechsler Intelligence Scale for Children - Third Edition. The Psychological Corporation, Harcourt Brace Jovanovich, Inc., San Antonio [Google Scholar]
- 22.Wechsler D (1997) Manual for the Wechsler Adult Intelligence Scale - Third Edition. Psychological Corporation, San Antonio, TX [Google Scholar]
- 23.Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence (WASI). 4th edn. The Psychological Corporation, San Antonio, Tx [Google Scholar]
- 24.Hollingshead AB (1975) Four factor index of social status. Four factor index of social status. Yale University Press, New Haven, CT [Google Scholar]
- 25.Stata Statistical Software: Release 15 (2017) StataCorp LLC. [Google Scholar]
- 26.Keyes KM, Hamilton A, Kandel DB (2016) Birth Cohorts Analysis of Adolescent Cigarette Smoking and Subsequent Marijuana and Cocaine Use. Am J Public Health 106 (6):1143–1149. doi: 10.2105/AJPH.2016.303128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturm A, Kasari C (2019) Academic and psychosocial characteristics of incoming college freshmen with autism spectrum disorder: The role of comorbidity and gender. Autism Res 12 (6):931–940. doi: 10.1002/aur.2099 [DOI] [PubMed] [Google Scholar]