Abstract
PURPOSE
We aimed to evaluate the efficacy and feasibility of patient-reported outcome (PRO)-based symptom management in the early period after lung cancer surgery.
METHODS
Before surgery, patients with clinically diagnosed lung cancer were randomly assigned 1:1 to receive postoperative PRO-based symptom management or usual care. All patients reported symptoms on MD Anderson Symptom Inventory-Lung Cancer presurgery, daily postsurgery, and twice a week after discharge for up to 4 weeks via an electronic PRO system. In the intervention group, treating surgeons responded to overthreshold electronic alerts driven by any of the five target symptom scores (score ≥ 4 on a 0-10 scale for pain, fatigue, disturbed sleep, shortness of breath, and coughing). The control group patients received usual care and no alerts were generated. The primary outcome was the number of symptom threshold events (any target symptom with a score of ≥ 4) at discharge. Per-protocol analyses were conducted.
RESULTS
Of the 166 participants, 83 were randomly allocated to each group. At discharge, the intervention group reported fewer symptom threshold events than the control group (median [interquartile range], 0 [0-2] v 2 [0-3]; P = .007). At 4 weeks postdischarge, this difference was maintained between the intervention and control groups (median [interquartile range], 0 [0-0] v 0 [0-1]; P = .018). The intervention group had a lower complication rate than the control group (21.5% v 40.6%; P = .019). Surgeons spent a median of 3 minutes managing an alert.
CONCLUSION
PRO-based symptom management after lung cancer surgery showed lower symptom burden and fewer complications than usual care for up to 4 weeks postdischarge.
INTRODUCTION
Lung cancer is the second most common cancer worldwide.1 The number of patients with lung cancer who are eligible for surgery is increasing owing to the use of computed tomography in screening.2 The symptom burden of patients undergoing lung cancer surgery is high, especially in the early postoperative phase.3,4 Usual symptom management is reactive and heavily reliant on routine ward rounds or hospital visits; thus, clinicians often fail to detect patients' severe symptoms timely,5 especially after discharge.6,7
CONTEXT
Key Objective
Timely and effective symptom management after major surgery is crucial for providing high-quality, patient-centered postoperative care. This randomized multicenter trial, the first patient-reported outcome (PRO)-based intervention study in a surgical setting in China, aimed to identify the benefits and feasibility of proactive symptom management after lung cancer surgery.
Knowledge Generated
Patients receiving PRO-based symptom management after lung cancer surgery had lower symptom burden, better functional status, and fewer complications for up to 4 weeks postdischarge than those who received usual care. This approach, comprising electronic symptom monitoring and response to overthreshold alerts driven by targeted symptoms, had an acceptable clinician burden, high clinician acceptability, and high patient satisfaction.
Relevance
PRO-based proactive symptom management might be the preferred postoperative care approach for patients undergoing lung cancer surgery.
Using patient-reported outcomes (PROs) to capture patients' symptoms is crucial to provide value-based, high-quality, and patient-centered care.8,9 Previous studies have reported that PRO-based proactive symptom monitoring can reduce symptom burden,10,11 improve physical well-being,12 enhance quality of life (QOL),6 reduce emergency room visits,6,7 and prolong survival time.13,14 Nevertheless, only two randomized controlled trials (RCTs) have been conducted in a surgical population.11,15 Both trials focused on discharge settings and only used PROs to evaluate the efficacy of symptom monitoring. In addition, given that both trials were conducted in Western countries, it remains unknown whether such results can be replicated in Eastern countries.
Thus, we conducted a multicenter RCT in China to evaluate the efficacy and feasibility of PRO-based symptom management in the early postoperative period (up to 4 weeks postdischarge) after lung cancer surgery.16 We hypothesized that patients receiving PRO-based symptom management would have a lower symptom burden than those receiving usual care.
METHODS
Study Design
This multicenter RCT was conducted in three tertiary hospitals in China. The initial study Protocol (online only) has been published previously.16 This trial was approved by the institutional review board of the three hospitals and was registered in the Chinese Clinical Trial Registry (CN-PRO-Lung 2; identifier: ChiCTR1900020846). Participants provided written informed consent.
Patients
Before patient enrollment, investigators at each center were trained using a standard operating procedure handbook.16 Eligible patients were age 18-75 years, had a clinical diagnosis of lung cancer with stage I-IIIA (8th edition),17 were scheduled to undergo surgery, and were willing and able to fill out the electronic questionnaire (e-questionnaire) on their smartphones or tablets. Exclusion criteria were previous neoadjuvant therapy, other malignancies, inability to understand the research contents, previous chest surgery, and daily analgesics use. Enhanced recovery after surgery pathway was not part of perioperative care in the participating centers.18
Random Assignment and Blinding
At enrollment (typically 1-3 days before surgery), eligible patients were randomly assigned in a 1:1 ratio to receive postoperative PRO-based symptom management (the intervention group) or usual care (the control group). We used a predefined random assignment module on the Research Electronic Data Capture (REDCap)19 platform for random assignment, which ensured allocation concealment. Random assignment was stratified by participating centers. Surgeons had patients in both groups. Because of the nature of the study, patients and surgeons delivering the interventions were not blinded, but research nurses assisting with PRO data collection and data analysts were blinded to group allocation.
Trial Interventions
After random assignment, patients and participating surgeons were interconnected by an electronic Symptom Monitoring, Alerting, and Response System (SMARS).16 SMARS was developed by our team, which involves a data platform (REDCap)19 hosted in Sichuan Cancer Hospital since 2017, an electronic PRO (ePRO) system, and a communication service application (WeChat mini program, telephone or message).20 Each patient filled out the e-questionnaires of the MD Anderson Symptom Inventory-Lung Cancer module (MDASI-LC)21 and single-item QOL scale (SIQOL)22 through password-protected accounts on a personal electronic device; this was done once preoperatively (baseline), daily during postoperative hospitalization, and twice weekly postdischarge until 4 weeks or when adjuvant therapy was commenced.
MDASI-LC is a validated lung cancer–specific scale that includes 16 symptom items with scores ranging from 0 (no symptom) to 10 (worst symptom imaginable) and six functional items with scores ranging from 0 (no interference) to 10 (complete interference). SIQOL uses a 0-10 scale, with 0 representing worst QOL and 10 representing best QOL. Automatic short message reminders were sent to patients at 7 am and 2 pm. Additional manual reminders were delivered up to two times if a patient failed to complete the e-questionnaires at the scheduled time.
Patients in the intervention group received PRO-based symptom management postoperatively, wherein real-time electronic alerts were sent to treating surgeons if their reported scores reached the preset threshold (score ≥ 4 on a 0-10 scale, indicating moderate-to-severe symptom severity)23,24 in any of the predefined five target symptom scores (pain, fatigue, disturbed sleep, shortness of breath, and coughing). The surgeons responded to the alerts within 24 hours. On the basis of the alert information, interventions were usually carried out in person during morning and afternoon ward rounds in the hospitalization period and by means of messages or phone calls after discharge. Interventions mainly included consultation, patient education, medication prescription, and hospital visit suggestions, which were conducted according to relevant guidelines and consensus.23-27 Additionally, patients were allowed to seek medical help through the usual channels.
Patients in the control group received usual care. They filled out the e-questionnaires, but the reported symptoms did not generate alerts, and the surgeons could not access the reported scores. During hospitalization, the surgeons assessed the patients' symptoms through patient complaints during morning and afternoon ward rounds, and managed patients' symptom on the basis of the same guidelines and consensus for the intervention group.23-27 After discharge, patients did not receive proactive symptom management from their treating surgeons unless they actively sought medical help. For example, when they had severe symptoms, they could contact their treating team, seek online consultations, or go to a local hospital.
Outcomes and Measures
The primary outcome was the number of symptom threshold events at discharge. A symptom threshold event was defined as a target symptom score of ≥ 4 on a 0-10 scale. Hence, if on the day of discharge, a patient reported a score of 5 on pain, 6 on fatigue, 4 on disturbed sleep, 2 on shortness of breath, and 3 on coughing, then the number of symptom threshold events for this patient would be counted as 3.
The secondary outcomes included the following: the number of symptom threshold events at 4 weeks postdischarge, composite symptom score (average score of the five target symptoms), composite physical interference score (average score of MDASI-LC interference items of general activity, work, and walking), composite affective interference score (average score of MDASI-LC interference items of mood, relations with others, and enjoyment of life), QOL score, and revisit rate after discharge. All these scores range from 0 to 10, with high scores indicating more severe symptoms, more severe functional interference, or better QOL. The revisit rate after discharge was defined as the ratio of the number of patients who were readmitted to the inpatient department or visited the emergency room or clinic (because of problems related to previous surgery during the 4 weeks postdischarge study period) divided by the total number of patients. Other outcomes included postoperative complications, surgeon workload, surgeon acceptability, and patient satisfaction. Postoperative complications during the study period were recorded and assessed using the Clavien-Dindo classification system.28 Surgeon acceptability and patient satisfaction with the interventions were measured by surveys we specifically designed for this trial, with a 0-10 numeric rating scale and a 5-point Likert scale, respectively. The response time of each alert was calculated by the start time and the end time of the interventions as reported by the surgeons.
Statistical Analyses
The null hypothesis was rejected if the between-group difference in the number of symptom threshold events at discharge was ≥ 0.5 standard deviation. A sample size of 64 patients in each group was calculated using the Student's t-test for the primary outcome on the basis of a two-tailed α level of .05 and β error of .2. Considering the 20% attrition rate, 80 cases were needed for each group. However, the sample size was finally increased to 83 patients per group to meet the minimum number of 64 cases per group as the attrition rate in the PRO-based care group exceeded 20%.
This trial used per-protocol analyses.16 Patients were excluded from the final analysis on the basis of the withdrawal criteria if their surgery was canceled, were histologically diagnosed with nonprimary lung cancer after surgery, were hospitalized for > 14 days after surgery or readmitted to an intensive care unit (ePRO data collection might not be feasible in severe illness condition), had poor compliance to the interventions more than three times, withdrew their consent, or were lost to follow-up.
Available PRO data for the 14 time points were included in the analyses, including presurgery, postoperative in-hospital day 1-5, and postdischarge week 0.5-4. The primary outcome was compared between the two groups using the Wilcoxon-Mann-Whitney test, because of the non-normal distribution. The secondary outcomes of PRO scores between groups over time were analyzed using linear mixed-effects models. Patient group, time (days-from-surgery or days-from-discharge), and the interaction between patient group and time were specified as fixed effects. Subject and time were specified as random effects. Maximum likelihood estimation was used. Other outcomes were analyzed using chi-squared test, two-tailed Fisher's exact test, or descriptive statistics, as appropriate. Analyses were adjusted for participating center, categorized as cancer hospital and general hospital.29 Subgroup analyses were conducted in different types of participating centers. Intention-to-treat analyses were performed as sensitivity analyses. Two-sided P values < .05 were considered statistically significant. All analyses were conducted using SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Patients
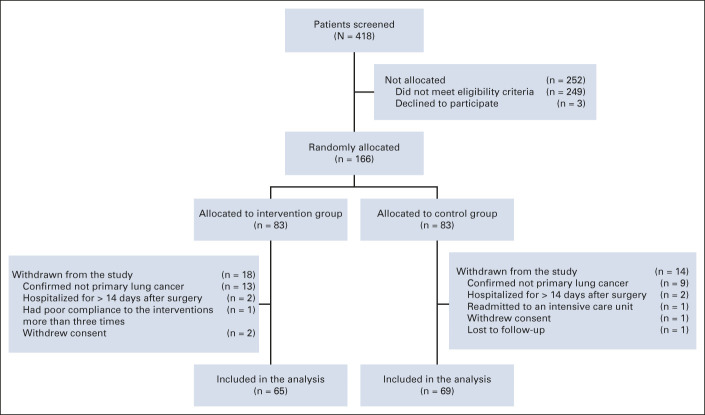
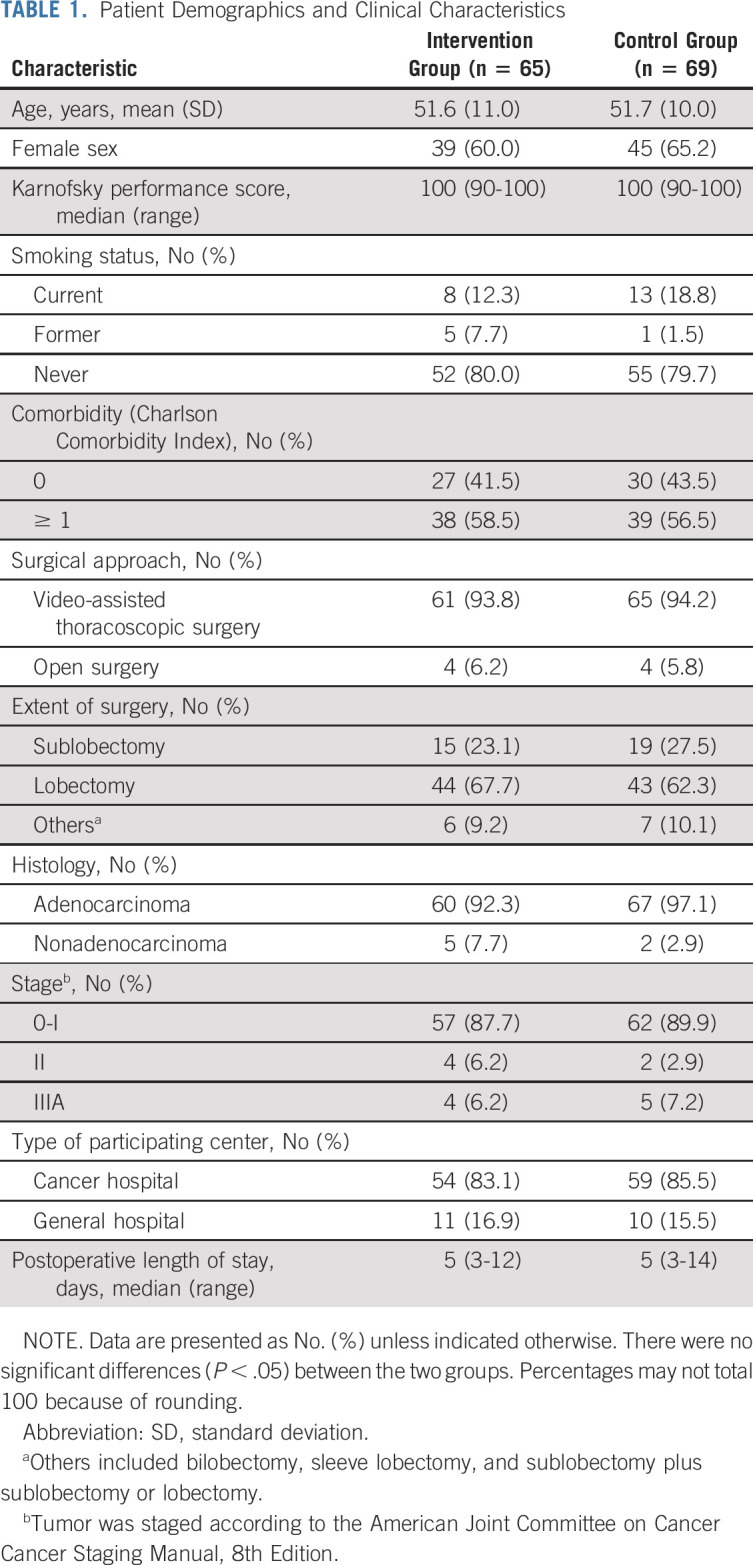
Among the 418 patients assessed for eligibility between November 2019 and August 2020, 249 were ineligible (Data Supplement, online only) and three declined to participate. Overall, 166 patients were randomly assigned, with 83 in each group. After random assignment, 32 (19.3%) patients met the withdrawal criteria, resulting in 65 patients in the intervention group and 69 patients in the control group (Fig 1). Table 1 shows the demographic and clinical characteristics of patients included in the analysis. There were no significant between-group differences. Comparison of demographic and clinical characteristics between patients included in and those excluded from the analysis did not show any statistically significant differences (Data Supplement). The median postoperative length of hospital stay was 5 days in both groups.
FIG 1.
CONSORT diagram.
TABLE 1.
Patient Demographics and Clinical Characteristics
Response Rates and Symptom Alerts
At baseline and discharge, the response rates to MDASI-LC were 100% (Data Supplement). During the postoperative hospitalization and 4 weeks after discharge, the intervention group generated 968 symptom threshold events that brought 417 alerts. One alert represented 1-5 symptom threshold events. Surgeons responded to 100% of the symptom alerts, and 71.7% (299 of 417) of the alert response times were recorded to identify the surgeon's burden.
Primary and Secondary Outcomes
At discharge, the number of symptom threshold events of the five target symptoms in the intervention group was significantly lower than in the control group (median [interquartile range], 0 [0-2] v 2 [0-3]; P = .007; Fig 2). Subgroup analyses showed a similar trend in both the cancer hospital (n = 113) and general hospital (n = 21), with P values of .004 and .971, respectively (Fig 2).
FIG 2.
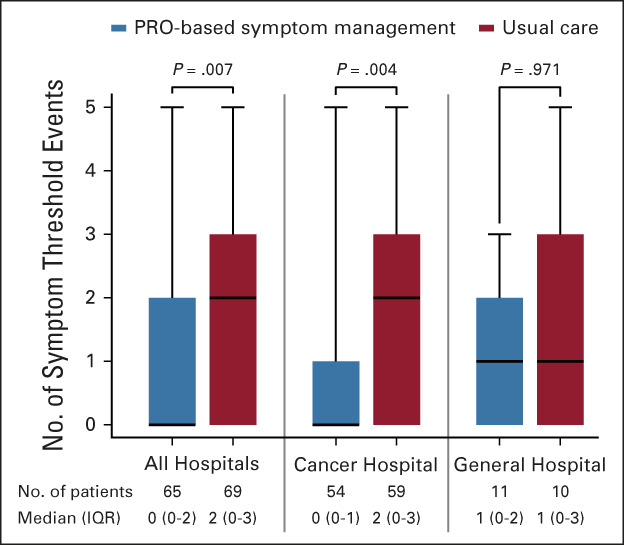
Number of symptom threshold events at discharge in all hospitals, the cancer hospital, and the general hospital. The box plot shows the median (horizontal line in the box), 25th and 75th quartiles (box limits), and minimum and maximum (bars). PRO, patient-reported outcome; IQR, interquartile range. PRO-based symptom management may be the preferred patient care approach following lung cancer surgery.
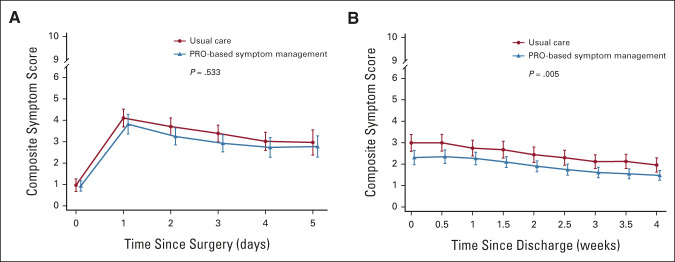
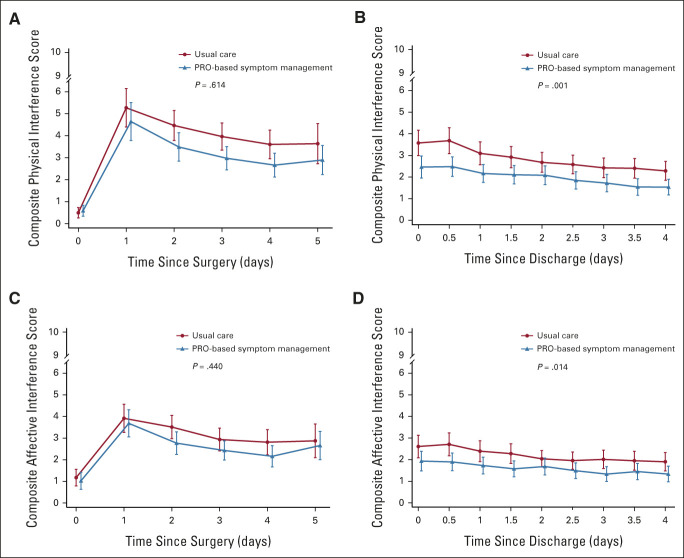
At 4 weeks postdischarge, the number of symptom threshold events in the intervention group was also significantly lower than that in the control group (median [interquartile range], 0 [0-0] v 0 [0-1]; P = .018). The composite symptom score of the five target symptoms was significantly lower in the intervention group than that in the control group (adjusted mean difference, –0.63; 95% CI, –1.07 to –0.19; P = .005) during the 4 weeks after discharge but was similar during the 5-day postoperative hospitalization (Fig 3). The composite physical interference score (adjusted mean difference, –1.09; 95% CI, –1.74 to –0.43; P = .001) and composite affective interference score (adjusted mean difference, –0.72; 95% CI, –1.28 to –0.15; P = .014) were significantly lower in the intervention group than in the control group during the 4 weeks after discharge; however, all were similar during the 5-day postoperative hospitalization period (Fig 4). The mean QOL score was not significantly different between the two groups during the 4 weeks after discharge (adjusted mean difference, –0.10; 95% CI, –0.85 to 0.65; P = .790) and the 5-day postoperative hospitalization period (adjusted mean difference, 0.004; 95% CI, –0.70 to 0.71; P = .992). No between-group differences were found in the revisit rate after discharge (intervention group v control group; 21.5% v 20.3%; adjusted relative risk, 1.07; 95% CI, 0.47 to 2.47; P = .868).
FIG 3.
Symptom severity over time. (A) Composite symptom score of the target symptoms (pain, fatigue, disturbed sleep, shortness of breath, and coughing) during hospitalization. (B) Composite symptom score of the target symptoms after discharge. High scores indicate more severe symptoms. I bars represent 95% CIs. PRO, patient-reported outcome.
FIG 4.
Functional interference over time. (A) Composite physical interference score (MDASI-LC general activity, work, and walking) during hospitalization. (B) Composite physical interference score after discharge. (C) Composite affective interference score (MDASI-LC mood, relations with others, and enjoyment of life) during hospitalization. (D) Composite affective interference score after discharge. High scores indicate more severe functional interference. I bars represent 95% CIs. MDASI-LC, MD Anderson Symptom Inventory-Lung Cancer module; PRO, patient-reported outcome.
Intention-to-treat analyses generated similar results as the per-protocol analyses for primary and secondary outcomes (Data Supplement).
Other Outcomes and Feasibility Report
The intervention group reported a lower postoperative complication rate (Clavien-Dindo grade I-IIIA) than the control group (21.5% v 40.6%; adjusted relative risk, 0.40; 95% CI, 0.19 to 0.86; P = .019). Surgeons spent a median of 3 (range, 1-27) minutes managing an alert. Overall, 24.7% of alerts took 5 or more minutes to respond. The acceptability of the PRO-based symptom management approach and SMARS among the surgeons was high, with a response rate of 100% and a minimum median score of 8 on 0-10 scales for questions 1-6 (higher scores represent higher acceptability; Data Supplement).
In the intervention group, 96.4% of patients thought that the PRO-based symptom management approach was helpful. The overall median score of satisfaction with this approach was 9 (range, 5-10; higher scores indicate greater satisfaction). Patients reported that this approach was very necessary (median score: 10; higher scores represent better outcomes) and that it did not interfere with their lives at all (median score: 0; lower scores represent less interference; Data Supplement).
DISCUSSION
This multicenter RCT examined the efficacy and feasibility of PRO-based symptom management in a surgical setting in China. Our data indicated that PRO-based symptom management after lung cancer surgery was associated with lower symptom burden, better functional status, and fewer complications in the early postoperative period. Moreover, this patient care approach—comprising electronic symptom monitoring and rapid response to the overthreshold alerts—had an acceptable surgeon burden, high surgeon acceptability, and high patient satisfaction from the current study.
Compared with the two previous RCTs of symptom monitoring in surgical settings,11,15 we further investigated the ePRO utility for postoperative care during the in-hospital period rather than only the postdischarge period, which gives a more comprehensive picture of PRO-based symptom management. In addition, we reported postoperative complications to validate the clinical benefit of symptom monitoring and intervention. Our primary findings were consistent with those of a previous RCT conducted in the United States.11 However, in our trial, the web-based ePRO system was used rather than the interactive voice response system, and the alerts were automatically sent to the treating surgeon rather than a nurse.
Two potential mechanisms may explain the benefits of the PRO-based symptom management approach. First, PRO-based symptom management proactively prompts clinicians to intervene early, before symptoms worsen and complications develop.6 Second, PRO-based symptom management can be administered remotely and in real time. Such a management system using telemedicine is especially helpful during the discharge period. Currently, the use of thoracoscopic techniques has shortened the length of hospital stay. However, patients are not fully recovered at discharge and may need continuing care after discharge.3 The usual postdischarge care hardly provides timely and remote care on patients' symptom.9 The use of ePRO monitoring and intervention may effectively fill this gap.30
Reducing the workload of clinicians and patients is crucial for the application of the PRO-based symptom management in practice.31,32 In this trial, treating surgeons had high acceptability for this approach, and the time spent on managing alerts was acceptable. This high acceptance and high response rate may be attributed to the use of an efficient ePRO system and to the integration of ePRO assessments into daily ward rounds during the in-hospital phase. More importantly, the PRO-based symptom management reduced complications and improved workflow efficiency, thus potentially saving clinicians' time rather than increasing it.33 Additionally, patients were also satisfied with this approach and reported that it did not interfere with their lives. It is noteworthy that the safety of large amount of ePRO data was well addressed by the institution-owned system, which communicated with the personal password–protected account under specific applications on an individual's device and the hospital's server.16
In previous RCTs of symptom monitoring that reported positive results,6,11,12 health care providers responded to 59.9%-84% of alerts, whereas in another RCT that reported negative results,34 health care providers rarely responded to alerts (only 1.9%). In the current trial, clinicians responded to all alerts. This suggests that the response to alerts by health care providers may be the key to the success of PRO-based symptom management. Moreover, the alert-direct-to-surgeon model maybe more efficient and beneficial to patients, given that surgeons can cover more professional concerns, and only doctors have the right to prescribe medications in China. However, to further reduce the burden on doctors and improve real-world feasibility, the ideal model may be one in which symptom alerts are intelligently triaged and then automatically fed into an appropriate pathway for intervention by self-management35 or a collaborative team of nurses and doctors.
This study has some limitations. First, a relatively large number of patients (28.7%) were excluded because of challenges in completing the ePRO, thus limiting the interpretation of our results to patients who were network users. Future studies should consider multiple PRO data collection methods (eg, paper, web, or telephone-based) to broaden the application of PRO-based intervention for real-world patient care. In addition, enhancing patient education, providing adequate support (eg, informative pamphlets), and developing a more user-friendly interface may facilitate the use of the ePRO system.36 Second, the strict criteria for inclusion and exclusion limited the generalizability of the trial results. Implementation of PRO-based symptom management in a more heterogeneous population is warranted in the future. Third, the recruitment and random assignment processes were performed before surgery in this trial considering that a substantial number of patients might be too sick to consent immediately after surgery. Potential bias might have been generated in the analyses because of excluding patients after random assignment.37 However, we did not find significant differences in demographic and clinical characteristics between patients included in and those excluded from the final analysis. In addition, the results for primary and second outcomes generated from intention-to-treat analyses were consistent with those from per-protocol analyses. Fourth, instruments for measuring surgeon acceptability and patient satisfaction were developed using an expert panel. Although we only used these scales for exploratory purposes, their validity and reliability need to be tested in future studies. Fifth, the trial focused on early postoperative recovery. Whether patients would benefit in the long term (ie, 3 months or 1 year postoperatively) needs further investigation.
In conclusion, PRO-based symptom management showed better symptom control than did usual care for patients undergoing lung cancer surgery in the early postoperative period. This approach also had fewer complications and high feasibility. Our findings suggest that PRO-based proactive symptom monitoring and intervention may be the preferred patient care approach following lung cancer surgery.
ACKNOWLEDGMENT
The authors thank all the trial participants and their families for their contributions. They also appreciate Ms Zhen Dai for her help with the data analyses, Dr Charles S. Cleeland of MD Anderson Cancer Center for his valuable comments on the manuscript, and Mr Zila Bi Que, Mr Xu Wang, and Mr Qing Guo for their help on technical development and following support of the Symptom Monitoring, Alerting and Response System (SMARS).
Xin Shelley Wang
Patents, Royalties, Other Intellectual Property: Symptom Assessment Systems, LLC
Cecilia Pompili
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: BD Medical, Medela
No other potential conflicts of interest were reported.
Listen to the podcast by Dr Sun at jcopodcast.libsynpro.com
PRIOR PRESENTATION
Presented at the 2020 Korean Association for Lung Cancer International Conference Virtual, November 19, 2020 (oral presentation).
SUPPORT
Supported by the Bethune Charitable Foundation (HZB-20181119-5), Sichuan Science and Technology Program (2019YFH0070), and National Natural Science Foundation of China (81872506).
Q.S., Q.L. and G.C. contributed equally to this work as senior authors.
DATA SHARING STATEMENT
The study Protocol was published and available for public read (https://bmjopen.bmj.com/content/9/8/e030041). Individual participant data that reported in this article will be available after deidentification, beginning 6 months after article publication. For purposes of nonprofit research or regulatory decision making, the data access will be granted with a signed agreement after approval of a proposal. All data request will be reviewed by the research committee at Sichuan Cancer Hospital to verify whether the request is subject to any intellectual property or confidentiality obligations.
AUTHOR CONTRIBUTIONS
Conception and design: Wei Dai, Wenhong Feng, Xing Wei, Bin Hu, Xiaozun Yang, Xin Wang, Qiang Li, Qiuling Shi
Financial support: Wei Dai, Qiuling Shi
Administrative support: Wenhong Feng, Xiaozun Yang, Xiaoqin Liu, Zhong Wu, Guowei Che, Qiang Li
Provision of study materials or patients: Wei Dai, Jia Liao, Xing Wei, Bin Hu, Bo Tian, Xiang Wang, Ping Xiao, Xin Wang, Fang Liu, Tianpeng Xie, Xiaojun Yang, Xiang Zhuang
Collection and assembly of data: Wei Dai, Yuanqiang Zhang, Shaohua Xie, Yaqin Wang, Xing Wei
Data analysis and interpretation: Wei Dai, Wenhong Feng, Yuanqiang Zhang, Xin Shelley Wang, Yangjun Liu, Cecilia Pompili, Wei Xu, Shaohua Xie, Jia Liao, Xing Wei, Bin Hu, Bo Tian, Xiaozun Yang, Xiang Wang, Ping Xiao, Qi Lai, Xin Wang, Bangrong Cao, Qifeng Wang, Fang Liu, Xiaoqin Liu, Tianpeng Xie, Xiaojun Yang, Xiang Zhuang, Zhong Wu, Guowei Che, Qiang Li, Qiuling Shi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: A Multicenter Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Xin Shelley Wang
Patents, Royalties, Other Intellectual Property: Symptom Assessment Systems, LLC
Cecilia Pompili
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: BD Medical, Medela
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Fan Y, Wang Y, et al. : China national lung cancer screening guideline with low-dose computed tomography (2018 version) [in Chinese]. Zhongguo Fei Ai Za Zhi 21:67-75, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagundes CP, Shi Q, Vaporciyan AA, et al. : Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 150:613-619.e612, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S, Chen Y, Yang L, et al. : Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs 22:1281-1290, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Di Maio M, Gallo C, Leighl NB, et al. : Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol 33:910-915, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girgis A, Durcinoska I, Arnold A, et al. : Web-based patient-reported outcome measures for personalized treatment and care (PROMPT-Care): Multicenter pragmatic nonrandomized trial. J Med Internet Res 22:e19685, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E: Patient-reported outcomes - Harnessing patients' voices to improve clinical care. N Engl J Med 376:105-108, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Sun V, Kim J: Symptom and functional recovery monitoring in thoracic surgery. J Thorac Dis 12:6931-6939, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nipp RD, El-Jawahri A, Ruddy M, et al. : Pilot randomized trial of an electronic symptom monitoring intervention for hospitalized patients with cancer. Ann Oncol 30:274-280, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleeland CS, Wang XS, Shi Q, et al. : Automated symptom alerts reduce postoperative symptom severity after cancer surgery: A randomized controlled clinical trial. J Clin Oncol 29:994-1000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Absolom K, Warrington L, Hudson E, et al. : Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol 39:734-747, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Basch E, Deal AM, Dueck AC, et al. : Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318:197-198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbera L, Sutradhar R, Seow H, et al. : The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: Results of a population-based retrospective matched cohort analysis. Cancer Med 9:7107-7115, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graetz I, Anderson JN, McKillop CN, et al. : Use of a web-based app to improve postoperative outcomes for patients receiving gynecological oncology care: A randomized controlled feasibility trial. Gynecol Oncol 150:311-317, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Dai W, Zhang Y, Feng W, et al. : Using patient-reported outcomes to manage postoperative symptoms in patients with lung cancer: Protocol for a multicentre, randomised controlled trial. BMJ Open 9:e030041, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstraw P, Chansky K, Crowley J, et al. : The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol 11:39-51, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Wei X, Yu H, Dai W, et al. : Patient-reported outcomes of video-assisted thoracoscopic surgery versus thoracotomy for locally advanced lung cancer: A longitudinal cohort study. Ann Surg Oncol 28:8358-8371, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377-381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montag C, Becker B, Gan C: The multipurpose application WeChat: A review on recent research. Front Psychol 9:2247, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza TR, Wang XS, Lu C, et al. : Measuring the symptom burden of lung cancer: The validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist 16:217-227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloan JA, Loprinzi CL, Kuross SA, et al. : Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol 16:3662-3673, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Swarm RA, Paice JA, Anghelescu DL, et al. : Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17:977-1007, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Bower JE, Bak K, Berger A, et al. : Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol 32:1840-1850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molassiotis A, Smith JA, Bennett MI, et al. : Clinical expert guidelines for the management of cough in lung cancer: Report of a UK task group on cough. Cough 6:9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denlinger CS, Ligibel JA, Are M, et al. : Survivorship: Sleep disorders, version 1.2014. J Natl Compr Canc Netw 12:630-642, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parshall MB, Schwartzstein RM, Adams L, et al. : An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185:435-452, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dindo D, Demartines N, Clavien PA: Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205-213, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleeland CS, Mendoza TR, Wang XS, et al. : Levels of symptom burden during chemotherapy for advanced lung cancer: Differences between public hospitals and a tertiary cancer center. J Clin Oncol 29:2859-2865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basch E, Mody GN, Dueck AC: Electronic patient-reported outcomes as digital therapeutics to improve cancer outcomes. JCO Oncol Pract 16:541-542, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Smith AB, Schwarze ML: Translating patient-reported outcomes from surgical research to clinical care. JAMA Surg 152:811-812, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Philpot LM, Barnes SA, Brown RM, et al. : Barriers and benefits to the use of patient-reported outcome measures in routine clinical care: A qualitative study. Am J Med Qual 33:359-364, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Rotenstein LS, Huckman RS, Wagle NW: Making patients and doctors happier—The potential of patient-reported outcomes. N Engl J Med 377:1309-1312, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Mooney KH, Beck SL, Friedman RH, et al. : Automated monitoring of symptoms during ambulatory chemotherapy and oncology providers' use of the information: A randomized controlled clinical trial. Support Care Cancer 22:2343-2350, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. : Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: A randomised, controlled trial. Lancet Oncol 21:80-94, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen H, Butow P, Dhillon H, et al. : A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci 68:186-195, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nüesch E, Trelle S, Reichenbach S, et al. : The effects of excluding patients from the analysis in randomised controlled trials: Meta-epidemiological study. BMJ 339:b3244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study Protocol was published and available for public read (https://bmjopen.bmj.com/content/9/8/e030041). Individual participant data that reported in this article will be available after deidentification, beginning 6 months after article publication. For purposes of nonprofit research or regulatory decision making, the data access will be granted with a signed agreement after approval of a proposal. All data request will be reviewed by the research committee at Sichuan Cancer Hospital to verify whether the request is subject to any intellectual property or confidentiality obligations.