Abstract
PURPOSE
Distant metastases are present in 6% or more of patients with newly diagnosed breast cancer. In this context, locoregional therapy for the intact primary tumor has been hypothesized to improve overall survival (OS), but clinical trials have reported conflicting results.
METHODS
Women presenting with metastatic breast cancer and an intact primary tumor received systemic therapy for 4-8 months; if no disease progression occurred, they were randomly assigned to locoregional therapy for the primary site (surgery and radiotherapy per standards for nonmetastatic disease) or continuing sysmetic therapy. The primary end point was OS; locoregional control and quality of life were secondary end points. The trial design provided 85% power to detect a 19.3% absolute difference in the 3-year OS rate in randomly assigned patients. The stratified log-rank test and Cox proportional hazards model were used to compare OS between arms. Cumulative incidence of locoregional progression was compared using Gray's test. Quality-of-life assessment used standard instruments.
RESULTS
Of 390 participants enrolled, 256 were randomly assigned: 131 to continued systemic therapy and 125 to early locoregional therapy. The 3-year OS was 67.9% without and 68.4% with early locoregional therapy (hazard ratio = 1.11; 90% CI, 0.82 to 1.52; P = .57). The median OS was 53.1 months (95% CI, 47.9 to not estimable) in the systemic therapy arm and 54.9 months (95% CI, 46.7 to not estimable) in the locoregional therapy arm. Locoregional progression was less frequent in those randomly assigned to locoregional therapy (3-year rate: 16.3% v 39.8%; P < .001). Quality-of-life measures were largely similar between arms.
CONCLUSION
Early locoregional therapy for the primary site did not improve survival in patients presenting with metastatic breast cancer. Although it was associated with improved locoregional control, this had no overall impact on quality of life.
INTRODUCTION
Locoregional therapy (surgery, accompanied by radiotherapy when indicated) is a potentially curative treatment in patients with nonmetastatic breast cancer. However, 6% or more of new breast cancer diagnoses in the United States and globally1,2 occur in the presence of concurrent distant metastases. In this setting, where systemic therapy is the primary treatment, the role of locoregional therapy is uncertain unless required for local palliation.
CONTEXT
Key Objective
For patients presenting at initial diagnosis with metastatic breast cancer, does the use of locoregional therapy for the primary site result in improved survival? Recent randomized trials have produced conflicting results.
Knowledge Generated
After a period of initial systemic therapy that either stabilized or decreased distant disease burden, patients receiving locoregional therapy for the primary tumor did not experience improved overall survival, compared with patients treated with continued systemic therapy. Although local progression occurred significantly less frequently among patients randomly assigned to locoregional therapy, their overall quality of life was similar to those randomly assigned to continued systemic therapy.
Relevance
Locoregional therapy for the primary breast tumor should not be recommended to patients with an asymptomatic primary tumor and distant metastases, with the expectation of improved survival. If such a treatment is contemplated to decrease the risk of future local progression, the lack of evidence for improved quality of life should also be discussed.
About 15 years ago, the possibility that locoregional therapy may aid survival was entertained on the basis of biologic and clinical data. At the biologic level, it appeared that mesenchymal stem cells in the primary tumor promote metastasis,3,4 suggesting benefit from primary tumor resection. At the clinical level, re-examination of the value of locoregional therapy for the intact primary site was prompted by a trial in patients with metastatic renal cell carcinoma, reporting modest survival improvement with nephrectomy.5 Subsequently, a series of retrospective analyses using breast cancer data6-11 generated the hypothesis that early locoregional therapy for the primary site may improve survival for patients with stage IV breast cancer.6 However, biases in the retrospective data (women receiving primary site local therapy were younger and had biologically favorable tumors and lower volume metastatic disease10,12) made clear that randomized trials were needed to test this hypothesis. Three such trials have been reported,13-15 showing conflicting results.
We hypothesized that among those presenting initially with metastatic breast cancer, the use of early locoregional treatment for the primary tumor would prolong overall survival (OS) only if the metastatic disease was not resistant to initial systemic therapy. To test this hypothesis, we performed a prospective, randomized phase III trial.
METHODS
Protocol Conduct and Eligibility Criteria
The trial (E2108) was coordinated by the ECOG-ACRIN Cancer Research Group and sponsored by the National Cancer Institute. Adults with pathologically confirmed locoregional breast cancer and distant metastases were eligible for registration, if systemic therapy had been initiated within 8 weeks after diagnosis. Written informed consent, approved by the local institutional review board, was required. This confirmed willingness to be randomly assigned to either early locoregional therapy after initial systemic therapy or continuation of systemic therapy. Biopsy confirmation of a suspected solitary metastatic lesion was required for eligibility. Patients who had already initiated systemic therapy were eligible for registration if the duration of systemic therapy was ≤ 30 weeks. Patients with a history of invasive malignancy ≥ 5 years previously were included (if recurrence-free), but not those with synchronous contralateral breast cancer. The full Protocol (online only) is available at the JCO website.
Initial Systemic Therapy
All registered participants received systemic therapy for 16-32 weeks, guided by National Comprehensive Cancer Center Network (NCCN) guidelines, according to age, menopausal status, metastatic sites, and tumor marker profile of the primary tumor. Those whose disease progressed while receiving initial systemic therapy were not randomly assigned but were followed for survival for up to 5 years. Progression was defined as the occurrence of new lesions, enlargement of existing sites by ≥ 20% in longest diameter, or symptomatic deterioration.
Random Assignment
After a maximum of 32 weeks of systemic therapy, those with stable or responsive disease and a resectable primary tumor were randomly assigned to continuation of systemic therapy or locoregional therapy. Random assignment was conducted using permuted blocks within strata with dynamic balancing within main institutions and their affiliated networks. Institutions obtained treatment assignments through the ECOG-ACRIN web registration program. Random assignment was stratified by the number of involved organ systems (≤ 1 v > 1) and by marker status (hormone receptor and human epidermal growth factor receptor 2 [HER2]) along with corresponding treatment plan (endocrine therapy alone, chemotherapy alone, or chemotherapy with HER2-targeting agents).
Locoregional Therapy
Those randomly assigned to locoregional therapy underwent breast-conserving surgery with free surgical margins or mastectomy. Sentinel node biopsy was allowed for clinically uninvolved nodes, with axillary dissection reserved for those with involved lymph nodes. Radiotherapy use followed NCCN guidelines, on the basis of surgical procedure and nodal involvement. After completion of locoregional treatment, systemic therapy was continued as clinically indicated. For participants randomly assigned to continued systemic therapy, delayed locoregional therapy was permitted for palliation at the discretion of the treating physician and patient; this and could include surgery, radiotherapy, or both. Follow-up data were returned every 3 months for years 0-2 and every 6 months for years 2-5.
Health-Related Quality of Life
Health-related quality of life was assessed using the FACT-B (including the 27-item FACT-G and the 10-item Breast Cancer subscale). The primary end point was the FACT-B Trials Outcome Index (TOI), calculated by summing the physical well-being, functional well-being, and breast cancer–specific scales from the FACT-B.16 We also evaluated the FACT-B and FACT-G total scores and FACT breast cancer subscale score. Given the lack of an available scale to assess differences in arm symptoms and discomfort or worry related to locoregional disease in a tailored and concise manner, nine additional items were added to address these aspects.
Trial End Points and Statistical Methods
The primary end point of the trial was OS, defined as time from random assignment to death from any cause. We planned to randomly assign 660 patients to identify an absolute 3-year survival improvement from 30% to 45%. A low accrual rate necessitated a revised plan in 2013, to register 368 eligible patients, assuming that 70% of these would demonstrate nonprogressive disease after systemic therapy. Allowing for 15% probability of crossover between arms after random assignment, with 258 randomly assigned patients and total information of 152 deaths, the trial had 85% power to detect a 19.3% absolute difference in the 3-year survival rates using the stratified log-rank test, with a one-sided type I error rate of 5%. The study was monitored using group sequential method by an independent Data and Safety Monitoring Committee (DSMC). To preserve the overall type I error rate, critical values at the interim analyses were determined using a truncated version of the Lan-DeMets error spending function corresponding to the O'Brien-Fleming boundary. The study was also monitored for early stopping in favor of the null hypothesis using Jennison-Turnbull repeated CI methodology. The primary comparison was an intent-to-treat analysis including all randomly assigned patients. Survival was analyzed using the Kaplan-Meier method, stratified log-rank test, and stratified Cox proportional hazards models. The trial crossed its futility boundary at the interim analysis at about 57% information time, at which time the DSMC recommended the trial be terminated. Patients continued to be followed; however, the data cutoff date for the present report was December 10, 2019 (information time 80%). All significance tests were two-sided.
A secondary end point was time to locoregional progression (in the continued systemic therapy arm) or recurrence (if prior locoregional therapy had been used); this was defined as time from random assignment to date of first locoregional progression or recurrence and was followed separately from distant progression. The diagnosis of locoregional progression was based on physician assessment of clinical or radiologic parameters. Patients who were still alive and had no reported locoregional recurrence or progression in the last submitted follow-up form were censored. Patients without any follow-up data were censored at random assignment. Death before occurrence of locoregional recurrence or progression was a competing risk. Cumulative incidence of locoregional recurrence or progression was estimated, and the Gray test was used to compare time to locoregional recurrence or progression between treatment groups. The Fine-Gray competing risk model was used to estimate the hazard ratio (HR).
FACT-B TOI score, FACT-B total score, FACT-G total score, and FACT breast cancer subscale score were compared between arms using the Wilcoxon rank-sum test (left skewed). Score change between follow-up and baseline visits was compared between arms using the two-sample t test. The significance level was 5% for all analyses without adjustment of multiple comparisons.
RESULTS
Patient Population
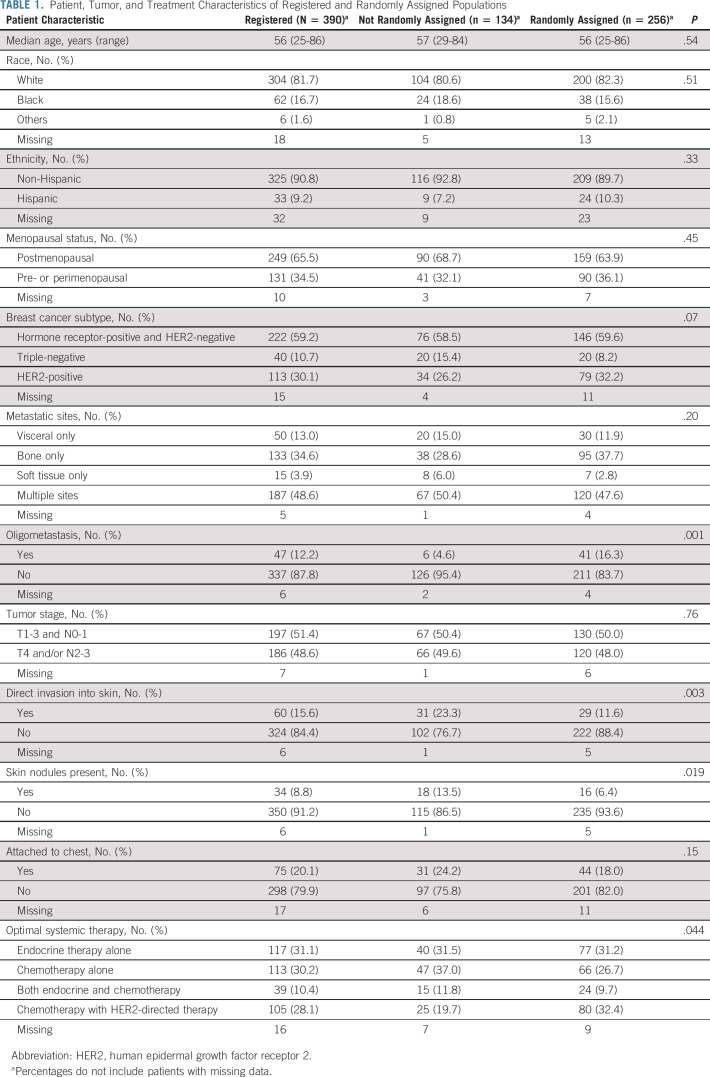
Between February 2011 and July 2015, we enrolled 389 women and one man. Of these, 256 demonstrated nonprogressive disease after systemic therapy and were randomly assigned. The demographics and disease characteristics of the entire population, with 134 being not randomly assigned and 256 randomly assigned, are shown in Table 1. All randomly assigned patients were women. Skin involvement by the primary tumor was significantly more frequent in the nonrandomized participants. Among 384 patients with assessable sites of disease, 47 (12.2%) had oligometastatic disease, defined as ≤ 3 lesions in a single organ site (29 bone, 12 liver, three distant nodal sites, two lung, and one brain); 41 (87.2%) of those with oligometastatic disease proceeded to random assignment.
TABLE 1.
Patient, Tumor, and Treatment Characteristics of Registered and Randomly Assigned Populations
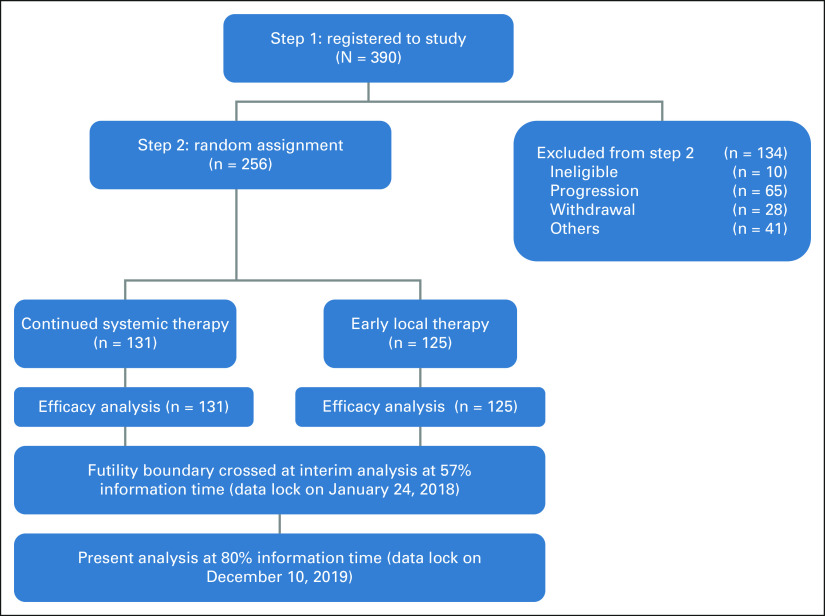
The study CONSORT diagram is shown in Figure 1. Among the 134 not randomly assigned, reasons for not proceeding to random assignment included disease progression during systemic therapy in 65 (48.5%), withdrawal from study in 28 (20.9%), unsuitability for surgery in 10 (7.5%), death during systemic therapy in seven (5.2%), ineligibility for random assignment in 5 (3.7%), and various other reasons in 19 (14.2%).
FIG 1.
CONSORT diagram shows the progression of patients through the study. Per protocol, only those showing stable or responsive disease after optimal systemic therapy were randomly assigned to the two arms. Results were presented at the American Society of Clinical Oncology Annual Meeting in June 2020.
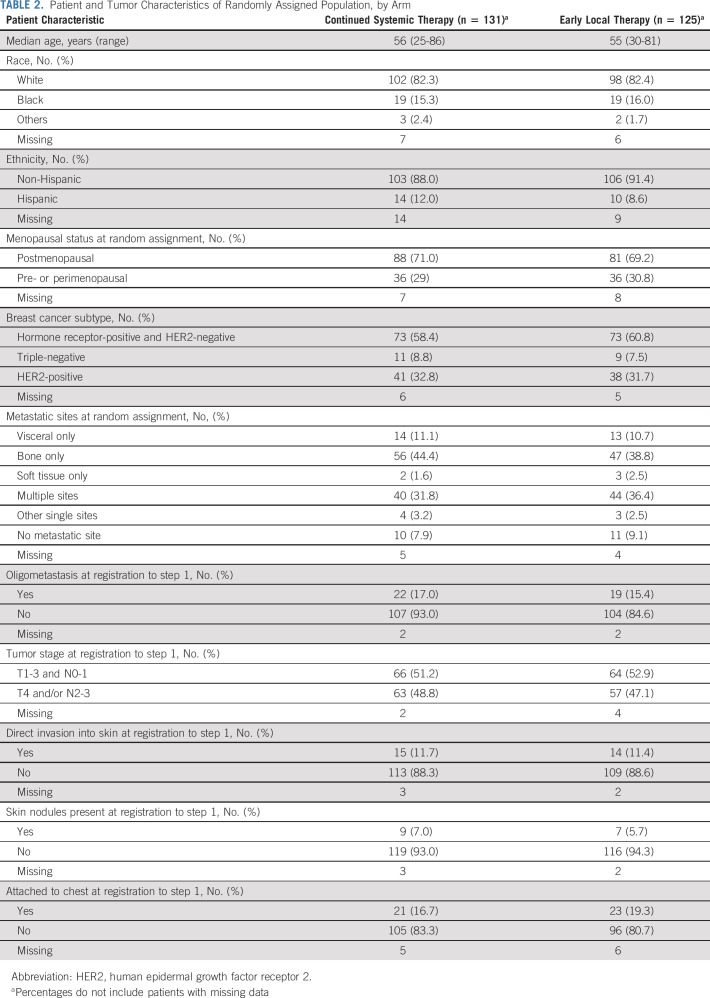
Among 256 randomly assigned patients, known prognostic factors were well balanced across treatment arms (Table 2). This included menopausal status and the distribution of breast cancer subtype by immunohistochemistry or fluorescence in situ hybridization (used to clarify HER2 status).
TABLE 2.
Patient and Tumor Characteristics of Randomly Assigned Population, by Arm
Optimal Systemic Therapy
Among 390 registered participants, optimal systemic therapy details were reported for 374, of whom 306 (82%) began therapy before registration, as allowed by the protocol. As shown in Table 1, systemic treatments included endocrine therapy (n = 117), chemotherapy (n = 113), chemotherapy with endocrine therapy (n = 39), or chemotherapy with HER2-directed therapy (n = 105). The median duration of optimal systemic therapy was 21 weeks (range, 5-32 weeks) for randomly assigned patients.
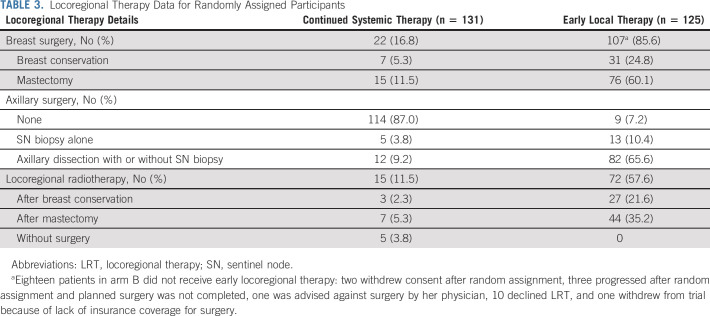
Locoregional Therapy
Locoregional therapy is described in Table 3. Among 125 randomly assigned to the locoregional therapy arm, 107 (85.6%) received surgical treatment: mastectomy in 75 (70.1%) and breast conservation in 32 (29.9%). Surgical margins were tumor-free in 98 (91.6%). Postoperative radiotherapy was given for 27 of 32 patients (84.4%) after breast conservation and 44 of 75 (58.7%) postmastectomy. Eighteen participants randomly assigned to locoregional therapy (14.4%) did not receive it. For 11, this was a matter of choice, with varying reasons for four others (physician advice, lack of insurance, progression between random assignment, and surgery). The reason was unknown for three patients. Surgery was completed within 3 months of random assignment in 101 of 107 patients (94.4%). For the remainder, locoregional therapy was completed within 6 months of random assignment.
TABLE 3.
Locoregional Therapy Data for Randomly Assigned Participants
Among 131 women randomly assigned to continued systemic therapy, 22 (16.8%) received surgery, followed by radiotherapy in 10. Surgery was performed within 3 months after random assignment in five patients, within 12 months in seven patients, and more than 12 months for the remaining 10. Five patients received local radiation without surgery, all more than 12 months after random assignment.
OS
After a median follow-up of 53 months among the 256 randomly assigned women, 121 (47.3%) died (63 in the continued systemic therapy arm and 58 in the locoregional therapy arm), including 94 (36.7%) from breast cancer, 10 (3.9%) from other causes, and cause unconfirmed in 17 (6.6%). The 3-year OS rate was 67.9% (95% CI, 58.8 to 75.5) for those randomly assigned to continued systemic therapy and 68.4% (95% CI, 59.0 to 76.1) for the locoregional therapy arm. The median OS was 53.1 months (95% CI, 47.9 to not estimable) in the systemic therapy arm and 54.9 months (95% CI, 46.7 to not estimable) in the locoregional therapy arm (HR 1.11; 90% CI, 0.82 to 1.52, stratified log-rank P = .57).
Exploratory post hoc subgroup analyses were performed, including demographic factors, body mass index, tumor and nodal status, metastatic sites at random assignment, oligometastasis at registration, and disease subtype (Data Supplement, online only). The results were similar across all the subgroups except for disease subtype, where the HR for 20 women with hormone receptor–negative and HER2-negative disease was 3.33 (95% CI, 1.09 to 10.12; Data Supplement). Notably, there was no survival difference across arms among women who presented with oligometastases (HR, 1.18; 95% CI, 0.38 to 3.67).
For the 134 patients who were not randomly assigned, 80 patients died (63 breast cancer deaths, eight because of other causes, and cause unknown for nine patients). The median follow-up time was 53 months. The 3-year and 5-year OS rates were 44.8% (95% CI, 35.6 to 53.5) and 29.7% (95% CI, 21.0 to 38.9), respectively. The median OS was 32.7 months (95% CI, 24.8 to 40.8 months).
Locoregional Progression
At the time of the analysis, 79 women had experienced locoregional progression on physical examination or imaging, 57 in the systemic therapy arm and 22 in the locoregional therapy arm. The 3-year cumulative incidence of locoregional progression was 39.8% (95% CI, 31.8 to 49.1) in the systemic therapy arm and 16.3% (95% CI, 10.7 to 24.4) in the locoregional therapy arm (Gray test P < .001, unadjusted HR = 0.34; 95% CI, 0.21 to 0.56). In a competing risk model analysis in the locoregional therapy arm, free margin status was associated with a significantly lower hazard for locoregional recurrence (HR = 0.17; 95% CI, 0.06 to 0.51), whereas postoperative radiation use was not (HR = 0.55; 95% CI, 0.18 to 1.70).
Health-Related Quality of Life
Among 131 patients who completed the FACT-B TOI at baseline and 18 months postrandomization, the mean TOI was significantly higher in the systemic therapy arm (mean score 74.2, standard deviation 11.5 v 68.0, standard deviation 13.7, P = .01; Fig 2C and the Data Supplement). There was no significant difference between arms at other time points. Multivariable linear mixed effect model analysis showed similar results (data not shown).
FIG 2.
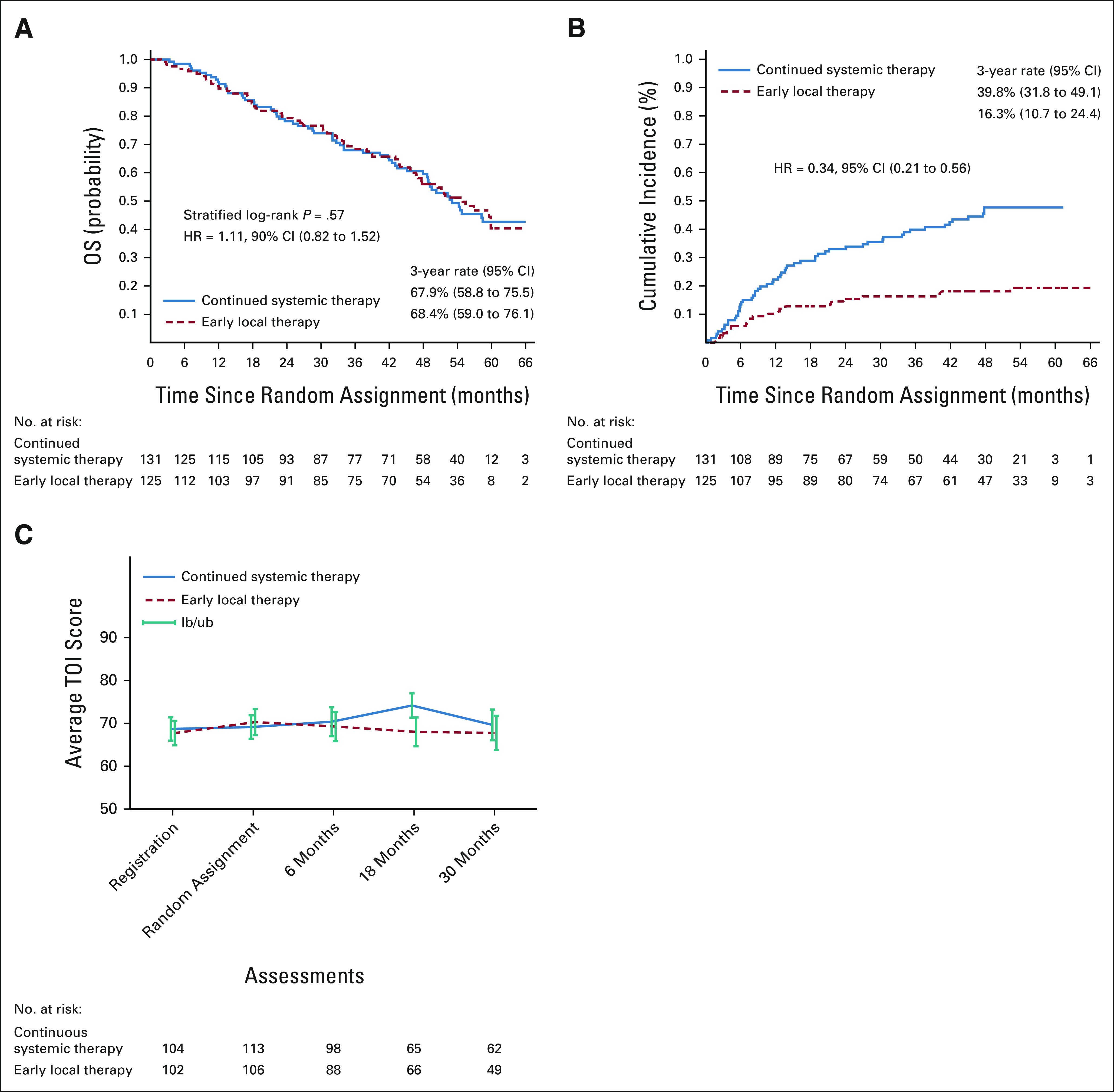
Kaplan-Meier plots of (A) OS and (B) locoregional progression, comparing the systemic therapy arm with the early locoregional therapy arm, among patients who demonstrated stable or responsive disease after initial systemic therapy and were randomly assigned. (C) Health-related quality of life as measured by the FACT-B TOI Score. HR, hazard ratio; OS, overall survival; TOI, Trials Outcome Index. If breast cancer is diagnosed after distant spread has occurred, surgery and radiation for the breast tumor do not improve survival.
Several other items were also evaluated, including items related to arm and chest wall symptoms and worry about primary tumor recurrence or progression. There were no significant differences in symptoms, worry, or functionality between the two arms (Data Supplement).
DISCUSSION
We performed a prospective randomized trial, designed to determine whether locoregional therapy with therapeutic rather than palliative intent improves clinical and patient-reported outcomes in newly diagnosed patients with synchronous distant metastases. Our design incorporated the primacy of systemic therapy, allowed flexibility in the choice of systemic therapy regimens according to tumor biology and contemporary therapies, and tested the value of locoregional therapy, as presently used with curative intent in those with localized breast cancer. Our results do not provide any support for the use of early locoregional treatment as a means of achieving improved survival, with a HR of 1.11 (90% CI, 0.82 to 1.52; P = .57). However, we did observe improved local progression-free survival in patients who received early locoregional therapy.
Exploratory subset analyses on the basis of age, hormone receptor and HER2 status, and metastatic patterns found no subgroup that derived an OS benefit, including those with oligometastases (ie, fewer than four lesions in a single organ system). In this subgroup, the majority of patients (41 of 47) proceeded to random assignment, but their survival did not differ by the treatment arm. Although our original trial design aimed to randomly assign 660 patients, to identify an absolute 3-year survival improvement of 15%, low accrual rate necessitated a revised plan with 258 randomly assigned patients and 152 deaths, to detect a 19% survival advantage. In fact, the futility boundary was crossed at interim analysis with 89 reported deaths.
Four other randomized trials have addressed this question; three have been previously reported, and another has completed accrual.17 The design of these trials followed two models: one prescribing systemic therapy before random assignment13 and the other with random assignment before systemic therapy.14,15 We reasoned that early locoregional therapy is highly unlikely to be beneficial in the context of resistance of distant disease to systemic therapy and used the first model. Our trial is most similar to a prospective trial conducted in Mumbai, randomly assigning 350 women.13 However, the systemic therapy regimen in that trial was less flexible; the use of initial endocrine therapy for hormone receptor–positive disease was rare, and HER2-directed therapy was generally unavailable for patients with HER2-positive tumors. These circumstances are reflected in the 3-year OS rates, which were approximately 65% in both arms of our trial, compared with about 20% at 20 months in the Mumbai study. Thus, even with more tailored systemic therapy in the resource-rich North American environment in which patients presented with less advanced disease and had access to optimal systemic therapy, the use of early locoregional therapy did not lead to improved survival.
Of the three published trials, only one (the Turkish Federation's MF07-01) reported improved OS with locoregional therapy. In this trial, 274 eligible patients were immediately randomly assigned to locoregional or systemic therapy14; 40-month survival favored the locoregional therapy arm (70% v 55%; HR, 0.66; 95% CI, 0.49 to 0.88). However, substantial imbalances in prognostic covariates likely contributed to better outcomes in the locoregional therapy arm. The Austrian POSYTIVE trial, with similar design, did not reach full accrual and reported results on 90 randomly assigned patients with a follow-up of 37.5 months 15; the survival results favored the systemic therapy arm nonsignificantly (HR, 0.69; 95% CI, 0.36 to 1.33).
A secondary objective of our trial was to evaluate the frequency of locoregional progression between the two arms, and we did observe substantially improved locoregional control in the locoregional therapy arm (unadjusted HR, 0.34; P < .001). This was achieved with surgical resection of the primary tumor, nodal staging, resection of involved axillary lymph nodes, and postoperative radiotherapy when indicated. In the systemic therapy arm, 27 patients (20.6%) received surgery or radiotherapy; this was considered to be palliative in 17 (13%), comparable with other trials (10%,13 11%,14 and 18%15).
We also assessed patient-reported outcomes using a standardized instrument with additional questions regarding local symptoms. In the 18-month evaluation, when 50% of randomly assigned participants completed the instrument, the FACT-B TOI was significantly better in the systemic therapy arm. The scores did not differ at other time points (baseline, 6 months, and 30 months). Our findings are limited by an incomplete response rate, but are supported by the POSYTIVE trial.18 They suggest that the symptomatic impact of locoregional therapy is substantial and the burden of worry about local tumor growth is not relieved by locoregional therapy. Recommendations regarding the use of early locoregional therapy to enhance local control must, therefore, disclose the lack of survival benefit and the morbidities of locoregional therapy, as well as the fact that for the great majority of women, locoregional disease remains asymptomatic with the use of systemic therapy.
Our main results, consistent with two of the three published trials, support the conclusion that the use of early locoregional therapy for patients with distant metastases at initial presentation does not provide a survival benefit and should not be offered with this expectation. For individuals with distant metastasis who defer early locoregional therapy, about 20% may eventually require this for local palliation. As systemic therapy improves further, this conclusion is likely to remain true.
ACKNOWLEDGMENT
Dr Lawrence Solin made enormous contributions to the design and conduct of this trial. He passed away in March 2020, at which time he was professor emeritus, Department of Radiation Oncology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Lori J. Goldstein
Stock and Other Ownership Interests: CureVac
Honoraria: Daiichi Sankyo, Roche/Genentech, Amgen, Mylan, Merck, Eisai, Immunomedics, Exact Sciences
Consulting or Advisory Role: Genentech, Genomic Health, Merck, Mylan, Immunomedics, Amgen, Eisai, Exact Sciences
Research Funding: Merck (Inst), Genentech/Roche (Inst)
Other Relationship: Daiichi Sankyo
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Asahi Kasei, Ipsen, Mei Pharma
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
Mark Basik
Honoraria: Roche Canada
Research Funding: Pfizer, LabCorp
Mehra Golshan
Consulting or Advisory Role: AbbVie, Bertis
Research Funding: Breast Cancer Research Foundation
Christine A. Lee
Consulting or Advisory Role: Olympus
Speakers' Bureau: AstraZeneca/Merck, OncoCyte
Joseph A. Sparano
Stock and Other Ownership Interests: MetaStat
Consulting or Advisory Role: Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack, Adgero Biopharmaceuticals, Cardinal Health, Pfizer, GlaxoSmithKline, CStone Pharmaceuticals, Epic Sciences, Daiichi Sankyo, BMSi
Speakers' Bureau: Eisai, Certara
Research Funding: Prescient Therapeutics (Inst), Deciphera (Inst), Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Novartis (Inst), Merrimack (Inst), Radius Health (Inst), Olema Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Menarini Silicon Biosystems, Roche/Genentech, Adgero Biopharmaceuticals, Myriad Genetics, Pfizer, AstraZeneca, Rhenium Medical
Gildy V. Babiera
Stock and Other Ownership Interests: PolyPid
Honoraria: Insightec, Gleolan
Consulting or Advisory Role: Insightec, Gleolan, Theracal, Polypid, Nektar
Patents, Royalties, Other Intellectual Property: Patent Holder for DNX 2401 and DNX2440 Owned by DNAtrix
Travel, Accommodations, Expenses: Gleolan, Insightec
Sarika Jain
Employment: G1 Therapeutics
Stock and Other Ownership Interests: G1 Therapeutics
Carla S. Fisher
Consulting or Advisory Role: Biom'Up
Travel, Accommodations, Expenses: Biom'Up
Amye J. Tevaarwerk
Other Relationship: Epic Systems
Lynne I. Wagner
Stock and Other Ownership Interests: Johnson & Johnson, Lilly, Gilead Sciences
Consulting or Advisory Role: Celgene, Athenex
Travel, Accommodations, Expenses: Celgene
George W. Sledge
Leadership: Syndax, Tessa Therapeutics
Stock and Other Ownership Interests: Syndax, Tessa Therapeutics, Pionyr
Consulting or Advisory Role: Symphogen, Synaffix, Syndax, Verseau Therapeutics, GRAIL, AstraZeneca, G1 Therapeutics
Research Funding: Genentech/Roche (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Verseau Therapeutics, Tessa Therapeutics
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 927
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part at the ASCO Virtual Annual Meeting, May 29-31, 2020.
SUPPORT
This study was conducted by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD, and Mitchell D. Schnall, MD, PhD, group cochairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: U10CA180821, U10CA180863, Canadian Cancer Society #704970, U10CA180820, U10CA180868, U10CA180822, U10CA180888, U10CA180794, UG1CA189830, UG1CA189859, UG1CA189953, UG1CA232760, UG1CA233180, UG1CA233193, UG1CA233234, UG1CA233277, UG1CA233320, UG1CA233329, and UG1CA233341.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Seema A. Khan, Lori J. Goldstein, Mehra Golshan, Thomas B. Julian, Joseph A. Sparano, Gildy V. Babiera, Lynne I. Wagner, George W. Sledge
Financial support: Lynne I. Wagner
Administrative support: Seema A. Khan, Mehra Golshan, Joseph A. Sparano
Provision of study materials or patients: Seema A. Khan, Lori J. Goldstein, Mark Basik, Thomas B. Julian, Joseph A. Sparano, Irene A. Dy, Paula Silverman, Amye J. Tevaarwerk
Collection and assembly of data: Seema A. Khan, Fengmin Zhao, Mark Basik, Mehra Golshan, Barbara A. Pockaj, Wajeeha Razaq, Gildy V. Babiera, Irene A. Dy, Sarika Jain, Paula Silverman, Carla S. Fisher, Amye J. Tevaarwerk
Data analysis and interpretation: Seema A. Khan, Fengmin Zhao, Lori J. Goldstein, David Cella, Mehra Golshan, Thomas B. Julian, Christine A. Lee, Joseph A. Sparano, Gildy V. Babiera, Amye J. Tevaarwerk, Lynne I. Wagner, George W. Sledge
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Early Local Therapy for the Primary Site in De Novo Stage IV Breast Cancer: Results of a Randomized Clinical Trial (EA2108)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lori J. Goldstein
Stock and Other Ownership Interests: CureVac
Honoraria: Daiichi Sankyo, Roche/Genentech, Amgen, Mylan, Merck, Eisai, Immunomedics, Exact Sciences
Consulting or Advisory Role: Genentech, Genomic Health, Merck, Mylan, Immunomedics, Amgen, Eisai, Exact Sciences
Research Funding: Merck (Inst), Genentech/Roche (Inst)
Other Relationship: Daiichi Sankyo
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Asahi Kasei, Ipsen, Mei Pharma
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
Mark Basik
Honoraria: Roche Canada
Research Funding: Pfizer, LabCorp
Mehra Golshan
Consulting or Advisory Role: AbbVie, Bertis
Research Funding: Breast Cancer Research Foundation
Christine A. Lee
Consulting or Advisory Role: Olympus
Speakers' Bureau: AstraZeneca/Merck, OncoCyte
Joseph A. Sparano
Stock and Other Ownership Interests: MetaStat
Consulting or Advisory Role: Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack, Adgero Biopharmaceuticals, Cardinal Health, Pfizer, GlaxoSmithKline, CStone Pharmaceuticals, Epic Sciences, Daiichi Sankyo, BMSi
Speakers' Bureau: Eisai, Certara
Research Funding: Prescient Therapeutics (Inst), Deciphera (Inst), Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Novartis (Inst), Merrimack (Inst), Radius Health (Inst), Olema Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Menarini Silicon Biosystems, Roche/Genentech, Adgero Biopharmaceuticals, Myriad Genetics, Pfizer, AstraZeneca, Rhenium Medical
Gildy V. Babiera
Stock and Other Ownership Interests: PolyPid
Honoraria: Insightec, Gleolan
Consulting or Advisory Role: Insightec, Gleolan, Theracal, Polypid, Nektar
Patents, Royalties, Other Intellectual Property: Patent Holder for DNX 2401 and DNX2440 Owned by DNAtrix
Travel, Accommodations, Expenses: Gleolan, Insightec
Sarika Jain
Employment: G1 Therapeutics
Stock and Other Ownership Interests: G1 Therapeutics
Carla S. Fisher
Consulting or Advisory Role: Biom'Up
Travel, Accommodations, Expenses: Biom'Up
Amye J. Tevaarwerk
Other Relationship: Epic Systems
Lynne I. Wagner
Stock and Other Ownership Interests: Johnson & Johnson, Lilly, Gilead Sciences
Consulting or Advisory Role: Celgene, Athenex
Travel, Accommodations, Expenses: Celgene
George W. Sledge
Leadership: Syndax, Tessa Therapeutics
Stock and Other Ownership Interests: Syndax, Tessa Therapeutics, Pionyr
Consulting or Advisory Role: Symphogen, Synaffix, Syndax, Verseau Therapeutics, GRAIL, AstraZeneca, G1 Therapeutics
Research Funding: Genentech/Roche (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Verseau Therapeutics, Tessa Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Agarwal G, Pradeep PV, Aggarwal V, et al. Spectrum of breast cancer in Asian women World J Surg 311031–10402007 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A.Cancer statistics, 2020 CA Cancer J Clin 707–302020 [DOI] [PubMed] [Google Scholar]
- 3.Norton L, Massague J.Is cancer a disease of self-seeding? Nat Med 12875–8782006 [DOI] [PubMed] [Google Scholar]
- 4.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis Nature 449557–5632007 [DOI] [PubMed] [Google Scholar]
- 5.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer N Engl J Med 3451655–16592001 [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, Stewart AK, Morrow M.Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 132620–6262002 [DOI] [PubMed] [Google Scholar]
- 7.Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis J Clin Oncol 242743–27492006 [DOI] [PubMed] [Google Scholar]
- 8.Gnerlich J, Jeffe DB, Deshpande AD, et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: Analysis of the 1988-2003 SEER data Ann Surg Oncol 142187–21942007 [DOI] [PubMed] [Google Scholar]
- 9.Babiera GV, Rao R, Feng L, et al. Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor Ann Surg Oncol 13776–7822006 [DOI] [PubMed] [Google Scholar]
- 10.Bafford AC, Burstein HJ, Barkley CR, et al. Breast surgery in stage IV breast cancer: Impact of staging and patient selection on overall survival Breast Cancer Res Treat 1157–122008 [DOI] [PubMed] [Google Scholar]
- 11.Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients Ann Surg 247732–7382008 [DOI] [PubMed] [Google Scholar]
- 12.Khan SA.Primary tumor resection in stage IV breast cancer: Consistent benefit, or consistent bias? Ann Surg Oncol 143285–32872007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: An open-label randomised controlled trial Lancet Oncol 161380–13882015 [DOI] [PubMed] [Google Scholar]
- 14. Soran A, Ozmen V, Ozbas S, et al. A randomized controlled trial evaluating resection of the primary breast tumor in women presenting with de novo stage IV breast cancer. J Clin Oncol. 2016;34 suppl 15; abstr 1005. [Google Scholar]
- 15. Fitzal F, Balic M, Bjelic-Radisic V, et al. Primary operation in synchronous metastasized breast cancer patients: First oncologic outcomes of the prospective randomized phase III ABCSG28 POSYTIVE trial. J Clin Oncol. 2017;35 suppl 15; abstr 557. [Google Scholar]
- 16.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale J Clin Epidemiol 57898–9102004 [DOI] [PubMed] [Google Scholar]
- 17.Shien T, Nakamura K, Shibata T, et al. A randomized controlled trial comparing primary tumour resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (PRIM-BC): Japan Clinical Oncology Group Study JCOG1017 Jpn J Clin Oncol 42970–9732012 [DOI] [PubMed] [Google Scholar]
- 18. Bjelic-Radisic V, Fitzal F, Knauer M, et al. Primary surgery versus no surgery in synchronous metastatic breast cancer: Patient-reported quality-of-life outcomes of the prospective randomized multicenter ABCSG-28 Posytive Trial. BMC Cancer. 2020;20:392. doi: 10.1186/s12885-020-06894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]