Abstract
PURPOSE
CD19-targeted chimeric antigen receptor T cells (CD19-CAR) and blinatumomab effectively induce remission in relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) but are also associated with CD19 antigen modulation. There are limited data regarding the impact of prior blinatumomab exposure on subsequent CD19-CAR outcomes.
PATIENTS AND METHODS
We conducted a multicenter, retrospective review of children and young adults with relapsed or refractory ALL who received CD19-CAR between 2012 and 2019. Primary objectives addressed 6-month relapse-free survival (RFS) and event-free survival (EFS), stratified by blinatumomab use. Secondary objectives included comparison of longer-term survival outcomes, complete remission rates, CD19 modulation, and identification of factors associated with EFS.
RESULTS
Of 420 patients (median age, 12.7 years; interquartile range, 7.1-17.5) treated with commercial tisagenlecleucel or one of three investigational CD19-CAR constructs, 77 (18.3%) received prior blinatumomab. Blinatumomab-exposed patients more frequently harbored KMT2A rearrangements and underwent a prior stem-cell transplant than blinatumomab-naïve patients. Among patients evaluable for CD19-CAR response (n = 412), blinatumomab nonresponders had lower complete remission rates to CD19-CAR (20 of 31, 64.5%) than blinatumomab responders (39 of 42, 92.9%) or blinatumomab-naive patients (317 of 339, 93.5%), P < .0001. Following CD19-CAR, blinatumomab nonresponders had worse 6-month EFS (27.3%; 95% CI, 13.6 to 43.0) compared with blinatumomab responders (66.9%; 95% CI, 50.6 to 78.9; P < .0001) or blinatumomab-naïve patients (72.6%; 95% CI, 67.5 to 77; P < .0001) and worse RFS. High-disease burden independently associated with inferior EFS. CD19-dim or partial expression (preinfusion) was more frequently seen in blinatumomab-exposed patients (13.3% v 6.5%; P = .06) and associated with lower EFS and RFS.
CONCLUSION
With the largest series to date in pediatric CD19-CAR, and, to our knowledge, the first to study the impact of sequential CD19 targeting, we demonstrate that blinatumomab nonresponse and high-disease burden were independently associated with worse RFS and EFS, identifying important indicators of long-term outcomes following CD19-CAR.
INTRODUCTION
CD19-targeted immunotherapies have revolutionized outcomes for children and young adults with chemotherapy-refractory or multiply relapsed B-cell acute lymphoblastic leukemia (B-ALL).1,2 CD19-directed chimeric antigen receptor T cells (CD19-CAR), in particular, have produced remarkably high complete remission (CR) rates of 70%-97%.3-11 These successes led to the approval of tisagenlecleucel, a murine-based CD19-CAR, by the US Food and Drug Administration (FDA) in 2017.12 This approval added an additional tool to target B-ALL along with other immunotherapeutic agents such as the CD3-CD19 bispecific T-cell engager antibody construct, blinatumomab. As monotherapy, blinatumomab has induced CR rates of 34%-69% in adults and 39% in children.13-17 Blinatumomab is also FDA-approved for children with relapsed or refractory B-ALL or for those in CR with minimal residual disease (MRD) ≥ 0.1%.18
CONTEXT
Key Objective
To describe the relationship between blinatumomab exposure and outcomes for children and young adults with relapsed or refractory acute lymphoblastic leukemia (ALL) who subsequently receive CD19-chimeric antigen receptor (CAR).
Knowledge Generated
We demonstrate an association between blinatumomab nonresponse and worse outcomes with subsequent CD19-CAR independent of other prognostic factors and not solely explained by higher rates of antigen escape. For patients receiving blinatumomab who proceed with CD19-CAR, those not-responding to blinatumomab likely represent a high-risk population predisposed to worse outcomes and may be enriched for patients with an inherent resistance to immunotherapy, as evidenced by the lower complete remission and event-free survival rates after CD19-CAR among blinatumomab nonresponders.
Relevance
The registration study leading to US Food and Drug Administration approval of tisagenlecleucel for children and young adults with relapsed or refractory B-ALL specifically excluded patients who had received prior blinatumomab. With increasing utilization of both blinatumomab and CD19-CAR, it is critical to understand the intersection of these therapies on long-term outcomes.
Despite impressive CR rates with CD19-targeted therapies, relapse remains a significant challenge. In the global registration trial of tisagenlecleucel, among patients who achieved CR, the 24-month relapse-free survival (RFS) rate was 62% (95% CI, 47 to 75).3,4 A common mechanism of relapse after either CD19-CAR or blinatumomab is target antigen downregulation or escape.17,19-23 Given the potential for CD19 modulation, concerns have been raised about increased risk of nonresponse or relapse with sequential targeting of CD19. The global registration trial of tisagenlecleucel, thus, excluded patients with prior blinatumomab exposure.4 Therefore, limited data exist regarding the impact of blinatumomab on subsequent CD19-CAR outcomes. A recent single-center analysis found that prior blinatumomab did not preclude CAR response but raised concerns for impact on CD19 expression and risk of antigen-negative relapses. The study size, however, was insufficient to address impact on survival outcomes.24
Understanding the impact of sequential CD19 immunotherapeutic targeting is critical in the current treatment era, particularly given the commercial availability of blinatumomab and its incorporation earlier in treatment regimens.25,26 Several trials have demonstrated that blinatumomab in first relapse is a more well-tolerated, less toxic, and highly effective regimen compared with traditional chemotherapy.27,28 Additionally, pediatric consortia, including the Children's Oncology Group (NCT03914625), the International Berlin-Frankfurt-Muenster Consortium (NCT03643276), and the St Jude Children's Research Hospital Consortium (NCT03117751), are testing blinatumomab with combination chemotherapy in newly diagnosed patients. Because of concerns over CD19-negative escape, relapse risk, and CD19-CAR nonresponse with selective pressure and increasing utilization of both blinatumomab and CD19-CAR, we sought to evaluate the relationships between blinatumomab exposure and subsequent CD19-CAR outcomes.
PATIENTS AND METHODS
Study Design and Patient Population
We conducted a retrospective, multicenter study of children and young adults who received CD19-CAR, either the FDA-approved commercial product, tisagenlecleucel, or another murine-based CD19-CAR T-cell therapy available on a clinical trial (NCT01626495, NCT02906371, NCT02028455, NCT02625480, NCT02435849, and NCT01593696) for relapsed or refractory B-ALL across seven centers in the United States. CAR constructs included two CD19/4-1BB constructs (CTL019/tisagenlecleucel and SCRI-CAR19) and a CD19/CD28 construct, all of which have been previously described.4,6,29 Patients were included if they were age ≤ 25 years at B-ALL diagnosis, had ≥ 1 disease assessment evaluation after CAR infusion and 30 days of follow-up, or had an event (nonresponse, disease progression, or treatment-related mortality) before 30 days. Patients who received a prior CAR product were excluded. All patients were infused between January 1, 2012, and December 31, 2019. Data cutoff was June 1, 2020. The study was reviewed and approved or considered exempt by each center's Institutional Review Board.
Disease Assessment
All disease assessments were conducted per protocol or institutional practice. The pre-CD19-CAR assessment was typically performed within 14 days of initiating lymphodepleting chemotherapy. First disease assessment post-CD19-CAR infusion was generally performed 21-28 days after infusion. Pre-blinatumomab disease assessment generally occurred within 30 days before blinatumomab administration. Response criteria were based on standardized definitions (Data Supplement, online only). MRD negativity was analyzed by multiparameter flow cytometry and was defined as < 0.01% of bone marrow mononuclear cells consistent with B-ALL. High-disease burden was defined as ≥ 5% marrow blasts (≥ M2 marrow), and low-disease burden was defined as < 5% bone marrow blasts, inclusive of MRD-negative disease. Additional flow cytometry review was performed by each institutional team for patients who had (1) prior blinatumomab or (2) concern for CD19-negative or dim populations on the basis of descriptive flow cytometry reports at any time point. Definitions for CD19 surface expression are in the Data Supplement.
Objectives
The primary objective was to evaluate RFS and event-free survival (EFS) rates 6 months after CD19-CAR infusion, stratified by blinatumomab exposure. Secondary objectives included evaluation of the following: CR rates at first assessment after infusion; EFS, RFS, and overall survival (OS) rates at 12 and 24 months; and incidence of CD19 modulation, defined as CD19-negative, dim, or partial expression at post-CAR relapse. Exploratory objectives included analyses of additional factors associated with EFS and outcomes following consolidative hematopoietic stem-cell transplant (HSCT).
Statistical Analysis
Descriptive statistics were calculated for patient demographic and disease characteristics. Blinatumomab-exposed patients (and blinatumomab responders v nonresponders) were compared with blinatumomab-naïve patients using the Wilcoxon rank-sum test for continuous variables or Fisher's exact test for categorical variables. Time-to-event end points were estimated by the Kaplan-Meier method, and the two strata were compared using the log-rank test. RFS was estimated for all evaluable patients who achieved a CR after CD19-CAR and was defined as the time from CR attainment to relapse, with censoring at last contact or death without relapse. EFS was defined as the time from CD19-CAR infusion to no response, relapse, or death from any cause. Patients without one of these events were censored at last contact. OS was defined as the time from CD19-CAR infusion to death from any cause or last contact. Cumulative incidence of relapse (CIR) was determined using death as the competing risk, with Gray's test to compare CIR curves. Follow-up was estimated using the reverse Kaplan-Meier method. Risk factors for EFS were evaluated for their joint effect using multivariable Cox proportional hazards analysis. Factors of interest were initially identified by univariate analysis; factors associated with EFS at P < .10 were included in the multivariable model. HSCT outcomes were defined from day of stem-cell infusion (day 0).
RESULTS
Study Population
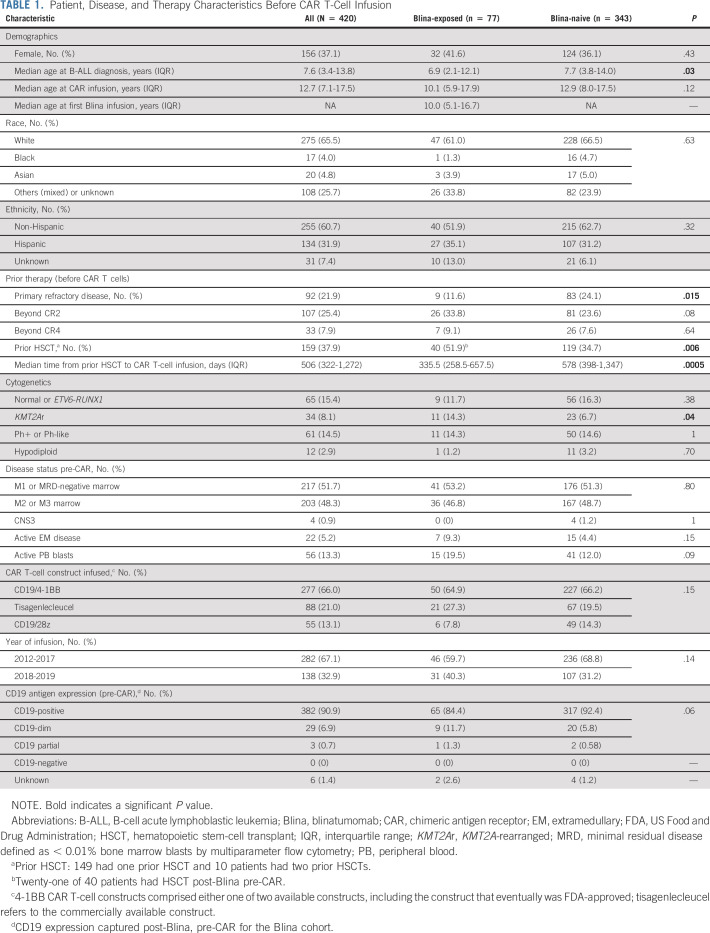
Among 420 patients treated with CD19-CAR, 412 (98.1%) were evaluable for response (Table 1). Eight (1.9%) patients died before day 28 (Data Supplement). The majority received a 4-1BB CAR (86.9%, n = 365) and were treated before 2018 (67.1%, n = 282), before commercial tisagenlecleucel was broadly available (Figs 1A and 1B). The median age at diagnosis was 7.6 years (interquartile range [IQR], 3.4-13.8 years) and at CAR infusion was 12.7 years (IQR, 7.1-17.5 years). Ninety-two (21.9%) patients had primary refractory disease, 159 (37.9%) had received a prior HSCT, and 203 (48.3%) had ≥ M2 marrow pre-CAR infusion. Additional patient, disease, and treatment characteristics are listed in Table 1.
TABLE 1.
Patient, Disease, and Therapy Characteristics Before CAR T-Cell Infusion
FIG 1.
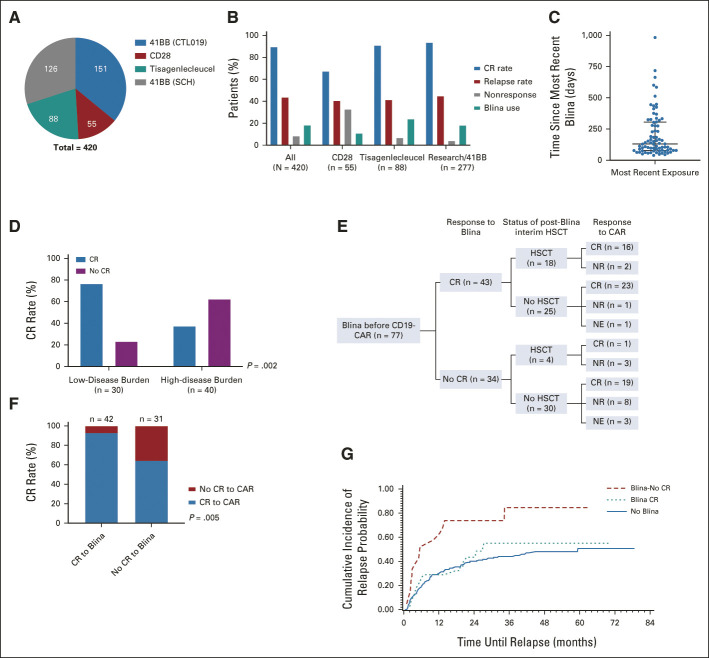
Characteristics of blinatumomab-naive and blinatumomab-exposed patients. (A) Distribution of full patient cohort (N = 420) by CD19-CAR construct. (B) Median and interquartile range of days from most recent blinatumomab exposure to CAR infusion among blinatumomab-exposed patients (n = 77). (C) Outcomes of patients by CAR construct. (D) CR rate to blinatumomab on the basis of pre-blinatumomab disease burden (data only available on 70 patients, missing data for seven patients). Among the low-disease burden patients (n = 30), 11 were in an MRD-negative CR at the time of blinatumomab administration. (E) Flowchart of blinatumomab-exposed patients (n = 77), including best response to blinatumomab, status of post-blinatumomab interim HSCT, and response to CAR. (F) CR rates to CD19-CAR among blinatumomab-exposed patients who were evaluable for response (n = 73), stratified by response to prior blinatumomab. (G) CIR, stratified by blinatumomab-naïve (solid line), blinatumomab-exposed prior responders (Blina-CR, dotted line), and blinatumomab-exposed prior nonresponders (Blina-No CR, dashed line). alloHSCT, allogeneic hematopoietic stem-cell transplant; Blina, blinatumomab; CAR, chimeric antigen receptor; CIR, cumulative incidence of relapse; CR, complete remission; MRD, minimal residual disease; NE, not evaluable; NR, no response.
Blinatumomab Utilization
Seventy-seven of 420 (18.3%) patients received prior blinatumomab (Table 1). The median time from most recent blinatumomab administration to CAR infusion was 131 days (range, 39-983 days; Fig 1C). Blinatumomab-exposed patients were more likely than blinatumomab-naïve patients to have had a prior HSCT (51.9% v 34.7%; P = .006), were younger at diagnosis (median age 6.9 years v 7.7 years; P = .03), and were more likely to have KMT2A-rearranged (KMT2Ar) B-ALL (14.3% v 6.7%; P = .04). They were less likely to have primary refractory disease (11.6% v 24.1%; P = .01). Forty-three (55.8%) patients achieved a CR with blinatumomab, with response rates varying by pre-blinatumomab disease burden (Fig 1D). Additional details regarding blinatumomab utilization are shown in Figure 1E and the Data Supplement.
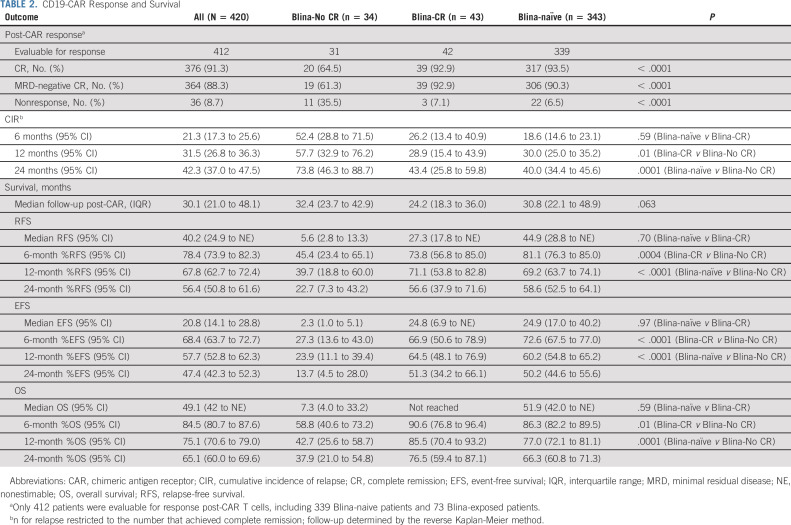
Response to CD19-CAR
Of 412 evaluable patients, 376 (91.3%) achieved a CR, of whom 364 (96.8%) were MRD-negative by flow cytometry (Table 2). Summaries of response, relapse, and HSCT in blinatumomab-exposed patients are in Figure 1E. CR rates following CD19-CAR were comparable between blinatumomab-naïve patients (317 of 339, 93.5%) and blinatumomab-exposed patients who responded to blinatumomab (39 of 42, 92.9%) but worse in blinatumomab-exposed nonresponders (20 of 31, 64.5%; P < .0001; Fig 1F). Eleven of 73 (15.1%) blinatumomab-exposed patients did not achieve a CR with either product.
TABLE 2.
CD19-CAR Response and Survival
The CIR was higher among blinatumomab-exposed nonresponders than either blinatumomab-exposed responders or blinatumomab-naïve patients with a 6-month CIR of 52.4% (95% CI, 28.8 to 71.5), 26.2% (95% CI, 13.4 to 40.9), and 18.6% (95% CI, 14.6 to 23.1), respectively (Fig 1G).
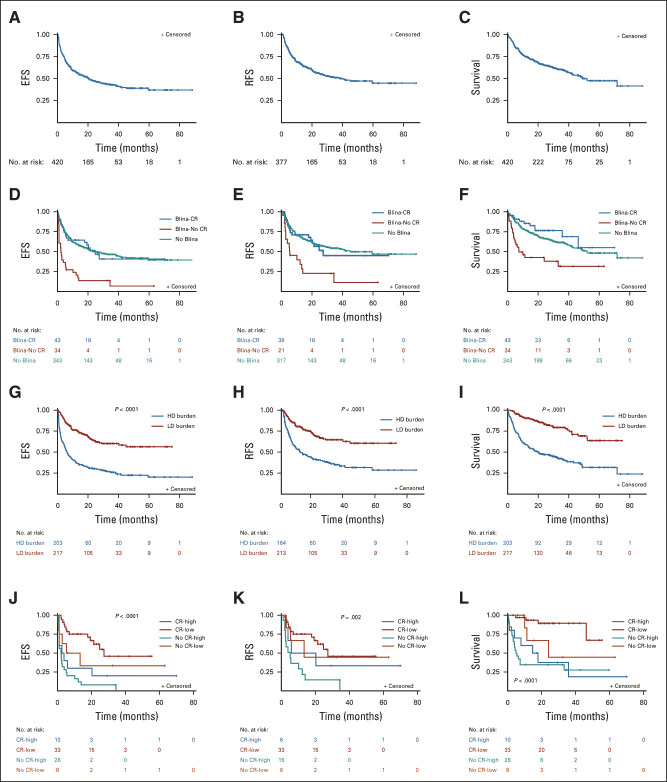
Survival
With a median follow-up of 30.1 months (IQR, 21.0-48.1 months), the median EFS, RFS, and OS among all patients were 20.8 (14.1-28.8), 40.2 (24.9 to nonestimable), and 49.1 (42 to non-estimable) months, respectively (Table 2, Figs 2A-2C). RFS, EFS, and OS were shorter in blinatumomab-exposed nonresponders than blinatumomab-exposed responders or blinatumomab-naïve patients (Figs 2D-2F). The 6-month RFS for blinatumomab-exposed versus blinatumomab-naïve patients was 64.1% (95% CI, 50.4 to 74.9) versus 81.1% (95% CI, 76.3 to 85.0; P = .02 overall) and the 6-month EFS was 49.7% (95% CI, 38.0 to 60.4) versus 72.6% (67.5 to 77.0; P = .001 overall) with blinatumomab-exposed nonresponders experiencing worse outcomes. Outcomes were similar when restricted to 4-1BB CAR constructs (Data Supplement).
FIG 2.
EFS, RFS, and OS. (A-C Kaplan-Meier survival curves for all patients. (A) EFS, defined as the time from CD19-CAR infusion to one of the following events: no response, relapse, or death. (B) RFS, defined as the time from the first response to relapse or death. (C) OS, (D) EFS, (E) RFS, and (F) OS, stratified by blinatumomab-naïve patients (No Blina—teal) versus blinatumomab-exposed and achieved CR to Blina (Blina-CR—blue) versus blinatumomab-exposed and did not achieve a CR to Blina (Blina-No CR—red). P values for EFS curve: .59 (No Blina v Blina-CR); .01 (Blina-CR v Blina-No CR); .0001 (No Blina v Blina-No CR). P values for RFS curve: .70 (No Blina v Blina-CR); .0004 (Blina-CR v Blina-No CR); < .0001 (No Blina v Blina-No CR). P values for OS curve: .97 (No Blina v Blina-CR); < .0001 (Blina-CR v Blina-No CR); < .0001 (No Blina v Blina-No CR). (G) EFS, (H) RFS, and (I) OS, stratified by high-disease burden (≥ 5% bone marrow blasts—blue [high]) versus low-disease burden (< 5% bone marrow blasts—red [low]). (J) EFS, (K) RFS, and (L) OS, restricted to blinatumomab-exposed patients only, stratified by blinatumomab exposure, blinatumomab response, and disease burden (high-disease burden [≥ 5% bone marrow blasts] v low-disease burden [< 5% bone marrow blasts]). Blina-CR-low—red; Blina-CR-high—blue; Blina-No CR-low—brown; Blina-No CR-high—teal. P value shown represents the global P value. Blina, blinatumomab; CAR, chimeric antigen receptor; CR, complete remission; EFS, event-free survival; HD, high disease; high, high-disease burden; LD, low disease; low, low-disease burden; OS, overall survival; RFS, relapse-free survival.
Additional Factors Associated with EFS
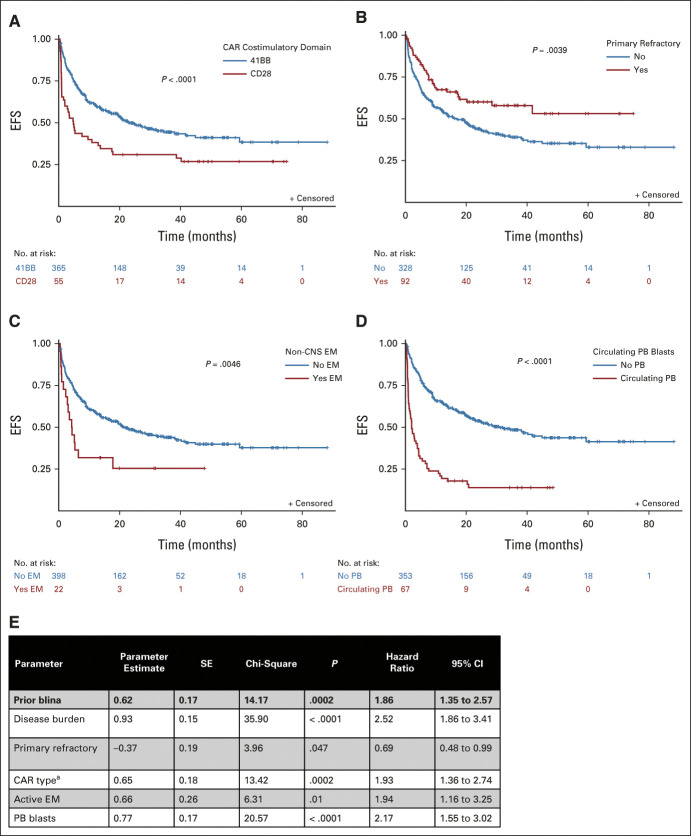
Similar to recent reports,10,30 we also found that high-disease burden was associated with worse survival (Figs 2G-2I). Blinatumomab utilization remained an independent factor, further contributing to the adverse impact of disease burden on EFS and RFS but not OS (Data Supplement). High-disease burden pre-CAR further amplified poor outcomes for blinatumomab-exposed nonresponders (Figs 2J-2L, Data Supplement). Additional univariate analyses were performed to evaluate the association of other factors with EFS. Factors not associated with EFS included sex, ethnicity, race, and prior HSCT. Factors associated with EFS at P < .1, in addition to prior blinatumomab and disease burden, included CAR construct type, cytogenetics, primary refractory disease, active non-CNS extramedullary disease, and circulating peripheral blood (PB) blasts (Figs 3A-3D).
FIG 3.
Factors associated with EFS after CD19-CAR. EFS, stratified by (A) CD19-CAR construct (41BB v CD28), (B) primary refractory disease versus relapsed disease, (C) active extramedullary disease at CAR infusion versus no extramedullary disease, and (D) active peripheral blasts at CAR infusion versus no blasts. (E) Multivariable comparisons of EFS. aAssociated with worse EFS with CD19/CD28 CAR. Active EM, active extramedullary disease at infusion; Active PB, detection of peripheral blasts at infusion. Blina, blinatumomab, CAR, chimeric antigen receptor; EFS, event-free survival.
Collectively, the factors associated with EFS by univariate analysis were included in a Cox multivariable model (Fig 3E) with exception for cytogenetics (because of multiple missing values; Data Supplement). The following factors remained independently associated with worse outcomes: prior blinatumomab use, high-disease burden, active non-CNS extramedullary disease, circulating PB blasts, and CAR construct type (CD28 costimulatory domains were associated with worse EFS; see the Data Supplement). Patients with primary refractory disease had marginally favorable outcomes. In an analysis restricted to 4-1BB CAR constructs, prior blinatumomab, high-disease burden, CNS status, and circulating PB blasts remained in the model.
CD19 Evolution
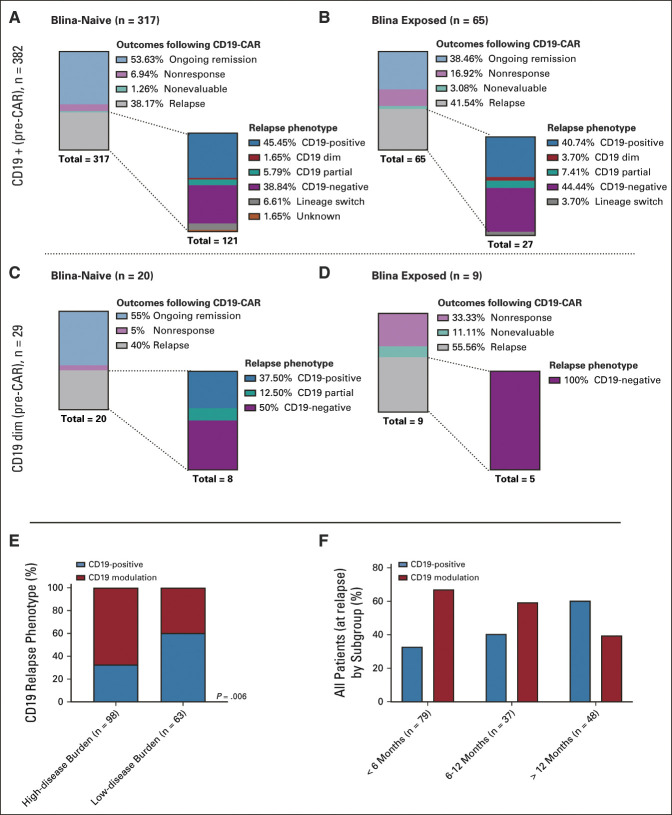
Among 414 patients with pre-CAR CD19 expression analysis, CD19-dim or partial expression was more common in blinatumomab-exposed than blinatumomab-naïve patients (10 of 75 [13.3%] v 22 of 339 [6.5%]; P = .06; Fig 4). For evaluable patients with robust CD19-positive expression pre-CAR (n = 376), CR rates were lower in blinatumomab-exposed (52 of 63, 82.5%) than blinatumomab-naïve patients (291 of 313, 92.9%; P = .01). Among patients achieving CR, CD19 immunophenotype at subsequent relapse did not differ between the two strata (Figs 4A and 4B). For those with CD19-dim or partial expression, 5 of 8 (62.5%) blinatumomab-exposed patients achieved CR, compared with 19 of 20 (95.0%) blinatumomab-naive patients (P = .06, Figs 4C and 4D). However, all five blinatumomab-exposed patients who achieved CR had subsequent CD19-negative relapse. Among the 28 evaluable patients with CD19-dim disease pre-CAR, the majority experienced relapse or nonresponse (17 of 28, 60.7%) with particularly dismal outcomes in those receiving blinatumomab who became CD19-dim (Data Supplement).
FIG 4.
CD19 modulation. Top: Patients with CD19-positive expression pre-CAR. (A) Outcomes following CD19-CAR for blinatumomab-naïve patients and subsequent CD19 immunophenotype at relapse. (B) Outcomes following CD19-CAR for blinatumomab-exposed patients and subsequent CD19 immunophenotype at relapse. Middle: Patients with CD19 dim expression pre-CD19-CAR. (C) Outcomes following CD19-CAR for blinatumomab-naïve patients and subsequent CD19 immunophenotype at relapse. (D) Outcomes following CD19-CAR for blinatumomab-exposed patients and subsequent CD19 immunophenotype at relapse. (Outcomes for patients with CD19 partial [n = 3] and CD19 status unknown [n = 6] are not shown. Among the CD19 partial population, one patient had received prior blinatumomab, and all three remain in an ongoing CR. Among patients with CD19 unknown expression, two had prior blinatumomab, three remain in an ongoing remission, three relapsed with CD19-positive disease [one had prior blinatumomab], one relapsed with CD19-negative disease [n = 1], and one relapsed with unknown CD19 expression. One patient was considered nonevaluable for response but emerged with CD19-negative relapse following initial disease assessment.) Bottom: CD19 expression at relapse. (E) CD19 expression at relapse, stratified by disease burden. (F) CD19 expression at relapse, stratified by time point of relapse post-CAR infusion. CAR, chimeric antigen receptor; CR, complete remission.
CD19 immunophenotype at relapse varied by disease burden and time to relapse. A greater proportion of patients with low-disease burden (38 of 63, 60.3%) were CD19-positive at relapse than those with high-disease burden (32 of 98, 32.7%; P = .0006). Late relapses (> 12 months after CAR infusion) were more frequently associated with CD19-positive relapse than early relapses (< 6 months after infusion) (Figs 4E and 4F).
HSCT Outcomes
A total of 146 patients proceeded to HSCT after CD19-CAR. Restricting analysis to patients that underwent HSCT within the first year after CD19-CAR to consolidate a CAR-induced remission without interval therapy (n = 92), indications for HSCT included the following: (1) planned CAR as a bridge to HSCT (n = 52, 56.5%), (2) loss of CAR T cells or loss of B-cell aplasia (n = 39, 42.4%), and (3) myeloid aplasia and delayed count recovery (n = 1, 1.1%). Among the 92 patients, 13 had a prior transplant, and only six had received blinatumomab. The median time from CAR infusion to HSCT was 93 days (range, 42-301 days). For consolidative HSCT performed for loss of B-cell aplasia, the median time from CAR infusion to HSCT was 145.5 days (range, 62-301). The 2-year OS (from HSCT day 0) was 78.3% (95% CI, 67.6 to 85.8); the corresponding 2-year EFS and RFS were 70.8% (95% CI, 59.5 to 79.5) and 82.6% (95% CI, 71.1 to 89.8), respectively (Data Supplement). Thirteen of 92 (13.4%) patients experienced post-HSCT relapse, for two of whom this represented relapse following second HSCT.
DISCUSSION
With availability of highly effective CD19-targeted strategies, the treatment paradigm for B-ALL has shifted dramatically in recent years. Both blinatumomab and CD19-CAR are more readily used in patients' treatment algorithms, particularly to achieve remission in chemotherapy-refractory disease. As CD19 targeting continues to be used and moved into earlier lines of treatment, there is little known about the impact of sequential CD19-directed immunotherapy. With CD19 antigen loss and downregulation as mechanisms of relapse after either approach,31-34 it is imperative to understand the intersection and interactions between various treatment strategies.
Using the largest multicenter retrospective series in pediatric and young adult CAR T-cell therapy to date with substantial follow-up, we present outcomes across various CD19-CARs and clearly demonstrate that blinatumomab-exposed nonresponders have inferior outcomes to either blinatumomab-naïve patients or those who previously responded to blinatumomab. Blinatumomab-exposed nonresponders had lower CR rates and higher relapse rates than blinatumomab-naïve patients, leading to inferior EFS and RFS. This impact was evident even when accounting for disease burden, which we, among others,10,30 demonstrate is highly associated with outcomes. Given the concern for a direct impact of blinatumomab on CD19 expression,19,21,35 this negative association was hypothesized. However, on the basis of the relatively comparable outcomes between blinatumomab-exposed responders and blinatumomab-naïve patients, our results suggest that emergence of CD19 escape is contributory but not the primary mechanism of poor outcomes in the blinatumomab-exposed CD19-CAR patients.
Our data support the notion that blinatumomab may lead to CD19 downregulation. For patients in whom CD19 expression diminished or changed following blinatumomab, the risk of post-CAR relapse with CD19-negative disease was high. Indeed, blinatumomab-exposed patients with CD19-dim disease pre-CD19-CAR had the worst outcomes, albeit represented by a limited sample size. However, for those with normal CD19 expression pre-CAR, CD19 immunophenotype at relapse and rates of antigen escape between blinatumomab-naïve and blinatumomab-exposed patients did not substantially differ. This result suggests that alternative mechanisms, including intrinsic T-cell dysfunction, immunotherapy resistance, or the adverse impact of extensive prior therapy, may contribute to the poorer outcomes for blinatumomab-exposed patients, particularly in this very heavily pretreated patient population.
The CR rate to blinatumomab in our cohort aligned with published experiences demonstrating that high-disease burden adversely affected blinatumomab CR rates.17 Moreover, blinatumomab responders had high CR rates to CD19-CAR (90.6%), in line with CD19-CAR CR rates for blinatumomab-naive patients. However, the lower CR rates to CD19-CAR in blinatumomab nonresponders raises the possibility that blinatumomab nonresponse serves as a proxy for identifying those with either intrinsic resistance to CD19 immunotherapeutic targeting or T-cell dysfunction and/or other high-risk features such as high-disease burden. Indeed, blinatumomab-exposed patients were younger, more frequently harbored KMT2Ar (who may be predisposed to antigen modulation following CD19 targeting),22,31 and more frequently received a prior HSCT compared with blinatumomab-naïve patients. Thus, patients who require additional therapy following blinatumomab are more likely to be refractory, are predisposed to worse outcomes, are more likely to relapse, and are ultimately less likely to achieve cure with CD19-CAR alone.
The observation of high-disease burden associating with CD19-negative relapse, which was also seen in a smaller cohort,36 is both noteworthy and problematic. Although factors predisposing high-disease burden patients to CD19 negativity are unknown, it is possible that miniscule populations of preexisting CD19-negative disease hiding amid more prominent CD19-positive populations are undetectable by standard flow cytometry but emerge only after clearance of CD19-positive disease, as recently demonstrated by single-cell analysis.37 Although additional exploration is needed to fully understand this association, it is clear that high-disease burden patients are more likely to relapse, relapse early post-CAR, are less likely to respond to blinatumomab, and also experience antigen loss, making the curative potential of CD19-CAR alone further limited in this group.
Since remission durability was shortened in blinatumomab-exposed patients, post-CAR consolidation with HSCT, especially in patients with high-disease burden or in those with less persistent CD19-CAR constructs,9 may be one approach to prevent relapse. We demonstrated favorable EFS, RFS, and OS for patients receiving consolidative HSCT. Although we were unable to address whether consolidative HSCT could improve outcomes in blinatumomab-exposed patients given limited numbers and variable prior HSCT exposure, consolidative HSCT likely has a role in achieving long-term cure in high-risk patients and needs to be prospectively studied.
The primary limitation of this study is its retrospective design, but no prospective trial is planned to address sequential CD19 targeting. Additionally, this study contains a heterogeneous patient population, treated with three different CD19-CAR constructs across seven institutions. However, by virtue of this large experience, with data from centers highly experienced in pediatric CD19-CAR and experts in B-ALL flow cytometry, outcomes were consistent across CAR constructs, providing generalizability of our data set. Furthermore, our study has a selection bias for more refractory patients, by selecting for blinatumomab nonresponders or multiply relapsed patients who may have inherent resistance to CD19 targeting, high-disease burden, or both. Finally, and most importantly, our data do not capture patients who emerge with CD19 escape post-blinatumomab who could not be referred for CD19-CAR. Therefore, the actual incidence of CD19 modulation post-blinatumomab cannot be ascertained from this data set.
Ultimately, our data inform one of the most vexing unanswered current questions in the clinical management of patients with B-ALL, namely, the impact of sequential CD19 targeting strategies. We found that blinatumomab exposure did not preclude CD19-CAR response. However, blinatumomab nonresponders had lower CR rates with subsequent CD19-CAR and blinatumomab exposure was associated with lower EFS and RFS, particularly for those with high-disease burden. Our data shed light on an important new risk factor for post-CD19-CAR outcomes. Additional studies are warranted to decipher the mechanisms for blinatumomab nonresponse and how they may influence CAR T-cell response, independent of antigen escape. Additionally, given the preliminary observation that blinatumomab-exposed patients with CD19-dim disease had poor outcomes, prospective serial monitoring of CD19 expression pre- and post-blinatumomab is needed to understand the impact on future single or sequential multiple antigen-targeted therapy to capture outcomes of blinatumomab-exposed patients not referred for CD19-CAR.
ACKNOWLEDGMENT
The authors would like to thank the treating and referring centers, care providers, supportive staff, and referring physicians who cared for the patients included in this study.
Agne Taraseviciute
Employment: Johnson & Johnson/Janssen
Alexandra E. Kovach
Stock and Other Ownership Interests: Lixte Biotechnology
Brent Wood
Honoraria: Amgen, Seattle Genetics, Abbvie, Janssen, Astellas Pharma, Roche Diagnostics, Beckman Coulter
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx¸ Biosight (Inst), Janssen Oncology (Inst), Novartis (Inst), Kite, a Gilead company, Macrogenics (Inst)
Travel, Accommodations, Expenses: Amgen
Michael J. Borowitz
Consulting or Advisory Role: Amgen, Blueprint Medicines
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Daniel W. Lee
Employment: Karyopharm Therapeutics (I)
Consulting or Advisory Role: Harpoon therapeutics, Amgen, Celgene
Research Funding: Kite/Gilead (Inst)
Patents, Royalties, Other Intellectual Property: CAR T cell for pediatric and adult high-grade gliomas and other tumors
Travel, Accommodations, Expenses: Kite/Gilead
Stephan A. Grupp
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, Cellular Biomedicine Group, TCR2 Therapeutics, Humanigen, Roche, Adaptimmune, Alimera Sciences, Cabaletta Bio, Crispr therapeutics/Vertex
Research Funding: Novartis (Inst), Kite/Gilead (Inst), Servier (Inst), Jazz Pharmaceuticals (Inst), Vertex (Inst)
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent
Expert Testimony: Juno Therapeutics
Samuel John
Patents, Royalties, Other Intellectual Property: UT Southwestern Medical Center, patent pending on novel anti-LILRB CAR-T cell
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Kite, a Gilead company, Servier
Theodore W. Laetsch
Consulting or Advisory Role: Bayer, Novartis, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicans' Education Resource, Y-mAbs Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst)
Lia Gore
Employment: Anchiano (I)
Leadership: Anchiano (I), Vedantra (I)
Stock and Other Ownership Interests: Amgen, Sanofi, Celgene, Anchiano (I), Mirati Therapeutics (I), OnKure, ITOS Oncology (I)
Consulting or Advisory Role: Novartis, Amgen, Roche/Genentech, Syndax, OnKure, Janssen Oncology, Pfizer
Patents, Royalties, Other Intellectual Property: Patent held for diagnostic discovery and treatment response methodology tools in the use of MR spectroscopy for leukemia
Rebecca A. Gardner
Honoraria: Janssen, Novartis
Patents, Royalties, Other Intellectual Property: IP and royalties are received from BMS related to CAR technology patents
Travel, Accommodations, Expenses: Novartis
Susan R. Rheingold
Employment: OptiNose (I)
Stock and Other Ownership Interests: OptiNose (I)
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer (Inst)
Michael A. Pulsipher
Honoraria: Amgen, Bellicum Pharmaceuticals, Miltenyi Biotec, Novartis, CSL Behring, Jasper Therapeutics, Novartis, Medexus, Equillium, Mesoblast
Speakers' Bureau: Novartis
Research Funding: Adaptive Biotechnologies, Miltenyi Biotec
Travel, Accommodations, Expenses: Medac, Bellicum Pharmaceuticals, Miltenyi Biotec
Nirali N. Shah
Research Funding: Lentigen (Inst)
No other potential conflicts of interest were reported.
See accompanying editorial on page 921
DISCLAIMER
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
PRIOR PRESENTATION
Presented at the American Society of Hematology Virtual Annual Meeting, December 5-8, 2020.
SUPPORT
Supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the Warren Grant Magnuson Clinical Center (ZIA BC 011823, N.N.S.).
R.M.M. and A.T. contributed equally as co-first authors.
P.A.B., T.W.L., L.G., R.A.G., S.R.R., and M.A.P. contributed equally to this work.
DATA SHARING STATEMENT
Contact the corresponding author for additional information.
AUTHOR CONTRIBUTIONS
Conception and design: Regina Myers, Agne Taraseviciute, Seth M. Steinberg, Deepa Bhojwani, Patrick A. Brown, Lia Gore, Rebecca A. Gardner, Susan R. Rheingold, Michael A. Pulsipher, Nirali N. Shah
Financial support: Stephan A. Grupp, Theodore W. Laetsch
Administrative support: Stephan A. Grupp, Susan R. Rheingold, Michael A. Pulsipher, Nirali N. Shah
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Agne Taraseviciute
Employment: Johnson & Johnson/Janssen
Alexandra E. Kovach
Stock and Other Ownership Interests: Lixte Biotechnology
Brent Wood
Honoraria: Amgen, Seattle Genetics, Abbvie, Janssen, Astellas Pharma, Roche Diagnostics, Beckman Coulter
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx¸ Biosight (Inst), Janssen Oncology (Inst), Novartis (Inst), Kite, a Gilead company, Macrogenics (Inst)
Travel, Accommodations, Expenses: Amgen
Michael J. Borowitz
Consulting or Advisory Role: Amgen, Blueprint Medicines
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Daniel W. Lee
Employment: Karyopharm Therapeutics (I)
Consulting or Advisory Role: Harpoon therapeutics, Amgen, Celgene
Research Funding: Kite/Gilead (Inst)
Patents, Royalties, Other Intellectual Property: CAR T cell for pediatric and adult high-grade gliomas and other tumors
Travel, Accommodations, Expenses: Kite/Gilead
Stephan A. Grupp
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, Cellular Biomedicine Group, TCR2 Therapeutics, Humanigen, Roche, Adaptimmune, Alimera Sciences, Cabaletta Bio, Crispr therapeutics/Vertex
Research Funding: Novartis (Inst), Kite/Gilead (Inst), Servier (Inst), Jazz Pharmaceuticals (Inst), Vertex (Inst)
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent
Expert Testimony: Juno Therapeutics
Samuel John
Patents, Royalties, Other Intellectual Property: UT Southwestern Medical Center, patent pending on novel anti-LILRB CAR-T cell
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Kite, a Gilead company, Servier
Theodore W. Laetsch
Consulting or Advisory Role: Bayer, Novartis, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicans' Education Resource, Y-mAbs Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst)
Lia Gore
Employment: Anchiano (I)
Leadership: Anchiano (I), Vedantra (I)
Stock and Other Ownership Interests: Amgen, Sanofi, Celgene, Anchiano (I), Mirati Therapeutics (I), OnKure, ITOS Oncology (I)
Consulting or Advisory Role: Novartis, Amgen, Roche/Genentech, Syndax, OnKure, Janssen Oncology, Pfizer
Patents, Royalties, Other Intellectual Property: Patent held for diagnostic discovery and treatment response methodology tools in the use of MR spectroscopy for leukemia
Rebecca A. Gardner
Honoraria: Janssen, Novartis
Patents, Royalties, Other Intellectual Property: IP and royalties are received from BMS related to CAR technology patents
Travel, Accommodations, Expenses: Novartis
Susan R. Rheingold
Employment: OptiNose (I)
Stock and Other Ownership Interests: OptiNose (I)
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer (Inst)
Michael A. Pulsipher
Honoraria: Amgen, Bellicum Pharmaceuticals, Miltenyi Biotec, Novartis, CSL Behring, Jasper Therapeutics, Novartis, Medexus, Equillium, Mesoblast
Speakers' Bureau: Novartis
Research Funding: Adaptive Biotechnologies, Miltenyi Biotec
Travel, Accommodations, Expenses: Medac, Bellicum Pharmaceuticals, Miltenyi Biotec
Nirali N. Shah
Research Funding: Lentigen (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Davis KL, Mackall CL: Immunotherapy for acute lymphoblastic leukemia: From famine to feast. Blood Adv 1:265-269, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers RM, Dolan J, Teachey DT: Chimeric antigen receptor T cell therapy for pediatric and young adult B cell acute lymphoblastic leukemia. Expert Rev Clin Immunol 16:1029-1042, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupp S, Maude S, Rives S, et al. : Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood abstract 895, ASH Meeting, 2019
- 4.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner RA, Finney O, Annesley C, et al. : Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129:3322-3331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran KJ, Margossian SP, Kernan NA, et al. : Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134:2361-2368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquini MC, Hu ZH, Curran K, et al. : Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4:5414-5424, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah NN, Lee DW, Yates B, et al. : Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol 39:1650-1659, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadauke S, Myers RM, Li Y, et al. : Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: A prospective clinical trial. J Clin Oncol 39:920-930, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Leary MC, Lu X, Huang Y, et al. : FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res 25:1142-1146, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Gokbuget N, Kelsh M, Chia V, et al. : Blinatumomab vs historical standard therapy of adult relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J 6:e473, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong WH: Comment on 'Blinatumomab vs historical standard therapy of adult relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J 6:e509, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topp MS, Gokbuget N, Stein AS, et al. : Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol 16:57-66, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Topp MS, Gokbuget N, Zugmaier G, et al. : Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 32:4134-4140, 2014 [DOI] [PubMed] [Google Scholar]
- 17.von Stackelberg A, Locatelli F, Zugmaier G, et al. : Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381-4389, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Jen EY, Xu Q, Schetter A, et al. : FDA approval: Blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin Cancer Res 25:473-477, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Ruella M, Maus MV: Catch me if you can: Leukemia escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J 14:357-362, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topp MS, Kufer P, Gokbuget N, et al. : Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29:2493-2498, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Mejstrikova E, Hrusak O, Borowitz MJ, et al. : CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J 7:659, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby E, Nguyen SM, Fountaine TJ, et al. : CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun 7:12320, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotillo E, Barrett DM, Black KL, et al. : Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 5:1282-1295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillai V, Muralidharan K, Meng W, et al. : CAR T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy. Blood Adv 3:3539-3549, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeer JL, Rau RE, Gupta S, et al. : Cutting to the front of the line: Immunotherapy for childhood acute lymphoblastic leukemia. Am Soc Clin Oncol Ed Book 40:1-12, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Winters A, Gore L: Moving immunotherapy into the front line in ALL. Hematol Am Soc Hematol Educ Program 2019:209-217, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown PA, Ji L, Xu X, et al. : Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 325:833-842, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locatelli F, Zugmaier G, Rizzari C, et al. : Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 325:843-854, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grupp SA, Kalos M, Barrett D, et al. : Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509-1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz LM, Baggott C, Prabhu S, et al. : Disease burden impacts outcomes in pediatric and young adult B-cell acute lymphoblastic leukemia after commercial tisagenlecleucel: Results from the Pediatric Real World CAR Consortium (PRWCC). Blood 136:14-15, 2020 [Google Scholar]
- 31.Gardner R, Wu D, Cherian S, et al. : Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 127:2406-2410, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majzner RG, Mackall CL: Tumor antigen escape from CAR T-cell therapy. Cancer Discov 8:1219-1226, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Mikhailova E, Semchenkova A, Illarionova O, et al. : Relative expansion of CD19-negative very-early normal B-cell precursors in children with acute lymphoblastic leukaemia after CD19 targeting by blinatumomab and CAR-T cell therapy: Implications for flow cytometric detection of minimal residual disease. Br J Haematol 193:602-612, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Demosthenous C, Lalayanni C, Iskas M, et al. : Extramedullary relapse and discordant CD19 expression between bone marrow and extramedullary sites in relapsed acute lymphoblastic leukemia after blinatumomab treatment. Curr Probl Cancer 43:222-227, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Libert D, Yuan CM, Masih KE, et al. : Serial evaluation of CD19 surface expression in pediatric B-cell malignancies following CD19-targeted therapy. Leukemia 34:3064-3069, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dourthe M-E, Rabian F, Yakouben K, et al. : Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia 35:3383-3393, 2021 [DOI] [PubMed] [Google Scholar]
- 37.Rabilloud T, Potier D, Pankaew S, et al. : Single-cell profiling identifies pre-existing CD19-negative subclones in a B-ALL patient with CD19-negative relapse after CAR-T therapy. Nat Commun 12:865, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Contact the corresponding author for additional information.