Abstract
PURPOSE
Children's Oncology Group trial AALL1621 was conducted to prospectively determine the safety and efficacy of inotuzumab ozogamicin (InO) in pediatric and adolescent patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL).
PATIENTS AND METHODS
This single-arm phase II trial enrolled patients age 1-21 years with R/R CD22-positive B-ALL. In cycle 1, InO dosing was 0.8 mg/m2 intravenously on day 1 and 0.5 mg/m2 on days 8 and 15 of a 28-day cycle with response evaluation at day 28. Using a two-stage design, the trial was continuously monitored for dose-limiting toxicities and sinusoidal obstruction syndrome (SOS). CD22 expression was retrospectively evaluated by central flow cytometry.
RESULTS
Forty-eight patients were evaluable for response and toxicity; 19 had complete response (CR) and nine CR with incomplete count recovery (CRi) after cycle 1 (CR/CRi rate: 58.3%; two-sided 90% CI, 46.5 to 69.3). Twenty-seven of 28 patients with CR or CRi had minimal residual disease measured by flow cytometry; 18 (66.7%) had minimal residual disease < 0.01%. Seven of 28 patients (25%) with CR or CRi had delayed count recovery past day 42 in cycle 1. Three (6.3%) patients had grade 3 ALT elevation and one patient had grade 3 hyperbilirubinemia in cycle 1. Of 21 patients undergoing hematopoietic stem-cell transplantation after InO, 6 (28.6%) developed grade 3 SOS. Partial CD22 expression and lower CD22 site density were associated with lower likelihood of response to InO.
CONCLUSION
InO is effective and well tolerated in heavily pretreated children and adolescents with R/R CD22-positive B-ALL. SOS after hematopoietic stem-cell transplantation and prolonged cytopenias were notable. CD22 modulation was identified as a mechanism of resistance. Expanded study of InO combined with chemotherapy is underway.
INTRODUCTION
Approximately 10%-20% of children and young adults with B-cell acute lymphoblastic leukemia (B-ALL) will relapse.1,2 Effective therapies that bridge to hematopoietic stem-cell transplantation (HSCT) or chimeric antigen receptor (CAR) T-cell therapy with manageable toxicities are critical. Inotuzumab ozogamicin (InO; Pfizer Inc, New York, NY) is a CD22-directed antibody-drug conjugate covalently linked to N-Ac-γ-calicheamicin dimethylhydrazide.3 Upon binding to surface CD22, InO is internalized and traffics to the lysosome where calicheamicin is released.3 InO was superior to chemotherapy in adults with relapsed or refractory (R/R) B-ALL in the INO-VATE trial.4 Toxicities include myelosuppression, infection, and sinusoidal obstruction syndrome (SOS).5,6
CONTEXT
Key Objective
Effective therapies that bridge to potentially curative hematopoietic stem-cell transplantation (HSCT) or chimeric antigen receptor (CAR) T-cell therapy with manageable toxicities are critical to improving outcomes for patients with relapsed or refractory B-cell acute lymphoblastic leukemia. Although inotuzumab ozogamicin (InO) is effective in adults, studies in children are limited. This phase II trial evaluated InO in pediatric relapsed or refractory B-cell acute lymphoblastic leukemia.
Knowledge Generated
InO was effective with high response rates and minimal residual disease < 0.01% in two thirds of responders. Importantly, a significant proportion of responding patients proceeded to potentially curative HSCT and/or CAR T-cell therapy. For patients proceeding to HSCT after InO, sinusoidal obstruction syndrome is a significant toxicity.
Relevance
The study demonstrates the utility of InO as a bridge to HSCT or CAR T-cell therapy and highlights the need to identify modifiable sinusoidal obstruction syndrome risk factors for patients receiving HSCT after InO.
Studies of InO in children are limited. Among 51 children treated via compassionate access, the rate of complete response (CR) or CR with incomplete count recovery (CRi) was 67%; 11 of 21 children (51%) receiving HSCT after InO developed SOS.7 The European Innovative Therapies for Children with Cancer consortium phase I trial of InO in children with R/R B-ALL confirmed the US Food and Drug Administration (FDA)–approved adult dose of 1.8 mg/m2 per course (fractionated schedule) as the pediatric recommended phase II dose.8 The AALL1621 phase II trial sought to define the efficacy and safety in pediatric patients with R/R B-ALL. We measured rates of morphologic response after one and two cycles, minimal residual disease (MRD) by flow cytometry, dose-limiting toxicities (DLTs), and SOS incidence and severity during InO therapy and subsequent HSCT. Exploratory studies included correlation of response with InO pharmacokinetics (PK) and surface CD22 expression.
PATIENTS AND METHODS
Children's Oncology Group (COG) AALL1621 (ClinicalTrials.gov identifier: NCT02981628), a single-arm, open-label phase II trial, enrolled from June 2017 to May 2019. The trial was temporarily closed twice, once for planned interim response assessment and once to assess SOS cases after stopping rules were triggered. The study was approved by the National Cancer Institute Pediatric Central Institutional Review Board and local institutional review boards according to institutional policy. All participants or legally authorized guardians provided consent. Eligibility criteria are detailed in the Data Supplement (online only). Patients with prior SOS were excluded. Patients with Down syndrome were eligible.
Treatment and Response Assessment
Patients received FDA-approved fractionated dosing for adults with InO administered intravenously over 1 hour on days 1, 8, and 15 of each 28-day cycle.4 The Data Supplement details treatment and supportive care guidelines. Bone marrow morphologic response was evaluated on day 28 of cycles 1, 2, 4, and 6. The Data Supplement details response definitions. MRD was measured by flow cytometry at a COG-approved laboratory. MRD-negativity was defined as < 0.01% lymphoblasts.
Safety Analysis
Patients who received at least one dose of InO were evaluable for toxicity. Adverse events (AEs) were assessed using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 and version 5.0 (after November 13, 2018). Grade 3 and higher nonhematologic AEs were collected. The Data Supplement defines DLTs. Continuous safety monitoring for a cycle 1 DLT rate of ≥ 30% and SOS rate of ≥ 15% was performed.9 SOS was defined by the modified Seattle criteria10; SOS grading reported herein uses Common Terminology Criteria for Adverse Event version 5.0. The monitoring boundary for SOS was exceeded in stage 1. Following review of stage 1 data (see the Results section), separate SOS monitoring rules were instituted for: (1) any grade SOS during first two cycles; (2) grade ≥ 4 SOS up to 12 months from last InO dose; and (3) grade ≥ 4 SOS after initiation of HSCT conditioning until 12 months from last InO dose. Peripheral blood lymphocyte subpopulations were assessed every one to two cycles.
PK and CD22 Expression
InO PK and central evaluation of surface CD22 expression (CD22%) and site density (CD22 antibody bound per cell [ABC, sites per cell]) were performed in a subset of patients (Data Supplement).
Efficacy Analysis
The primary end point of rate of CR or CRi after cycle 1 was estimated among all eligible patients enrolled with a two-sided 90% Agresti-Coull CI reported.11 Patients who received at least one dose of InO were evaluable for response. A two-stage admissible design was used (detailed in the Data Supplement).12 Secondary or exploratory efficacy end points included event-free survival (EFS) and overall survival (OS), with EFS events including treatment failure, relapse, second malignancy neoplasm, or death because of any cause, whichever occurred first, and OS events being death because of any cause (detailed in the Data Supplement). Data cutoff was December 31, 2020.
RESULTS
Patients
Forty-nine patients enrolled; one was inevaluable because of elevated hepatic transaminases on repeat testing before starting therapy and was not treated. Forty-eight patients received at least one InO dose and were evaluable for response and toxicity (Table 1). The Data Supplement details patient flow. In cycle 1, 45 patients (93.8%) received all prescribed doses of InO without dose modification. Two patients progressed within two doses and one patient received two doses because of DLT (drug rash with eosinophilia and systemic symptoms). The median number of cycles was 2 (range, 1-6).
TABLE 1.
Patient Characteristics
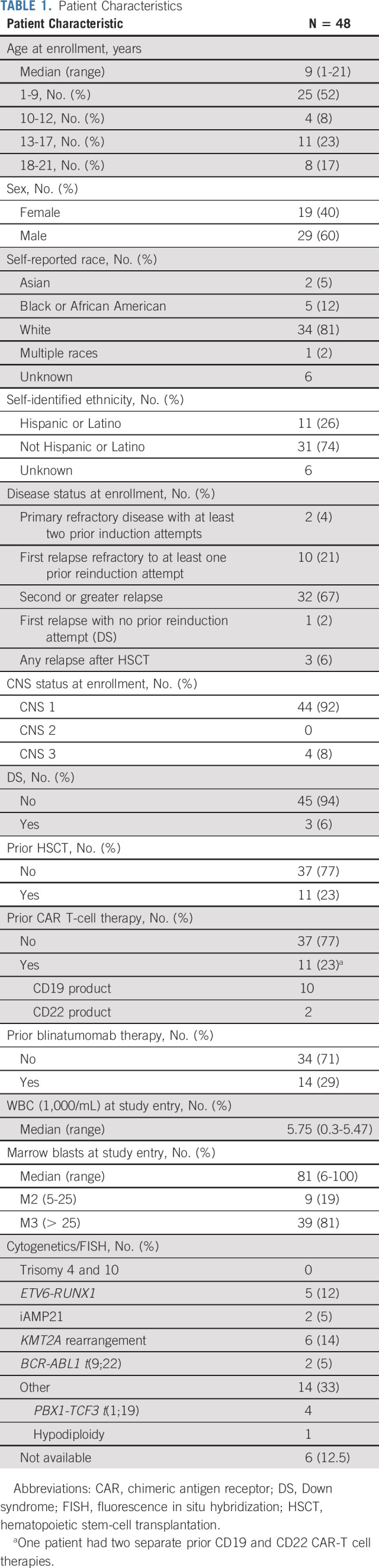
Efficacy
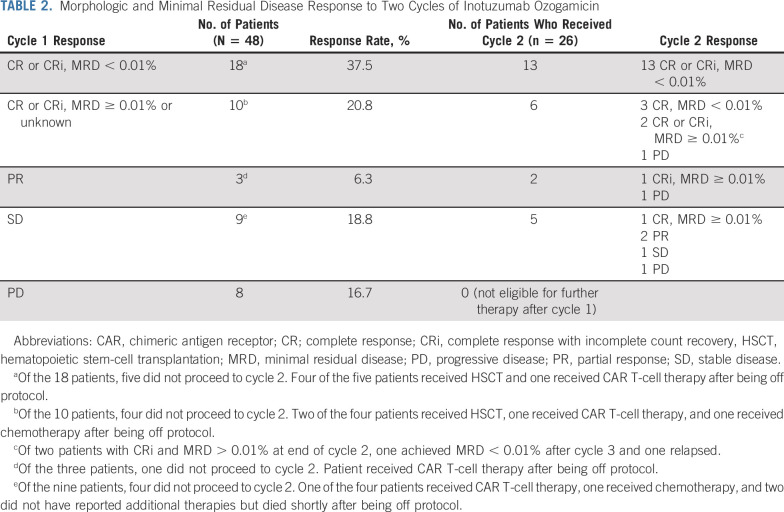
Of 24 patients enrolled in stage 1, 10 had CR and five had CRi after cycle 1, meeting the Protocol (online only)–defined threshold to proceed to stage 2. Among all patients (N = 48) following cycle 1, 19 had CR and nine had CRi (58.3%; two-sided 90% CI, 46.5 to 69.3). With 28 patients achieving CR or CRi after cycle 1, the null hypothesis was rejected and InO deemed promising. MRD was evaluated for 27 of 28 patients (96.4%) with cycle 1 CR or CRi, of whom 18 (66.7%) had MRD < 0.01% and three (11.1%) had MRD 0.01%-0.099%. Three patients had partial response (PR), nine had stable disease (SD), and eight had progressive disease (PD). Two patients with PD because of CNS progression achieved bone marrow CR with MRD < 0.01% and 0.02%.
Forty patients without PD were eligible for cycle 2. Table 2 details response for the 26 patients who received cycle 2 and subsequent treatment received by the 14 patients who elected to come off study. Thirty of 48 patients (62.5%; two-sided 90% CI, 50.6 to 73.0) achieved CR or CRi within the first two cycles; 21 (70%) had MRD < 0.01%. Thus, 21 of 48 patients (43.8%) achieved MRD-negative CR or CRi within two cycles. All 13 patients with MRD-negative CR or CRi after cycle 1 who received a second cycle maintained MRD-negative remission, and three of six with CR or CRi and positive or unknown MRD after cycle 1 achieved MRD < 0.01% after cycle 2. Seven patients with PR or SD after cycle 1 received cycle 2, of whom two achieved CR or CRi with detectable MRD and one cleared MRD after cycle 3. In univariate analysis, age was significantly associated with response after cycle 1 (P = .023) with a higher response rate for those age 7-17 years (Data Supplement). Cytogenetics did not correlate with response, although analysis is limited by small sample size. Notably, no patient with KMT2A rearrangement (KMT2A-R, n = 6) achieved MRD-negative CR or CRi.
TABLE 2.
Morphologic and Minimal Residual Disease Response to Two Cycles of Inotuzumab Ozogamicin
Subsequent Therapy and Survival
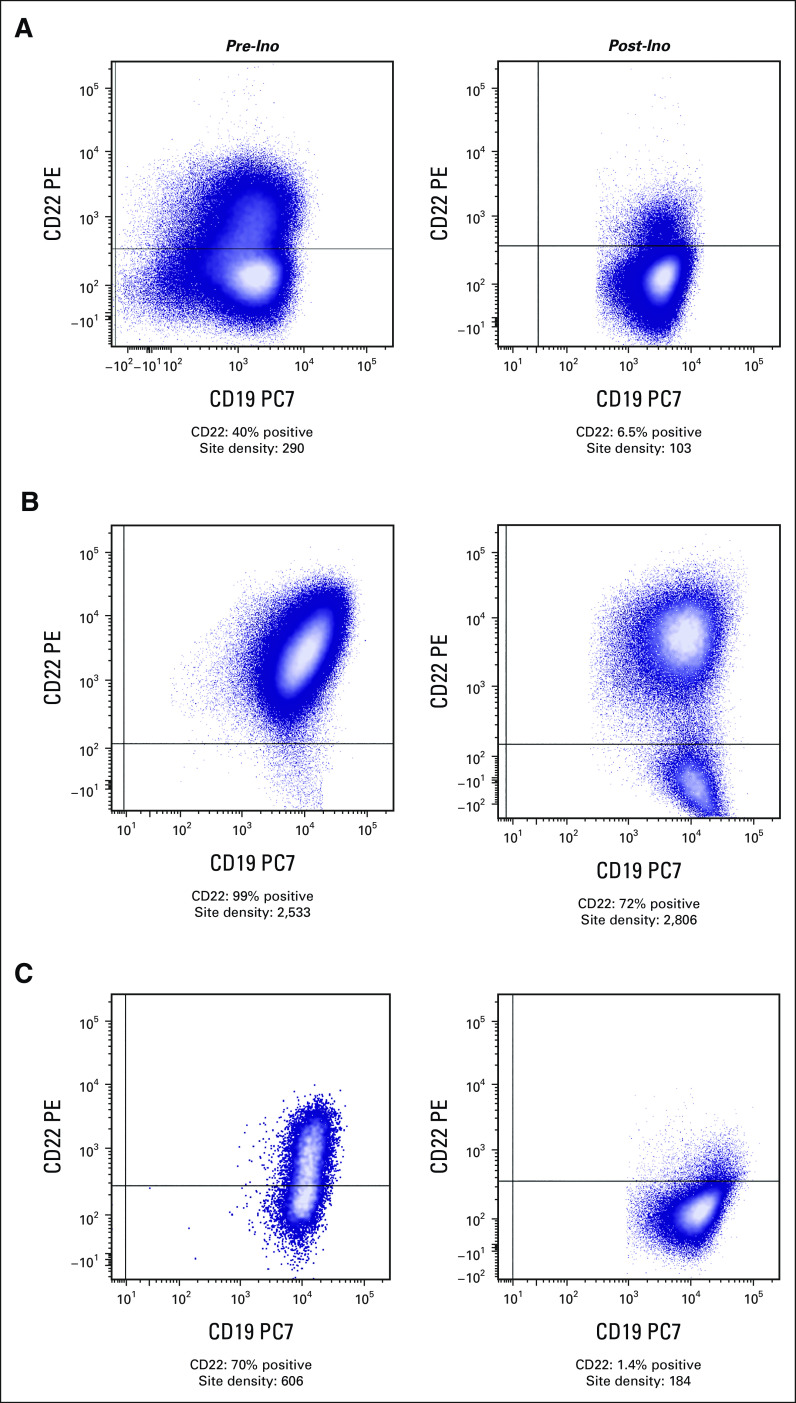
Response, subsequent therapy, and individual patient outcomes are illustrated in Figure 1. Twenty-one patients (43.8%) underwent allogeneic HSCT following study therapy; 17 patients with CR or CRi underwent HSCT within 3 months of the last InO dose and four (two CR, one PR, and one PD) underwent HSCT 6-12 months after InO following either chemotherapy (n = 1) or CAR T-cell therapy (n = 3). Fourteen patients (29.2%) received CAR T-cell therapy following InO including nine within 3 months (five CR or CRi, two PR, and two SD), three during 3-6 month follow-up (all CR or CRi), and two after > 2 years of follow-up. Seven of eight patients with CR or CRi who received CAR T-cell therapy within the first 6 months of follow-up did not receive other bridging therapy. One patient with KMT2A-R had MRD-positive CR with loss of CD22 expression after InO (Fig 2C), proceeded to CD19 CAR T-cell therapy, and subsequently had myeloid lineage switch.
FIG 1.
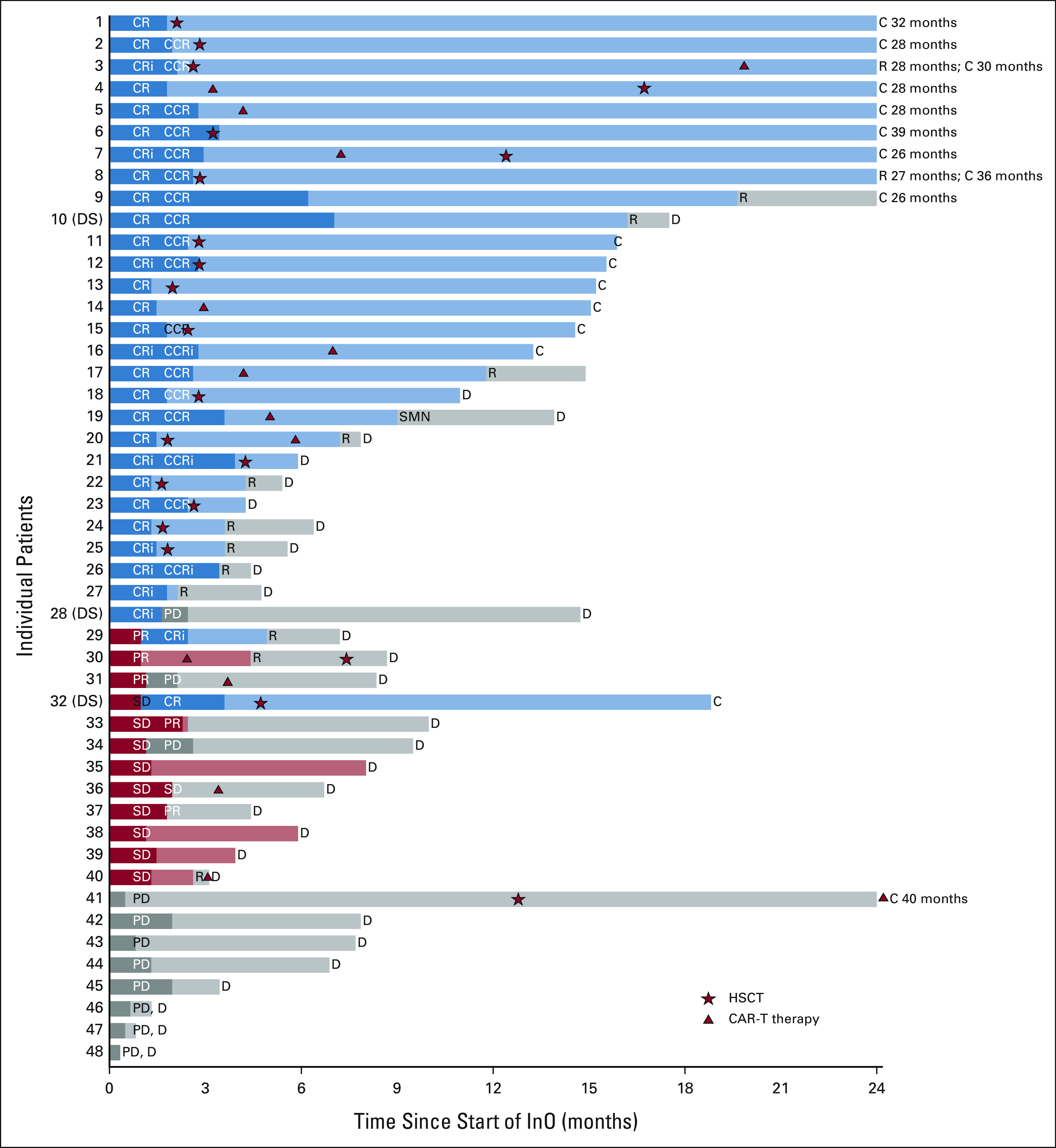
Swimmer's plot depicting individual patient response, subsequent therapy, and outcomes for 48 patients. Data are censored as of December 31, 2020. Patients with Down syndrome are denoted as DS. Dark bars indicate InO given on study. Initial response to cycle 1 is indicated by CR, CRi, PR, SD, or PD. For patients who received cycle 2, response is indicated as CCR or CCRi if previously achieved CR or CRi with cycle 1. Duration of initial response is indicated by color (CR or CRi = blue; PR or SD = red, PD = gray). HSCT is indicated by star, and CAR T-cell therapy is indicated by triangle. Patient 9 received six cycles of therapy on study and continued to receive commercial InO for an additional year before relapse. Patient 19 with KMT2A rearrangement received CD19 CAR T-cell therapy following three cycles of InO and subsequently developed lineage switch to acute myeloid leukemia (denoted as SMN). C, censored; CAR, chimeric antigen receptor; CCR, continuous complete response; CCRi, continuous complete response with incomplete count recovery; CR, complete response with or without count recovery; CRi, complete response with incomplete count recovery; D, death; DS, Down syndrome; HSCT, hematopoietic stem-cell transplantation; InO, inotuzumab ozogamicin; PD, progressive disease; PR, partial response; R, relapse; SD, stable disease; SMN, second malignant neoplasm.
FIG 2.
Flow cytometry evaluation of CD22 expression in three patients pretreatment (left column) and post-treatment (right column) with InO. The CD22 site density shown reflects that of the entire blast population, irrespective of percentage of CD22 positivity. (A) Partial CD22 expression pre-InO, which is further decreased post-InO. (B) Uniformly positive expression of CD22 pre-InO, with partial/bimodal expression of CD22 post-InO. (C) CD22 expression ranging from moderate to dim pre-InO with diminished CD22 expression post-InO (KMT2A-rearrangment with subsequent lineage switch to acute myeloid leukemia after CD19-directed therapy). InO, inotuzumab ozogamicin.
Among 48 treated patients, 18 were alive at last follow-up (median follow-up duration 2.2 years, range, 1.1-3.4 years). Thirty died; 26 from disease and four from toxicity (three post-HSCT). Thirty-five patients had an EFS event: 13 treatment failures, 15 relapses, one second malignancy neoplasm, and six deaths. The estimated 2-year EFS and OS rates were 28.6% (95% CI, 15.9 to 42.8) and 36.0% (95% CI, 22.3 to 49.9), respectively (Fig 3A). Patients with MRD-negative CR or CRi within two cycles (n = 21) had 2-year EFS rate 57.7% (95% CI, 31.9 to 76.8; Fig 3B). For 24 patients with CR or CRi who proceeded to HSCT (n = 17) or CAR T-cell therapy (n = 7) without additional bridging therapy, 2-year EFS rate was 58.8% (95% CI, 32.5 to 77.8) for HSCT and 68.6% (95% CI, 21.3 to 91.2) for CAR T-cell therapy (overall 62.0% [95% CI, 39.6 to 78.1], Fig 3C). Patients with ≥ 2 relapses (n = 32) had superior 2-year EFS rate (41.7%; 95% CI, 23.7 to 58.7) compared with 10 patients with first relapse refractory to ≥ 1 prior reinduction attempt (0%, P = .001) despite similar response rates (Data Supplement).
FIG 3.
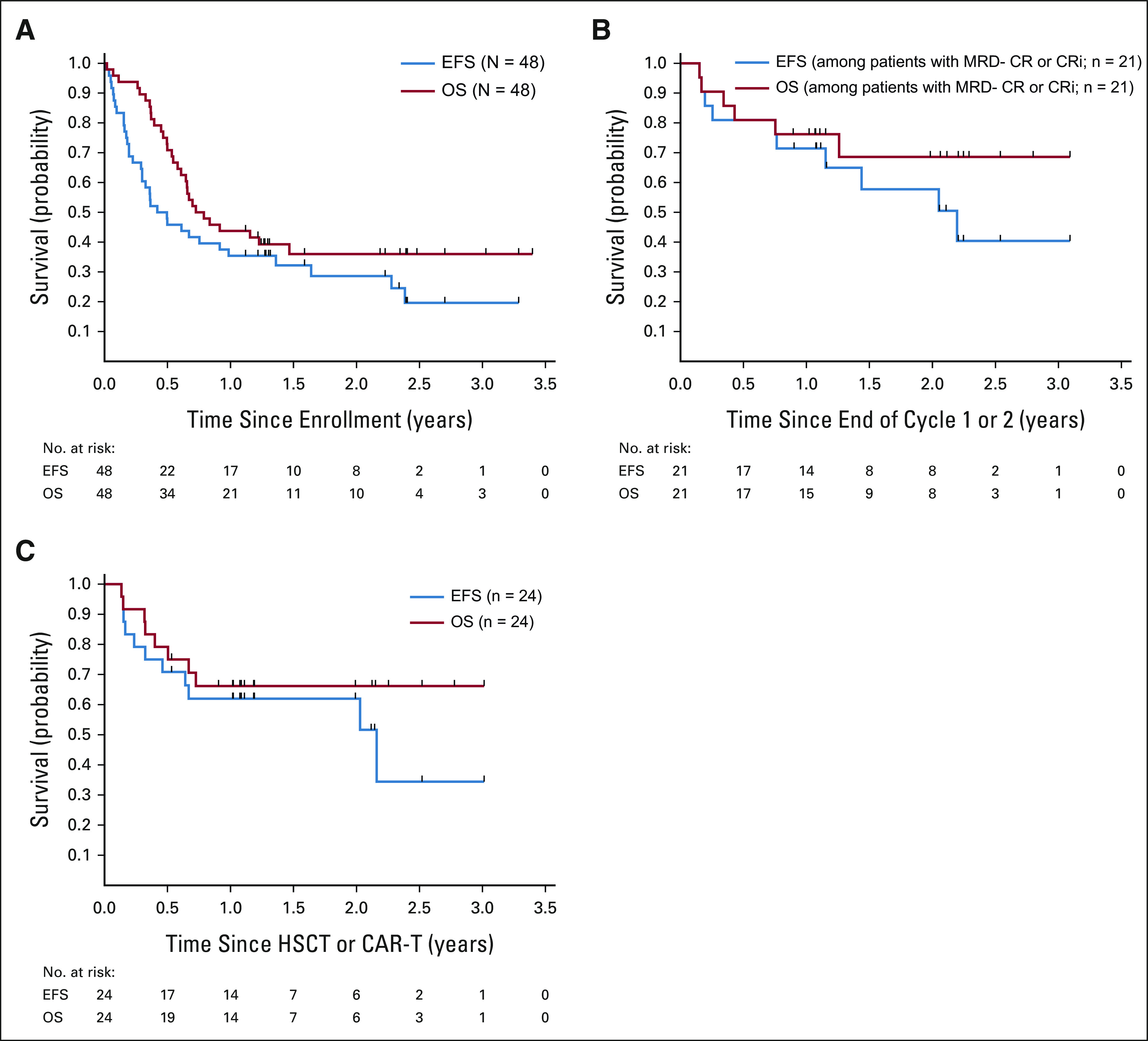
Outcomes after InO monotherapy. (A) EFS and OS of the 48 patients treated on study. The estimated EFS rate was 28.6% (95% CI, 15.9 to 42.8) at 2 years and 19.6% (95% CI, 7.9 to 35.1) at 3 years. The estimated OS rate was 36.0% (95% CI, 22.3 to 49.9) at 2 years or 3 years. (B) EFS and OS of patients with morphologic CR or CRi and MRD < 0.01% by flow cytometry with one or two cycles of treatment. Among patients with best response of CR or CRi and MRD < 0.01%, EFS was 71.4% (95% CI, 47.2 to 86) at 1 year and 57.7% (95% CI, 31.9 to 76.8) at 2 year, and the 2-year OS rate was 68.6% (95% CI, 41.8 to 84.9). (C) EFS and OS of patients who received HSCT or CART without other bridging therapy following a best response of CR or CRi after up to two cycles of InO. Of the 30 patients who achieved CR or CRi after up to two cycles of InO, four patients had an event at the end of InO therapy or shortly after being off protocol, two patients did not have HSCT or CART within 6 months without other therapy, and the remaining 24 patients had HSCT or CART following CR or CRi. Of the 24 patients who received HSCT or CART following CR or CRi, 2-year EFS and OS rates were 62.0%% (95% CI, 39.6 to 78.1) and 66.2% (95% CI, 43.6 to 81.5), respectively. CART, chimeric antigen receptor T-cell therapy; CR, complete response; CRi, complete response with incomplete count recovery; EFS, event-free survival; HSCT, hematopoietic stem-cell transplantation; InO, inotuzumab ozogamicin; MRD, minimal residual disease; OS, overall survival.
Safety and Toxicity
Nine patients experienced DLT in cycle 1, not crossing predefined stopping bounds. Hematologic DLT was the most common, reported in seven of 28 patients (25%) with CR or CRi; one additional patient was inevaluable because of platelet transfusion just before day 42. Nonhematologic DLTs included one grade 3 pulmonary hemorrhage with concurrent thrombocytopenia, one grade 3 stroke-like event proximate to intrathecal methotrexate, one grade 3 respiratory distress, and one grade 3 drug rash with eosinophilia and systemic symptoms. This patient tolerated rechallenge with dose-reduced InO in cycle 2 without toxicity, suggesting an alternate drug cause.
Grade 3 or higher AEs in cycles 1 and 2 are summarized in the Data Supplement. The most common AEs in cycle 1 were febrile neutropenia (29.2%) and infection (16.7%), predominantly bacterial. AEs were uncommon in subsequent courses, with grade 3 or higher neutropenia and/or thrombocytopenia noted in 19.2% of patients in cycle 2. No InO-related deaths occurred. Three (6.3%) patients had ALT increase (maximum grade 3), five (10.4%) had AST increase (maximum grade 3), and one had grade 3 bilirubin in cycle 1. In cycle 2, one patient had grade 3 ALT. No patient required InO dose modification for hepatic toxicity. No SOS occurred during InO monotherapy. Peripheral B-cell aplasia was observed in all evaluated patients. No impact on peripheral T cells was observed (Data Supplement).
Among 24 patients enrolled in stage 1, four developed grade 3 SOS after HSCT, triggering protocol-defined stopping rule (any grade SOS rate > 15%). As the rate of post-HSCT SOS was similar to prior studies, stopping rules were modified to capture severe events (≥ grade 4) after HSCT or any grade events during InO therapy. In stage 2, two patients developed grade 3 SOS after HSCT. Overall, SOS occurred in 12.5% (six of 48) of all patients and 28.6% (six of 21) of those undergoing HSCT. All six received early intervention including defibrotide; five recovered and one died from other HSCT complications (Data Supplement). Clinical features were evaluated for the 21 patients who underwent HSCT. No statistically significant associations with SOS were observed, with analysis limited by small numbers (Data Supplement).
Pharmacokinetics
InO trough levels were obtained in 22 patients in cycle 1 and 12 patients in cycle 2. Median trough levels increased with each dose, with trough levels during cycle 2 higher than the corresponding time points in cycle 1 (Data Supplement), consistent with reported PK data.8,13 Trough levels did not differ by response to cycle 1 (Data Supplement). For 10 patients with trough levels on day 1 of cycle 2, InO was detectable at low levels (median 9.2 ng/mL, range, 0.99-35.3 ng/mL), although cycle 2 day 1 started at least three weeks from the last dose of InO in cycle 1 in 80% of patients (median time between cycle 1 last InO dose and start of cycle 2 = 20 days, range, 7-29 days; Data Supplement).
CD22 Site Density and Expression
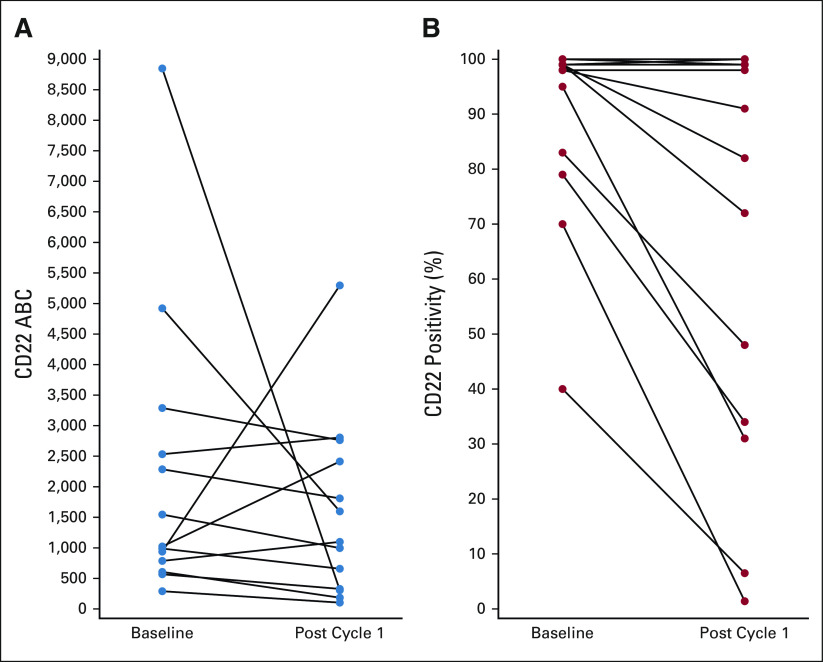
Among 27 patients with paired pre- and post-cycle 1 samples, median baseline CD22 ABC was 1,022 sites per cell (range, 290-8,848 sites per cell) for nonresponders (n = 13) and 4,123 sites per cell (range, 762-10,715 sites per cell) for responders (n = 14; P = .008). Median baseline CD22% was similar but ranged more widely in nonresponders (98%; range, 40%-100%) versus responders (99%; range, 92.9%-100%; P = .014). For nonresponders, ABC did not change significantly pre- and post-cycle 1 (Fig 4A), but CD22% decreased in eight of 13 patients (median, 82%; range, 1.4%-100%) compared with baseline (median, 98%; range, 40%-100%, P = .007; Fig 4B). For the four patients with baseline CD22% < 90%, evaluations after cycle 1 revealed emergence of predominantly CD22-negative populations. Representative modulation of CD22 expression is depicted in Figure 2. Among four patients with KMT2A-R who were evaluated centrally, three had partial CD22% at baseline and all had ABC < 1,500 sites per cell.
FIG 4.
CD22 site density. (A) Surface CD22 site density measured using ABC at baseline and after cycle 1 in 13 patients with residual leukemia after cycle 1. (B) Surface CD22 expression (CD22%) at baseline and after cycle 1 in 13 patients with residual leukemia after cycle 1, demonstrating decrease from baseline in eight of 13 patients. ABC, antibody bound per cell.
DISCUSSION
In this phase II single-arm trial, the rate of CR or CRi with one cycle of InO was 58.3% with MRD < 0.01% in 66.7% of responders. On the ITCC-059 phase I trial, the rate of CR or CRi for 11 patients at this dose was 85%.8 The difference may be because of sample size, given overlapping CIs as trial populations were similar. Collectively, these response rates compare favorably with blinatumomab and tisagenlecleucel, CD19-targeted immunotherapies that are FDA-approved for children with R/R B-ALL. The RIALTO phase II trial of blinatumomab had rate of CR or CRi 59.2% (95% CI, 48.4 to 69) with 78% of responders MRD-negative and the ELIANA phase II trial of tisagenlecleucel had rate of CR or CRi of 81% (95% CI, 71 to 89) with 100% of responders MRD-negative.14,15 Tisagenlecleucel has superior survivor outcomes with EFS 50% (95% CI, 35 to 64) at 12 months and unlike blinatumomab or InO has the potential for long-term cure without subsequent HSCT. The side effect profiles of these CD19-targeted immunotherapies include cytokine release syndrome and neurologic toxicity.16,17 Administration of these therapies can be logistically challenging as blinatumomab requires continuous intravenously infusion for 28 days and tisagenlecleucel requires T-cell collection, manufacturing, and infusion at specialized treatment centers. By contrast, InO has easier administration but a significant risk of SOS for patients proceeding to HSCT.
Although not curative alone, InO is highly effective as a bridge to potentially curative therapy. Among 30 patients with CR or CRi, 24 (80%) proceeded to HSCT (n = 17) or CAR T-cell therapy (n = 7) within the first 6-month follow-up period with 2-year EFS rate of 53.1%. Despite concern that patients with MRD-negative remission and peripheral B-cell aplasia after InO therapy might impair CAR T-cell expansion because of lack of antigen target, patients in this study received CAR-T cell therapy without prolonged delay.18,19 No impact on peripheral blood CD3 counts was observed, suggesting that T-cell collection after InO is feasible, although T-cell function after InO requires further study. Investigations regarding duration of B-cell aplasia after InO, optimal timing of T-cell collection, and outcomes after CAR T-cell therapy are ongoing.
Central evaluation of surface CD22 expression confirmed that pre-existing partial CD22-positivity is associated with poor response. In INO-VATE, only two of five patients with CD22% < 70% achieved MRD-negative CR.4 Pharmacodynamic analysis found that patients with high CD22% benefited most from InO.4,20 In this study, two of four patients with CD22% < 90% achieved CR or CRi but neither had MRD < 0.01%. For CD22 site density, patients without CR or CRi had lower median baseline CD22 ABC. However, some patients with low ABC did respond; no clear threshold was identified for dim expression that would preclude InO response. Patients with KMT2A-R, who commonly have lower ABC and/or partial CD22 expression,21,22 had poor responses; zero of six patients achieved MRD-negative CR or CRi, consistent with adult outcomes.23 One patient with KMT2A-R demonstrated myeloid lineage switch following subsequent CD19 CAR T-cell therapy; it is unknown whether InO exposure contributed as lineage switch has been reported with CD19-targeted therapy.24,25 Further research is needed to understand mechanisms of CD22 modulation in response to InO. Furthermore, predictors of response to InO beyond CD22 expression remain incompletely understood and may include host factors and cell intrinsic mechanisms of resistance similar to the calicheamicin-conjugated antibody-drug conjugate gemtuzumab ozogamicin.26
InO was well tolerated in these heavily pretreated patients. Delayed count recovery beyond day 42 occurred in 25% of patients achieving CR or CRi in cycle 1, consistent with prior trials demonstrating myelosuppression as a significant toxicity.4,8 The rates of febrile neutropenia and infection were much lower than reported with intensive chemotherapy for similar patients and are consistent with trials demonstrating decreased toxicity with immunotherapy.27-29 Although significant hepatic toxicity including SOS was not observed during InO therapy, lower-grade ALT and bilirubin elevations were not captured on this trial. The SOS rate with subsequent HSCT was significant (28.6%), similar to prior studies.6-8 With analysis limited by small numbers, no other risk factors were identified, including use of dual alkylator conditioning regimens or abnormal ALT or bilirubin before HSCT, which have been associated with SOS in adults.6 While prophylactic ursodiol and/or defibrotide peri-HSCT did not appear to affect SOS risk, larger studies of prophylaxis in patients undergoing HSCT after calicheamicin-based therapies are needed. Although most SOS cases resolved quickly in this trial, physician awareness of prior InO exposure and early intervention including defibrotide may be helpful, as SOS has the potential for significant post-HSCT morbidity and mortality. Reduced InO dosing before HSCT to mitigate SOS risk is under investigation in adults (ClinicalTrials.gov identifier: NCT03677596). Another strategy to optimize the risk-benefit ratio is to incorporate InO into frontline acute lymphoblastic leukemia therapy for patients who are unlikely to need HSCT in the immediate future (ClinicalTrials.gov identifier: NCT03959085, NCT03150693).
Trough PK sampling in a subset of patients showed accumulation over the course of each cycle consistent with target-mediated clearance and increasing half-life with cumulative dosing as published.8,13 There was a nonsignificant trend toward higher troughs in patients with CR or CRi in cycle 1, consistent with exposure-response modeling in adults where InO exposure correlated with response and MRD‐negativity.30 Trough InO levels at cycle 2 day 1 were detectable in most patients, albeit at low levels, even in patients with significant delay from the last InO dose in cycle 1. This may affect clinical trial designs incorporating blocks of InO because of potential toxicity in subsequent chemotherapy cycles.
Although the response rate with InO is encouraging in this heavily pretreated population, combining InO with chemotherapy has the potential to improve the rate, depth, and duration of remission, allowing more patients to proceed to curative therapies and potentially decrease the emergence/selection of CD22-negative blasts. Adult trials have demonstrated the success of this approach with dose reductions of both InO and chemotherapy.31,32 COG AALL1621 is now evaluating InO combined with augmented Berlin-Frankfurt-Munster consolidation chemotherapy used in frontline COG trials, establishing the safety for future incorporation of this promising agent.
Maureen M. O'Brien
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Pfizer (Inst)
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Amgen (Inst), Celgene (Inst), BTG (Inst), Incyte (Inst), AbbVie (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst)
Nirali N. Shah
Research Funding: Lentigen (Inst)
Susan R. Rheingold
Employment: OptiNose (I)
Stock and Other Ownership Interests: OptiNose (I)
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer (Inst)
Andrew C. Harris
Consulting or Advisory Role: Mesoblast, Horizon Therapies
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead Company, Amgen, Kura Oncology, Takeda
Michael J. Borowitz
Consulting or Advisory Role: Amgen, Blueprint Medicines
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
John Kairalla
Stock and Other Ownership Interests: Editas Medicine, ACADIA Pharmaceuticals Inc, Johnson & Johnson/Janssen
Meenakshi Devidas
Honoraria: Novartis
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: Celgene
Lia Gore
Employment: Anchiano (I)
Leadership: Anchiano (I), Vedantra (I)
Stock and Other Ownership Interests: Amgen, Sanofi, Celgene, Anchiano (I), Mirati Therapeutics (I), OnKure, ITOS Oncology (I)
Consulting or Advisory Role: Novartis, Amgen, Roche/Genentech, Syndax, OnKure, Janssen Oncology, Pfizer
Patents, Royalties, Other Intellectual Property: Patent held for diagnostic discovery and treatment response methodology tools in the use of MR spectroscopy for leukemia
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part in abstract form at the American Society of Hematology annual meeting, Orlando, FL, December 7-10, 2019, and the American Society of Clinical Oncology annual meeting (virtual), May 29-31, 2020.
SUPPORT
Supported by NCTN Operations Center and Statistics and Data Center (SDC) Grant Nos. (U10CA180886, U10CA180899, and 1U24CA-CA196173), the St Baldrick's Foundation, and Pfizer Inc. M.L.L. is the Benioff Chair of Children's Health and the Deborah and Arthur Ablin Endowed Chair for Pediatric Molecular Oncology at Benioff Children's Hospital. E.A.R. is a KiDS of NYU Foundation Professor at NYU Langone Health. L.G. is the Ergen Family Chair in Pediatric Oncology at Children's Hospital Colorado and supported by University of Colorado Cancer Center P30 CA046934.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The Children's Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase III studies, individual-level deidentified data sets that would be sufficient to reproduce the results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For nonphase III studies, data are available following the primary publication. An individual-level deidentified data set containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use. For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.
AUTHOR CONTRIBUTIONS
Conception and design: Maureen M. O'Brien, Lingyun Ji, Nirali N. Shah, Susan R. Rheingold, Deepa Bhojwani, Olga Militano, John Kairalla, Elizabeth A. Raetz, Lia Gore, Mignon L. Loh
Administrative support: Lia Gore
Provision of study materials or patients: Nirali N. Shah, Susan R. Rheingold, Lia Gore
Collection and assembly of data: Maureen M. O'Brien, Nirali N. Shah, Susan R. Rheingold, Constance M. Yuan, Patrick A. Brown, Michael J. Borowitz, Lia Gore
Data analysis and interpretation: Maureen M. O'Brien, Lingyun Ji, Nirali N. Shah, Susan R. Rheingold, Deepa Bhojwani, Constance M. Yuan, Xinxin Xu, Joanna S. Yi, Andrew C. Harris, Patrick A. Brown, Michael J. Borowitz, Meenakshi Devidas, Lia Gore, Mignon L. Loh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of Inotuzumab Ozogamicin in Children and Adolescents With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia: Children's Oncology Group Protocol AALL1621
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Maureen M. O'Brien
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Pfizer (Inst)
Consulting or Advisory Role: Jazz Pharmaceuticals
Research Funding: Amgen (Inst), Celgene (Inst), BTG (Inst), Incyte (Inst), AbbVie (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst)
Nirali N. Shah
Research Funding: Lentigen (Inst)
Susan R. Rheingold
Employment: OptiNose (I)
Stock and Other Ownership Interests: OptiNose (I)
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer (Inst)
Andrew C. Harris
Consulting or Advisory Role: Mesoblast, Horizon Therapies
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead Company, Amgen, Kura Oncology, Takeda
Michael J. Borowitz
Consulting or Advisory Role: Amgen, Blueprint Medicines
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
John Kairalla
Stock and Other Ownership Interests: Editas Medicine, ACADIA Pharmaceuticals Inc, Johnson & Johnson/Janssen
Meenakshi Devidas
Honoraria: Novartis
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: Celgene
Lia Gore
Employment: Anchiano (I)
Leadership: Anchiano (I), Vedantra (I)
Stock and Other Ownership Interests: Amgen, Sanofi, Celgene, Anchiano (I), Mirati Therapeutics (I), OnKure, ITOS Oncology (I)
Consulting or Advisory Role: Novartis, Amgen, Roche/Genentech, Syndax, OnKure, Janssen Oncology, Pfizer
Patents, Royalties, Other Intellectual Property: Patent held for diagnostic discovery and treatment response methodology tools in the use of MR spectroscopy for leukemia
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hunger SP, Mullighan CG.Acute lymphoblastic leukemia in children N Engl J Med 3731541–15522015 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study Leukemia 222142–21502008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shor B, Gerber HP, Sapra P.Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies Mol Immunol 67107–1162015 [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia N Engl J Med 375740–7532016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study Cancer 1252474–24872019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian HM, DeAngelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: Results from the open-label, randomised, phase 3 INO-VATE study Lancet Haematol 4e387–e3982017 [DOI] [PubMed] [Google Scholar]
- 7.Bhojwani D, Sposto R, Shah NN, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia Leukemia 33884–8922019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brivio E, Locatelli F, Lopez-Yurda M, et al. A phase I study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study) Blood 1371582–15902020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanova A, Qaqish BF, Schell MJ.Continuous toxicity monitoring in phase II trials in oncology Biometrics 61540–5452005 [DOI] [PubMed] [Google Scholar]
- 10.Shulman HM, Hinterberger W.Hepatic veno-occlusive disease—Liver toxicity syndrome after bone marrow transplantation Bone Marrow Transplant 10197–2141992 [PubMed] [Google Scholar]
- 11.Agresti A, Coull BA.Approximate is better than “exact” for interval estimation of binomial proportions Am Stat 52119–1261998 [Google Scholar]
- 12.Jung SH, Lee T, Kim K, et al. Admissible two-stage designs for phase II cancer clinical trials Stat Med 23561–5692004 [DOI] [PubMed] [Google Scholar]
- 13.Garrett M, Ruiz-Garcia A, Parivar K, et al. Population pharmacokinetics of inotuzumab ozogamicin in relapsed/refractory acute lymphoblastic leukemia and non-Hodgkin lymphoma J Pharmacokinet Pharmacodyn 46211–2222019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Locatelli F, Zugmaier G, Mergen N, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: Results of the RIALTO trial, an expanded access study. Blood Cancer J. 2020;10:77. doi: 10.1038/s41408-020-00342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia N Engl J Med 378439–4482018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viardot A, Locatelli F, Stieglmaier J, et al. Concepts in immuno-oncology: Tackling B cell malignancies with CD19-directed bispecific T cell engager therapies Ann Hematol 992215–22292020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8:e001511. doi: 10.1136/jitc-2020-001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of chimeric antigen receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)—An expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT) Bone Marrow Transplant 541868–18802019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults Blood 1293322–33312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarjian HM, Stock W, Cassaday RD, et al. Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia in the INO-VATE trial: CD22 pharmacodynamics, efficacy, and safety by baseline CD22 Clin Cancer Res 272742–27542021 [DOI] [PubMed] [Google Scholar]
- 21.Shah NN, Stevenson MS, Yuan CM, et al. Characterization of CD22 expression in acute lymphoblastic leukemia Pediatr Blood Cancer 62964–9692015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, Advani AS, Stelljes M, et al. Prognostic implications of cytogenetics in adults with acute lymphoblastic leukemia treated with inotuzumab ozogamicin Am J Hematol 94408–4162019 [DOI] [PubMed] [Google Scholar]
- 23.Jabbour E, O'Brien S, Huang X, et al. Prognostic factors for outcome in patients with refractory and relapsed acute lymphocytic leukemia treated with inotuzumab ozogamicin, a CD22 monoclonal antibody Am J Hematol 90193–1962015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oberley MJ, Gaynon PS, Bhojwani D, et al. Myeloid lineage switch following chimeric antigen receptor T-cell therapy in a patient with TCF3-ZNF384 fusion-positive B-lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e27265. doi: 10.1002/pbc.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayes A, McMasters RL, O'Brien MM.Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy Pediatr Blood Cancer 631113–11152016 [DOI] [PubMed] [Google Scholar]
- 26.Lamba JK, Chauhan L, Shin M, et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: Report from randomized phase III Children's Oncology Group trial AAML0531 J Clin Oncol 352674–26822017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: A Children's Oncology Group study[corrected] J Clin Oncol 263971–39782008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messinger YH, Gaynon PS, Sposto R, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) study Blood 120285–2902012 [DOI] [PubMed] [Google Scholar]
- 29.Brown PA, Ji L, Xu X, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial JAMA 325833–8422021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Haughey M, Vandendries E, et al. Characterization of the relationship of inotuzumab ozogamicin exposure with efficacy and safety end points in adults with relapsed or refractory acute lymphoblastic leukemia Clin Transl Sci 14184–1932021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jabbour E, Ravandi F, Kebriaei P, et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory philadelphia chromosome-negative acute lymphoblastic leukemia: A phase 2 clinical trial JAMA Oncol 4230–2342018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with philadelphia chromosome-negative acute lymphoblastic leukaemia: A single-arm, phase 2 study Lancet Oncol 19240–2482018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Children's Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase III studies, individual-level deidentified data sets that would be sufficient to reproduce the results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For nonphase III studies, data are available following the primary publication. An individual-level deidentified data set containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use. For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.