Abstract
PURPOSE
Dual targeting of the gastrointestinal stromal tumor (GIST) lineage-specific master regulators, ETV1 and KIT, by MEK and KIT inhibitors were synergistic preclinically and may enhance clinical efficacy. This trial was designed to test the efficacy and safety of imatinib plus binimetinib in first-line treatment of GIST.
METHODS
In this trial (NCT01991379), treatment-naive adult patients with confirmed advanced GISTs received imatinib (400 mg once daily) plus binimetinib (30 mg twice daily), 28-day cycles. The primary end point was RECIST1.1 best objective response rate (ORR; complete response plus partial response [PR]). The study was designed to detect a 20% improvement in the ORR over imatinib alone (unacceptable rate of 45%; acceptable rate of 65%), using an exact binomial test, one-sided type I error of 0.08 and type II error of 0.1, and a planned sample size of 44 patients. Confirmed PR or complete response in > 24 patients are considered positive. Secondary end points included Choi and European Organisation for Research and Treatment of Cancer Response Rate, progression-free survival (PFS), overall survival (OS), pathologic responses, and toxicity.
RESULTS
Between September 15, 2014, and November 15, 2020, 29 of 42 evaluable patients with advanced GIST had confirmed RECIST1.1 PR. The best ORR was 69.0% (two-sided 95% CI, 52.9 to 82.4). Thirty-nine of 41 (95.1%) had Choi PR approximately 8 weeks. Median PFS was 29.9 months (95% CI, 24.2 to not estimable); median OS was not reached (95% CI, 50.4 to not estimable). Five of eight patients with locally advanced disease underwent surgery after treatment and achieved significant pathologic response (≥ 90% treatment effect). There were no unexpected toxicities. Grade 3 and 4 toxicity included asymptomatic creatinine phosphokinase elevation (79.1%), hypophosphatemia (14.0%), neutrophil decrease (9.3%), maculopapular rash (7.0%), and anemia (7.0%).
CONCLUSION
The study met the primary end point. The combination of imatinib and binimetinib is effective with manageable toxicity and warrants further evaluation in direct comparison with imatinib in frontline treatment of GIST.
INTRODUCTION
Gastrointestinal stromal tumor (GIST) represents one of the most common subtypes of sarcoma.1 The majority of GISTs harbor activating mutations in KIT or PDGFRA receptor tyrosine kinases and less frequently in BRAF and FGFR1, or inactivating mutations in NF1 and succinate dehydrogenase complex core components.1-6 They have provided the scientific rationale for the clinical success of targeting mutant KIT and alpha-platelet-derived growth factor receptor, alpha (PDGFRα) with tyrosine kinase inhibitors (TKIs) in GIST. Imatinib mesylate (Gleevec; 400 mg once daily), a TKI that targets mutant KIT and PDGFRα, is the standard-of-care (SOC) first-line therapy in advanced GIST with an objective response rate (ORR) of 45%-52%, a median progression-free survival (mPFS) of 18-20.4 months, and approximately 10%-17% of patients on first-line imatinib remain nonprogressors in the long term (> 9 years).7-12 Despite the clinical success, most GISTs develop resistance to imatinib within 2 years of treatment9; among them, 15% develop early resistance within 3 months and 5%-10% demonstrate primary resistance. The development of imatinib resistance often leads to a rapid clinical decline and eventual death,13 as subsequent therapies, including second-line sunitinib and third-line regorafenib, have limited efficacies with mPFS of 5.6 months and 4.8 months and ORR of 7% and 4.5%, respectively.13,14 Recently, US Food and Drug Administration approved ripretinib, a new generation of TKI targeting a broad spectrum of imatinib-resistant mutations in KIT and PDGFRA, which demonstrated mPFS of 6.3 months and ORR of 9% in the fourth-line treatment of advanced GIST.15
CONTEXT
Key Objective
Despite the clinical success of imatinib, most patients with advanced gastrointestinal stromal tumor (GIST) develop resistance to imatinib and succumb to their disease. We aim to identify a therapeutic strategy that can enhance the efficacy of imatinib in frontline treatment of GIST.
Knowledge Generated
We conducted a phase II trial of the novel combination of imatinib and binimetinib in treatment-naive advanced GIST. This trial met its prespecified primary end point, demonstrating an objective response rate of 69.0% (29 confirmed RECIST1.1 partial response), 95.1% response rate (RR) by Choi, longer progression-free survival, deeper pathologic responses, insights toward therapy resistance, and manageable toxicity.
Relevance
Our study highlights the importance of targeting the GIST lineage dependence on ETV1 and KIT. To our knowledge, this is the first trial testing a tyrosine kinase inhibitor combination in the frontline treatment of GIST. This study puts forth the combination as a novel therapeutic strategy and warrants further evaluation to directly challenge imatinib in the first-line treatment of advanced GIST.
Imatinib resistance mechanisms in GIST are heterogeneous. Imatinib-resistant KIT mutations are rare in primary-resistance setting, but are found in 50%-67% of patients with acquired resistance.16-18 Emerging data indicate that the KIT-low and KIT-independent GIST stem or progenitor cells may play a role in both primary and secondary imatinib resistance.19 Early adaptive responses to imatinib use developmentally programmed cell-autonomous mechanisms that lead to decreased dependence on KIT and MAPK signaling20 and create opportunities for persistent disease and development of therapy resistance. Furthermore, genetic tumor heterogeneity that exists within a single tumor, among tumors from different patients (interpatient) or within the same patient (intrapatient), have been increasingly appreciated as mechanisms of cancer progression through therapeutic resistance. In GIST, it remains controversial whether tumor heterogeneity is present at initial presentation. Nevertheless, interpatient and intrapatient genetic tumor heterogeneity is well recognized in advanced GIST upon progression on imatinib (unpublished).1 The tumor heterogeneity in the imatinib-resistant setting poses significant challenges for next-generation therapeutic development. Imatinib resistance remains the most significant problem in the current management of advanced GIST.9,16,21-26 It is imperative to develop novel therapeutic strategies that can enhance the efficacy and forestall the resistance of imatinib in frontline treatment of GIST.
KIT and ETV1 are well-established master regulators of GIST.2,27,28 The ETV1 protein is stabilized by active MAPK signaling downstream of KIT and PDGFRα signaling and that stabilized ETV1 cooperates with activated KIT in GIST pathogenesis through enhanced transcriptional regulation of KIT expression by ETV1.20,28-31 Furthermore, ETV1 maintains the homeostasis of the MAPK signaling in GIST and regulates the early adaptive response and resistance to imatinib treatment in GIST, through MAPK-dependent COP1-mediated protein degradation of ETV1.20 In preclinical models, dual lineage targeting of ETV1 protein stability by an MEK inhibitor (binimetinib) and of KIT by imatinib synergistically inhibited GIST tumor growth and survival in vitro and in vivo.20,31
Reasoning that the dual targeting of ETV1 and KIT by the combination of binimetinib and imatinib may have the potential to induce enhanced therapeutic responses in GIST, we designed a phase Ib and II clinical trial to evaluate the safety and tolerability of the combination of imatinib and binimetinib and to define the recommended phase II dose (RP2D) in patients with refractory GIST (phase Ib, reported separately) and to evaluate the efficacy in patients with treatment-naive advanced GIST (phase II).
METHODS
See additional details in the Supplemental Methods (Appendix 1, online only) and Protocol (online only).
Patients
Adult patients (age ≥ 18 years) who had histologically confirmed advanced GIST, an Eastern Cooperative Oncology Group (ECOG) performance score of 0-1, treatment-naive, or who had previously been treated with adjuvant imatinib but has been off imatinib for at least 3 months, or who had started SOC imatinib within 4 weeks, and had adequate end-organ function were eligible to consent and participate.
Study Design, Treatment, and End Points
This is a single-center, single-arm, phase II study to evaluate the safety and efficacy of imatinib plus binimetinib in patients with treatment-naive advanced histologically confirmed GIST.
All eligible patients received a 2-week lead-in of imatinib alone (400 mg once daily) followed by imatinib (400 mg once daily) plus binimetinib (30 mg twice daily) on the basis of the RP2D defined in a phase Ib study,32 continuously on every 28-day cycle.
Disease assessments with computed tomography (CT) or magnetic resonance imaging were performed at baseline, every 8 weeks for initial 32 weeks and every 12 weeks until surgery, disease progression, death, or withdrawal. Combined positron emission tomography (PET)-CT was performed at baseline and at the end of cycle 1 of the combination therapy. Adverse events (AEs) were graded by the investigator according to the Common Terminology Criteria for Adverse Events (4.03) until 28 days after discontinuation of treatment.
The primary end point was best ORR by RECIST1.133 (complete response [CR] plus partial response [PR]). Secondary end points included RR by Choi34 and European Organisation for Research and Treatment of Cancer (EORTC),35 PFS, overall survival (OS), pathologic responses, and treatment-associated AE.
Statistical Analysis
The trial is designed to detect a 20% improvement in the RECIST ORR of imatinib (400 mg once daily) alone,8,9,36 with an unacceptable rate of 45% and acceptable rate of 65%, on the basis of the exact binomial test and one-sided type I error of 0.08 and a type II error of 0.1, and a planned sample size of 44 patients. If > 24 patients have confirmed CR or PR by RECIST1.1, the trial will be considered positive.
Trial Oversight
The study was performed in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. The protocol, protocol amendments, and informed-consent documents were approved by the institutional review board (IRB) at Memorial Sloan Kettering Cancer Center. All participants provided written informed consent. All biopsies and molecular testing were performed in accordance with the IRB-approved protocol.
RESULTS
Study Participants
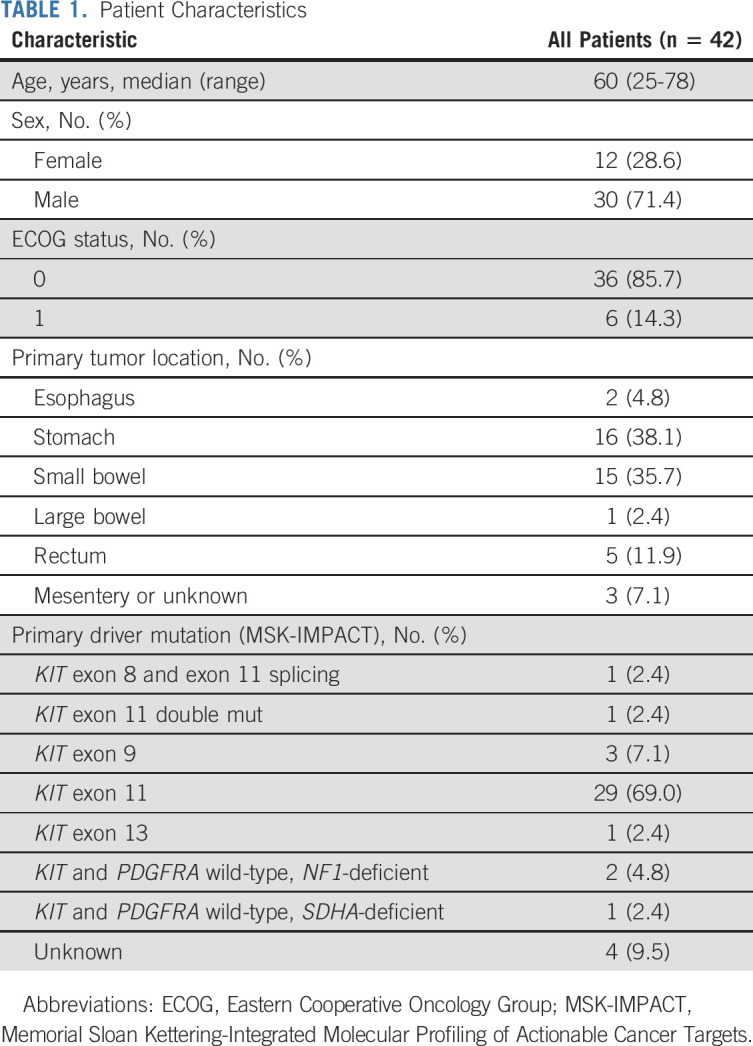
Between September 15, 2014, and November 15, 2020, 54 patients were screened and 50 patients were consented and enrolled in the phase II study (Fig 1). Forty-three patients who received at least two doses of the combination treatment were evaluable for safety; 42 patients who had at least one follow-up imaging study were evaluable for efficacy. The median age of the efficacy analytic cohort was 60 years (range, 25-78 years), 28.6% female, and 85.7% patients were ECOG 0 (Table 1). Primary tumors are localized throughout the GI tract. The primary driver mutations included KIT exon 11 (n = 29, 69%), exon 9 (n = 3, 7.1%), exon 13 (n = 1, 2.4%), KIT exons 8 of 11 (n = 1) and 11 of 11 (n = 1) double mutations, and NF1- (n = 2, 4.8%) or SDHA- (n = 1, 2.4%) deficiency (Table 1).
FIG 1.
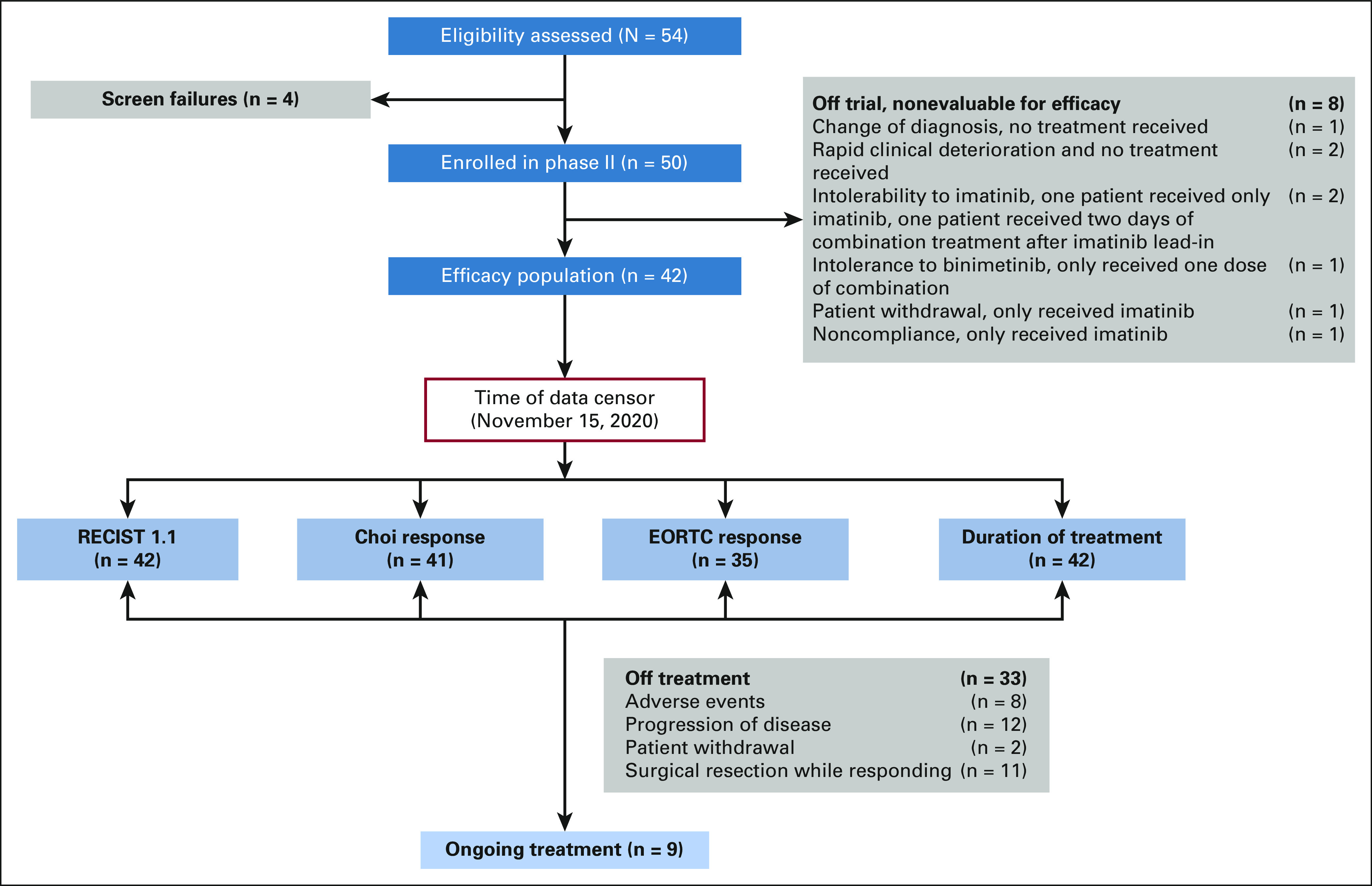
CONSORT flow diagram of patients in the phase II study of imatinib in combination with binimetinib (September 15, 2014-November 15, 2020, data cutoff). EORTC, European Organisation for Research and Treatment of Cancer.
TABLE 1.
Patient Characteristics
Efficacy
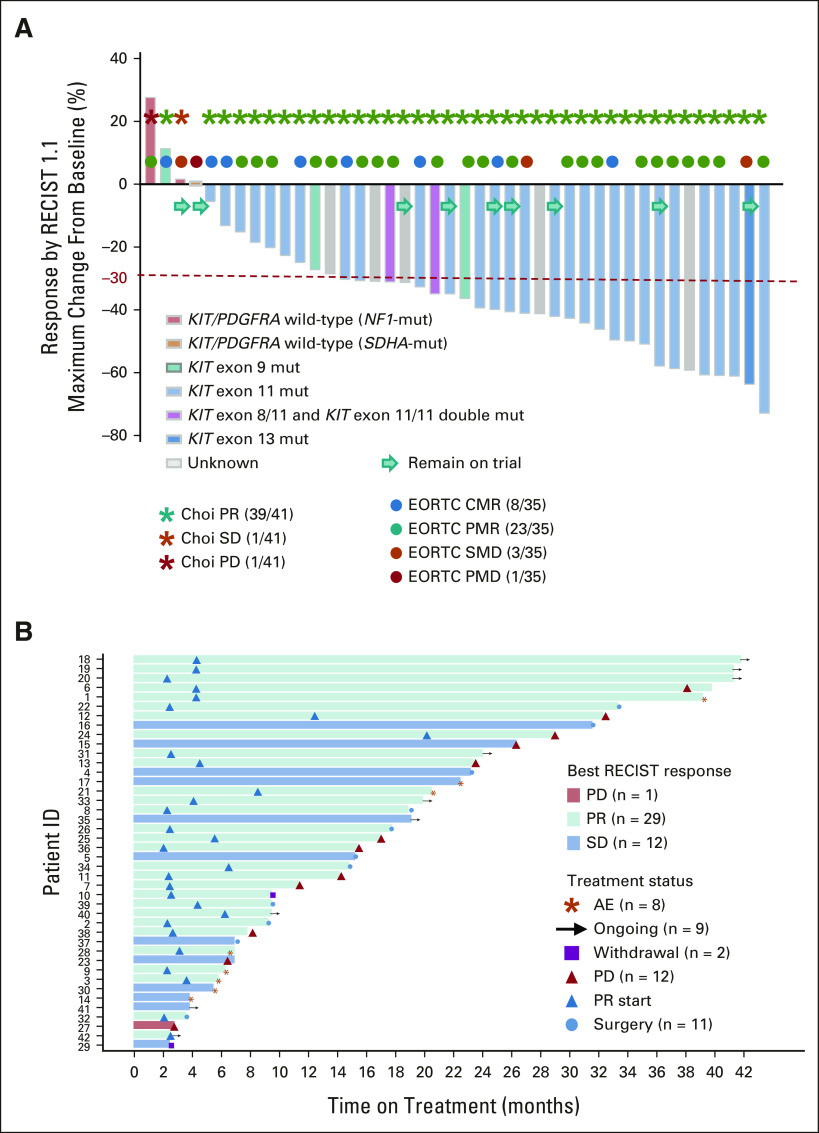
At data cutoff, 29 of 42 evaluable patients have confirmed RECIST1.1 PR. The study met its prespecified primary end point (> 24 confirmed RECIST1.1 PR). The best ORR was 69.0% (95% CI, 52.9 to 82.4; Fig 2A). Thirty-nine of 41 (95.1%; 95% CI, 83.5 to 99.4) evaluable patients had a Choi PR approximately 8 weeks. Eight of 35 (22.9%) patients with PET scans evaluable by EORTC at 4 weeks of combination treatment achieved complete metabolic response and 23 of 35 (65.7%) achieved partial metabolic response (Fig 2A). Nine patients have remained on trial (range, 2.4-39.0 months), with seven RECIST PR and two RECIST stable disease (SD); 11 responding patients went to surgery (range, 3.4-31.2 months); 12 patients progressed (range, 2.6-37.2 months); eight patients discontinued trial because of treatment-associated toxicity; and two patients withdrew consent (Fig 2B). The clinical benefit rate (CR plus PR plus SD) was 83.3% (95% CI, 68.6 to 93.0) at 12 months, and 73.8% (95% CI, 58.0 to 86.1) at 24 months. Although not stipulated in the protocol, we also performed an intention-to-treat analysis including the three patients who had intolerance to imatinib (n = 2) and binimetinib (n = 1); the best ORR was 64.4% (29 of 45; 95% CI, 48.8 to 78.1) by RECIST1.1 and 88.6% (39 of 44; 95% CI, 75.4 to 96.2) by Choi.
FIG 2.
Response rates (RECIST1.1, Choi, and EORTC) and duration of response. (A) Best objective responses by RECIST1.1 (n = 42), Choi responses (n = 41) around 8 weeks (end of cycle 2, first post-treatment scan), and EORTC responses (n = 35) by PET at 4 weeks (end of cycle 1) on combination imatinib and binimetinib treatment. The best RECIST1.1 responses are shown as % of change from baseline for patients who received the combination of imatinib and binimetinib and with at least one postbaseline scan. The known associated primary driver mutations in KIT, PDGFRA, and others are shown. The best ORR was 69.0% (29 of 42 confirmed PR), two-sided 95% CI: 52.9 to 82.4. (B) Duration of the response. AE, adverse events; CMR, complete metabolic response; EORTC, European Organisation for Research and Treatment of Cancer; mut, mutant; ORR, objective response rate; PD, progression of disease; PET, positron emission tomography; PMD, progressive metabolic disease; PMR, partial metabolic response; PR, partial response; SD, stable disease; SMD, stable metabolic response.
At data cutoff, the median follow-up among survivors was 35.6 months (range, 2.4-73.8 months). The mPFS was 29.9 months (95% CI, 24.2 to not estimable [NE]); 69.7% (95% CI, 53.3 to 91.2) and 47.8% (95% CI, 28.7 to 79.6) patients remained progression-free at 24 and 30 months, respectively (Fig 3A). The mOS was NE (95% CI, 50.4 to -NE); 83.0% (95% CI, 70.4 to 97.8) and 72.8% (95% CI, 56.9 to 93.1) patients were alive at 30 and 48 months, respectively (Fig 3B).
FIG 3.
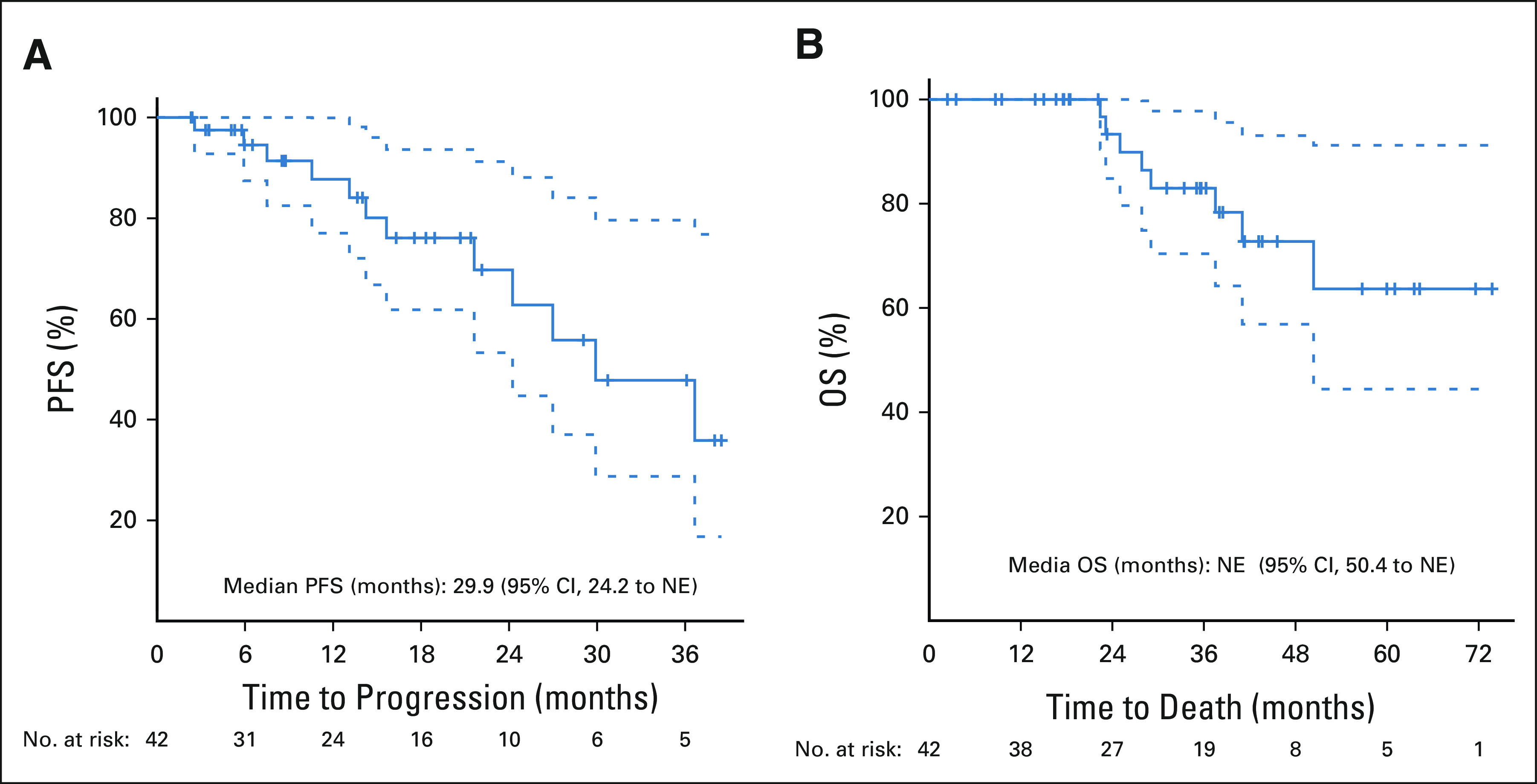
Kaplan-Meier estimates of PFS and OS: (A) PFS by RECIST1.1 and (B) OS. Median PFS is based on a Kaplan-Meier estimate of PFS, per investigator assessment. The median PFS was 29.9 months (95% CI, 24.2 to NE). The median OS was not reached (95% CI, 50.4 to NE). NE, not estimable; OS, overall survival; PFS, progression-free survival.
There were nine patients who initially presented with locally advanced GIST (n = 8) or locally advanced GIST with a solitary liver metastasis (n = 1). We analyzed the pathologic responses of eight patients (treatment duration: 6.6-31.2 months); one patient was excluded because of protocol nonadherence. All eight patients achieved at least 70% confirmed pathologic response of the primary tumors localized across the GI tract; 5 of 8 patients achieved significant pathologic response (SPR; ≥ 90% treatment-effect17; Figs 4A and 4B). One patient with KIT exon9 (pA502_Y503dup)-mutant primary esophageal GIST and a solitary liver metastasis achieved 90% and 100% pathologic response in the primary and metastatic lesions, respectively (Fig 4A). Compared with conventional RECIST1.1, the pathologic responses demonstrated more profound and robust treatment effects (Figs 4A and 4B).
FIG 4.
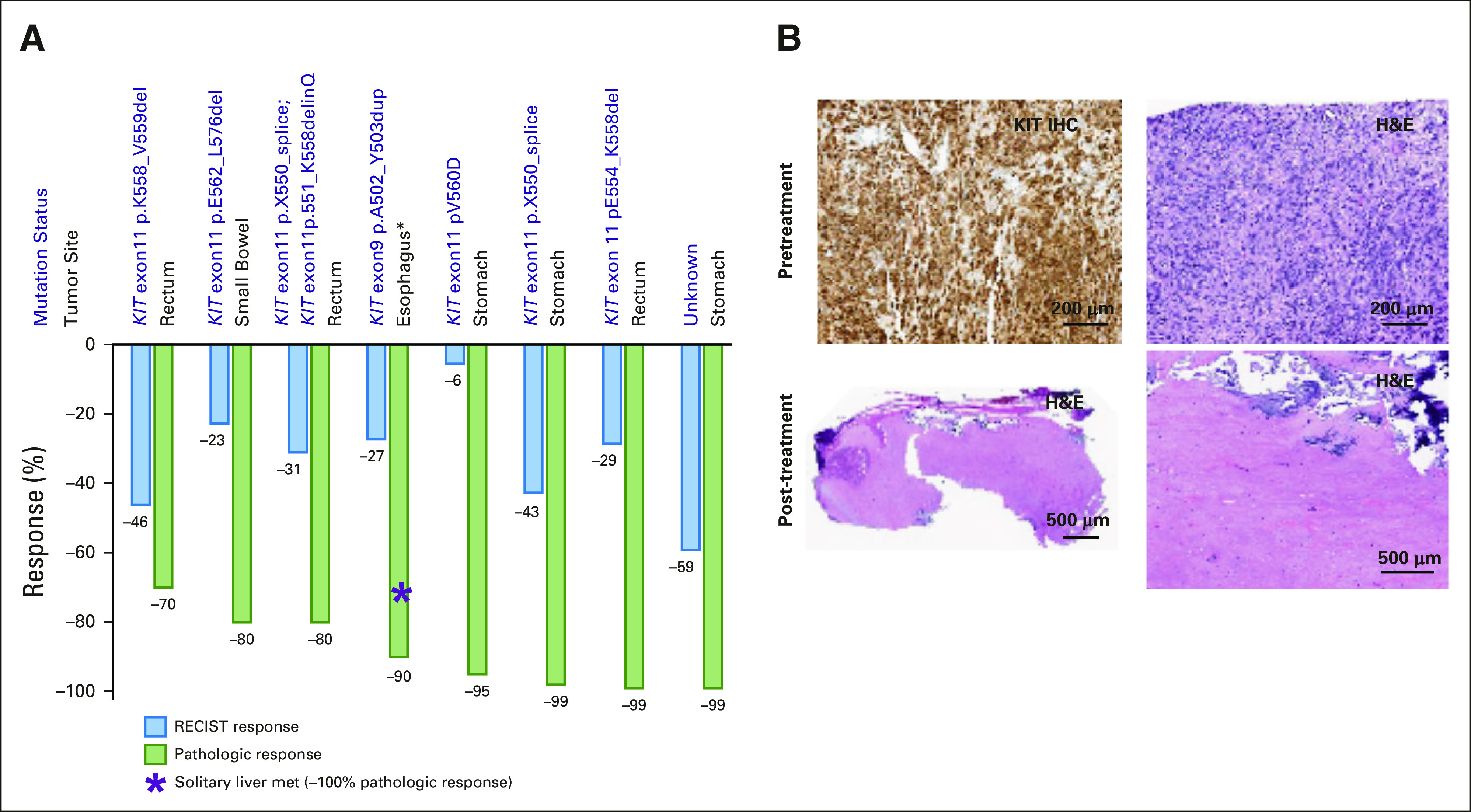
Pathologic responses. (A) Comparison of pathologic and RECIST1.1 response. (B) Representative images of KIT IHC (top left) and H&E (top right) of a patient with rectal GIST (KIT exon 11 pE554_K558del) pretreatment samples demonstrating 100% viable tumor tissue, and representative H&E images of post-treatment H&Es (bottom left: lower magnification image; bottom right: calcified scar) demonstrating 99% treatment effect. H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Genomic Analysis
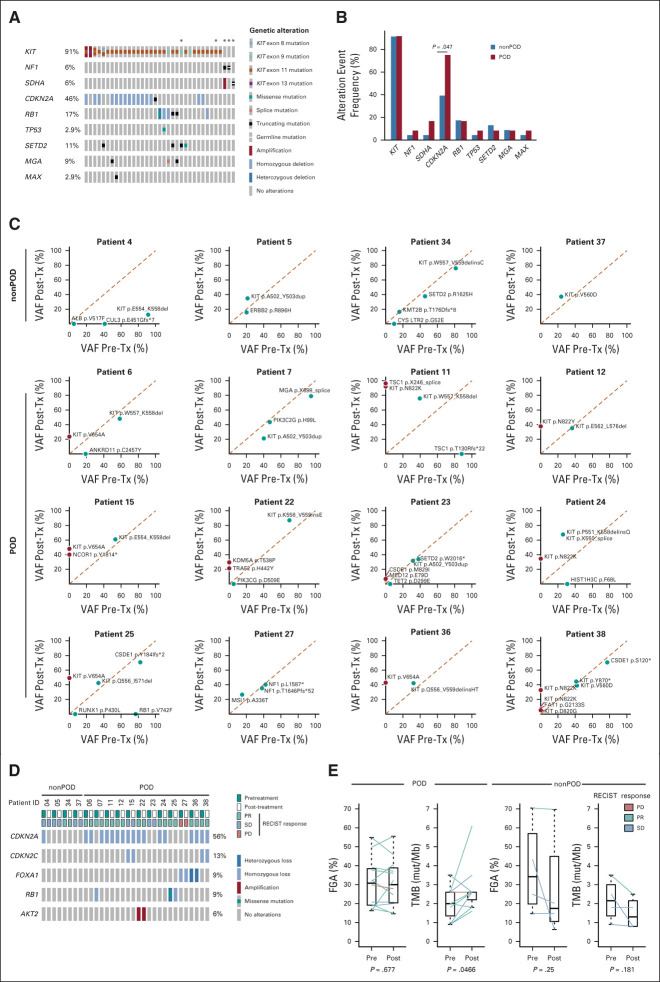
Thirty-five patients had genetic analysis of pretreatment tumors by Memorial Sloan Kettering-Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT),37 which demonstrated activating mutations in KIT, and inactivating mutations in NF1 and SDHA, all were known oncogenic drivers in GIST.1,38 Furthermore, we observed recurrent co-occurring mutations in cell cycle regulators (CDKN2A, RB1, and TP53), chromatin-modifying enzyme (SETD2), and basic helix-loop-helix transcription factors (MGA and MAX; Fig 5A). Twelve of 35 patients had progression of disease (POD), and 23 patients did not and therefore they were designated as nonprogressors (nonPOD). Comparing the baseline genomic profiles between POD and nonPOD patients, inactivation in CDKN2A (P = .047) was significantly enriched in the POD patients (Fig 5B). There was a trend of association of baseline CDKN2A genetic inactivation with shorter PFS by RECIST1.1 response in POD compared with nonPOD patients (Appendix Fig A1, online only).
FIG 5.
Genomic analysis of resistant disease. (A) Oncogenic driver mutations and concurrent genetic alterations in pretreatment patient samples (n = 35). *Indicates patients who consented to germline testing. (B) Comparison of frequency of common genetic alteration events in pretreatment samples of patients who have progressed on imatinib and binimetinib combination treatment (POD, n = 12) and of those without disease progression (nonPOD, n = 23). P = .047 for CDKN2A by Fisher's exact test. Comparison of (C) VAF of mutation and (D) CNA of paired pretreatment and post-treatment biopsy samples from patients with (POD, n = 12) and without (nonPOD, n = 4) disease progression. (E) Comparison of FGA and TMB changes of paired pretreatment and post-treatment tumor samples from POD and nonPOD patients. P values were calculated using Student's paired-samples t-test. RECIST response: on the basis of best RECIST1.1 response. CNA, copy-number alteration; FGA, fraction of genome altered; nonPD, nonprogression of disease; PD, progression of disease; PR, partial response; SD, stable disease; TMB, tumor mutational burden; Tx, treatment; VAF, variant allele frequency.
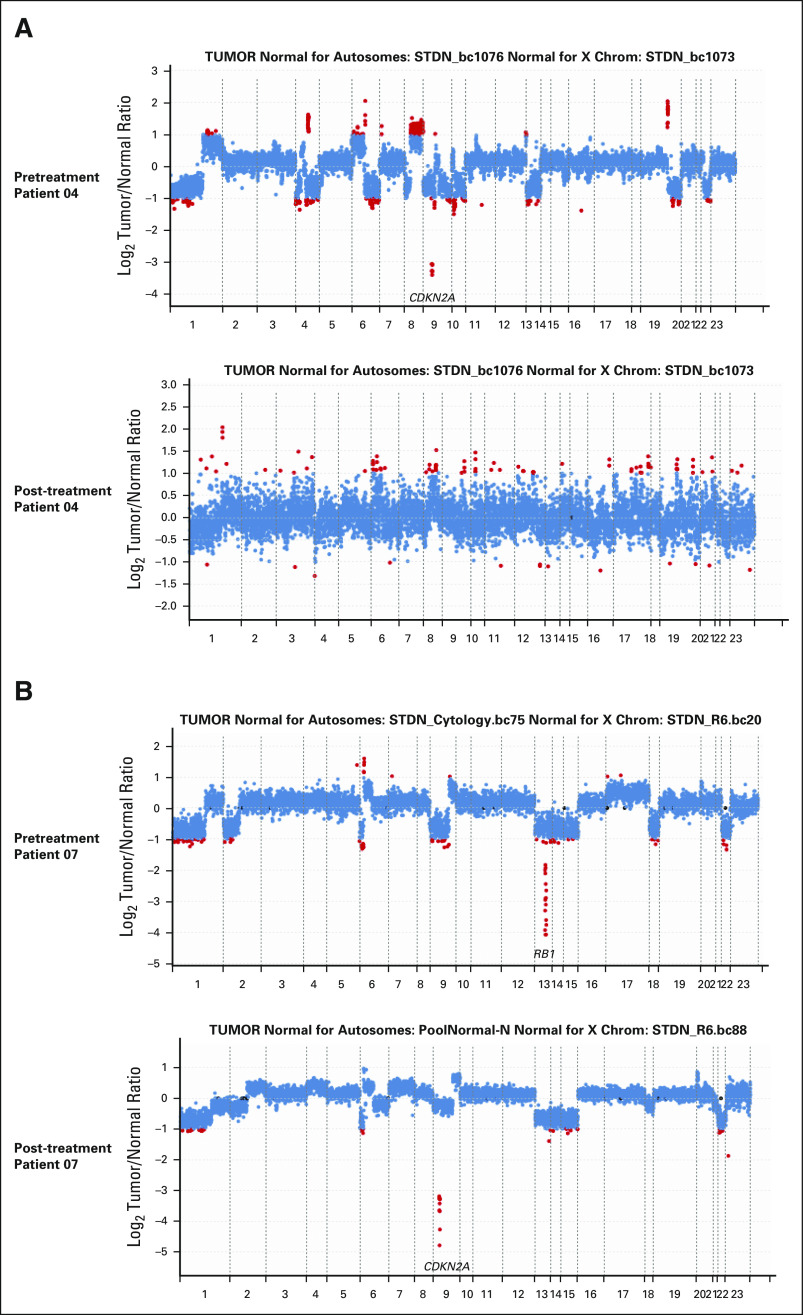
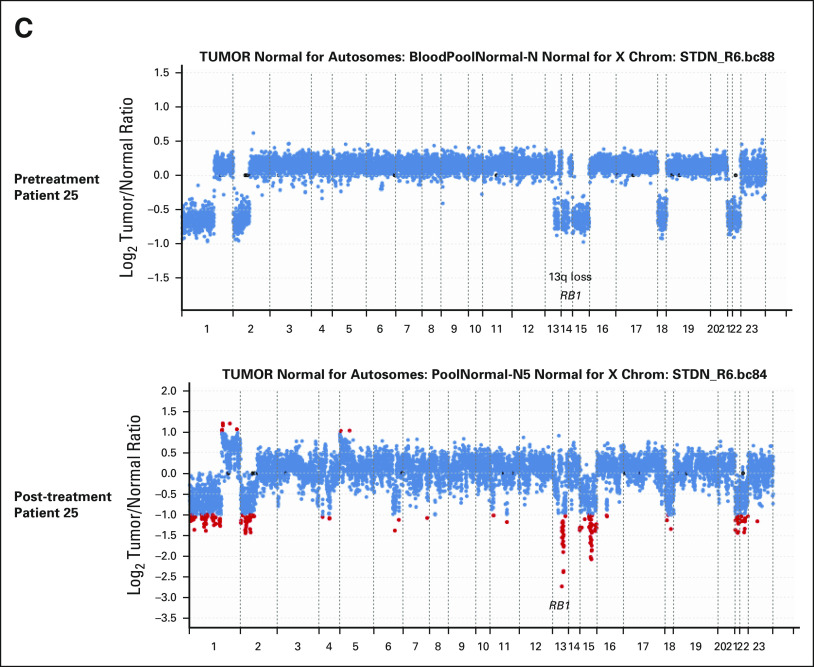
All POD (n = 12) and four nonPOD patients had paired pretreatment and post-treatment samples available for comparative genomic analysis by MSK-IMPACT.37 From nonPOD patients, we observed no significant changes in somatic mutation, copy-number alteration, fraction of genome altered (FGA), and tumor mutational burden (TMB) between pretreatment and post-treatment samples (Figs 5C-5E). For patient 04, CDKN2A homozygous deletion, and CUL3 and ALB point mutations were present in the pretreatment sample, but not called in the post-treatment sample because of significant drop in tumor purity, low coverage, and high background noise (Figs 5C and 5D and Appendix Fig A2A, online only). By contrast, most POD patients had emergent genomic alterations. One POD patient (patient 27) with sporadic NF1-mutant GIST had progressed within 2 months of treatment, indicating primary resistance. Eight of nine POD patients with KIT exon 11-mutant GIST developed secondary resistant mutations in KIT exon 13 (pV654) or exon 17 (pN822K/Y, pD820G). One patient (patient 38) had multiple subclonal secondary resistant KIT mutations (eg, pN822K and pD820G) emergent in post-treatment samples (Fig 5C). Notably, examination of the raw data revealed that the pN822K subclone was present at low variant allele frequency (VAF, below mutation call threshold) in pretreatment sample (data not shown), and the pN822K VAF was enriched to 6.9% in post-treatment samples.
Interestingly, POD patients with KIT exon 9-mutant GISTs (patients 07 and 23) had no detectable secondary resistant mutations in KIT or PDGFRA, but had emergence of new mutations in MED12 (exon 3, pE79D) and CSDE1 (exon 20, pM829I; patient 23) or new copy-number loss in CDKN2A (patient 07; Fig 5C and Appendix Fig A2B, online only). In one patient (patient 25), we also observed emergent homozygous loss of RB1 (RB1 heterozygous loss plus missense mutation in pretreatment sample) along with secondary resistant KIT exon 13 mutation (pV654; Figs 5C and 5D and Appendix Fig A2C, online only). There were no significant changes of FGA comparing pretreatment and post-treatment samples in POD patients (Fig 5E). The baseline pretreatment TMB was low in GIST, approximately 2 mut/Mb, and there was no significant difference between POD and nonPOD patients (Fig 5E). However, TMB was significantly increased in post-treatment samples comparing to pretreatment samples only in POD patients (P = .0466), consistent with emergent mutations in KIT and other genes in resistant post-treatment samples (Figs 5C and 5E).
Safety
Overall, the imatinib plus binimetinib combination has manageable toxicities over long term (range, 1.5-73.8 months). No unexpected toxicities were observed. The most commonly observed toxicity of any grade and investigator-attributed as possibly, probably, or definitely associated with either imatinib and/or binimetinib, included peripheral (79.1%) and periorbital (69.8%) edema, acneiform (74.4%) and maculopapular (48.8%) rash, diarrhea (60.5%), asymptomatic creatinine phosphokinase (CPK) elevation (100%), anemia (88.4%), white blood cell decrease (58.1%), platelet count decrease (51.2%), hypophosphatemia (46.5%), ALT (55.8%), and AST (86.1%) increase. Most common grade 3 or 4 toxicity included asymptomatic CPK elevation (79.1%), hypophosphatemia (14.0%), neutrophil decrease (9.3%), anemia (7.0%), and maculopapular rash (7.0%; Table 2).
TABLE 2.
Treatment-Associated AEs
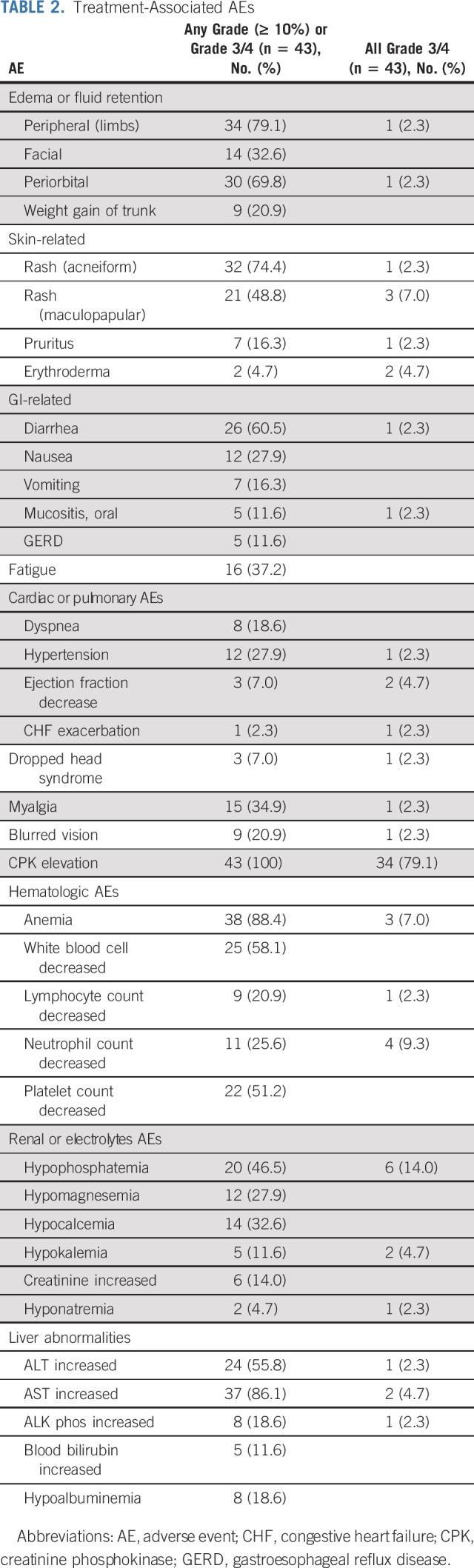
Three patients developed dropped head syndrome with one grade 3 event, who had returned to baseline with either dose reduction (n = 2) or discontinuation of binimetinib (n = 1). Three patients experienced a decrease in left ventricular ejection fraction (LVEF); 2 of 3 patients had grade 3 events and one of them had congestive heart failure exacerbation with LVEF decrease during the imatinib lead-in phase; this patient discontinued trial after two dose of combination of imatinib and binimetinib because of intolerability to imatinib. Two of three patients with LVEF decrease were attributed to binimetinib; both patients had returned to their baseline cardiac function after dose reduction of binimetinib and continued trial. There was no clinically significant grade 4 or 5 AEs at least possibly associated with study medications in this trial.
DISCUSSION
Imatinib has remained the first-line treatment of advanced GIST. However, the clinical benefit of imatinib is not indefinite and patients eventually develop imatinib and other TKI resistance and succumb to their disease. Previous efforts of directly challenging imatinib with newer generations of TKIs in the first-line setting have been unsuccessful.11 Considering the intertumor and intratumor heterogeneity in imatinib-resistant GIST, identifying an upfront therapeutic strategy that addresses the heterogeneous resistance mechanisms is particularly challenging. Extensive preclinical work supports a novel combination of binimetinib and imatinib to durably target the GIST lineage-specific master regulator ETV1 protein stability and the signaling master regulator KIT.19,20,28-31,39 This combination strategy has the potential to enhance the efficacy of imatinib by inducing cytotoxicity and more extensive tumor responses while preventing early adaptive TKI resistance. Because the combination therapy relies on imatinib to block the feedback reactivation of upstream KIT, we reasoned that the combination therapy will be relatively ineffective in second- or third-line settings, where the majority of GISTs harbor heterogeneous secondary imatinib-resistant KIT mutations (eg, KIT exon 13 of 14 or exon 17 of 18 mutations).1,16-18 Therefore, after establishing the RP2D of the combination,32 the phase II portion of the trial was specifically offered to patients with treatment-naive advanced GIST. A randomized proof-of-principle, investigator-initiated trial was not feasible as GIST is a rare and underfunded disease. We therefore chose a stringent test of ORR by RECIST1.1, selecting a high-level of ORR improvement (20%) in the well-established historical ORR (45%-52%) of imatinib (400 mg once daily).7-11 This phase II study met its prespecified primary end point (29 patients with confirmed RECIST1.1 PR) before full accrual. Consistently, the mPFS of the combination treatment was 29.9 months (95% CI, 24.2 to NE) and mOS was NE (95% CI, 50.4 to NE). In previous phase III trials with large number of GIST patients treated in the first-line TKI setting with a subset of patients treated with prior chemotherapy, the ORR is approximately 45%-50% for imatinib 400 mg once daily (SWOG) and 45%-54% for imatinib 800 mg/day (European EORTC, Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group),7,8 and 52% for imatinib 400-800 mg/day in the more contemporary frontline ENESTg1 phase III trial with no patients exposed to prior systemic chemotherapy.11 The ORR for imatinib in smaller phase II trials has been more variable and often superior to the phase III trials, 64.9%-71%, mostly with the use of higher doses (600-800 mg/day) of imatinib.36,40 Despite the superior ORR, the mPFS of these trials are relatively comparable, approximately 20 months for imatinib 400 mg once daily, and 26 months for imatinib 800 mg/day.8,9,36 Understanding the complexities of cross-trial comparisons, the data with the use of imatinib plus binimetinib presented here are encouraging compared with historical mPFS of imatinib 400 mg once daily (18-20.4 months) or 800 mg/day (20-26 months) and mOS of imatinib 400 mg once daily (46.8-57 months) or 800 mg/day (46.8-57 months).8,9,36 Understandably, patients in historical phase III trials7-9 had relatively worse ECOG status, higher disease burden, and less sophisticated modern-day TKI management compared with the current phase II trial among other uncontrolled factors, which could all contribute to inferior clinical outcome.
One of the goals of the study is to see whether the combination therapy can forestall therapeutic resistance and explore resistance mechanisms. Although many patients responded well, 12 (range, 2.6-36.7 months, median time to progression: 14.9 months) of the 42 evaluable patients developed resistant disease. Most had large disease burden and one received 1 and 3 years of imatinib adjuvant therapy during two prior relapses. Interestingly, we observed interpatient, intrapatient, and intratumoral subclonal secondary resistant KIT mutations in exons 13 and 17, only in the setting of GIST with KIT exon 11, but not with KIT exon 9 primary mutations, indicating divergent resistant mechanisms, although acknowledging the limitation that only three exon 9 patients were enrolled. The preclinical data suggest that imatinib is essential to block MEK inhibitor–induced feedback activation of KIT and PDGFRα pathway signaling. It is possible that these secondary resistant mutations are pre-existing as subclones or they emerged under treatment pressure. These data would argue for combination therapy of an MEK inhibitor with a newer generation of TKI that can target multiple secondary resistant KIT and PDGFRA mutations. Furthermore, cell cycle regulators, eg, CDKN2A and RB1, are significantly enriched in patients who eventually progress on therapy and/or emerging in treatment-resistant samples, indicating the importance of their function in disease control and overall prognosis.
Although no unexpected toxicity was seen, we observed several MEK inhibitor–associated class effects (G3 and G4 toxicity), including LVEF decrease and dropped head syndrome, all of which were reversible with dose reduction or discontinuation of drug.41,42 The most bothersome side effects were binimetinib-associated acneiform rash and binimetinib- and/or imatinib-associated periorbital and peripheral edema. These were managed with prophylactic antibiotics, topical steroids, and ancillary support without the need for dose modifications. Overall, the combination therapy is reasonably tolerated with manageable toxicity.
This is one of the first clinical trials combining a TKI and an MEK inhibitor in the frontline treatment of GIST. The combination of imatinib and binimetinib is effective in treatment-naive advanced GIST. Deep and durable responses were noted. However, addition of binimetinib to imatinib has increased toxicity mostly related to rash and peripheral edema. Imatinib and binimetinib or a similar combination should be evaluated in a randomized trial in direct comparison with the SOC, imatinib alone, in the first-line treatment of advanced GIST, with careful consideration of the efficacy end points and toxicity profiles.
ACKNOWLEDGMENT
The authors thank patients and their families for participating in the study.
APPENDIX 1. SUPPLEMENTAL METHODS
Patients
Additional key inclusion criteria were patients with measurable lesion(s) by RECIST1.1, were able to take oral medications, and sign informed consents. Key exclusion criteria included severe and/or uncontrolled medical diseases, active brain metastasis, history of retinal degenerative disease or central serious retinopathy or retinal vein occlusion, or neuromuscular disorders associated with elevated creatinine phosphokinase (CPK; eg, inflammatory myopathies, muscular dystrophy, and spinal muscular atrophy). Complete inclusion and exclusion criteria are available in the study protocol.
Study Design, Treatment, and End Points
Eligible patient with advanced gastrointestinal stromal tumor (GIST) of all genotypes, including mutations in KIT, PDGFRA, NF1, and subunits of the SDH complex could enroll in the study.
Pathologic responses were reported by standard pathology review and independently reviewed by study pathologist, Dr Cristina Antonescu (Memorial Sloan Kettering Cancer Center [MSKCC]). Correlative analysis included tumor genomics by MSK-Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT).
Genomic Studies
Samples.
All tumors were profiled using the MSK-IMPACT clinical sequencing assay, a hybridization capture, next-generation sequencing platform amenable to DNA from both fresh-frozen and formalin-fixed, paraffin-embedded samples for targeted sequencing as described.37,43 The library construction and sequencing were performed by the MSKCC Integrated Genomics Operation Facility, Marie-Josée and Henry R. Kravis Center for Molecular Oncology. Alignment and single nucleotide variant, indel, and copy-number alteration calling were performed as described previously.37,43
Genomic analysis.
Somatic alterations were annotated using OncoKB for oncogenicity and clinical actionability44 (Data version: v3.2, released on March 12, 2021). Tumor mutational burden was calculated for each sample as the total number of nonsynonymous mutations, divided by the number of bases sequenced. Fraction of genome altered was calculated for each sample as the percentage of the genome with absolute log2 copy ratios > 0.2.
Statistics.
Comparisons between groups were done using the nonparametric Mann-Whitney U test or the Fisher's exact test for continuous and categorical variables, respectively. Statistical tests comparing pretreatment and post-treatment paired values were done using the Student's paired-samples t-test. All reported P values are two-tailed and a P value < .05 was considered significant. All analyses were performed using R v3.5.245 and Bioconductor v3.4. Association with progression-free survival was assessed for each IMPACT marker using the Kaplan-Meier curve and the log-rank test.
Statistical Analysis
All patients who received at least one dose of the combination of imatinib and binimetinib were included in the safety and toxicity analysis. All patients who received combination therapy and were evaluated by at least one follow-up scan were included in the efficacy analysis. All data reflect an interim data-cut on November 15, 2020, from patients enrolled between September 15, 2014, and November 15, 2020 (Fig 1). Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. The study is registered at ClinicalTrials.gov identifier: NCT01991379.
FIG A1.
Association of CDKN2A genetic alteration with PFS by the Kaplan-Meier estimates. Log-rank P = .14. mut, mutation; PFS, progression-free survival; WT, wild-type.
FIG A2.
Examples of changes in CNA comparing pretreatment and post-treatment samples. Each dot represents a probe set, and the values on the y-axis show the log2 transformed ratio of tumor versus normal. Red dots denote fold changes ≥ 2. (A) Patient 04 is a nonPOD patient; there was a deep deletion of CDKN2A in the pretreatment samples (tumor purity approximately 70%, sequence coverage 577×), which was lost in the post-treatment sample because of low tumor purity (approximately 10%) and low sequence coverage (95×) and high background noise. (B and C) CNA changes in POD patients demonstrating loss of RB1 deep deletion and emergence of (B) CDKN2A deep deletion in patient 07 resistant tumor samples, and (C) emergence of RB1 deep deletion in patient 25 resistant tumor samples (C). CNA, copy-number alteration; POD, progression of disease.
SUPPLEMENTAL DISCUSSION
Although RECIST1.1 has been the standard evaluation method of objective responses in GIST, it has been shown to underestimate treatment responses, especially during early treatment course. Evaluations of changes in computed tomography (CT) density by Choi criteria34 and positron emission tomography (PET)-metabolic changes by European Organisation for Research and Treatment of Cancer (EORTC)35 are used within the first 4-8 weeks in GIST as ancillary corroborating imaging studies to identify potential early responses or signs of therapy resistance. 39 of 41 (95.1%) evaluable patients had a Choi PR approximately 8 weeks, and 31 of 35 (88.6%) evaluable patients had either complete metabolic response or partial metabolic response by PET scan at 4 weeks. The responses by Choi and EORTC criteria are largely concordant with RECIST (PR plus SD). EORTC noted complete responses in eight patients, which were PR or SD by other response criteria, which is consistent with the finding that metabolic activity may decrease to background in solid tumors, despite residual CT lesions.46,47 Furthermore, we were able to compare the pathologic response with RECIST1.1 measurement in the eight patients who underwent surgery after treatment. Five of eight (62.5%) patients achieved significant pathologic response (SPR; ≥ 90% treatment effects; Fig 4), which appeared more superior comparing with historical SPR consistently < 50% in patients with GIST treated with first-line imatinib (400-600 mg/day).17,48 The SPR was not restricted to gastric GIST or KIT exon11-mutant GIST that are known to have better responses to imatinib than GIST from other GI locations or KIT mutations. The SPR also indicates that the imatinib and binimetinib combination can induce enhanced cytotoxicity and deep tumor treatment responses that are often not seen with single agent imatinib. The pathologic responses are consistently higher than RECIST responses in the same patient and there was no specific correlation of RECIST PR versus SPR. These observations indicate that for GIST therapeutic assessment, RECIST1.1 RR consistently underestimated the treatment responses. RECIST PFS can be an effective measure of clinical benefit and as predictive as RECIST RR in first-line metastatic GIST treatment assessment. However, as GIST tumors have different shapes and can manifest treatment response with fibrosis and/or necrosis without significant change in tumor size, a multimodality assessment algorithm that integrates novel imaging modalities, such as 3D volumetric measurement, genetic/molecular and histopathologic features of GIST would be ideal for accurate assessment of treatment response to novel therapeutics in future trials.
One patient with sporadic NF1-deficient GIST had primary resistance and progressed within 2 months; no new mutations were discovered. Notably, SDH-deficient GIST is primarily resistant to imatinib. We had one patient with SDH-deficient GIST on phase II trial who had stable disease by RECIST1.1 for 16 weeks at the time of data cutoff. There were five patients with SDH-deficient GIST on the phase Ib portion of the trial, all with either SD (n = 4) or PR (n = 1) for at least 8 months (unpublished). These data indicate that the combination therapy has the potential to target the interstitial cell of Cajal and/or GIST lineage-dependent survival pathways beyond KIT-activating mutations.
The RP2D was initially defined as imatinib 400 mg once daily plus binimetinib 45mg twice daily in phase Ib.32 Although higher doses of imatinib had been shown to be more efficacious in KIT exon 9-mutant GIST, considering that (1) KIT exon 9 patients account for a small fraction of GIST (approximately 10%) and the trial is not restrictive on the basis of mutational status,1 (2) randomized phase III trials including all GISTs irrespective of mutational status demonstrated no significant difference in PFS and OS between high-dose and standard-dose of imatinib,7,9,36 and (3) preclinical studies indicated significant synergist antitumor response of the imatinib and binimetinib combination in GIST even at significantly reduced doses of imatinib or binimetinib,31 we decided not to include imatinib dose levels higher than standard-of-care dose (400 mg once daily) in the dose-escalation phase Ib study. Furthermore, for durable tolerability and reduced toxicity, we treated the phase II patients with imatinib 400 mg once daily plus binimetinib 30 mg twice daily (one dose level lower than RP2D).
Ping Chi
Stock and Other Ownership Interests: ORIC Pharmaceuticals (I)
Consulting or Advisory Role: Deciphera, Exelixis, Merck (I)
Research Funding: Deciphera (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from ORIC (I)
Li-Xuan Qin
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Viela Bio (I), Sironax (I)
Leadership: Viela Bio (I), Sironax (I)
Stock and Other Ownership Interests: Viela Bio (I), Sironax (I)
Bastien Nguyen
Employment: Loxo/Lilly
Ciara M. Kelly
Employment: Daichii Sankyo (I)
Stock and Other Ownership Interests: Daichii Sankyo (I)
Consulting or Advisory Role: Exicure, Immunicum
Research Funding: AGIOS (Inst), Amgen (Inst), Merck (Inst), Incyte (Inst), Kartos Therapeutics (Inst), Exicure (Inst), Xencor (Inst)
Sandra P. D'Angelo
Consulting or Advisory Role: EMD Serono, Amgen, Nektar, Immune design, GlaxoSmithKline, Incyte, Merck, Adaptimmune, Immunocore
Research Funding: EMD Serono (Inst), Amgen (Inst), Merck (Inst), Incyte (Inst), Nektar (Inst), Bristol Myers Squibb (Inst), Deciphera (Inst)
Travel, Accommodations, Expenses: Adaptimmune, EMD Serono, Nektar
Mark A. Dickson
Consulting or Advisory Role: Celgene
Research Funding: Lilly (Inst), AADi (Inst)
Mrinal M. Gounder
Honoraria: Flatiron Health, PER, Medscape, Guidepoint Global, touchIME, Med Learning Group, More Health, PER
Consulting or Advisory Role: Daiichi Sankyo, Karyopharm Therapeutics, Epizyme, Bayer, Springworks Therapeutics, Boehringer Ingelheim, TYME, Ayala Pharmaceuticals
Speakers' Bureau: Amgen, Karyopharm Therapeutics, Boehringer Ingelheim
Patents, Royalties, Other Intellectual Property: UpToDate, GODDESS PRO Desmoid Tumor (Inst)
Travel, Accommodations, Expenses: Epizyme
Other Relationship: Desmoid Tumor Research Foundation
Uncompensated Relationships: Foundation Medicine, Rain Therapeutics, Athenex
Open Payments Link: https://openpaymentsdata.cms.gov/physician/459583
Sujana Movva
Consulting or Advisory Role: Genmab
Research Funding: Hutchison MediPharma (Inst)
Benjamin A. Nacev
Uncompensated Relationships: Delfi Diagnostics, Rapafusyn Pharmaceuticals, QuadW Foundation
Katherine A. Thornton
Consulting or Advisory Role: More Health, Adaptimmune, Macrogenics, Deloitte, GlaxoSmithKline, Epizyme
Travel, Accommodations, Expenses: Adaptimmune
Aimee M. Crago
Stock and Other Ownership Interests: Gilead Sciences
Honoraria: Wolters Kluwer, WebMD
Consulting or Advisory Role: Springworks Therapeutics
Patents, Royalties, Other Intellectual Property: Patent assigned to MSKCC for a companion diagnostic to CDK4 inhibitors—Patent number 9,889,135 (Inst)
Sam Yoon
Stock and Other Ownership Interests: Attis Lab
Gary Ulaner
Consulting or Advisory Role: GE Healthcare, ImaginAb
Speakers' Bureau: GE Healthcare
Research Funding: Sanofi, Genentech, Puma Biotechnology, GE Healthcare, Lantheus Medical Imaging
Expert Testimony: GE Healthcare
Michael F. Berger
Consulting or Advisory Role: Lilly, PetDx
Research Funding: Grail
Patents, Royalties, Other Intellectual Property: Provisional patent pending for Systems and Methods for Detecting Cancer via cfDNA Screening
Yu Chen
Stock and Other Ownership Interests: ORIC Pharmaceuticals
Honoraria: Merck
Patents, Royalties, Other Intellectual Property: Invention related to glucocorticoid inhibitors for treatment of prostate cancer (SK2013-045), Invention related to glucocorticoid inhibitors for treatment of prostate cancer (SK2013-045)
William D. Tap
Leadership: Certis Oncology Solutions, Atropos, Innova Therapeutics
Stock and Other Ownership Interests: Certis Oncology Solutions, Atropos
Consulting or Advisory Role: EMD Serono, Lilly, Daiichi Sankyo, Deciphera, C4 Therapeutics, Mundipharma, Adcendo, Ayala Pharmaceuticals, Kowa Pharmaceutical, Servier, Bayer, Epizyme, Cogent, Medpacto, Foghorn Therapeutics, Amgen
Research Funding: Novartis, Lilly, Plexxikon, Daiichi Sankyo, TRACON Pharma, Blueprint Medicines, Immune Design, BioAtla, Deciphera
Patents, Royalties, Other Intellectual Property: Companion Diagnostic for CDK4 inhibitors—14/854,329, Enigma and CDH18 as companion Diagnostics for CDK4 inhibition SKI2016-021-03
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO 2020 Virtual Meeting, May 29-31, 2020.
SUPPORT
Supported by Array BioPharma/Pfizer, and supported by grants from the National Institute of Health/National Cancer Institute (Cancer Center Support Grant P30CA008748 to P.C., L.-X.Q., C.R.A., Y.C., W.D.T., S.S., A.M.C., S.P.D., M.G., Sarcoma SPORE P50CA217694 to P.C., L.-X.Q., C.R.A., W.D.T., S.S., A.M.C., S.P.D.; P50CA140146 to C.R.A.), Orphan Products Grants Program/US Food and Drug Administration (R01FD005731 to P.C., L.-X.Q., M.F.B., C.R.A., W.D.T.; R01FD005105 to M.G.), Cycle for Survival and Geoffrey Beene Research Fund to P.C., Shuman Fund to P.C., C.R.A., W.D.T., GIST Cancer Research Fund to P.C., C.R.A., GIST Cancer Awareness Fund to P.C., Grail to M.F.B.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Ping Chi, Li-Xuan Qin, Mark A. Dickson, Yu Chen, William D. Tap
Administrative support: Ping Chi, William D. Tap
Provision of study materials or patients: Ping Chi, Ciara M. Kelly, Sandra P. D'Angelo, Mark A. Dickson, Mary L. Keohan, Aimee M. Crago, Samuel Singer, Sinchun Hwang, William D. Tap
Collection and assembly of data: Ping Chi, Sandra P. D'Angelo, Mark A. Dickson, Mark A. Dickson, Mary L. Keohan, Sujana Movva, Evan Rosenbaum, Sam Yoon, Randy Yeh, Moriah Martindale, Haley T. Phelan, Matthew D. Biniakewitz, Sarah Warda, Cindy J. Lee, Samuel Singer, Sinchun Hwang, William D. Tap
Data analysis and interpretation: Ping Chi, Li-Xuan Qin, Bastien Nguyen, Ciara M. Kelly, Mark A. Dickson, Mark A. Dickson, Benjamin A. Nacev, Katherine A. Thornton, Aimee M. Crago, Sam Yoon, Michael F. Berger, Nikolaus D. Schultz, Samuel Singer, Yu Chen, Cristina R. Antonescu, William D. Tap
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of Imatinib Plus Binimetinib in Patients With Treatment-Naive Advanced Gastrointestinal Stromal Tumor
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ping Chi
Stock and Other Ownership Interests: ORIC Pharmaceuticals (I)
Consulting or Advisory Role: Deciphera, Exelixis, Merck (I)
Research Funding: Deciphera (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from ORIC (I)
Li-Xuan Qin
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Viela Bio (I), Sironax (I)
Leadership: Viela Bio (I), Sironax (I)
Stock and Other Ownership Interests: Viela Bio (I), Sironax (I)
Bastien Nguyen
Employment: Loxo/Lilly
Ciara M. Kelly
Employment: Daichii Sankyo (I)
Stock and Other Ownership Interests: Daichii Sankyo (I)
Consulting or Advisory Role: Exicure, Immunicum
Research Funding: AGIOS (Inst), Amgen (Inst), Merck (Inst), Incyte (Inst), Kartos Therapeutics (Inst), Exicure (Inst), Xencor (Inst)
Sandra P. D'Angelo
Consulting or Advisory Role: EMD Serono, Amgen, Nektar, Immune design, GlaxoSmithKline, Incyte, Merck, Adaptimmune, Immunocore
Research Funding: EMD Serono (Inst), Amgen (Inst), Merck (Inst), Incyte (Inst), Nektar (Inst), Bristol Myers Squibb (Inst), Deciphera (Inst)
Travel, Accommodations, Expenses: Adaptimmune, EMD Serono, Nektar
Mark A. Dickson
Consulting or Advisory Role: Celgene
Research Funding: Lilly (Inst), AADi (Inst)
Mrinal M. Gounder
Honoraria: Flatiron Health, PER, Medscape, Guidepoint Global, touchIME, Med Learning Group, More Health, PER
Consulting or Advisory Role: Daiichi Sankyo, Karyopharm Therapeutics, Epizyme, Bayer, Springworks Therapeutics, Boehringer Ingelheim, TYME, Ayala Pharmaceuticals
Speakers' Bureau: Amgen, Karyopharm Therapeutics, Boehringer Ingelheim
Patents, Royalties, Other Intellectual Property: UpToDate, GODDESS PRO Desmoid Tumor (Inst)
Travel, Accommodations, Expenses: Epizyme
Other Relationship: Desmoid Tumor Research Foundation
Uncompensated Relationships: Foundation Medicine, Rain Therapeutics, Athenex
Open Payments Link: https://openpaymentsdata.cms.gov/physician/459583
Sujana Movva
Consulting or Advisory Role: Genmab
Research Funding: Hutchison MediPharma (Inst)
Benjamin A. Nacev
Uncompensated Relationships: Delfi Diagnostics, Rapafusyn Pharmaceuticals, QuadW Foundation
Katherine A. Thornton
Consulting or Advisory Role: More Health, Adaptimmune, Macrogenics, Deloitte, GlaxoSmithKline, Epizyme
Travel, Accommodations, Expenses: Adaptimmune
Aimee M. Crago
Stock and Other Ownership Interests: Gilead Sciences
Honoraria: Wolters Kluwer, WebMD
Consulting or Advisory Role: Springworks Therapeutics
Patents, Royalties, Other Intellectual Property: Patent assigned to MSKCC for a companion diagnostic to CDK4 inhibitors—Patent number 9,889,135 (Inst)
Sam Yoon
Stock and Other Ownership Interests: Attis Lab
Gary Ulaner
Consulting or Advisory Role: GE Healthcare, ImaginAb
Speakers' Bureau: GE Healthcare
Research Funding: Sanofi, Genentech, Puma Biotechnology, GE Healthcare, Lantheus Medical Imaging
Expert Testimony: GE Healthcare
Michael F. Berger
Consulting or Advisory Role: Lilly, PetDx
Research Funding: Grail
Patents, Royalties, Other Intellectual Property: Provisional patent pending for Systems and Methods for Detecting Cancer via cfDNA Screening
Yu Chen
Stock and Other Ownership Interests: ORIC Pharmaceuticals
Honoraria: Merck
Patents, Royalties, Other Intellectual Property: Invention related to glucocorticoid inhibitors for treatment of prostate cancer (SK2013-045), Invention related to glucocorticoid inhibitors for treatment of prostate cancer (SK2013-045)
William D. Tap
Leadership: Certis Oncology Solutions, Atropos, Innova Therapeutics
Stock and Other Ownership Interests: Certis Oncology Solutions, Atropos
Consulting or Advisory Role: EMD Serono, Lilly, Daiichi Sankyo, Deciphera, C4 Therapeutics, Mundipharma, Adcendo, Ayala Pharmaceuticals, Kowa Pharmaceutical, Servier, Bayer, Epizyme, Cogent, Medpacto, Foghorn Therapeutics, Amgen
Research Funding: Novartis, Lilly, Plexxikon, Daiichi Sankyo, TRACON Pharma, Blueprint Medicines, Immune Design, BioAtla, Deciphera
Patents, Royalties, Other Intellectual Property: Companion Diagnostic for CDK4 inhibitors—14/854,329, Enigma and CDH18 as companion Diagnostics for CDK4 inhibition SKI2016-021-03
No other potential conflicts of interest were reported.
REFERENCES
- 1.Corless CL, Barnett CM, Heinrich MC.Gastrointestinal stromal tumours: Origin and molecular oncology Nat Rev Cancer 11865–8782011 [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors Science 279577–5801998 [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, Fletcher JA, Heinrich MC.Biology of gastrointestinal stromal tumors J Clin Oncol 223813–38252004 [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors Science 299708–7102003 [DOI] [PubMed] [Google Scholar]
- 5. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in "Wild-Type" gastrointestinal stromal tumors. J Transl Med. 2016;14:339. doi: 10.1186/s12967-016-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors Genes Chromosomes Cancer 47853–8592008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial Lancet 3641127–11342004 [DOI] [PubMed] [Google Scholar]
- 8.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033 J Clin Oncol 26626–6322008 [DOI] [PubMed] [Google Scholar]
- 9.Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: Long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group intergroup phase III randomized trial on imatinib at two dose levels J Clin Oncol 351713–17202017 [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors N Engl J Med 347472–4802002 [DOI] [PubMed] [Google Scholar]
- 11.Blay JY, Shen L, Kang YK, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): A randomised phase 3 trial Lancet Oncol 16550–5602015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: Analysis of phase 3 SWOG intergroup trial S0033 JAMA Oncol 3944–9522017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial Lancet 3681329–13382006 [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial Lancet 381295–3022013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial Lancet Oncol 21923–9342020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer S, Duensing A, Demetri GD, et al. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway Oncogene 267560–75682007 [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation Clin Cancer Res 114182–41902005 [DOI] [PubMed] [Google Scholar]
- 18.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST J Pathol 21664–742008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors Gastroenterology 139942–9522010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Cao Z, Wong EW, et al. COP1/DET1/ETS axis regulates ERK transcriptome and sensitivity to MAPK inhibitors J Clin Invest 1281442–14572019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanke CD.Perforation and stage-II colon cancer: Is it always high risk? Gastrointest Cancer Res 2103–1042008 [PMC free article] [PubMed] [Google Scholar]
- 22.Demetri GD.Identification and treatment of chemoresistant inoperable or metastatic GIST: Experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571) Eur J Cancer 38S52–S592002suppl 5 [DOI] [PubMed] [Google Scholar]
- 23.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor J Clin Oncol 214342–43492003 [DOI] [PubMed] [Google Scholar]
- 24.Blay JY, Reichardt P.Advanced gastrointestinal stromal tumor in Europe: A review of updated treatment recommendations Expert Rev Anticancer Ther 9831–8382009 [DOI] [PubMed] [Google Scholar]
- 25.Debiec-Rychter M, Dumez H, Judson I, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group Eur J Cancer 40689–6952004 [DOI] [PubMed] [Google Scholar]
- 26.de Jong FA, Verweij J.Role of imatinib mesylate (Gleevec/Glivec) in gastrointestinal stromal tumors Expert Rev Anticancer Ther 3757–7662003 [DOI] [PubMed] [Google Scholar]
- 27.Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity Nature 373347–3491995 [DOI] [PubMed] [Google Scholar]
- 28.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours Nature 467849–8532010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ran L, Chen Y, Sher J, et al. FOXF1 defines the core-regulatory circuitry in gastrointestinal stromal tumor Cancer Discov 8234–2512018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran L, Murphy D, Sher J, et al. ETV1-positive cells give rise to BRAFV600E-mutant gastrointestinal stromal tumors Cancer Res 773758–37652017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran L, Sirota I, Cao Z, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth Cancer Discov 5304–3152015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chi P, Qin L, D'Angelo SP, et al. A phase Ib/II study of MEK162 (binimetinib [BINI]) in combination with imatinib in patients with advanced gastrointestinal stromal tumor (GIST) J Clin Oncol. 2015;33 suppl; abstr 10507. [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer 45228–2472009 [DOI] [PubMed] [Google Scholar]
- 34.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria J Clin Oncol 251753–17592007 [DOI] [PubMed] [Google Scholar]
- 35.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group Eur J Cancer 351773–17821999 [DOI] [PubMed] [Google Scholar]
- 36.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT J Clin Oncol 26620–6252008 [DOI] [PubMed] [Google Scholar]
- 37. Won HH, Scott SN, Brannon AR, et al. Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. J Vis Exp. 2013;80:e50710. doi: 10.3791/50710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: Current standard and investigational therapeutics. J Hematol Oncol. 2021;14:2. doi: 10.1186/s13045-020-01026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi Y, Bardsley MR, Toyomasu Y, et al. Platelet-derived growth factor receptor-alpha regulates proliferation of gastrointestinal stromal tumor cells with mutations in KIT by stabilizing ETV1 Gastroenterology 149420–432.e162015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study Eur J Cancer 392006–20112003 [PubMed] [Google Scholar]
- 41.Chen X, Schwartz GK, DeAngelis LM, et al. Dropped head syndrome: Report of three cases during treatment with a MEK inhibitor Neurology 791929–19312012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger M, Amini-Adle M, Maucort-Boulch D, et al. Left ventricular ejection fraction decrease related to BRAF and/or MEK inhibitors in metastatic melanoma patients: A retrospective analysis Cancer Med 92611–26202020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology J Mol Diagn 17251–2642015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A precision oncology knowledge base JCO Precis Oncol 11–162017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The R Project for Statistical Computing. www.R-project.org
- 46.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors J Nucl Med 50122S–50S2009suppl 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulaner GA, Saura C, Piha-Paul SA, et al. Impact of FDG PET imaging for expanding patient eligibility and measuring treatment response in a genome-driven basket trial of the pan-HER kinase inhibitor, neratinib Clin Cancer Res 257381–73872019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agaram NP, Besmer P, Wong GC, et al. Pathologic and molecular heterogeneity in imatinib-stable or imatinib-responsive gastrointestinal stromal tumors Clin Cancer Res 13170–1812007 [DOI] [PubMed] [Google Scholar]