Abstract
The effectiveness of gatifloxacin therapy (15 mg/kg every 5 h [q5h]) was compared with that of meropenem (75 mg/kg q5h) and cefotaxime (75 mg/kg q5h) therapy in experimental meningitis caused by a β-lactamase-producing strain of Escherichia coli. Gatifloxacin therapy was more rapidly bactericidal than cefotaxime but similar to meropenem therapy (bacterial killing rates at 5 h, 0.83 ± 0.26, 0.46 ± 0.3, and 0.73 ± 0.17 CFU/ml/h, respectively; P = 0.03 for gatifloxacin versus cefotaxime). At 10 h, seven of eight animals treated with gatifloxacin had <10 CFU/ml in their cerebrospinal fluid, compared with one of seven treated with cefotaxime therapy (P = 0.01). Gatifloxacin was at least as effective as currently available antibiotics in this model of E. coli meningitis.
Gram-negative meningitis is rare but is associated with significant morbidity and mortality (22). Currently, cefotaxime alone or in combination with an aminoglycoside is a recommended option for the therapy for Escherichia coli meningitis. However, therapeutic failures have occurred, especially in neonates (10, 18). The fluoroquinolones are active against gram-negative bacilli, and ciprofloxacin, pefloxacin, and ofloxacin have been shown to be effective in the therapy of gram-negative meningitis in experimental animals and in humans (19).
Gatifloxacin (AM-1155) is a new 8-methoxy fluoroquinolone. It is well absorbed and widely distributed into body fluids and tissues and is primarily excreted unchanged in the urine (3). Gatifloxacin has excellent bactericidal activity against most gram-negative and gram-positive microorganisms and, because of its lipophilicity, penetrates cerebrospinal fluid (CSF) better (8, 9, 21, 24). We have shown previously that gatifloxacin is highly effective as a single agent in experimental cephalosporin-resistant pneumococcal meningitis (17). The present study was conducted to compare the effectiveness of gatifloxacin therapy with meropenem and cefotaxime therapy in experimental meningitis caused by a β-lactamase-producing strain of E. coli.
Methods.
E. coli 77-436, a K1:O18 strain (β-lactamase positive) originally isolated from a neonate with bacterial meningitis, was grown overnight on blood agar. The plates were flooded with endotoxin-free phosphate-buffered saline, and aliquots of the resultant suspension were frozen at −70°C. The MICs and MBCs of antibiotics for this strain were measured by standard National Committee for Clinical Laboratory Standards methods (20).
Overnight Mueller-Hinton broth cultures of E. coli were diluted in fresh broth to final concentrations of 105 to 108 CFU/ml. Antibiotics were added to achieve concentrations similar to those in the CSF of patients with meningitis, i.e., cefotaxime at 4 μg/ml, meropenem at 3 μg/ml, and gatifloxacin at 1 μg/ml. Resultant suspensions were incubated at 37°C for 24 h; serial 100-fold dilutions were plated on blood agar at 6 and 24 h and incubated overnight at 37°C. The lower limit of detection was 100 CFU/ml.
A rabbit meningitis model originally described by Dacey and Sande was used (4). Meningitis was induced in young New Zealand White male rabbits by intracisternal inoculation of 0.25 ml of the E. coli suspension (approximately 105 CFU/ml).
Antibacterial therapy was started 14 h after inoculation of bacteria. All antibiotics were given intravenously via a marginal ear vein. The antibiotics studied were gatifloxacin (Bristol-Myers-Squibb, Wallingford, Conn.) at 15 mg/kg, cefotaxime (Hoechst-Roussel, Somerville, N.J.) at 75 mg/kg, and meropenem (Zeneca, Wilmington, Del.) at 75 mg/kg. Dosages were chosen to simulate concentrations achieved in human CSF (5, 23). Three doses of each antibiotic were given 5 h apart. Animals were killed by pentobarbital overdose 24 h after the initiation of antibacterial therapy. Each treatment group consisted of 7 to 10 animals.
CSF samples for measurement of peak and trough drug concentrations were collected 1 and 10 h after administration of the first antibiotic dose. Bacterial concentrations were measured before therapy and at 5, 10, and 24 h after initiation of therapy by plating undiluted CSF and serial dilutions of CSF on sheep blood agar and incubating them at 35°C for 24 h. The lower limit of detection was 10 CFU/ml.
Meropenem and cefotaxime concentrations were measured by high-performance liquid chromatography (1). Gatifloxacin concentrations were determined by disk diffusion bioassay using Bacillus subtilis ATCC 6633 (17). Standard curves were prepared with rabbit CSF and were linear in the ranges of 1.0 to 10 μg/ml for meropenem and cefotaxime and 0.1 to 2.0 μg/ml for gatifloxacin. Concentrations of each antibiotic were measured in one run. Intra-assay coefficients of variance were <5%.
Continuous variables are expressed as means ± standard deviations (SD). Student’s t test and analysis of variance were used to compare continuous variables. The Fisher exact test was used to compare categorical values.
Results.
The respective MICs and MBCs for E. coli are presented in Table 1. Table 2 shows the effect of bacterial concentration on the bacterial killing rate (BKR) in vitro (inoculum effect). Gatifloxacin was more rapidly bactericidal than meropenem and cefotaxime. The BKR of cefotaxime decreased as bacterial concentrations increased; this did not occur with meropenem or gatifloxacin. At 24 h, all cultures, other than the controls, were sterile.
TABLE 1.
MICs and MBCs for E. coli K1:O18 and drug concentrations in CSF
| Antibiotic (dose [mg/kg]) | MIC (μg/ml) | MBC (μg/ml) | Mean concn in CSF (μg/ml) ± SD
|
|
|---|---|---|---|---|
| Peak | Trough | |||
| Cefotaxime (75) | 0.06 | 0.06 | 4.1 ± 2.5 | 1.5 ± 1.1 |
| Meropenem (75) | 0.02 | 0.02 | 3.8 ± 0.8 | 1.4 ± 1.0 |
| Gatifloxacin (15) | 0.06 | 0.06 | 0.9 ± 0.2 | 0.1 ± 0.1 |
TABLE 2.
Effect of bacterial concentration on in vitro BKR
| Initial bacterial concn (CFU/ml) | BKR (Δlog10 CFU/ml/h) at 6 ha
|
||
|---|---|---|---|
| Cefotaxime (4 μg/ml) | Meropenem (3 μg/ml) | Gatifloxacin (1 μg/ml) | |
| 108 | 0.16 | 0.6 | >1 |
| 107 | 0.25 | 0.58 | >0.83 |
| 106 | 0.33 | 0.58 | >0.66 |
| 105 | 0.6 | >0.6 | >0.6 |
Antibiotics were added to achieve concentrations similar to those in the CSF of patients with meningitis. The antibiotic concentration/MIC ratios for cefotaxime, meropenem, and gatifloxacin were 67, 150, and 16.7, respectively.
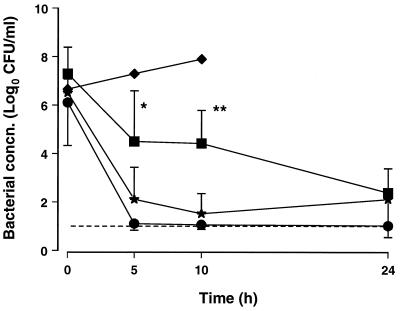
Antibiotic concentrations in CSF are shown in Table 1. The bacteriologic effectiveness of the different therapies is demonstrated in Fig. 1. The BKRs in the first 5 h were 0.83 ± 0.26, 0.73 ± 0.17, and 0.46 ± 0.3 CFU/ml/h with the gatifloxacin, meropenem, and cefotaxime therapies, respectively (P = 0.03 for gatifloxacin versus cefotaxime). As a result of its greater BKR, more animals had negative (<10 CFU/ml) CSF cultures at 5 and 10 h with gatifloxacin therapy than with cefotaxime therapy (7 of 9 versus 2 of 10 at 5 h [P = 0.02] and 7 of 8 versus 1 of 7 at 10 h [P = 0.01], respectively). Meropenem and gatifloxacin were similarly effective; four of seven animals had <10 CFU/ml at 5 and 10 h with meropenem therapy. At 24 h, the bacterial concentrations in CSF were 1.0 ± 0.1, 2.4 ± 1.0, and 2.1 ± 1.6 CFU/ml with the gatifloxacin, meropenem, and cefotaxime therapies, respectively, and the numbers of animals with negative cultures were similar in all groups. However, only 5 of 10 animals treated with cefotaxime survived compare with 8 of 9 and 7 of 7 treated with gatifloxacin and meropenem, respectively. The in vivo BKRs of the three antibiotics studied were not influenced by the initial bacterial concentrations in CSF.
FIG. 1.
Bacterial concentrations (mean and SD) in CSF of rabbits with E. coli meningitis treated with cefotaxime (■, 75 mg/kg), meropenem (★, 75 mg/kg), and gatifloxacin (●, 15 mg/kg) at 0, 5, and 10 h. Symbols: ∗, P = 0.02; and ∗∗, P = 0.01 (for gatifloxacin versus cefotaxime therapy). Control animals (⧫) were euthanized after 10 h. The dashed line indicates the lower limit of detection.
Discussion.
Gatifloxacin, a new fluoroquinolone, demonstrated rapid bacterial killing in this experimental model of E. coli meningitis; its effectiveness was comparable to that of meropenem and was superior to that of cefotaxime therapy.
In vitro, higher MICs of cefotaxime for E. coli are measured in the presence of high initial bacterial concentrations (14). This is thought to be caused by the overproduction of β-lactamase by dense bacterial populations (16). This inoculum effect occurs to a much lesser extent with gatifloxacin or meropenem (8, 11). Our time-kill studies confirmed that the BKR of cefotaxime was reduced when the initial bacterial concentrations were large whereas the BKR of gatifloxacin was independent of the initial bacterial concentration. Similarly, in experimental E. coli meningitis, a reduction in the effectiveness of cefotaxime therapy in the presence of high bacterial concentrations has been shown (12–14) and was confirmed in this study. In contrast to its BKR in vitro, the BKR of cefotaxime was not affected by bacterial concentrations in the meningitis model. However, the BKR of cefotaxime was relatively slow compared with that of gatifloxacin therapy, and in the presence of high bacterial concentrations, this translates into delayed CSF sterilization and increased mortality. A similar effect may occur in humans with meningitis because patients who have bacterial concentrations in the CSF of >106 CFU/ml tend to have delayed CSF sterilization (2, 6).
The MBCs of the three antibiotics studied for the E. coli strain used were low and antibiotic concentrations in CSF many fold greater than the respective MBCs were achieved. Thus, we believe the slower bacterial clearance with cefotaxime therapy was related not to low drug concentrations in CSF but to the drug’s mode of action; the lower BKR observed in vitro supports this supposition.
Whether a more rapid BKR, as occurs with gatifloxacin or meropenem therapy, is of clinical benefit in gram-negative meningitis is unproven, although available evidence suggests that this is likely. In experimental meningitis, persistence of E. coli in the CSF eventually results in much greater endotoxin release than that induced by effective antibacterial therapy (7). In the present and previous experimental studies (13), lower BKRs were associated with increased mortality. In children with Haemophilus influenzae or enteric gram-negative meningitis, failure to sterilize the CSF within 24 h of antibacterial therapy is associated with a poorer outcome (15, 18, 25).
In conclusion, gatifloxacin was rapidly bactericidal in this experimental E. coli meningitis model. Whether this will translate into improved outcome of gram-negative enteric meningitis in humans warrants further investigation.
Acknowledgments
This study was supported by a grant from Bristol-Myers Squibb Pharmaceuticals.
REFERENCES
- 1.Bergan T, Solberg R. Assay of cefotaxime by high-performance-liquid chromatography. Chemotherapy. 1981;27:155–165. doi: 10.1159/000237972. [DOI] [PubMed] [Google Scholar]
- 2.Bingen E, Lambert-Zechovsky N, Mariani-Kurkdjian P, Doit C, Aujard Y, Fournerie F, Mathieu H. Bacterial counts in cerebrospinal fluid of children with meningitis. Eur J Clin Microbiol Infect Dis. 1990;9:278–281. doi: 10.1007/BF01968060. [DOI] [PubMed] [Google Scholar]
- 3.Bristol-Myers-Squibb Pharmaceutical Research Institute. Investigator’s brochure: gatifloxacin. Princeton, N.J: Bristol-Myers-Squibb Pharmaceutical Research Institute; 1997. pp. 35–41. [Google Scholar]
- 4.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivates. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan R, Velghe L, Rodda J L, Klugman K P. Penetration of meropenem into cerebrospinal fluid of patients with inflamed meninges. J Antimicrob Chemother. 1994;34:175–179. doi: 10.1093/jac/34.1.175. [DOI] [PubMed] [Google Scholar]
- 6.Feldman W E. Relation of concentration of bacteria and bacterial antigen in cerebrospinal fluid to prognosis in patients with bacterial meningitis. N Engl J Med. 1977;296:433–435. doi: 10.1056/NEJM197702242960806. [DOI] [PubMed] [Google Scholar]
- 7.Friedland I R, Jafari H, Ehrett S, Rinderknecht S, Paris M, Coulthard M, Saxen H, Olsen K, McCracken G H., Jr Comparison of endotoxin release by different antimicrobial agents and the effect on inflammation in experimental Escherichia coli meningitis. J Infect Dis. 1993;168:657–662. doi: 10.1093/infdis/168.3.657. [DOI] [PubMed] [Google Scholar]
- 8.Hosaka M, Kinoshita S, Toyama A, Otsuki M, Nishino T. Antibacterial properties of AM-1155, a new 8-methoxy quinolone. J Antimicrob Chemother. 1995;36:293–301. doi: 10.1093/jac/36.2.293. [DOI] [PubMed] [Google Scholar]
- 9.Hosaka M, Yasue T, Fukuda H, Tomizawa H, Aoyama H, Hirai K. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1992;36:2108–2117. doi: 10.1128/aac.36.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan S L, Patrick C C. Cefotaxime and aminoglycoside treatment of meningitis caused by gram-negative enteric organisms. Pediatr Infect Dis J. 1990;9:810–814. doi: 10.1097/00006454-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kessler R E, Fung-Tomc J, Kolek B, Minassian B, Huczko E, Gradelski E, Bonner D P. In vitro activity of BMS-181139, a new carbapenem with potent antipseudomonal activity. Antimicrob Agents Chemother. 1995;39:380–385. doi: 10.1128/aac.39.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K S. Comparison of cefotaxime, imipenem-cilastin, ampicillin-gentamicin, and ampicillin-chloramphenicol in the treatment of experimental Escherichia coli bacteremia and meningitis. Antimicrob Agents Chemother. 1985;28:433–436. doi: 10.1128/aac.28.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K S. Efficacy of cefomenoxime in experimental Escherichia coli bacteremia and meningitis. Antimicrob Agents Chemother. 1985;28:389–392. doi: 10.1128/aac.28.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K S, Manocchio M, Bayer A S. Efficacy of cefotaxime and latamoxef for Escherichia coli bacteriemia and meningitis in newborn rats. Chemotherapy. 1984;30:262. doi: 10.1159/000238278. [DOI] [PubMed] [Google Scholar]
- 15.Lebel M H, McCracken G H., Jr Delayed cerebrospinal fluid sterilization and adverse outcome of bacterial meningitis in infants and children. Pediatrics. 1989;83:161–167. [PubMed] [Google Scholar]
- 16.Levinson M E. Pharmacodynamics of antimicrobial agents. Infect Dis Clin N Am. 1995;9:483–495. [PubMed] [Google Scholar]
- 17.Lutsar I, Friedland I R, Wubbel L, McCoig C C, Jafri H S, Ng W, Ghaffar F, McCracken G H., Jr Pharmacodynamics of gatifloxacin in cerebrospinal fluid in experimental cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1998;42:2650–2655. doi: 10.1128/aac.42.10.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCracken G H, Mize S G. A controlled study of intrathecal antibiotic therapy in gram-negative enteric meningitis of infancy. Report of the National Meningitis Cooperative Study Group. J Pediatr. 1976;89:66–72. doi: 10.1016/s0022-3476(76)80929-8. [DOI] [PubMed] [Google Scholar]
- 19.Modai J. Potential role of fluoroquinolones in the treatment of bacterial meningitis. Eur J Clin Microbiol Infect Dis. 1991;10:291–295. doi: 10.1007/BF01967002. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard. NCCLS publication no. M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 21.Ooie T, Suzuki H, Terasaki T, Sugiyama Y. Comparative distribution of quinolone antibiotics in cerebrospinal fluid and brain in rats and dogs. J Pharmacol Exp Ther. 1996;278:590–596. [PubMed] [Google Scholar]
- 22.Paap C M, Bosso J A. Treatment options for the pharmacological therapy of neonatal meningitis. Drugs. 1992;43:700–712. doi: 10.2165/00003495-199243050-00006. [DOI] [PubMed] [Google Scholar]
- 23.Trang J M, Jacobs R F, Kearns G L, Brown A L, Wells T G, Underwood F L, Kluza R B. Cefotaxime and desacetylcefotaxime pharmacokinetics in infants and children with meningitis. Antimicrob Agents Chemother. 1985;28:791–795. doi: 10.1128/aac.28.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakabayashi E, Mitsuhashi S. In vitro antibacterial activity of AM-1155, a novel 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1994;38:594–601. doi: 10.1128/aac.38.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson H D, Hatalin K C. Ampicillin in Haemophilus influenzae meningitis. Clinicopharmacologic evaluation of intramuscular vs intravenous administration. Am J Dis Child. 1975;129:208–215. doi: 10.1001/archpedi.1975.02120390042009. [DOI] [PubMed] [Google Scholar]