To the Editor:
The coronavirus disease (COVID-19) pandemic has resulted in significant changes in the delivery of care, accelerating the shift toward telehealth models (1), especially in the area of cystic fibrosis (CF) (2). Generally, these changes have been embraced by persons with CF and physicians (3), particularly because of the greater flexibility and reduced travel requirements (4, 5).
Although there is little doubt that remote healthcare delivery offers many practical benefits to patients and providers, some aspects of routine care can become more complicated. With regards to CF and non-CF bronchiectasis, this includes sputum microbiology surveillance for the timely detection of new organisms.
In response to the COVID-19 pandemic, our center rapidly transitioned to quarterly telehealth reviews, similar to most CF clinics in Canada and abroad, with requisition forms for quarterly sputum culture sent to patients in advance of scheduled telehealth appointments. Patients throughout our province can deposit sputum samples at their local laboratory, and the samples are subsequently shipped to our center’s clinical microbiology laboratory, thus removing the need to travel.
Nonetheless, we were concerned that the inconvenience of collecting sputum at home and the reduction of in-clinic cough swabs would result in a reduction in respiratory samples analyzed, creating a potential impact on timely detection of significant new pathogens. We performed an audit to determine the effect of the shift to remote monitoring on the number of samples collected and estimated the potential impact in terms of missed detection of new, clinically significant organisms.
Methods
We reviewed patients’ electronic charts at St. Paul’s Hospital Adult CF Clinic in Vancouver to identify all respiratory samples received between January 1, 2015, and September 11, 2020. Lung transplant recipients were excluded as were individuals receiving CFTR (cystic fibrosis transmembrane conductance regulator) modulators on the basis that Canadian persons with CF have not had widespread access to these therapies, and the inclusion of individuals recently commenced on modulators between 2015 and 2020 could have confounded our findings because of subsequent reduction in sputum production. To ensure our observations were made on a cohort of historically adherent individuals, inclusion required attendance at our center from 2015 to 2020 and provision of ⩾1 respiratory sample every 6 months in the prepandemic period. We defined our pandemic observational period as March 11, 2020 (World Health Organization declaration of the COVID-19 pandemic) to September 10, 2020. Pandemic data was compared with the prepandemic data (March 11, 2016, to March 10, 2020) in aggregate (mean values for 2016–2020) and as separate 6-month blocks (March–September and September–March for each year). Respiratory sample reports from 2015 were used to ascertain significant new growths on cultures received from March 11, 2016, and onward. Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, Burkholderia cepacia complex species, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans were considered significant pathogens. We defined a culture result as “new” when it revealed the first detected growth of a significant pathogen within a given individual or first recurrence when not seen for ⩾1 year and absence in ⩾3 intercurrent samples. The presence of the aforementioned organisms in all samples was recorded together with all other documented species. Nontuberculous mycobacteria cultures were not included in our analysis. Statistical analysis was performed using R version 4.0.4 (The R Foundation for Statistical Computing). Normally and nonnormally distributed data were compared by the Student’s t test and Mann-Whitney U test. Categorical outcomes were compared by the Chi-square or Fisher’s exact test with odds ratios (OR) calculated by comparing mean prepandemic versus pandemic proportions, and 95% confidence intervals were calculated from the standard error of the log(OR). A significance threshold of P < 0.05 was used for all analyses.
Results
Out of 269 individuals followed at our clinic, we excluded 61 receiving CFTR modulators, 32 because of prior transplantation and 74 because of irregular sputum analysis before the pandemic. In total, 102 individuals (median age 31, 60% male, Table 1) provided 1,904 respiratory samples between March 11, 2016, and September 11, 2020. The most prevalent organisms were methicillin-susceptible S. aureus (range 33–45%) and P. aeruginosa (range 25–34%), with no significant difference between prepandemic and pandemic samples (P = 0.61and P = 0.72, respectively, by Chi-square test).
Table 1.
Baseline population characteristics at the start of the pandemic and compared by those who did versus did not provide sputum during the pandemic period
| Total (N = 102) | No Samples (n = 63) | ⩾1 Sample (n = 39) | P Value* | |
|---|---|---|---|---|
| Age, yr | 31.0 (25.0–45.3) | 35.5 (26.3–45.0) | 31.0 (23.0–45.3) | 0.367 |
| Sex, male | 62 (60.1) | 40 (63.5) | 22 (57.9) | 0.669 |
| Genotype | — | — | — | 0.367 |
| F508del heterozygous | 45 (44.1) | 28 (44.4) | 17 (43.6) | — |
| F508del homozygous | 39 (38.3) | 22 (34.9) | 16 (41.0) | — |
| Other | 18 (17.6) | 13 (20.1) | 6 (15.4) | — |
| FEV1PP | 77 ± 21 | 77 ± 22 | 75 ± 19 | 0.509 |
| BMI, kg/m2 | 23.1 ± 3.6 | 23.4 ± 3.7 | 22.6 ± 3.5 | 0.252 |
| Pancreatic insufficiency | 76 (74.5) | 45 (71.5) | 31 (79.5) | 0.435 |
| CF-related diabetes | 30 ± 29.4 | 20 ± 31.7 | 10 ± 25.7 | 0.686 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; FEV1PP = percent predicted of forced expiratory volume in 1 second.
Data are presented as median (interquartile range) when nonnormally distributed, mean ± standard deviation when normally distributed data, and n (%) when categorical.
For the comparisons of individuals who did versus did not provide sputum during the pandemic.
We observed a threefold reduction in the number of respiratory samples received, from a mean of 229 per 6-month period before the pandemic to 76 per 6-month period during the pandemic, with no clear baseline differences noted between those individuals who did (n = 39) versus did not (n = 63) provide samples (Table 1) during the pandemic.
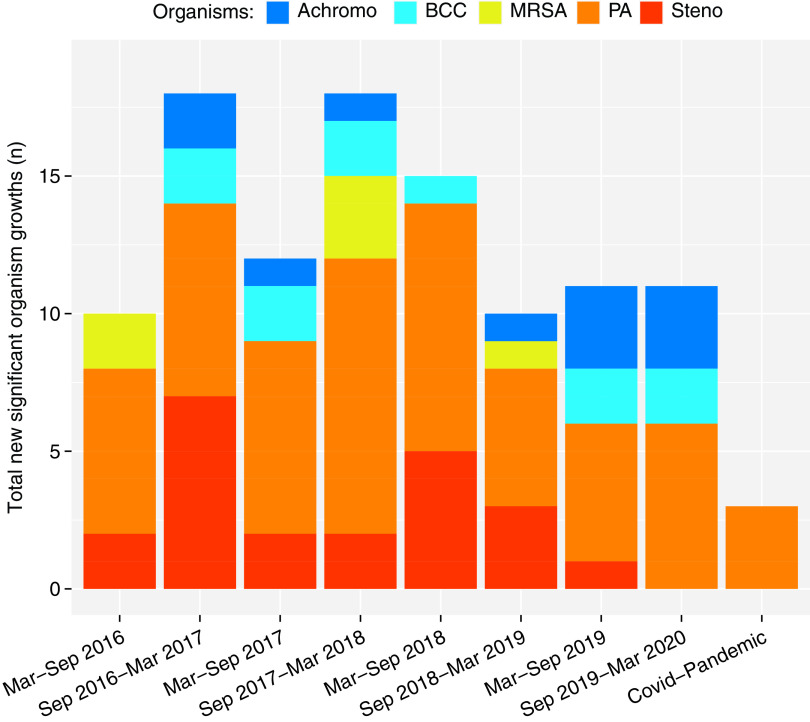
This was associated with a reduction in the absolute number of clinically significant new organisms identified, from 13 (mean) per 6-month period in 102 individuals before the pandemic (12.7% of individuals in 6 months, range 10–17%) to 3 (1.7% of individuals per 6-month period) during the pandemic. The OR of new significant organism identification during the pandemic compared with the 6-month mean of before the pandemic was 0.21 (95% confidence interval, 0.04–0.79; P = 0.016 by Fisher’s exact test) (Figure 1).
Figure 1.
Total new significant respiratory cultures in each 6-month block before the pandemic (March 2016 to March 2020) and the 6-month block during the pandemic. Identification of clinically significant new organisms dropped from a mean of 13 per 6-month period in 102 individuals before the pandemic to 3 during the pandemic (odds ratio of new significant organism identification during the pandemic compared with prepandemic 6-monthly mean = 0.21; 95% confidence interval, 0.04–0.79; P = 0.016 by Fisher’s exact test). Achromo = Achromobacter xylosoxidans; BC = Burkholderia Cepacia complex; MRSA = methicillin-resistant Staphylococcus aureus; PA = Pseudomonas aeruginosa; Steno = Stenotrophomonas maltophilia.
Discussion
Social distancing and efforts aimed at reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure among high-risk individuals have accelerated the transition to telehealth consultation, a change that will now, undoubtedly, have a more permanent place in clinical care beyond the COVID-19 pandemic given the unquestionable associated advantages. Nevertheless, the lessons we can learn from this enforced shift in practice should not be underestimated and can help optimize quality of care going forward.
Our experience highlights the challenges arising from a reduction of in-person contact for CF. Without appropriate adjustments to remote care models, reduced frequency of sputum monitoring may lead to missed or delayed opportunities to detect new pathogens that may require timely eradication, such as Pseudomonas. Home-performed cough swabs may be an option for monitoring younger patients and those not producing sputum regularly (6), whereas induced sputum production should be encouraged for those patients already established on nebulized saline. Specialist centers should be aware of the transport requirements for each sample type and plan service modifications to ensure adequate microbiology monitoring is maintained during the shift to telehealth care (7). The limitations of our study include the single-center nature of this analysis as well as the exclusion of CFTR modulator–treated patients, both of which might limit the generalizability of our findings. CFTR modulator therapies are rapidly becoming the standard of care in CF, but we believed they would represent a significant confounder in our analysis (especially if started just before or early during the pandemic) because they can reduce spontaneous sputum production; the primary focus of our audit was to assess the impact of the pandemic on microbiological acquisition owing to a change in the care delivery model from in-person care to telehealth.
Conclusions
Despite the advantages of telehealth monitoring, a reduction of in-person patient visits may be associated with suboptimal longitudinal monitoring of CF sputum microbiology. Regardless of specialty, all centers adopting new telehealth practices should be cognizant of the need to facilitate remote monitoring protocols that achieve parity with traditional in-person models. Ongoing assessment of adherence to these protocols in the longer term will be of paramount importance to optimize patient outcomes as care delivery models shift.
Footnotes
Supported by a Michael Smith Foundation for Health Research Trainee Award (RT-2020-0493 to A.N.F.) and a Michael Smith Foundation for Health Research Scholar Award 16414 (B.S.Q.).
Author Contributions: A.N.F., P.G.W., and B.S.Q. proposed the study. A.N.F. performed data collection, analysis, and manuscript writing. P.G.W. edited the final manuscript. B.S.Q. edited the manuscript and is the senior author.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Smith AC, Thomas E, Snoswell CL, Haydon H, Mehrotra A, Clemensen J, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19) J Telemed Telecare . 2020;26:309–313. doi: 10.1177/1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Compton M, Soper M, Reilly B, Gettle L, List R, Bailey M, et al. A feasibility study of urgent implementation of cystic fibrosis multidisciplinary telemedicine clinic in the face of COVID-19 pandemic: single-center experience. Telemed J E Health . 2020;26:978–984. doi: 10.1089/tmj.2020.0091. [DOI] [PubMed] [Google Scholar]
- 3. Perkins RC, Davis J, NeSmith A, Bailey J, Powers MR, Chaudary N, et al. Favorable clinician acceptability of telehealth as part of the cystic fibrosis care model during the COVID-19 pandemic. Ann Am Thorac Soc . 2021;18:1588–1592. doi: 10.1513/AnnalsATS.202012-1484RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ketchell RI. Telemedicine is the way forward for the management of cystic fibrosis—the case in favour. Paediatr Respir Rev . 2018;26:19–21. doi: 10.1016/j.prrv.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 5. Wood J, Mulrennan S, Hill K, Cecins N, Morey S, Jenkins S. Telehealth clinics increase access to care for adults with cystic fibrosis living in rural and remote Western Australia. J Telemed Telecare . 2017;23:673–679. doi: 10.1177/1357633X16660646. [DOI] [PubMed] [Google Scholar]
- 6. Lenhart-Pendergrass PM, Anthony M, Sariyska S, Andrews A, Scavezze H, Towler E, et al. Detection of bacterial pathogens using home oropharyngeal swab collection in children with cystic fibrosis. Pediatr Pulmonol . 2021;56:2043–2047. doi: 10.1002/ppul.25421. [DOI] [PubMed] [Google Scholar]
- 7.Moore JE, Millar BC, McCaughan J, O’Neill D, Bell J, Crossan A, et al. The virtual CF clinic: implications for sputum microbiology J Cyst Fibros 202120699–701.. [DOI] [PMC free article] [PubMed] [Google Scholar]