Abstract
Tissue damage in the upper and lower airways caused by mechanical abrasion, noxious chemicals, or pathogenic organisms must be followed by rapid restorative processes; otherwise, persistent immunopathology and disease may ensue. This review will discuss evidence for the important role served by trefoil factor (TFF) family members in healthy and diseased airways of humans and rodents. Collectively, these peptides serve to both maintain and restore homeostasis through their regulation of the mucous layer and their control of cell motility, cell differentiation, and immune function in the upper and lower airways. We will also discuss important differences in which trefoil member tracks with homeostasis and disease between humans and mice, which poses a challenge for research in this area. Moreover, we discuss new evidence supporting newly identified receptor binding partners in the leucine-rich repeat and immunoglobulin-like domain–containing NoGo (LINGO) family in mediating the biological effects of TFF proteins in mouse models of epithelial repair and infection. Recent advances in our knowledge regarding TFF peptides suggest that they may be reasonable therapeutic targets in the treatment of upper and lower airway diseases of diverse etiologies. Further work understanding their role in airway homeostasis, repair, and inflammation will benefit from these newly uncovered receptor–ligand interactions.
Keywords: trefoil factor family, LINGO receptors, mucosal epithelium repair, CXCR4, CXCR7
Clinical Relevance
This translational review highlights the role of trefoil factor (TFF) family peptides in repair of the airway mucosa. Collectively, these peptides serve to both maintain and restore homeostasis through their regulation of the mucous layer and control of cell motility, cell differentiation, and immune function in the upper and lower airways. Recent advances in our knowledge regarding TFF peptides and their newly uncovered receptor–ligand interactions suggest that they may be reasonable therapeutic targets in the treatment of upper and lower airway diseases of diverse etiologies.
Humans inspire up to 20,000 L of air per day (1), which is filtered by the nasal cavity and airway. This filtration is performed by a mucosal barrier containing ciliated epithelial cells interspersed with mucus-producing secretory cells (2). The damaged mucosal epithelium can be repaired by peptides in the TFF (trefoil factor) family: TFF1, TFF2, and TFF3 (also known as breast cancer–associated peptide ps2, spasmolytic peptide, and intestinal TFF, respectively) (3). TFFs have a cloverleaf structure created by disulfide bonds at six conserved cysteine residues (4, 5). These peptides are expressed in the airway by various glandular and mucus-secreting epithelia and hematopoietic cells in the sinonasal tract (6, 7), trachea, and lungs (8).
In this review, we first discuss expression patterns of TFFs 1, 2, and 3 in healthy and diseased human respiratory tissue (Table 1 and Figure 1) and evidence from mouse model systems supporting a role for TFF2 in airway restoration. Next, we discuss the biological functions of TFFs in the airway, including their contribution to cell motility, signaling-cell proliferation and differentiation, and modulation of inflammation (Figure 2). Finally, we will discuss evidence of receptors for TFFs, including previously orphaned members of the LINGO (leucine-rich repeat and immunoglobulin-like domain–containing NoGo) receptor family (Figure 3), which could open the possibility for novel therapeutics in airway disease.
Table 1.
Evidence of Increased TFF Expression in Human Airway Diseases
| Disease | TFFs | Tissue or Fluid Localization | Measured | Severity or Treatment | Citation |
|---|---|---|---|---|---|
| Lung cancer | TFFs 1, 2, and 3 | TFFs 1 and 2, BALF; TFF3, tissue | Protein | TFF2 = metastasis | 14, 40 |
| CF | TFFs 1 and 3 | Lung, nasal polyps, sputum | Both | — | 6 |
| COPD | TFFs 1*, 2, and 3* | BALF*, serum | Protein | Stage II, III > stage I | 14, 15 |
| COPD | TFFs 1 and 3 | Sputum | Protein | — | 15 |
| Asthma, pediatric, attack | TFF2 | Nasal mucosa brushings | mRNA | — | 16 |
| Asthma, adult, controlled | None | Serum, sputum | Protein | Control = asthma < COPD | 15 |
| CRSwNP | TFF3† | Middle nasal turbinate† | mRNA | Treatment reversed | 12 † |
| CRSsNP | TFFs 1 and 3 | Sinonasal tract | Both | Eosinophilia had no effect | 9 |
| Nasal allergy | TFFs 1 and 3 | Inferior turbinate | mRNA | — | 13 |
Definition of abbreviations: BALF = BAL fluid; CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; TFF = trefoil factor.
TFFs 1 and 3 were higher in BALF of patients with stage II or III than stage I COPD.
TFF1 mRNA decreased in all CRSwNP sites sampled (nasal polyps, middle nasal turbinate, bulla ethmoidalis), versus controls (inferior turbinate).
Figure 1.
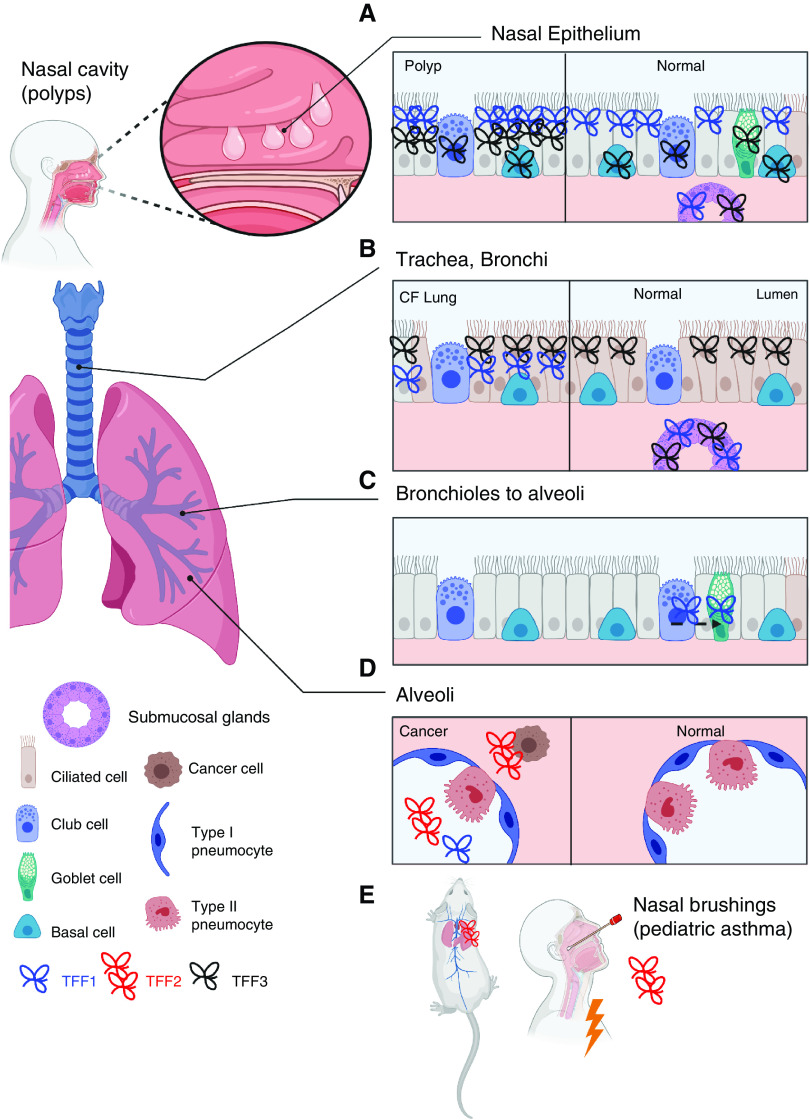
Localization of TFFs (trefoil factors) in the healthy and diseased airway. TFFs are found in the (A) nasal epithelium, (B) tracheal/bronchial epithelium, and (C) bronchiolar–alveolar transition (club-cell transdifferentiation into goblet cells) and (D) are mostly absent from healthy alveoli but may be located there in the context of cancer. (E) TFF2 is dominant in the mouse lung and is expressed in nasal brushings from pediatric patients with asthma during an attack. CF = cystic fibrosis.
Figure 2.
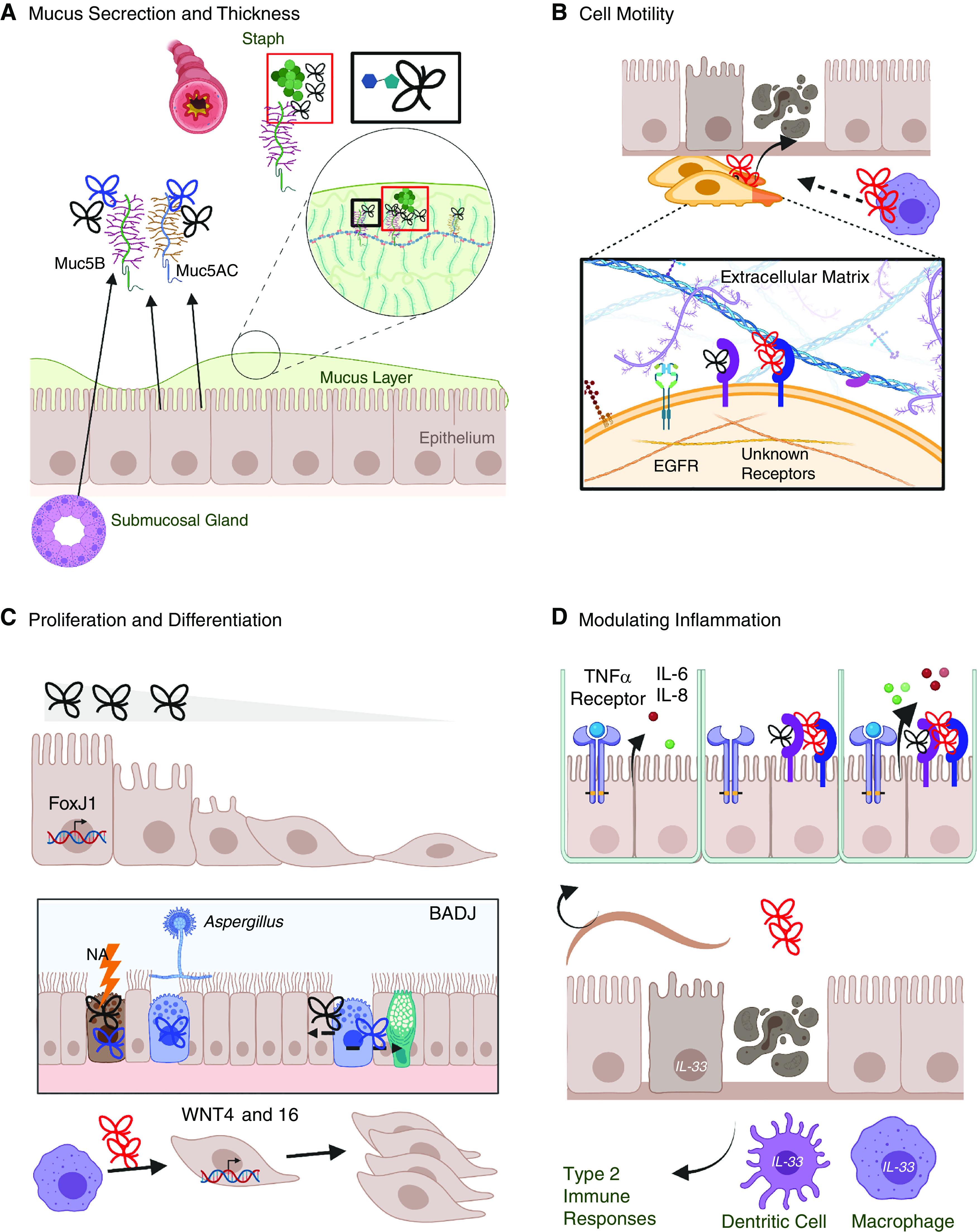
Contribution of TFFs to mucosal barrier function and repair. (A) TFFs bind the terminal disaccharide of mucin glycoproteins to thicken the mucous layer (box outlined in black), which can constrict the airway. TFF1 (blue) and TFF3 (black) are associated with Muc5AC (epithelial) and Muc5B (epithelial and submucosal). TFF1 has been found around Staph, and Muc5B is required for bacterial clearance in the lung (box outlined in red). (B) Cell migration into a damaged site is required for tissue repair. TFF2 (red) and TFF3 (black) bind with unknown receptors to enhance cell motility synergistically with EGF (epidermal growth factor) and extracellular matrix components. (C) TFF3 and FoxJ1 transcription induce ciliogenesis. In the BADJ, NA injury or allergen exposure induces TFF1 and/or TFF3 in club cells, which can differentiate into epithelial or goblet cells. TFF2 from macrophages induces WNT epithelial expression, which is associated with cell proliferation. (D) TFFs contribute to immune function, including enhancing TNFα-evoked release of IL-6 and IL-8; TFF2-dependent induction of IL-33 in epithelial cells, dendritic cells, and macrophages; type 2 responses; and worm clearance during helminth infection. BADJ = bronchoalveolar duct junctions; EGFR = EGF receptor; NA = naphthalene; Staph = Staphylococcus aureus.
Figure 3.
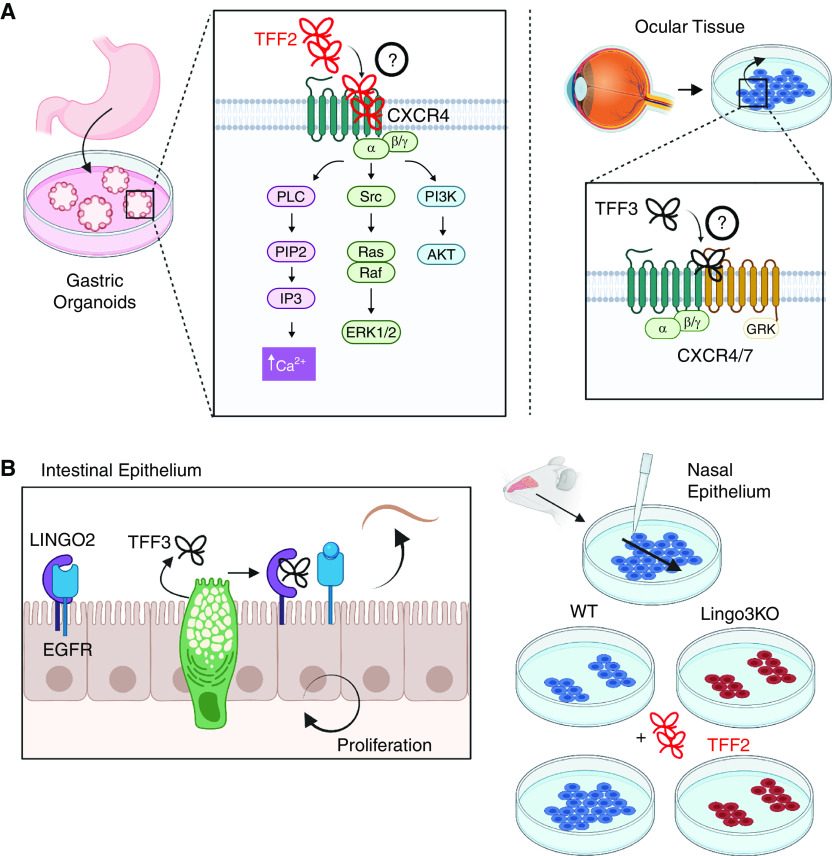
CXCR (CXC receptor) and LINGO (leucine-rich repeat and immunoglobulin-like domain–containing NoGo) family members as TFF receptors. (A) TFF2 requires CXCR4 for calcium mobilization, ERK1/2 activation, and AKT activation. Antagonism of CXCR4–CXCR7 dimer prevents TFF3-mediated cell migration but does not prevent cell proliferation or MAPK activity. Direct binding has not been demonstrated, indicated by the circled question mark. (B) TFF3 interacts with LINGO2 to competitively reverse LINGO2–EGFR binding, enhancing cell proliferation and protection against helminths. LINGO3 is required for TFF2-mediated repair of nasal epithelial cultures. ERK1/2 = extracellular regulated kinase 1/2; KO = knockout; PLC = primary liver cancer; Src = scrambled; WT = wild-type.
Localization of TFFs in Airway Tissue
TFF family members are found throughout human mucosal tissues (8). The second-highest TFF3 expression occurs in the trachea, especially in the submucosal glands, which also express TFF1, and the pseudostratified epithelium in the bronchus (8) (Figure 1). Healthy human nasal and lung tissue has a higher basal mRNA and protein expression for TFF3 relative to TFF1, whereas TFF2 is undetectable (6–9).
TFF1 and TFF3 (6.5 kD and 7 kD) share a common single trefoil domain and carboxy-terminal cysteine and differ in tissue-specific glycosylation patterns (10). TFF2 is larger (12 kD) and has multiple trefoil domains and no carboxy-terminal cysteine (11). TFF1 and TFF3 immunostaining localized in ciliated epithelial layers that included mucus-secreting cells and were absent from alveolar cells that lacked mucin expression (6, 8) (Figure 1). TFF levels in sampled tissues or fluids are often increased during disease and are associated with disease severity (Table 1) (6, 9, 12–16). Specifically, TFF1 and TFF3 are associated with functional impairment during chronic obstructive pulmonary disease (COPD), with greater levels than those measured in controlled adult patients with asthma or healthy control subjects being demonstrated (14, 15). TFF2 levels increase moderately during acute pediatric asthma and during metastatic lung cancer (Table 1) (14, 16). There is some conflicting information regarding the direction of changes in chronic rhinosinusitis (CRS), depending on whether nasal polyps are present (CRS with nasal polyps [CRSwNP]) or not (CRS without nasal polyps [CRSsNP]) (9, 12). Both TFF1 and TFF3 levels were higher in nasal tissue from patients with CRSsNP (9), whereas TFF1 was decreased and TFF3 was increased in CRSwNP (12). Surgical removal of the polyps with steroid treatment was associated with a reversal of altered TFF1 and TFF3 mRNA expression (12). Some of the discrepant findings may be related to different sampling regions in the sinonasal tract, but more work is needed to clarify the biological importance of TFFs in both CRSwNP and CRSsNP (Table 1).
TFF2 in Murine Model Systems: Potential for Translation
Most clinical data do not support the presence of TFF2 in healthy human lungs or noncancerous disease (Table 1). In culture systems using human bronchial epithelial cell lines, TFF2 can promote features of wound healing, specifically cell migration and restoration of mono- or multilayered cells (17). It is possible that TFF2 may be play a role at lower expression levels; is rapidly turned over, depending on age; or is impacted by specific diseases (e.g., asthma in Table 1). In contrast, TFF2 is highly expressed in the mouse lung (18), which lacks submucosal glands in the distal airways (19) (Figure 1E). TFF2 immunostaining is limited to “occasional” bronchial epithelial cells but becomes more intense and localized to cytoplasm and mucous vacuoles of bronchial epithelial cells after OVA (ovalbumin) exposure (20). Deletion experiments suggest that immune-cell expression of TFF2 also contributes to lung function at a steady state (21). TFF2 is upregulated during helminth infection, early allergen exposure (20, 22), or chemical injury (21, 23), suggesting that it plays an important role in airway repair.
TFF2 deficiency did not impair goblet-cell numbers, epithelial thickness, or normal immune-cell content in BAL fluid (BALF) but did cause significant thickening of the subepithelial layer because of increased deposition of collagen (20, 22), although this was not replicated by others (23). We have observed that TFF2 deficiency is also associated with enlarged alveoli and hypoxia at a steady state, likely because of the combined lack in hematopoietic and nonhematopoietic cells (21). TFF2 deficiency has different consequences depending on the allergic airway model examined. In the OVA model of allergic asthma, a lack of TFF2 did not alter tissue remodeling, mucus secretion, or lung function (22). TFF2 deficiency reduced airway hyperresponsiveness, eosinophil infiltration, and mucus production in allergic asthma models (house dust mite and exogenous IL-13) (16) and exacerbated naphthalene-induced lung injury (23). That exogenous TFF2 in healthy mice produced cellular features reminiscent of allergic airway responses (16) but alleviated pathology in the OVA model (20) suggests that a deeper understanding of TFF2’s biological functions is needed to determine its therapeutic potential.
TFFs, Mucins, and Airway Resistance
TFFs are co-secreted with mucins (6, 8) and are believed to enhance mucociliary clearance. TFFs can increase mucous viscosity by binding with the terminal disaccharide residue of mucin glycoproteins (24) (Figure 2A). However, thick mucus obstructs small airways and increases resistance, impeding airflow in diseases such as COPD, in which high TFF3 and TFF1 levels in BALF are associated with low forced expiratory volume (14). Both TFF1 and TFF3 colocalized with the mucin MUC5AC in the nasal epithelium and colocalized with MUC5B in the epithelium and submucosal glandular cells (13) (Figure 2A). MUC5B, rather than MUC5AC, is indispensable for bacterial clearance from the lungs (25), which may suggest that the association of TFFs 1 and 3 with MUC5B promotes this function. TFFs accumulate around bacterial pathogens in tissues or sputum, and TFF upregulation is associated with the presence of specific bacteria (6, 12). In cystic fibrosis, TFF1 was found in sputum and TFF3 was found in bronchus tissue surrounding Staphylococcus aureus (6) (Figure 2A). In patients with CRSwNP, TFF3 upregulation in the polyp and middle turbinate tissue was associated with the presence of S. epidermis as compared with patients with sterile nasal swabs (12). Thus, either alone or with mucins, TFFs may augment bacterial clearance but exacerbate COPD.
TFFs and Cell Motility
Newly generated cells migrate into damaged regions (26) (Figure 2B). TFF2 and TFF3 induce the migration of cultured human bronchial epithelial cells synergistically with EGF (epidermal growth factor) (17), using different intracellular signaling cascades (27), suggesting that TFFs do not signal through EGFR (EGF receptor). Moreover, TFF-induced migration is enhanced by extracellular matrix substrates like fibronectin and collagen type I (27). During regeneration from H1N1 influenza infection in mice, there is a dramatic upregulation of TFF2 transcripts in extremely mobile progenitor cells that give rise to a dysplastic epithelium (28, 29). Whether TFF2 expression is also elevated in progenitors that contribute to euplastic regeneration remains unknown.
TFFs, Cell Proliferation and Differentiation, and Cancer
Cell proliferation and differentiation are also critical for the repair of damaged mucosal epithelium (26). Exogenous TFF3 promoted ciliogenesis in the primary human airway epithelia via induction of the transcription factor FOXJ1 (30) (Figure 2C). Club cells are nonciliated secretory cells (Figure 1) vulnerable to naphthalene injury (31) and have a stem-cell function that regenerates the lung epithelium in vitro (32, 33). Early after naphthalene injury, TFF1 and TFF3 are highly expressed in degenerating club cells within the bronchoalveolar duct junctions (18), a stem-cell niche (34). TFF-expressing stem cells could aid repair after injury. Club cells also express TFF1 after OVA or Aspergillus fumigatus allergen exposure, which may implicate TFF1 in their differentiation into goblet cells (35, 36). TFF2 released by alveolar macrophages is required for epithelial repair during bleomycin-induced injury and parasitic helminth infection and is dependent on Wnt4 and Wnt16 expression (37) (Figures 2B and 2C). Taken together, these findings suggest that TFFs may contribute to airway epithelial repair by signaling proliferation and differentiation of progenitors into mucus-secreting cells.
Unchecked regeneration produces cancer, which is also associated with TFFs (14, 38–41). TFF3 immunoreactivity is found in adenocarcinoma (AD), but not squamous cell carcinoma, and is required to maintain the AD phenotype (40). The combination of novel TFF3 and MEK1/2 kinase inhibitors were able to suppress AD cell growth (41). TFF1 expression is also preferentially associated with AD, but overexpression in cancer cell lines may suppress proliferation (38). TFF2 is downregulated in lung ADs from nonsmokers as compared with those from smokers (39). TFF1 and TFF2, but not TFF3, were elevated in BALF from patients with lung cancer (mostly smokers), and for TFF2, this was associated with metastatic cancer (14) (Table 1). TFF2 may be a biomarker for invasive mucinous AD, a rare but highly lethal subtype of non–small-cell lung cancer (42). Tumors lack the lung-specifying transcription factor NKX2.1, and co-deletion of NKX2.1 and K-Ras recapitulates this cancer in mice, which appear to revert to a gastric-like architecture (42). TFF2 might contribute to invasiveness by enhancing cell motility. It has been suggested that chronic inflammation and overblown TFF repair could contribute to colorectal cancer (43). Perhaps this could also occur in the airway. Disentangling the mechanisms of mucosal healing from cancer progression will be critically important to determine how TFFs may be targeted to best therapeutic effect.
TFFs and Inflammation
TFF2 expression can be acutely induced by type 2 cytokines (IL-4 and IL-13) or Aspergillus in the lung by STAT6-dependent processes and can be maintained by STAT6-independent mechanisms during chronic exposure (44). In gastrointestinal mucus-producing cell lines, IL-4 and IL-13 similarly upregulate TFF3 in a STAT6-dependent manner (45), but whether this occurs in the airway mucosa is unknown. TFFs can also modulate inflammation (16, 21, 46, 47) (Figure 2D). When TNFα is present in culture, both TFF2 and TFF3 augment IL-6 and IL-8 release by human bronchiolar epithelial cells but do not do so alone (46). TFFs are believed to augment intracellular signaling cascades that drive IL-6 and IL-8 secretion (e.g., phosphorylation of ERK1/2) (46). Evidence in mouse parasitic infections suggests that TFF2 may help to skew responses toward type 2 immune responses and may hinder some aspects of type 1 immune responses, characterized by IL-12 signaling (16, 21, 47). Given that type 2 cytokines induce TFF2, this may constitute an autocrine circuit. TFF modulation of mucosal immune responses may have consequences for airway function in the context of infection or disease.
TFF Receptors
TFF signaling through canonical receptor–ligand interactions has been long debated. TFF2 requires CXCR4 (CXC receptor 4) for calcium mobilization and ERK1/2 and AKT activation in vitro, two downstream signaling cascades of TFFs (48, 49) (Figure 3A). CXCR4 dimerizes with CXCR7, and these receptors are required for TFF3-mediated cell migration but do not mediate cell proliferation or MAPK activation (50). However, direct binding between TFFs and CXCRs has not been demonstrated. TFF3 binds to CD147 (basigin or emmprin), which seems to mediate both mucosal restitution after injury and promotion of colorectal cancer (43). Both of these effects were attributed to a TFF3-enhanced CD147–CD44 interaction, leading to phosphorylation of STAT3 and expression of PTGS2 (prostaglandin G/H synthase 2) (43). Given that CD147, CD44, and EGFR form a signaling complex that promotes invasion of breast cancer cells (51), such associations may be important to examine in the context of lung carcinomas.
We show several lines of evidence implicating LINGO2 and LINGO3 as the receptors of TFF3 and TFF2, respectively (52, 53), including verification of direct LINGO2–TFF3 binding (52) (Figure 3B). In the gastrointestinal tract, TFF3 binding to LINGO2 removes it from EGFR, allowing EGFR signaling to occur (52). We also demonstrate that LINGO3 is required for exogenous TFF2 to speed airway epithelial repair in culture and that both are located in the nasal polyp epithelium (53). Given the newly identified TFF3–CD147 association and a known role for CD147–CD44–EGFR in cancer, it will be important to know how TFF–LINGO interactions affect these dynamics and if they might promote healing without increased risk of cancer.
Conclusions/Outstanding Questions
TFFs contribute to tissue repair by regulating mucus secretion and viscosity, promoting cell motility and differentiation and regulating inflammation. To perform these functions, TFFs can bind to mucins and membrane-bound receptors. We still do not completely understand how TFF secretion is regulated or how far TFFs travel from the cell of origin to perform effector signaling functions. This could have implications for targeting mucous viscosity versus immune signaling. Canonically, we think of TFFs as being secreted from epithelial or mucus-secreting cells, but immune-cell expression also seems to be critical to epithelial repair and should be explored further.
A major roadblock to therapeutic use is our poor understanding of mechanisms responsible for appropriate injury repair versus cancer progression. If one mechanism is responsible for both, how can we enhance repair without increasing the risk of cancer? Direct activation or inhibition of TFFs may not be the best approach. It may be better to target binding partners or combine TFF-targeted drugs with others, as in the study by Zhang and colleagues (41). Antagonizing TFF signaling could abrogate tumor growth or reduce mucus thickening in airway disease, whereas potentiating TFF signaling could promote tissue repair during discrete stages of injury. However, more questions need to be answered to move forward safely.
Acknowledgments
Acknowledgment
Figures were generated using BioRender.
Footnotes
Supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grants U01AI163062 and R01AI164715 (D’B.R.H.).
Originally Published in Press as DOI: 10.1165/rcmb.2021-0373TR on November 16, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gilmour MI, Koren HS. In: Gehr P, Heyder J, editors. New York, NY: Marcel Dekker; 2000. Interaction of inhaled particles with the immune system; pp. 626–652. [Google Scholar]
- 2. Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science . 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thim L. A new family of growth factor-like peptides. ‘Trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins) FEBS Lett . 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- 4. Muskett FW, May FE, Westley BR, Feeney J. Solution structure of the disulfide-linked dimer of human intestinal trefoil factor (TFF3): the intermolecular orientation and interactions are markedly different from those of other dimeric trefoil proteins. Biochemistry . 2003;42:15139–15147. doi: 10.1021/bi030182k. [DOI] [PubMed] [Google Scholar]
- 5. Williams MA, Westley BR, May FE, Feeney J. The solution structure of the disulphide-linked homodimer of the human trefoil protein TFF1. FEBS Lett . 2001;493:70–74. doi: 10.1016/s0014-5793(01)02276-1. [DOI] [PubMed] [Google Scholar]
- 6. dos Santos Silva E, Ulrich M, Döring G, Botzenhart K, Gött P. Trefoil factor family domain peptides in the human respiratory tract. J Pathol . 2000;190:133–142. doi: 10.1002/(SICI)1096-9896(200002)190:2<133::AID-PATH518>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7. Lee SH, Lee SH, Oh BH, Lee HM, Choi JO, Jung KY. Expression of mRNA of trefoil factor peptides in human nasal mucosa. Acta Otolaryngol . 2001;121:849–853. doi: 10.1080/00016480152602320. [DOI] [PubMed] [Google Scholar]
- 8. Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem . 2007;55:505–513. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 9. Li P, Turner JH. Chronic rhinosinusitis without nasal polyps is associated with increased expression of trefoil factor family peptides. Int Forum Allergy Rhinol . 2014;4:571–576. doi: 10.1002/alr.21334. [DOI] [PubMed] [Google Scholar]
- 10. Sands BE, Podolsky DK. The trefoil peptide family. Annu Rev Physiol . 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 11. Stürmer R, Müller S, Hanisch FG, Hoffmann W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell Physiol Biochem . 2014;33:895–904. doi: 10.1159/000358662. [DOI] [PubMed] [Google Scholar]
- 12. Mihalj M, Bujak M, Butković J, Zubčić Ž, Tolušić Levak M, Čes J, et al. Differential expression of TFF1 and TFF3 in patients suffering from chronic rhinosinusitis with nasal polyposis. Int J Mol Sci . 2019;20:5461. doi: 10.3390/ijms20215461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyahara N, Ishino T, Kono T, Go K, Takeno S, Takumida M, et al. Expression of trefoil factor family peptides in the nasal allergic mucosa. Rhinology . 2012;50:408–416. doi: 10.4193/Rhino11.221. [DOI] [PubMed] [Google Scholar]
- 14. Viby NE, Nexø E, Kissow H, Andreassen H, Clementsen P, Thim L, et al. Trefoil factors (TFFs) are increased in bronchioalveolar lavage fluid from patients with chronic obstructive lung disease (COPD) Peptides . 2015;63:90–95. doi: 10.1016/j.peptides.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 15. Viby NE, Pedersen L, Lund TK, Kissow H, Backer V, Nexø E, et al. Trefoil factor peptides in serum and sputum from subjects with asthma and COPD. Clin Respir J . 2015;9:322–329. doi: 10.1111/crj.12146. [DOI] [PubMed] [Google Scholar]
- 16. Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med . 2012;209:607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oertel M, Graness A, Thim L, Bühling F, Kalbacher H, Hoffmann W. Trefoil factor family-peptides promote migration of human bronchial epithelial cells: synergistic effect with epidermal growth factor. Am J Respir Cell Mol Biol . 2001;25:418–424. doi: 10.1165/ajrcmb.25.4.4429. [DOI] [PubMed] [Google Scholar]
- 18. Greeley MA, Van Winkle LS, Edwards PC, Plopper CG. Airway trefoil factor expression during naphthalene injury and repair. Toxicol Sci . 2010;113:453–467. doi: 10.1093/toxsci/kfp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dajani R, Zhang Y, Taft PJ, Travis SM, Starner TD, Olsen A, et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol . 2005;32:548–552. doi: 10.1165/rcmb.2005-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Royce SG, Lim C, Muljadi RC, Samuel CS, Ververis K, Karagiannis TC, et al. Trefoil factor-2 reverses airway remodeling changes in allergic airways disease. Am J Respir Cell Mol Biol . 2013;48:135–144. doi: 10.1165/rcmb.2011-0320OC. [DOI] [PubMed] [Google Scholar]
- 21. Hung LY, Oniskey TK, Sen D, Krummel MF, Vaughan AE, Cohen NA, et al. Trefoil factor 2 promotes type 2 immunity and lung repair through intrinsic roles in hematopoietic and nonhematopoietic cells. Am J Pathol . 2018;188:1161–1170. doi: 10.1016/j.ajpath.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikolaidis NM, Wang TC, Hogan SP, Rothenberg ME. Allergen induced TFF2 is expressed by mucus-producing airway epithelial cells but is not a major regulator of inflammatory responses in the murine lung. Exp Lung Res . 2006;32:483–497. doi: 10.1080/01902140601059547. [DOI] [PubMed] [Google Scholar]
- 23. Royce SG, Li X, Tortorella S, Goodings L, Chow BS, Giraud AS, et al. Mechanistic insights into the contribution of epithelial damage to airway remodeling: novel therapeutic targets for asthma. Am J Respir Cell Mol Biol . 2014;50:180–192. doi: 10.1165/rcmb.2013-0008OC. [DOI] [PubMed] [Google Scholar]
- 24. Järvå MA, Lingford JP, John A, Soler NM, Scott NE, Goddard-Borger ED. Trefoil factors share a lectin activity that defines their role in mucus. Nat Commun . 2020;11:2265. doi: 10.1038/s41467-020-16223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature . 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci . 2005;62:2932–2938. doi: 10.1007/s00018-005-5481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chwieralski CE, Schnurra I, Thim L, Hoffmann W. Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am J Respir Cell Mol Biol . 2004;31:528–537. doi: 10.1165/rcmb.2003-0433OC. [DOI] [PubMed] [Google Scholar]
- 28. Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature . 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol . 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LeSimple P, van Seuningen I, Buisine MP, Copin MC, Hinz M, Hoffmann W, et al. Trefoil factor family 3 peptide promotes human airway epithelial ciliated cell differentiation. Am J Respir Cell Mol Biol . 2007;36:296–303. doi: 10.1165/rcmb.2006-0270OC. [DOI] [PubMed] [Google Scholar]
- 31. Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, et al. Relationship of cytochrome P450 activity to Clara cell cytotoxicity: IV. metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol . 1995;47:74–81. [PubMed] [Google Scholar]
- 32. Hegab AE, Kubo H, Fujino N, Suzuki T, He M, Kato H, et al. Isolation and characterization of murine multipotent lung stem cells. Stem Cells Dev . 2010;19:523–536. doi: 10.1089/scd.2009.0287. [DOI] [PubMed] [Google Scholar]
- 33. Rokicki W, Rokicki M, Wojtacha J, Dżeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol . 2016;13:26–30. doi: 10.5114/kitp.2016.58961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet . 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cui YH, Wang YY, Liu Z. Transdifferentiation of Clara cell 10-kDa protein secreting cells in experimental allergic rhinitis. Am J Rhinol Allergy . 2011;25:145–151. doi: 10.2500/ajra.2011.25.3596. [DOI] [PubMed] [Google Scholar]
- 36. Kouznetsova I, Chwieralski CE, Bälder R, Hinz M, Braun A, Krug N, et al. Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model. Am J Respir Cell Mol Biol . 2007;36:286–295. doi: 10.1165/rcmb.2006-0008OC. [DOI] [PubMed] [Google Scholar]
- 37. Hung LY, Sen D, Oniskey TK, Katzen J, Cohen NA, Vaughan AE, et al. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a trefoil factor 2-dependent mechanism. Mucosal Immunol . 2019;12:64–76. doi: 10.1038/s41385-018-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Minegishi K, Dobashi Y, Tsubochi H, Hagiwara K, Ishibashi Y, Nomura S, et al. TFF-1 functions to suppress multiple phenotypes associated with lung cancer progression. OncoTargets Ther . 2021;14:4761–4777. doi: 10.2147/OTT.S322697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sui Q, Liang J, Hu Z, Chen Z, Bi G, Huang Y, et al. Genetic and microenvironmental differences in non-smoking lung adenocarcinoma patients compared with smoking patients. Transl Lung Cancer Res . 2020;9:1407–1421. doi: 10.21037/tlcr-20-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang XN, Wang SJ, Pandey V, Chen P, Li Q, Wu ZS, et al. Trefoil factor 3 as a novel biomarker to distinguish between adenocarcinoma and squamous cell carcinoma. Medicine (Baltimore) . 2015;94:e860. doi: 10.1097/MD.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang M, Wang B, Chong QY, Pandey V, Guo Z, Chen RM, et al. A novel small-molecule inhibitor of trefoil factor 3 (TFF3) potentiates MEK1/2 inhibition in lung adenocarcinoma. Oncogenesis . 2019;8:65. doi: 10.1038/s41389-019-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tata PR, Chow RD, Saladi SV, Tata A, Konkimalla A, Bara A, et al. Developmental history provides a roadmap for the emergence of tumor plasticity. Dev Cell . 2018;44:679–693, e5. doi: 10.1016/j.devcel.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cui HY, Wang SJ, Song F, Cheng X, Nan G, Zhao Y, et al. CD147 receptor is essential for TFF3-mediated signaling regulating colorectal cancer progression. Signal Transduct Target Ther . 2021;6:268. doi: 10.1038/s41392-021-00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolaidis NM, Zimmermann N, King NE, Mishra A, Pope SM, Finkelman FD, et al. Trefoil factor-2 is an allergen-induced gene regulated by Th2 cytokines and STAT6 in the lung. Am J Respir Cell Mol Biol . 2003;29:458–464. doi: 10.1165/rcmb.2002-0309OC. [DOI] [PubMed] [Google Scholar]
- 45. Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, et al. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol . 2004;172:3775–3783. doi: 10.4049/jimmunol.172.6.3775. [DOI] [PubMed] [Google Scholar]
- 46. Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffmann W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem . 2002;277:18440–18446. doi: 10.1074/jbc.M200468200. [DOI] [PubMed] [Google Scholar]
- 47. McBerry C, Egan CE, Rani R, Yang Y, Wu D, Boespflug N, et al. Trefoil factor 2 negatively regulates type 1 immunity against Toxoplasma gondii. J Immunol . 2012;189:3078–3084. doi: 10.4049/jimmunol.1103374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem . 2009;284:3650–3662. doi: 10.1074/jbc.M804935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Engevik KA, Hanyu H, Matthis AL, Zhang T, Frey MR, Oshima Y, et al. Trefoil factor 2 activation of CXCR4 requires calcium mobilization to drive epithelial repair in gastric organoids. J Physiol . 2019;597:2673–2690. doi: 10.1113/JP277259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dieckow J, Brandt W, Hattermann K, Schob S, Schulze U, Mentlein R, et al. CXCR4 and CXCR7 mediate TFF3-induced cell migration independently from the ERK1/2 signaling pathway. Invest Ophthalmol Vis Sci . 2016;57:56–65. doi: 10.1167/iovs.15-18129. [DOI] [PubMed] [Google Scholar]
- 51. Grass GD, Tolliver LB, Bratoeva M, Toole BP. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J Biol Chem . 2013;288:26089–26104. doi: 10.1074/jbc.M113.497685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Belle NM, Ji Y, Herbine K, Wei Y, Park J, Zullo K, et al. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat Commun . 2019;10:4408. doi: 10.1038/s41467-019-12315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zullo KM, Douglas B, Maloney NM, Ji Y, Wei Y, Herbine K, et al. LINGO3 regulates mucosal tissue regeneration and promotes TFF2 dependent recovery from colitis. Scand J Gastroenterol . 2021;56:791–805. doi: 10.1080/00365521.2021.1917650. [DOI] [PMC free article] [PubMed] [Google Scholar]