Abstract
Background
Although clinical trials showed that vaccines have high efficacy and safety, differences in study designs and populations do not allow for comparison between vaccines and age groups. The objective of this study was to evaluate the effectiveness of vaccines against COVID-19 in real-world conditions in adults aged 60 years and older in Colombia.
Methods
In this retrospective, population-based, matched cohort study, we evaluated the effectiveness of vaccines against COVID-19-related hospitalisation and death in people aged 60 years and older. The full cohort consisted of every person who was eligible to receive a COVID-19 vaccine in Colombia (the ESPERANZA cohort). The exposed cohort consisted of older adults who were fully vaccinated with Ad26.COV2-S, BNT162b2, ChAdOx1 nCoV-19, or CoronaVac, and who did not have a history of confirmed SARS-CoV-2 infection. The unexposed cohort were people aged 60 years and older who had not received any dose of a COVID-19 vaccine during the study period. Participant follow-up was done between March 11, 2021, and Oct 26, 2021. Vaccine effectiveness was estimated as 1– hazard ratio from cause-specific proportional hazards models in the presence of competing risks. We estimated the overall effectiveness of being fully vaccinated, as well as effectiveness for each vaccine, adjusting by main potential confounders. The effectiveness of each vaccine was also assessed by age groups (ages 60–69 years, 70–79 years, and ≥80 years).
Findings
2 828 294 participants were assessed between March 11 and Oct 26, 2021. For all ages, the overall effectiveness across all assessed COVID-19 vaccines at preventing hospitalisation without subsequent death was 61·6% (95% CI 58·0–65·0, p<0·0001), 79·8% (78·5–81·1, p<0·0001) for preventing death after hospitalisation with COVID-19, and 72·8% (70·1–75·3, p<0·0001) for preventing death without previous COVID-19 hospitalisation. The effectiveness of all vaccines analysed at preventing death after hospitalisation for COVID-19 was 22·6% lower in adults who were aged 80 and older (68·4% [65·7–70·9], p<0·0001) compared with adults aged between 60 and 69 years (91·0% [89·0–92·6], p<0·0001).
Interpretation
All vaccines analysed in this study were effective at preventing hospitalisation and death from COVID-19 in fully vaccinated older adults, which is a promising result for the national vaccination programme against COVID-19 in Colombia and in countries where these biologics have been applied. Efforts should be improved to increase coverage among older adults. In addition, given that we observed that the effectiveness of vaccines declined with increasing age, a booster dose is also justified, which should be prioritised for older adults.
Funding
Colombian Ministry of Health and Social Protection.
Introduction
In addition to high rates of mortality,1 the COVID-19 pandemic has generated one of the largest social, economic, and health crises in recent history, exacerbating social inequalities between and within countries. With the availability of vaccines with proven safety and efficacy, which were developed in record time, vaccination, together with non-pharmacological measures, became an essential resource to manage the pandemic and to control the spread of the virus.
People who are 60 years and older have been shown to be more likely than younger people to have severe COVID-19, require hospitalisation, and die from COVID-19.2, 3 On Feb 23, 2021, Colombia's national vaccination plan against COVID-19 prioritised vaccinating health-care staff and older adults, with the first doses for this age group given to people aged 80 years and older.
Colombia has a diverse portfolio of vaccines for COVID-19 that were procured mainly on the basis of delivery timelines and the number of doses that producers offered. Colombia has also negotiated supply agreements with five pharmaceutical companies and has adhered to the COVAX procurement mechanism. Among the purchased vaccines are Ad26.COV2-S, BNT162b2, ChAdOx1 nCoV-19, CoronaVac, and mRNA-1273. Vaccine roll-out was done according to availability in Colombia. Although BNT162b2 was the first vaccine to arrive (in February, 2021), CoronaVac was the first to be available in considerable amounts to immunise people aged 60 years and older, therefore it became the most frequently used biologic in this age group. On the contrary, the mRNA-1273 vaccine was the last to be available (in July, 2021), and although some older adults were immunised with this biologic, we did not include these adults in this study because of the short time window available to observe the outcomes of interest.
Research in context.
Evidence before this study
We searched OVID MEDLINE and MedRxiv on Dec 15, 2021, to identify studies on vaccine effectiveness against COVID-19 in people aged ≥60 years using the search terms “Effectiveness”, “COVID-19”, “cohort studies”, and “Older adults”, without date, language, or article type restrictions. In Spain, a large cohort study found that the effectiveness of mRNA COVID-19 vaccines at preventing hospitalisations in institutionalised people aged ≥60 years when fully vaccinated was 88·4% (95% CI 74·9–94·7) and 97·0% (91·7–98·9) for preventing deaths. Similar results were found in Catalonia in a retrospective cohort study that analysed 28 456 nursing home residents who were vaccinated with BNT162b2, which showed an adjusted effectiveness in people who were fully vaccinated of 95·0% (93·0–96·0) for preventing hospital admission and 97·0% (96·0–98·0) for preventing death by COVID-19. Also, a cohort study was conducted in Portugal in 1·8 million people who were aged 65 years and older to evaluate the effectiveness of mRNA vaccines against COVID-19 hospitalisations and deaths. This study found a reduction in the risk of hospitalisation in fully vaccinated older adults of 59·0% (95% CI 32·0–76·0) and a reduction in the risk of death in the same population of 81·0% (73·0–87·0). In a national cohort of people aged 16 years or older in Chile who were immunised with CoronaVac, the subgroup of older adults (aged ≥60 years) who were fully immunised had an adjusted effectiveness of 89·2% (87·6–90·6) for preventing the admission to the intensive care unit, and 86·5% (84·6–88·1) for preventing COVID-19-related death. Other studies that evaluated the effectiveness of vaccines in older adults did not analyse effectiveness in fully vaccinated older adults or did not have a sufficient follow-up time to assess the effectiveness in preventing death. The studies found present methodological differences (including the study population and predominant variant during the period of analysis). Furthermore, published articles focused on the effectiveness of mRNA COVID-19 vaccines. Only few studies were available that compared the effectiveness of various vaccines in older populations, including various vaccine platforms.
Added value of this study
This study presents real-world evidence for the effectiveness of Ad26.COV2-S, BNT162b2, ChAdOx1 nCoV-19, and CoronaVac vaccines, disaggregated by vaccine and age group, in people aged 60 years and older who were fully vaccinated and had no previous confirmed history of SARS-CoV-2 infection. Our results suggest that increasing age reduces the effectiveness of immunisation against COVID-19 and that the vaccine or vaccine platform used might also be associated with reduced effectiveness.
Implications of all the available evidence
In this study we provide evidence of reduced effectiveness of COVID-19 vaccines in people aged 70 years and older in Colombia, after adjusting for many confounders. Other studies have shown that additional doses rapidly increase antibody titters, hence offering an additional dose to those at higher risk of breakthrough infection might increase protection. These results support the use of a booster dose in people aged 60 years and older, regardless of the vaccine used.
These vaccines have shown their efficacy and safety in several clinical trials, hence being approved for emergency use in Colombia.4, 5, 6, 7, 8 All of these vaccines showed a wide range in efficacy in preventing a range of COVID-19 severities, but estimates for preventing severe COVID-19 and death were highly uncertain because of the low sample size and low frequency of these outcomes in these trials. Therefore, it is necessary to study the effectiveness of vaccines in uncontrolled real-life conditions, especially in a highly clinically vulnerable population. Colombia's diverse portfolio of vaccines makes it a good setting for evaluating the effectiveness of various vaccines across age groups.
In our study, we aimed to compare the effectiveness of Ad26.COV2-S, BNT162b2, ChAdOx1 nCoV-19, and CoronaVac at preventing hospitalisation and death in people aged 60 years and older who were fully vaccinated and who had no previously confirmed history of SARS-CoV-2 infection (according to the health information systems of Colombia), when the mu variant (B.1.621) was the most prevalent variant in the country. We aimed for evidence generated in this study to inform the comprehensive evaluation of the national vaccination plan against COVID-19 in Colombia and to support decisions made by the Ministry of Health.
Methods
Study design and participants
We conducted a population-based, match-paired cohort study to assess the effectiveness of a complete scheme (ie, all required doses recommended in the manufacturer's guidelines) of COVID-19 vaccination in people aged 60 years and older in Colombia without a confirmed history of SARS-CoV-2 infection. The data used in this study was collected by the Ministry of Health and Social Protection of Colombia and the National Institute of Health.
The full cohort consisted of every person who was eligible to receive a COVID-19 vaccine in Colombia (the ESPERANZA cohort). In this study, we included people aged 60 years and older, which, in 2021, was projected to be 7 107 914 individuals, according to the National Administrative Department of Statistics. Data for each cohort member was identified by searching each individual's personal identification number (using an anonymised code) across several databases: (1) MiVacuna, which collected sociodemographic data of all people who were eligible to receive a COVID-19 vaccine; (2) PAIWEB, an individual-level vaccine registry; (3) SEGCOVID, which provides follow-up data of confirmed COVID-19 cases; (4) RUAF-ND, which provides a registry of all deaths, including the cause of death; and (5) the high-cost disease registry (Cuenta de Alto Costo), which provides data for patients by disease diagnosis (eg, chronic kidney disease, hypertension, diabetes, cancer, and HIV). These information sources are part of the integrated information system for social protection and satisfy the information quality standards defined in this framework. A flowchart of the database preparation is included in the appendix (p 1).
In this study, we included all people aged 60 years and older who had a complete scheme of COVID-19 vaccination or who had not received any dose of a COVID-19 vaccine. Therefore, the exposed (vaccinated) individuals in the cohort included people who had been immunised with two doses of BNT162b2, ChAdOx1 nCoV-19, or CoronaVac, or with one dose of Ad26.COV2-S; the unexposed (non-vaccinated) individuals in the cohort consisted of people who had not received any dose of a COVID-19 vaccine during the entire study period. We excluded individuals with a confirmed diagnosis of COVID-19 before enrolment in the cohort (we used SEGCOVID to identify those individuals who had a previous confirmed SARS-CoV-2 infection), as well as individuals with heterologous vaccination, and those who were diagnosed, hospitalised, or who had died from COVID-19 within the 14 days following any dose of a vaccine. We also excluded individuals who had incomplete records.
This study complies with the scientific, technical, and administrative regulation for human health research in Colombia, which classifies this study as research without risk as it only used secondary data sources of anonymised information. This study does not therefore require the review or approval of a research ethics committee.
Procedures
Individuals who met the inclusion criteria were first allocated to groups according to their characteristics (ie, potential confounders): sex; age at the time of vaccination (for those vaccinated; in one-year categories); being diagnosed with cancer, diabetes, chronic kidney disease, hypertension, or HIV, which have been shown to be risk factors for severe COVID-19 and mortality from COVID-19;9, 10 health system regime affiliation (contributory or subsidised); and municipality of residence (or department of residence when municipality was missing). For identifying the covariates that should be considered in the matching and adjusting process, we created a directed acyclic graph. These groups were then separated into the exposed and the unexposed groups. Individuals in each group (exposed and unexposed) were assigned a random number from a uniform distribution (from zero to the last number, indicating the last individual in each subgroup that shares the same characteristics given by the matching variables). For each group, random numbers were generated, and a couple was generated by matching each individual of the exposed cohort, as the individual was vaccinated, with an individual randomly selected from the unvaccinated cohort group that had the same characteristics given by the variables used for matching (ie, sex, age at the time of vaccine for those vaccinated [in 1-year categories]; being diagnosed with cancer, diabetes, chronic kidney disease, hypertension, or HIV diagnosis; health system affiliation regime [contributory or subsidised]; and municipality of residence [or department of residence when municipality was missing]). The unvaccinated individual was therefore assigned the same start of follow-up as their vaccinated counterpart. When the unvaccinated individual had an event (hospitalisation or death) before 14 days after the vaccination of their vaccinated counterpart, that unvaccinated individual was replaced by the next unvaccinated individual in the defined order. This process was done iteratively until all the possible pairs were formed within each group. Individuals who were not matched were excluded from the analysis and no one was matched more than once.
Outcomes
The primary outcomes of interest in this study were hospitalisations and deaths from COVID-19. We used the definitions recommended by WHO for surveillance of COVID-19.11 Death from COVID-19 was defined as death that resulted from clinically compatible illness in a probable or confirmed COVID-19 case, unless there was a clear alternative cause of death that could not be related to COVID-19, without a defined period of complete recovery between illness and death.12
During the observation period, each individual provided a specific follow-up duration. The follow-up duration for each vaccinated–unvaccinated pair began 15 days after the vaccinated individual in the pair received their last dose (the time period for the vaccine to induce the immune response), the day of the event (hospitalisation or death from COVID-19), or individual censoring. We had three types of right-censoring: people who died from causes other than COVID-19, those who received a booster dose of the vaccine, and those who finished the follow-up and observation period without having presented the outcome of interest. Furthermore, to control for immortal time bias, outcomes that occurred within 14 days of completion of the vaccination scheme were not considered in the analysis.
Statistical analysis
We conducted a descriptive analysis of participants according to the exposure (ie, whether a vaccine was received, and type of vaccine received). Quantitative variables were summarised as median and IQR, and qualitative variables as percentages. We also used Kaplan-Meier estimates and risk tables to describe time to hospitalisation and time to death from COVID-19 across the entire cohort, by age groups (ie, ages 60–69 years, 70–79 years, and ≥80 years), and by vaccine received.
Because events compete over time (ie, hospitalisation with death as a competing risk), we used a competing risk survival analysis by creating a cause-specific Cox regression model with time of the event or the censored time-to-event. From these data we identified three possible outcomes: (1) hospitalisation without subsequent death; (2) death after hospitalisation for COVID-19; and (3) death without a previous record of hospitalisation for COVID-19.
Vaccine effectiveness was estimated as 1 – hazard ratio (HR) from cause-specific proportional hazards models in the presence of competing risks. First, we estimated overall effectiveness (across all vaccines) by including a single exposure variable in the model (ie, being vaccinated or unvaccinated) and the matched pairs as a stratum, which allowed us to include the correlation structure among pairs. Thereafter, given that the specific vaccine was not included in the matching process, we estimated the effectiveness for each vaccine and age group in separate models that were adjusted for sex, age, diagnosis of hypertension, diabetes, cancer, kidney disease, or HIV, and health system affiliation regime. Municipality of residence was included as a random intercept (in these models, we did not include the matched pairs as a stratum). For identifying the covariates that should be considered in the matching and adjusting process, we created a directed acyclic graph.
We verified the proportional hazards assumptions by checking the logarithms of the accumulated risks for vaccinated and unvaccinated cohorts, confirming that differences between the curves were constant over time. We also evaluated if the log (survival) and log (t) curves were parallel, and we did two sensitivity analyses for misclassification bias. For the first analysis, we randomly changed a proportion of non-vaccinated individuals to being fully vaccinated with any vaccine, which was done to explore how much vaccine effectiveness would change if we had 25%, 50%, and 75% of registered non-vaccinated individuals as fully vaccinated, which addresses problems such as a lag in reporting vaccination. The second sensitivity analysis tested the assumption that the 206 607 individuals with missing information on the first vaccination dose (who were excluded from the main analysis), were, in fact, fully vaccinated individuals (appendix pp 2–5).
We used R (version 4.1.0), survival packages 3.2 to 11 (to estimate Kaplan-Meier functions) and risk regression (version 2020.12.08, for the competing risk Cox model) for the analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The 230-day observation period ran from March 11 to Oct 26, 2021, during which each individual provided a specific follow-up time between the 15th day after completion of the vaccination schedule of the exposed individual in the couple and the day of the individual event or date of censoring. The median time of follow-up was 118 days (IQR 88–156), with longer follow-up time for individuals vaccinated with CoronaVac and shorter time for individuals who received ChAdOx1 nCoV-19 and Ad26.COV2-S. Of the individuals aged 60 years and older who were prioritised to receive a COVID-19 vaccine, we excluded 1 564 092 records because these individuals died before the study observation period, had data quality issues, or met at least one exclusion criterion (appendix p 1). The last consultation of any of these databases was made on Nov 3, 2021.
We analysed a total of 2 828 294 individuals, who were assigned in a 1:1 ratio to the exposed group or to the unexposed group matched by sex, age, health system affiliation status, presence of comorbidities (ie, hypertension, diabetes, chronic kidney disease, HIV, or cancer), municipality of residence, and an approximation of follow-up time.
Hence, because of the matching process, vaccinated and unvaccinated individuals had comparable sociodemographic and clinical characteristics. However, there were differences in these characteristics according to the vaccine administered (table 1 ).
Table 1.
Social, demographic, and medical characterisation of study participants by vaccine
| Ad26.COV2-S (n=64 997) | BNT162b2 (n=400 136) | ChAdOx1 nCoV-19 (n=265 730) | CoronaVac (n=683 284) | Fully vaccinated with any vaccine (n=1 414 147) | Unvaccinated (n=1 414 147) | ||
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Median, years | 65·0 (62·0–70·0) | 66·0 (63·0–69·0) | 66·0 (63·0–70·0) | 72·0 (64·0–80·0) | 68·0 (63·0–75·0) | 68·0 (63·0–75·0) | |
| Age group | |||||||
| 60–69 years | 48 553 (74·7%) | 301 302 (75·3%) | 188 934 (71·1%) | 282 196 (41·3%) | 820 205 (58·0%) | 820 205 (58·0%) | |
| 70–79 years | 13 064 (20·1%) | 87 630 (21·9%) | 69 621 (26·2%) | 219 334 (32·1%) | 390 304 (27·6%) | 390 304 (27·6%) | |
| ≥80 years | 3380 (5·2%) | 11 204 (2·8%) | 7175 (2·7%) | 181 754 (26·6%) | 203 638 (14·4%) | 203 638 (14·4%) | |
| Sex | |||||||
| Male | 34 118 (52·8%) | 175 260 (43·8%) | 128 879 (48·5%) | 308 161 (45·1%) | 646 265 (45·7%) | 64 626 (45·7%) | |
| Female | 30 679 (47·2%) | 224 876 (56·2%) | 136 851 (51·5%) | 375 123 (54·9%) | 767 882 (54·3%) | 767 882 (54·3%) | |
| Affiliation regime to the health system | |||||||
| Contributory | 12 674 (19·5%) | 194 066 (48·5%) | 104 698 (39·4%) | 263 748 (38·6%) | 575 558 (40·7%) | 575 558 (40·7%) | |
| Subsidised | 52 323 (80·5%) | 206 070 (51·5%) | 161 032 (60·6%) | 419 536 (61·4%) | 838 589 (59·3%) | 838 589 (59·3%) | |
| Comorbidities | |||||||
| At least one comorbidity | 10 725 (16·5%) | 102 435 (25·6%) | 67 230 (25·3%) | 204 302 (29·9%) | 384 648 (27·2%) | 384 648 (27·2%) | |
| Cancer | 325 (0·5%) | 4401 (1·1%) | 2657 (1·0%) | 6833 (1·0%) | 14 141 (1·0%) | 14 141 (1·0%) | |
| Diabetes | 2665 (4·1%) | 28 810 (7·2%) | 18 601 (7·0%) | 51 246 (7·5%) | 101 818 (7·2%) | 101 818 (7·2%) | |
| Chronic kidney disease | 1235 (1·9%) | 14 405 (3·6%) | 9034 (3·4%) | 38 264 (5·6%) | 62 222 (4·4%) | 62 222 (4·4%) | |
| Hypertension | 9880 (15·2%) | 93 232 (23·3%) | 61 915 (23·3%) | 192 686 (28·2%) | 357 779 (25·3%) | 357 779 (25·3%) | |
| HIV | 65 (0·1%) | 400 (0·1%) | 266 (0·1%) | 0 | 1414 (0·1%) | 1414 (0·1%) | |
Data are median (IQR) or n (%). On account of the matching process, data for vaccinated and unvaccinated cohorts are identical.
The cohort consisted of mostly women (1 535 764 [54·3%] of 2 828 294 participants), with a median age of 68 years (IQR 12 years [63–75]). 1 677 178 (59·3%) of 2 828 294 participants were affiliated to the subsidised health system regime. 769 296 (27·2%) had at least one underlying disease identified as a risk factor for becoming seriously ill and dying from COVID-19. As CoronaVac was the first vaccine to be available in the country for mass use, people vaccinated with CoronaVac were older than people who received the other vaccines (table 1).
Table 2 shows the occurrence of each main outcome by vaccine manufacturer. The median follow-up time was 118 days (IRQ 89–156) for all individuals who were fully vaccinated with any vaccine and 117 days (87–156) for all unvaccinated individuals. As Coronovac was the first vaccine available to older adults in Colombia, the longest follow-up time was for this vaccine (median 146 days; IQR 112–171). The shortest follow-up time was for ChAdOx1 nCoV-19 (70 days; 60–78 days), owing to the longer interval needed between doses to finish the schedule.
Table 2.
Occurrence of main studied outcomes through the study period by vaccine
| Ad26.COV2-S (n=64 997) | BNT162b2 (n=400 136) | ChAdOx1 nCoV-19 (n=265 730) | CoronaVac (n=683 284) | Fully vaccinated with any vaccine (n=1 414 147) | Unvaccinated (n=1 414 147) | |
|---|---|---|---|---|---|---|
| Outcomes | ||||||
| Hospitalisation without death | 17 (<1%) | 71 (<1%) | 19 (<1%) | 555 (<1%) | 662 (<1%) | 1684 (<1%) |
| Death after hospitalisation | 17 (<1%) | 56 (<1%) | 15 (<1%) | 1061 (<1%) | 1149 (<1%) | 5413 (<1%) |
| Death without hospitalisation | 2 (<1%) | 42 (<1%) | 12 (<1%) | 511 (<1%) | 567 (<1%) | 1987 (<1%) |
| Time of follow-up | ||||||
| Median, days | 88·0 (74·0–94·0) | 135·0 (110·0–151·0) | 70·0 (60·0–78·0) | 146·0 (112·0–171·0) | 118·0 (89·0–156·0) | 117·0 (87·0–156·0) |
Data are n (%) or median (IQR).
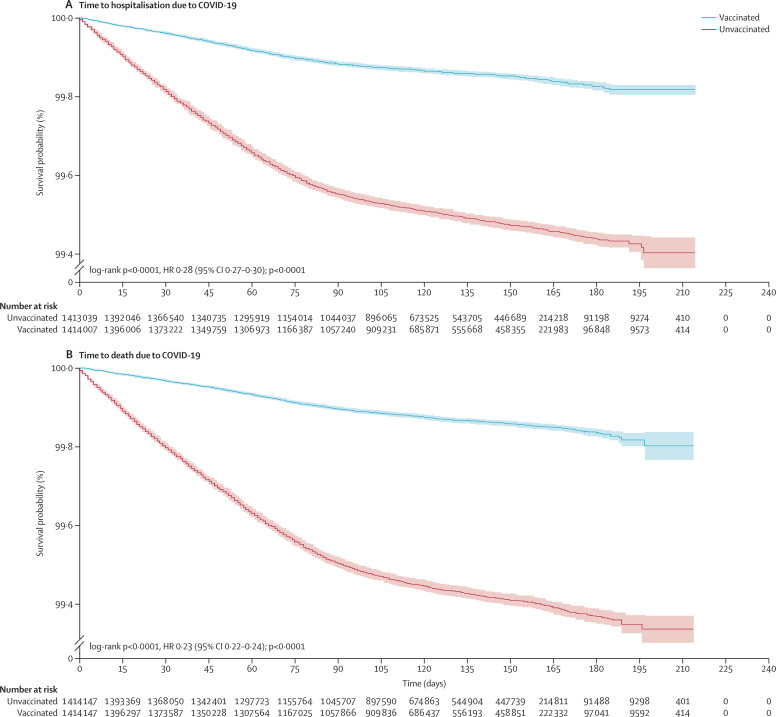
The risk of hospitalisation and death due to COVID-19 was higher in the unvaccinated cohort, as shown in the Kaplan-Meier survival curves (long rank test p<0·0001; figure 1 ).
Figure 1.
Kaplan-Meier survival curves for adults aged 60 years and older in Colombia
(A) Time to hospitalisation due to COVID-19. (B) Time to death due to COVID-19. HR=hazard ratio.
For people aged 60 years and older, the effectiveness of the COVID-19 vaccines in preventing hospitalisations and deaths ranged between 61·6% and 79·8% (table 3 ). For any vaccine, the effectiveness for preventing hospitalisation without death was 61·6% (95% CI 58·0–65·0, p<0·0001) across all age groups, with the highest effectiveness in adults aged 60–69 years (76·1% ([71·2–80·2], p<0·0001) and the lowest in adults aged 80 years and older (46·9% [38·5–54·1], p<0·0001).
Table 3.
Effectiveness of vaccines in preventing hospitalisation and death due to COVID-19 in adults aged 60 years and older in Colombia
| Hospitalisation without death (95% CI); n=2 828 294 | Death after hospitalisation (95% CI); n=2 828 294 | Death without hospitalisation (95% CI); n=2 828 294 | |
|---|---|---|---|
| Any vaccine | |||
| Total | 61·6% (58·0–65·0) | 79·8% (78·5–81·1) | 72·8% (70·1–75·3) |
| 60–69 years | 76·1% (71·2–80·2) | 91·0% (89·0–92·6) | 87·6% (83·4–90·7) |
| 70–79 years | 60·8% (54·6–66·2) | 85·0% (83·1–86·7) | 78·9% (74·6–82·4) |
| ≥80 years | 46·9% (38·5–54·1) | 68·4% (65·7–70·9) | 61·2% (56·3–65·6) |
| Ad26.COV2-S | |||
| Total | 60·9% (36·8–75·8) | 85·8% (77·1–91·2) | 95·5% (82·0–98·9) |
| 60–69 years | 45·8% (7·5–68·2) | 85·0% (69·9–92·5) | 95·0% (64·2–99·3) |
| 70–79 years | 77·9% (31·1–92·9) | 88·6% (72·5–95·3) | 93·4% (52·7–99·1) |
| ≥80 years | .. | 81·9% (51·7–93·2) | .. |
| BNT162b2 | |||
| Total | 83·0% (78·4–86·6) | 94·8% (93·3–96·0) | 88·3% (84·1–91·4) |
| 60–69 years | 84·0% (77·8–88·5) | 94·0% (91·4–95·8) | 88·1% (81·3–92·4) |
| 70–79 years | 81·3% (72·5–87·3) | 96·2% (93·9–97·6) | 89·9% 82·9–94·1) |
| ≥80 years | 79·3% (49·9–91·4) | 92·7% (85·4–96·4) | 83·4% (66·6–91·7) |
| ChAdOx1 nCoV-19 | |||
| Total | 90·8% (85·5–94·2) | 97·5% (95·8–98·5) | 93·9% (89·3–96·6) |
| 60–69 years | 88·4% (79·4–93·5) | 98·3% (95·4–99·4) | 93·7% (84·9–97·4) |
| 70–79 years | 92·4% (84·0–96·4) | 96·6% (93·7–98·2) | 95·7% (88·4–98·4) |
| ≥80 years | .. | 98·0% (85·7–99·7) | 86·5% (57·9–95·7) |
| CoronaVac | |||
| Total | 47·3% (41·9–52·3) | 72·1% (70·1–73·9) | 64·9% (61·2–68·2) |
| 60–69 years | 63·4% (52·8–71·6) | 83·3% (78·5–87·1) | 82·5% (73·7–88·3) |
| 70–79 years | 44·0% (34·5–52·2) | 78·1% (75·1–80·7) | 70·7% (64·4–76·0) |
| ≥80 years | 43·4% (34·5–51·2) | 66·3% (63·4–69·0) | 59·1% (53·8–63·7) |
All estimators were statistically significant (p<0·0001). The results for any vaccine were obtained from a cause-specific Cox regression model, in which each pair of vaccinated and unvaccinated individuals represented a stratum within the model, according to the study design. The results for each vaccine were obtained from multivariate cause-specific Cox regression models, which were adjusted by age, sex, affiliation regime to the Colombian health system, cancer, diabetes, hypertension, kidney disease, and HIV, with a random effect for municipality of residence. The reference group corresponds to people who have not received any dose of the COVID-19 vaccine.
The effectiveness across all vaccines analysed for preventing death after hospitalisation was 79·8% (95% CI 78·5–81·1, p<0·0001) across all ages, with the highest effectiveness in adults aged 60–69 years (91·0% [89·0–92·6], p<0·0001) and the lowest effectiveness in adults aged 80 years and older (68·4% [65·7–70·9], p<0·0001). Finally, the effectiveness across all vaccines for preventing death without hospitalisation was 72·8% (70·1–75·3, p<0·0001), being higher in adults aged 60–69 years (87·6% [83·4–90·7], p<0·0001), reducing to 78·9% (74·6%–82·4%, p<0·0001) in adults aged 70–79 years, and 61·2% (56·3–65·6, p<0·0001) in those aged 80 years and older.
Table 3 also shows the effectiveness of each vaccine by age group. We observed high effectiveness for every vaccine analysed, particularly for preventing death. BNT162b2 and ChAdOx1 nCoV-19 were most effective at preventing all outcomes of interest, with overlapping CIs in people aged 60–79 years. Across all age groups, the effectiveness of BNT162b2 in preventing hospitalisation without death was 83·0% (95% CI 78·4–86·6, p<0·0001), 94·8% (93·3–96·0, p<0·0001) in preventing death after hospitalisation, and 88·3% (84·1–91·4, p<0·0001) in preventing death without hospitalisation. Regarding ChAdOx1 nCoV-19, across all age groups, effectiveness in preventing hospitalisation without death was 90·8% (85·5–94·2, p<0·0001), 97·5% (95·8–98·5, p<0·0001) in preventing death after hospitalisation, and 93·9% (89·3–96·6, p<0·0001) in preventing death without hospitalisation. For ChAdOx1 nCoV-19, the estimators of effectiveness for each outcome were very similar for the different age groups.
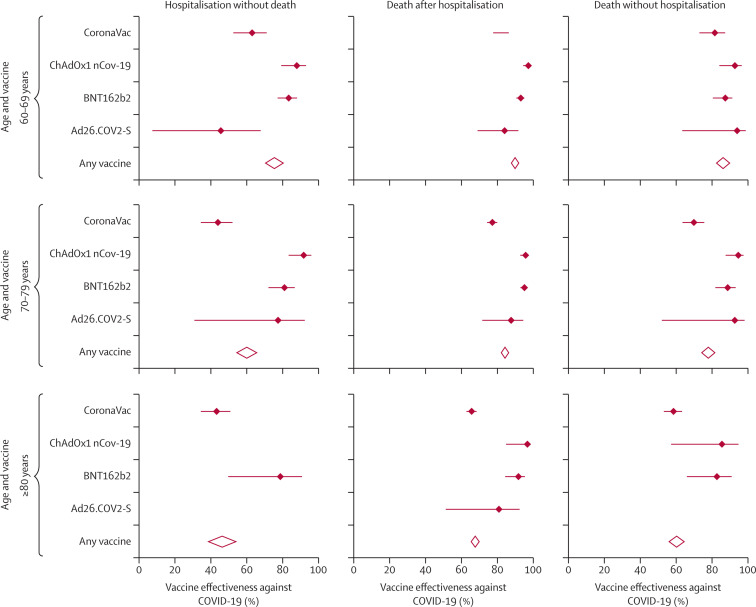
CoronaVac, although showing a high effectiveness in preventing all outcomes, had a lower effectiveness compared with BNT162b2 and ChAdOx1 nCoV-19, with some of the CIs not overlapping (table 3, figure 2 ). CoronaVac prevented hospitalisation without death in 47·3% (95% CI 41·9–52·3, p<0·0001) of participants, prevented death after hospitalisation in 72·1% (70·1–73·9, p<0·0001) of participants, and prevented death without hospitalisation in 64·9% (61·2–68·2, p<0·0001) of participants. The effectiveness of the CoronaVac vaccine showed a clear tendency to decrease with age. For instance, for adults aged 80 years and older, CoronaVac was 43·4% (34·5–51·2, p<0·0001) effective at preventing hospitalisation without death, 66·3% (63·4–69·0, p<0·0001) effective at preventing death after hospitalisation, and 59·1% (53·8–63·7, p<0·0001) effective at preventing death without hospitalisation.
Figure 2.
Forest plot of vaccine effectiveness at preventing hospitalisation and death due to COVID-19 in adults aged 60 years and older in Colombia
Finally, regarding Ad26.COV2-S, this vaccine prevented hospitalisation without death in 60·9% (95% CI 36·8–75·8, p<0·0001) of participants, prevented death after hospitalisation in 85·8% (77·1–91·2, p<0·0001) of participants, and prevented death without hospitalisation in 95·5% (82·0–98·9, p<0·0001) of participants. It is possible that the estimates by interval of the effectiveness by age group of this vaccine were imprecise given the small sample size and low numbers of participants vaccinated by this vaccine, so it was not possible to establish if there were significant differences.
In brief, the effectiveness of the vaccines evaluated decreased with age. We found better outcomes among people aged 60–69 years, followed by those aged 70–79 years, with the lowest effectiveness in people aged 80 years and older (figure 2). Although all the vaccines analysed showed a reduction in effectiveness as age increased, this reduction was greater for the CoronaVac vaccine.
For some of the vaccines evaluated, a point estimate of vaccine effectiveness could not be estimated because no outcomes occurred in that age group. In other instances, the CIs were wide because of the low number of people who received that specific vaccine, or the short duration of follow-up. Results of both sensitivity analyses were consistent with results of our primary analyses, suggesting that results are robust and consistent (appendix pp 4–5).
Discussion
We conducted a retrospective, national-cohort study to evaluate the effectiveness of vaccines against COVID-19 in people aged 60 years and older in Colombia. We included individuals who received the complete vaccination schedule between March 11 and Oct 26, 2021, when the mu variant was the most prevalent in the country. The results of this study confirm the high effectiveness of the available vaccines in Colombia for preventing hospitalisations and deaths due to COVID-19. These results are congruous with those reported by vaccine manufacturers, obtained from controlled clinical trials.4, 5, 6, 7
For all vaccines studied, we found an overall effectiveness of 61·6% for preventing hospitalisation without subsequent death, 79·8% for preventing death after hospitalisation, and 72·8% for preventing death without previous hospitalisation. These results are similar to those found in similar populations in other countries. A study13 done in fully vaccinated older long-term care residents in Spain found that the effectiveness of mRNA COVID-19 vaccines in preventing hospitalisations was 88·4% (95% CI 74·9–94·7) and 97·0% (91·7–98·9) for preventing death. Similarly, a retrospective cohort study14 in Catalonia, which analysed 28 456 nursing home residents who were vaccinated with BNT162b2, had an adjusted effectiveness in those who were fully vaccinated of 95·0% (93·0–96·0) for preventing hospital admission and 97·0% (96·0–98·0) for preventing death. Also, a cohort study15 done in Portugal evaluated the effectiveness of mRNA vaccines against COVID-19 hospitalisations and deaths in 1·8 million people who were aged 65 years and older and found a reduction in the risk of hospitalisation (59·0% [32·0–76·0]) and death (81·0% [73·0–87·0]) in fully vaccinated older adults.
In our study, vaccine effectiveness was negatively correlated with older age, regardless of the vaccine used, but not all vaccine platforms were affected the same. Among the vaccines included in this study, viral vector and mRNA vaccines were associated with higher effectiveness with increasing age than inactivated virus vaccines. Colombia used CoronaVac for the prioritised older population because it was the first vaccine to be available in sufficient quantities. Although mRNA vaccines have a higher vaccine effectiveness than CoronaVac, waiting several months for them to become available in Colombia would have been an unnecessary risk. This rationale had already been suggested by other authors, in which early vaccination with less effective biologics outweighed the advantages of waiting for other, more effective vaccines to become available.16
In Chile, one study17 analysed a national cohort of people aged 16 years or older who were immunised with CoronaVac. In the subgroup of older adults (aged 60 years and older) who were fully immunised, CoronaVac was 89·2% (95% CI 87·6–90·6) effective at preventing older adults from admission to the intensive care unit, and 86·5% (84·6–88·1) effective at preventing COVID-19-related death. Other studies that evaluated the effectiveness of vaccines in older people did not analyse vaccine effectiveness in older adults who were fully vaccinated or did not have a sufficient follow-up time to assess the effectiveness of preventing death.18
These studies present methodological differences, including the study population and predominant variant during the period of analysis, thus the above studies should be compared against our results carefully. Furthermore, these other studies focused on mRNA COVID-19 vaccines.5, 8, 13, 15 Only a small number of studies are available for comparing the effectiveness of various vaccines in older populations, including various vaccine platforms.18, 19
We observed that the effectiveness across vaccines in preventing death after hospitalisation decreased by 22·6% and decreased by 26·4% in preventing death without previous hospitalisation for those aged 80 years and older compared with adults aged 60–69 years. Possible explanations for this lower effectiveness include the greater probability of pre-existing conditions in older people, in conjunction with age-related frailty20 and immunosenescence, which can cause poor responses to vaccination.21 These results are congruent with those presented in other studies.22, 23
Older adults have already been identified as the population with the highest risk of severe illness and death from COVID-19. Our findings show the need for additional prevention strategies for this age group. A booster dose has been found to increase the immune response, and therefore represents a potential solution to the decreased effectiveness of vaccines in older people.24 On the basis of our findings, Colombia started offering booster doses to people aged 50 years and older from early October, 2021.
The mu variant was predominant in Colombia throughout the observation period. Although there is no information on genomic sequencing in instances of breakthrough infections, the high observed effectiveness of vaccines indirectly suggests that all vaccines used in Colombia confer adequate protection against this variant and other variants circulating during the study period. However, our study was not able to assess the effectiveness of vaccines for any specific variant of SARS-CoV-2, given that the evaluated outcomes did not distinguish the variant involved in the clinical course of the patients. Therefore, our results could not be totally extrapolated to other contexts in which other variants are predominant.
The differences in self-selection to access the application of the vaccines could be a source of confounding in this study (eg, an unvaccinated adult might also be less likely to wear a mask or take precautions, thereby exposing themselves to greater risk of infection, which could contribute to the observed differences). However, we consider that this bias, if present, would have only a small effect on the estimations. This is because, first, according to previous surveys of vaccination intention in Colombia, older adults had a high willingness to get vaccinated. Second, vaccination coverage in older adults at the end of December, 2021, was above 90%, which confirms that vaccine hesitancy was not an issue in this age group. Finally, according to international evidence, one of the factors associated with refusal to be vaccinated is social class. This factor was partly controlled for in this study by adjusting for affiliation regime to the health system, which is a strong proxy indicator of social class in Colombia.
However, as any cohort study, this different propensity to be exposed (vaccinated in this instance) could be related to unobserved (or unobservable) variables that could affect the estimates. It should be considered that this might mean that unvaccinated people did not get vaccinated because of individual characteristics that are also associated with a higher risk of infection, such as lower self-care, which could lead to overestimating the effect.
Thus, because there are many factors that influence whether people are vaccinated, and the bias could either lead to overestimation or underestimation of the observed effectiveness estimates, this is a limitation of the study. However, this limitation is minimised because of the high willingness and rate of vaccination in Colombia, as well as the study indirectly controlling for social class in this age group and by the matching process in the design.
We identified delays in reporting to the individual-level vaccination registry system (PAIWEB), which introduced a high probability of misclassification bias. The vaccinated population who had not been registered as such is likely to have reduced the differences in risk for outcomes of interest between the exposed cohort and the unexposed cohort. This bias tended to underestimate the observed effectiveness, so we believe that the presented estimators are lower than the actual effectiveness values. We conducted a sensitivity analysis in which we randomly changed a proportion of non-vaccinated individuals as being fully vaccinated with any vaccine, equal to the estimated proportion of people fully vaccinated but not registered as such (appendix p 4). By the cutoff date (Oct 26), approximately 24% of those vaccinated had not been registered, although people aged 60 years and older were more likely to be reported because they were the first to be vaccinated. Results of this analysis suggest a higher vaccine effectiveness when individuals in the unexposed cohort were randomly assigned as being exposed, suggesting that our observed effectiveness was underestimated.
Another limitation of our study was that 2 06 607 people had a record labelled as a second dose, but without the first dose being registered. These records were excluded from our analysis. A sensitivity analysis that included all records, assuming that all individuals were fully vaccinated, showed similar results to our primary analysis and did not show a significant increase in overall vaccination effectiveness, suggesting that these records might correspond to mislabelling and that our estimates are robust.
Another limitation of this study is the short follow-up period for people vaccinated with Ad26.COV2-S and ChAdOx1 nCoV-19, which led to a small number of observed events, especially for Ad26.COV2-S, and therefore lower precision of the estimators. For future studies, we recommend repeating this analysis with a longer follow-up period. Moreover, it was not possible to control for other possible confounding variables, such as frailty or residing in nursing homes, among others. So, it is possible that our results have some residual confounding.
Our study had several strengths. To the best of our knowledge, this study is the first investigation to analyse and report the effectiveness of four different vaccines against COVID-19 in older adults disaggregated by age group. There have only been few publications that compare inactivated virus vaccines with mRNA and viral vector vaccines in the same country in high-risk populations. This makes our study relevant for countries where inactivated virus vaccines were widely used in older populations to act towards better protecting those at higher risks of severe illness from COVID-19. In addition, the competing risk analysis we used had the advantage that, instead of estimating a separate model for each outcome, it fitted a joint model for all outcomes. This analysis is especially useful since outcomes (hospitalisation and death from COVID-19 in this instance) might occur at different points in time, especially when one of them might have affected the censoring and risk of the other.25 Also, we conducted several sensitivity analyses, which allowed us to confirm the robustness of our findings (appendix pp 4–5).
Our study generates solid evidence on vaccination effectiveness in older people in Colombia and might inform the administration of these vaccines in older populations across the world and decision-making that considers risk stratification approaches to target the most vulnerable people according to their demographic and epidemiological characteristics.
This online publication has been corrected. The corrected version first appeared at thelancet.com/healthy-longevity on July 19, 2022
Declaration of interests
FR-G, LA-C, and JF-N are members of the Colombian COVID-19 vaccine advisory committee. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was conducted by the Ministry of Health and Social Protection of Colombia. Workers were from the Directorate of Medicines and Health Technologies, and Directorate of Epidemiology and Demography. Funding from the Ministry was given indirectly through the wage of and payments to the statistician of the project. In Colombia, public data from health information systems at the individual level are managed and safeguarded by the Ministry of Health and Social Protection. In the case of information on vaccination and covariates at the individual level, these will be publicly available once the technical and regulatory requirements of the Ministry have been adjusted, which is currently in process. The databases are not yet available to the public because authorisation from the Ministry has not been obtained. However, according to regulations, these data can be requested by researchers who ask for the information, who must also send the objective of the investigation, a plan of analysis, and a publication plan to the Ministry of Health (https://www.minsalud.gov.co/atencion/Paginas/Solicitudes-sugerencias-quejas-o-reclamos.aspx). Each request will be evaluated in accordance with the regulations and legal requirements defined in the country.
Contributors
LA-C and JF-N conceptualised the effectiveness evaluation of COVID-19 vaccines strategy. LA-C, MR-B, and JF-N conceptualised the design and statistical analysis plan for the present study. LR-M built the dataset. BT-V advised the study design and the statistical analysis. AP-C and LR-M cleaned the data. AP-C and MR-B did the statistical analysis. LA-C, JF-N, LR-M, MR-B, and MG-P verified the data underlying the study. LA-C, MR-B, and MP-Á wrote the first draft. All authors reviewed and discussed the results and approved the final version of the manuscript. JF-N, AP-C, and LA-C accessed and verified the data. All authors agree with the viewpoints expressed in the article and contributed to the review and editing of the manuscript.
Supplementary Material
References
- 1.Johns Hopkins University & Medicine Coronavirus resource center. Global map. 2021. https://coronavirus.jhu.edu/map.html
- 2.Ho FK, Petermann-Rocha F, Gray SR, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifuentes MP, Rodriguez-Villamizar LA, Rojas-Botero ML, et al. Socioeconomic inequalities associated with mortality for COVID-19 in Colombia: a cohort nationwide study. J Epidemiol Community Health. 2021;75:610–615. doi: 10.1136/jech-2020-216275. [DOI] [PubMed] [Google Scholar]
- 10.Nasiri MJ, Haddadi S, Tahvildari A, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med. 2020;7:459. doi: 10.3389/fmed.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Public health surveillance for COVID-19. Interim guidance. 2020. https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020.8
- 12.Pan American Health Organization Case definitions for COVID-19 surveillance. 2020. https://www.paho.org/en/case-definitions-covid-19-surveillance-16-december-2020
- 13.Mazagatos C, Monge S, Olmedo C, et al. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabezas C, Coma E, Mora-Fernandez N, et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ. 2021;374 doi: 10.1136/bmj.n1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes B, Rodrigues AP, Kislaya I, et al. mRNA vaccine effectiveness against COVID-19-related hospitalisations and deaths in older adults: a cohort study based on data linkage of national health registries in Portugal, February to August 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.38.2100833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahuja A, Athey S, Baker A, et al. National Bureau of Economic Research; 2021. Preparing for a pandemic: accelerating vaccine availability.https://www.nber.org/papers/w28492 [Google Scholar]
- 17.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerqueira-Silvaa T, de Araújo V, Boaventura V, et al. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: a population-based study. Lancet Reg Health Am. 2022;6 doi: 10.1016/j.lana.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 21.Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in the elderly population during a gamma variant-associated epidemic of COVID-19 in Brazil: a test-negative case-control study. BMJ. 2021;374 doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Yang J, Wang L, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv. 2021 doi: 10.1101/2021.05.19.21257472. published online July 21. (preprint). [DOI] [Google Scholar]
- 24.Flaxman A, Marchevsky N, Jenkin D, et al. Tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 (AZD1222) SSRN. 2021 doi: 10.2139/ssrn.3873839. published online June 28. (preprint). [DOI] [Google Scholar]
- 25.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.