Abstract
Introduction
Active surveillance (AS) is increasingly used for favorable intermediate-risk (FIR) prostate cancer (PCa). Our objective was to determine oncological and sociodemographic predictors of deferred definitive therapy and decision for radical prostatectomy (RP) vs. radiotherapy (RT).
Methods
The Surveillance, Epidemiology, and End Results (SEER) Prostate with Watchful Waiting database was used to identify all FIR PCa diagnosed between 2010 and 2015 opting for AS for at least one year following diagnosis. We sought to determine predictors of treatment and treatment type using multivariable logistic regression.
Results
A total of 20 334 patients were identified. An annual decrease in incident FIR patients managed initially with AS between 2010 (4061) and 2015 (2947) was noted (p for trend <0.001); 17 895 (88.0%) patients underwent deferred RP and/or RT. Patients with higher baseline cancer volume and clinical stage were significantly more likely to discontinue AS. Patients of higher socioeconomic status were more likely to undergo deferred therapy, with increased odds for RT over RP. African American patients had lower odds of undergoing definitive intervention (odds ratio 0.83, p=0.030) and were significantly more likely to opt for XRT. Oncological characteristics leading to FIR classification influenced treatment choice at the time of deferred intervention: RT was treatment of choice in 86.3% and 86.0% of Gleason group 2 and prostate-specific antigen 10–20 FIR patients, respectively; 96.1% of treated cT2b-c FIR patients opted for RP.
Conclusions
Most FIR PCa patients initially managed with AS eventually undergo deferred definitive therapy, with choice of treatment significantly influenced by patients’ baseline oncological and sociodemographic characteristics.
Introduction
Active surveillance (AS) is currently the standard of care for very low- and low-risk prostate cancer (PCa).1,2 This management strategy has proven its long-term oncological safety in these cohorts and simultaneously maintains patient quality of life,3,4 and thus, there has been increased interest in expanding the indications for AS to the intermediate-risk cohort. This is supported by results from the Prostate Testing for Cancer and Treatment (ProtecT) trial demonstrating that low- and intermediate-risk PCa patients managed conservatively with active monitoring had long-term PCa mortality outcomes similar to those managed with radical prostatectomy (RP) or radiotherapy (RT).5 Furthermore, a large, multinational autopsy study demonstrated that more than half of Asian men with incidental PCa at autopsy harbored evidence of Grade Group (GG) 2 disease or worse, confirming the indolent nature of a subset of this risk group.6 These findings have contributed to multiple governing bodies supporting AS use for patients with favorable intermediate-risk (FIR) PCa,1,2 defined per the National Comprehensive Cancer Network (NCCN) as predominant GG1 disease, percentage of positive biopsy cores <50%, and a single NCCN intermediate risk factor.7 These recommendations have been reflected in an increased uptake of AS for patients with intermediate-risk disease, with a recent analysis of the Surveillance, Epidemiology, and End Results (SEER) Prostate with Watchful Waiting (WW) database demonstrating that use of AS for such patients has significantly increased over time, from 3.7% in 2010 to 7.3% in 2015.8
With the increased uptake of AS for FIR PCa patients, understanding the factors influencing the decision to discontinue AS in favor of deferred definitive intervention, as part of a shared decision-making process between the patient and physician, becomes important. Our objective was to evaluate both oncological and sociodemographic predictors of undergoing deferred definitive intervention after a period of AS, and in patients undergoing deferred intervention, choice of RP vs. RT.
Methods
Patient population
Men with NCCN FIR PCa were identified using the SEER WW database, which is a nationally representative database supported by the National Cancer Institute that captures patients with incident PCa from 18 population-based registries, accounting for approximately 30% of the U.S. population. Study patients were diagnosed with PCa between 2010 and 2015 and all underwent documented AS or WW for a period of at least one year, as per records from the treating institutions.9 Such patients did not receive definitive therapy for at least one year following diagnosis and were managed with AS or WW for at least one year following diagnosis. Thus, patients initially managed with AS or WW but who subsequently opted for definitive therapy within one year of diagnosis and those that simply deferred treatment by one year were not included in the cohort. Patients older than 80 years at time of diagnosis were excluded from our cohort, as the majority of such patients would be expected to fall under the WW category.10 Patients were not excluded if they had a prior diagnosis of another non-PCa related malignancy and were noted as such.
FIR patients were subdivided into one of three groups based on which risk factor categorized them as having intermediate-risk disease, per NCCN criteria: GG2, prostate-specific antigen (PSA) 10–20 ng/ml, and cT2b-c. Each patient thus had only one intermediate risk factor and the three FIR groups were mutually exclusive.
Given the deidentified and public availability of the dataset, research ethics board approval for this study was not required by the participating institutions.
Study outcomes
The two primary study outcomes were: 1) a definitive intervention event, defined as RP or definitive RT; and 2) choice of RP vs. definitive RT among those who opted for intervention. Definitive RT includes both external beam radiotherapy and/or brachytherapy treatment. These two outcomes were each operationalized as a binary variable (yes vs. no), with time to intervention not available from this dataset.
Study variables
Patient oncological and sociodemographic variables were available at time of PCa diagnosis only. Oncological variables included: clinical T, N, and M stages, serum PSA level, Gleason score on prostate biopsy or transurethral resection of the prostate (TURP) specimens, and number of positive and sampled biopsy cores/specimens. The percent positive cores variable was calculated from the number of positive and sampled cores/specimens for each patient. Followup serum PSA levels, clinical exam, imaging findings, and repeat biopsy results were not available, and thus the trigger for discontinuing AS in favor of deferred therapy was not available.
Baseline patient-level sociodemographic variables included: year of diagnosis, age at diagnosis, race, insurance status, marital status, and SEER registry. Individual socioeconomic status (SES) was derived from the following five county-level variables: percentage of individuals: 1) below the poverty line; 2) unemployed; 3) median household income; 4) foreign-born; and 5) with less than a high school education.11–13
Statistical analysis
Continuous variables were reported using medians and interquartile ranges (IQR). Categorical variables were reported using frequency counts and proportions and were compared using the Chi-squared test. Predictors of deferred therapy and decision for RP vs. definitive RT were each evaluated using univariable and multivariable logistic regression analyses. All of the aforementioned oncological and sociodemographic variables, operationalized as categorical variables, were included a priori in the multivariable analyses to control for potential sources of confounding. The variance inflation factor test was used to test for variable multicollinearity. A cutoff value of five was used to exclude variables on the basis of a high degree of multicollinearity. The Cuzick’s test for trend, an extension of the Wilcoxon rank-sum test,14 was used to evaluate trends in AS uptake by year for each FIR group. A p<0.05 denoted statistical significance. R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) was used to perform all statistical analyses.
Results
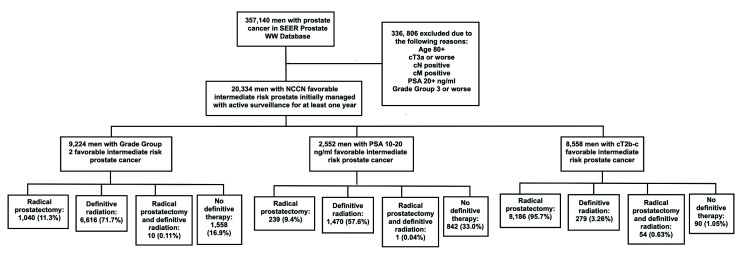
Of 357 140 men with PCa in the SEER WW database, we identified 20 334 men with NCCN FIR PCa who were managed with AS for at least one year following diagnosis. Of these 20 334 men, 9224 (45.4%), 2552 (12.6%), and 8558 (42.1%) were in the GG2, PSA 10–20 ng/ml, and cT2b-c groups, respectively (Fig. 1). Baseline sociodemographic and oncological characteristics for the overall cohort and by FIR group are presented in Table 1. Median age at diagnosis was 64.0 years (IQR 58.0–69.0). Caucasian and African American patients accounted for 14 182 (69.7%) and 3344 (16.4%) patients, respectively. Median serum PSA level at diagnosis was 5.60 ng/ml and median percent positive cores was 21.4% (IQR 12.5–33.3%) (Table 1).
Fig. 1.
Study flow chart. PSA: prostate-specific antigen; SEER: Surveillance, Epidemiology, and End Results. WW: watchful waiting
Table 1.
Baseline demographic and oncological characteristics for overall cohort and by risk group
| Variable | Overall favorable intermediate-risk cohort (n=20 334) | GG2 favorable intermediate-risk cohort (n=9224) | PSA 10–20 ng/ml favorable intermediate-risk cohort (n=2552) | cT2b-c favorable intermediate-risk cohort (n=8558) |
|---|---|---|---|---|
| Year of diagnosis | ||||
| 2010 | 4061 (20.0%) | 1454 (15.8%) | 502 (19.7%) | 2105 (24.6%) |
| 2011 | 3707 (18.2%) | 1438 (15.6%) | 466 (18.3%) | 1803 (21.1%) |
| 2012 | 3603 (17.7%) | 1651 (17.9%) | 435 (17.0%) | 1517 (17.7%) |
| 2013 | 3163 (15.6%) | 1547 (16.8%) | 409 (16.0%) | 1207 (14.1%) |
| 2014 | 2853 (14.0%) | 1459 (15.8%) | 379 (14.9%) | 1015 (11.9%) |
| 2015 | 2947 (14.55%) | 1675 (18.2%) | 361 (14.1%) | 911 (10.6%) |
| Age at diagnosis, median (IQR) | 64.0 (58.0–69.0) | 66.0 (61.0–71.0) | 67.0 (61.0–72.0) | 60.0 (55.0–65.0) |
| Race | ||||
| Caucasian | 14 182 (69.7%) | 6302 (68.3%) | 1590 (52.3%) | 6290 (73.5%) |
| African American | 3344 (16.4%) | 1772 (19.2%) | 473 (18.5%) | 1099 (12.8%) |
| Hispanic | 1567 (7.71%) | 590 (6.40%) | 252 (9.87%) | 725 (8.47%) |
| Asia/Pacific Islander | 864 (4.25%) | 367 (3.98%) | 166 (6.50%) | 331 (3.87%) |
| American Indian/Alaska Native | 68 (0.33%) | 31 (0.34%) | 8 (0.31%) | 29 (0.34%) |
| Unknown | 309 (1.52%) | 162 (1.76%) | 63 (2.47%) | 84 (0.98%) |
| Marital status | ||||
| Married | 14 173 (69.7%) | 6046 (65.5%) | 1548 (60.7%) | 6579 (76.9%) |
| Not married | 4144 (20.4%) | 2105 (22.8%) | 631 (24.7%) | 1408 (16.5%) |
| Unknown | 2017 (9.9%) | 1073 (11.6%) | 373 (14.6%) | 571 (6.67%) |
| SEER registry | ||||
| New Jersey | 2816 (13.8%) | 1361 (14.7%) | 295 (11.6%) | 1160 (13.6%) |
| San Francisco-Oakland | 1123 (5.52%) | 559 (6.06%) | 196 (7.68%) | 368 (4.30%) |
| Los Angeles | 1142 (5.62%) | 374 (4.05%) | 146 (5.72%) | 622 (7.27%) |
| Louisiana | 1106 (5.44%) | 503 (5.45%) | 139 (5.45%) | 464 (5.42%) |
| Connecticut | 982 (4.83%) | 506 (5.49%) | 102 (4.00%) | 374 (4.37%) |
| Detroit (metropolitan) | 1709 (8.40%) | 1044 (11.3%) | 119 (4.66%) | 546 (6.38%) |
| Seattle (Puget Sound) | 1071 (5.27%) | 470 (5.10%) | 98 (3.84%) | 503 (5.88%) |
| Rural Georgia | 53 (0.26%) | 30 (0.33%) | 12 (0.47%) | 11 (0.13%) |
| Atlanta (metropolitan) | 801 (3.94%) | 504 (5.46%) | 101 (4.0%) | 196 (2.29%) |
| California (excluding SF/SJM/LA) | 4052 (19.9%) | 1556 (16.9%) | 650 (25.5%) | 1846 (21.6%) |
| Greater Georgia | 1828 (9.0%) | 963 (10.4%) | 237 (9.29%) | 628 (7.34%) |
| Kentucky | 1235 (6.07%) | 398 (4.31%) | 140 (5.49%) | 697 (8.14%) |
| San Jose-Monterey | 725 (3.57%) | 327 (3.55%) | 123 (4.82%) | 275 (3.21%) |
| Utah | 511 (2.51%) | 189 (2.05%) | 36 (1.41%) | 286 (3.34%) |
| Hawaii | 271 (1.33%) | 107 (1.16%) | 41 (1.61%) | 123 (1.44%) |
| Iowa | 584 (2.87%) | 233 (2.53%) | 62 (2.43%) | 289 (3.38%) |
| New Mexico | 321 (1.58%) | 100 (1.08%) | 54 (2.12%) | 167 (1.95%) |
| Alaska Natives | 4 (0.02%) | 0 (0.0%) | 1 (0.04%) | 3 (0.035%) |
| Insurance status | ||||
| Insured | 18 239 (89.7%) | 8074 (87.5) | 2106 (82.5%) | 8059 (94.2%) |
| Uninsured | 212 (1.04%) | 86 (0.93%) | 52 (2.04%) | 74 (0.86%) |
| Medicaid | 681 (3.35%) | 318 (3.45%) | 150 (5.88%) | 213 (2.49%) |
| Unknown | 1202 (5.91%) | 746 (8.09%) | 244 (9.56%) | 212 (2.48%) |
| Socioeconomic status | ||||
| 1 (lowest) | 4620 (22.7%) | 1991 (21.6%) | 664 (26.0%) | 1965 (23.0%) |
| 2 | 4580 (22.5%) | 2055 (22.3%) | 656 (25.7%) | 1869 (21.8%) |
| 3 | 5724 (28.1%) | 2674 (29.0%) | 691 (27.1%) | 2359 (27.6%) |
| 4 (highest) | 5410 (26.6%) | 2504 (27.1%) | 541 (21.2%) | 2365 (27.6%) |
| PSA at diagnosis, median (IQR) | 5.60 (4.40–7.70) | 5.60 (4.50–7.10) | 12.30 (10.90–14.60) | 5.00 (4.10–6.30) |
| Percent cores positive, median (IQR) | 21.4 (12.5–33.3) | 25.0 (16.7–33.3) | 16.7 (8.33–25.0) | 18.8 (11.1–33.3) |
| cT Stage | ||||
| cT1 | 9603 (47.2%) | 7383 (80.0%) | 2220 (87.0%) | 0 (0.0%) |
| cT2a | 2173 (10.7%) | 1841 (20.0%) | 332 (13.0%) | 0 (0.0%) |
| cT2b | 373 (1.83%) | 0 (0.0%) | 0 | 373 (4.36%) |
| cT2c | 8185 (40.3%) | 0 (0.0%) | 0 | 8185 (95.6%) |
| Prostate cancer as first diagnosed malignancy | ||||
| Yes | 19 046 (93.7%) | 8543 (92.6%) | 2364 (92.6%) | 8139 (95.1%) |
| No (i.e., previous, non-prostate cancer diagnosis) | 1288 (6.33%) | 681 (7.38%) | 188 (7.37%) | 419 (4.90%) |
GG: grade group; IQR: interquartile range; PSA: prostate-specific antigen; SF/SJM/LA: San Francisco, San Jose-Monterey, Los Angeles.
A total of 4061 patients (20.0% of all patients in the study cohort) were diagnosed in 2010; this figure decreased to 2947 (14.55%) in 2015 (p for trend <0.001). Of the 20 334 patients in the cohort, 17 895 (88.0%) eventually underwent deferred definitive therapy with either RP and/or RT. The corresponding figures in the GG2, PSA 10–20 ng/ml, and cT2b-c groups were 7666 (83.1%), 1710 (67.0%), and 8519 (99.5%), respectively (p<0.001). Of the 7666 and 1710 patients in the GG2 and PSA 10–20 ng/ml groups, respectively, definitive RT was the treatment of choice in 6616 (86.3%) and 1470 (86.0%) patients, respectively. Conversely, 8186 patients (96.1% of treated patients) in the cT2b-c group opted for RP (p across FIR groups <0.001).
Predictors of undergoing deferred therapy on univariable logistic regression analyses are presented in Supplementary Table 1 (available at cuaj.ca). On multivariable analysis, baseline oncological variables predicting increased odds of deferred intervention included higher volume disease on biopsy/TURP specimens in the GG2 and PSA 10–20 ng/ml groups (odds ratio [OR] for 37.6–49.9% vs 0–12.5%: 1.33 and 2.10, p=0.008 and p<0.001, respectively) and more advanced clinical stage in all three FIR groups (OR 3.00 and 5.26 for cT2a vs. cT1, p<0.001 in the GG2 and PSA 10–20 ng/ml groups, respectively; OR 15.5 for cT2c vs. cT2b in the cT2b-c group, p<0.001).
With regards to baseline sociodemographic variables, patients of higher SES were significantly more likely to undergo definitive therapy in all three FIR groups (ORs for highest vs. lowest quartiles: 1.72, 1.50, and 1.60, p<0.001, p=0.013, and p=0.03, for the GG2, PSA 10–20 ng/ml, and cT2b-c groups, respectively). In the GG2 group, African American patients (vs. Caucasian, OR 0.83, p=0.03) and those diagnosed in a Western region (vs. Northeastern, OR 0.81, p=0.014) had significantly lower odds of undergoing definitive therapy, whereas patients who were not married were significantly more likely to opt for definitive therapy (OR 1.43, p<0.001). In the cT2b-c group, older patients (OR for 70–79 vs. 30–49: 0.16, p=0.004), those who were uninsured (vs. insured, OR 0.18, p=0.009), and those diagnosed in a Southeastern region (vs. Northeastern, OR 0.42, p=0.022) all had significantly lower odds of undergoing definitive therapy (Table 2).
Table 2.
Predictors of receiving deferred therapy (radical prostatectomy or radiation therapy) on multivariable logistic regression analysis for favorable intermediate prostate cancer patients by group
| GG2 favorable intermediate-risk cohort (n=9224) | PSA 10–20 ng/ml favorable intermediate-risk cohort (n=2552) | cT2b-c favorable intermediate-risk cohort (n=8558) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Year of diagnosis (reference: 2010–11) | |||||||||
| 2012–13 | 0.88 | 0.75–1.03 | 0.12 | 0.80 | 0.64–1.02 | 0.071 | 0.45 | 0.25–0.78 | 0.005 |
| 2014–15 | 0.89 | 0.75–1.04 | 0.14 | 0.76 | 0.59–0.97 | 0.028 | 0.64 | 0.33–1.22 | 0.17 |
| Age at diagnosis (reference: 30–49 years) | |||||||||
| 50–59 | 1.22 | 0.75–1.91 | 0.40 | 0.91 | 0.27–2.66 | 0.87 | 1.17 | 0.26–3.68 | 0.81 |
| 60–69 | 1.43 | 0.89–2.20 | 0.12 | 1.08 | 0.33–3.10 | 0.90 | 0.44 | 0.10–1.25 | 0.18 |
| 70–79 | 1.30 | 0.81–2.03 | 0.26 | 0.88 | 0.27–2.54 | 0.82 | 0.16 | 0.036–0.50 | 0.004 |
| Race (reference: Caucasian) | |||||||||
| African American | 0.83 | 0.70–0.98 | 0.03 | 0.85 | 0.65–1.13 | 0.27 | 0.69 | 0.36–1.43 | 0.29 |
| Hispanic | 0.97 | 0.75–1.28 | 0.85 | 0.96 | 0.68–1.36 | 0.83 | 0.61 | 0.29–1.38 | 0.20 |
| Asia/Pacific Islander/American Indian/Alaska Native | 0.94 | 0.69–1.30 | 0.69 | 0.95 | 0.64–1.43 | 0.81 | 2.86 | 0.57–52.2 | 0.31 |
| Insurance status (reference: Insured) | |||||||||
| Uninsured | 0.58 | 0.39–1.11 | 0.096 | 0.59 | 0.32–1.12 | 0.10 | 0.18 | 0.058–0.82 | 0.009 |
| Medicaid | 0.88 | 0.65–1.20 | 0.40 | 0.78 | 0.53–1.17 | 0.23 | 0.72 | 0.27–2.52 | 0.55 |
| Marital status (reference: married) | |||||||||
| Not married | 1.43 | 1.24–1.65 | <0.001 | 1.17 | 0.94–1.46 | 0.16 | 1.61 | 0.91–2.76 | 0.087 |
| SEER registry region (reference: Northeast) | |||||||||
| Southeast | 1.10 | 0.91–1.34 | 0.31 | 1.15 | 0.82–1.60 | 0.43 | 0.42 | 0.20–0.87 | 0.022 |
| Midwest | 1.21 | 0.86–1.73 | 0.28 | 1.00 | 0.62–1.63 | 1.00 | 0.79 | 0.28–2.56 | 0.66 |
| West | 0.81 | 0.68–0.96 | 0.014 | 0.76 | 0.57–1.02 | 0.068 | 0.79 | 0.38–1.60 | 0.53 |
| SES quartiles (reference: 1 [lowest]) | |||||||||
| 2 | 1.30 | 1.08–1.57 | <0.001 | 1.26 | 0.95–1.67 | 0.11 | 1.78 | 0.89–3.68 | 0.11 |
| 3 | 1.11 | 0.93–1.32 | 0.24 | 1.07 | 0.81–1.41 | 0.64 | 0.93 | 0.50–1.72 | 0.83 |
| 4 (highest) | 1.72 | 1.41–2.10 | <0.001 | 1.50 | 1.09–2.08 | 0.013 | 1.60 | 1.12–3.52 | 0.03 |
| PCa as first cancer diagnosis (reference: previously diagnosed with other cancer) | 1.20 | 0.95–1.52 | 0.12 | 1.46 | 1.01–2.09 | 0.042 | 0.50 | 0.12–1.45 | 0.27 |
| PSA (references: 0–5 ng/ml for groups 1 and 3; 10–15 ng/ml for group 2) | |||||||||
| 5–10 ng/ml | 1.20 | 0.99–1.45 | 0.053 |

|
1.17 | 0.65–2.00 | 0.59 | ||
| 10–20 ng/ml |

|
1.13 | 0.69–1.81 | 0.62 |

|
||||
| Percent cores positive (reference: 0–12.5%) | |||||||||
| 12.6–25.0% | 1.06 | 0.88–1.28 | 0.51 | 1.22 | 0.96–1.55 | 0.10 | 0.92 | 0.42–1.94 | 0.83 |
| 25.1–37.5% | 1.44 | 1.20–1.72 | <0.001 | 1.58 | 1.22–2.07 | <0.001 | 0.67 | 0.23–0.10 | 0.13 |
| 37.6–49.9% | 1.33 | 1.08–1.65 | 0.008 | 2.10 | 1.34–3.25 | <0.001 | 0.76 | 0.32–1.82 | 0.53 |
| cT stage (cT1 as reference for groups 1 and; cT2b for group 3) | |||||||||
| cT2a | 3.00 | 2.44–3.72 | <0.001 | 5.26 | 3.49–8.29 | <0.001 |

|
||
| cT2c |

|

|
15.5 | 9.40–25.2 | <0.001 | ||||
CI: confidence interval; NA: not available; OR: odds ratio; PSA: prostate-specific antigen; SEER: Surveillance, Epidemiology, and End Results; SES: socioeconomic status.
Among patients who underwent deferred definitive therapy, patients with larger tumor volume at time of diagnosis were significantly more likely to opt for definitive RT over RP in all FIR groups (OR for 37.6–49.9% vs. 0–12.5%: 0.34, 0.21, and 0.33, p<0.001, p=0.014, and p<0.001 for the GG2, PSA 10–20 ng/ml, and cT2b-c groups, respectively). Conversely, patients with a more advanced clinical stage were significantly more likely to opt for RP (OR 167.2 and 239.8 for cT2a vs. cT1, p<0.001 in the GG2 and PSA 10–20 ng/ml groups, respectively, and OR 38.6 for cT2c vs. cT2b in the cT2b-c group, p<0.001). In the GG2 group, patients with a PSA of 5–10 ng/ml (OR 1.60, p=0.033) and no prior non-PCa malignancy (OR 1.60, p=0.033) were both significantly more likely to opt for RP over definitive RT. With regards to sociodemographic variables, older patients (ORs for 70–79 vs. 30–49: 0.034 and 0.027, p<0.001 for the GG2 and cT2b-c groups, respectively) and those of higher SES in the GG2 (OR for second vs. lowest: 0.63, p=0.013) and cT2b-c groups (OR for third vs. lowest: 0.32, p=0.034) were significantly more likely to choose RT over RP. Similarly, African American patients in the GG2 and PSA 10–20 ng/ml groups were significantly more likely to undergo XRT (OR vs. Caucasian: 0.58 and 0.34, p=0.003 and p=0.012, respectively). Conversely, patients who were not married were significantly more likely to undergo RP vs. RT in the GG2 (OR 1.39, p=0.016) and cT2b-c groups (OR 2.45, p<0.001) (Table 3) (Supplementary Table 2; available at cuaj.ca).
Table 3.
Predictors of receiving radical prostatectomy vs. radiation therapy on multivariable logistic regression analysis for favorable intermediate prostate cancer patients by group
| GG2 favorable intermediaterisk cohort (n=9224) | PSA 10–20 ng/ml favorable intermediate-risk cohort (n=2552) | cT2b-c favorable intermediate-risk cohort (n=8558) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Year of diagnosis (reference: 2010–11) | |||||||||
| 2012–13 | 0.96 | 0.74–1.25 | 0.75 | 1.47 | 0.79–2.75 | 0.23 | 1.05 | 0.72–1.54 | 0.80 |
| 2014–15 | 1.03 | 0.79–1.34 | 0.83 | 1.46 | 0.76–2.86 | 0.26 | 1.29 | 0.84–2.03 | 0.25 |
| Age at diagnosis (reference: 30–49 years) | |||||||||
| 50–59 | 0.42 | 0.20–0.88 | 0.025 | 0.46 | 0.04–5.34 | 0.56 | 0.48 | 0.11–1.45 | 0.25 |
| 60–69 | 0.12 | 0.059–0.25 | <0.001 | 0.40 | 0.040–4.50 | 0.49 | 0.12 | 0.028–0.34 | <0.001 |
| 70–79 | 0.034 | 0.015–0.071 | <0.001 | 0.12 | 0.010–1.37 | 0.11 | 0.027 | 0.006–0.082 | <0.001 |
| Race (reference: Caucasian) | |||||||||
| African American | 0.58 | 0.41–0.83 | 0.003 | 0.34 | 0.14–0.78 | 0.012 | 0.69 | 0.41–1.19 | 0.16 |
| Hispanic | 1.15 | 0.75–1.77 | 0.54 | 0.76 | 0.33–1.75 | 0.51 | 0.96 | 0.49–2.02 | 0.90 |
| Asia/Pacific Islander/American Indian/Alaska Native | 0.96 | 0.59–1.57 | 0.88 | 0.62 | 0.23–1.72 | 0.36 | 0.54 | 0.27–1.19 | 0.10 |
| Insurance status (reference: insured) | |||||||||
| Uninsured | 1.50 | 0.45–4.83 | 0.51 | 1.37 | 0.16–9.18 | 0.77 | 0.87 | 0.18–15.6 | 0.89 |
| Medicaid | 0.56 | 0.28–1.08 | 0.087 | 0.62 | 0.18–2.11 | 0.44 | 0.78 | 0.36–1.88 | 0.56 |
| Marital status (reference: married) | |||||||||
| Not married | 1.39 | 1.06–1.81 | 0.016 | 0.84 | 0.46–1.53 | 0.57 | 2.45 | 1.67–3.57 | <0.001 |
| SEER registry region (reference: Northeast) | |||||||||
| Southeast | 1.38 | 0.98–1.94 | 0.069 | 1.16 | 0.46–2.94 | 0.76 | 0.71 | 0.42–1.17 | 0.18 |
| Midwest | 1.36 | 0.91–2.03 | 0.14 | 1.33 | 0.42–4.17 | 0.63 | 0.81 | 0.42–1.63 | 0.54 |
| West | 1.09 | 0.82–1.45 | 0.54 | 0.82 | 0.37–1.80 | 0.62 | 1.07 | 0.66–1.71 | 0.79 |
| SES quartiles (reference: 1 [lowest]) | |||||||||
| 2 | 0.63 | 0.44–0.91 | 0.013 | 0.81 | 0.37–1.78 | 0.60 | 1.11 | 0.66–1.89 | 0.70 |
| 3 | 0.78 | 0.57–1.08 | 0.13 | 0.68 | 0.32–1.44 | 0.32 | 0.61 | 0.38–0.96 | 0.034 |
| 4 (highest) | 0.74 | 0.53–1.04 | 0.084 | 0.46 | 0.20–1.00 | 0.052 | 1.00 | 0.59–1.69 | 1.00 |
| PCa as first cancer diagnosis (reference: previously diagnosed with other cancer) | 1.60 | 1.04–2.46 | 0.033 | 0.97 | 0.33–2.90 | 0.96 | 1.61 | 0.88–2.79 | 0.10 |
| PSA (references: 0–5 ng/ml for groups 1 and 3; 10–15 ng/ml for group 2) | |||||||||
| 5–10 ng/ml | 1.35 | 1.04–1.74 | 0.023 |

|
1.29 | 0.87–1.89 | 0.20 | ||
| 10–20 ng/ml |

|
1.41 | 0.35–5.76 | 0.63 |

|
||||
| Percent cores positive (reference: 0–12.5%) | |||||||||
| 12.6–25.0% | 0.77 | 0.57–1.05 | 0.10 | 0.69 | 0.37–1.29 | 0.25 | 0.74 | 0.42–1.26 | 0.27 |
| 25.1–37.5% | 0.51 | 0.38–0.69 | <0.001 | 0.42 | 0.20–0.85 | 0.017 | 0.33 | 0.20–0.54 | <0.001 |
| 37.6–49.9% | 0.34 | 0.23–0.48 | <0.001 | 0.21 | 0.058–0.70 | 0.014 | 0.33 | 0.18–0.58 | <0.001 |
| cT Stage (cT1 as reference for groups 1 and 2; cT2b for group 3) | |||||||||
| cT2a | 167.2 | 122.65–233.09 | <0.001 | 239.8 | 133.8–459.3 | <0.001 |

|
||
| cT2c |

|

|
38.6 | 26.9–55.6 | <0.001 | ||||
CI: confidence interval; NA: not available; OR: odds ratio; PSA: prostate-specific antigen; SEER: Surveillance, Epidemiology, and End Results; SES: socioeconomic status.
Discussion
In this population-based analysis of 20 334 men with FIR PCa managed with AS for at least one year following diagnosis, we determined that most (88.0%) patients eventually discontinued AS in favor of deferred definitive therapy. This figure is significantly higher than that previously reported for low-risk PCa patients from the SEER WW database (65.7%).15 It is also higher than the proportion treated in a recent, single-center experience of AS in intermediate-risk cancer, where it was 49% at 10 years.16 Notably, choice of deferred definitive therapy differed by FIR risk group in our cohort. RT was the treatment of choice for patients with GG2 and PSA 10–20 ng/ml FIR PCa (86.3% and 86.0%, respectively), whereas RP was the treatment of choice for 96.1% of patients with cT2b-c FIR PCa.
A significant annual decrease in number of FIR PCa patients managed with AS was observed for the overall cohort, which was secondary to an absolute decrease in number of PSA 10–20 ng/ml and cT2b-c FIR patients managed with AS. There was a concurrent increase in the number of GG2 FIR patients managed with AS. Despite this overall decrease, it is not possible to infer that there has been a decreased uptake of AS for FIR patients without considering the number of such patients managed with definitive therapy during the same timeframe. This decrease may, in part, reflect the overall decrease in PCa incidence following the 2012 United States Preventive Services Task Force recommendations.17
Advanced clinical stage was consistently found to be strongly associated with increased odds of undergoing deferred definitive intervention, and among those who underwent intervention, higher rates of RP over RT (ORs of 38.6–239.8 across all three FIR groups). These findings may be related to the increased uptake of multiparametric magnetic resonance imaging (mpMRI) in the followup of AS patients since 2010, resulting in increased detection of extraprostatic extension.18 This would plausibly trigger discontinuation of AS and may also explain the decrease in numbers of cT2b-c FIR patients managed with AS between 2010 and 2015 in our cohort.
Patients of higher SES were significantly more likely to undergo deferred definitive therapy across FIR subgroups. Patients of higher SES are known to be more likely to follow up with their physicians,19,20 and thus be more compliant with repeat PSA, clinical exam, and biopsy protocols. This increases the likelihood of detecting signs of disease progression/understaging, which act as triggers for intervention. Interestingly, such patients were also more likely to opt for definitive RT over RP in two of the FIR groups. Similarly, FIR African American patients with GG2 or PSA 10–20 ng/ml were more likely to opt definitive RT over RP, even after controlling for baseline oncological and sociodemographic variables, such as SES and insurance status. These findings may reflect African American patients’ known distrust of the medical system21–23 and their desire to avoid invasive interventions. This is further reflected in GG2 FIR African American patients being 17% less likely to undergo definitive intervention.
This is the first population-based study evaluating sociodemographic and oncological predictors of deferred definitive therapy in AS FIR PCa patients. Our study is strengthened by our use of a large, validated,24,25 nationally representative dataset.9 It is important to note, however, that our deferred intervention rate of 88.0% is significantly higher than those previously reported in other series, which have ranged from 31–49% over a 5–10-year followup period.16,26–28 This difference is likely related in part to differences in cohort eligibility criteria, with previously reported series applying stricter eligibility criteria. In these series, AS was often restricted to FIR patients older than 65 years26,28 or a life expectancy less than 10 years.28 Furthermore, these series originated in academic tertiary centers, where practice patterns are likely to differ from those seen in population-based settings. However, without followup oncological data (e.g., PSA changes, mpMRI findings, GS upgrading, or increased PCa volume on repeat biopsies), exact triggers for intervention, and information regarding patients’ medical comorbidities, it is not possible to discern the exact reasons for these disparities. AS protocols (i.e., timing of confirmatory biopsy, frequency of PSA measurements, etc.) were not available for these patients.
Practice patterns have also evolved since the study time period of 2010–2015. There has been an increased uptake of mpMRI29 and prognostic genetic biomarkers30 in the AS setting, as well as changes to the GS scoring system.31 Thus, the results of this study must be interpreted in light of these recent advances.
Further limitations to this study include the absence of timing of interventions, which precluded us from performing time-to-event analyses with Cox proportional hazard modeling, and inability to differentiate patients initially managed with AS or WW. However, by excluding patients older than 80 years at time of diagnosis, we attempted to minimize the number of patients in the WW group.10 Similar to other studies originating from population-based registries, this analysis is subject to the limitations and inherent biases characteristic of population-based registries, particularly with regards to missing data, which has been found to be as high as 46% in validation studies of this dataset.24,25 Patients in the SEER database are from population-based cancer registries covering approximately 35% of the U.S. population, and thus results from this dataset may not be generalizable to the entire U.S. population.9
Conclusions
Most FIR PCa patients initially managed with AS eventually undergo deferred definitive therapy, with choice of treatment significantly influenced by patients’ baseline oncological and sociodemographic characteristics.
Supplementary Information
Footnotes
Appendix available at cuaj.ca
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:242–62. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part 1: Risk stratification, shared decision-making, and care options. J Urol. 2018;199:683–90. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Carter HB, Lepor A, et al. Active surveillance for prostate cancer: Current evidence and contemporary state of practice. Nat Rev Urol. 2016;13:205–15. doi: 10.1038/nrurol.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term followup of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 5.Hamdy FC, Donovan JL, Lane A, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Eng J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 6.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: Cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013;105:1050–8. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 8.Butler SS, Mahal BA, Lamba N, et al. Use and early mortality outcomes of active surveillance in patients with intermediate-risk prostate cancer. Cancer. 2019;125:3164–71. doi: 10.1002/cncr.32202. [DOI] [PubMed] [Google Scholar]
- 9.NIH. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Prostate with Watchful Waiting database (2010–2016) [Accessed Jan 2, 2021]. Available at: https://seer.cancer.gov/seerstat/databases/prostate-ww/index.html/
- 10.Steinberg GD, Bales GT, Brendler CB. An analysis of watchful waiting for clinically localized prostate cancer. J Urol. 1998;159:1431–6. doi: 10.1097/00005392-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Chandrasekar T, Klaassen Z, Goldberg H, et al. High competing risks minimize real-world utility of adjuvant targeted therapy in renal cell carcinoma: A population-based analysis. Oncotarget. 2018;9:16731–43. doi: 10.18632/oncotarget.24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekar T, Klaassen Z, Goldberg H, et al. Metastatic renal cell carcinoma: Patterns and predictors of metastases — a contemporary, population-based series. Urol Oncol. 2017;35:661.e7–14. doi: 10.1016/j.urolonc.2017.06.060. [DOI] [PubMed] [Google Scholar]
- 13.Sayyid RK, Wilson B, Benton JZ, et al. Upgrading on radical prostatectomy specimens of very low- and low-risk prostate cancer patients on active surveillance: A population-level analysis. Can Urol Assoc J. 2021;15:E335–9. doi: 10.5489/cuaj.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 15.Sayyid RK, Klotz L, Benton JZ, et al. Influence of sociodemographic factors on definitive intervention among very low- and low-risk active surveillance patients. Urology. 2021;155:117–23. doi: 10.1016/j.urology.2021.01.053. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson S, Benfante N, Alvim R, et al. Risk of metastasis in men with grade group 2 prostate cancer managed with active surveillance at a tertiary cancer center. J Urol. 2020;203:1117–21. doi: 10.1097/JU.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14:26–37. doi: 10.1038/nrurol.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fam MM, Yabes JG, Macleod LC, et al. Increasing utilization of multiparametric magnetic resonance imaging in prostate cancer active surveillance. Urology. 2019;130:99–105. doi: 10.1016/j.urology.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olah ME, Gaisano G, Hwang SW. The effect of socioeconomic status on access to primary care: An audit study. CMAJ. 2013;185:E263–9. doi: 10.1503/cmaj.121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong MKY, Wang JT, Czarnecki A, et al. Factors associated with physician followup among patients with chest pain discharged from the emergency department. CMAJ. 2015;187:E160–8. doi: 10.1503/cmaj.141294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs EA, Rolle I, Ferrans CE, et al. Understanding African Americans’ views of the trustworthiness of physicians. J Gen Intern Med. 2006;21:642–7. doi: 10.1111/j.1525-1497.2006.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajakumar K, Thomas SB, Musa D, et al. Racial differences in parents’ distrust of medicine and research. Arch Pediatr Adolesc Med. 2009;163:108–14. doi: 10.1001/archpediatrics.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbie-Smith G, Thomas SB, StGeorge DMM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–63. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 24.Jeong CW, Washington SL, Herlemann A, et al. The new Surveillance, Epidemiology, and End Results Prostate with Watchful Waiting database: Opportunities and limitations. Eur Urol. 2020;78:335–44. doi: 10.1016/j.eururo.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Laviana AA, Luckenbaugh AN, Wallis CJD. Seeking the truth: Understanding the impact of missing data on the validity of the new Surveillance, Epidemiology and End Results Prostate with Watchful Waiting database. Eur Urol. 2020;78:345–6. doi: 10.1016/j.eururo.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localized prostate cancer. Eur Urol. 2013;64:981–7. doi: 10.1016/j.eururo.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–34. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musunuru HB, Yamamoto T, Klotz L, et al. Active surveillance for intermediate-risk prostate cancer: Survival outcomes in the Sunnybrook experience. J Urol. 2016;16:1651–8. doi: 10.1016/j.juro.2016.06.102. [DOI] [PubMed] [Google Scholar]
- 29.Fam MM, Yabes JG, Macleod LC, et al. Increasing utilization of multiparametric magnetic resonance imaging in prostate cancer active surveillance. Urology. 2019;130:99–105. doi: 10.1016/j.urology.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin DW, Nelson PS. Prognostic genomic biomarkers in patients with localized prostate cancer. JAMA Onco. 2021;7:59–60. doi: 10.1001/jamaoncol.2020.6045. [DOI] [PubMed] [Google Scholar]
- 31.Swanson GP, Trevathan S, Hammonds KAP, et al. Gleason score evolution and the effect on prostate cancer outcomes. Am J Clin Pathol. 2021;155:711–7. doi: 10.1093/ajcp/aqaa130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.