Abstract
Ibrutinib is associated with durable responses in patients with Waldenström macroglobulinemia (WM). We hypothesized that response depth is predictive of progression-free survival (PFS) in WM patients treated with ibrutinib. Using landmark analyses, we evaluated response depth in two cohorts of WM patients treated with ibrutinib monotherapy. The learning cohort was composed of 93 participants from two clinical trials, and the validation cohort of 190 consecutive patients treated off clinical trial. Rates of partial response (PR) or better at 6 months in learning and validation cohorts were 64% and 71%, respectively (p=0.29). In the learning cohort, 3-year PFS rates for patients who attained PR or better at 6 months vs. not were 81% and 57%, respectively (p=0.009). In the validation cohort, 3-year PFS rates for patients who attained PR or better at 6 months vs. not were 83% and 54%, respectively (p=0.008). In multivariate analyses, attaining PR or better at 6 months was associated with superior PFS in the learning (HR 0.38; p=0.01) and validation cohorts (HR 0.18; p=0.004). Attaining PR at 6 months on ibrutinib emerges as an intermediate outcome of interest and should be validated as surrogate for PFS in clinical trials evaluating Bruton tyrosine kinase inhibitors in WM.
INTRODUCTION
The oral Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved in Europe and the United States (US) for the treatment of patients with symptomatic Waldenström macroglobulinemia (WM). The approval was based on the results of a prospective phase II study in which 63 previously treated patients with WM were treated with ibrutinib monotherapy, with an overall response rate (ORR) of 91%, a major response (partial response [PR] or better) rate of 73% and a 5-year progression-free survival (PFS) of 54% (Treon, et al 2015). In two other prospective studies in previously untreated or those refractory to rituximab therapy similar response rates were seen, with a 18-month PFS rate in previously untreated vs rituximab-refractory of 92% vs 86%, respectively (Dimopoulos, et al 2017, Treon, et al 2018). WM is an indolent malignancy, and with the use of effective therapies, PFS can be substantially prolonged, thereby requiring protracted patient follow-up in randomized controlled clinical trials.
Prior studies have suggested that depth of response correlates with PFS in WM patients treated with chemoimmunotherapy (Castillo, et al 2018, Treon, et al 2014, Treon, et al 2011). However, it is unclear if depth of response can be used as a surrogate for PFS in patients with WM treated with ibrutinib monotherapy. This analysis is timely, as the recently published open-label, randomized phase III ASPEN study comparing zanubrutinib and ibrutinib, has the proportion of patients achieving very good partial response (VGPR) or better as the primary endpoint (Tam, et al 2020).
We hypothesized that the depth of response at a specific time point following treatment initiation is predictive of PFS in patients with WM treated with ibrutinib. We tested our hypothesis by evaluating two separate cohorts (learning and validation) of WM patients treated with ibrutinib monotherapy using a landmark analysis.
PATIENT AND METHODS
Patient selection
The patients who participated in two prospective clinical trials (NCT01614821 and NCT02604511) at Dana-Farber Cancer Institute (DFCI), Memorial Sloan Kettering Cancer Center and Stanford Cancer Center composed the learning cohort. The validation cohort was composed of consecutive WM patients treated with ibrutinib monotherapy off clinical trial at DFCI and Mayo Clinic. All patients provided consent to having their data collected for research, and met clinicopathological criteria for a diagnosis of WM requiring treatment based on guidelines from the 2nd International Workshop on WM (IWWM-2) (Kyle, et al 2003, Owen, et al 2003). Patients with central nervous system involvement by WM were excluded from the study.
Data collection
Pertinent clinicopathological data were collected at the time of ibrutinib therapy initiation. Categorical responses were assessed based on modified IWWM-6 criteria (Owen, et al 2013), in which assessment of extramedullary disease was not required for attainment of a minor response (MR; 25-50% decrease in serum IgM from baseline), PR (50-90% decrease in serum IgM from baseline),VGPR (>90% decrease in serum IgM from baseline, or normalization of serum IgM levels with persistence of a detectable IgM monoclonal paraprotein in serum protein electrophoresis) but was mandated for attainment of complete response (CR; normalization of serum IgM level and serum protein electrophoresis and complete resolution of extramedullary disease as well as bone marrow involvement). This modification to IWWM-6 response criteria is widely used for clinical trial design. We evaluated two depths of response: attainment of PR or better, and VGPR or better. As the depth of response to ibrutinib monotherapy is time dependent, with rates of response increasing over time, we chose two response assessment time points, at 6 (± 1month, in the validation cohort) and 12 months (± 2 months, in the validation cohort) from treatment initiation. At DFCI and for patients from clinical trials, MYD88 and CXCR4 mutational status was assessed in CD19-selected bone marrow samples using allele-specific polymerase chain reaction (AS-PCR) for MYD88 L265P and nonsense CXCR4 mutations. Frameshift CXCR4 mutations were assessed by Sanger sequencing. MYD88 and CXCR4 mutational detection techniques have been previously reported (Hunter, et al 2014, Treon, et al 2012, Xu, et al 2016, Xu, et al 2013). At Mayo Clinic, MYD88 mutational status was assessed by the amplification-refractory mutation system (ARMS), a variant of AS-PCR. DNA is extracted using the Qiagen DNeasy kit (Qiagen, Valencia, CA) from archived unsorted bone marrow aspirate sample pellets fixed in methanol–acetic acid. A single-tube multiplex ARMS is performed using primers situated in exon 5 of MYD88 (NM_002468.4), including one primer specifically targeting the L256P alteration. Reaction products are analyzed using capillary electrophoresis (QIAxcel; Qiagen). MYD88 wild-type control amplification yields a PCR product of 141 base pairs (bp), and if present, an additional specific 72-bp product denotes the L265P mutation. CXCR4 mutational testing was not performed in Mayo Clinic patients.
Statistical analysis
Patients’ characteristics and response rates are presented using descriptive statistics. Differences between categorical variables were assessed using the Chi-square test or the Fisher exact test, based on the number of observations. Depending on the time of the landmark analyses, PFS was estimated starting at the 6-month mark (for patients with response assessment at 6 months) and starting at the 12-month mark (for patients with response assessment at 12 months). Survival curves were generated using the Kaplan-Meier method for incomplete observations and compared using the log-rank test. Since we performed four separate landmark analyses evaluating two depths of response (PR or better, and VGPR or better) at two difference time points (6 and 12 months), p-values <0.0125 were considered statistically significant (p<0.05 divided by 4, to adjust for multiplicity). We then fitted univariate and multivariate Cox proportional-hazard regression models for PFS at each landmark. Outcomes are reported using hazard ratio (HR) with 95% confidence interval (CI). Differences in overall survival or survival after first treatment initiation were not assessed due to a small number of deaths in this cohort (n=37; 13%) at the time of this report. Calculations were obtained using STATA 15 (StataCorp, College Station, TX, USA).
RESULTS
Patients’ characteristics
Ninety-three and 190 patients comprised the learning and validation cohorts, respectively. The distribution of patients’ characteristics as well as differences between groups are shown in Table 1. In the validation vs. learning cohort, there was a statistically lower proportion of male patients (62% vs. 74%; p=0.04), and bone marrow involvement ≥50% (48% vs. 68%; p=0.003) and a trend towards a higher proportion of previously untreated patients (78% vs. 68%; p=0.07), respectively. There were no statistical differences between cohorts in the proportion of patients age >65 years, hemoglobin level ≤11.5 g/dl, platelet count ≤100 K/uL, serum β2-microglobulin ≤3 mg/l, serum IgM level >7,000 mg/dl, IPPSWM distribution, MYD88 and CXCR4 mutational status. CXCR4 mutational status data were not available in 1 patient from the learning and 83 patients from the validation cohort.
Table 1.
Baseline characteristics of Waldenström macroglobulinemia patients treated with ibrutinib from the learning and validation cohorts
| Characteristic | Learning cohort (n=93) |
Validation cohort (n=190) |
p-value |
|---|---|---|---|
| Age >65 years | 50 (54%) | 116 (61%) | 0.24 |
| Male sex | 69 (74%) | 118 (62%) | 0.04 |
| Hemoglobin level ≤11.5 g/dl | 66 (71%) | 131/179 (73%) | 0.70 |
| Platelet count ≤100 K/uL | 9 (10%) | 23/176 (13%) | 0.41 |
| Serum β2-microglobulin >3 mg/l | 65/91 (71%) | 77/124 (62%) | 0.15 |
| Serum IgM level >7,000 mg/dl | 6 (5%) | 10/189 (5%) | 0.98 |
| Bone marrow involvement ≥50% | 63 (68%) | 78/161 (48%) | 0.003 |
| IPSSWM | |||
| Low risk | 20/91 (22%) | 30/134 (22%) | 0.54 |
| Intermediate risk | 34/91 (38%) | 41/134 (31%) | |
| High risk | 37/91 (41%) | 63/134 (47%) | |
| MYD88 L265P mutation present | 89 (96%) | 137/146 (94%) | 0.54 |
| CXCR4 mutation present | 36/92 (39%) | 42/107 (39%) | 0.99 |
| Previously treated | 63 (68%) | 148 (78%) | 0.07 |
IPSSWM: International Prognostic Scoring System for Waldenström macroglobulinemia
Response to ibrutinib therapy
Categorical response rates at 6 and 12 months in patients from the learning and validation cohorts are shown in Table 2.
Table 2.
Categorical response rates to ibrutinib at 6 and 12 months in Waldenström macroglobulinemia patients from the learning and validation cohorts
| At 6 months | Learning cohort (n=86) |
Validation cohort (n=150) |
p-value |
|---|---|---|---|
| VGPR | 8 (9%) | 28 (19%) | 0.18 |
| PR | 47 (55%) | 78 (52%) | |
| MR | 18 (21%) | 30 (20%) | |
| NR | 13 (15%) | 14 (9%) | |
| At 12 months | Learning cohort (n=76) |
Validation cohort (n=126) |
p-value |
| VGPR | 15 (20%) | 31 (25%) | 0.41 |
| PR | 41 (54%) | 71 (56%) | |
| MR | 12 (16%) | 18 (14%) | |
| NR | 8 (11%) | 6 (5%) |
VGPR: very good partial response; PR: partial response; MR: minor response; NR: no response
At 6 months, data on response were available in 86 patients (92%) from the learning cohort and in 150 patients (79%) from the validation cohort. In the learning cohort, 7 patients were not evaluable for response as the follow-up time was shorter than 6 months, and therefore there were no missing data. In the validation cohort, 26 were not evaluable for response due to follow-up shorter than 6 months, leaving 14 patients (7%) with missing data. The rates of PR or better at 6 months in the learning and validation cohorts were 64% (55/86) and 71% (106/150), respectively (p=0.29), and the rates of VGPR or better were 9% (8/86) and 19% (28/150), respectively (p=0.06).
At 12 months, data on response were available in 76 patients (82%) from the learning cohort and in 126 patients (66%) from the validation cohort. In the learning cohort, 16 patients were not evaluable for response as follow-up time was shorter than 12 months, leaving 1 patient (1%) with missing data. In the validation cohort, 54 patients were not evaluable for response due to follow-up time shorter than 12 months, leaving 10 patients (8%) with missing data. The rates of PR or better at 12 months in the learning and validation cohorts were 74% (56/76) and 81% (102/126), respectively (p=0.23), and the rates of VGPR or better were 20% (15/76) and 25% (31/126), respectively (p=0.42). There was one patient in the validation cohort who attained CR.
Landmark analyses at 6 months
The median follow-up times for the learning cohort was 40 months (95% CI 38-49 months) while it was 31 months (95% 28-34 months) for the validation cohort (p<0.001).
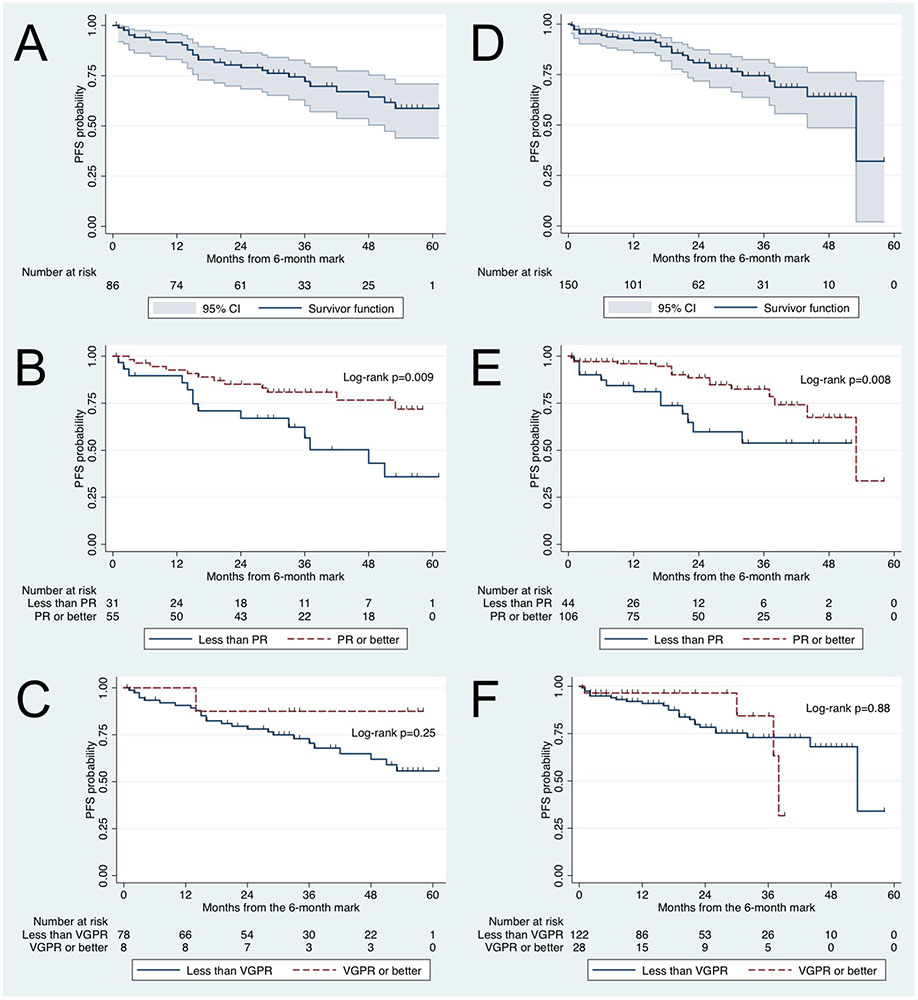
In the learning cohort, the 3-year PFS rate starting at the 6-month mark was 72% (95% CI 60-81%; Figure 1A). For patients who attained PR or better at 6 months vs. not, the 3-year PFS rate starting at the 6-month mark was 81% (95% CI 67-89%) vs. 57% (95% CI 35-74%) respectively (p=0.009; Figure 1B). For patients who attained VGPR or better vs. not at 6 months, the 3-year PFS rate was 88% (95% CI 39-98%) vs. 70% (95% CI 58-80%), respectively (p=0.25; Figure 1C).
Figure 1.
Kaplan-Meier progression-free survival (PFS) curves starting at the 6-month mark in Waldenström macroglobulinemia patients. Learning cohort: (A) PFS, (B) PFS according to attaining partial response (PR) at 6 months or not, and (C) PFS according to attaining very good partial response (VGPR) at 6 months or not. Validation cohort: (D) PFS, (E) PFS according to attaining PR at 6 months or not, and (F) PFS according to attaining VGPR at 6 months or not.
In the validation cohort, the 3-year PFS rate starting at the 6-month mark was 76% (95% CI 66-84%; Figure 1D). For patients who attained PR or better at 6 months vs. not, the 3-year PFS rate starting at the 6-month mark was 83% (95% CI 71-90%) vs. 54% (95% CI 32-71%), respectively (p=0.008; Figure 1E). For patients who attained VGPR or better vs. not at 6 months, the 3-year PFS rate starting at the 6-month mark was 84% (95% CI 43-97 vs. 73% (95% CI 61-82%), respectively (p=0.88; Figure 1F).
Landmark analyses at 12 months
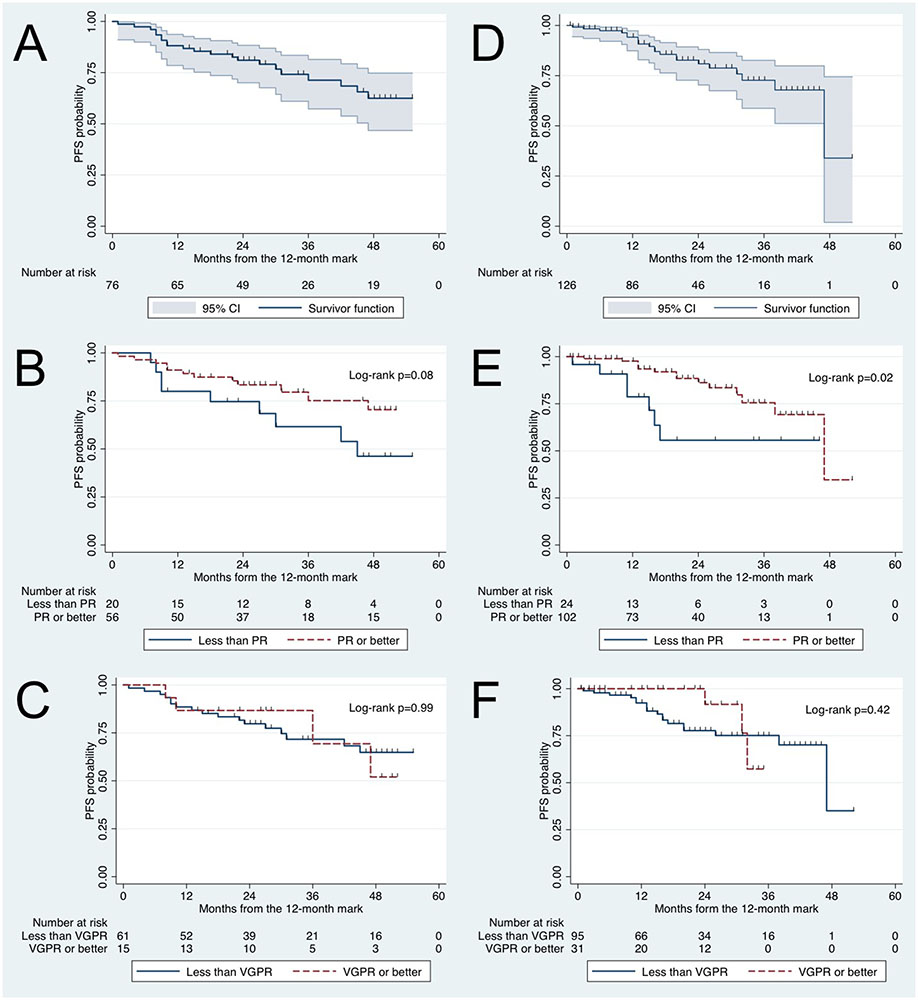
In the learning cohort, the 3-year PFS rate starting at the 12-month mark was 71% (95% CI 57-81%; Figure 2A). For patients who attained PR or better at 12 months vs. not, the 3-year PFS rate starting at the 12-month mark was 75% (95% CI 58-86%) vs. 62% (95% CI 35-80%), respectively (p=0.08; Figure 2B). For patients who attained VGPR or better vs. not at 12 months, the 3-year PFS rate starting at the 12-month mark was 69% (95% CI 26-91%) vs. 72% (95% CI 57-82%), respectively (p=0.99; Figure 2C).
Figure 2.
Kaplan-Meier progression-free survival (PFS) curves starting at the 12-month mark in Waldenström macroglobulinemia patients. Learning cohort: (A) PFS, (B) PFS according to attaining partial response (PR) at 12 months or not, and (C) PFS according to attaining very good partial response (VGPR) at 12 months or not. Validation cohort: (D) PFS, (E) PFS according to attaining PR at 12 months or not, (F) PFS according to attaining VGPR at 12 months or not.
In the validation cohort, the 3-year PFS rate starting at the 12-month mark was 71% (95% CI 57-81%; Figure 2D). For patients who attained PR or better at 12 months vs. not, the 3-year PFS was 76% (95% CI 58-86%) vs. 56% (95% CI 27-77%), respectively (p=0.02; Figure 2E) and therefore did not meet the pre-defined study criteria for statistical significance. For patients who attained VGPR or better at 12 months vs. not, the 3-year PFS rate starting at the 12-month mark was 56% (95% CI 27-77%) vs. 75% (95% CI 62-84%), respectively (p=0.42; Figure 2F).
Univariate and multivariate analysis
We fitted univariate models to identify predictive factors for PFS starting at the 6-month mark in the learning and validation cohorts separately. These models are shown in Table 3.
Table 3.
Univariate Cox proportional-hazard regression models for progression-free survival in the learning and validation cohorts
| Learning cohort | Validation cohort | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age >65 years | 1.36 (0.63-2.96) | 0.43 | 1.28 (0.60-2.77) | 0.52 |
| Male sex | 1.02 (0.41-2.56) | 0.96 | 1.80 (0.77-4.22) | 0.18 |
| Hemoglobin level ≤11.5 g/dl | 1.03 (0.45-2.38) | 0.94 | 1.54 (0.58-4.09) | 0.39 |
| Platelet count ≤100 K/uL | 2.76 (0.82-9.26) | 0.10 | 4.73 (1.94-11.5) | 0.001 |
| Serum β2-microglobulin >3 mg/l | 0.53 (0.24-1.20) | 0.13 | 1.39 (0.54-3.60) | 0.49 |
| Serum IgM level >7,000 mg/dl | 4.65 (1.36-16.0) | 0.01 | 1.23 (0.29-5.23) | 0.78 |
| Bone marrow involvement ≥50% | 0.49 (0.23-1.06) | 0.07 | 1.02 (0.47-2.24) | 0.95 |
| High vs. low IPSSWM | 1.45 (0.52-4.02) | 0.47 | 3.77 (0.85-16.8) | 0.08 |
| Intermediate vs. low IPSSWM | 0.87 (0.30-2.51) | 0.80 | 3.00 (0.61-14.7) | 0.18 |
| MYD88 L265P mutation present | 0.20 (0.03-1.58) | 0.13 | UTC | |
| CXCR4 mutation present | 2.47 (1.13-5.39) | 0.02 | 3.01 (1.09-8.30) | 0.03 |
| Previously treated | 1.65 (0.60-4.52) | 0.33 | 1.69 (0.51-5.63) | 0.39 |
| PR or better at 6 months | 0.37 (0.17-0.80) | 0.01 | 0.38 (0.18-0.80) | 0.01 |
IPSSWM: International Prognostic Scoring System for Waldenström macroglobulinemia; PR: Partial response; HR: hazard ratio; CI: confidence interval; UTC: unable to calculate
In the learning cohort, attaining a major response at 6 months was associated with better PFS (HR 0.37, 95% CI 0.17-0.80; p=0.01), while serum IgM ≥7,000 mg/dl (HR 4.65, 95% CI 1.36-16.0; p=0.01) and CXCR4 mutations (HR 2.47, 95% CI 1.13-5.39; p=0.02) were associated with worse PFS. No other variables were associated with either a better or worse PFS. In a multivariate model including attaining PR or better at 6 months and CXCR4 mutations, attaining a major response at 6 months was an independent prognostic factor of adverse PFS (HR 0.38, 95% CI 0.15-0.84; p=0.01). None of these variables violated the proportionality assumption (p=0.85 and p=0.78, respectively). The interaction term between PR or better at 6 months and CXCR4 mutations was not significant (p=0.38).
In the validation cohort, attaining PR or better at 6 months was associated with better PFS (HR 0.38, 95% CI 0.18-0.80; p=0.01), while platelet count ≤100 K/uL (HR 4.73, 95% CI 1.94-11.5; p=0.001) and CXCR4 mutations (HR 3.01, 95% CI 1.09-8.30; p=0.03) were associated with worse PFS. No other variables were associated with either a better or worse PFS. In a multivariate model including attaining PR or better at 6 months, platelet count ≤100 K/uL and CXCR4 mutations, attaining PR or better at 6 months was an independent prognostic factor for better PFS (HR 0.18, 95% CI 0.05-0.58; p=0.004). None of these variables violated the proportionality assumption (p=0.55, p=71 and p=0.39, respectively). The interaction term between PR or better at 6 months and CXCR4 mutations was not significant (p=0.97).
DISCUSSION
Ibrutinib is the only drug formally approved for the treatment of patients with symptomatic WM in the United States and Europe, and a number of prospective and retrospective studies have shown ibrutinib monotherapy to be safe, effective and able to induce durable responses in treatment naïve as well previously treated patients with WM (Abeykoon, et al 2019, Castillo, et al 2019, Dimopoulos, et al 2017, Treon, et al 2018, Treon, et al 2015). Data, however, are limited regarding prognostic factors for PFS on ibrutinib therapy. Furthermore, the association between depth of response to and PFS on ibrutinib has not been previously evaluated.
To the best of our knowledge, this is the first study evaluating the prognostic value of depth of response on PFS in patients with WM treated with ibrutinib monotherapy. All, the patients in the validation cohort were treated at academic institutions with experience in WM. Using a landmark analysis, we evaluated two depths of response, PR or better and VGPR or better, at two different time points, 6 and 12 months. The only combination of depth and timing of response that met our predetermined criteria, and therefore the only factor prognostic of a superior PFS in both the learning and the validation cohorts, was attaining PR or better at 6 months. As the median follow-up time for the learning cohort was 40 months and for the validation cohort was 31 months, we presented PFS rates at 3 years. The 3-year PFS rates for attaining PR or better versus not in the learning cohort were 81% and 57%, respectively, while in the validation cohort the 3-year PFS rates for attaining PR or better versus not were 83% and 54%, respectively.
The baseline characteristics between our learning and validation cohorts were relatively similar, with higher proportion of men and bone marrow involvement ≥50% in the validation cohort with no other differences. The patients included had a similar distribution to WM patients in the general population, with more than half of the patients being older than 65 years and an approximate 2-to-1 male predominance (Castillo, et al 2014, Castillo, et al 2015, Kastritis, et al 2015). The laboratory values for serum hemoglobin, β2-microglobulin and IgM levels as well as platelet counts were consistent with previous retrospective and prospective studies (Abeykoon, et al 2019, Castillo, et al 2018, Treon, et al 2014). Consistently, the proportion of patients deemed to be of low, intermediate and high risk based on the IPSSWM was similar to the seminal report by Morel and colleagues (Morel, et al 2009). Finally, the distribution of the genomic alterations in MYD88 and CXCR4 are consistent with prior reports from our research group and others (Ballester, et al 2016, Castillo, et al 2019, Hunter, et al 2014, Poulain, et al 2016, Schmidt, et al 2015). Overall, we believe the characteristics of the patients reported in the present study are representative of the WM population, which would make our results generalizable.
We also assessed univariate and multivariate Cox proportional-hazard regression models to better understand the independent impact of attaining PR or better at 6 months on PFS. As data on prognostic factors in WM patients on ibrutinib therapy are limited, we constructed the statistical models by evaluating clinically relevant factors such as age, serum IgM levels and hemoglobin levels, among others. Attaining PR or better at 6 months was an independent favorable prognostic factor for PFS in both the learning and validation cohorts, associated with an 60-80% lower risk of progression and/or death than in patients who did not attain PR or better at 6 months.
We believe our findings of impact of attaining PR or better at 6 months are of clinical relevance and could help practitioners counseling patients and family members, and more importantly, serve as a surrogate for PFS in clinical trial design evaluating BTK inhibitors. The covalent, irreversible BTK inhibitors acalabrutinib, zanubrutinib and tirabrutinib are undergoing clinical development in WM, and have shown encouraging safety and efficacy data (Munakata, et al 2019, Owen, et al 2020, Trotman, et al 2019). The results of the randomized ASPEN study have been recently published (Tam, et al 2020). In this open-label study, 201 WM patients were randomized to receive zanubrutinib or ibrutinib. The main objective of the study was to show superiority in VGPR rates of zanubrutinib over ibrutinib. In this regard, the study did not meet its endpoint, as zanubrutinib and ibrutinib were associated with VGPR rates of 28% and 19%, respectively, and were not statistically different. Rates of PR or better were comparable at 77% and 78%, and with a median follow-up of 18 months, the 18-month PFS rates were also similar at 85% and 84%, respectively.
The presence of CXCR4 mutations emerged as an adverse prognostic factor for PFS in WM patients treated with ibrutinib monotherapy. We had previously reported an adverse impact of CXCR4 mutations in depth of response and PFS in WM patients treated with ibrutinib monotherapy (Castillo, et al 2019, Treon, et al 2018, Treon, et al 2019, Treon, et al 2015). However, not all CXCR4 mutations appear to weigh equally on outcome, as there is mounting evidence than nonsense CXCR4 mutations, especially with a clonality higher than 25%, might have a more adverse impact on outcomes to ibrutinib than frameshift CXCR4 mutations (Castillo, et al 2019, Gustine, et al 2019). These findings support the development of therapeutic strategies targeting CXCR4, as one avenue for clinical research in patients with WM. Prospective clinical trials evaluating the combination of ibrutinib and the anti-CXCR4 monoclonal antibody ulocuplumab (NCT03225716) and ibrutinib and the CXCR4-targeting small molecule mavorixafor (NCT04274738) are underway in WM patients who harbor a CXCR4 mutation. Akin to chronic lymphocytic leukemia, BTK C481S mutations have shown to confer resistance to irreversible, covalent BTK inhibitors in patients with WM (Chen, et al 2018, Woyach, et al 2014). A new generation of reversible, noncovalent BTK inhibitors that do not interact with the C481 loci, such as vecabrutinib (NCT03037645), LOXO-305 (NCT03740529) and ARQ513 (NCT03162536), are also being evaluated in clinical trials.
Our study has limitations. First, the total sample size of 283 patients could be considered small, when compared to other malignancies. However, WM is a rare disease with an incidence of 1,500 new cases per year in the United States. Taking this aspect into account, our cohort is the largest reporting clinical experience in WM patients on ibrutinib. Second, there were missing data on response in the validation and in the learning cohort. Yet, the rates of missing data were low, at 1% in the learning cohort and less than 10% in the validation cohort. Despite the difference in missing response data, the rates of response at 6 and 12 months were not statistically different between the learning and validation cohorts. Data on CXCR4 mutational status were missing in 30% of patients, and the results of the subgroup analyses with regards to CXCR4 mutational status should be taken with caution. Nevertheless, the clinical features of the patients in our cohorts (e.g. age and sex distribution as well as median serum IgM levels) were consistent with and representative of the general population of patients with WM.
We should note that the purpose of our study was to identify a predictive marker of PFS in WM patients that could be later validated as a surrogate marker in clinical trials. Our purpose was not to identify a time point in which ibrutinib therapy should be modified, and therefore not attaining PR or better at 6 months on ibrutinib monotherapy should not be factored into the decision of discontinuing or changing ibrutinib therapy in patients with WM. We conclude that attaining PR or better at 6 months on ibrutinib monotherapy was associated with better PFS and should be validated as a surrogate endpoint for PFS in clinical trials evaluating BTK inhibitors in WM patients.
Acknowledgements
Portions of this research were presented at the 24th European Hematology Association Congress in Amsterdam, Netherlands, in June 2019, and at the 61st American Society of Hematology Meeting in Orlando, Florida, in December 2019. Dr. Castillo would like to acknowledge the support of the WMR Fund.
Footnotes
Disclosures
JJC received honoraria and/or research funding from Abbvie, Beigene, Janssen, Kymera, Millennium, Pharmacyclics and TG Therapeutics. RHA received research funding from Janssen and Pharmacyclics. MLP received honoraria and/or research funding from Celgene, Kite, Merck, Novartis and Pharmacyclics. SMA received research funding from Affimed, AI Therapeutics, Bristol Myers Squibb, Pfizer, Regeneron, Seattle Genetics and Trillium. MAG received honoraria and/or research funds from Abbvie, Alnylam, Amgen, Annexon, Appellis, Celgene, Ionis/Akcea, Janssen, Johnson & Johnson, Pharmacyclics, Prothena, Teva and Spectrum. PK received research funding from Amgen, Celgene and Takeda Pharmaceuticals. SPT received research funding and/or consulting fees from Bristol Myers Squibb, Janssen and Pharmacyclics. All other authors have no conflicts of interest to disclose.
References
- Abeykoon JP, Zanwar S, Ansell SM, Gertz MA, Kumar S, Manske M, Novak AJ, King R, Greipp P, Go R, Inwards D, Muchtar E, Habermann T, Witzig TE, Thompson CA, Dingli D, Lacy MQ, Leung N, Dispenzieri A, Gonsalves W, Warsame R, Kyle RA, Rajkumar V, Parikh SA & Kapoor P (2019) Ibrutinib monotherapy outside of clinical trial setting in Waldenstrom macroglobulinaemia: practice patterns, toxicities and outcomes. Br J Haematol. [DOI] [PubMed] [Google Scholar]
- Ballester LY, Loghavi S, Kanagal-Shamanna R, Barkoh BA, Lin P, Medeiros LJ, Luthra R & Patel KP (2016) Clinical Validation of a CXCR4 Mutation Screening Assay for Waldenstrom Macroglobulinemia. Clin Lymphoma Myeloma Leuk, 16, 395–403 e391. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Meid K, Gustine JN, Dubeau T, Severns P, Hunter ZR, Yang G, Xu L & Treon SP (2018) Prospective Clinical Trial of Ixazomib, Dexamethasone, and Rituximab as Primary Therapy in Waldenstrom Macroglobulinemia. Clin Cancer Res, 24, 3247–3252. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Olszewski AJ, Cronin AM, Hunter ZR & Treon SP (2014) Survival trends in Waldenstrom macroglobulinemia: an analysis of the Surveillance, Epidemiology and End Results database. Blood, 123, 3999–4000. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Olszewski AJ, Kanan S, Meid K, Hunter ZR & Treon SP (2015) Overall survival and competing risks of death in patients with Waldenstrom macroglobulinaemia: an analysis of the Surveillance, Epidemiology and End Results database. Br J Haematol, 169, 81–89. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Xu L, Gustine JN, Keezer A, Meid K, Dubeau TE, Liu X, Demos MG, Kofides A, Tsakmaklis N, Chen JG, Munshi M, Guerrera ML, Chan GG, Patterson CJ, Yang G, Hunter ZR & Treon SP (2019) CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenstrom macroglobulinaemia treated with ibrutinib. Br J Haematol, 187, 356–363. [DOI] [PubMed] [Google Scholar]
- Chen JG, Liu X, Munshi M, Xu L, Tsakmaklis N, Demos MG, Kofides A, Guerrera ML, Chan GG, Patterson CJ, Meid K, Gustine J, Dubeau T, Severns P, Castillo JJ, Hunter ZR, Wang J, Buhrlage SJ, Gray NS, Treon SP & Yang G (2018) BTK(Cys481Ser) drives ibrutinib resistance via ERK1/2 and protects BTK(wild-type) MYD88-mutated cells by a paracrine mechanism. Blood, 131, 2047–2059. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Trotman J, Tedeschi A, Matous JV, Macdonald D, Tam C, Tournilhac O, Ma S, Oriol A, Heffner LT, Shustik C, Garcia-Sanz R, Cornell RF, de Larrea CF, Castillo JJ, Granell M, Kyrtsonis MC, Leblond V, Symeonidis A, Kastritis E, Singh P, Li J, Graef T, Bilotti E, Treon S, Buske C, iNnovate Study G & the European Consortium for Waldenstrom's, M. (2017) Ibrutinib for patients with rituximab-refractory Waldenstrom's macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol, 18, 241–250. [DOI] [PubMed] [Google Scholar]
- Gustine JN, Xu L, Tsakmaklis N, Demos MG, Kofides A, Chen JG, Liu X, Munshi M, Guerrera ML, Chan GG, Patterson CJ, Keezer A, Meid K, Dubeau T, Yang G, Hunter ZR, Treon SP & Castillo JJ (2019) CXCR4 (S338X) clonality is an important determinant of ibrutinib outcomes in patients with Waldenstrom macroglobulinemia. Blood Adv, 3, 2800–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C, Patterson CJ, Sheehy P & Treon SP (2014) The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood, 123, 1637–1646. [DOI] [PubMed] [Google Scholar]
- Kastritis E, Gavriatopoulou M, Kyrtsonis MC, Roussou M, Hadjiharissi E, Symeonidis A, Repoussis P, Michalis E, Delimpasi S, Tsatalas K, Tsirigotis P, Vassou A, Vervessou E, Katodritou E, Gika D, Terpos E & Dimopoulos MA (2015) Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenstrom macroglobulinemia: final analysis of a phase 2 study. Blood, 126, 1392–1394. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Treon SP, Alexanian R, Barlogie B, Bjorkholm M, Dhodapkar M, Lister TA, Merlini G, Morel P, Stone M, Branagan AR & Leblond V (2003) Prognostic markers and criteria to initiate therapy in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol, 30, 116–120. [DOI] [PubMed] [Google Scholar]
- Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, Crowley J, Ocio EM, Garcia-Sanz R, Treon SP, Leblond V, Kyle RA, Barlogie B & Merlini G (2009) International prognostic scoring system for Waldenstrom macroglobulinemia. Blood, 113, 4163–4170. [DOI] [PubMed] [Google Scholar]
- Munakata W, Sekiguchi N, Shinya R, Suzuki K, Handa H, Shibayama H, Endo T, Terui Y, Iwaki N, Fukuhara N, Tatetsu H, Iida S, Ishikawa T, Shiibashi R & Izutsu K (2019) Phase 2 Study of Tirabrutinib (ONO/GS-4059), a Second-Generation Bruton's Tyrosine Kinase Inhibitor, Monotherapy in Patients with Treatment-Naïve or Relapsed/Refractory Waldenström Macroglobulinemia. Blood, 134, 345–345. [Google Scholar]
- Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G, Garcia-Sanz R, Ocio EM, Morra E, Morel P, Anderson KC, Patterson CJ, Munshi NC, Tedeschi A, Joshua DE, Kastritis E, Terpos E, Ghobrial IM, Leleu X, Gertz MA, Ansell SM, Morice WG, Kimby E, Treon SP & macroglobulinaemia, V.I.I.W.o.W. (2013) Response assessment in Waldenstrom macroglobulinaemia: update from the VIth International Workshop. Br J Haematol, 160, 171–176. [DOI] [PubMed] [Google Scholar]
- Owen RG, McCarthy H, Rule S, D'Sa S, Thomas SK, Tournilhac O, Forconi F, Kersten MJ, Zinzani PL, Iyengar S, Kothari J, Minnema MC, Kastritis E, Aurran-Schleinitz T, Cheson BD, Walter H, Greenwald D, Chen DY, Frigault MM, Hamdy A, Izumi R, Patel P, Wei H, Lee SK, Mittag D & Furman RR (2020) Acalabrutinib monotherapy in patients with Waldenstrom macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol, 7, e112–e121. [DOI] [PubMed] [Google Scholar]
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR & Dimopoulos MA (2003) Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol, 30, 110–115. [DOI] [PubMed] [Google Scholar]
- Poulain S, Roumier C, Venet-Caillault A, Figeac M, Herbaux C, Marot G, Doye E, Bertrand E, Geffroy S, Lepretre F, Nibourel O, Decambron A, Boyle EM, Renneville A, Tricot S, Daudignon A, Quesnel B, Duthilleul P, Preudhomme C & Leleu X (2016) Genomic Landscape of CXCR4 Mutations in Waldenstrom Macroglobulinemia. Clin Cancer Res, 22, 1480–1488. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Federmann B, Schindler N, Steinhilber J, Bonzheim I, Fend F & Quintanilla-Martinez L (2015) MYD88 L265P and CXCR4 mutations in lymphoplasmacytic lymphoma identify cases with high disease activity. Br J Haematol, 169, 795–803. [DOI] [PubMed] [Google Scholar]
- Tam CS, Opat S, D'Sa S, Jurczak W, Lee HP, Cull G, Owen RG, Marlton P, Wahlin BE, Garcia-Sanz R, McCarthy H, Mulligan S, Tedeschi A, Castillo J, Czyz J, Fernandez de Larrea C, Belada D, Libby E, Matous JV, Motta M, Siddiqi T, Tani M, Trneny M, Minnema MC, Buske C, Leblond V, Chan WY, Schneider JY, Ro S, Cohen A, Huang J & Dimopoulos MA (2020) A Randomized Phase 3 Trial of Zanubrutinib Versus Ibrutinib in Symptomatic Waldenstrom Macroglobulinemia:The Aspen Study. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon SP, Gustine J, Meid K, Yang G, Xu L, Liu X, Demos M, Kofides A, Tsakmaklis N, Chen JG, Munshi M, Chan G, Dubeau T, Raje N, Yee A, O'Donnell E, Hunter ZR & Castillo JJ (2018) Ibrutinib Monotherapy in Symptomatic, Treatment-Naive Patients With Waldenstrom Macroglobulinemia. J Clin Oncol, 36, 2755–2761. [DOI] [PubMed] [Google Scholar]
- Treon SP, Meid K, Gustine J, Yang G, Xu L, Patterson CJ, Ghobrial I, Laubach JP, Hunter ZR, Dubeau T, Palomba L, Advani R & Castillo JJ (2019) IBRUTINIB MONOTHERAPY PRODUCES LONG-TERM DISEASE CONTROL IN PREVIOUSLY TREATED WALDENSTROM'S MACROGLOBULINEMIA. FINAL REPORT OF THE PIVOTAL TRIAL (NCT01614821). Hematological Oncology, 37, 184–185. [Google Scholar]
- Treon SP, Tripsas CK, Meid K, Kanan S, Sheehy P, Chuma S, Xu L, Cao Y, Yang G, Liu X, Patterson CJ, Warren D, Hunter ZR, Turnbull B, Ghobrial IM & Castillo JJ (2014) Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenstrom's macroglobulinemia. Blood, 124, 503–510. [DOI] [PubMed] [Google Scholar]
- Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV, Yang G, Cao Y, Xu L, Patterson CJ, Rodig S, Zehnder JL, Aster JC, Harris NL, Kanan S, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR, Salman Z, Li J, Cheng M, Clow F, Graef T, Palomba ML & Advani RH (2015) Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med, 372, 1430–1440. [DOI] [PubMed] [Google Scholar]
- Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL, Laramie JM, Skifter DA, Lincoln SE & Hunter ZR (2012) MYD88 L265P somatic mutation in Waldenstrom's macroglobulinemia. N Engl J Med, 367, 826–833. [DOI] [PubMed] [Google Scholar]
- Treon SP, Yang G, Hanzis C, Ioakimidis L, Verselis SJ, Fox EA, Xu L, Hunter ZR, Tseng H, Manning RJ, Patterson CJ, Sheehy P & Turnbull B (2011) Attainment of complete/very good partial response following rituximab-based therapy is an important determinant to progression-free survival, and is impacted by polymorphisms in FCGR3A in Waldenstrom macroglobulinaemia. Br J Haematol, 154, 223–228. [DOI] [PubMed] [Google Scholar]
- Trotman J, Opat S, Marlton P, Gottlieb D, Simpson D, Cull G, Ritchie D, Verner E, Munoz J, Tedeschi A, Huang J, Novotny W, Osman M, Atwal S, Seymour JF, Roberts AW & Tam CS (2019) UPDATED SAFETY AND EFFICACY DATA IN A PHASE 1/2 TRIAL OF PATIENTS WITH WALDENSTRÖM MACROGLOBULINAEMIA (WM) TREATED WITH THE BRUTON TYROSINE KINASE (BTK) INHIBITOR ZANUBRUTINIB (BGB-3111). HemaSphere, 3, 192–193. [Google Scholar]
- Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, Xue L, Li DH, Steggerda SM, Versele M, Dave SS, Zhang J, Yilmaz AS, Jaglowski SM, Blum KA, Lozanski A, Lozanski G, James DF, Barrientos JC, Lichter P, Stilgenbauer S, Buggy JJ, Chang BY, Johnson AJ & Byrd JC (2014) Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med, 370, 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hunter ZR, Tsakmaklis N, Cao Y, Yang G, Chen J, Liu X, Kanan S, Castillo JJ, Tai YT, Zehnder JL, Brown JR, Carrasco RD, Advani R, Sabile JM, Argyropoulos K, Lia Palomba M, Morra E, Trojani A, Greco A, Tedeschi A, Varettoni M, Arcaini L, Munshi NM, Anderson KC & Treon SP (2016) Clonal architecture of CXCR4 WHIM-like mutations in Waldenstrom Macroglobulinaemia. Br J Haematol, 172, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y, Liu X, Morra E, Trojani A, Greco A, Arcaini L, Varettoni M, Brown JR, Tai YT, Anderson KC, Munshi NC, Patterson CJ, Manning RJ, Tripsas CK, Lindeman NI & Treon SP (2013) MYD88 L265P in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood, 121, 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]