Abstract
Streptococcus agalactiae or Group B Streptococcus (GBS) is a gram-positive bacterial pathobiont that is the etiological cause of severe perinatal infections. GBS can colonize the vagina of pregnant patients and invade tissues causing ascending infections of the gravid reproductive tract that lead to adverse outcomes including preterm birth, neonatal sepsis, and maternal or fetal demise. Additionally, transmission of GBS during labor or breastfeeding can also cause invasive infections of neonates and infants. However, human milk has also been shown to have protective effects against infection; a characteristic that is likely derived from antimicrobial and immunomodulatory properties of molecules that comprise human milk. Recent evidence suggests that human milk oligosaccharides (HMOs), short-chain sugars that comprise 8–20% of breast milk, have antimicrobial and anti-biofilm activity against GBS and other bacterial pathogens. Additionally, HMOs have been shown to potentiate the activity of antibiotics against GBS. This review presents the most recent published work that studies the interaction between HMOs and GBS.
Keywords: Group B Streptococcus, human milk oligosaccharides, glycobiology, bacterial pathogenesis, Streptococcus agalactiae
Graphical Abstract
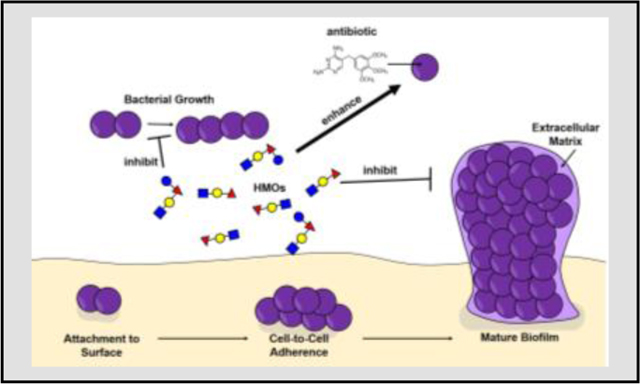
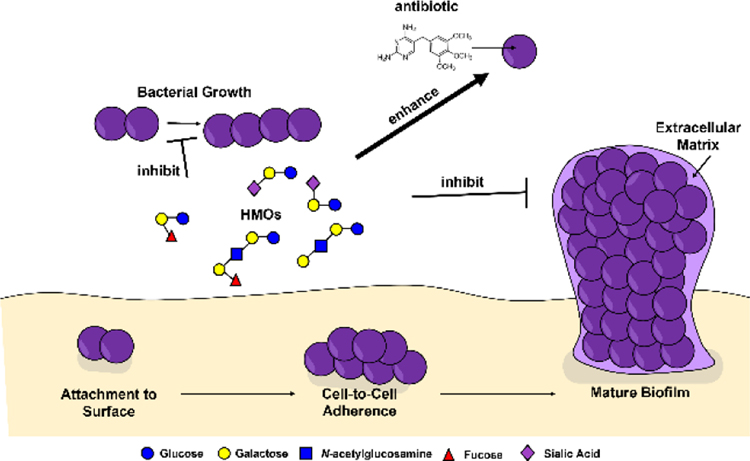
Table of Contents: Human milk oligosaccharides (HMOs) exert various activities against Group B Streptococcus including inhibition of bacterial growth, enhanced penetrance and efficacy of antibiotics via bacterial cell permeabilization, and inhibition of bacterial biofilm formation.
1. Group B Streptococcus
Streptococcus agalactiae, otherwise known as Group B Streptococcus (GBS) is a gram-positive diplococcus that typically colonizes the gastrointestinal and lower genital tracts. While GBS is typically asymptomatic in healthy individuals, it can be quite detrimental to the elderly, newborns, and those with a compromised immune system. Since 10–40% of pregnant women are colonized with GBS during the late stages of pregnancy, it is standard to screen for the presence of the bacteria between 35 and 37 weeks.[1a,b] GBS is most commonly passed on to neonates through vertical transmission prior to, or during labor and delivery from a GBS-infected mother and is one of the leading causes of neonatal sepsis and meningitis.[2a,b] However, if a patient test positive during GBS screening, intrapartum antibiotic prophylaxis can be administered.[3]
1.1. Group B Streptococcus is associated with adverse perinatal outcomes
Preterm birth
Preterm birth is defined as parturition before 37 weeks gestation. There are approximately 15 million preterm births globally each year; an estimated 11% of all live births (ref).[4] Complications surrounding preterm birth are the most common direct cause of death in children under 5 years of age (ref).[5a, b, c] Preterm birth is associated with 15% of child deaths and 35% of neonatal deaths worldwide. Additionally, preterm birth can result in lifelong disability among survivors including neurodevelopmental complications, cognitive disorders, hearing and visual impairment, motor disorders, severe infections, pulmonary disorders, metabolic disorders, cardiovascular disease, and mental health issues.[6a,b,c,d,e, f]
One of the leading causes of preterm birth is intrauterine infection during pregnancy.[6e] GBS is a leading cause of bacterial infection of the gravid reproductive tract, and colonization of the rectum or vagina has been associated as a strong risk factor for invasive infection during pregnancy and cognate disease outcomes including preterm birth.[6e]
Early-onset vs. late-onset GBS disease
GBS disease in infants is either classified as early onset disease (EOD) or late onset disease (LOD) dependent on when the neonate acquires the infection. EOD is more common than LOD and typically presents itself within the first 24 hours of life but can occur up to one week after birth.[7] These affected babies usually contract GBS from their mothers during birth, with premature newborns more likely to acquire infection.[7] EOD is more often associated with mortality than LOD, and typically presents itself as sepsis, pneumonia, and less often meningitis. LOD occurs anywhere between seven days and up to three months.[8] It is not usually clear how GBS is transmitted to the baby with LOD, but typically from the mother, other family members, or hospital caregivers. LOD typically manifests as bacteremia, meningitis, or less often organ or soft tissue infection.[9]
2. Breastmilk and Breastfeeding
2.1. Protective biochemical properties of breastmilk
Most health experts including the American Academy of Pediatrics recommend exclusive breast feeding for the first six months of life as a result of the well-established benefits breast milk provides to the baby.[10] Breast feeding is endorsed by health organizations, doctors, and even formula companies themselves. And while universally breast milk should still be the first choice to meet the nutritional needs of the baby through at least the first six months of life, formula companies have advanced the formula contents to a level where it is a safe and healthy alternative when necessary. Formula design is meant to duplicate the mother’s milk. Formula companies however are still unable to match the complexity involved in milk production. Breast milk is constantly changing along with the needs of the baby as it provides protective antibiotics and bioactive components that cannot to be added.[11] Cow milk and soy milk are two most common bases for infant formula. The FDA regulates the composition of proteins, fats, carbohydrates, vitamins, and minerals to ensure proper nutrition. Iron fortification of formula is recommended for the prevention of anemia.
Human milk oligosaccharides which are abundantly present in breast milk will be discussed in more detail below. While it is not synthetically or financially feasible to include all the known HMOs found in breast milk, many formula companies supplement prebiotics and/or probiotics to attempt to mimic the beneficial properties the HMOs provide. Prebiotics in formula are designed to stimulate the growth of beneficial bacteria species including Bifidobacterium and Lactobacillus.[12] The most commonly supplemented oligosaccharides are short-chain galacto-OS (GOS), long-chain fructo-OS (FOS), polydextrose, and 2’-fucosyllactose (2’-FL).[13] However, it is important to note that GOS, FOS, and polydextrose are plant polymers that are not native to human milk.
2.2. Transmission of GBS via breastmilk
Although the protective properties of human milk and breastfeeding are well-supported,[10] there are also risks associated with breast feeding. One example is that breast milk has been demonstrated to be a vehicle of transmission of GBS infection.[14] GBS can cause invasive infections of mammary tissues that lead to mastitis, and consequently, GBS can be transmitted from mother to infant via ingestion of GBS-contaminated breastmilk.[14]
2.3. Antimicrobial molecules in milk including HMOs
The proteins found in the highest concentrations are casein, α-lactalbumin, lactoferrin, immunoglobulin IgA, lysozyme and serum albumin. Proteins in breast milk provide a wide array of functions including serving as an important source of amino acids necessary for growth and development; protection against bacterial infections; absorption of both micro and macronutrients; and shaping the immature microbiome.
A portion of milk secretory IgA (sIgA) is tightly bound to and can phosphorylate oligosaccharides and polysaccharides. Furthermore, sIgA has also been shown to have kinase activities against both proteins and lipids.[15] Thus, sIgA is considered an “abzyme” or an antibody-enzyme hybrid. Mannose-containing oligosaccharides of secreted IgA have also been shown to possess antimicrobial and activities, including the ability to inhibit Vibrio cholerae biofilm formation.[16]
The iron-binding glycoprotein lactoferrin is one of the most abundant proteins found in breast milk, comprising of 15 to 20% of total protein content.[17] The antimicrobial properties of lactoferrin against harmful pathogens arise from its ability to sequester iron thus rendering it unavailable for the bacteria to proliferate. Lactoferrin also possesses anti-inflammatory and immunomodulatory properties necessary for the developing gastrointestinal tract of infants.[18a,b]
Another major whey protein encompassing 20 to 25% of the total protein content is α-lactalbumin which binds Ca2+ and Zn2+ ions driving the absorption of essential minerals.[19] In the mammary gland, α-lactalbumin promotes milk production as a critical factor in the lactose synthase complex. In addition to its bactericidal properties, it most importantly provides a rich source of essential amino acids including tryptophan, lysine, cysteine, leucine, isoleucine, and valine.[20]
Human Milk Oligosaccharides
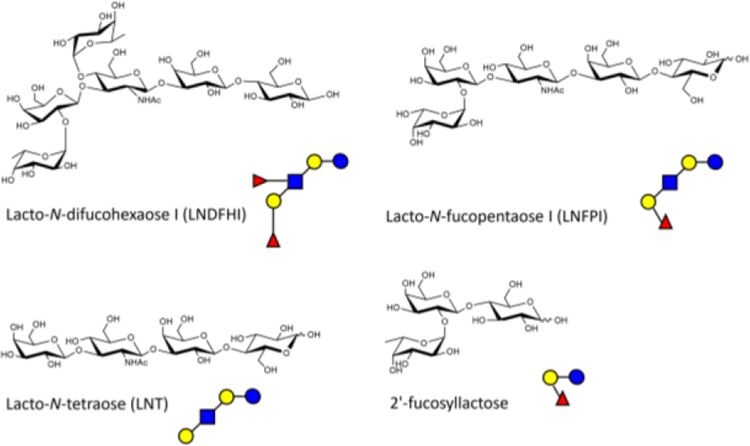
In the last decade, researchers have examined the effects of HMOs on modulating the outcome of infectious diseases. In separate studies by the Le Doare and Bode groups, they both demonstrated the ability of HMOs to directly inhibit the growth of GBS both in vitro and in vivo.[21a,b,c] In 2016, Le Doare and coworkers found a correlation between the mother’s Lewis secretor status and GBS colonization in infants since HMO expression is related to Lewis blood type.[21c] Interestingly, maternal Lewis secretor status has also been shown to shape infant gut microbiota; a result likely shaped by HMO presence or absence. The predominant HMO in secretor milk samples were 2’-fucosyllactose (2’FL) and lacto-N-fucopentaose I, whereas non-secretor milk was characterized by lacto-N-fucopentaose and lacto-N-difucohexaose (Figure 1). Differences in microbiota composition and quantity were found depending on secretor/non-secretor status. For example: Lactobacillus spp, Enterococcus spp, and Streptococcus spp were lower in non-secretor than secretor samples. Bifidobacterium were less prevalent in non-secretor samples compared to secretor samples. Despite no differences on diversity and richness, non-secretor samples had lower Actinobacteria and higher relative abundance of Enterobacteriaceae, Lactobacillaceae, and Staphylococcaceae.[22] There are four milk groups defined: Lewis-positive Secretors and non-Secretors, and Lewis-negative Secretors and non-Secretors. Using 1H nuclear magnetic resonance spectroscopy methods, they were able to assign a milk group based on the type of fucosylated HMO residues present in the milk sample. In their study they found that babies born to Lewis-positive mothers were less likely to acquire GBS infections as well as more likely to clear infection up to 90 days after birth when compared to infants born to Lewis-negative women. However, they were not able to find a correlation between Secretor status and incidences of GBS colonization in either mother or baby. In addition to the in vivo work, they found that the presence of lacto-N-difucohexaose I (LNDFHI), a branched fucosylated HMO in the mother’s milk sample was linked to an inhibition of GBS growth.[21c]
Figure 1.
Structures of common human milk oligosaccharides (HMOs). The structures of Lacto-N-difucohexaose I (LNDFHI), Lacto-N-fucopentaose I (LNFPI), Lacto-N-tetraose (LNT), and 2’-fucosyllactose (2’-FL). Glucose (blue circles), galactose (yellow circles), N-acetyl-glucosamine (blue squares), fucose (red triangles).
In 2017, Bode and coworkers discovered that pooled samples of HMO extracts directly inhibited the growth of GBS in a dose-dependent manner.[21b] This was preceded by a study in which the Bode lab uncovered that HMOs provide protection to bladder epithelial cells through preventing the colonization of uropathogenic Escherichia coli (UPEC), the primary pathogen responsible for urinary tract infections.[22] In addition to pooled HMOs, they also fractionated the HMOs into sialylated, acidic HMOs and non-sialylated, neutral HMOs using multidimensional chromatography. They concluded that while the sialylated HMOs did not inhibit GBS growth, the neutral, non-sialylated HMO moieties provided narrow spectrum bacteriostatic activity against GBS growth. Specifically, lacto-N-tetraose (LNT) and the fucosylated lacto-N-fucopentaose I (LNFPI) both inhibited GBS growth, while the isomer of LNT, lacto-N-neotetraose surprisingly did not. After expanding their studies to other species of bacteria including UPEC, Streptococcus pyogenes (Group A Streptococcus) Pseudomonas aeruginosa, and Staphylococcus aureus, they found that HMOs did not inhibit the growth of these pathogens.
3. HMOs and Group B Streptococcus
3.1. HMOs have antimicrobial activity against GBS
Following the results of the Le Doare and Bode groups, our interdisciplinary team focused on the synthesis and analysis of HMOs in infectious disease. Initial research was based on the hypothesis that HMOs possess both antimicrobial and antibiofilm activities against GBS. Bacterial growth was quantified using a plate-based assay using spectrophotometric techniques measuring optical density at 600 nm (OD600) over a period of 24 hours. In this assay, a heterogenous mixture of HMOs pooled from five donors was found to possess bacteriostatic activity against GBS, inhibiting growth up to 89%.[23] The next steps following the elucidation of this impressive antimicrobial activity involved uncovering the most active single-entity HMOs present in the mixture. As expected, no single-entity HMOs were as potent as the pooled mixture with the understanding there are likely multiple mechanisms responsible for this inhibition.[24a,b]
3.2. HMOs have anti-biofilm activity against GBS
Biofilm formation is often directly associated with the pathogenesis of an organism and its’ ability to persist in hostile environments. To test our hypothesis that in addition to possessing antimicrobial activity, HMOs also inhibit biofilm production in GBS. Using a colorimetric, plate-based assay, we assessed biofilm production by spectrophotometrically measuring absorbance at OD560. Importantly, after 24 hours, we also measured bacterial growth at OD600. The ratio of biofilm/biomass allows us to account for any differences in bacterial growth amongst samples. We uncovered that a heterogeneous mixture of HMOs inhibited biofilm formation in GBS up to 93%.[24] Additionally, we found that HMOs also inhibited biofilm production up to 60% in another gram-positive species, methicillin-resistant Staphylococcus aureus.[24]
Initially, the antimicrobial and antibiofilm activities of a heterogenous mixture of HMOs were studied. However, there was profound interest in teasing out which single-entity HMOs were responsible for eliciting these responses. Subsequent studies interrogated the anti-biofilm activities of 17 single-entity HMOS and found that as a general trend that no single HMO inhibited biofilm production nearly as well as the heterogenous mixture of HMOs.[25a,b] We hypothesized that we could convert these single-entity HMOs that lacked antibiofilm activity into antibiofilm compounds by incorporating a positive charge on the reducing end of these sugars. This hypothesis was formed based on the knowledge that cationic molecules are known to disrupt even the most rigid biofilm structures. Using the Kochetkov amination, we could readily convert an alcohol at the anomeric position at the reducing end of the sugar to an amino group which is positively charged at physiological pH. Four native HMOs were converted into their β-amino variants and their ability to inhibit bacterial growth and biofilm in GBS was assessed. While neither the parent single-entity HMOs or β-amino HMOS exhibited antimicrobial activity, all four of the β-amino HMOs significantly inhibited quantifiable biofilm production, and these results were confirmed using field emission gun-scanning electron microscopy (FEG-SEM).[26]
3.2. HMOs potentiate antibiotic activity against GBS
Following up initial studies in which it was found that a heterogeneous mixture of HMOs possessed antimicrobial and antibiofilm activity against GBS, it was hypothesized that HMOs could potentiate antibiotic activity. One of the strategies employed to treat virulent bacterial infections is combination therapy in which an adjuvant is administered along with the drug as not only helps increase its efficacy, but also helps with the antibiotic resistance problem by lowering the dosages of the drugs required. Through testing this hypothesis, we hoped to not only enhance the antimicrobial activity of the existing antibiotics but also to elucidate the mechanism of action of the HMOs.
In a first-generation study of combination therapy of HMOs with select antibiotics, the HMOs were found to help potentiate the activity of a variety of antibiotics, including ribosomal targeting drugs and decrease the MICs of these drugs by up to 32-fold (Table 1).[27] To determine if there was strain specificity, the HMOs were assayed against three strains of GBS across various serotypes: GB2 (serotype Ia), GB590 (serotype III), and CNCTC 10/84 (serotype V). While a panel of antibiotics was chosen, interestingly β-lactams and glycopeptide antibiotics which inhibit cell-wall synthesis exhibited much more subtle effects compared to the ribosomal-targeting drugs in conditions of HMO supplementation. The ribosomal targeting antibiotics in this study were three antibiotics in which GBS has evolved resistance towards. They were aminoglycosides, macrolides, and tetracyclines which was promising as this combination therapy could help to repurpose these antibiotics (Table 1). Based on the antibiotic/HMO combination studies, we further hypothesized that HMOs increase cell membrane permeability thereby increasing the efficacy of intracellular-targeting antibiotics. This was initially confirmed using a LIVE/DEAD Baclight assay which found that HMOs increase cell membrane permeability up to 30%.[27] In an effort to validate the hypothesis that HMOs increase cell membrane permeability, the panel of antibiotics was expanded to include additional intracellular targeting antibiotics.
Table 1.
Enhanced efficacy of antibiotics against GBS (strain CNTC 10/84) in the presence of HMOs.
| Antibiotics | MIC in medium alone | MIC in medium + HMOs | Fold Change in antibiotic efficacy |
|---|---|---|---|
|
| |||
| Penicillin[a] | 0.03 | 0.015 | 2 |
| ampicillin[a] | .0625 | 0.0312 | 2 |
| cefazolin[a] | 0.125 | 0.0625 | 2 |
| vancomycin[a] | 2 | 1 | 2 |
| clindamycin[a] | 0.0325 | 0.0156 | 2 |
| gentamicin[a] | 16 | 2 | 8 |
| erythromycin[a] | 0.0156 | 0.0019 | 8 |
| linezolid[a] | 2 | 1 | 2 |
| minocycline[a] | 0.0625 | 0.0019 | 32 |
| Trimethoprim[b] | >1,024 | 5.12 | >256 |
Craft, K.M. et al. (2018) ACS Chemical Biology
Chamber, S.A. and Moore, R.E. et al. (2020) mBio.
In a related study, the powerful activity of HMOs in combating antifolate antibiotic resistance in GBS was discovered. Results indicated that pooled HMOs could be co-dosed with trimethoprim (TMP) to synergistically increase GBS susceptibility to TMP. Representative GBS strains from five distinct capsular serotypes were tested and exhibited between 16–512-fold reduction in TMP MIC when dosed in coordination with HMOs.[28] The HMO-TMP combination was more efficacious than a TMP-sulfadiazine combination and was also demonstrated to be due to increased on-target activity of TMP in inhibiting folate biosynthesis. Consequently, we hypothesized that HMOs facilitate increased GBS cell permeability that can restore TMP cell penetrance and antibiotic activity within GBS strains that are initially resistant to TMP. We further validated this hypothesis through untargeted metabolomic analyses, which revealed that HMOs significantly effect fatty acid and lipid metabolism within GBS. Specifically, linoleic acid metabolism is the metabolic pathway most impacted when GBS is exposed to HMOs. Linoleic acid metabolites play a crucial role in cellular signaling, response to stress, and proper membrane construction. All identified linoleic acid precursors were accumulated in the HMO-treated population, with several metabolites having a 100-fold increase from the untreated controls. Two epoxyoctadecanoic acid metabolites were of particular interest, epoxyoctadecanoic acids (EpOMEs) and dihydroxyoctadecanoic acids (DiHOMEs). Accumulation of these metabolites is linked to changes in cell membrane fluidity and cell membrane construction. [28] These perturbations support that HMOs significantly alter cell membrane associated metabolites, likely causing increased GBS cell permeability. Taken together, these results support HMOs as effective antibiotic adjuvants, capable of reversing antibiotic resistant GBS phenotypes.
4. Conclusions
In conclusion, this review demonstrates that molecules within human breast milk, especially human milk oligosaccharides have potential utility as antimicrobial and anti-biofilm compounds against perinatal pathogens such as S. agalactiae (Figure 2). Furthermore, HMOs represent potential adjuvants to enhance the penetrance and efficacy of antibiotics, providing a chemotherapeutic mechanism to circumnavigate antimicrobial resistance mechanisms.
Figure 2.
Conceptual model of the diverse activities of human milk oligosaccharides (HMOs) against Group B Streptococcus bacteria. HMOs such as Lacto-N-fucopentaose I (LNFPI), Lacto-N-tetraose (LNT), and 2’-fucosyllactose (2’-FL) exert various activities against GBS including inhibition of bacterial growth, enhanced penetrance and efficacy of antibiotics via bacterial cell permeabilization, and inhibition of bacterial biofilm formation. Symbols include: glucose (blue circles), galactose (yellow circles), N-acetyl-glucosamine (blue squares), fucose (red triangles), and sialic acid (purple diamonds).
Acknowledgements
This work was funded by National Institutes of Health grant NICHD R01 HD090061 (to J.A.G.) and a Merit Review Award I01 BX005352-01 (to J.A.G) from the Office of Medical Research, Department of Veterans Affairs, and also by the National Science Foundation (1847804) to S.D.T.
Biography

Rebecca Moore is a 4th year Ph.D. Candidate in the Department of Chemistry at Vanderbilt University. Her graduate research focuses on the biological evaluation of human milk oligosaccharides against Group B Streptococcus.

Dr. Steven Townsend, Ph.D. is an Associate Professor of Chemistry at Vanderbilt University. His research focuses on leveraging synthetic chemistry and chemical biology techniques to study antimicrobial molecules. Dr. Townsend is a Camille Dreyfus Teacher Scholar and a fellow of the Alfred P. Sloan Foundation.

Dr. Jennifer Gaddy, Ph.D. is an Assistant Professor of Medicine at Vanderbilt University Medical Center and a Research Scientist at the Tennessee Valley Healthcare Systems. Her research focuses on determining the mechanisms of host-pathogen interactions with a specific interest in bacterial pathogenesis. Dr. Gaddy was awarded Young Investigator of the Year in 2017 from the American Society for Microbiology
References
- [1].a) Kwatra G, Adrian P, Shiri T, Buchmann E, Cutland C, Madhi S, PLoS One. 2014, 9, e98778; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Russell N, Seale A, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, Lawn J, Baker C, Bartlett L, Cutland C, Gravett M, Heath P, Le Doare K, Madhi S, Rubens C, Schrag S, Sobanjo-Ter Meulen A, Vekemans J, Saha S, Ip M, Clin. Infect. Dis. 2017, 65, S100–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Schuchat A, Wenger J, Epidemiol. Rev. 1994, 16, 374–402; [DOI] [PubMed] [Google Scholar]; b) Simonsen K, Anderson-Berry A, Delair S, Davies H, Clin. Microbiol. Rev. 2014, 27, 21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Verani J, McGee L, Schrag S, Morb. Mortal. Wkly. Rep. 2010, 59, 1–36. [Google Scholar]
- [4].Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A, Kinney M, Lawn J, Reprod. Health. 2013, 10, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Levels and Trends in Child Mortality, 2015; [Google Scholar]; b) Oza S, Lawn J, Hogan D, Mathers C, Cousens S. Bull. World. Health. Organ. 2015, 93, 19–28; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Liu L, Oza S, Hogan D, Lancet, 2015, 385, 430–40. [DOI] [PubMed] [Google Scholar]
- [6].a) Blencowe H, Lee A, Cousens S, Pediatr. Res. 2013, 74, 17–34; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lin F, Weisman L, Troendle J, Adams K, J. Infect. Dis. 2003, 188, 267–7; [DOI] [PubMed] [Google Scholar]; c) Schuchat A, Lancet, 1999, 353, 51–6; [DOI] [PubMed] [Google Scholar]; d) Oddie S, Embleton N, BMJ. 2002, 325, 308; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Goldenberg R, Culhane J, Iams J, Romero R,. Lancet, 2008, 371, 75–84; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Cunnington M, Kortsalioudaki C, Heath P, Curr. Opin. Infect. Dis. 2013, 26, 219–30. [DOI] [PubMed] [Google Scholar]
- [7].Morgan J, Zafar N, Cooper D. StatPearls, 2021. [Google Scholar]
- [8].Jordan H, Farley M, Craig A, Ped. Infect. Dis. J. 2008, 27, 1057–1064. [DOI] [PubMed] [Google Scholar]
- [9].Hon K, Chan K, Ko P, So K, Leung A, Case. Rep. Pediatr. 2017, 8418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gartner L, Morton J, Lawrence R, Pediatrics. 2005, 115, 496–506. [DOI] [PubMed] [Google Scholar]
- [11].Ballard O, Morrow A, Pediatr. Clin. North. Am. 2013, 60, 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Borewicz K, Suarez-Diez M, Hechler C, Sci. Rep. 2019, 9, 2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vandenplas Y, De Greef E, Veereman G. Gut Microbes. 2014, 5, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ueda N, Nakamura K, Go H, Takehara H, Kashiwabara N, Arai K, Takemura H, Namai Y, Kanemitsu K. Int J Infect Dis. 2018, 74, 13–15. [DOI] [PubMed] [Google Scholar]
- [15].Karataeva N, Gorbunov D, Prokudin I, Buneva V, Kulminskaya A, Neustroev K, Nevinsky G, Immunol Lett. 2006, 103, 58–67. [DOI] [PubMed] [Google Scholar]
- [16].Murthy A, Chaganty B, Troutman T, Guentzel M, Yu J, Ali S, Lauriano C, Chambers J, Klose K, Arulanandam B, PLoS One. 2011, 6, e16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang Z, Jiang R, Chen Q, Nutrients. 2018, 10, 1235. [Google Scholar]
- [18].a) Vogel H, Biochem.. Cell Biol. 2012, 90, 233–244; [DOI] [PubMed] [Google Scholar]; b) Lönnerdal B, Curr. Opin. Clin. Nutr. Metab. Care. 2009, 12, 293–297. [DOI] [PubMed] [Google Scholar]
- [19].Lönnerdal B, Lien E, Nutr. Rev. 2003, 61, 295–305. [DOI] [PubMed] [Google Scholar]
- [20].Layman D, Lönnerdal B,Fernstrom J, Nutr. Rev. 2018, 76, 444–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Bode L, Early Hum. Dev. 2015, 91, 619–622; [DOI] [PubMed] [Google Scholar]; b) Lin A, Autran C, Szyszka A, Escajadillo T, Huang M, Godula K, Prudden A, Boons G, Lewis A, Doran K, Nizet V, Bode L, J. Biol. Chem. 2017, 292, 11243–11249.; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Andreas N, Al-Khalidi A, Jaiteh M, Clarke E, Hyde M, Modi N, Holmes E, Kampmann B, Le Doare K, Clin. Transl. Immunol. 2016, 5, e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cabrera-Rubio R, Kunz C, Rudloff S, García-Mantrana I, Crehuá-Gaudiza E, Martínez-Costa C, Collado M. J Pediatr Gastroenterol Nutr. 2019, 68, 256–263. [DOI] [PubMed] [Google Scholar]
- [23].Lin A, Autran C, Espanola S, Bode L, Nizet V, Am. J. Infect. Dis. 2014, 209, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ackerman D, Craft K, Doster R, Weitkamp J, Aronoff D, Gaddy J, Townsend S, ACS Infect. Dis. 2018, 4, 315–324, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) Craft K, Thomas H, Townsend S, ACS Infect. Dis. 2018, 4, 1755–1765; [DOI] [PubMed] [Google Scholar]; b) Craft K, Thomas H, Townsend S, Org. Biomol. Chem. 2019.1893–1900, [DOI] [PubMed] [Google Scholar]
- [26].Moore R, Craft K, Xu L, Chambers S, Nguyen J, Marion K, Gaddy J, Townsend S, J. Org. Chem. 2020, 85, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Craft K, Gaddy J, Townsend S, ACS Chem. Bio. 2018, 13, 2020–2026. [DOI] [PubMed] [Google Scholar]
- [28].Chambers S, Moore R, Craft K, Thomas H, Das R, Manning S, Codreanu S, Sherrod S, Aronoff D, McLean J, Gaddy J, Townsend S, mBio. 2020, 11, 00076–20. [DOI] [PMC free article] [PubMed] [Google Scholar]