Abstract
(+)-Calanolide A (NSC 650886) has previously been reported to be a unique and specific nonnucleoside inhibitor of the reverse transcriptase (RT) of human immunodeficiency virus (HIV) type 1 (HIV-1) (M. J. Currens et al., J. Pharmacol. Exp. Ther., 279:645–651, 1996). Two isomers of calanolide A, (−)-calanolide B (NSC 661122; costatolide) and (−)-dihydrocalanolide B (NSC 661123; dihydrocostatolide), possess antiviral properties similar to those of calanolide A. Each of these three compounds possesses the phenotypic properties ascribed to the pharmacologic class of nonnucleoside RT inhibitors (NNRTIs). The calanolide analogs, however, exhibit 10-fold enhanced antiviral activity against drug-resistant viruses that bear the most prevalent NNRTI resistance that is engendered by amino acid change Y181C in the RT. Further enhancement of activity is observed with RTs that possess the Y181C change together with mutations that yield resistance to AZT. In addition, enzymatic inhibition assays have demonstrated that the compounds inhibit RT through a mechanism that affects both the Km for dTTP and the Vmax, i.e., mixed-type inhibition. In fresh human cells, costatolide and dihydrocostatolide are highly effective inhibitors of low-passage clinical virus strains, including those representative of the various HIV-1 clade strains, syncytium-inducing and non-syncytium-inducing isolates, and T-tropic and monocyte-tropic isolates. Similar to calanolide A, decreased activities of the two isomers were observed against viruses and RTs with amino acid changes at residues L100, K103, T139, and Y188 in the RT, although costatolide exhibited a smaller loss of activity against many of these NNRTI-resistant isolates. Comparison of cross-resistance data obtained with a panel of NNRTI-resistant virus strains suggests that each of the three stereoisomers may interact differently with the RT, despite their high degree of structural similarity. Selection of viruses resistant to each of the three compounds in a variety of cell lines yielded viruses with T139I, L100I, Y188H, or L187F amino acid changes in the RT. Similarly, a variety of resistant virus strains with different amino acid changes were selected in cell culture when the calanolide analogs were used in combination with other active anti-HIV agents, including nucleoside and nonnucleoside RT and protease inhibitors. In assays with combinations of anti-HIV agents, costatolide exhibited synergy with these anti-HIV agents. The calanolide isomers represent a novel and distinct subgroup of the NNRTI family, and these data suggest that a compound of the calanolide A series, such as costatolide, should be evaluated further for therapeutic use in combination with other anti-HIV agents.
The structurally diverse class of nonnucleoside reverse transcriptase (RT) inhibitors (NNRTIs) includes compounds which are among the most potent anti-human immunodeficiency virus (anti-HIV) agents identified (for a review, see references 17 and 18). The therapeutic utility of these anti-HIV compounds, however, is severely compromised by the rapid appearance of drug-resistant virus isolates in patients (26). Similarly, growth of HIV in cell culture in the presence of the NNRTIs rapidly selects for drug-resistant viruses (26). The high specificity of the interaction of these compounds at the hydrophobic nonnucleoside binding site on the HIV-1 RT results in the ability of single amino acid changes in the NNRTI binding pocket to reduce or eliminate the inhibitory activity of the compound (14, 15, 20, 29). Amino acid changes in the RT which affect drug sensitivity include A98G, L100I, K101E, K103N, V106A, V108I, E138K, T139I, Y181C, Y188C, G190A, F227L, and P236L (26).
The effective use of NNRTIs in patients is dependent on the definition of appropriate combinations of agents which will prevent or retard the selection of drug-resistant viruses or which will result in the selection of drug-resistant virus isolates in which mutation of critical amino acid residues renders the RT less fit to support virus reproduction. NNRTIs may also be useful as part of a combination anti-HIV strategy with a highly potent NNRTI and additional anti-HIV type 1 (anti-HIV-1) agents in therapy-naive patients. The potential for the therapeutic use of NNRTIs in patients has recently been reviewed (17, 18). Results of clinical trials with nevirapine as a component of a three-drug regimen in patients have highlighted the possible benefits of the development of additional novel or more potent NNRTIs (13). Although the use of NNRTIs alone is not warranted, these compounds may be used in other ways, for example, as topical microbicides for the prevention of the sexual transmission of HIV, for postexposure prophylaxis, or as a first-line therapeutic option for the treatment of patients without the elimination of future therapy options.
It has become increasingly apparent that the HIV-1-specific inhibitor class of compounds is quite diverse (2, 3, 19). This diversity may allow the identification of therapeutically beneficial combinations of anti-HIV compounds, including the NNRTIs. A variety of structurally distinct NNRTIs have been identified through the efforts of the National Cancer Institute’s (NCI’s) high-capacity anti-HIV drug screening program (1, 6–11, 23, 24). Evaluation of the activity of calanolide A against RT and NNRTI-resistant viruses, as well as detailed evaluation of the kinetics of RT inhibition, has suggested that calanolide A represents a novel new class of HIV-1-specific inhibitor (7, 16, 22, 23). Like other members of the NNRTI family of inhibitors, calanolide A exhibits potent anti-HIV activity in established and fresh human cells and synergistically inhibits HIV when it is used in combination with nucleoside anti-HIV compounds. NNRTI-resistant virus isolates were determined to possess a unique profile of sensitivity to the compound, and the compound predominantly selected for drug-resistant virus isolates with a previously unknown mutation at amino acid residue 139 (T139I) (7). These data, as well as those presented here, indicate that the calanolide class of NNRTIs may interact in a mechanistically different fashion with the HIV-1 RT and therefore may be useful inhibitors when used in combination with other anti-HIV agents, including other NNRTIs.
MATERIALS AND METHODS
Cells and viruses.
The established human cells, laboratory-derived virus isolates (including drug-resistant virus isolates), and low-passage clinical virus isolates used in these evaluations have previously been described in detail (11, 12). These cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Fresh human cells were obtained from the American Red Cross (Baltimore, Md.).
Materials.
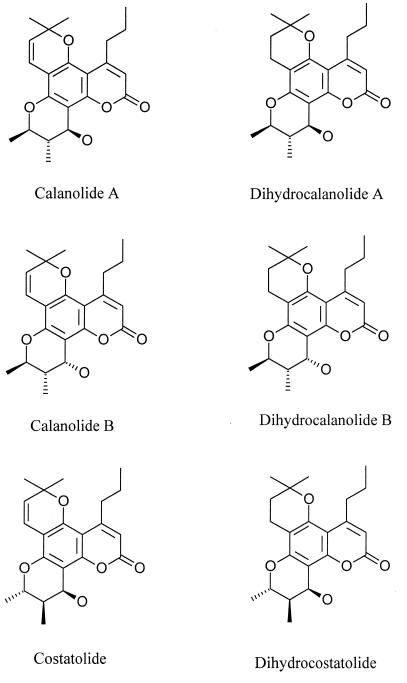
(+)-Calanolide A, (−)-calanolide B (costatolide), and (−)-dihydrocalanolide B (dihydrocostatolide) (NSC 654086, NSC 661122, and NSC 661123, respectively) were obtained from the Developmental Therapeutics Program, Division of Cancer Treatment, Diagnosis and Centers, NCI. The structures of each of these molecules are provided in Fig. 1. Crystalline stock materials were stored at −70°C and were solubilized in 100% dimethyl sulfoxide. All stocks were diluted at least 400-fold prior to performance of drug susceptibility assays. All of the compounds used in these studies were obtained from NCI, including zidovudine (AZT; NSC 602670), didanosine (ddI), zalcitabine (ddC), lamivudine (3TC), stavudine (d4T), nevirapine, E-EBTU (8), diphenylsulfone (25), UC10 and UC781 (5), KNI272, and resobene (21). 2′,5′-Bis-O--(tert-butyldimethylsilyl)-3′-spiro-5"-(4"-amino-1",2"-oxathiole-2", 2"-dioxide-β-d-pentofuranosyl (TSAO) and α-APA were obtained from Jan Balzarini and Janssen Pharmaceuticals, respectively. Enzyme-linked immunosorbent assay plates were purchased from Coulter Immunotech (Hialeah, Fla.). Materials required for the performance of RT inhibition assays and anti-HIV assays and for the growth and maintenance of established and fresh human cells have been described previously (4, 11).
FIG. 1.
Structures of calanolide A analogs.
Antiviral and cross-resistance assays.
The inhibitory activities of the compounds against HIV were evaluated as described previously (11) by microtiter anti-HIV assays with CEM-SS cells or fresh human peripheral blood mononuclear cells (PBMCs); these assays quantify the ability of a compound to inhibit HIV-induced cell killing or HIV replication. Quantification was performed by the tetrazolium dye XTT assay (CEM-SS, 174×CEM, MT2, and AA5 cell-based assays), which is metabolized to a colored formazan product by viable cells, RT assay (U937- and PBMC-based assays), and/or p24 enzyme-linked immunosorbent assay (monocyte-macrophage assays). Antiviral and toxicity data are reported as the quantity of drug required to inhibit virus-induced cell killing or virus production by 50% (EC50) and the quantity of drug required to reduce cell viability by 50% (IC50).
Anti-HIV assays with drug combinations.
Analysis of drug combinations was performed with CEM-SS cells acutely infected with the IIIB strain of HIV-1 as described previously (11) by the anti-HIV assay methodology described above. Statistical evaluations were performed with MacSynergy II software (28). The results of the assays with drug combinations are presented three dimensionally for each combination concentration, yielding a surface of activity extending above (synergy) or below (antagonism) the plane of additive interaction. The volume of the surface is calculated and expressed as a synergy volume (square micromolar percent) calculated at the 95% confidence interval (28). For these studies, synergy is defined as drug combinations with synergy volumes of greater than 50 μM2%. Slightly synergistic and highly synergistic anti-HIV activities have been defined as synergy volumes of 50 to 100 and >100 μM2%, respectively. Synergy volumes of between 0 and 50 μM2% are considered additive, and synergy volumes of less than 0 μM2% are considered antagonistic.
Selection of drug-resistant strains.
Resistant virus isolates were selected in cell culture by serial passage of the IIIB strain of HIV-1 in CEM-SS, MT2, H9, or AA5 cells in the presence of increasing concentrations of antiviral compound. Identical selections were performed with the clinical isolate ROJO in fresh human peripheral blood cells. The initial selection was performed with two times the EC50 of the compound as determined by the microtiter anti-HIV assay. With successive passages the drug concentration was increased twofold to enhance the selective pressure on the virus. The final drug concentrations used in the selection of viruses resistant to calanolide A, costatolide, and dihydrocostatolide were 2.7, 5.4, and 5.4 μM, respectively. Upon selection of a drug-resistant virus isolate, cross-resistance testing was performed by the methods described above for the performance of antiviral assays. Resistance has been defined in this study as a greater than fivefold increase in the EC50 compared to the concentration of the compound with activity against the wild-type (IIIB) isolate. Two drug-resistant strains were selected exactly as described above by beginning with two times the EC50 of each drug and increasing the drug concentration twofold at each passage.
Analysis of RT mutations.
Resistance-engendering mutations were identified by the direct sequencing of PCR products amplified from the RT region of proviral DNA obtained from acutely infected CEM-SS cells. A PCR-amplified product was prepared from the first 750 bp of the RT gene with the A′-NE1′ primer set. Single-stranded, biotinylated DNA was purified from this product with avidin-conjugated supraparamagnetic beads (Dynabeads, M280; Dynal). Direct sequencing with dideoxynucleotide chain termination was performed with each of the appropriate G, A, C, and T dideoxy sequencing mixes with the Sequenase T7 polymerase kit (United States Biochemicals) with 7-deaza-dGTP to resolve compression artifacts, α-33P, and five sets of overlapping primers obtained with a primer analysis software package (Oligo 4.04; National Bioscience, Inc.). Evaluation of the resulting sequencing gels was accomplished with Millipore’s automated gel scanning system. RT sequences from drug-resistant isolates were aligned with the parental wild-type HIV-IIIIB and HXB2 RTs with Millipore and Bioimage’s software run on a Sun Microsystems Sparc 10 Station microcomputer.
RT inhibition assays.
Analysis of the drug sensitivity of RT containing defined amino acid substitutions was performed as described previously (4). Evaluation of the activities of the compounds with homopolymer and heteropolymer templates was performed as described previously (7). For the Ki studies, HIV-1 RT activity was measured in 50-μl reaction mixtures containing 50 mM Tris (pH 8.0), 50 mM KCl, 10 mM MgCl2, 4 mM 2-mercaptoethanol, 3% glycerol, 1 mg of bovine serum albumin per ml, 6.6 μg of primed 16S rRNA from Escherichia coli per ml, 10 μM dATP, 10 μM dCTP, 10 μM dGTP, and various concentrations of [3H]dTTP (27). Purified recombinant HIV-1BH10 RT was used for these experiments (30). The Km for dTTP was 1.67 μM, and for the rRNA template it was 0.66 μg/ml.
RESULTS
Range of activity and mechanisms of action of calanolide isomers.
The activities of calanolide A, costatolide, and dihydrocostatolide were evaluated with established and fresh human cells infected with both laboratory-derived and clinical strains of HIV-1, HIV-2, and simian immunodeficiency virus (SIV). Nevirapine (an NNRTI) and AZT (a nucleoside RT inhibitor [NRT]) were used as positive anti-HIV control compounds. These data are summarized in Table 1. Each of the compounds was determined to be active against HIV-1 in the T-cell lines CEM-SS, H9, and MT2, in the B-cell line AA5, in the monocytic line U937, and in the T-cell–B-cell hybrid line 174×CEM. The activities (EC50) of the compounds against HIV-1 ranged from 0.08 to 0.5, 0.06 to 1.4, and 0.1 to 0.8 μM for calanolide A, costatolide, and dihydrocostatolide, respectively. None of the compounds was found to have activity against HIV-2 or SIV. AZT and nevirapine exhibited the expected levels of activity in each of the cell lines.
TABLE 1.
Range of anti-HIV activities of calanolide analogs in established human cell lines
| Virus (isolate) | Cellsa | EC50 (μM)b
|
||||
|---|---|---|---|---|---|---|
| Calanolide A | Costatolide | Dihydrocostatolide | Nevirapine | AZT | ||
| HIV-1 (IIIB) | CEM-SS | 0.08 | 0.2 | 0.1 | 0.1 | 0.003 |
| HIV-1 (RF) | CEM-SS | 0.1 | 0.7 | 0.6 | 0.2 | 0.004 |
| HIV-1 (SK1) | CEM-SS | 0.09 | 0.06 | 0.3 | 0.2 | 0.005 |
| HIV-2 (ROD) | CEM-SS | >10 | >10 | >10 | >38 | 0.005 |
| SIV (DeltaB670) | 174×CEM | >10 | >10 | >10 | >38 | 0.02 |
| HIV-1 (IIIB) | 174×CEM | 0.5 | 0.15 | 0.8 | 0.1 | 0.03 |
| HIV-1 (IIIB) | MT2 | 0.4 | 0.8 | 0.8 | 0.1 | 0.04 |
| HIV-1 (IIIB) | U937 | 0.2 | 0.3 | 0.8 | 0.3 | 0.003 |
| HIV-1 (IIIB) | AA5 | 0.2 | 1.4 | 0.6 | 0.7 | 0.01 |
| HIV-1 (WEJO) | PBMC | 0.03 | 0.2 | 0.2 | 0.06 | 0.02 |
| ROJO (SI) | PBMC | 0.07 | 1.5 | 0.5 | 0.3 | 0.01 |
| TEKI (SI) | PBMC | 0.3 | 0.2 | 0.5 | 0.1 | 0.05 |
| SLKA (SI) | PBMC | 0.3 | 0.03 | 0.3 | 0.2 | 0.02 |
| Clade A | PBMC | 0.07 | 0.1 | 0.5 | 0.06 | 0.008 |
| Clade B | PBMC | 0.2 | 0.2 | 0.4 | 0.07 | 0.01 |
| Clade C | PBMC | 0.02 | 0.09 | 0.06 | 0.05 | 0.007 |
| Clade D | PBMC | 0.06 | 0.2 | 0.2 | 0.1 | 0.01 |
| Clade E | PBMC | 0.03 | 0.4 | 0.5 | 0.007 | 0.01 |
| Clade F | PBMC | 0.04 | 0.1 | 0.1 | 0.2 | 0.05 |
| BaL | MM | 0.05 | 0.01 | 0.1 | 0.4 | 0.03 |
| ADA | MM | 0.07 | 0.05 | 0.1 | 0.2 | 0.01 |
Cellular phenotypes are as follows: CEM-SS, T cell; 174×CEM, T cell–B cell fusion; U937, macrophage; AA5, Epstein-Barr virus-infected B cell; PBMC, fresh PBMCs, MM, fresh peripheral blood monocytes.
EC50s are the mean for a minimum of three replicate evaluations. The reproducibilities of the EC50s obtained between replicate experiments in this highly optimized anti-HIV assay have been determined to be such that the standard errors averaged less than 10% of the respective mean value.
Each of the compounds was also evaluated in fresh human peripheral blood leukocytes and monocytes-macrophages infected with a variety of low-passage clinical virus isolates (Table 1). The compounds were determined to be equally active against clinical virus strains, including viruses representative of the various HIV-1 clades (clades A through F) found worldwide, syncytium-inducing and non-syncytium-inducing viruses, and T-tropic and monocyte-macrophage tropic viruses. The toxicity of each of the compounds was determined in parallel in each cell line. The toxic concentrations (IC50s) of each of the three compounds were reached at approximately 10 to 20 μM for all cell lines, yielding a therapeutic index (which is equal to IC50/EC50) of approximately 100 to 200 for each of the compounds.
Mechanistic assays indicated that each of the calanolide analogs inhibited RT when they were evaluated in a biochemical RT inhibition assay with either a homopolymeric poly(rC)-oligo(dG) or a heteropolymeric rRNA template-primer assay system. The compounds did not inhibit virus attachment, integrase, protease, or cell-cell fusion (data not shown). Also, the compounds did not inhibit late-stage virus reproduction events on the basis of their inability to suppress virus production in chronically infected cells (data not shown). Limited pretreatment assays demonstrated that compound had to be continuously present in order to be effective.
Evaluation of the abilities of the compounds to inhibit the enzymatic activity of purified RT was performed in order to define the modes of action of the calanolide analogs. When the rRNA template was present at saturating concentrations and the dTTP was present at unsaturating concentrations, costatolide and dihydrocostatolide inhibited the enzyme with IC50s of 0.003 to 0.01 μM. Inhibition could be reversed by increasing the dTTP concentration in the assay 10-fold (IC50s, >1 μM) at either saturating or nonsaturating concentrations of template. Consistent with the results reported by Currens et al. (16) for calanolide A, costatolide and dihydrocostatolide inhibited HIV-1 RT by a complex mechanism. At concentrations of between 0.02 and 0.15 μM, the inhibition approximates a mixed type of inhibition in which the inhibitor affects both the Km for dTTP and the Vmax of the enzyme (data not shown). In addition to this complexity, our data for the highest concentration tested (0.2 μM) suggest that there is a second mechanism of inhibition for both compounds. Considering that NNRTIs bind in a pocket of the enzyme outside of the catalytic site, the complexity in the mechanism that the data demonstrate is not surprising. These data provide inhibition constants (Ki) for costatolide and dihydrocostatolide of 0.06 and 0.03 μM, respectively.
Interaction of calanolide isomers with other anti-HIV agents.
Anti-HIV assays were performed with each of the calanolide analogs in combination with a variety of anti-HIV agents including the NRTIs, AZT, ddC, ddI, 3TC, and d4T, the NNRTIs UC10, UC781, and diarylsulfone, the protease inhibitors ritonavir, indinavir, nelfinavir, and saquinavir, and the surface-active attachment inhibitor resobene. A summary of the data obtained with combinations of anti-HIV agents from the MacSynergy II evaluations is presented in Table 2. Data obtained from the MacSynergy II evaluations are presented in synergy volume units (square micromolar percent) at the 95% confidence interval as described above. Each of the compounds exhibited a synergistic interaction with the NRTIs, with synergy volumes ranging from approximately 100 to over 500 μM2%. The interactions of the calanolides in combination with ritonavir and saquinavir were also determined to be synergistic. Additive interactions were obtained with the compounds in combination with other NNRTIs (with the exception of UC781), the attachment inhibitor resobene, and the protease inhibitors indinavir and nelfinavir. Costatolide in combination with the nucleoside analogs generally exhibited the highest levels of synergy. Calanolide A was less synergistic than costatolide, and dihydrocostatolide exhibited lower levels of synergy than calanolide A. Antagonistic anti-HIV drug interactions or synergistic toxicity was not observed with any of the drug combinations evaluated.
TABLE 2.
Activities of compounds in combination with other anti-HIV agents
| Agent used in combination (mechanism of action)a | Synergy vol (μM2% [SD])b
|
||
|---|---|---|---|
| Calanolide A | Costatolide | Dihydrocostatolide | |
| Resobene (AFI) | 12 (2) | 10 (1) | 4 (2) |
| AZT (NRTI) | 136 (15) | 223 (29) | 111 (8) |
| ddI (NRTI) | 95 (10) | 152 (12) | 37 (6) |
| ddC (NRTI) | 74 (7) | 525 (49) | 67 (8) |
| 3TC (NRTI) | 129 (18) | 156 (29) | 25 (3) |
| d4T (NRTI) | 115 (5) | 185 (9) | 58 (3) |
| UC10 (NNRTI) | 12 (3) | 30 (2) | 50 (9) |
| UC781 (NNRTI) | 123 (10) | 152 (12) | 143 (12) |
| Ritonavir (PI) | 112 (8) | 182 (15) | NDc |
| Indinavir (PI) | 12 (1) | 20 (1) | ND |
| Nelfinavir (PI) | 49 (5) | 15 (10) | ND |
| Saquinavir (PI) | 95 (15) | 120 (12) | ND |
Abbreviations for mechanism of action: NRTI, nucleoside RT inhibitor; NNRTI, nonnucleoside RT inhibitor; PI, protease inhibitor; AFI, attachment-fusion inhibitor.
Mean synergy volume was calculated from two replicate anti-HIV assays with drug combinations. Synergy volumes were calculated by the Prichard and Shipman (28) MacSynergy II program at the 95% confidence interval.
ND, not determined.
Sensitivities of calanolide isomers to NNRTI-resistant viruses.
The three calanolide stereoisomers and the positive control compounds nevirapine and AZT were evaluated for their antiviral activities against viruses selected in cell culture for resistance to a variety of NNRTIs (Table 3). Virus isolates containing an L100I, K103N, or Y188H amino acid change in the RT were resistant to the antiviral effects of the compounds. Calanolide A also had lower levels of activity when it was used to challenge viruses that possess either the V108I or the T139I amino acid change. Costatolide and dihydrocostatolide were not adversely affected by the V108I change, and costatolide remained partially active against viruses with the T139I amino acid change in the RT.
TABLE 3.
Activities of compounds against viruses resistant to HIV-1-specific inhibitors
| Drug(s) to which isolates are resistant (mutation) | EC50 (μM)a
|
||||
|---|---|---|---|---|---|
| Calanolide A | Costatolide | Dihydrocostatolide | Nevirapine | AZT | |
| IIIB (control) | 0.1 | 0.2 | 0.2 | 0.01 | 0.05 |
| Oxathiin carboxanilide (L100I) | >27 | >270 | >20 | 0.1 | 0.04 |
| UC10-costatolide (K103N) | >27 | >270 | >20 | NDb | 0.003 |
| Thiazolobenzimidazole (V108I) | 24.0 | 4.4 | 3.5 | 0.3 | 0.04 |
| TIBO-R82150 (A98G-V108I) | 22.0 | 1.6 | 5.1 | 0.6 | 0.05 |
| Calanolide A (T139I) | >27 | 4.5 | >20 | 0.01 | 0.01 |
| Diphenylsulfone (Y181C) | 0.08 | 0.08 | <0.01 | 5.9 | 0.01 |
| Nevirapine (Y181C) | <0.01 | <0.01 | 0.09 | >38 | 0.03 |
| Pyridinone (Y181C-L103N) | 0.12 | 0.8 | 0.8 | >38 | 0.01 |
| E-BPTU (Y181C) | 0.1 | <0.08 | <0.06 | 1.9 | 0.03 |
| UC38 (Y181C) | 0.2 | <0.03 | 0.1 | 1.9 | 0.01 |
| 3TC (M184V) | 0.3 | 1.3 | 1.0 | 0.01 | 0.02 |
| Costatolide (Y188H) | >27 | >27 | >27 | ND | 0.004 |
| HEPT (P236L) | 0.6 | 1.1 | 0.2 | 0.02 | 0.01 |
EC50s are the means for a minimum of two replicate evaluations. The reproducibilities of the EC50s obtained between replicate experiments in this highly optimized anti-HIV assay have been determined to be such that the standard errors averaged less than 10% of the respective mean value. EC50s indicated as greater than indicate that the compound was inactive at greatest nontoxic concentration tested.
ND, not determined.
A striking enhanced activity of each of these compounds against mutant virus with the Y181C amino acid change was observed. This enhanced anti-HIV activity was absent against a mutant containing both the Y181C and the K103N changes. However, viruses with these changes remained sensitive to the calanolide analogs, whereas viruses with the K103N amino acid change alone were completely resistant to the compounds. Calanolide A remained active against viruses that possessed the M184I and P236L amino acid changes, while slight but reproducible lower levels of activity were observed with costatolide and dihydrocostatolide when they were used to challenge these isolates.
Confirmation of the results of these assays was obtained by evaluating the activity of each compound against viruses or purified RT with single amino acid changes introduced by site-directed mutagenesis. As noted above, viruses with the L100I, K101E, K103N, T139I, and Y188C amino acid changes were resistant to the calanolide analogs (Table 4). The calanolide A-specific amino acid change T139I resulted in a 4- to 20-fold loss of activity with calanolide A and dihydrocostatolide but had a much greater effect on costatolide (60-fold loss of activity). Enhanced sensitivity was detected with the Y181C amino acid change in the RT, and further enhancement (two- to threefold) was observed upon introduction of AZT resistance-engendering mutations into the RT with the Y181C mutation. As expected, each of the compounds was inactive against purified HIV-2 RT.
TABLE 4.
Cross-resistance of virus isolates with defined amino acid changes
| Virus isolate or amino acid change | EC50 (μM)a
|
||||
|---|---|---|---|---|---|
| Calanolide A | Costatolide | Dihydrocostatolide | Nevirapine | AZT | |
| NL4-3 | 0.1 | 0.2 | 0.5 | 0.02 | 0.001 |
| L74V | 0.1 | 0.2 | 0.6 | 0.02 | 0.005 |
| A98G | 0.2 | 0.3 | 0.2 | 0.2 | 0.01 |
| L100I | 22.0 | 5.0 | >20 | 0.1 | 0.003 |
| K101E | 1.7 | 2.9 | 3.5 | 0.3 | 0.002 |
| K103N | 1.7 | 1.2 | 3.8 | >2 | 0.004 |
| V106I | 0.2 | 0.2 | 0.8 | >2 | 0.003 |
| V108I | 0.3 | 0.2 | 1.2 | 0.06 | 0.008 |
| T139I | 1.7 | 11.9 | 2.9 | 0.002 | 0.003 |
| V179D | 0.05 | 0.1 | 0.4 | 0.03 | 0.003 |
| Y181C | 0.02 | 0.02 | <0.06 | 1.3 | 0.01 |
| Y188C | 1.7 | 1.7 | 1.0 | 3.2 | 0.006 |
| 4 × AZT | 0.2 | 0.2 | 0.3 | 0.02 | 0.8 |
| 4 × AZT + Y181C | 0.01 | 0.01 | <0.06 | 0.8 | 0.04 |
| 4 × AZT + L100I | 1.5 | 3.0 | >20 | 0.03 | 0.02 |
EC50s are the means for a minimum of two replicate evaluations. The reproducibilities of the EC50s obtained between replicate experiments in this highly optimized anti-HIV assay have been determined to be such that the standard errors averaged less than 10% of the respective mean value.
Selection and characterization of drug-resistant virus isolates.
Viruses resistant to the calanolide isomers were selected in CEM-SS cells infected with the IIIB strain of HIV-1 after a short time of culture in the presence of increasing concentrations of each compound (four to six passages) (Table 5). Selection with calanolide A yielded a virus isolate with the T139I amino acid change in the RT. This virus was >100-fold more resistant than the wild type to calanolide was but less resistant to costatolide and dihydrocostatolide. Selection with costatolide yielded a virus that possessed both T139I and L100I amino acid changes and that was highly cross-resistant to each of the calanolide analogs. A virus strain selected with dihydrocostatolide contains the L100I amino acid change. This virus was >100-fold more resistant than the wild type to costatolide and dihydrocostatolide but was less cross-resistant to calanolide A.
TABLE 5.
Cross-resistance phenotype of calanolide-resistant viruses
| Compound | EC50 (μM) for viruses with following phenotypes (resistance mutation)a
|
|||
|---|---|---|---|---|
| Calanolide A resistant (T139I) | Costatolide resistant (L100I, T139I) | Dihydrocostatolide resistant (L100I) | Wild type (strain IIIB) | |
| Calanolide A | >27 | >27 | 7.2 | 0.1 |
| Costatolide | 4.5 | >27 | >27 | 0.1 |
| Dihydrocostatolide | >27 | >27 | >27 | 0.2 |
| UC10 | 0.3 | 2.6 | 1.0 | 0.1 |
| NSC 676509 | 0.4 | >2 | 1.0 | 0.05 |
| UC781 | 0.04 | 0.04 | 0.3 | 0.005 |
| NSC 675187 | 0.03 | 0.08 | 0.03 | 0.05 |
| Thiazolobenzimidazole | 3.9 | >69 | 22.8 | 2.5 |
| Oxathiin carboxanilide | 1.6 | >28 | >28 | 0.5 |
| TIBO | 0.4 | >35 | 11.7 | 0.1 |
| Diphenylsulfone | 16.0 | 85 | 53.1 | 3.7 |
| TSAO | 0.5 | 0.3 | 0.05 | 0.05 |
| α-APA | 0.07 | 0.09 | 0.02 | 0.03 |
| E-BPTU | 0.01 | 3.0 | 0.08 | 0.04 |
| Nevirapine | 0.03 | 0.2 | 0.1 | 0.01 |
| AZT | 0.01 | 0.06 | 0.003 | 0.005 |
| 3TC | 0.1 | 0.04 | 0.03 | 0.07 |
EC50s are the means for a minimum of two replicate evaluations. The reproducibilities of the EC50s obtained between replicate experiments in this highly optimized anti-HIV assay have been determined to be such that the standard errors averaged less than 10% of the respective mean value.
To examine the influence of the cell type in selecting resistant virus, costatolide was used to treat a variety of different established human cell lines and fresh human cells infected with HIV-1IIIB. A variety of amino acid changes were observed in viruses isolated from different cell types. In CEM-SS cells, the resistant isolate contained both the T139 and L100I amino acid changes. In both MT2 and AA5 cells, costatolide selected for virus that possessed the Y188H amino acid change. In H9 cells, the resistant virus isolate possessed an L187F amino acid change. Each of these substitutions was determined to yield high-level resistance to costatolide (>27-fold greater resistance than the wild type). Fresh human cells infected with a low-passage clinical strain (ROJO) were also used to select for a costatolide-resistant isolate by the methodology identical to that described above. In this experiment the virus selected contained the L100I amino acid change.
Finally, calanolide A and costatolide were used in combination with a second NNRTI in strategies for the isolation of drug-resistant virus. The compounds chosen for these assays with drug combinations were selected on the basis of their ability to effectively inhibit the replication of calanolide A-resistant viruses, as well as on the basis of the ability of calanolide A to inhibit drug-resistant viruses selected by the second compound. The compounds 3TC, diphenylsulfone, E-BPTU, α-APA, UC10, TSAO, and diarylsulfone were used in these studies. In each drug combination, a virus strain with a mutation that conferred resistance to both compounds was obtained in cell culture (Table 6). These amino acid changes differed from the changes selected by either compound when it was used alone in these in vitro selection assays. These viruses possessed changes in the amino acid sequence of the RT which included L100I, K101E, V108I, K103N, V106I, and Y188H. On the basis of a direct comparison of the number of passages required to select for a drug-resistant virus, the NNRTIs most capable of suppressing virus reproduction appeared to be a calanolide analog (which was active against Y181C mutants) in combination with agents that were inhibitory to viruses with the L100I amino acid change (α-APA and diarylsulfone). With these combinations, selection for viruses with either K103N or Y188H amino acid changes conferred resistance to both antiviral agents after approximately 13 to 14 passages in cell culture.
TABLE 6.
Selection of virus strains resistant to two compounds (compounds 1 and 2)
| Compound 2 | Amino acid change (no. of passages in cell culture) with the following compound 1:
|
|||
|---|---|---|---|---|
| None | Calanolide A | Costatolide | Dihydrocostatolide | |
| None | T139I | T139I, L100I | L100I | |
| 3TC | M184V | M184V, L100I (5) | M184V, L100I (6) | M184V, L100I (5) |
| Diphenylsulfone | Y181C | V108I (6) | Y188H (6) | NDa |
| E-BPTU | Y181C | K103N, V106I (6) | ND | ND |
| α-APA | Y181C | ND | K103N (14) | ND |
| UC10 | K101E, Y181C | Y188H (6) | K103N (5) | K103N (5) |
| TSAO | Y181C | K101E (6) | K101E (6) | K101E (6) |
| Diarylsulfone | Y181C | Y188H (13) | Y188H (11) | Y188H (8) |
ND, not determined.
DISCUSSION
The stereoisomers (+)-calanolide A, (−)-calanolide B (costatolide), and (−)-dihydrocalanolide B (dihydrocostatolide) were determined to be highly effective nonnucleoside inhibitors of RT. Although less potent than many members of the NNRTI class, several unique features regarding the anti-HIV activities of calanolide compounds have been defined, and these features suggest that the calanolides represent a novel and potentially useful subclass of the NNRTI family of inhibitors. The major difference between the calanolide isomers and other NNRTIs involves the activities of the compounds against viruses that possess the Y181C amino acid change in the RT. In most cases, the isomers exhibited approximately 10-fold enhanced activity against these strains. This activity appears to be a result of a general structural feature of the molecule since (−)-calanolide A, which failed to inhibit wild-type HIV-1, was determined to be active, albeit at a higher concentration, against the Y181C-containing viruses (EC50 = 1.5 μM) (4a). This enhanced level of activity is more pronounced when the Y181C change is present in the background of an AZT-resistant virus. In addition, although the K103N amino acid change resulted in the loss of activity of the compounds, introduction of the K103N change into the Y181C-possessing RT yielded a virus which remained sensitive to the antiviral activities of all three compounds. This suggests that the conformational change in the RT pocket associated with the K103N amino acid change is compensated for by the Y181C change. Enzymatic data and studies with chimeric HIV-1 and HIV-2 RT have suggested that calanolide A may bind to two sites on the RT (16, 22). One of the proposed sites is within the hydrophobic NNRTI binding pocket, while the second site was determined to be outside the pocket. Our kinetic data confirm that each of the calanolides has a complex pattern of interaction with the RT. Our data are consistent with those reported for calanolide A by Currens et al. (16).
Sensitivity testing of the calanolide isomers also suggests that fine specificity differences exist in the interaction of the three isomers with the RT. For example, although only slightly resistant, costatolide appears to be reproducibly affected by amino acid changes M184I-M184V and P236L. Anti-HIV assays with drug combinations also suggest that differences may exist between the isomers in regard to their interactions with other compounds for the inhibition of HIV. Of the three stereoisomers, costatolide exhibited the greatest level of synergy with each of the nucleoside analogs. Unlike the majority of the NNRTIs, each of the calanolide analogs was determined to have synergistic activity with the NNRTI UC781. Most importantly, for further clinical development of these compounds, all of the anti-HIV assays performed with combinations of the calanolide isomers failed to detect either synergistic toxicity or antagonism with respect to virus reproduction.
The selection of drug-resistant isolates in cell culture yielded important information regarding the interaction of the isomers with the RT and with other anti-HIV agents. Calanolide A selected for viruses with a previously unidentified T139I amino acid change in the RT (7). Costatolide, the only one of the three compounds which remained active against viruses with the T139I change, selected for a resistant isolate with both the T139I and L100I amino acid changes or with the L100I change alone. The calanolide A-resistant virus with the T139I change was not cross-resistant to any of the other NNRTIs evaluated. Introduction of the L100I amino acid change, however, yielded a virus that was highly cross-resistant to a variety of NNRTIs with the exception of α-APA, which remained completely active against the strain. In addition, the virus with the L100I change exhibited only 10-fold reductions in sensitivity to UC781 and TSAO.
Our drug efficacy, drug combination, and viral resistance data suggest that costatolide may be superior to calanolide A as an inhibitor of HIV. Thus, costatolide was used to select for additional resistant strains in different cell lines in order to define the range of mutations which might be expected to appear in patient isolates during therapy with this compound. In addition to the diagnostic T139I change, a variety of amino acid changes were observed, including L100I, Y188H, and L187F. Viruses with these changes likely represent subpopulations of virus which exist in the wild-type virus pool with growth advantage in the particular cell line used for selection.
In order to further explore the interaction of costatolide with other NNRTIs in the context of resistant virus selection, compounds were chosen for use in combination with the calanolide isomers on the basis of their ability to inhibit the replication of viruses resistant to the isomers. Resistant virus strains were selected with each of these drug combinations. The amino acid changes observed in each resistant virus were different from those selected with the individual compounds. Selection of calanolide-resistant viruses by using a drug-resistant strain as the starting material rapidly yielded drug-resistant virus populations. Use of an AZT-resistant strain (strain G910-6) yielded viruses with K103N and K122E amino acid changes upon selection with costatolide and dihydrocostatolide in MT2 cells. The most difficult selections to perform on the basis of a direct comparison of the number of passages required to obtain a resistant strain included the calanolide isomers with compounds that inhibited viruses with L100I amino acid changes. In these cases, the number of passages required to select for a resistant strain were approximately double the number of passages required for other combinations of agents (11 to 14 passages versus 5 to 6 passages).
Our results demonstrate that the calanolide isomers can be clearly distinguished from other members of the NNRTI class, and thus, that the compounds may be useful in combination with other anti-HIV agents. Nonetheless, resistance to a single calanolide isomer or an isomer in combination with a second active compound was easily achieved in cell culture. Thus, the use of two NNRTIs may be ineffective in patients unless sufficiently high concentrations of the compounds are maintained to suppress the ability of the virus to replicate. The use of two NNRTIs in combination will require the addition of a third anti-HIV compound with a different mechanism of anti-HIV action in order to be effective. The possibility of using two NNRTIs in a cocktail with a third NRTI may be clinically useful from the viewpoint of preserving the possibility of using more potent future therapies with the protease inhibitors.
ACKNOWLEDGMENTS
This work was supported by contract NO1-CM-37818 to the Southern Research Institute from NCI.
We gratefully acknowledge Larry Ross and Cathi Pyle for technical laboratory support, Barbara Toyer and Michelle Wenzel for drug preparation support, and Diana Markle for assistance with the preparation of the manuscript.
REFERENCES
- 1.Bader J P, McMahon J B, Schultz R J, Narayanan V L, Pierce J B, Harrison W A, Weislow O S, Midelfort C F, Stinson S F, Boyd M R. Oxathiin carboxanilide, a potent inhibitor of human immunodeficiency virus reproduction. Proc Natl Acad Sci USA. 1991;88:6740–6744. doi: 10.1073/pnas.88.15.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, Karlsson A, Perez-Perez M J, Camarasa M J, Tarpley W G, De Clercq E. Treatment of human immunodeficiency virus type 1 (HIV-1)-infected cells with combinations of HIV-1-specific inhibitors results in a different resistance pattern than does treatment with single-drug therapy. J Virol. 1993;67:5353–5359. doi: 10.1128/jvi.67.9.5353-5359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini J, Karlsson A, Vandamme A M, Perez-Perez M J, Zhang H, Vrang L, Oberg B, Backbro K, Unge T, San-Felix A. Human immunodeficiency virus type 1 (HIV-1) strains selected for resistance against the HIV-1-specific [2′,5′-bis-O--(tert-butyldimethylsilyl)-3′-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide)]-beta-D-pentofuranosyl (TSAO) nucleoside analogues retain sensitivity to HIV-1-specific nonnucleoside inhibitors. Proc Natl Acad Sci USA. 1993;90:6952–6956. doi: 10.1073/pnas.90.15.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer P L, Currens M J, McMahon J B, Boyd M R, Hughes S H. Analysis of nonnucleoside drug-resistant variants of human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1993;67:2412–2420. doi: 10.1128/jvi.67.4.2412-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Buckheit, R. W., Jr. Unpublished results.
- 5.Buckheit, R. W., Jr., M. J. Snow, V. Fliakas-Boltz, T. L. Kinjerski, J. D. Russell, L. A. Pallansch, W. G. Brouwer, and S. S. Yang. Highly potent oxathiin carboxanilide derivatives with efficacy against human immunodeficiency virus nonnucleoside reverse transcriptase inhibitor-resistant virus isolates. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 6.Buckheit R W, Jr, Fliakas-Boltz V, Decker W D, Roberson J L, Pyle C A, White E L, Bowdon B J, McMahon J B, Boyd M R, Bader J P, Nickell D G, Barth H, Antonucci T K. Biological and biochemical anti-HIV activity of the benzothiadiazine class of nonnucleoside reverse transcriptase inhibitors. Antivir Res. 1994;25:43–56. doi: 10.1016/0166-3542(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 7.Buckheit R W, Jr, Fliakas-Boltz V, Decker W D, Roberson J L, Stup T L, Pyle C A, White E L, McMahon J B, Currens M J, Boyd M R, Bader J P. Comparative anti-HIV evaluation of diverse HIV-1-specific reverse transcriptase inhibitor-resistant virus isolates demonstrates the existence of distinct phenotypic subgroups. Antivir Res. 1995;2•:117–132. doi: 10.1016/0166-3542(94)00069-k. [DOI] [PubMed] [Google Scholar]
- 8.Buckheit R W, Jr, Fliakas-Boltz V, Yeagy-Bargo S, Weislow O, Mayers D L, Boyer P L, Hughes S H, Pan B C, Chu S H, Bader J P. Resistance to 1-[2-hydroxyethoxy)methyl]-6-(phenylthio)thymine derivatives is generated by mutations at multiple sites in the HIV-1 reverse transcriptase. Virology. 1995;210:186–193. doi: 10.1006/viro.1995.1330. [DOI] [PubMed] [Google Scholar]
- 9.Buckheit R W, Jr, Germany-Decker J, Hollingshead M G, Allen L B, Shannon W M, Janssen P A, Chirigos M A. Differential antiviral activity of two TIBO derivatives against the human immunodeficiency and murine leukemia viruses alone and in combination with other anti-HIV agents. AIDS Res Hum Retroviruses. 1993;9:1097–1106. doi: 10.1089/aid.1993.9.1097. [DOI] [PubMed] [Google Scholar]
- 10.Buckheit R W, Jr, Hollingshead M G, Germany-Decker J, White E L, McMahon J B, Allen L B, Ross L J, Decker W D, Westbrook L, Shannon W M. Thiazolobenzimidazole: biological and biochemical anti-retroviral activity of a new nonnucleoside reverse transcriptase inhibitor. Antivir Res. 1993;21:247–265. doi: 10.1016/0166-3542(93)90031-d. [DOI] [PubMed] [Google Scholar]
- 11.Buckheit R W, Jr, Kinjerski T L, Fliakas-Boltz V, Russell J D, Stup T L, Pallansch L A, Brouwer W G, Dao D C, Harrison W A, Schultz R J, Bader J P, Yang S S. Structure-activity and cross-resistance evaluations of a series of human immunodeficiency virus type 1-specific compounds related to oxathiin carboxanilide. Antimicrob Agents Chemother. 1995;39:2718–2727. doi: 10.1128/aac.39.12.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrnes V W, Sardana V V, Schleif W A, Condra J H, Waterbury J A, Wolfgang J A, Long W J, Schneider C L, Schlabach A J, Wolanski B S. Comprehensive mutant enzyme and viral variant assessment of human immunodeficiency virus type 1 reverse transcriptase resistance to nonnucleoside inhibitors. Antimicrob Agents Chemother. 1993;37:1576–1579. doi: 10.1128/aac.37.8.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr A, Cooper D A. Current clinical experience with nevirapine for HIV infection. Adv Exp Med Biol. 1998;394:299–304. doi: 10.1007/978-1-4757-9209-6_27. [DOI] [PubMed] [Google Scholar]
- 14.Cohen K A, Hopkins J, Ingraham R H, Pargellis C, Wu J C, Palladino D E, Kinkade P, Warren T C, Rogers S, Adams J. Characterization of the binding site for nevirapine (BI-RG-587), a nonnucleoside inhibitor of human immunodeficiency virus type-1 reverse transcriptase. J Biol Chem. 1991;266:14670–14674. [PubMed] [Google Scholar]
- 15.Condra J H, Emini E A, Gotlib L, Graham D J, Schlabach A J, Wolfgang J A, Colonno R J, Sardana V V. Identification of the human immunodeficiency virus reverse transcriptase residues that contribute to the activity of diverse nonnucleoside inhibitors. Antimicrob Agents Chemother. 1992;36:1441–1446. doi: 10.1128/aac.36.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currens M J, Gulakowski R J, Mariner J M, Moran R A, Buckheit R W, Jr, Gustafson K R, McMahon J B, Boyd M R. Antiviral activity mechanism of action of calanolide A against the human immunodeficiency virus. J Pharmacol Exp Ther. 1996;279:645–651. [PubMed] [Google Scholar]
- 17.De Clercq E. What can be expected from non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the treatment of human immunodeficiency virus type 1 (HIV-1) infections? Med Virol. 1996;6:97–117. doi: 10.1002/(SICI)1099-1654(199606)6:2<97::AID-RMV168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antivir Res. 1998;38:153–179. doi: 10.1016/s0166-3542(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 19.Dueweke T J, Pushkarskaya T, Poppe S M, Swaney S M, Zhao J Q, Chen I S, Stevenson M, Tarpley W G. A mutation in reverse transcriptase of bis(heteroaryl)piperazine-resistant human immunodeficiency virus type 1 that confers increased sensitivity to other nonnucleoside inhibitors. Proc Natl Acad Sci USA. 1993;90:4713–4717. doi: 10.1073/pnas.90.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grob P M, Wu J C, Cohen K A, Ingraham R H, Shih C K, Hargrave K D, McTague T L, Merluzzi V J. Nonnucleoside inhibitors of HIV-1 reverse transcriptase: nevirapine as a prototype drug. AIDS Res Hum Retroviruses. 1992;8:145–152. doi: 10.1089/aid.1992.8.145. [DOI] [PubMed] [Google Scholar]
- 21.Halliday S M, Lackman-Smith C S, Decker W D, Bader J P, Rice W G, Clanton D J, Buckheit R W., Jr Inhibition of HIV replication by the sulfonated dye resobene. Antivir Res. 1996;33:41–53. doi: 10.1016/s0166-3542(96)00994-1. [DOI] [PubMed] [Google Scholar]
- 22.Hizi A, Tal R, Shaharabany M, Currens M J, Boyd M R, Hughes S H, McMahon J B. Specific inhibition of the reverse transcriptase of human immunodeficiency virus type 1 and the chimeric enzymes of human immunodeficiency virus type 1 and type 2 by nonnucleoside inhibitors. Antimicrob Agents Chemother. 1993;37:1037–1042. doi: 10.1128/aac.37.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashman Y, Gustafson K R, Fuller R W, Cardellina J H, McMahon J B, Currens M J, Buckheit R W, Jr, Hughes S H, Cragg G M, Boyd M R. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem. 1992;35:2735–2743. doi: 10.1021/jm00093a004. . (Erratum, 36:1110, 1993.) [DOI] [PubMed] [Google Scholar]
- 24.McMahon J B, Buckheit R W, Jr, Gulakowski R J, Currens M J, Vistica D T, Shoemaker R H, Stinson S F, Russell J D, Bader J P, Narayanan V L, Shultz R J, Brouwer W G, Felauer E E, Boyd M R. Biological and biochemical anti-human immunodeficiency virus activity of UC-38, a new nonnucleoside reverse transcriptase inhibitor. J Pharmacol Exp Ther. 1995;276:298–305. [PubMed] [Google Scholar]
- 25.McMahon J B, Gulakowski R J, Weislow O S, Schultz R J, Narayanan V L, Clanton D J, Pedemonte R, Wassmundt F W, Buckheit R W, Jr, Decker W D. Diarylsulfones, a new chemical class of nonnucleoside antiviral inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993;37:754–760. doi: 10.1128/aac.37.4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellors J W, Larder B A, Schinazi R F. Mutations in HIV-1 reverse transcriptase and protease associated with drug resistance. Int Antivir News. 1995;3:8–13. [Google Scholar]
- 27.Parker W B, White E L, Shaddix S C, Ross L J, Buckheit R W, Jr, Germany J M, Secrist J A, Vince R, Shannon W M. Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase and human DNA polymerases α,β, and gamma by the 5′-triphosphates of carbovir, 3′-azido-3′-deoxythymidine, 2′,3′-dideoxyguanosine and 3′-deoxythymidine. A novel RNA template for the evaluation of antiretroviral drugs. J Biol Chem. 1991;266:1754–1762. [PubMed] [Google Scholar]
- 28.Prichard M N, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antivir Res. 1990;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 29.Saag M S, Emini E A, Laskin O L, Douglas J, Lapidus W I, Schleif W A, Whitley R J, Hildebrand C, Byrnes V W, Kappes J C. A short-term clinical evaluation of L-697,661, a non-nucleoside inhibitor of HIV-1 reverse transcriptase. L-697,661 Working Group. N Engl J Med. 1993;329:1065–1072. doi: 10.1056/NEJM199310073291502. [DOI] [PubMed] [Google Scholar]
- 30.White E L, Parker W B, Ross L J, Shannon W M. Lack of synergy in the inhibition of HIV-1 reverse transcriptase by combinations of the 5′-triphosphates of various anti-HIV nucleoside analogs. Antivir Res. 1993;22:295–308. doi: 10.1016/0166-3542(93)90039-l. [DOI] [PubMed] [Google Scholar]