Abstract
Surgery can be highly effective for treating certain cases of drug resistant epilepsy. The current study tested a novel, non-invasive, surgical strategy for treating seizures in a rat model of temporal lobe epilepsy. The surgical approach uses magnetic resonance-guided, low-intensity focused ultrasound (MRgFUS) in combination with intravenous microbubbles to open the blood-brain barrier (BBB) in a transient and focal manner. During the period of BBB opening, a systemically administered neurotoxin (Quinolinic Acid: QA) that is normally impermeable to the BBB gains access to a targeted area in the brain, destroying neurons where the BBB has been opened. This strategy is termed Precise Intracerebral Non-invasive Guided Surgery (PING). Spontaneous recurrent seizures induced by pilocarpine were monitored behaviorally prior to and after PING or under control conditions. Seizure frequency in untreated animals or animals treated with MRgFUS without QA exhibited expected seizure rate fluctuations frequencies between the monitoring periods. In contrast, animals treated with PING targeting the intermediate-temporal aspect of the hippocampus exhibited substantial reductions in seizure frequency, with convulsive seizures being eliminated entirely in two animals. These findings suggest that PING could provide a useful alternative to invasive surgical interventions for treating drug resistant epilepsy, and perhaps for treating other neurological disorders in which aberrant neural circuitries play a role.
Keywords: Epilepsy surgery, Non-invasive, Focused ultrasound, Quinolinic acid, Magnetic resonance-guided, Neuronal loss
1. Introduction
Temporal lobe epilepsy (TLE) is a chronic disorder of the nervous system characterized by spontaneous, recurrent, focal seizures that originate in the temporal lobe of the brain and last about one or two minutes. TLE is the most common form of epilepsy with focal seizures, and mesial temporal lobe epilepsy (MTLE) accounts for almost 80% of cases. MTLE involves the medial or internal structures of the temporal lobe, with seizures typically being initiated in the hippocampus or neighboring areas. MTLE is often associated with abnormal findings on magnetic resonance imaging (MRI), with one of the most common findings being hippocampal sclerosis involving one or both sides of the brain (Cendes et al., 2016).
The majority of patients with epilepsy achieve excellent seizure control with anti-seizure drugs. However, almost a third of patients do not respond to drug therapy (Kwan and Brodie, 2000), and epilepsy surgery may then become an option. Temporal lobectomy leads to a significant reduction in seizure or complete seizure control in approximately 70% to 80% of cases in patients with hippocampal sclerosis and with seizures originating from the same area (Dupont et al., 2006; Sperling et al., 1996). However, memory and language can be affected if this procedure is performed on the dominant hemisphere. Selective amygdalohippocampectomy results in less risk for language function and fewer neuropsychological sequelae (Spencer and Burchiel, 2012). Nonetheless, complications from surgical interventions may still include psychiatric disorders, visual field defects, cognitive disorders, cranial nerve deficits, etc. Therefore, minimally invasive or non-invasive techniques, such as stereotactic radiofrequency (Boling, 2018), laser-induced thermal therapy (Widjaja et al., 2019), or radiosurgery (Feng et al., 2016) are increasingly being utilized to mitigate these potential risks and increase the pool of potential surgical candidates.
MRI-guided high-intensity focused ultrasound (HIFU) ablation is another promising therapeutic approach because of its precise and non-invasive nature. However, HIFU’s treatment envelope is currently restricted to more central areas of the brain, due to the risk of thermal injury to the skull, meninges, and superficial portions of the brain parenchyma.
Previous studies have shown that low-intensity focused ultrasound (MRgFUS) combined with intravenous microbubbles can transiently and focally open the BBB in targeted areas of the brain (Zhang et al., 2019). The period of reversible BBB opening is then exploited to deliver a systemically-administered neurotoxin (Quinolinic Acid: QA) that is normally BBB-impermeable to the targeted area of the brain parenchyma where the BBB has been opened. Quinolinic acid poorly penetrates the intact BBB. The neurotoxic effects of QA are primarily dependent on direct access to neural NMDA receptors. Systemic administration of QA is relatively innocuous (Foster et al., 1984). Even when very high dosages of 60 mmol per day for 8 days were administered, only moderate CNS changes such as the neuronal cell bodies in the arcuate nucleus had features of increased cellular activity, and some evident damage of neuronal cell were observed (Beskid et al., 1997). Previous studies using this approach, which is termed PING, demonstrate that focal delivery to the brain of systemically-administered QA produces neuronal loss in the area of BBB opening (Zhang et al., 2019; Zhang et al., 2016). In a recent preliminary study, PING targeting the intermediate-temporal aspect of the hippocampus reduced seizure frequency in pilocarpine-treated mice(Zhang et al., 2020). A point of clarification is warranted here regarding neuroanatomical terminology. Owing to their relative degrees of neocortical expansion, the anterior aspect of the human hippocampus corresponds to the more intermediate and temporal aspects of the rodent hippocampus. Thus, targeting the intermediate-temporal aspect of the rodent brain corresponds generally to the typical targeting for human epilepsy surgery. One of the limitations of the previous study was the lack of control groups. In the current study, we included three control groups: control group of animals with TLE which received no FUS/no QA, control group of animals without TLE which received FUSit/QA, and control group of animals without TLE which received FUSs-it/QA. The current study was carried out with these control groups to confirm the conclusion that the destruction of neurons in the intermediate-temporal hippocampus was effective in suppressing seizures in another species of rat pilocarpine model of temporal lobe epilepsy.
2. Methods
2.1. Study design
The animal protocol for this study was approved by the Institutional Administrative Panel on Laboratory Animal Care (APLAC). All experiments were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. The basic timeline for the experiment is shown in Fig. 1.
Fig. 1.
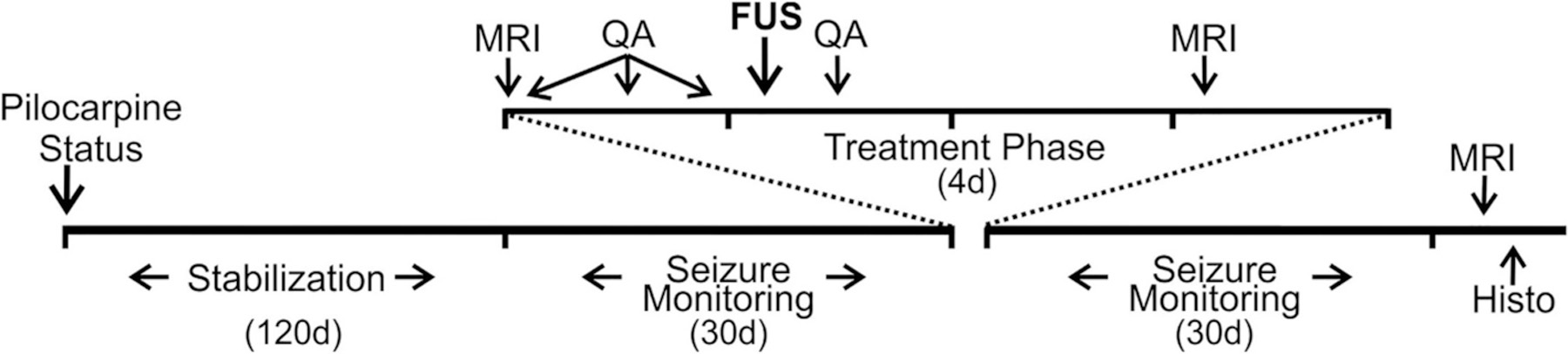
Timeline of the study. Pilocarpine-induced status epilepticus (Status) was followed by a stabilization period of 120 days. Behavioral seizures were then monitored for 30 days. A 60-day period then intervened (not shown) prior to initiating treatment. A 4-day treatment phase included two episodes of imaging (MRI), four intraperitoneal injections of Quinolinic Acid (QA), and the delivery of focused ultrasound (FUS). Behavioral seizures were then monitored for 30 days. At the end of seizure monitoring, imaging was again performed, after which animals were euthanized and prepared for histological analyses (Histo).
2.2. Induction of status epilepticus (SE)
An established model of temporal lobe epilepsy involving pilocarpine-induced status epilepticus (SE) followed by the development of spontaneous, recurrent seizures was utilized for this study (Toyoda et al., 2013). Status epilepticus was induced in 30 male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 100–150 g each. Pilocarpine hydrochloride (Sigma) was dissolved in bacteriostatic 0.9% NaCl to a final concentration of 200 mg/ml. 380 mg/kg of the pilocarpine solution was injected intraperitoneally into each rat. Thirty minutes prior to pilocarpine treatment, 1 mg/kg of scopol-amine methyl bromide (Sigma) was administered subcutaneously, in order to antagonize peripheral side effects of pilocarpine. Status Epileptics was gauged by the presence of continuous and/or repetitive motor convulsions. Thirteen animals died during or soon after pilocarpine treatment. After 2 h of SE, seizures were suppressed with diazepam administered intraperitoneally at 5–10 mg/kg. Diazepam treatment was repeated every 2–3 h for up to 6 h, in order to maintain seizure suppression. After SE, lactated Ringers solution was administered subcutaneously to maintain hydration. Sixteen of the surviving animals developed spontaneous, recurrent seizures, weighed 350–425 g each, and were retained for testing. 6 male Sprague Dawley rats with similar weight and without pilocarpine injection were included in two control groups. The study was comprised of the following 7 groups:
No FUS/No QA (n = 4)
FUSit/No QA: FUS targeting the intermediate-temporal hippocampus in rats with pilocarpine injection (n = 4)
FUSit/QA: FUS targeting the intermediate-temporal hippocampus with QA administered in rats with pilocarpine injection (n = 4)
FUSs-it/No QA: FUS targeting septal and intermediate-temporal hippocampus in rats with pilocarpine injection (n = 2)
FUSs-it/QA: FUS targeting septal and intermediate-temporal hippocampus with QA administered in rats with pilocarpine injection (n = 2)
FUSit/QA control: FUS targeting the intermediate-temporal hippocampus with QA administered in rats without pilocarpine injection (n = 3)
FUSs-it/QA control: FUS targeting septal and intermediate-temporal hippocampus with QA administered in rats without pilocarpine injection (n = 3)
The rationale for assigning fewer animals to the groups in which the septal hippocampus was affected was that evidence from prior work indicated that damage to that area produced by FUS together with QA severely aggravated seizure activity, and adverse outcomes could be anticipated (Zhang et al., 2020).
2.3. Monitoring of behavioral seizures
For the animals injected with pilocarpine, a post-SE period of 120 days was allowed for stabilizing the rats’ condition and allowing for the development of spontaneous, recurrent seizures. After this period, rats were video-recorded daily for 30 days to detect and quantify behavioral seizures. A 30-day recording period for the seizure frequency baseline was selected because previous evidence from this model has shown that seizure rates are either stable or increase slightly after this post-SE time frame. Recordings were begun at approximately 8:00 a.m. each day, and were continued for 10–11 h per day. Video recordings were reviewed in a fast-forward playback setting for identifying behavioral convulsive seizures of grade 3 (forelimb clonus) or greater (Arida et al., 1999; Racine, 1972). The motor seizures were rated on a five-point scale with respect to strength: (1) Mouth and facial movements. (2) Head nodding. (3) Forelimb clonus. (4) Rearing. (5) Rearing and falling. A full motor seizure, with loss of postural control, will be referred to as a Class 5 motor seizure. Seizure frequency was measured by an investigator who was blinded to the group identity of the animals. The same video monitoring procedure was repeated for an additional 30 days post-treatment. The 6 animals in FUSit/QA control and FUSs-it/QA control groups were monitored for 30 days post-treatment.
2.4. Magnetic resonance imaging and experimental treatments
At the end of the initial 30 days of video monitoring, baseline MRI scans were performed. T2-weighted fast spin echo (FSE) images (repetition time/echo time [TR/TE] =4800/68 milliseconds, 2 averages, field of view = 50 mm, matrix size = 248 × 256, slice thickness = 1.0 mm) were obtained for structural assessment of the rat brains.
Administration of QA was then initiated with a total of 4 injections given at 6-h intervals. Quinolinic Acid (Santa Cruz Biotechnology, Dallas, TX, USA) was dissolved in saline (10 mg/mL) and injected intraperitoneally at 80 mg/kg body weight per injection. This injection protocol was designed to achieve a relatively stable plasma concentration of QA during the period of BBB opening. Previous findings demonstrate that MR guided low-intensity focused ultrasound combined with similar dosages of QA induced focal neuronal loss in the hippocampus (Zhang et al., 2016). Two hours after the third QA injection, rats were anesthetized with isoflurane (4% induction and 2% maintenance), and microbubbles were administered. Definity® Microbubbles (300 uL/kg, mean diameter range: 1.1–3.3 μm, 1:20 diluted to a concentration of 5.0–8.0 × 108 bubbles per ml; Lantheus Medical Imaging, MA, USA) were injected through the tail vein.
Magnetic Resonance-guided Focused Ultrasound was then delivered at an acoustic peak negative pressure of 0.4 MPa characterized using a fiber-optic hydrophone in free water (Precision Acoustics, Dorset, UK), in order to open the BBB. Sonication parameters were 650KHz, pulse duration 20-ms, duty cycle of 2%, 1-Hz pulse repetition frequency, 90-s duration per sonication. Multiple sonications were administered in the vicinity of the targeted area of the hippocampus by moving the sonication zones slightly rostro-caudally and medio-laterally. After sonicating a target on one side of the brain, the system was re-directed to sonicate the same target on the contralateral side of the brain. The MRgFUS system (Image Guided Therapy, Pessac, France) was configured as previously described (Zhang et al., 2019; Zhang et al., 2020). The system includes an MR-compatible, pre-focused, six-element annular array, 650-kHz transducer (spherical radius = 30±2 mm, active diameter = 30 mm [focal ratio = 0.8]; Imasonic, Voray surl’Ognon, France), which was connected to a phased array generator and radiofrequency power amplifier. An MR-compatible motorized positioning stage was used to move the transducer in the rostral-caudal and medial-lateral directions. The membrane in front of the transducer was filled with degassed water and inflated to ensure good ultrasonic coupling between the membrane and the head of the animal. For sonication, the animals were placed in a prone position and maintained in that position using a bite bar and ear bars. The scalp hair was shaved and removed with depilatory cream. Acoustic gel was applied between the transducer and skin. The experimental apparatus of this study is shown in Fig. 2.
Fig. 2.
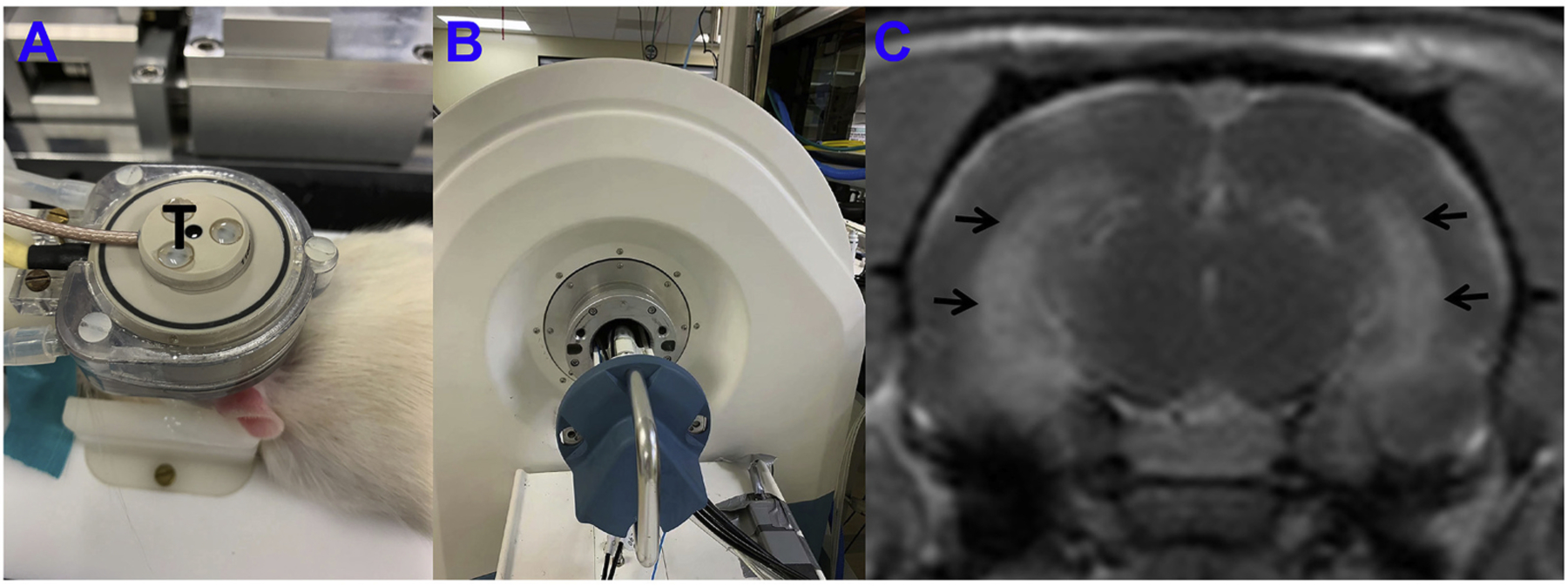
Experimental apparatus and post contrast T1-weighted image immediately after sonication. A: FUS system, a 650-kHz transducer (T) in brown rests upon the top of the rat head and can move in X-Y planes and be focused in the Z axis. B: A 3 T MRI scanner was used to detect the BBB-opening after sonication. C: Post contrast T1-weighted image immediately post sonication; enhancement of the bilateral intermediate-temporal hippocampus (black arrows) indicated BBB opening.
2.5. Tissue preparation and analysis
Rats were euthanized one day after completion of the second round of behavioral monitoring with pentobarbital (>100 mg/kg, i.p.), and perfused through the ascending aorta at 30 mL/min for 1 min with 0.9% NaCl and then for 30 min with 4% formaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were post-fixed overnight at 4 °C and then transferred into 30% (w/v) sucrose in PB. After equilibrating in the 30% sucrose solution, the brains were sectioned coronally (40 μm) with a sliding microtome. Serial sections were collected in 30% ethylene glycol and 25% glycerol in 50 mM PB and stored at 20 °C until use. A 1-in-6 series of sections were processed for Nissl staining. The sites of lesions were identified in Nissl-stained sections by an investigator blinded to the seizure frequency outcomes and to the identity of the group to which the animal belonged. The hippocampus was present in approximately 40 sections in each animal. Five areas were examined (CA1, CA2, CA3, dentate gyrus, and subiculum), and each area was assessed in three aspects of the longitudinal axis of the hippocampus (septal, intermediate, and temporal). Hippocampal damage was measured using previously reported methods (Zhang et al., 2020). For each rat, series of Nissl-stained sections were evaluated with a 10 × objective and a Neuro-lucida system (MBF Bioscience, Williston, VT, USA). Contours were drawn around the hippocampus, and hippocampal volume was calculated (Fig. 3). Lines were drawn along the pyramidal cell layer and granule cell layer where those cell layers were populated by neurons, but not along gaps where neuron loss occurred. The total lengths of cell layers were recorded and calculated for analysis.
Fig. 3.
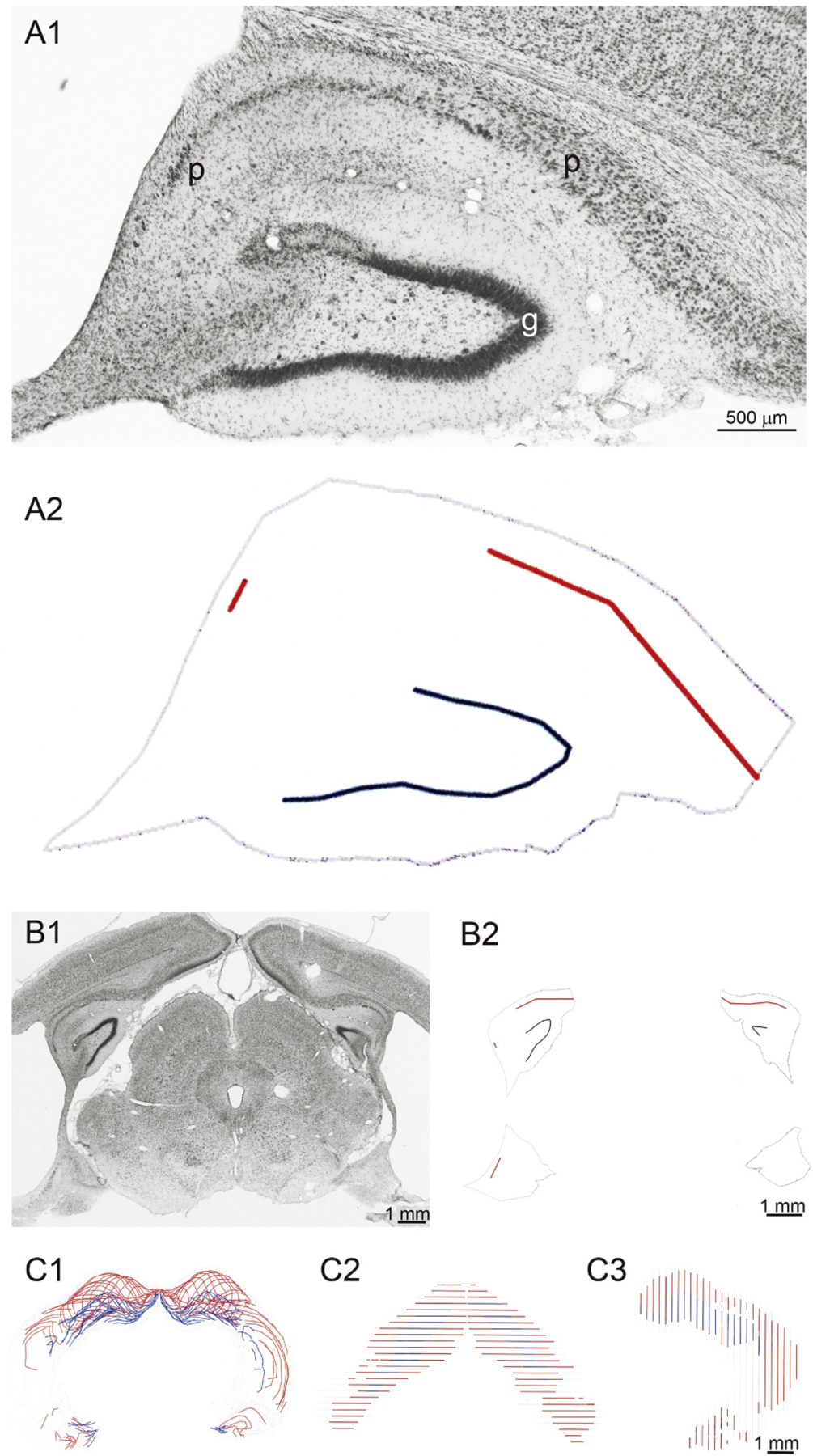
Method for measuring hippocampal cell layer loss and volume loss. A1: Nissl-stained hippocampus from a rat in the FUSit/QA group. Intact cell layers in the subiculum and Ammon’s horn (p) and in the granule cell layer (g) could be distinguished from gaps in those layers, where neuronal loss had occurred. A2: Reconstruction shows intact pyramidal cell layer (red), intact granule cell layer (blue), and outline of hippocampus (grey). B1: Nissl-stained coronal brain section from same rat. The left, dorsal hippocampus is same as that shown in panel A. B2: Reconstructed intact cell layers and hippocampal outline. Reconstruction of entire 1-in-6 series of sections shown from the coronal view (C1), dorsal view (C2), and side view (C3).
3. Results
3.1. Effect of PING on seizure frequency
Video recordings initiated 120 days after SE showed spontaneous, recurrent convulsive seizures during the 30-day baseline (pre-treatment) recording period. Fig. 4 presents the daily seizure frequencies in individual animals recorded before and after treatment. Animals in the No FUS/No QA group exhibited similar seizure frequencies during the two recording periods. Animals in the FUSit/No QA group, in which FUS was delivered to the intermediate and temporal aspects of the hippocampus, also exhibited similar seizure frequencies prior to and post-treatment. Notably, seizure frequencies in animals in the FUSit/QA group, which received both FUS and QA, exhibited profound reductions in seizure frequencies post-treatment. The graph in Fig. 5 illustrates this effect by presenting the average values for each of these groups; and, the post-treatment reduction in seizure frequency in the FUSit/QA group was highly significant.
Fig. 4.
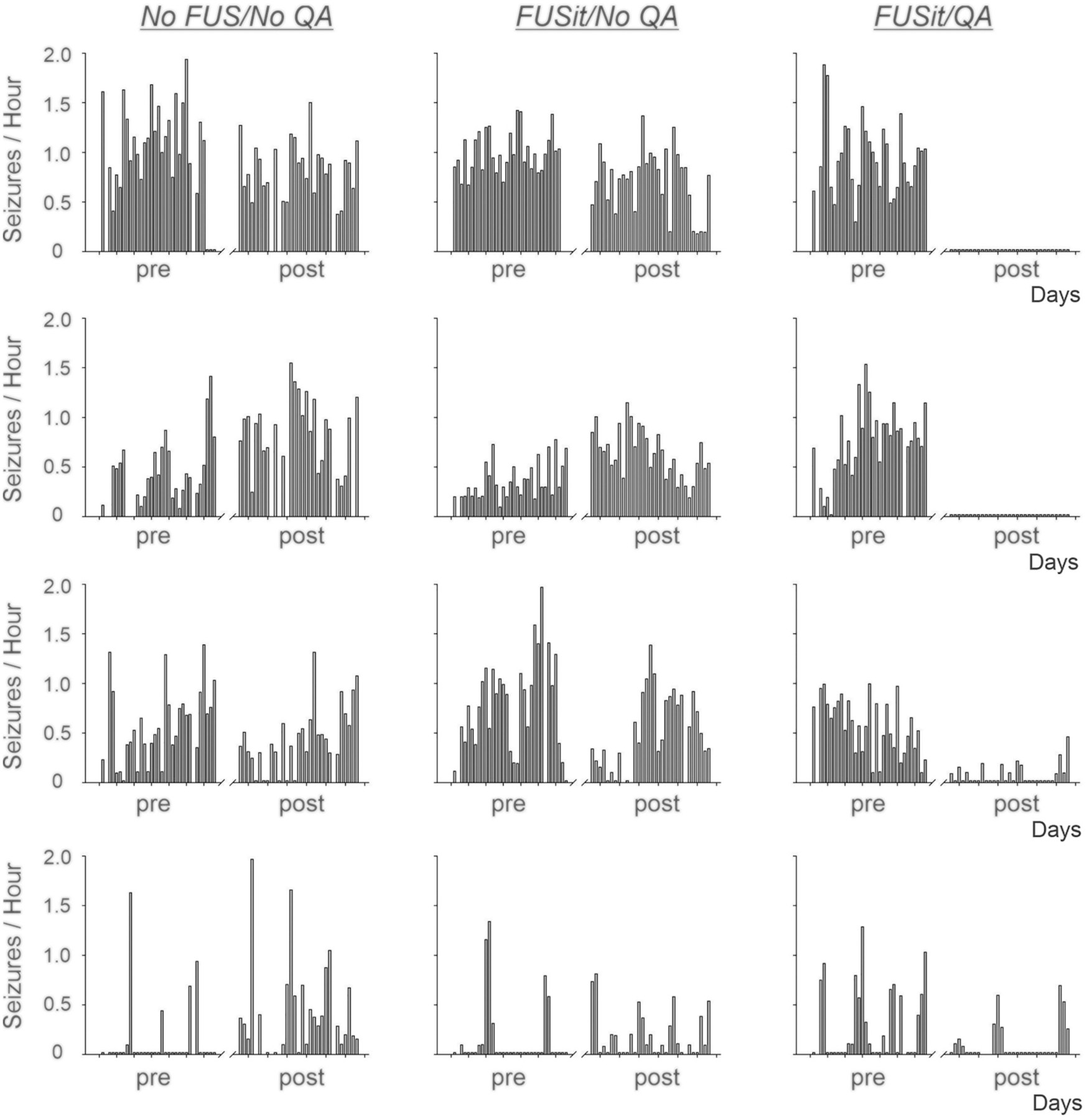
Daily convulsive seizure frequencies in individual animals before and after treatment. Daily convulsive seizure frequencies (seizures/Hour) recorded prior to (pre) and after (post) treatment are shown for animals in the No FUS/NoQA, FUSit/No QA, and FUSit/QA groups. Seizure frequencies were relatively stable in the pre- and post-treatment recording periods for animals in the No FUS/No QA and FUSit/No QA groups. In contrast, seizure frequencies were reduced substantially after treatment in the animals in the FUSit/QA group. In two of these animals, convulsive seizures were eliminated entirely.
Fig. 5.
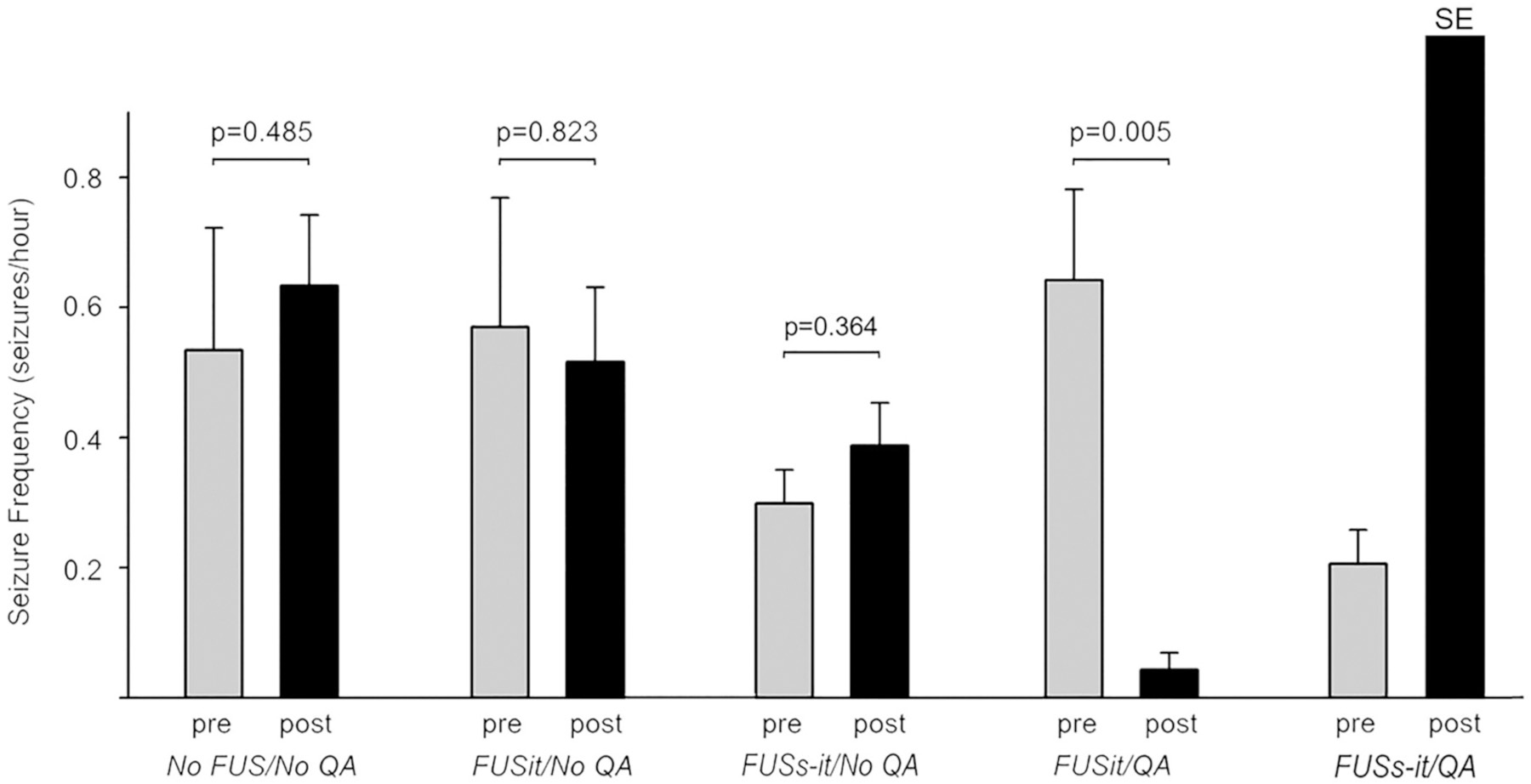
Group seizure frequencies before (pre) and after (post) treatment. Average seizure frequencies (mean +/− SEM) are shown. Seizure frequencies did not differ significantly between pre- and post-treatment recording periods in either the No FUS/No QA or FUSit/NoQA groups. In contrast, seizure frequency was reduced substantially and significantly post-treatment in the FUSit/QA group. The animals from FUSs-it/QA group showed status epilepticus (SE) immediately after treatment. P values are from Students’ t-test comparing pre and post-treatment values for individual groups.
The animals in the FUSs-it/QA group (n = 2), which received sonication in both the septal and intermediate-temporal, aspects of the hippocampus together with QA, exhibited SE after treatment. One of these animals exhibited SE upon recovery from anesthetic after the FUS procedure, and the other exhibited SE a day later. These animals were euthanized after the episodes of SE based on humane endpoints criteria. The animals in the FUSs-it/QA control group (n = 3) that received sonication in both the septal and intermediate-temporal, aspects of the hippocampus together with QA, exhibited SE immediately after treatment only, but not subsequently. In contrast, neither of the animals in the FUSs-it/No QA group (n = 2) and FUSit/QA control group (n = 3) exhibited SE after treatment.
3.2. MR imaging
MR images obtained prior to any of the treatments were unremarkable, providing no evidence of lesions, edema, or hemorrhage. Immediately after FUS, post-contrast T1 imaging showed evidence of BBB opening in all animals (Fig. 6, Supplementary Table 1). All the 18 animals that received FUS showed hyperintensity on post-contrast T1 imaging, which indicated BBB opening. Opening of the BBB in the intermediate-temporal hippocampus was observed in all 18 of the animals that received FUS. In the 5 animals receiving QA together with FUS targeting both the septal and intermediate-temporal aspects of the hippocampus, the septal hippocampus and parts of the underlying thalamus also displayed hyperintensity on post contrast T1 MRI. Two animals from FUSit/QA group and one animal from FUSit/No QA group showed slightly hyperintensity on T2-weighted MRI immediately after sonication (Fig. 6, Supplementary Table 1), which indicates edema, and no evidence of bleeding was observed on the gradient echo images at any of the three imaging time points post FUS (data not shown). T2 images at 24 h after sonication demonstrated enlargement of intermediate-temporal hippocampus in 4 of 4 animals from the FUSit/QA group and 2 of 4 from the FUSit/No QA group. In addition, hyperintensity was observed at 24 h post FUS in 4 of 4 animals in the FUSit/QA group and in 1 of 4 animals in the FUSit/No QA group. T2 images from the FUSit/No QA animals appeared normal at 1 month post-treatment, while animals in the FUSit/QA group were severely affected indicated with hyperintensity and atrophy of hippocampus area (Fig. 6). T2 images from the FUSs-it/No QA animals appeared normal at 1 month post-treatment, while imaging was not possible at 1 month for the FUSit/QA animals because they were euthanized at an earlier time point.
Fig. 6.
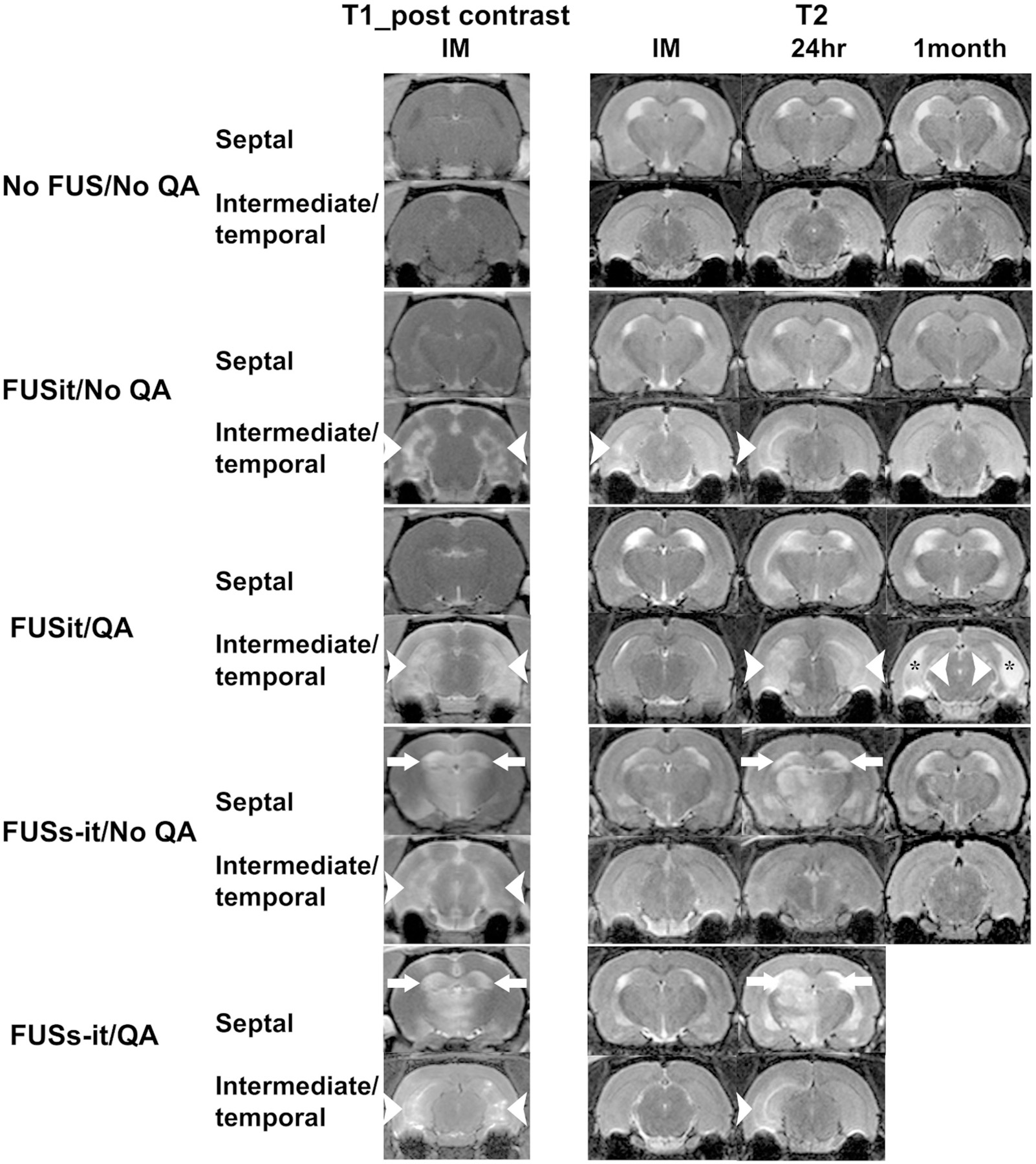
MRI evidence of BBB opening and tissue damage post-treatment. Post-contrast T1 images show areas of hyperintensity (BBB opening) in the regions targeted by FUS. BBB opening is indicated by white arrowheads in the intermediate-temporal hippocampus and by white arrows in the septal hippocampus. The untreated (No FUS/No QA) animal did not show signs of BBB opening. Examples of T2 images obtained immediately, 24 h, and 1 month post-FUS are shown for each of the five groups of animals. The untreated (No FUS/No QA) animal did not show signs of damage (abnormal bright or dark signal) at any of the post-treatment time points. Evidence of unilateral hyperintensity which indicates edema was seen immediately and at 24 h post-treatment (white arrowheads), but no abnormal signal was seen at the1 month time point in the FUSit/No QA example. Evidence of both edema (higher intensity signal indicated by white arrowheads and arrows) at the 24 h and atrophy (the thickness of hippocampus was significantly reduced compared to the ones on T2 images acquired immediately and 24 h post-FUS, the area that had used to be occupied by hippocampus was filled with cerebrospinal fluid indicated with black stars) was observed and 1 month time points for the FUSit/QA example. The one animal in the FUSs-it/QA group that survived the initial post-treatment period showed signs of edema in both the intermediate-temporal and septal hippocampus at 24 h. Part of the thalamus underlying the septal hippocampus in the FUSs-it/QA example also exhibited unilateral BBB opening, as well as evidence of edema at 24 h. No images are available at the 1-month time point for the FUSs-it/QA animals because they were euthanized prior to that time point.
3.3. Location of PING-induced neuronal loss
The location of neuronal loss in each animal was identified by analyzing semi-serial, Nissl-stained sections. Subfields CA1, CA3, and dentate gyrus were examined along the longitudinal axis of the hippocampus at septal, intermediate, and temporal sites. Examples of the patterns of neuronal loss observed across the longitudinal axis of the hippocampus are shown in Fig. 7A and B. The pattern of hippocampal neuron loss in rats that had experienced pilocarpine-induced status epilepticus was similar to that previously reported (Mello et al., 1993). Obvious neuron loss was evident in the hilus of all rats. However, gaps in the pyramidal cell layer and/or granule cell layer were common in rats that also experienced focused ultrasound, but not in control rats that had experienced status epilepticus. All of the animals receiving FUS and QA (i.e. FUSit/QA and FUSs-it/QA groups) exhibited substantial neuronal loss in the intermediate-temporal hippocampus, with damage located in CA1, CA3, and/or dentate gyrus. The two animals in the FUSs-it/QA group also exhibited damage in the septal hippocampus with involvement of the underlying thalamus. Relatively minor and more sporadic neuronal loss was observed in some of the animals receiving FUS without QA (i.e. the FUSit/No QA and FUSs-it/No QA groups).
Fig. 7.
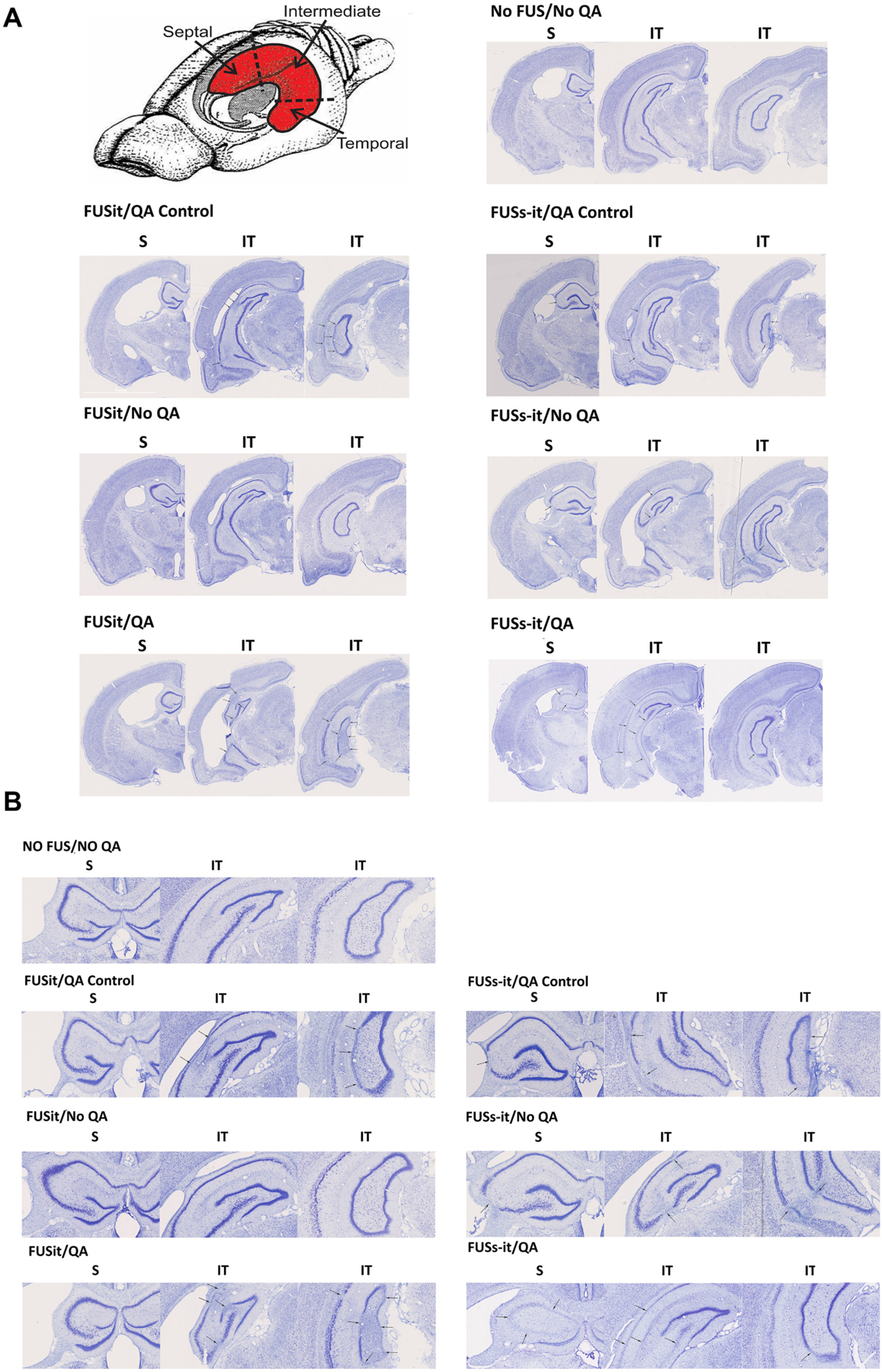
Location of PING-induced neuronal loss. A drawing of the rodent brain with the hippocampus (in red) shows the aspects of the hippocampus that were assessed for neuronal loss. Examples of the types of neuronal loss that occurred in the different groups of animals are shown in Nissl-stained, coronal sections taken along the longitudinal axis of the hippocampus (arrows, 7A and 7B with different magnification). Obvious neuron loss was evident in the hilus of all rats including the untreated animals (No FUS/No QA) and the animal receiving FUS-only targeting the intermediate-temporal (it) aspect of the hippocampus (FUSit/No QA). In contrast, the animals induced epilepsy with pilocarpine injection and receiving QA together with FUS in the ‘it’ hippocampus (FUSit/QA), displayed substantial neuronal loss, involving CA1, CA3, and the dentate gyrus. The animals that did not receive pilocarpine injection, receiving QA together with FUS (FUSit/QA Control and FUSs-it/QA Control) all showed neuronal loss involving CA1 and CA3. The animal receiving FUS-only to both the it and septal aspects of the hippocampus without QA (FUSs-it/No QA) exhibited limited, sporadic cell loss. In contrast, the animal receiving FUS to both the it and septal aspects of the hippocampus together with QA (FUSs-it/QA) exhibited substantial neuronal loss in both the septal and it aspects of the hippocampus.
The size of the hippocampus and neuronal loss were quantified in the No FUS/No QA, FUSit/No QA, and FUSit/QA groups (Fig. 8). Hippocampal area was reduced significantly in the FUSit/QA group, as compared to the untreated control group (No FUS/No QA). In addition, the length of the granule cell layer was reduced significantly in the FUSit/QA group, as compared to both the No FUS/No QA and FUS/No QA groups (Fig. 8). The length of the pyramidal cell layer showed a strong trend for reduction in the FUSit/QA group, as compared to the No FUS/No QA group (Fig. 7). The hippocampal volume decreased significantly in the FUSit/QA group, as compared to both the No FUS/No QA and FUS/No QA groups (Fig. 8).
Fig. 8.
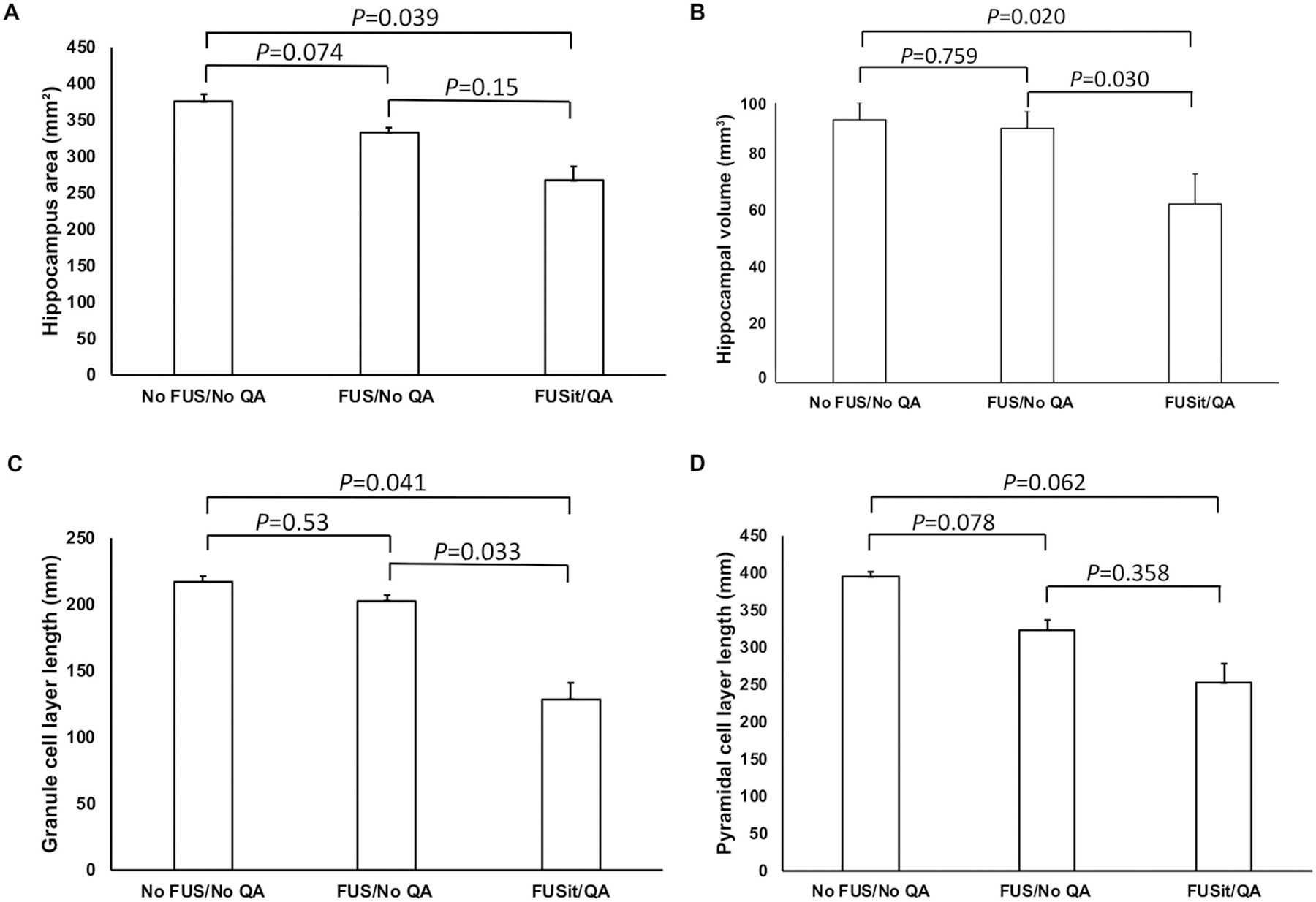
Quantification of hippocampal size and the length of neuronal cell body laminae. A. The area of the hippocampus was reduced significantly in the FUSit/QA group (all p values are from comparisons using Students’ t-test). B. The length of the granule cell layer was also reduced significantly in the FUSit/QA group. C. The length of the pyramidal cell layer also showed a strong trend toward reduction in the FUSit/QA group. D. The hippocampal volume decreased significantly in the FUSit/QA group.
4. Discussion
Surgical treatment of drug resistant epilepsy (DRE) can be very effective in carefully-selected candidates with focal epilepsy. And, in recent years, advances in neurosurgical procedures and the dedicated efforts of the entire teams including neurosurgeons, neurologists and neuroradiologists have improved the accuracy and reduced the invasiveness for treating DRE(Baud et al., 2018b). Nonetheless, considerable opportunity still exists for the further improvement of epilepsy surgery. Accordingly, the goal of the current study was to test a targeted drug therapy approach (PING) that: a) is non-invasive, b) is capable of precise conformal targeting, c) produces lesions of targeted cells while sparing non-target cells, and d) effectively reduces or eliminates convulsive seizures. Our findings from previous study (Zhang et al., 2020) indicate that PING is capable of non-invasively producing targeted neuronal loss that reduces or eliminates seizures in a model of temporal lobe epilepsy. Nevertheless, one of the limitations of our previous study was the lack of control groups. In the current study, we included three control groups: control group of animals with TLE which received no FUS/no QA, control group of animals without TLE which received FUSit/QA, and control group of animals without TLE which received FUSs-it/QA. Also, in order to reinforce the conclusions drawn from previous study, we reproduced our results in a different species—a rat TLE model was utilized.
This study utilized a well-established model of TLE in which pilocarpine-induced SE leads to spontaneous recurrent seizures. The pilocarpine model has been used in many laboratories since its first description over thirty years ago (Turski et al., 1983a; Turski et al., 1983b). Electrophysiological, behavioral, histopathological, and other features of this model have been characterized in detail (Cifelli and Grace, 2012; Curia et al., 2008; Santos et al., 2000; Thind et al., 2010). Importantly, this model has also been used extensively for testing the efficacy of antiepileptic drugs (AEDs) (Leite et al., 2002) because of the chronic and relatively stable rates of spontaneous, recurrent seizures. The advantages of the model for the present study include spontaneous convulsive seizures that last about 1 min, that can be measured with video recording alone, and that occur at a relatively high frequency for quantification. In animals treated intrahippocampally, on the other hand, seizures are shorter (most <10 s duration), they do not always resemble the evolving waveform pattern typical of human temporal lobe epilepsy, and they are usually not convulsive (Krook-Magnuson et al., 2013; Krook-Magnuson et al., 2014). By using pilocarpine-treated rats, we were able to non-invasively evaluate seizures more similar to those of patients with temporal lobe epilepsy.
Our baseline seizure monitoring results were consistent with previous findings demonstrating expected seizure rate fluctuations across recording periods in untreated (No FUS/ No QA) animals, which are the multidien rhythms discovered by Baud MO and the coauthors recently in humans (Baud et al., 2019; Baud et al., 2018a). Seizure frequencies were also unchanged between pre- and post-treatment monitoring periods in animals that received FUS targeting the intermediate-temporal hippocampus in the absence systemically-administered toxin (No FUS/No QA). This is notable because it indicates that targeted, low-intensity FUS by itself does not alter seizure frequency. However, when delivered in conjunction with systemically-administered QA, FUS targeting the bilateral intermediate-temporal hippocampus, reduced or eliminated convulsive seizures. Average seizure frequency was significantly reduced by 93% in the FUSit/QA group, with two animals exhibiting a complete elimination of convulsive seizures. The efficacy of our intervention was stronger compared to the previous study. This may at least be partially explained by the different species used in this study. Bilateral sonication was selected because evidence strongly suggests that seizures usually start bilaterally and mainly from the ventral hippocampus in this model (Wyeth et al., 2020). Post-FUS MRI showed edema in the animals from FUSit/QA, FUSs-it/QA, FUSs-it/No QA, and one of the animals from FUSit/No QA group. This edema may be caused by enhanced water transfer into the tissue after BBB opening as well as sterile inflammation (Klibanov and McDannold, 2019; Kovacs et al., 2017). Histological analyses demonstrated substantial neuronal loss in the intermediate and temporal aspects of the hippocampus in the FUSit/QA group, but not in the No FUS/No QA or FUS/No QA groups. Together, these findings indicate that targeted, focal delivery of a neurotoxin to the intermediate-temporal hippocampus produces neuronal loss and sustained suppression of seizures. This conclusion is consistent with that of a previous preliminary study in mice in which the frequency of pilocarpine-induced seizures was reduced by PING targeting the intermediate-temporal hippocampus (Zhang et al., 2020).
Interestingly, when the targeting of FUS (together with QA) was expanded to include the septal aspect of the hippocampus in two animals, both animals exhibited SE after recovery. Based on humane endpoints criteria, these animals were euthanized prior to post-treatment monitoring. The finding of increased seizure activity after lesions affecting the septal aspect of the hippocampus is consistent with that of a pilot study in mice, in which neuronal lesions in the septal hippocampus increased seizure activity (Zhang et al., 2020). The reason for this increasement was not examined in the current study. However, evidence from previous studies indicates that the septal and the temporal aspects of the hippocampus are morphologically, anatomically, biochemically and functionally distinct (Fanselow and Dong, 2010). (Fanselow and Dong, 2010; Moser and Moser, 1998; Strange et al., 2014). Moreover, regional characteristics of neurons and circuits appear to predispose more temporal aspects of the hippocampus to heightened excitability, as compared with the septal aspect of the hippocampus (Bragdon et al., 1986; Derchansky et al., 2004; Kouvaros and Papatheodoropoulos, 2017; Lee et al., 1983; Toyoda et al., 2013). An important goal for future studies will be to characterize the underpinnings of the differential effects of neuronal lesions across the longitudinal axis of the hippocampus. A caveat for the finding of increased aberrant activity in the FUSs-it/QA group is that both animals in that group also exhibited injury to parts of the underlying thalamus. Thus, a contribution by non-hippocampal injury to post-treatment SE cannot be ruled out.
We acknowledge the following limitations to our study: (1) The sample size was small. Stable convulsive seizures were induced in only 16 rats. In future studies, we will allocate more animals into different groups to test the effect of PING through targeting different portions of the hippocampus. (2) 24 h EEG-video was not included in this study because the electrodes might get in the way of ultrasound beam and interfere with the PING treatment. Therefore, the effect of PING could be only on the secondary generalization of the seizure and focal seizure could still be present (Wang et al., 2017). The approaches to perform PING around the site of electrode implantation will be investigated in future studies. (3) Unilateral versus bilateral sonication was not tested in this study, and in a translational perspective, bilateral sonication of the hippocampi in humans might result in severe amnestic syndromes. The effect of unilateral sonication will be investigated in future studies. (4) QA was delivered focally to destroy the neurons; other approaches such as targeted delivery of anti-epileptic drugs to rescue the neurons or neuromodulation to modulate their activity represent future avenues of research holding a lot of potential.
In conclusion, the results of the current study provide support for the potential utility of PING as a non-invasive surgical modality for treating drug-resistant, focal epilepsy. Surgery for medically-refractory epilepsy remains one of the most underutilized treatments that is effective for the treatment of a major neurological disorder. The development and refinement of a precise, non-invasive surgical procedure for this purpose could potentially increase the utilization of this important therapeutic intervention.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants R01 CA217953-01 and R01 NS102194.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2021.113761.
Declaration of Competing Interest
These are no financial and non-financial competing interests.
References
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA, 1999. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res 34, 99–107. [DOI] [PubMed] [Google Scholar]
- Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, Rao VR, 2018a. Multi-day rhythms modulate seizure risk in epilepsy. Nat. Commun 9, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud MO, Perneger T, Racz A, Pensel MC, Elger C, Rydenhag B, Malmgren K, Cross JH, McKenna G, Tisdall M, Lamberink HJ, Rheims S, Ryvlin P, Isnard J, Mauguiere F, Arzimanoglou A, Akkol S, Deniz K, Ozkara C, Lossius M, Rektor I, Kalviainen R, Vanhatalo LM, Dimova P, Minkin K, Staack AM, Steinhoff BJ, Kalina A, Krsek P, Marusic P, Jordan Z, Fabo D, Carrette E, Boon P, Rocka S, Mameniskiene R, Vulliemoz S, Pittau F, Braun KPJ, Seeck M, 2018b. European trends in epilepsy surgery. Neurology 91, e96–e106. [DOI] [PubMed] [Google Scholar]
- Baud MO, Ghestem A, Benoliel JJ, Becker C, Bernard C, 2019. Endogenous multidien rhythm of epilepsy in rats. Exp. Neurol 315, 82–87. [DOI] [PubMed] [Google Scholar]
- Beskid M, Rozycka Z, Taraszewska A, 1997. Quinolinic acid: effect on the nucleus arcuatus of the hypothalamus in the rat (ultrastructural evidence). Exp. Toxicol. Pathol 49, 477–481. [DOI] [PubMed] [Google Scholar]
- Boling WW, 2018. Surgical considerations of intractable mesial temporal lobe epilepsy. Brain Sci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon AC, Taylor DM, Wilson WA, 1986. Potassium-induced epileptiform activity in area CA3 varies markedly along the septotemporal axis of the rat hippocampus. Brain Res 378, 169–173. [DOI] [PubMed] [Google Scholar]
- Cendes F, Theodore WH, Brinkmann BH, Sulc V, Cascino GD, 2016. Neuroimaging of epilepsy. Handb. Clin. Neurol 136, 985–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli P, Grace AA, 2012. Pilocarpine-induced temporal lobe epilepsy in the rat is associated with increased dopamine neuron activity. Int. J. Neuropsychopharmacol 15, 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M, 2008. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 172, 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derchansky M, Shahar E, Wennberg RA, Samoilova M, Jahromi SS, Abdelmalik PA, Zhang L, Carlen PL, 2004. Model of frequent, recurrent, and spontaneous seizures in the intact mouse hippocampus. Hippocampus 14, 935–947. [DOI] [PubMed] [Google Scholar]
- Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M, 2006. Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia 47, 2115–2124. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ES, Sui CB, Wang TX, Sun GL, 2016. Stereotactic radiosurgery for the treatment of mesial temporal lobe epilepsy. Acta Neurol. Scand 134, 442–451. [DOI] [PubMed] [Google Scholar]
- Foster AC, Miller LP, Oldendorf WH, Schwarcz R, 1984. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp. Neurol 84, 428–440. [DOI] [PubMed] [Google Scholar]
- Klibanov AL, McDannold NJ, 2019. Moving toward noninvasive, focused ultrasound therapeutic delivery of drugs in the brain: prolonged opening of blood-brain barrier may not be needed. Radiology 291, 467–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvaros S, Papatheodoropoulos C, 2017. Prominent differences in sharp waves, ripples and complex spike bursts between the dorsal and the ventral rat hippocampus. Neuroscience 352, 131–143. [DOI] [PubMed] [Google Scholar]
- Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA, 2017. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. U. S. A 114, E75–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I, 2013. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun 4, 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I, 2014. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ, 2000. Early identification of refractory epilepsy. N. Engl. J. Med 342, 314–319. [DOI] [PubMed] [Google Scholar]
- Lee KS, Reddington M, Schubert P, Kreutzberg G, 1983. Regulation of the strength of adenosine modulation in the hippocampus by a differential distribution of the density of A1 receptors. Brain Res 260, 156–159. [DOI] [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA, 2002. New insights from the use of pilocarpine and kainate models. Epilepsy Res 50, 93–103. [DOI] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM, 1993. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34, 985–995. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, 1998. Functional differentiation in the hippocampus. Hippocampus 8, 608–619. [DOI] [PubMed] [Google Scholar]
- Racine RJ, 1972. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol 32, 281–294. [DOI] [PubMed] [Google Scholar]
- Santos NF, Marques RH, Correia L, Sinigaglia-Coimbra R, Calderazzo L, Sanabria ER, Cavalheiro EA, 2000. Multiple pilocarpine-induced status epilepticus in developing rats: a long-term behavioral and electrophysiological study. Epilepsia 41 (Suppl. 6), S57–S63. [DOI] [PubMed] [Google Scholar]
- Spencer D, Burchiel K, 2012. Selective amygdalohippocampectomy. Epilepsy Res. Treat 2012, 382095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, O’Connor MJ, Saykin AJ, Plummer C, 1996. Temporal lobectomy for refractory epilepsy. JAMA 276, 470–475. [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI, 2014. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS, 2010. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J. Comp. Neurol 518, 647–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS, 2013. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J. Neurosci 33, 11100–11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L, 1983a. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav. Brain Res 9, 315–335. [DOI] [PubMed] [Google Scholar]
- Turski WA, Czuczwar SJ, Kleinrok Z, Turski L, 1983b. Cholinomimetics produce seizures and brain damage in rats. Experientia 39, 1408–1411. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, Chen B, Wu X, Gao F, Wang S, Guo Y, Li X, Luo J, Duan S, Chen Z, 2017. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron 95, 1221. [DOI] [PubMed] [Google Scholar]
- Widjaja E, Papastavros T, Sander B, Snead C, Pechlivanoglou P, 2019. Early economic evaluation of MRI-guided laser interstitial thermal therapy (MRgLITT) and epilepsy surgery for mesial temporal lobe epilepsy. PLoS One 14, e0224571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth M, Nagendran M, Buckmaster PS, 2020. Ictal onset sites and gamma-aminobutyric acidergic neuron loss in epileptic pilocarpine-treated rats. Epilepsia 61, 856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tan H, Bertram EH, Aubry JF, Lopes MB, Roy J, Dumont E, Xie M, Zuo Z, Klibanov AL, Lee KS, Wintermark M, 2016. Non-invasive, focal disconnection of brain circuitry using magnetic resonance-guided low-intensity focused ultrasound to deliver a neurotoxin. Ultrasound Med. Biol 42, 2261–2269. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liao C, Qu H, Huang S, Jiang H, Zhou H, Abrams E, Habte FG, Yuan L, Bertram EH, Lee KS, Pauly KB, Buckmaster PS, Wintermark M, 2019. Testing different combinations of acoustic pressure and doses of quinolinic acid for induction of focal neuron loss in mice using transcranial low-intensity focused ultrasound. Ultrasound Med. Biol 45, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou H, Qu H, Liao C, Jiang H, Huang S, Ghobadi SN, Telichko A, Li N, Habte FG, Doyle T, Woznak JP, Bertram EH, Lee KS, Wintermark M, 2020. Effects of non-invasive, targeted, neuronal lesions on seizures in a mouse model of temporal lobe epilepsy. Ultrasound Med. Biol 46, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


