Abstract
Frequencies of mutation to resistance with trovafloxacin and four other quinolones were determined with quinolone-susceptible Staphylococcus aureus RN4220 by a direct plating method. First-step mutants were selected less frequently with trovafloxacin (1.1 × 10−10 at 2 to 4× the MIC) than with levofloxacin or ciprofloxacin (3.0 × 10−7 to 3.0 × 10−8 at 2 to 4× the MIC). Mutants with a change in GrlA (Ser80→Phe or Tyr) were most commonly selected with trovafloxacin, ciprofloxacin, levofloxacin, or pefloxacin. First-step mutants were difficult to select with sparfloxacin; however, second-step mutants with mutations in gyrA were easily selected when a preexisting mutation in grlA was present. Against 29 S. aureus clinical isolates with known mutations in gyrA and/or grlA, trovafloxacin was the most active quinolone tested (MIC at which 50% of isolates are inhibited [MIC50] and MIC90, 1 and 4 μg/ml, respectively); in comparison, MIC50s and MIC90s were 32 and 128, 16 and 32, 8 and 32, and 128 and 256 μg/ml for ciprofloxacin, sparfloxacin, levofloxacin, and pefloxacin, respectively. Strains with a mutation in grlA only were generally susceptible to all of the quinolones tested. For mutants with changes in both grlA and gyrA MICs were higher and were generally above the susceptibility breakpoint for ciprofloxacin, sparfloxacin, levofloxacin, and pefloxacin. Addition of reserpine (20 μg/ml) lowered the MICs only of ciprofloxacin fourfold or more for 18 of 29 clinical strains. Topoisomerase IV and DNA gyrase genes were cloned from S. aureus RN4220 and from two mutants with changes in GrlA (Ser80→Phe and Glu84→Lys). The enzymes were overexpressed in Escherichia coli GI724, purified, and used in DNA catalytic and cleavage assays that measured the relative potency of each quinolone. Trovafloxacin was at least five times more potent than ciprofloxacin, sparfloxacin, levofloxacin, or pefloxacin in stimulating topoisomerase IV-mediated DNA cleavage. While all of the quinolones were less potent in cleavage assays with the altered topoisomerase IV, trovafloxacin retained its greater potency relative to those of the other quinolones tested. The greater intrinsic potency of trovafloxacin against the lethal topoisomerase IV target in S. aureus contributes to its improved potency against clinical strains of S. aureus that are resistant to other quinolones.
Trovafloxacin is a new fluoronaphthyridone agent that belongs to the quinolone class of antimicrobial agents. Trovafloxacin differs from ciprofloxacin, ofloxacin, sparfloxacin, and levofloxacin in that it demonstrates greater in vitro potency against gram-positive cocci and anaerobes (3, 5, 8, 19, 33, 34, 40).
The new fluoroquinolones including trovafloxacin have been proposed for use in the treatment of respiratory tract infections, in which gram-positive bacteria such as Streptococcus pneumoniae and Staphylococcus aureus can be involved. Resistance to beta-lactams and macrolides in these organisms is occurring with increased frequency worldwide, and physicians are looking for new, alternative therapies (4, 26, 37, 39). While resistance to the new fluoroquinolones occurs infrequently in S. pneumoniae (4, 39), S. aureus isolates are often resistant to ciprofloxacin, and such strains have reduced susceptibility to the new quinolones, although cross-resistance is incomplete (13, 18, 41). New agents such as clinafloxacin, trovafloxacin, gatifloxacin, sparfloxacin, and moxifloxacin have MICs within clinically achievable levels in blood for some S. aureus and S. pneumoniae isolates that are resistant to ciprofloxacin (5, 18, 41). The clinical utility of the new fluoroquinolones for the treatment of infections caused by ciprofloxacin-resistant gram-positive cocci has yet to be established, however. In order to further understand their potential against such strains, numerous studies have characterized the activities of the new fluoroquinolones against both ciprofloxacin-susceptible and -resistant cocci (5, 18, 41).
Resistance to ciprofloxacin in gram-positive cocci can arise by several mechanisms, the most prevalent of which involves mutations in genes encoding DNA gyrase and topoisomerase IV. Mutations associated with increased resistance to quinolones in S. aureus have been documented in conserved regions of gyrA, gyrB, and grlA, which are referred to as the quinolone resistance-determining region (QRDR). Ser80→Phe or Tyr and Glu84→Lys changes in GrlA and GyrA Ser84→Leu changes are among the most frequently encountered changes associated with ciprofloxacin resistance in S. aureus (10, 12, 32, 35, 36, 42). Studies by Blanche et al. (2) indicate that S. aureus topoisomerase IV with an alteration in GrlA of Ser80 to Tyr was 10- to 50-fold less potent than the parent enzyme at generating topoisomerase IV-mediated DNA cleavage with ciprofloxacin or sparfloxacin. Efflux of ciprofloxacin via the NorA efflux pump may also contribute to ciprofloxacin resistance in S. aureus (25). While numerous studies have characterized these mechanisms of resistance to ciprofloxacin in S. aureus, relatively little is known concerning their effects on the activities of the new fluoroquinolones such as trovafloxacin.
In one study (12) single mutations in the genes encoding both GyrA (Ser84 to Leu) and GrlA (Ser80 to Tyr, or Phe) were found in S. aureus clinical strains that were resistant (MICs, ≥8 μg/ml) to ciprofloxacin, levofloxacin, and sparfloxacin. Such strains remained susceptible to trovafloxacin (MIC, 0.75 μg/ml). A third alteration (GrlA, Glu84→Lys or Gly) was required in order to make the strain resistant to trovafloxacin (MIC, ≥4 μg/ml). In their study of 66 clinical isolates of methicillin-resistant S. aureus (MRSA), Fitzgibbon et al. (12) found that 89% (59 of 66) were highly resistant to ciprofloxacin, while trovafloxacin MICs were substantially elevated (MICs, 8 to 16 μg/ml) for only 4 strains. Such differences in levels of resistance to ciprofloxacin and trovafloxacin have prompted interest in studying the relative sensitivities of the gyrase and topoisomerase IV from gram-positive organisms to these agents.
Recent studies involving S. pneumoniae (15, 17, 20) indicate that trovafloxacin, like ciprofloxacin, preferentially targets topoisomerase IV in this species, since first-step mutants selected with either drug possessed alterations in the A subunit (ParC) of this enzyme. In contrast, sparfloxacin has been shown to preferentially target DNA gyrase in S. pneumoniae (30), although another report seems to contradict that observation (28).
The purpose of the current work is to study the interactions of trovafloxacin with both topoisomerase IV and DNA gyrase in another gram-positive organism, S. aureus. The activity of trovafloxacin against first-step mutants selected with quinolones in vitro as well as against a collection of S. aureus clinical isolates containing single and multiple topoisomerase mutations was studied (24). The relative potency of trovafloxacin against wild-type and mutant topoisomerase IV and DNA gyrase from S. aureus RN4220 was also studied. Results suggest that the potent activity of trovafloxacin against purified topoisomerases from S. aureus parallels its improved activity against both wild-type and ciprofloxacin-resistant isolates of this species.
(Portions of this study were given at the 6th International Symposium of New Quinolones, Denver, Colo., 1998.)
MATERIALS AND METHODS
Bacterial strains.
S. aureus RN4220 was used for first-step mutant selection studies and for cloning of the grlA and grlB and the gyrA and gyrB genes of topoisomerase IV and DNA gyrase, respectively. In order to assess the relative activity of trovafloxacin against clinical isolates of S. aureus, we studied a collection of 29 isolates for which ciprofloxacin MICs varied, as characterized previously by Kaatz and Seo (24). The collection consists of 13 methicillin-susceptible S. aureus (MSSA) and 16 MRSA isolates collected between 1989 and 1996 from eight different U.S. cities. All 29 isolates have unrelated genomic DNA restriction patterns, as assessed by pulsed-field gel electrophoresis (24). Restriction fragment length polymorphism (RFLP) analysis identified mutations in the gyrA and/or grlA genes.
Antibiotics.
Trovafloxacin mesylate and sparfloxacin were prepared at Pfizer, Groton, Conn. Levofloxacin was obtained from R. W. Johnson, Raritan, N.J., and pefloxacin was from Rhone-Poulenc Rorer, Collegeville, Pa. Ciprofloxacin was obtained from Miles, West Haven, Conn.
Determination of MICs.
MICs were determined by a macrodilution method with cation-supplemented Mueller-Hinton broth (Difco, Detroit, Mich.) with a final bacterial inoculum of 5 × 105 CFU/ml. The MICs of the quinolones were also determined with and without 20 μg of reserpine (Sigma, St. Louis, Mo.) per ml as described previously (25).
Selection of quinolone-resistant mutants.
First-step mutants were selected from S. aureus RN4220 by plating ≥109 CFU on the surfaces of Mueller-Hinton agar plates containing from two to eight times the MIC of each quinolone. Selection plates were incubated at 37°C, and the number of resistant colonies was counted at 48 h. The susceptibilities of the resistant mutants to quinolones were determined and were compared to those of the parent strain RN4220.
Amplification of QRDRs of grlA and grlB and of gyrA and gyrB by PCR.
PCR amplification of the QRDRs of the genes for gyrase and topoisomerase IV was carried out with S. aureus RN4220 chromosomal DNA with a Gene Amp PCR system 9600 (Perkin-Elmer Cetus) and high-fidelity Platinum Taq DNA polymerase (Gibco-BRL). The nucleotide primers used for PCR, based on published sequences, included the following: for grlA, 5′ primer 2107-ATTCAAGAGCGTGCATTGCC-2126 and 3′ primer 2488-CTTGATGGCAATAACATTGG-2507 (11); for grlB, 5′ primer 1520-CGATTAAAGCACAACAAGCAAG-1541 and 3′ primer 1874-CATCAGTCATAATAATTACTC-1894 (32); for gyrA, 5′ primer 2358-GCGATGAGTGTTATCGTTGC-2377 and 3′ primer 2912-CAGGACCTTCAATATCCTCC-2931 (27); and for gyrB, 5′ primer 1400-CAGCGTTAGATGTAGCAAGC-1419 and 3′ primer 1631-CCGATTCCTGTACCAAATGC-1650 (32).
The PCR included an initial 4-min denaturation step at 94°C, followed by a 3-min annealing step at 53°C. This was followed by 30 cycles of elongation (30 s at 72°C), denaturation (30 s at 94°C), and annealing (30 s at 53°C), followed by a final cycle of elongation (5 min at 72°C). The sizes of the PCR products obtained for the QRDR of each gene were as follows: for grlA, 401 bp; for grlB, 375 bp; for gyrA, 574 bp; and for gyrB, 251 bp.
PCR products were purified with Qiagen PCR purification spin columns, and the PCR-amplified DNA was sequenced by the dye terminator method in both the forward and reverse directions with the Perkin-Elmer ABI 373 system (Norwalk, Conn.). All PCR-amplified fragments were generated in two separate experiments, and both strands of each fragment were sequenced. We were able to obtain reliable sequence data for GrlA (amino acids 27 to 159) and GyrA (amino acids 29 to 210). These QRDRs are somewhat longer than those that are routinely analyzed.
Measurement of [3H]thymidine incorporation into S. aureus RN4220 clones.
Logarithmic-phase cells of the S. aureus RN4220 parent, single mutant RN4220-20 (GrlA Ser80→Phe), and double mutant RN4220-20-48 (GrlA Ser80→Phe and GyrA Ser84→Leu) that had been grown in minimal medium (22) were labeled with 0.03 μCi of [3H]thymidine (80 Ci/mmol; Amersham, Arlington Heights, Ill.) per ml for 10 min at 37°C in the presence of one of the quinolones being tested. Incorporation was interrupted by the addition of 0.5 mg of bovine serum albumin per ml in 22.5% ethanol (final concentrations), followed by the addition of 10% trichloroacetic acid, and the mixture was held at 4°C for 30 min. The cells were filtered (Packard Unifilter 96, GF/B plate; Packard, Meriden, Conn.) and were then washed with water and ethanol. The counts on the filters were determined on a Packard Topcount microplate scintillation counter, and the percent thymidine incorporation was determined. The effects of the quinolones on the growth of the double mutant S. aureus RN4220-20-48 were determined over 24 h in cation-supplemented Mueller-Hinton broth at concentrations of 2 to 4× the MIC of each drug. Samples were removed over timed intervals, washed, diluted in phosphate-buffered saline (pH 7.4), and plated in duplicate on drug-free plates containing brain heart infusion agar. The colonies were counted after 24 h of incubation at 35°C. The minimal number of viable cells detectable under these conditions was approximately 102 CFU/ml.
Cloning of grlA and grlB and of gyrA and gyrB genes from S. aureus RN4220 and overexpression in E. coli GI724.
On the basis of previously published work (6, 11), genomic fragments containing grlA, grlB, gyrA, and gyrB were subcloned into the general cloning vector pZero-2.1 (Invitrogen Corporation, San Diego, Calif.) by standard methods (31). Putative transformants were screened by colony hybridization with a 400-bp PCR-generated fragment (corresponding to amino acids 26 to 157) and with the QRDR of grlA as a probe. Positive clones were verified by DNA sequence analysis. An expression vector containing the trp promoter (14) was used to overexpress the grlB gene in Escherichia coli HB101. The grlA, gyrA, and gyrB genes were overexpressed in E. coli GI724 by using the expression vector pLEX (purchased from Invitrogen Corporation, San Diego, Calif.). Expression of these subunits was under the control of the pL promoter. Expression levels were evaluated by polyacrylamide gel electrophoresis. All four protein subunits were expressed as native Met proteins.
Cloning and overexpression of quinolone-resistant grlA genes.
Genomic DNAs were isolated from two quinolone-resistant mutants (mutants 4220-16 and 4220-19) derived from S. aureus RN4220. A 600-bp PCR product containing the QRDR was isolated from each mutant with the following forward and reverse PCR primers: 5′-CCGGCATATGAGTGAAATAATTCAAGATTTATCA-3′ and 5′-GGTATATCTGTCGCGTAACCTGC-3′, respectively. The amplification mixture consisted of 100 ng of template DNA, 0.2 mM each deoxynucleoside triphosphate, 0.5 μM each primer, 1.5 mM MgCl2, and 2.5 U of Taq DNA polymerase. Conditions for the PCR were as follows: denaturation for 4 min at 94°C and then 3 min at 53°C, followed by 30 cycles of 72°C for 30 s, 94°C for 30 s, and 53°C for 30 s. This was followed by another cycle at 72°C for 5 min for final elongation. In strain 4220-16 this fragment contained a single base pair change (GAA to AAA) that changes amino acid Glu84 to Lys and that is associated with elevated levels of resistance to quinolones. In strain 4220-19 this change is also a single base pair (TCC to TTC), which results in a change of amino acid Ser80 to Phe. The PCR fragments were restricted with NdeI and PstI, which resulted in fragments of approximately 0.5 kb. The previously described plasmid vector that contained most of the grlB and all of the grlA gene on a 4.2-kb KpnI genomic fragment was restricted with PstI-KpnI, and the 1.9-kb 3′ end of the grlA gene was isolated. The expression vector pLEX was restricted with NdeI-KpnI and was isolated. The three fragments were ligated together and were transformed into recipient host strain GI724, and putative clones were identified by restriction digestion. Clones were then positively identified by DNA sequence analysis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis confirmed overexpression of the mutant alleles in E. coli. Each protein was expressed as the native Met protein.
Purification of S. aureus DNA gyrase and topoisomerase IV.
The GyrA and GyrB proteins of DNA gyrase and the GrlA and GrlB proteins of topoisomerase IV were purified separately after overexpression in E. coli. Cells from logarithmic-phase cultures were pelleted and resuspended in TED buffer (50 mM Tris-HCl [pH 7.6], 1 mM EDTA, 5 mM dithiothreitol). The cells were broken by sonication, followed by centrifugation at 18,000 × g for 30 min. Topoisomerases were extracted from both the soluble fraction and the cell pellet. The two soluble fractions were combined and dialyzed against TGED buffer (TED buffer plus 10% glycerol), and the subunits were purified by the method of Hallett et al. (23), with modification. In the first step, the crude fraction was applied to a heparin-Sepharose column and the column was washed with TGED plus 0.25 M NaCl, followed by elution with a linear gradient of 0.25 to 1.0 M NaCl in TGED. The active fractions were combined, concentrated and adjusted to 1 M (NH4)2SO4, and loaded onto a fast-performance liquid chromatography phenyl-superose column, where they were eluted with a linear gradient starting with 1 M (NH4)2SO4. The individual subunits were assayed for catalytic activity with an excess of the complementing subunit. The specific activity of S. aureus gyrase was 4.5 × 103 supercoiling units/mg of protein (conversion of 200 ng of relaxed pBR322 substrate in 30 min at 37°C). The specific activity of S. aureus topoisomerase IV was 3.1 × 104 decatenation units/mg of protein (decatenation of 200 ng of catenated substrate in 30 min at 37°C). E. coli DNA gyrase and topoisomerase IV were purchased from Enzyco, Denver, Colo.
Topoisomerase catalytic and DNA cleavage assays.
S. aureus RN4220 DNA gyrase and topoisomerase IV were evaluated in DNA supercoiling and decatenation assays, respectively, as described previously (2). Decatenation of kinetoplast DNA was conducted with 350 mM potassium glutamate added to 50 mM Tris-HCl (pH 7.7). Supercoiling of relaxed pBR322 DNA was conducted in the presence of 700 mM potassium glutamate (2). Topoisomerase IV-mediated DNA cleavage assays were conducted with unlabeled, supercoiled pBR322 as the substrate (2). In these tests, the minimum concentration of each fluoroquinolone that stimulated topoisomerase IV-mediated DNA cleavage was determined with densitometric scans of photographs of ethidium bromide-stained agarose gels. Linearized pBR322 labeled at the 3′ end with 32P was used in cleavage experiments designed to compare the cleavage patterns induced by topoisomerase IV treated with trovafloxacin or sparfloxacin.
RESULTS
Selection of first-step mutants from S. aureus RN4220.
Ciprofloxacin and levofloxacin (2 to 4× the MIC) selected first-step resistant mutants at frequencies higher than those observed for trovafloxacin (Table 1). First-step mutants selected with sparfloxacin grew as small-colony variants by 72 h. These clones grew slowly and were not evaluated further as mutants in MIC tests. Interestingly, mutants could readily be selected with 2 to 4× the MIC of sparfloxacin for two RN4220 clones containing a preexisting mutation in GrlA (Table 1; see data for mutants 4220-16 and 4220-19).
TABLE 1.
Selection of first- and second-step mutants
| Straina | MIC (μg/ml)
|
Frequency of mutation at:
|
Changes in:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trovafloxacin | Ciprofloxacin | Levofloxacin | Sparfloxacin | Pefloxacin | 2× the MIC | 4× the MIC | 8× the MIC | GrlA | GrlB | GyrA | GyrB | |
| Parent strain RN4220 | 0.015 | 0.25 | 0.125 | 0.063 | 0.25 | None | None | None | None | |||
| First-step mutants | ||||||||||||
| 4220-53T | 0.125 | 2 | 0.5 | 0.063 | 4 | 1.1 × 10−10 | 1.1 × 10−10 | <1.1 × 10−10 | Ser80 to Phe | None | None | None |
| 4220-54T | 0.125 | 2 | 0.5 | 0.063 | 4 | Ser80 to Tyr | None | None | None | |||
| 4220-55T | 0.063 | 2 | 0.5 | 0.063 | 4 | Ser80 to Tyr | None | None | None | |||
| 4220-56T | 0.125 | 2 | 0.5 | 0.063 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-16C | 0.063 | 1 | 0.5 | 0.125 | 4 | Glu84 to Lys | None | None | None | |||
| 4220-19C | 0.125 | 2 | 1 | 0.125 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-20C | 0.125 | 1 | 0.5 | 0.125 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-57C | 0.063 | 2 | 0.5 | 0.063 | 4 | 3.1 × 10−7 | 3.0 × 10−8 | <4.6 × 10−9 | Glu84 to Lys | None | None | None |
| 4220-58C | 0.125 | 2 | 0.5 | 0.063 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-5L | 0.063 | 2 | 0.5 | 0.125 | 4 | 3.0 × 10−7 | 3.2 × 10−8 | <1.4 × 10−9 | None | Pro451 to Gln | None | None |
| 4220-7L | 0.125 | 2 | 0.5 | 0.125 | 4 | Gly78 to Cys | None | None | None | |||
| 4220-9L | 0.125 | 2 | 0.5 | 0.125 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-11L | 0.125 | 2 | 0.5 | 0.125 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-1P | 0.125 | 2 | 0.5 | 0.125 | 4 | 1.03 × 10−7 | <4.4 × 10−9 | <4.4 × 10−9 | Ser80 to Phe | None | None | None |
| 4220-3P | 0.125 | 2 | 0.5 | 0.125 | 4 | Glu84 to Lys | None | None | None | |||
| 4220-5P | 0.125 | 2 | 0.5 | 0.125 | 4 | Ser80 to Phe | None | None | None | |||
| 4220-7P | 0.125 | 2 | 0.5 | 0.125 | 4 | Ser80 to Tyr | None | None | None | |||
| Second-step mutants | ||||||||||||
| 4220-16-3S | 0.25 | 8 | 8 | 8 | 32 | 3.5 × 10−8 | 3.3 × 10−8 | 2.1 × 10−8 | Glu84 to Lys | None | Ser84 to Leu | None |
| 4220-16-8S | 0.5 | 8 | 4 | 8 | 32 | Glu84 to Lys | None | Ser84 to Leu | None | |||
| 4220-16-10S | 0.25 | 8 | 8 | 4 | 32 | Glu84 to Lys | None | Glu88 to Lys | None | |||
| 4220-16-21S | 0.25 | 8 | 4 | 4 | 32 | Glu84 to Lys | None | Glu88 to Lys | None | |||
| 4220-19-1S | 1 | 8 | 4 | 8 | 32 | 1.6 × 10−8 | 1.3 × 10−8 | 1.8 × 10−8 | Ser80 to Phe | None | Ser84 to Leu | None |
| 4220-19-6S | 1 | 8 | 4 | 4 | 32 | Ser80 to Phe | None | Glu88 to Lys | None | |||
| 4220-19-12S | 1 | 8 | 4 | 8 | 32 | Ser80 to Phe | None | Ser84 to Leu | None | |||
| 4220-19-23S | 1 | 8 | 4 | 4 | 32 | Ser80 to Phe | None | Glu88 to Lys | None | |||
| 4220-20-48C | 1 | 8 | 4 | 4 | 32 | Ser80 to Phe | None | Ser84 to Leu | None | |||
The strain number followed by T, C, L, P, or S designates mutant selected with trovafloxacin, ciprofloxacin, levofloxacin, pefloxacin, or sparfloxacin, respectively. The first-step mutants were selected with trovafloxacin or perfloxacin at 2× the MIC for RN4220 or with ciprofloxacin or levofloxacin at 4× the MIC for RN4220.
While the frequency of selection of first-step mutants observed with trovafloxacin at 2 to 4× the MIC was lower than that observed with ciprofloxacin and levofloxacin, all three fluoroquinolones selected mutants with changes in GrlA of topoisomerase IV (Table 1). The MICs of all of the fluoroquinolones tested except sparfloxacin were four to eight times greater for the mutants than for the parent strain, S. aureus RN4220. The most common change in the QRDR of GrlA in these mutants was the Ser80→Phe or Tyr change. Glu84→Lys and Gly78→Cys changes were also detected in some mutants. One first-step mutant (4220-5L) selected with levofloxacin had a novel single change in GrlB (Pro451→Gln), with no change in GrlA. Quinolone MICs for this mutant were comparable to those for mutants with single changes in grlA. The MICs of trovafloxacin for the first-step mutants were generally 0.125 μg/ml. The MICs of ciprofloxacin were generally 2 μg/ml, which is above the susceptibility breakpoint of 1 μg/ml for this quinolone. Alterations in GrlA alone did not appear to affect susceptibility to sparfloxacin. The MICs for the first-step mutants increased fourfold for levofloxacin and 16-fold for pefloxacin.
Second-step mutants selected from mutant 4220-16 or 4220-19 with sparfloxacin had an additional mutation in GyrA, either a Ser84→Leu change or a Glu88→Lys change (Table 1). The frequency of second-step mutations was similar to that obtained with 2 to 8× the MIC of sparfloxacin (1.3 × 10−8 to 3.5 × 10−8). Trovafloxacin was the most potent of the fluoroquinolones tested against the double mutants (MICs, 0.25 to 1.0 μg/ml). The MICs of ciprofloxacin, levofloxacin, and sparfloxacin were 4 to 8 μg/ml. No additional mutations were detected in grlB or gyrB of these second-step mutants.
Inhibition of whole-cell DNA synthesis.
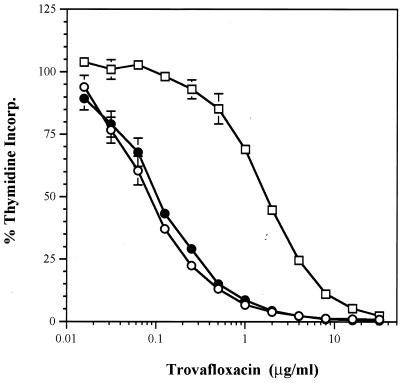
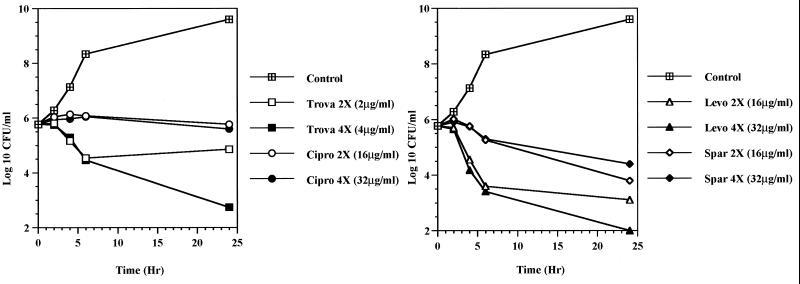
The effects of the fluoroquinolones on the synthesis of DNA were monitored by measuring [3H]thymidine incorporation in S. aureus RN4220 and the in vitro-selected mutants containing a single alteration in GrlA (S. aureus RN4220-20; Ser80 to Phe) or changes in both GrlA and GyrA (S. aureus RN4220-20-48, Ser80 to Phe in GrlA and Ser84 to Leu in GyrA). Figure 1 illustrates the incorporation of label into each clone in the presence of various concentrations of trovafloxacin. The sigmoidal shapes of the curves for inhibition of DNA synthesis observed for each clone with trovafloxacin were comparable for all of the quinolones tested. The increase in the 50% inhibitory concentration (IC50) of each quinolone for the parent and the first-step mutant containing the Ser80→Phe substitution in GrlA was less than twofold, while the MIC increased four- to eightfold with the exception of the MIC of sparfloxacin, for which no changes were noted (Table 2). In contrast, the MICs of trovafloxacin, ciprofloxacin, and levofloxacin increased eightfold and that of sparfloxacin increased 133-fold between the first- and second-step mutants, while the IC50 for inhibition of thymidine incorporation increased from 12-fold (levofloxacin) to 36-fold (sparfloxacin). The bactericidal effects of trovafloxacin, ciprofloxacin, levofloxacin, and sparfloxacin were examined against the double mutant, S. aureus RN4220-20-48. With the exception of ciprofloxacin, the quinolones at 4× the MIC decreased the viable counts of the double mutant; however, none of the quinolones were highly bactericidal at 24 h (Fig. 2). Ciprofloxacin showed only a bacteriostatic effect over 24 h, even with a test concentration of 32 μg/ml.
FIG. 1.
Inhibition of [3H]thymidine incorporation (Incorp.) into whole cells of the S. aureus RN4220 parent strain (○), the first-step mutant (mutant strain RN4220-20 [●]), and the second-step mutant (mutant strain RN4220-20-48 [□]) by trovafloxacin.
TABLE 2.
Inhibition of whole-cell [3H]thymidine incorporation in the S. aureus RN4220 parent and its derivatives
| Quinolone | RN4220 parent
|
RN4220-20 (GrlA)
|
RN4220-20-48 (GrlA and GyrA alterationsa)
|
|||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | IC50 (μg/ml) | MIC (μg/ml) | IC50 (μg/ml) | MIC (μg/ml) | IC50 (μg/ml) | |
| Trovafloxacin | 0.015 | 0.09 (0.01)b | 0.125 | 0.11 (0.02) | 1 | 1.8 (0.11) |
| Ciprofloxacin | 0.25 | 0.80 (0.1) | 1 | 1.35 (0.25) | 8 | 25.8 (5.4) |
| Levofloxacin | 0.125 | 0.47 (0.02) | 1 | 0.73 (0.14) | 8 | 8.7 (1.5) |
| Sparfloxacin | 0.063 | 0.10 (0.01) | 0.063 | 0.13 (0.02) | 8 | 4.7 (0.4) |
GrlA alteration, Ser80 to Phe; GyrA alteration, Ser84 to Leu.
Values in parentheses are standard deviations.
FIG. 2.
Killing curves for the S. aureus RN4220-20-48 double mutant (GrlA, Ser80→Phe; GyrA, Ser84→Leu) by trovafloxacin (Trova), ciprofloxacin (Cipro), levofloxacin (Levo), and sparfloxacin (Spar) at 2 or 4× the MIC of each drug.
Fluoroquinolone susceptibility of S. aureus clinical isolates containing mutations in topoisomerase genes.
In order to assess the relative activity of trovafloxacin against clinical strains of S. aureus, a collection of 29 isolates with various levels of resistance to ciprofloxacin, as characterized previously by Kaatz and Seo (24), was used. RFLP analysis identified mutations in the gyrA and/or grlA genes (Table 3). We have further characterized the mutations in these strains by PCR amplification and DNA sequence analysis of the QRDRs of their gyrA and gyrB and their grlA and grlB genes.
TABLE 3.
Quinolone susceptibilities of and topoisomerase changes in S. aureus clinical isolates
| Organism | Strain no. | Results of RFLP analysisa
|
Protein change
|
MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| grlA | gyrA | GyrA | GyrB | GrlA | GrlB | Trovafloxacin | Ciprofloxacin | Levofloxacin | Sparfloxacin | Pefloxacin | ||
| MSSA | 1081 | 79/80 | None | None | None | None | Glu422 to Asp | 0.031 | 0.25 | 0.25 | 0.125 | 0.5 |
| MSSA | 1039 | 79/80 | None | None | None | Ser80 to Phe | None | 0.125 | 1 | 1 | 0.125 | 4 |
| MSSA | 1066 | 84 | None | None | None | Glu84 to Lys | Ser410 to Pro, Glu422 to Asp | 0.125 | 2 | 0.5 | 0.25 | 4 |
| MSSA | 1035 | 79/80 | None | None | None | Ser80 to Phe | None | 0.125 | 1 | 1 | 0.25 | 8 |
| MSSA | 1249 | 79/80 | None | None | None | Ser80 to Phe | None | 0.125 | 2 | 0.5 | 0.25 | 8 |
| MSSA | 1305 | 84 | None | None | None | Glu84 to Lys | None | 0.125 | 1 | 1 | 0.25 | 8 |
| MSSA | 1134 | 79/80 | None | None | None | Ser80 to Tyr | None | 0.25 | 1 | 1 | 0.25 | 8 |
| MSSA | 872 | 84 | None | None | None | Glu84 to Lys | Glu422 to Asp | 0.125 | 1 | 0.5 | 0.125 | 4 |
| MSSA | 1642 | 79/80 | None | None | None | Ser80 to Phe | None | 0.25 | 2 | 1 | 0.25 | 8 |
| MSSA | 1275 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | His478 to Tyr | 1 | 8 | 8 | 8 | 64 |
| MSSA | 1640 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr | None | 1 | 8 | 4 | 16 | 64 |
| MSSA | 1637 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | His478 to Tyr | 4 | 32 | 16 | 32 | 128 |
| MSSA | 1628 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | Glu422 to Asp | 4 | 256 | 16 | 8 | 256 |
| MRSA | 906 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr | Glu422 to Asp | 1 | 8 | 8 | 16 | 128 |
| MRSA | 970 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | Asp432 to Val | 1 | 32 | 32 | 16 | 128 |
| MRSA | 954 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | None | 2 | 64 | 8 | 16 | 128 |
| MRSA | 1326 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr | Glu422 to Asp | 2 | 32 | 8 | 16 | 128 |
| MRSA | 1617 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | None | 2 | 32 | 8 | 16 | 128 |
| MRSA | 1623 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | None | 1 | 16 | 4 | 16 | 128 |
| MRSA | 1626 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | None | 2 | 32 | 8 | 16 | 256 |
| MRSA | 1000 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | Glu422 to Asp | 1 | 32 | 8 | 16 | 128 |
| MRSA | 1020 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | Glu422 to Asp | 2 | 128 | 16 | 16 | 128 |
| MRSA | 1629 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr | None | 1 | 8 | 8 | 16 | 128 |
| MRSA | 1630 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr | None | 1 | 16 | 4 | 8 | 64 |
| MRSA | 1627 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | None | 2 | 32 | 8 | 16 | 256 |
| MRSA | 1151 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | None | 1 | 32 | 8 | 8 | 256 |
| MRSA | 1254 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Phe | Glu422 to Asp | 8 | 256 | 32 | 32 | 256 |
| MRSA | 1643 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr | Glu422 to Asp | 8 | 64 | 32 | 32 | 128 |
| MRSA | 983 | 79/80 | 83/84 | Ser84 to Leu | None | Ser80 to Tyr, Glu84 to Lys | Glu422 to Asp | 16 | 128 | 64 | 32 | 256 |
Codon(s) in which a mutation(s) resulting in loss or acquisition of recognition sites was found.
Nine of the MSSA strains had mutations in grlA only according to RFLP analysis; all nine were highly susceptible to trovafloxacin and sparfloxacin (MICs, 0.031 to 0.25 μg/ml). With the exception of isolate 1081, all MSSA strains had a single change in GrlA (Ser80→Phe or Tyr or Glu84→Lys), as determined by PCR analysis. Six of the MSSA strains examined by PCR had additional mutations in grlB (which usually led to a Glu422→Asp change); however, these additional changes did not influence the fluoroquinolone MICs. Four of the MSSA strains and all of the MRSA strains contained a mutation in both grlA and gyrA, as identified by RFLP analysis (24) and our PCR data. While the most common substitution in GrlA was Ser80→Phe or Tyr, all of the double mutants had a Ser84→Leu substitution in GyrA. Eight MRSA strains (50%) had a third mutation in GrlB, most commonly Glu422→Asp, but, again, this appeared to have little effect on the MIC. None of the strains had mutations in GyrB. Trovafloxacin MICs were 1 to 2 μg/ml for 15 of 20 S. aureus clinical isolates with alterations in both GrlA and GyrA. For four additional strains trovafloxacin MICs were in the range of 4 to 8 μg/ml; for one mutant (MRSA 983) with a second alteration in GrlA (Glu84→Lys), the trovafloxacin MIC was 16 μg/ml.
The MICs of all of the quinolones were elevated for all of the clinical strains with alterations in both GrlA and GyrA. Ciprofloxacin MICs ranged from 8 to 256 μg/ml; those of levofloxacin were 4 to 64 μg/ml, those of sparfloxacin were 8 to 32 μg/ml, and those of pefloxacin were ≥64 μg/ml. The magnitude of the increase in the MICs among the fluoroquinolones was not strain specific, nor were specific combinations of mutations in the topoisomerases associated with the highest levels of resistance (e.g., in MSSA 1628 and MRSA 1000). These observations suggest that undetected alterations in the topoisomerases or some other gene product affected the level of resistance in these clinical strains.
Fluoroquinolone MICs in the presence of 20 μg of reserpine per ml.
The MICs for the 29 clinical S. aureus isolates were determined with and without reserpine, a known inhibitor of the NorA efflux pump in S. aureus (25). The addition of reserpine lowered the MIC of ciprofloxacin at least fourfold for 18 of 29 (62%) of the strains. Without reserpine and in the presence of reserpine, the ciprofloxacin MICs at which 90% of isolates are inhibited (MIC90s) were 64 and 4 μg/ml, respectively. In contrast, the addition of reserpine had little effect on the MICs of trovafloxacin (MIC90s, 2 and 1 μg/ml without reserpine and in the presence of reserpine, respectively) or the other fluoroquinolones tested. Reserpine lowered the ciprofloxacin MICs 8- to 16-fold for MSSA 1640, MSSA 1628, MRSA 1000, and MRSA 1020. Interestingly, a presumed reserpine-sensitive efflux system exists in the majority of clinical S. aureus in this collection, but this system contributes to resistance only to ciprofloxacin. However, this second resistance mechanism did not account for the differences in the levels of resistance to the other fluoroquinolones observed in these clinical isolates.
Decatenation and supercoiling studies with purified DNA gyrase and topoisomerase IV.
Both the gyrA and gyrB and the grlA and grlB genes were cloned from quinolone-susceptible S. aureus RN4220 and were overexpressed in E. coli. Individual subunits were purified, and the catalytic activities of the reconstituted holoenzymes were compared to those of commercial E. coli topoisomerase IV and DNA gyrase. The relative potencies of trovafloxacin, ciprofloxacin, and sparfloxacin for inhibition of topoisomerase IV decatenation and DNA gyrase supercoiling activities are shown in Table 4. All three fluoroquinolones were approximately 15-fold more potent at inhibiting E. coli DNA gyrase supercoiling activity than at inhibiting topoisomerase IV decatenation. Ciprofloxacin was slightly more potent than trovafloxacin, consistent with the MICs of both quinolones previously reported for E. coli (18, 19). While DNA gyrase was the principal target in E. coli, both trovafloxacin and ciprofloxacin were preferentially inhibitory to the decatenation activity of topoisomerase IV from S. aureus (Table 4). Sparfloxacin was more inhibitory to the activity of DNA gyrase from S. aureus, consistent with reports that have described the occurrence of first-step mutants of S. pneumoniae with mutations in gyrA upon selection with sparfloxacin (20, 30). Trovafloxacin was 1.6- and 4.6-fold more potent than ciprofloxacin and sparfloxacin, respectively, against S. aureus topoisomerase IV decatenation activity.
TABLE 4.
Sensitivities of topoisomerase IV and gyrase to fluoroquinolones
| Drug | IC50 (μM)a
|
|||
|---|---|---|---|---|
|
E. coli
|
S. aureus
|
|||
| Topoisomerase IVb | Gyrasec | Topoisomerase IV | Gyrase | |
| Trovafloxacin | 8.0 (0.53) | 0.52 (0.02) | 8.7 (0.29) | 18.8 (1.7) |
| Ciprofloxacin | 5.0 (0.18) | 0.42 (0.02) | 13.7 (1.9) | 25 (0) |
| Sparfloxacin | 9.3 (0.17) | 0.57 (0.02) | 40 (1.8) | 22 (1.5) |
IC50 drug concentration for half-maximal inhibition of catalytic activity. Values are given as means (standard errors).
Decatenation of kinetoplast DNA.
Supercoiling of relaxed pBR322.
Topoisomerase IV-mediated DNA cleavage.
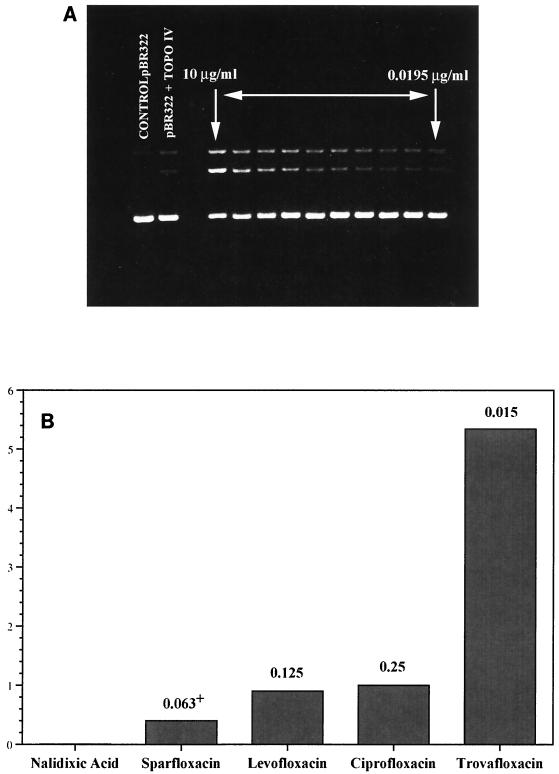
Studies have shown that the bactericidal activities of fluoroquinolones result from their stabilization of the cleavable complex formed between topoisomerases and DNA (7, 18). Since the data in Tables 1 and 4 indicate that topoisomerase IV is the primary target of trovafloxacin in S. aureus, trovafloxacin was tested for its relative potency at inducing topoisomerase IV-mediated DNA cleavage (Fig. 3). In these tests, the lowest concentration of drug that stimulated enzyme-mediated DNA cleavage was determined. Trovafloxacin induced DNA cleavage with the enzyme in a dose-proportional manner at drug concentrations of >0.078 μg/ml. When the relative cleavage-enhancing potency of trovafloxacin was compared to those of the other quinolones, it was found to be approximately five times more potent than ciprofloxacin, levofloxacin, and sparfloxacin, consistent with its lower MICs in vitro for S. aureus RN4220 (Table 1; Fig. 3).
FIG. 3.
Stimulation of topoisomerase IV (TOPO IV)-mediated DNA cleavage by trovafloxacin (A). Trovafloxacin concentrations of 0.0195, 0.039, 0.078, 0.156, 0.313, 0.625, 1.25, 2.5, 5.0, and 10 μg/ml were tested. (B) Relative potency (that of ciprofloxacin is given as a value of 1) of trovafloxacin for stimulation of topoisomerase IV-mediated DNA cleavage. The lowest concentration of drug required to stimulate enzyme-mediated DNA cleavage was determined by densitometric scanning of photographs of ethidium bromide-stained agarose gels. The numbers above the bars are MICs for S. aureus RN4220.
Cleavage tests with topoisomerase IV containing alterations in GrlA.
In cleavage tests with S. aureus topoisomerase IV containing an alteration in GrlA (Glu84→Lys), trovafloxacin was 3, 9, 18, and 27 times more potent than ciprofloxacin, levofloxacin, sparfloxacin, and pefloxacin, respectively (Table 5). Topoisomerase IV from a second construct that contained the GrlA change of Ser80 to Phe was similarly less sensitive to inhibition by all of the fluoroquinolones tested. In these experiments trovafloxacin was more potent than the other compounds at inducing topoisomerase IV-mediated DNA cleavage with both wild-type S. aureus topoisomerase IV and enzymes containing alterations in GrlA that are associated with increased fluoroquinolone MICs.
TABLE 5.
Cleavage with parent and mutant S. aureusa topoisomerase IV (GrlA alteration)
| Strain | Minimum concn (μM) of quinolone required to induce cleavage with topoisomeraseb IV
|
||||
|---|---|---|---|---|---|
| Trovafloxacin | Ciprofloxacin | Levofloxacin | Sparfloxacin | Pefloxacin | |
| S. aureus parent | 0.036 | 0.16 | 0.62 | 0.28 | 1.18 |
| 4220-19 (GrlA; Phe80) | 0.99 | 3.9 | 15 | 11.25 | 35 |
| 4220-16 (GrlA; Lys84) | 1.11 | 3.3 | 10 | 11.7 | 30 |
Mutant 4220-19, Ser80 to Phe; mutant 4220-16, Glu84 to Lys.
Values are means of multiple assays; standard deviations are ≤20% of the mean.
Radiolabeled DNA cleavage patterns with trovafloxacin and sparfloxacin.
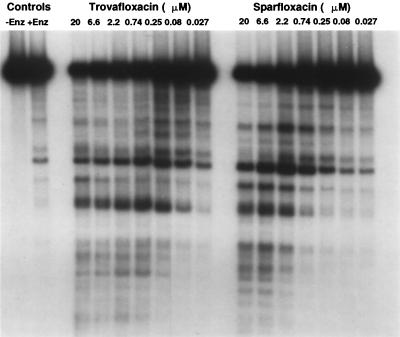
Previous studies with quinolone-resistant mutants of S. pneumoniae have indicated that sparfloxacin preferentially targets DNA gyrase rather than topoisomerase IV. We conducted studies with trovafloxacin and sparfloxacin using wild-type S. aureus topoisomerase IV and a linear pBR322 substrate that was end labeled with 32P. The cleavage patterns from the autoradiograph shown in Fig. 4 reflect similar banding patterns for the two quinolones. The drug concentrations that produced the most intense cleavage banding patterns were 0.25 and 2.2 μM for trovafloxacin and sparfloxacin, respectively. At these respective concentrations, the number of DNA fragments obtained was similar for both quinolones, although differences in the intensities of some cleavage bands are evident. Both fluoroquinolones induce marked DNA cleavage with topoisomerase IV from S. aureus RN4220. Any differences in the radiolabeled cleavage pattern appear to be due to the greater relative potency of trovafloxacin against this enzyme rather than to differences in cleavage site specificity.
FIG. 4.
Pattern of cleavage of 32P-end-labeled pBR322 DNA by S. aureus topoisomerase IV in the presence of trovafloxacin or sparfloxacin. Enz, enzyme.
DISCUSSION
The available literature indicates that some new fluoroquinolones including trovafloxacin, sparfloxacin, gatifloxacin, moxifloxacin, and clinafloxacin have improved in vitro potencies against ciprofloxacin-resistant gram-positive cocci (3, 8, 18, 19, 41). In the case of S. aureus, the new fluoroquinolones have MICs of ≤1 μg/ml for some ciprofloxacin-resistant strains, but they are not very active against those strains that are highly resistant to ciprofloxacin (32, 33, 41). Trovafloxacin is 8- to 16-fold more potent than ciprofloxacin against quinolone-susceptible staphylococci (5, 8, 19, 33) and has been reported to have MIC90s in the range of 1 to 4 μg/ml for ciprofloxacin-resistant S. aureus strains (MICs, ≥32 μg/ml).
The goal of this study was to characterize the improved potency of trovafloxacin against susceptible S. aureus RN4220 as well as first- and second-step mutants selected from this strain in vitro with a number of quinolones. The natures of the mutations found in these selected mutants were compared with those present in a previously characterized set of S. aureus clinical isolates with various levels of resistance to ciprofloxacin (24).
In agreement with previously published studies, trovafloxacin was 16-fold more potent than ciprofloxacin against the susceptible S. aureus RN4220 strain. It was fourfold more potent than the next most active quinolone, sparfloxacin. In direct plating experiments with RN4220, trovafloxacin at 2 to 4× the MIC selected first-step mutants less frequently (1.1 × 10−10) than ciprofloxacin or levofloxacin did. This lower frequency of resistance selection with trovafloxacin in S. aureus compared with that with ciprofloxacin has been detected by others (1, 9). This observation may have clinical significance, since trovafloxacin blood levels following the administration of doses of 200 to 300 mg in humans (3 to 4.5 μg/ml) remain well above the MIC for quinolone-susceptible S. aureus over the entire 24-h dosing period (5, 38).
When the alterations in the QRDRs of GrlA and GyrA in the first-step mutants selected with trovafloxacin, ciprofloxacin, levofloxacin, or pefloxacin were characterized, the most common changes were only in GrlA; these consisted of substitution at Ser80 of Phe or Tyr. One mutant had a novel change in GrlB (Pro451→Gln), which represented a CCA nucleotide change to CAA. A change in this residue to Ser (TCA) has been reported recently (32). No GyrA changes were detected in these first-step mutants. The MICs of these quinolones increased four- to eightfold for the first-step mutants, suggesting that the changes in GrlA and GrlB were responsible for resistance. Direct genetic evidence provided in two studies indicates that topoisomerase IV mutations result in increased MICs of quinolones (29, 42). Our data also suggest that topoisomerase IV is the primary target of these fluoroquinolones in S. aureus. This conclusion is also supported by the greater sensitivity of S. aureus topoisomerase IV decatenation activity to trovafloxacin compared with the sensitivity of gyrase supercoiling activity (Table 4). While sparfloxacin appeared to preferentially target S. aureus DNA gyrase in the catalytic assays, it was difficult to select first-step mutants with this compound. The small-colony variants obtained following selection with sparfloxacin grew slowly and were not analyzed for their susceptibility or for the presence of mutations in the topoisomerase QRDR.
Interestingly, we were able to select second-step mutants with sparfloxacin from two first-step mutants containing either a Glu84→Lys or a Ser80→Phe change in GrlA. All of the second-step mutants obtained with sparfloxacin had a single amino acid change in GyrA, either Ser84→Leu or Glu88→Lys. Similar results were obtained by Yamagishi et al. (42), who found that S. aureus RN4220 containing mutations in grlA had a 100-fold higher frequency of mutation to high-level sparfloxacin resistance than did RN4220 without changes in grlA. Those investigators suggested that mutations in grlA predispose cells to selection of second-step mutations in gyrA (Ser84→Leu). It is noteworthy that all of the first-step mutants remained highly susceptible to trovafloxacin (MICs, ≤0.125 μg/ml), and for all eight second-step mutants trovafloxacin MICs were 0.25 to 1.0 μg/ml.
While the enzymatic and mutational assay results appear to be conflicting, it may be that sparfloxacin equally targets both gyrase and topoisomerase IV in S. aureus. Alternatively, biochemical data with cell-free enzymes may be providing misleading information concerning identification of the primary site of mutation in the whole cell. In our study, sparfloxacin was less active at inducing cleavage with topoisomerase IV obtained from two mutant grlA alleles. This is in contrast to the lack of increase in the MIC of sparfloxacin for the corresponding mutant clones containing these changes. It is noteworthy that Blanche et al. (2) found sparfloxacin to be twofold more potent against S. aureus topoisomerase IV decatenation activity than against gyrase supercoiling activity. Controversy over the identity of the principal target of sparfloxacin in S. pneumoniae has recently been noted as well (15, 16, 28, 30).
In order to further understand the role that individual mutations play in conferring resistance to fluoroquinolones, S. aureus RN4220 and two mutant clones were evaluated for their susceptibilities to inhibition of DNA synthesis. The data given in Fig. 1 and Table 2 demonstrate that the single alteration in GrlA (Ser80→Phe) increased the MICs of ciprofloxacin, trovafloxacin, and levofloxacin four- to eightfold, while the IC50 for thymidine incorporation increased less than twofold. Neither the MIC nor the IC50 of sparfloxacin changed for the grlA mutant, lending further support to the hypothesis that gyrase is the more sensitive target of this fluoroquinolone in S. aureus. Both the MICs and the IC50s of all four quinolones were markedly increased for the second-step mutant containing an alteration in GyrA (Ser84→Leu). While the IC50 for inhibition of DNA synthesis paralleled the MIC, the IC50 of ciprofloxacin was over three times higher than the MIC for the double mutant. In bactericidal studies, ciprofloxacin at 2 to 4× the MIC showed no killing of this mutant; these drug concentrations approximated the IC50 for DNA synthesis inhibition. Trovafloxacin and levofloxacin demonstrated some killing at 4× the MIC, while the viable count of the double mutant exhibited only a slow decrease with high concentrations of sparfloxacin. These data are consistent with the notion first presented by Goss et al. (21) in studies with E. coli. They indicated that concentrations of quinolones sufficient for inhibition of DNA synthesis may not be rapidly lethal to the cell. More recently, it has been proposed that fluoroquinolone concentrations above those required to halt DNA replication fork progression around the chromosome are required to cause cell death (7, 18). An as yet unidentified component appears to be required in E. coli in order to produce lethal DNA strand breaks following arrest of the replication fork after it collides with the quinolone-stabilized complex of DNA and topoisomerase (7, 18). Our data from studies with S. aureus are consistent with this hypothesis.
Trovafloxacin and the other fluoroquinolones were also tested against a collection of 29 clinical S. aureus isolates evaluated previously by RFLP analysis for the presence of mutations in gyrA and grlA (24). When we obtained sequence data for the QRDRs of gyrA, gyrB, grlA, and grlB, trovafloxacin MICs for strains of MSSA with a mutation in grlA only were 0.125 or 0.25 μg/ml (Table 3). This level of susceptibility to trovafloxacin in nine of the MSSA strains examined equaled that observed in the first-step mutants selected from RN4220; the same GrlA changes were also noted in both groups of strains. As observed in previous studies (32), a second mutation in grlB that provides a Glu422-to-Asp change in GrlB did not appear to influence the MICs for the grlA mutants. Although they were from different geographic sources, four of the MSSA clinical isolates and all of the MRSA clinical isolates had an additional alteration in GyrA (all Ser84→Leu). For nine strains, this increased the trovafloxacin MIC an additional four- to eightfold (MICs, 1 μg/ml), as was observed for the second-step mutants derived from RN4220. For some of these strains, the ciprofloxacin MIC was 32 μg/ml. Again, even in the presence of changes in both GrlA and GyrA, a third alteration in GrlB did not appear to influence the MIC. In a comparison of the mutation profiles of strains such as MSSA 1275, MSSA 1637, MSSA 1628, and MRSA 1623, another determinant(s) of resistance appeared to affect the MICs of the fluoroquinolones. These may be mutations that occur outside of the QRDR that we examined or in some unrelated determinant. The strain for which the trovafloxacin MIC was the highest, MRSA 983 (16 μg/ml), had two changes in GrlA (Ser80→Tyr and Glu84→Lys) as well as the Ser84→Leu change in GyrA. Our results differ slightly from those of Fitzgibbon et al. (12), in that for some strains with single mutations in both grlA and gyrA trovafloxacin MICs were as high as 8 μg/ml. The previous study found that at least three mutations were required (two in grlA and one in gyrA) in order to reach this level of resistance to trovafloxacin. Our data suggest that other mutations may exist in these clinical isolates, adding to the effects of those documented in the topoisomerases. When MICs were redetermined in the presence of reserpine, ciprofloxacin MICs were fourfold or more lower for several strains, but no significant changes in the MICs of the other quinolones were observed. Reserpine is a known inhibitor of the NorA efflux pump in S. aureus, and it is known to decrease the MICs of ciprofloxacin for NorA-overproducing strains (25). Recent studies have shown that the MICs of some of the new quinolones are not affected by a reserpine-sensitive efflux pump in S. pneumoniae, presumably due to their more hydrophobic properties (4). Our data would support this for S. aureus as well.
Since trovafloxacin is more potent in vitro against both quinolone-sensitive and -resistant S. aureus strains than the other quinolones tested, studies were performed with purified DNA gyrase and topoisomerase IV obtained from RN4220. Inhibition of catalytic activity and first-step mutant selection results indicated that topoisomerase IV is the primary target of trovafloxacin in S. aureus. Formation of a fluoroquinolone-stabilized cleavable complex between topoisomerase and DNA is the initial step in the cessation of cellular DNA replication, which subsequently leads to cell death (7, 18). Trovafloxacin was shown to be up to five times more potent than ciprofloxacin or levofloxacin for the stimulation of topoisomerase IV-mediated DNA cleavage with the S. aureus enzyme. It was also 3, 9, 18, and 27 times more potent than ciprofloxacin, levofloxacin, sparfloxacin, and pefloxacin, respectively, in cleavage tests with topoisomerase IV containing a Glu-to-Lys change in GrlA. A similar relative potency was observed with enzyme containing a Ser-to-Phe change in GrlA. These data agree with those in Table 1 indicating that either change increases the MICs of the quinolones tested to approximately the same degree. The lower MICs of trovafloxacin obtained for many strains of S. aureus are likely due to this greater intrinsic potency against the topoisomerase IV lethal target. This may represent only a quantitative difference, since the qualitative DNA banding patterns observed between trovafloxacin and sparfloxacin (which appears to target the DNA gyrase) were similar when the S. aureus topoisomerase IV and a linear, end-labeled DNA substrate were used. Identification of any mechanistic differences between trovafloxacin and other quinolones will await additional studies with the purified enzyme.
While it is true that trovafloxacin is more potent than older fluoroquinolones against many strains of S. aureus, data from studies with our clinical strains indicate that an as yet undetected mutation exists in some strains and that this mutation makes them resistant to trovafloxacin. In this sense, the newer fluoroquinolones such as trovafloxacin cannot be used empirically for the treatment of serious infections caused by S. aureus until the MICs of a specific agent are determined. It is clear that class susceptibility testing with ciprofloxacin will not suffice for this purpose due to the incomplete cross-resistance that has been observed.
ACKNOWLEDGMENTS
We thank G. Kaatz for supplying the collection of 29 clinical isolates used in the study. We also thank K. Brighty for helpful comments in regard to the manuscript.
REFERENCES
- 1.Barry A L, Brown S D, Fuchs P C. In vitro selection of quinolone-resistant staphylococcal mutants by a single exposure to ciprofloxacin or trovafloxacin. J Antimicrob Chemother. 1996;38:324–327. doi: 10.1093/jac/38.2.324. [DOI] [PubMed] [Google Scholar]
- 2.Blanche F, Cameron B, Bernard F-X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II topoisomerases. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla H F, Zarins L T, Bradley S F, Kauffman C A. Susceptibility of ciprofloxacin-resistant staphylococci and enterococci to trovafloxacin. Diagn Microbiol Infect Dis. 1996;26:17–21. doi: 10.1016/s0732-8893(96)00146-0. [DOI] [PubMed] [Google Scholar]
- 4.Brenwald N P, Gill M J, Wise R. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2032–2035. doi: 10.1128/aac.42.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brighty K, Gootz T. Chemistry and biological profile of trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):1–14. doi: 10.1093/jac/39.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 6.Brockbank S M V, Barth P T. Cloning, sequencing, and expression of the DNA gyrase genes from Staphylococcus aureus. J Bacteriol. 1993;175:3269–3277. doi: 10.1128/jb.175.11.3269-3277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliopoulos G M, Klimm K, Eliopoulos C T, Ferraro M J, Moellering R C., Jr In vitro activity of CP-99,219, a new fluoroquinolone, against isolates of gram-positive bacteria. Antimicrob Agents Chemother. 1993;37:366–370. doi: 10.1128/aac.37.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans M E, Titlow W B. Selection of fluoroquinolone-resistant methicillin-resistant Staphylococcus aureus with ciprofloxacin and trovafloxacin. Antimicrob Agents Chemother. 1998;42:727. doi: 10.1128/aac.42.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrero L, Cameron B, Manse B, Lagneau D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgibbon J E, John J F, Delucia J L, Dubin D T. Topoisomerase mutations in trovafloxacin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2122–2124. doi: 10.1128/aac.42.8.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier B, Hooper D C. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob Agents Chemother. 1998;42:121–128. doi: 10.1128/aac.42.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke A E, Danley D E, Kaczmarek F S, Hawrylik S J, Gerard R D, Lee S E, Geoghegan K F. Expression of human plasminogen activator inhibitor type-1 (PAI-1) in E. coli as a soluble protein comprised of active and latent forms. Biochim Biophys Acta. 1990;1037:16–27. doi: 10.1016/0167-4838(90)90096-x. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410–412. doi: 10.1128/aac.43.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George J, Morrissey I. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Activity of levofloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against DNA gyrase from S. pneumoniae C3LN4, abstr. C-176; p. 120. [Google Scholar]
- 17.Gootz T, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer R J. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. doi: 10.1128/aac.40.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gootz T D, Brighty K E. Chemistry and mechanism of action of the quinolone antibacterials. In: Andriole V T, editor. The quinolones. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1998. pp. 29–80. [Google Scholar]
- 19.Gootz T D, Brighty K E, Anderson M R, Schmeider B J, Haskell S L, Sutcliffe J A, Castaldi M J, McGuirk P R. In vitro activity of CP-99,219, a novel 7-(3-azabicyclo[3.1.0]hexyl) naphthyridone antimicrobial. Diagn Microbiol Infect Dis. 1994;19:235–243. doi: 10.1016/0732-8893(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 20.Gootz T D, Haskell S, Zaniewski R. Program and abstracts of the 8th European Congress of Clinical Microbiology and Infectious Diseases. 1997. Comparison of ciprofloxacin, trovafloxacin, and sparfloxacin for selecting topoisomerase II mutations in Streptococcus pneumoniae, abstr. P315; p. 68. [Google Scholar]
- 21.Goss W A, Dietz W H, Cook T M. Mechanism of action of nalidixic acid on Escherichia coli. J Bacteriol. 1965;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guirard B M, Snell E E. Biochemical factors in growth. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. p. 95. [Google Scholar]
- 23.Hallett P, Grimshaw A J, Wigley D B, Maxwell A. Cloning of the DNA gyrase genes under tac promoter control: overproduction of gyrase A and B proteins. Gene. 1990;93:139–142. doi: 10.1016/0378-1119(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 24.Kaatz G, Seo S. Topoisomerase mutations in fluoroquinolone-resistant and methicillin-susceptible and -resistant clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:197–198. doi: 10.1128/aac.42.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaatz G, Seo S, Ruble C. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leggiadro R J. The clinical impact of resistance in the management of pneumococcal disease. Infect Dis Clin N Am. 1997;11:867–874. doi: 10.1016/s0891-5520(05)70394-9. [DOI] [PubMed] [Google Scholar]
- 27.Margerrison E E C, Hopewell R, Fisher L M. Nucleotide sequencing of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J Bacteriol. 1992;174:1596–1603. doi: 10.1128/jb.174.5.1596-1603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrissey I, George J T. Program and abstracts of the 6th International Symposium on New Quinolones. 1998. The unique equipotency of sitafloxacin against topoisomerase IV and DNA gyrase from Streptococcus pneumoniae; p. 55. [Google Scholar]
- 29.Ng E Y, Truckiss M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan X-S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Schmitz F-J, Jones M E, Hofman B, Hansen B, Scheuring S, Lückefahr M, Fluit A, Verhoef J, Hadding V, Heinz H-P, Köhrer K. Characterization of grlA, grlB, gyrA, and gyrB mutations in 116 unrelated isolates of Staphylococcus aureus and effects of mutations on ciprofloxacin MIC. Antimicrob Agents Chemother. 1998;42:1249–1252. doi: 10.1128/aac.42.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sefton A M, Maskell J P, Rafay A M, Whiley A, Williams J D. The in vitro activity of trovafloxacin, a new fluoroquinolone, against gram-positive bacteria. J Antimicrob Chemother. 1997;39(Suppl. B):57–62. doi: 10.1093/jac/39.suppl_2.57. [DOI] [PubMed] [Google Scholar]
- 34.Spangler S K, Jacobs M R, Appelbaum P C. Activity of CP-99,219 compared with those of ciprofloxacin, grepafloxacin, metronidazole, cefoxitin, piperacillin, and piperacillin-tazobactam against 489 anaerobes. Antimicrob Agents Chemother. 1994;38:2471–2476. doi: 10.1128/aac.38.10.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sreedharan S, Oram M, Jensen B, Peterson L, Fisher L M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990;172:7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahata M, Yonezawa M, Kurose S, Futakuchi N, Matsubara N, Watanabe Y, Narita H. Mutations in the gyrA and grlA genes of quinolone-resistant clinical isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1996;38:543–546. doi: 10.1093/jac/38.3.543. [DOI] [PubMed] [Google Scholar]
- 37.Takenouchi T, Ishii C, Sugawara M, Yokue Y, Ohya S. Incidence of various gyrA mutants in 451 Staphylococcus aureus strains isolated in Japan and their susceptibilities to 10 fluoroquinolones. Antimicrob Agents Chemother. 1995;39:1414–1418. doi: 10.1128/aac.39.7.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng R, Liston T E, Harris S C. Multiple-dose pharmacokinetics and safety of trovafloxacin in healthy volunteers. J Antimicrob Chemother. 1996;37:955–963. doi: 10.1093/jac/37.5.955. [DOI] [PubMed] [Google Scholar]
- 39.Thornsberry C, Ogilvie P, Kahn J, Mauriz Y. Surveillance of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States in 1996–1997 respiratory season. Diagn Microbiol Infect Dis. 1997;29:249–257. doi: 10.1016/s0732-8893(97)00195-8. [DOI] [PubMed] [Google Scholar]
- 40.Visalli M A, Jacobs M R, Appelbaum P C. Activity of trovafloxacin compared with ciprofloxacin, sparfloxacin, clinafloxacin, lomefloxacin, and cefuroxime against ten penicillin-susceptible and penicillin-resistant pneumococci by time-kill methodology. J Antimicrob Chemother. 1996;37:77–84. doi: 10.1093/jac/37.1.77. [DOI] [PubMed] [Google Scholar]
- 41.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamagishi J, Kojima T, Oyamada Y, Fujimoto K, Hattori H, Nakamura S, Inoue M. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1157–1163. doi: 10.1128/aac.40.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]